Abstract
Adipose stroma/stem cells (ASC) represent an ideal source of autologous cells for cell-based therapy. Their transplantation enhances neovascularization after experimental ischemic injury. Aging is associated with a progressive decrease in the regenerative potential of mesenchymal stem cells (MSCs) from bone marrow. This work aims to determine the aging effect on human ASC capacities. First, we show that aging impairs angiogenic capacities of human ASC (hASC) in a mouse ischemic hindlimb model. Although no change in hASC number, phenotype, and proliferation was observed with aging, several mechanisms involved in the adverse effects of aging have been identified in vitro combining a concomitant decrease in (i) ASC ability to differentiate towards endothelial cells, (ii) secretion of proangiogenic and pro-survival factors, and (iii) oxidative stress. These effects were counteracted by a hypoxic preconditioning that improved in vivo angiogenic capacities of hASC from older donors, while hASC from young donors that have a strong ability to manage hypoxic stress were not. Finally, we identified reactive oxygen species (ROS) generation as a key signal of hypoxia on hASC angiogenic capacities. This study demonstrates for the first time that age of donor impaired angiogenic capacities of hASC in ischemic muscle and change in ROS generation by hypoxic preconditioning reverse the adverse effect of aging.
Introduction
The use of regenerative stem cells in the treatment of cardiovascular and ischemic disease is viewed as a highly promising strategy to ensure revascularization of ischemic tissues. Several regenerative cells have been considered among them mesenchymal stem cells (MSCs) were taken into account. These cells initially obtained from adult bone marrow (BM) are able to differentiate into multiple mesodermal cell lineages, contribute to tissue repair in vivo,1,2 and supply robust vascular support.3
Nevertheless, as ischemic diseases are often related to aging, a key point concerns the putative change in therapeutic cell efficacy during this process. Indeed, human aging is associated with a progressive decrease in the regenerative abilities to maintain tissue homeostasis, notably in neovascularization.4 Mechanisms involved seem to be attributed to a reduction in number and/or regenerative potential of stem cells.5,6 The mechanisms of stem cell aging remain to be defined, however, the involvement of reactive oxygen species (ROS) and oxidative stress are mainly incriminated.7 For BM-MSC, a concomitant decrease in proliferation and differentiation potential, an increase in senescent cell number and ROS formation are reported during aging.8,9 Such changes could explain that BM-MSC of aged donors are less effective in myocardial infarction treatment in mice.10
Similar mesenchymal cells obtained from adipose tissue present the advantage of being accessible, abundant, and provided in higher number in comparison to BM-MSC.11,12 We and other groups have reported that such human adipose-derived stromal cells (hASCs) display strong angiogenic properties in vivo due to their potential to differentiate into endothelial-like cell and their paracrine activity.13,14,15 Furthermore, a strict comparison between BM and adipose-derived cells in similar setting of experiments, demonstrates the higher angiogenic efficacy of adipose-derived cells.16 Therefore, hASC represent an attractive stem cell source for regenerative medicine and are currently evaluated in phase I trial to treat critical leg ischemia for the treatment of ischemic diseases.
