Abstract
Linker for activation of T cells (LAT) is a transmembrane adaptor protein that links T cell receptor (TCR) engagement to downstream signaling events. While it is clear that LAT is essential in thymocyte development and initiation of T cell activation, its function during T cell expansion, contraction, and memory formation remains unknown. To study the role of TCR-mediated signaling in CD8 T cells during the course of pathogen infection, we used an inducible mouse model to delete LAT in antigen-specific CD8 T cells at different stages of Listeria infection and analyzed the effect of deletion on T cell responses. Our data showed that LAT is important for maintaining CD8 T cell expansion during the priming phase; however, it is not required for CD8 T cell contraction and memory maintenance. Moreover, LAT deficiency accelerates memory differentiation during the effector-to-memory transition, leading to a higher frequency of KLRG1lowIL-7RhighCD62Lhigh memory T cells. Nonetheless, these LAT-deficient memory T cells were unable to proliferate or produce cytokines upon secondary infection. Our data demonstrated that, while it is dispensable for contraction and memory maintenance, TCR-mediated signaling regulates CD8 T cell memory differentiation and is essential for the memory response against pathogens.
INTRODUCTION
Due to their ability to self-renew and differentiate into effector cells upon antigen re-exposure, memory CD8 T cells are essential to mounting effective immune responses against pathogen infections. After an initial pathogen infection, naïve CD8 T cells undergo a three-phase response comprised of expansion, contraction, and memory formation (1). Upon recognition of MHC class I-peptide complexes, antigen-specific CD8 T cells proliferate rapidly and acquire effector functions that are essential to the elimination of pathogen-infected cells. Following pathogen clearance, the majority of CD8 T cells undergo contraction by apoptosis; however, a small subset (5–10%) survives and converts into memory precursors. These precursor cells eventually become long-lived memory T cells that are able to rapidly respond to infection by the same pathogen.
The differentiation of memory CD8 T cells is a process during which the phenotypic and functional properties of memory T cells are acquired over time(2). After initial pathogen infection, activated CD8 T cells consist of a heterogeneous population that includes short-lived effector cells (SLECs: KLRG1highIL-7Rlow) and long-lived memory precursor cells (MPECs: KLRG1lowIL-7Rhigh)(3, 4). The fate of a particular cell to become a SLEC or MPEC is determined by the amount of inflammatory cytokines, transcriptional regulators, metabolic switches, and the strength of TCR signals (1). As MPECs develop into memory CD8 T cells, they fall into one of two subsets based on the expression of lymph node homing molecules: central memory T cells (TCM: CD62L+ CCR7+) and effector memory T cells (TEM: CD62L− CCR7−). It is thought that tissue–resident TEM cells provide effector function at the portal of pathogen entry, and TCM cells serve as the stem cell-like population that maintain lifelong immunological memory.
Engagement of the T cell receptor (TCR) with MHC molecules leads to activation of tyrosine kinases, such as Lck and ZAP-70 and phosphorylation of LAT and other signaling proteins. LAT is a transmembrane adaptor protein that is phosphorylated by ZAP-70 (5). Upon phosphorylation, it interacts with Grb2, Gads, and PLC-γ1 directly and SLP-76 indirectly to activate downstream signaling cascades. Despite the essential role of TCR signaling pathway in the activation of naïve T cells, published data indicate that TCR-mediated signaling seems to play different roles in memory T cells. For example, although naïve T cells require tonic TCR signaling for long-term survival (6, 7), maintenance of memory CD8 T cells is independent of persistent TCR-MHC engagement (8). Interestingly, the generation and maintenance of CD8 and CD4 memory T cells are still observed in MHC class I- and MHC class II-deficient mice, respectively (9, 10) Moreover, deletion of the TCR or essential signaling molecules, such as Lck and SLP-76, does not seem to impair the persistence of memory T cells (11–13). Increased frequencies of MPECs and TCM cells were observed when SLP-76 signaling was attenuated (13). How LAT functions in memory T cells has not been studied. Since LAT is essential in coupling TCR engagement to activation of downstream signaling events, such as Ras-MAPK activation and calcium flux(14), understanding the role LAT in CD8 memory T cells is essential for us to fully understand how TCR-mediated signaling regulates memory T cell differentiation and function.
In this study, we investigate the function of LAT in CD8 T cell responses following Listeria monocytogenes (Lm-Ova) infection. We performed a mixed adoptive transfer of wildtype and LAT-floxed OT-I TCR transgenic CD8 T cells and deleted LAT at different time points after infection by tamoxifen injection to assess the requirement of LAT in CD8 T cell priming, contraction, differentiation, maintenance, and memory response. Here, we show that LAT was essential for optimal CTL expansion during the priming phase, but was not required for contraction and memory maintenance. Moreover, deletion of LAT during the effector-to-memory transition led to the development of a higher frequency of memory precursor cells and consequently accelerated memory differentiation. Our data also indicated that LAT is necessary for memory CD8 T cells to proliferate and produce cytokines upon antigen re-exposure.
MATERIALS & METHODS
Mice
LAT−/− and LATf/f mice were generated as previously described (15, 16). LAT−/− mice were crossed with ERCre transgenic mice (kindly provided by Dr. Thomas Ludwig, Columbia University, New York, NY) to produce ERCre+LAT−/− mice. LATf/f mice were crossed with OT-I transgenic mice (Jackson lab, Bar Harbor, ME) to generate OT-I/LATf/f mice, which were then mated with ERCre+LAT−− mice to obtain ERCre+LATf/−OT-I or LATf/−OT-Imice. These mice have been backcrossed onto the C57/Bl6 background for more than ten generations. All mice were used in accordancewith the NIH guidelines. The proceduresin this study were approved by the DukeUniversity IACUC. Micewere housed in a specific pathogen-free facility.
Tamoxifen injection, adoptive transfer and infection
To induce the floxed lat deletion, mice received tamoxifen (10mg/ml in corn oil, 60 mg/kg body weight) intraperitoneally for two consecutive days and once every other week after the first two injections. For adoptive transfer of naïve OT-I cells, the indicated numbers (1×105 in T cell expansion experiments and 1×104 in other experiments including contraction, memory differentiation, maintenance, and secondary response) of WT and LATKO OT-I cells with different congenic markers were adoptively transferred into non-irradiated naïve recipient mice (CD45.1+ CD45.2+) at day 0. One day after OT-I cell transfer (day 1), recipient mice were intravenously injected with 1.5×104 CFU Listeria monocytogenes expressing ovalbumin (Lm-Ova). To induce a secondary response, mice were injected with 1.5×105 CFU Lm-Ova. For BrdU incorporation, mice received 1mg of BrdU (5mg/ml) intraperitoneally at day 6 and splenocytes were analyzed after 24 h. Single cell suspensions were stained with antibodies against CD8, CD45.1, and CD45.2, and BrdU incorporation was examined using a BrdU Flow kit (BD).