Several studies have reported extensive changes in ASC from aged rodents including a decrease in proliferation, adipogenesis17,18 as well as angiogenesis capacities.19 Despite controversial results on proliferation capacities of hASC,20,21 it appears that the age of donor does not affect adipogenesis but decreases osteogenesis capacities.21,22 The effect of aging on angiogenic capacities of ASC was evaluated in very scarce and partial studies. In humans, a single in vitro study performed by Madonna et al. reported that the availability of the adipose CD45−/CD34+/CD133+ cell population and its angiogenic differentiation in matrigel assay are impaired in elderly donors with variable degrees of cardiovascular risk.23 Recently, another in vitro study reported that ASC isolated from 12 months compared with 3 months old mice show a decrease in angiogenic properties with impaired cell proliferation and expression of several secreted proteins. They also observed that after 48 hours under hypoxia, mouse ASC display higher angiogenic gene expression.24 Indeed, hypoxia is described to play a beneficial role in neovascularization notably through ROS generation and expression of several angiogenic genes.25,26 Thus, the duality in ROS effects on ASC in vitro and in vivo remains to be elucidated, as in one hand ROS production is associated with the deleterious aging process leading to a loss in tissue regenerative properties, whereas on the other hand ROS production induced by hypoxic preconditioning is described as a positive effect promoting angiogenic potential.24
In the present work, we provide evidence that age of donor impairs in vitro as well as in vivo angiogenic capacities of hASC obtained after enzyme-based isolation and ex vivo expansion in full clinical grade conditions. We also demonstrated that hypoxia preconditioning rejuvenates angiogenic capacities of hASC from donors aged over 50 years in a mouse model of limb ischemia and identified ROS production as a key signal in the biology of hASC.
Results
Aging impaired in vivo angiogenic capacities of hASC
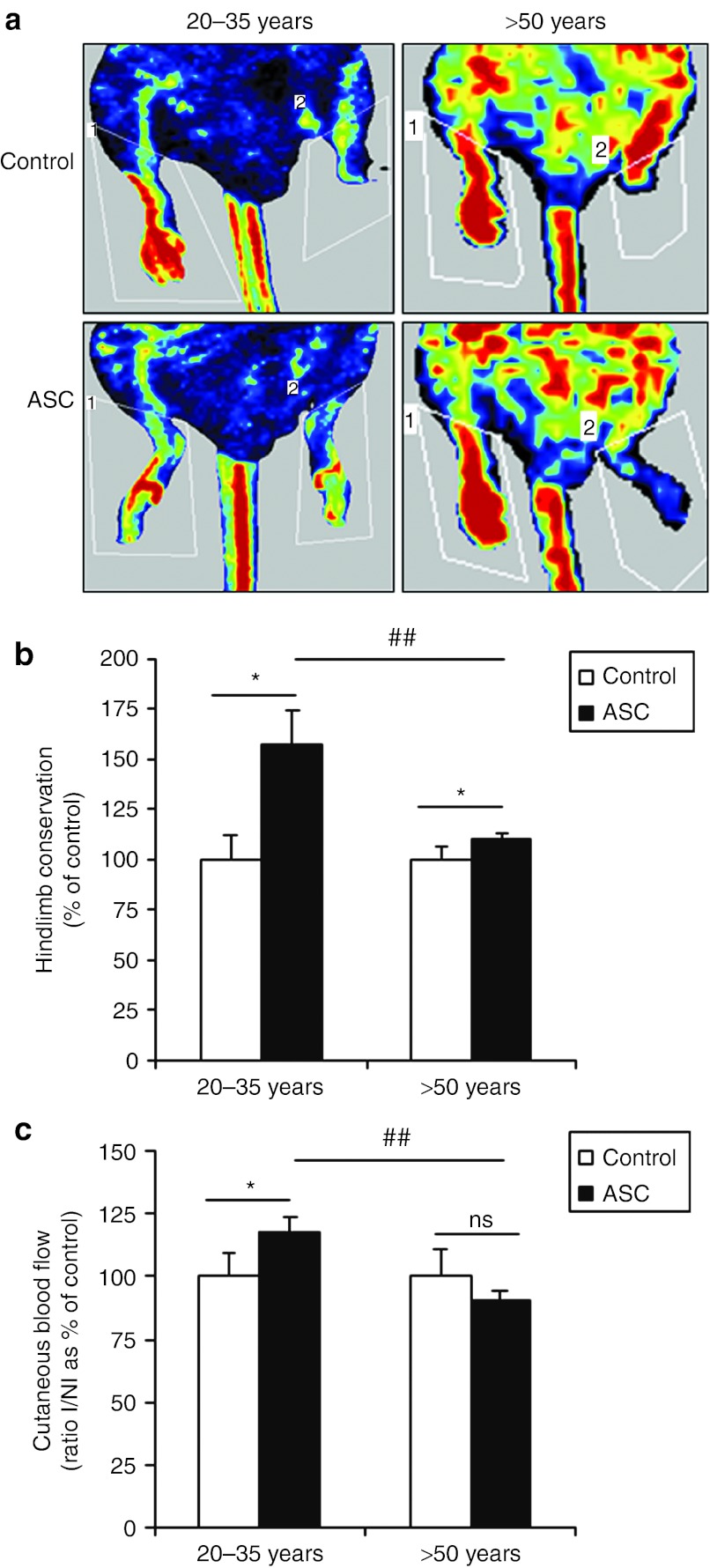
The aging effect on in vivo angiogenic capacities of hASC was evaluated as previously described in a mouse model of ischemic hindlimb.13 As expected intramuscular transplantation of hASC from donors aged 20–35 years resulted in a markedly increase in limb conservation (1.6-fold, Figure 1b) as well as an improvement of neovascularization in remaining ischemic tissues, revealed by a 1.2-fold rise in Doppler perfusion score compared with control (Figure 1a,c). Although hASC from donors over 50 years old improved hindlimb preservation compared with nontreated mice, this beneficial effect was significantly decreased (1.1-fold, Figure 1b). Cutaneous blood flow was also reduced after treatment with hASC from older donors when compared with younger ones (0.9- and 1.2–fold, respectively) and inefficient when compared with control (Figure 1c). Taken together, our results suggested that aging impaired angiogenic capacities of hASC in vivo.