FACS analysis
All of the antibodies used for FACS were purchased from eBioscience or BioLegend. To detect the percentage of OT-I T cells among total CD8 T cells, single cell suspensions were prepared from spleens or blood after lysing red blood cells with ACK lysis buffer. Cells were stained with PE-Cy7-anti-CD8, APC-Cy7-anti-CD45.1, Pacific Blue-anti-CD45.2, and 7AAD. To characterize the phenotypes of OT-I cells, they were further stained with PE- or APC-conjugated antibodies, such as PE- anti-IL7R, CD62L, CD122, and APC-anti-KLRG1, CXCR3, or TCRβ. To detect transcription factors or intracellular proteins, cells were first stained with surface markers, fixed and permeabilized using the Cytofix/Cytoperm kit (BD), and then stained with APC-anti-T-bet, perforin, or Bcl-2 and PE-anti-Eomes or anti-Ki-67. For intracellular staining of cytokines, splenocytes were re-stimulated with 1 μM Ova peptide (Ova257–264: SIINFEKL) for 5 h in the presence of Golgi-stop, fixed, permeabilized, and stained with anti-CD8, anti-CD45.1/CD45.2, as well as APC-anti-IFN-γ and PE-anti-TNF-α or anti-IL2. Samples were analyzedon FACSCanto II (BD Biosciences). FACS plots shownwere analyzed with Flowjo (Ashland, OR).
Calcium mobilization
Splenocytes were loaded with 2.5 μg/ml Indo-1 acetoxymethyl ester at 30 °C for 30 minutes and then stained with APC-Cy7-anti-CD8, PE-Cy7-anti-CD45.1 and PE-anti-CD45.2 antibodies. Calcium flux was initiated by the addition of anti-CD3-biotin (5 μg/ml) and anti-CD8-biotin antibodies (1 μg/ml) followed by crosslinking with streptavidin (25 μg/ml). Calcium flux was determined by monitoring the fluorescence emission ratio at 405/495 nm.
Statistical analyses
For all experiments, differences between groups were examined for statistical significance by the unpaired two-tailed Student’s t tests to assess data using Prism (GraphPad Software).
RESULTS
Inducible LAT deletion system
To study the function of LAT in CD8 T cells during in vivo immune responses against pathogens, we utilized LAT conditional knockout mice (LATf/f) to inducibly delete the lat gene in mature T cells at different stages of the immune response. In LATf/f mice, the functional exons 7–11 of the lat gene are flanked by two Loxp sites. Cre-mediated deletion results in the expression of a non-functional LAT-GFP fusion protein, thus accurately marking T cells with the lat gene deleted (16). We crossed ERCreLATf/f, OT-I TCR transgenic, and LAT−/− mice to generate ERCre+LATf/−OT-Imice in which only one floxed allele needs to be deleted (17). With tamoxifen treatment, the lat gene can be inducibly deleted in OT-I cells in vivo. LATf/−OT-I mice were used as controls. For simplicity, “LATKO” will be used to represent OT-I cells from ERCre+LATf/−OT-I mice and “WT” used to describe OT-I cells fromLATf/−OT-I controls after tamoxifen administration. The deletion of lat in OT-I cells was proven to be very efficient in our previous publication (17). Two days after tamoxifen injection, more than 95% of LATKO cells are GFP positive. Furthermore, the expression of LAT protein in these cells is undetectable by Western blotting and immunofluorescence 3–4 days following tamoxifen administration (17).
LAT mediated-signaling is essential for optimal CD8 T cell expansion
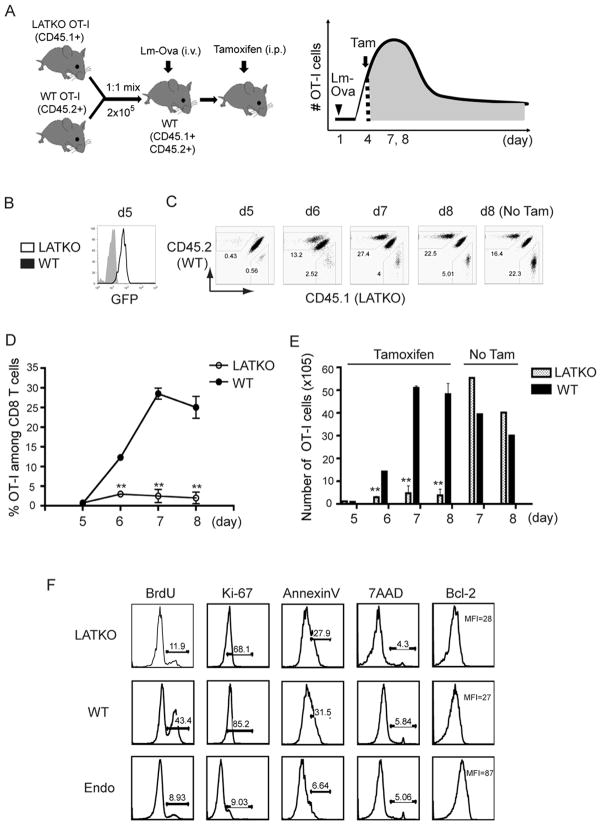
We previously demonstrated that LAT is required for the activation of naïve T cells (18); however, whether continuous LAT mediated-signaling during T cell priming phase is needed for optimal T cell expansion remains unclear. To assess the role of LAT in CD8 T cell proliferation upon pathogen infection, a dual adoptive transfer experiment followed by Listeria monocytogenes (Lm-Ova) infection was carried out (Figure 1A). A mixture of 1×105 naïveLATf/− OT-I cells (CD45.2+) and 1×105 naïve ERCre+LATf− OT-I cells (CD45.1+) were transferred into wildtype (CD45.1+ CD45.2+) mice (day 0). On the following day (day 1), these recipients were infected intravenously with 1.5×104 CFU Lm-Ova. To delete the floxed lat gene, recipient mice were treated with tamoxifen intraperitoneally starting at day 4 for two consecutive days. CD8 T cell expansion was monitored at day 5, 6, 7, and 8 post-infection. As shown in Figure 1B, more than 95% of LATKO T cells were GFP+ one day after tamoxifen injection as we described previously (17). The percentages of LATKO and WT OT-I T cells among total splenic CD8 T cells were similar at day 5. By day 6, the CD8 T cell population was composed of 13% WT T cells and only 2.5% LATKO T cells (Figure 1B). The difference in percentages between WT and LATKO OT-I cells was more drastic by day 7 and 8. Whereas the number of WT OT-I cells increased ~60-fold from day 5 to day 7, LATKO OT-I cells only expanded 7-fold (Figure 1C, D). Recipient mice without tamoxifen treatment showed comparable expansion of WT and LATKO OT-I cells (Figure 1C, E). These data demonstrate that the expansion of CD8 T cells following Lm-Ova infection is severely perturbed in the absence of LAT.
Figure 1. LAT in the expansion of CD8 T cells upon Lm-Ova infection.
(A) A schematic diagram of the experimental procedure. A mixture of 1×105 WT OT-I cells (CD45.2+) and 1×105 LATKO OT-I cells (CD45.1+) were adoptively transferred into WT (CD45.1+ CD45.2+) recipient mice. On the following day, these recipients were infected intravenously withLm-Ova at day 1. The mice then received tamoxifen to delete lat on day 4 and 5 during the T cell expansion phase. (B) Deletion efficiency. GFP expression in WT and LATKO cells were examined on day 5. Flow cytometry of WT cells is gated live CD8+CD45.2+ and LATKO cells is gated live CD8+ CD45.1+. (C) CD8 T cell expansion. Live CD8 T cells in the spleen were analyzed at the indicated times after infection. Numbers on the plots indicate the percentage of LATKO (CD45.1+) and WT (CD45.2+) OT-I cells in the total CD8 T cell population. (D) The percentages of OT-I cells among total CD8 T cells in the spleen after Lm-Ova infection. Data are shown as mean±SEM. (E) The number of OT-I cells in the spleen after infection with or without tamoxifen administration. (F) A representative plot gated on total splenic CD8 T cells at day 7. *, P<0.05; **, P<0.005. Data represent four (B–E) and two (F) independent experiments (n=3–4 mice per time point)
The reduced expansion of LATKO OT-I cells could be a consequence of either impaired proliferation or increased death. We examined the proliferative capacity of CD8 T cells using BrdU incorporation and expression of Ki-67, a marker that is positively associated with cell proliferation. Mice were treated as described in Figure 1A, injected with BrdU intraperitoneally at day 6, and sacrificed 24h later. As shown in Figure 1F, total splenic CD8 T cells were divided into three populations: LATKO (CD45.1+), WT (CD45.2+), and endogenous CD8 T cells (CD45.1+CD45.2+). Compared with WT OT-I cells, LATKO OT-I cells had less BrdU incorporation compared to WT OT-I cells (Figure 1F). Moreover, the expression of Ki-67 in LATKO OT-I cells was reduced compared to that seen in WT OT-I cells, further supporting a role for LAT in continuous T cell expansion during the priming phase (Figure 1F). To determine if LATKO T cells exhibited accelerated cell death, we quantified dying and dead cells among LATKO and WT OT-I cells by Annexin V and 7AAD staining at day 7. The frequency of dying and dead cells was similar between WT and LATKO OT-I cells (Figure 1F). Moreover, Bcl-2 expression was downregulated similarly in both LATKO and WT OT-I cells (Figure 1F), indicating that reduced expansion of LATKO T cells in recipient mice was not due to augmented cell death during the priming phase. Collectively, our data indicate that continuous LAT mediated-signaling is essential for optimal CD8 T cell proliferation during the T cell expansion phase.
LAT mediated-signaling is not required for CD8 T cell contraction
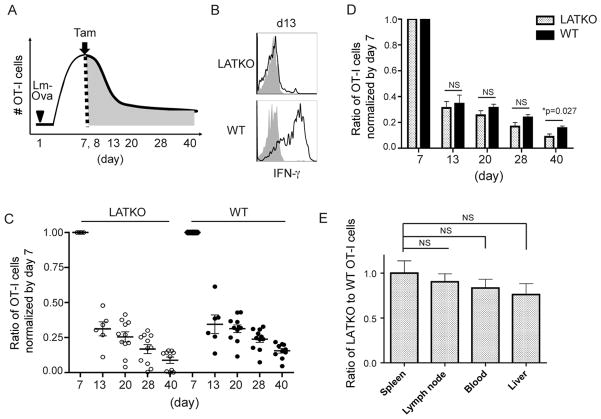
After the expansion phase and pathogen clearance, 90–95% of effector CD8 T cells undergo programmed cell death to re-establish homeostasis. To investigate whether LAT-mediated signaling regulates cell death during the contraction phase, we performed a similar transfer experiment as described above. A mixture of 1×104 LATf/− OT-I cells (CD45.1+) and 1×104 ERCre+LATf/− naïve OT-I cells (CD45.2+) were adoptively transferred into wildtype (CD45.1+ CD45.2+) recipients at day 0. Mice were infected with Lm-Ova at day 1 and treated with tamoxifen at the peak of CD8 T cell expansion, day 7 and 8 (Figure 2A). LATKO OT-I cells became GFP+ one day after tamoxifen administration, as described previously. To further confirm that LAT was deleted, and consequently that LAT mediated-signaling was abrogated, we stimulated T cells in vitro with the Ova peptide at day 13 and examined their ability to produce IFN-γ. As expected, LATKO cells were unable to produce IFN-γ upon stimulation (Figure 2B). To examine the contraction kinetics, antigen-specific CD8 T cells were monitored by serial bleeding about every 7–10 days after tamoxifen treatment. Because the percentages of WT and LATKO cells among total CD8 T cells before tamoxifen injection were not the same in each experiment, we normalized the percentage of cells at later time points to the percentage of cells at day 7 prior to tamoxifen injection. As seen in Figure 2C, 31% and 34% of cells survived by day 13 in the LATKO and WT groups, respectively. 25% of LATKO and 32% of WT CD8 T cells remained alive by day 20, indicating that the contraction kinetics between these two groups was comparable. The average percentage of remaining LATKO OT-I cells seemed to be slightly lower than WT OT-I cells during the contraction phase but the difference was not statistically significant at day 13, 20, or 28 (Figure 2D). To exclude the possibility that LATKO T cells do not properly migrate to lymphoid and/or non-lymphoid tissues, we compared the percentages of WT and LATKO OT-I cells among total CD8 T cells in the blood, spleens, lymph nodes, and livers at day 40. The ratios of LATKO T cells to their WT counterparts in these tissues were close to the ratio in the blood (Figure 2E), suggesting that the contraction kinetics of LATKO OT-I cells seen in the blood is representative of that in other tissues. Altogether, our data demonstrate that CD8 T cell contraction is largely LAT-independent.
Figure 2. LAT in the contraction of CD8 T cells.
(A) 1×104 WT OT-I cells (CD45.1+) and 1×104 LATKO OT-I cells (CD45.2+) were adoptively transferred into hosts (CD45.1+ CD45.2+) that were subsequently infected with Lm-Ova. The recipient mice received tamoxifen to delete lat on day 7 and 8 at the peak of T cell expansion and the CD8 T cell response was monitored every 7–10 days. (B) IFN-γ production by splenic OT-I cells at day 13. Splenocytes were stimulated with (open) or without (filled) 1μM Ova peptide for 4 h before intracellular staining of IFN-γ. Data are representative of three independent experiments, n=3–4 mice. (C, D) Contraction curves following Lm-Ova infection. The percentage of OT-I cells in the blood at the indicated time points was normalized by their percentage at day 7 before tamoxifen treatment. The percentage of WT OT-I is gated on live CD8+CD45.2+ and the percentage of LATKO OT-I is gated on live CD8+CD45.1+. Each dot represents one mouse and the horizontal lines depict the mean of the population. NS=not significant; *, P<0.05. Data are representative of three independent experiments (n=5–11 per time point). (E) Ratio of the percentages of LATKO to WT OT-I cells in blood, spleen, liver, and lymph nodes on day 40. Data are shown as mean±SEM. Data represent two independent experiments (n=3 per group).