Figure 1.
Effect of aging on in vivo angiogenic capacities of hASC. Transplantation of 1 × 106 hASC was performed by intramuscular injection in the ischemic hindlimb in comparison to control animals. Representative photomicrographs of (a) laser Doppler perfusion imaging, (b) percent of limb preservation, and (c) quantitative evaluation of cutaneous blood flow in preserved limb 14 days after treatment are presented. Cutaneous blood flow is expressed as the percentage normalized to control of the ratio ischemic leg perfusion/nonischemic leg perfusion (I/NI). n = 3–6 independent experiments. *P ≤ 0.05 in control versus hASC-treated animals. ##P ≤ 0.01 in 20–35 years versus >50 years hASC-treated animals. hASC, human adipose-derived stromal cell; ns, not significant.
Aging did not change the number, phenotype, cell growth, and senescence of hASC
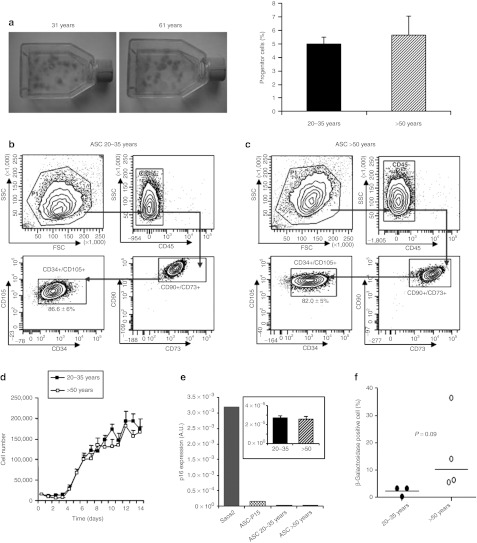
To identify the parameters involved in the adverse effect of aging on adipose tissue cells, the progenitor cell number was first estimated. The colony-forming unit fibroblast (CFU-F) assay revealed that the progenitor content of freshly isolated stromal vascular fraction (SVF) was similar between the group of younger versus older donors (5.0 ± 0.4 and 5.6 ± 0.7%, respectively; Figure 2a). Phenotype analysis showed no change in the percent of hASC, corresponding in total SVF to cells simultaneously negative for CD45 and positive for CD34 and the classical mesenchymal markers CD90, CD73, and CD105 (10.5 ± 3% for 20–35 years and 9.7 ± 1% for >50 years) (data not shown). After primary culture of SVF cells, hASC represents 86.6 ± 6% of adherent cells from 20–35 years old donors and 82.0 ± 5% from >50 years old donors (Figure 2b,c). The cell growth evaluated in hASC from both groups indicated no change in the number of cells recovered every day, suggesting that aging did not affect in vitro proliferative activity (Figure 2d). To determine whether age of donor was associated with cellular senescence, the expression of the senescence marker p16 was assessed.27 Compared with the human osteosarcoma cell line Saos2 expressing high level of endogenous p16 and used as positive control, there was no significant increase in p16 expression with hASC from both groups and a modest increase in hASC obtained after 15 passages (ASC-P15) (Figure 2e). This result was confirmed by the quantification of the senescence-associated β-galactosidase activity indicating no significant increase in perinuclear blue coloured cell number with age of donor (Figure 2f and Supplementary Figure S1).