LAT function during the effector-to-memory transition
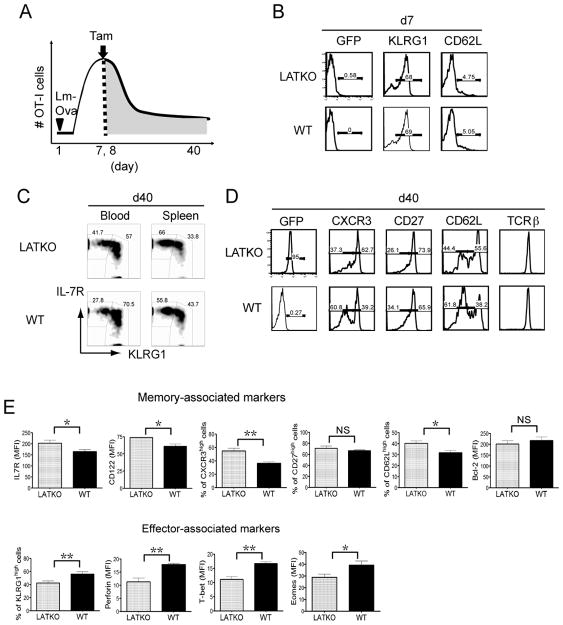
During the effector-to-memory transition phase, a small portion of effector cells survive and convert into functional memory cells that persist for a long time. To assess whether LAT-mediated signaling regulates memory formation, we deleted LAT at day 7 and 8 during the effector-to-memory transition and characterized the phenotype of LATKO and WT memory CD8 T cells at day 36–40 post-infection (Figure 3A). Before tamoxifen treatment at day 7, LATKO OT-I cells remained GFPnegative and they upregulated KLRG1 and downregulated CD62L similar to WT OT-I cells (Figure 3B). By day 40 after tamoxifen treatment, in comparison with their WT counterparts, LATKO memory OT-I cells acquired phenotypic properties that are associated with a better memory potential (Figure 3C, D, E). LATKO memory OT-I cells demonstrated increased expression of surface markers that are important for memory formation and maintenance, such as IL-7R, CD27, CD122, and CXCR3 (Figure 3C, E). Conversely, expression of markers that are associated with T cell effector function, such as KLRG1, perforin, T-bet, and Eomes, was decreased in LATKO memory OT-I cells (Figure 3E). We also wanted to evaluate the ability of LATKO T cells to form central memory cells, which express CD62L and are thought to be the stem cell-like subset that undergoes self-renewal and exhibits multipotency (1). 55% of LATKO memory T cells upregulated CD62L while only 38% of WT memory T cells re-expressed CD62L at day 40 post-infection (Figure 3D, E), demonstrating that a higher frequency of central memory T cells was generated in the absence of LAT. Collectively, these results indicate that LAT deficiency during the effector-to-memory transition accelerates CD8 T cell memory differentiation.
Figure 3. LAT in the memory differentiation of CD8 T cells.
(A) 1×104 WT OT-I cells (CD45.1+) and 1×104 LATKO OT-I cells (CD45.2+) were adoptively transferred into hosts (CD45.1+ CD45.2+) and followed with Lm-Ova infection. The recipient mice received tamoxifen to delete lat on day 7 and 8 during the effector-to-memory transition and were analyzed on day 36–40. (B) A representative histogram of GFP, KLRG1, and CD62L expression gated on LATKO (CD8+ CD45.2+), WT (CD8+ CD45.1+), and endogenous CD8 T cells (CD8+ CD45.1+ CD45.2+) in the peripheral blood at day 7 before tamoxifen injection. Data are representative of three independent experiments (n=3–5 mice per group) (C) A representative plot of KLRG1 and IL-7R expression on CD8 T cells in the blood and spleens at day 40. Data are representative of three independent experiments (n=4–6 mice per group) (D) A representative histogram of memory-associated markers CXCR3, CD27, CD62L, and surface TCR expression on CD8 T cells at day 40. Data are representative of three independent experiments (n=3–7 mice per group) (E) Expression of memory and effector associated markers at day 36–40 on LATKO and WT CD8 T cells. NS=not significant; *, P<0.05; **, P<0.005.
LAT deficiency affects T-bet expression in memory T cells
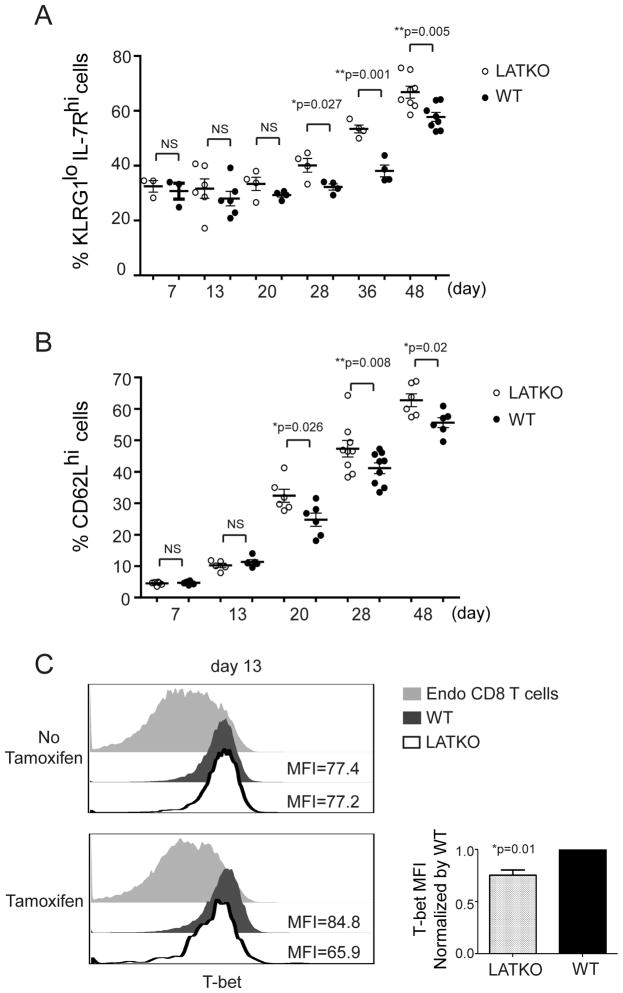
We sought to investigate the mechanism by which LAT deletion modulates memory differentiation. Published studies indicate that cells that continue to receive stimulation during the later stage of infection are more likely to become terminally differentiated effectors (2). Therefore, in our study, it is possible that some residual antigens are present during the contraction phase (day 7 to 20) and continue to stimulate T cells. We hypothesize that LATKO T cells are unable to receive stimulation at this stage, and they preferentially become memory precursors. To test this possibility, we closely monitored the kinetics of memory precursor formation after tamoxifen treatment from day 7 to day 48 in the peripheral blood. Based on expression of KLRG1 and IL-7R, KLRG1loIL-7Rhi CD8 T cells are referred as MPECs and KLRG1hiIL-7Rlo CD8 T cells as SLECs (3, 4). The percentages of MPECs among LATKO OT-I cells remained comparable to those among WT OT-I cells at early time points, day 7 and 13 (Figure 4A). By day 20, more LATKO MPECs than WT ones were observed and this increase was maintained over time.
Figure 4. LAT in the formation of memory precursor CD8 T cells.
Mice received OT-I cells at day 0, were infected with Listeria-Ova at day 1, and were treated with Tamoxifen at day 7. (A) Kinetics of MPECs formation upon tamoxifen administration. The percentage of KLRG1lowIL7Rhi cells (MPECs) in LATKO (live CD8+ CD45.2+), or WT (live CD8+ CD45.1+) OT-I cells in the blood was monitored over time by gating on live CD8 T cells. Each dot represents one mouse. Data are representative of two independent experiments (n=5–7 mice per time points). (B) Kinetics of TCM generation from day 7 to 48. The percentage of CD62Lhi cells (TCM) in the blood was gated on CD8 T cells from day 7 to 48. Data are representative of two independent experiments (n=5–8 mice per time point). (C) T-bet expression in LATKO OT-I, WT OT-I, and endogenous CD8 T cells at day 13 post-infection. Data are representative of three independent experiments (n=3 mice).
We also monitored the kinetics of CD62L upregulation as an indicator of TCM formation. In line with the kinetics of MPEC differentiation, LATKO OT-I cells displayed more CD62Lhi cells at day 20 and later time points in comparison to WT OT-I cells (Figure 4B), demonstrating that the generation of TCM cells occurred more rapidly in the absence of LAT. To assess the transcriptional mechanism driving TCM development, we analyzed T-bet expression in LATKO and WT cells. T-bet is a transcription factor that is induced in effector CD8 T cells and plays a critical role in determining CD8 T cell fate. Higher amounts of T-bet promote SLEC formation, whereas lower amounts of T-bet instruct MPEC development (4). To determine if T-bet expression was decreased at earlier time points, we depleted LAT at day7 and analyzed T-bet expression in OT-I cells 5–6 days after deletion. Indeed, T-bet expression was decreased in LATKO OT-I cells but not in the control group that did not receive tamoxifen treatment (Figure 4C). This observation suggests that LAT deficiency leads to diminished T-bet expression, which consequently promotes the formation of MPECs.
LAT-mediated signaling is dispensable for memory CD8 T cell maintenance
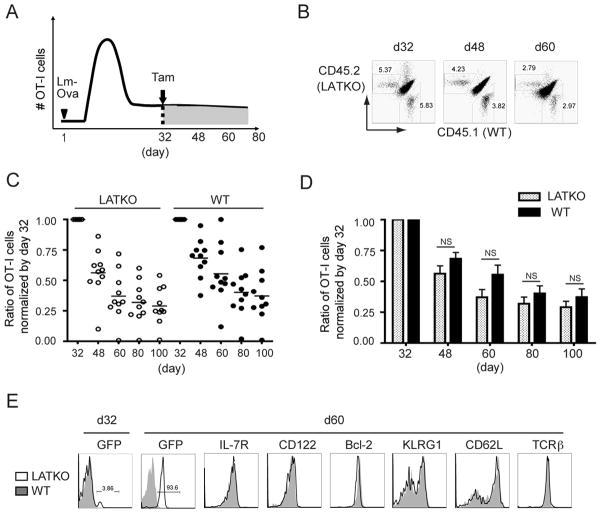
Since LAT-mediated signaling is important in naïve T cell survival (18), we were interested in determining whether LAT is also essential for memory T cell maintenance. To study the role of LAT in the homeostasis of memory CD8 T cells, we performed the same adoptive transfer experiment described previously in which LAT was deleted in memory OT-I cells starting at day 32 post-infection (Figure 5A). We analyzed CD8 T cells at day 48, 60, 80, and 100 in the peripheral blood and normalized the percentages of T cells to their percentages at day 32. As depicted in Figure 5C and D, the frequency of LATKO and WT memory OT-I cells was comparable after tamoxifen administration. 31.8% of LATKO memory OT-I cells and 40% of WT memory OT-I cells were observed by day 80 (Figure 5C, D). By day 100, 29% and 37% of LATKO and WT memory OT-I cells, respectively, remained. The average percentage of LATKO T cells was slightly lower than their WT counterparts but the difference was not statistically significant (Figure 5D). We also observed that few mice (4 out of 26) had significantly lower percentages of LATKO T cells in other set of experiments and we speculated that the depletion of LATKO OT-I cells in these mice might be due to the rejection of GFP+ cells over time. Except for these 4 outliers, memory CD8 T cells persisted in the absence of LAT, demonstrating that the maintenance of memory CD8 T cells was largely LAT-independent.
Figure 5. LAT in the maintenance of memory CD8 T cells.
(A) A mixture of LATKO and WT OT-I cells (104 each) was transferred and infected with Lm-Ova, mice received tamoxifen to delete lat on day 32, and the immune response was monitored about every 15 days. (B) Representative plots depict the percentage of LATKO (live CD8+ CD45.2+) and WT (live CD8+ CD45.1+) memory OT-I cells gated on total CD8 T cells in the blood at indicated time points. (C, D) Ratio of the percentages of memory OT-I cells in the blood. The percentage of LATKO or WT OT-I cells was normalized by its percentage at day 32 before tamoxifen injection. Each dot represents one mouse and data are shown as mean±SEM. Data are representative of three independent experiments (n=5–11 per time point). (E) Expression of GFP and indicated markers gated on LATKO or WT memory OT-I cells on day 60. Data are representative of three independent experiments (n=3–5 mice).
We also investigated the expression of IL-7 and IL-15 receptors because these two cytokines are essential for the survival and homeostasis of memory T cells. LATKO memory T cells displayed similar levels of IL-7R and CD122, the beta chain of IL-15R and IL-2R, as WT memory T cells (Figure 5E). Moreover, the expression of Bcl-2, a cell-intrinsic survival factor upregulated in memory cells to support their maintenance, was equivalent among WT and LATKO memory T cells (Figure 5E). Collectively, these observations suggest that LAT-mediated signaling is dispensable for the persistence of memory CD8 T cells.
LAT-mediated signaling is indispensable for a memory recall response
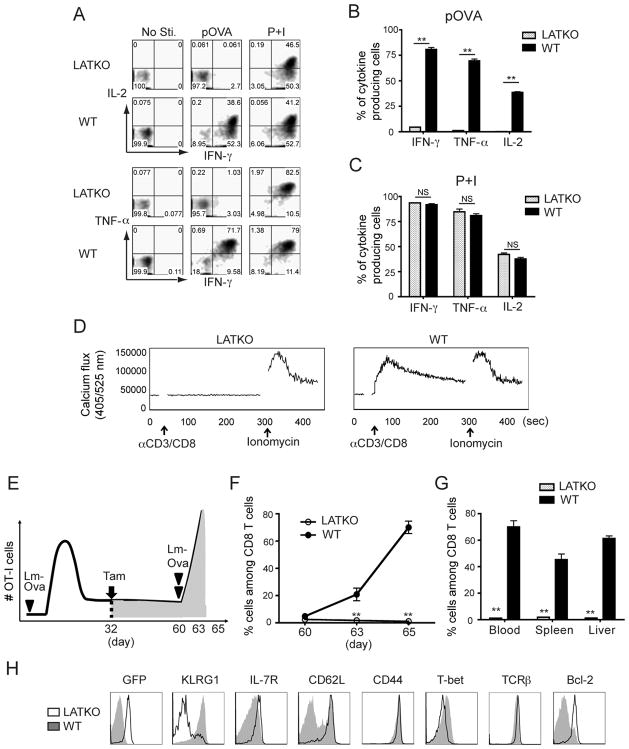
We next compared the functional potency of LATKO and WT memory OT-I cells by evaluating their ability to produce effector cytokines upon in vitro stimulation. Mice were treated with tamoxifen at day 7 and at day 48 splenocytes were cultured in the presence of Ova peptide or PMA and ionomycin (P+I) for 4 h before cytokine production by CD8 T cells was examined. As shown in Figure 6A and B, WT memory OT-I cells produced robust amounts of IFN-γ, TNF-α, and IL-2; however, LATKO memory OT-I cells produced insignificant amounts of cytokines upon Ova peptide stimulation. When cells were stimulated with P+I, which bypasses the TCR signaling complex, both LATKO and WT cells demonstrated a similar capability to produce cytokines (Figure 6A and C). We also examined the ability of LATKO memory OT-I cells to mobilize calcium. WT OT-I cells displayed strong calcium flux initiated by anti-CD3 and anti-CD8 crosslinking; however, calcium mobilization by LATKO memory OT-I cells was abrogated (Figure 6D). The defects in cytokine production and calcium mobilization were not due to a defect in TCR surface expression. Our previous study demonstrated that depletion of LAT in naïve T cells leads to a drastic downregulation of surface TCR (18). In contrast to naïve T cells, depletion of LAT did not affect TCR expression on memory OT-I cells (Figure 3D and 5E). Here, we show that LAT-mediated signaling is imperative for cytokine production and calcium flux in memory CD8 T cells. To examine whether LAT is also required for secondary immune responses in vivo, we directly re-infected recipient mice at day 60 with 1.5×105 Lm-Ova and monitored the T cell response at day 63 and 65 (Figure 6E). Compared with WT memory OT-I cells, LATKO memory OT-I cells failed to proliferate in response to re-challenge with Lm-Ova in both lymphoid organs and peripheral tissues (Figure 6F and G). Moreover, while WT memory OT-I cells converted to an effector phenotype characterized by CD62LlowIL-7RlowKLRG1high, LATKO memory cells remained CD62LhighIL-7Rhigh KLRG1low, indicating their inability to be reactivated (Figure 6H). In addition, LATKO memory T cells failed to upregulate T-bet and downregulate Bcl-2 like WT T cells (Figure 6H). Collectively, our data demonstrate that LATKO memory OT-I cells are unable to proliferate and produce cytokines in response to a recall challenge, indicating that LAT is required to trigger a secondary CD8 T cell immune response.
Figure 6. LAT in the cytokine production and recall response by memory CD8 T cells.
(A, B, C) Cytokine production by LATKO and WT memory OT-I cells upon Ova peptide (A) or P+I (B) stimulation. Splenocytes from day 48 were stimulated with 1 μM Ova peptide or P+I for 4 hr in vitro before ICS staining. Representative plots show IFNγ, TNFα, and IL-2 production by LATKO and WT OT-I cells. Data are representative of two independent experiments (n=3–4 mice). (D) Calcium mobilization by memory OT-I cells. Splenocytes from day 48 post-infection were loaded with the calcium indicator, Indo-1. Calcium flux was induced by anti-CD3 and anti-CD8 cross-linking followed by ionomycin stimulation and analyzed by flow cytometry. (E) Mice received mixed OT-I cells and 1.5×104 Lm-Ova were injected with tamoxifen on day 32. To induce a secondary response, the same mice were challenged with 1.5×105 Lm-Ova on day 60. The recall response was monitored on day 63 and 65. (F) T cell expansion upon re-infection of Lm-Ova. Percentage of LATKO and WT OT-I cells among total CD8 T cells in the spleen after infection. Data are shown as mean±SEM. Data are representative of three independent experiments (n=3 mice per time points). (G) Percentage of OT-I cells among total CD8 T cells in blood, spleen, and liver. Data are representative of three independent experiments (n=3 mice per time points). (H) Expression of indicated markers gated on LATKO or WT OT-I cells on day 65. Data are representative of three independent experiments (n=3 mice per group). NS=not significant; *, P<0.05; **, P<0.005
DISCUSSIONS
Using an inducible LAT deletion system, we investigated the requirement for TCR-mediated signaling in each step of the CD8 T cell response elicited by acute Listeria infection. We adoptively transferred LATKO and WT OT-I cells into the same hosts to ensure that OT-I cells received the same environmental stimuli, including the number of pathogens, CD4 T cell help, and inflammatory cytokines. This system allowed us to compare the quantitative and qualitative changes that occur during the immune response. Our data showed that LAT-mediated signaling is essential for the continuous expansion of CD8 T cells during T cell priming but is not required for contraction and memory maintenance of CD8 T cells. Deletion of LAT during the effector-to-memory transition results in enhanced development of memory precursors likely due to the downregulation of T-bet expression. Our data further indicated that LAT is essential for the recall response as LATKO memory CD8 T cells are unable to produce cytokines or respond to a secondary challenge.
When naïve T cells recognize foreign peptide-MHC complexes presented by antigen presenting cells, T cells and APCs interact for about 12–24 h in vivo to drive T cell activation (1). Afterward, T cells dissociate from APCs and undergo proliferation and differentiation. A single cell clone can undergo ~15 rounds of division in 7–8 days in a mouse model. Our data demonstrate that after the initial contact with APCs, CD8 T cell expansion is perturbed by LAT deficiency, indicating that sustained TCR signaling during the priming phase is required for optimal primary response magnitude. Although it was reported that the expression of ERCre might have a toxic effect on T cells after tamoxifen injection (19), impaired T cell expansion during priming is unlikely caused by the toxic effect of ERCre. The dose of tamoxifen we used in our experiments was much lower compared with the dose used in the published study. Moreover, thymocyte development and T cell activation are normal in ERCreLATf/+ mice treated with tamoxifen at the same dose (20). Our results are well in line with previous findings showing that varying the antigen dose or affinity can affect CTL expansion (21, 22). Depletion of epitope-presenting DCs during T cell primary responses dampens the magnitude of the responding CTL population (23). It has also been shown that the extent of CTL proliferation is proportional to the amount of Ova epitope produced by vaccinia virus (24). Together with these studies, our results provide additional evidence that the degree of CTL expansion correlates with the strength of TCR signaling.
Manipulating the amount and duration of external stimuli affects cell expansion in addition to influencing memory differentiation of CD8 T cells (2, 25). These data suggest that the strength of TCR signaling during the expansion phase plays a role in memory formation. Recently, analyses of SLP-76-deficient or –mutant knock-in mice showed that altered intrinsic TCR signaling affects the differentiation and function of endogenous memory CD8 T cells (13, 26). SLP-76-deficient or -mutated CD8 T cells differentiate into memory precursors and TCM cells at a faster rate than WT cells. Notably, a caveat of these systems is that CD4 T cells in these mice also have altered SLP-76 signaling and CD4 T cells play an important role in the differentiation of CD8 T cells during pathogen infection (27). Weak TCR signaling alters the differentiation of CD4 T cells from Th1 to Th2, thereby skewing the cytokines production (18). Furthermore, an additional study shows that the depletion of Treg cells leads to enhanced formation of memory CD8 T cells, and, similar to LAT-deficient Treg cells, SLP-76-deficient Treg cells seem to lose their inhibitory function (20). Therefore, the altered memory differentiation observed in SLP-76-deficient or -mutated CD8 T cells could be an indirect effect of altered CD4 T cells. In our study, by transferring LATKO OT-I cells into WT recipients, we were able to exclusively focus on LAT signaling in CD8 memory T cell formation while CD4 T cells remained unaffected. Our data revealed that LATKO memory CD8 T cells expressed higher levels of memory associated markers, such as IL-7R, CD122, CD27, CXCR3, and CD62L, indicating that dampened TCR signaling drives CD8 T cell development into memory precursors and TCM cells. It is thought that cells that receive a higher amount of antigen or are stimulated for longer periods of time during expansion are more likely to become terminally differentiated cells (2). Previous studies suggest that the decision for a CD8 T cell to become a short-lived effector or a self-renewing memory T cell occurs early in the stage of clonal expansion (1). In our study, the deletion of LAT happened at and after the peak of T cell expansion, allowing a normal primary response to occur. Our findings indicated that diminishing TCR signaling after the expansion phase still affected memory formation. Thus, our data suggested that intrinsic TCR signaling can still control fate of CD8 T cell differentiation after initial expansion.
To gain insight into the mechanism by which LAT-mediated signaling regulates memory differentiation, we examined the expression of T-bet, which controls the balance between MPEC and SLEC formation (4). Indeed, decreased expression of T-bet in LATKO OT-I cells was detected several days after LAT deletion (day 13), and this reduction occurred before the skewing of SLEC to MPEC was observed (day 20), suggesting that reduced T-bet expression in LATKO T cells was one of the driving forces that promote MPEC development. It is not yet clear how LAT regulates T-bet expression. One possibility is that the downstream signaling pathways of LAT, such as the mTOR pathway, regulate T-bet expression. mTOR has also been shown to modulate the balance between T-bet and Eomes expression through regulation of Foxo1 (28). Blockade of mTOR activity diminishes T-bet and enhances Eomes expression (29). However, since both T-bet and Eomes expression were reduced in LATKO memory OT-I cells, other LAT-dependent pathways are likely to be involved in controlling or mediating this balance. Another possible mechanism by which LAT regulates T-bet is through crosstalk between LAT and cytokine pathways. Since the production of IFN-γ is impaired by LAT deficiency, it is possible that IFN-γ-driven STAT1-mediated synthesis of T-bet is not as efficient in LATKO cells as it is in WT T cells. Additionally, IL-12 and IL-2 signaling are involved in determining CD8 T cell fate via regulation of T-bet expression. Prolonged IL-2 signals or higher expression of CD25 on CD8 T cells during the early expansion phase (day 3–5) favor the differentiation of CD8 T cells into SLECs (30). CD8 T cells convert to a central memory phenotype faster in the absence of IL-2 signaling (31). Therefore, it is possible that LAT deficiency affects IL-12 or IL-2 signaling; however, when we deleted LAT at day 7, CD25 expression had already decreased in both LATKO and WT CD8 T cells (data not shown). Hence, it is not likely that the altered memory differentiation observed in LATKO OT-I cells was caused by altered IL-2 signaling.
During the contraction phase, antigen-specific CD8 T cells undergo programmed cell death through a death receptor pathway, such as TNFR/TNF and Fas/FasL, and the mitochondrial pathway (1, 32). Without CD8 T cell contraction, prolonged accumulation of activated lymphocytes can lead to autoimmunity and immunopathology. Previous studies report that Fas does not appear to be important in CTL contraction (33); however, TNFRI and TNFRII double-deficient mice exhibited severely compromised contraction of virus-specific CD8 T cells (34). We observed that when LAT was depleted in CD8 T cells at the peak of T cell expansion (day 7 and 8), CTL contraction still occurred at a similar rate as WT CD8 T cells, indicating that the regulation of program cell death was largely LAT-independent. One possible explanation is that LATKO OT-I cells had already upregulated TNF receptors at day 7. After tamoxifen administration, LATKO CD8 T cells failed to produce TNF-α, while WT CD8 T cells and other cell types in the same environment were able to do so, thus inducing the death of LATKO cells to a similar extent as their WT counterparts. Moreover, it has been indicated that cytokine withdrawal-induced death plays an essential role in regulating CD8 T cell death during contraction (35). Withdrawal of IL-2 or other γ-chain cytokines activates Bim, which antagonizes the function of Bcl-2 family. Bim also activates Bax, which induces mitochondrial permeabilization and cell death(36). Our data show that Bcl-2 expression was comparable in LATKO and WT CD8 T cells during contraction, further demonstrating that LAT is dispensable for controlling cell death at this stage.
Memory CD8 T cells are capable of mounting a robust and efficient recall immune response upon secondary exposure to re-infection. Our results here show that although LATKO memory T cells acquire phenotypic markers associated with a high memory potential, they completely fail to be reactivated and produce IFN-γ, TNF-α, and IL-2 upon re-exposure to Listeria. This impaired memory response was not due to a lack of CD4 T cell help as LATKO OT-I cells were transferred to WT recipients containing normal CD4 T cells. One explanation is that LAT-mediated signaling is essential for T cell activation and cytokine production by memory CD8 T cells. Another possibility is that persistent LAT-mediated signaling is vital to maintain the functionality of memory CD8 T cells over time. We favor the former explanation because LATKO memory T cells were still fully capable of producing cytokines, especially IL-2, upon TCR-independent stimulation.
By assessing the role of LAT in the immunological response following microbial infection, we have demonstrated that continuous TCR-mediated signaling is essential to promote T cell expansion and cytokine production during both primary and secondary immune responses. However, contraction and maintenance of memory CD8 T cells do not require the presence of TCR signaling.
Acknowledgments
Funding: The National Institutes of Health grants AI048674 and AI093717. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
We thank the Duke University Cancer Center Flow Cytometry, DNA Sequencing, and Transgenic Mouse facilities for their excellent services.
References
- 1.Cui W, Kaech SM. Generation of effector CD8+ T cells and their conversion to memory T cells. Immunol Rev. 2010;236:151–166. doi: 10.1111/j.1600-065X.2010.00926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sarkar S, Kalia V, Haining WN, Konieczny BT, Subramaniam S, Ahmed R. Functional and genomic profiling of effector CD8 T cell subsets with distinct memory fates. J Exp Med. 2008;205:625–640. doi: 10.1084/jem.20071641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaech SM, Wherry EJ. Heterogeneity and cell-fate decisions in effector and memory CD8+ T cell differentiation during viral infection. Immunity. 2007;27:393–405. doi: 10.1016/j.immuni.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, Gapin L, Kaech SM. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang W, Sloan-Lancaster J, Kitchen J, Trible RP, Samelson LE. LAT: the ZAP-70 tyrosine kinase substrate that links T cell receptor to cellular activation. Cell. 1998;92:83–92. doi: 10.1016/s0092-8674(00)80901-0. [DOI] [PubMed] [Google Scholar]
- 6.Takeda S, Rodewald HR, Arakawa H, Bluethmann H, Shimizu T. MHC class II molecules are not required for survival of newly generated CD4+ T cells, but affect their long-term life span. Immunity. 1996;5:217–228. doi: 10.1016/s1074-7613(00)80317-9. [DOI] [PubMed] [Google Scholar]
- 7.Tanchot C, Lemonnier FA, Perarnau B, Freitas AA, Rocha B. Differential requirements for survival and proliferation of CD8 naive or memory T cells. Science. 1997;276:2057–2062. doi: 10.1126/science.276.5321.2057. [DOI] [PubMed] [Google Scholar]
- 8.Mullbacher A. The long-term maintenance of cytotoxic T cell memory does not require persistence of antigen. J Exp Med. 1994;179:317–321. doi: 10.1084/jem.179.1.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murali-Krishna K, Lau LL, Sambhara S, Lemonnier F, Altman J, Ahmed R. Persistence of memory CD8 T cells in MHC class I-deficient mice. Science. 1999;286:1377–1381. doi: 10.1126/science.286.5443.1377. [DOI] [PubMed] [Google Scholar]
- 10.Swain SL, Hu H, Huston G. Class II-independent generation of CD4 memory T cells from effectors. Science. 1999;286:1381–1383. doi: 10.1126/science.286.5443.1381. [DOI] [PubMed] [Google Scholar]
- 11.Tewari K, Walent J, Svaren J, Zamoyska R, Suresh M. Differential requirement for Lck during primary and memory CD8+ T cell responses. Proc Natl Acad Sci U S A. 2006;103:16388–16393. doi: 10.1073/pnas.0602565103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leignadier J, Hardy MP, Cloutier M, Rooney J, Labrecque N. Memory T- lymphocyte survival does not require T-cell receptor expression. Proc Natl Acad Sci U S A. 2008;105:20440–20445. doi: 10.1073/pnas.0806289106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wiehagen KR, Corbo E, Schmidt M, Shin H, Wherry EJ, Maltzman JS. Loss of tonic T-cell receptor signals alters the generation but not the persistence of CD8+ memory T cells. Blood. 2010;116:5560–5570. doi: 10.1182/blood-2010-06-292458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Finco TS, Kadlecek T, Zhang W, Samelson LE, Weiss A. LAT is required for TCR-mediated activation of PLCgamma1 and the Ras pathway. Immunity. 1998;9:617–626. doi: 10.1016/s1074-7613(00)80659-7. [DOI] [PubMed] [Google Scholar]
- 15.Zhang W, Sommers CL, Burshtyn DN, Stebbins CC, DeJarnette JB, Trible RP, Grinberg A, Tsay HC, Jacobs HM, Kessler CM, Long EO, Love PE, Samelson LE. Essential role of LAT in T cell development. Immunity. 1999;10:323–332. doi: 10.1016/s1074-7613(00)80032-1. [DOI] [PubMed] [Google Scholar]
- 16.Shen S, Zhu M, Lau J, Chuck M, Zhang W. The essential role of LAT in thymocyte development during transition from the double-positive to single-positive stage. J Immunol. 2009;182:5596–5604. doi: 10.4049/jimmunol.0803170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ou-Yang CW, Zhu M, Fuller DM, Sullivan SA, Chuck MI, Ogden S, Li QJ, Zhang W. Role of LAT in the granule-mediated cytotoxicity of CD8 T cells. Mol Cell Biol. 2012;32:2674–2684. doi: 10.1128/MCB.00356-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shen S, Chuck MI, Zhu M, Fuller DM, Yang CW, Zhang W. The importance of LAT in the activation, homeostasis, and regulatory function of T cells. J Biol Chem. 2010;285:35393–35405. doi: 10.1074/jbc.M110.145052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higashi AY, Ikawa T, Muramatsu M, Economides AN, Niwa A, Okuda T, Murphy AJ, Rojas J, Heike T, Nakahata T, Kawamoto H, Kita T, Yanagita M. Direct hematological toxicity and illegitimate chromosomal recombination caused by the systemic activation of CreERT2. J Immunol. 2009;182:5633–5640. doi: 10.4049/jimmunol.0802413. [DOI] [PubMed] [Google Scholar]
- 20.Chuck MI, Zhu M, Shen S, Zhang W. The role of the LAT-PLC-gamma1 interaction in T regulatory cell function. J Immunol. 2010;184:2476–2486. doi: 10.4049/jimmunol.0902876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaech SM, Ahmed R. Memory CD8+ T cell differentiation: initial antigen encounter triggers a developmental program in naive cells. Nat Immunol. 2001;2:415–422. doi: 10.1038/87720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zehn D, Lee SY, Bevan MJ. Complete but curtailed T-cell response to very low-affinity antigen. Nature. 2009;458:211–214. doi: 10.1038/nature07657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Stipdonk MJ, Lemmens EE, Schoenberger SP. Naive CTLs require a single brief period of antigenic stimulation for clonal expansion and differentiation. Nat Immunol. 2001;2:423–429. doi: 10.1038/87730. [DOI] [PubMed] [Google Scholar]
- 24.Wherry EJ, Puorro KA, Porgador A, Eisenlohr LC. The induction of virus-specific CTL as a function of increasing epitope expression: responses rise steadily until excessively high levels of epitope are attained. J Immunol. 1999;163:3735–3745. [PubMed] [Google Scholar]
- 25.Sarkar S, Teichgraber V, Kalia V, Polley A, Masopust D, Harrington LE, Ahmed R, Wherry EJ. Strength of stimulus and clonal competition impact the rate of memory CD8 T cell differentiation. J Immunol. 2007;179:6704–6714. doi: 10.4049/jimmunol.179.10.6704. [DOI] [PubMed] [Google Scholar]
- 26.Smith-Garvin JE, Burns JC, Gohil M, Zou T, Kim JS, Maltzman JS, Wherry EJ, Koretzky GA, Jordan MS. T-cell receptor signals direct the composition and function of the memory CD8+ T-cell pool. Blood. 2010;116:5548–5559. doi: 10.1182/blood-2010-06-292748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khanolkar A, V, Badovinac P, Harty JT. CD8 T cell memory development: CD4 T cell help is appreciated. Immunol Res. 2007;39:94–104. doi: 10.1007/s12026-007-0081-4. [DOI] [PubMed] [Google Scholar]
- 28.Rao RR, Li Q, Gubbels Bupp MR, Shrikant PA. Transcription factor Foxo1 represses T-bet-mediated effector functions and promotes memory CD8(+) T cell differentiation. Immunity. 2012;36:374–387. doi: 10.1016/j.immuni.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rao RR, Li Q, Odunsi K, Shrikant PA. The mTOR kinase determines effector versus memory CD8+ T cell fate by regulating the expression of transcription factors T-bet and Eomesodermin. Immunity. 2010;32:67–78. doi: 10.1016/j.immuni.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Obar JJ, Molloy MJ, Jellison ER, Stoklasek TA, Zhang W, Usherwood EJ, Lefrancois L. CD4+ T cell regulation of CD25 expression controls development of short-lived effector CD8+ T cells in primary and secondary responses. Proc Natl Acad Sci U S A. 2010;107:193–198. doi: 10.1073/pnas.0909945107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williams MA, Tyznik AJ, Bevan MJ. Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. Nature. 2006;441:890–893. doi: 10.1038/nature04790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hand TW, Kaech SM. Intrinsic and extrinsic control of effector T cell survival and memory T cell development. Immunol Res. 2009;45:46–61. doi: 10.1007/s12026-008-8027-z. [DOI] [PubMed] [Google Scholar]
- 33.Pellegrini M, Belz G, Bouillet P, Strasser A. Shutdown of an acute T cell immune response to viral infection is mediated by the proapoptotic Bcl-2 homology 3-only protein Bim. Proc Natl Acad Sci U S A. 2003;100:14175–14180. doi: 10.1073/pnas.2336198100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suresh M, Singh A, Fischer C. Role of tumor necrosis factor receptors in regulating CD8 T-cell responses during acute lymphocytic choriomeningitis virus infection. J Virol. 2005;79:202–213. doi: 10.1128/JVI.79.1.202-213.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Snow AL, Pandiyan P, Zheng L, Krummey SM, Lenardo MJ. The power and the promise of restimulation-induced cell death in human immune diseases. Immunol Rev. 2010;236:68–82. doi: 10.1111/j.1600-065X.2010.00917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Merino D, Giam M, Hughes PD, Siggs OM, Heger K, O’Reilly LA, Adams JM, Strasser A, Lee EF, Fairlie WD, Bouillet P. The role of BH3-only protein Bim extends beyond inhibiting Bcl-2-like prosurvival proteins. J Cell Biol. 2009;186:355–362. doi: 10.1083/jcb.200905153. [DOI] [PMC free article] [PubMed] [Google Scholar]