Figure 2.
Effect of aging on in vitro hASC properties. (a) Representative pictures of CFU-F assay as well as their quantification were performed to estimate progenitor cells amount in freshly prepared SVF, n = 14. The mesenchymal phenotype corresponding to the CD45−, CD34+, CD73+, CD90+, CD105+ cell population in hASC from (b) 20–35 and (c) >50 years old donors was presented, n = 3–5. (d) The growth of hASC was evaluated by cell counting over 14 days in culture, n = 7–9. Senescence was evidenced by (e) p16INK4A expression level, n = 9, and (f) β-galactosidase coloration in hASC and quantification of positive blue cells, n = 9–10. A.U., arbitrary unit; CFU-F, colony-forming unit fibroblast; FSC, forward scatter; hASC, human adipose-derived stromal cell; SSC, side scatter; SVF, stromal vascular fraction.
Aging impaired in vitro endothelial differentiation and paracrine secretions by hASC
The decrease in hASC angiogenesis capacities in vivo could be the consequence of a decrease in their endothelial differentiation potential and/or the release in paracrine secretion.13,15 We first assessed in vitro endothelial differentiation of hASC from donors of different age. After 10 days in angiogenic medium, hASC from younger donors displayed some typical endothelial-like organization and formed highly branched networks of cells expressing CD31. The quantification of the CD31+ network showed a 1.6-fold increase with hASC of younger donors in angiogenic versus control medium (Figure 3a,b) with no difference in total cell number (Figure 3c). In contrast, the angiogenic medium had no significant effect on differentiation of hASC from donors aged over 50 years (Figure 3a,b). We then investigated the aging effect on the paracrine activity of hASC. Our data showed that vascular endothelial growth factor (VEGF) and transforming growth factor (TGF)-β2 mRNA content were downregulated by 2.6- and 4.2-fold, respectively; in hASC from old compared with young donors (Figure 3d). Aging also decreased VEGF secretion, while TGF-β2 levels could not be detected (Figure 3e). No significant change in hepatocyte growth factor (HGF) and insulin-like growth factor (IGF)-1 mRNA level or protein secretion was observed with aging (Figure 3d,e). In addition, aging strongly decreased the content of mRNA coding for interleukin (IL)-8, IL-6, tumor necrosis factor (TNF)-α, and CXCL-1 (Figure 3f). These results suggested that in vitro endothelial differentiation potential of hASC as well as their paracrine activity were damaged with aging.
Figure 3.
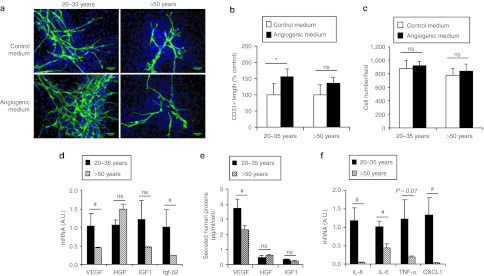
Effect of aging on in vitro endothelial differentiation and paracrine activity of hASC. (a) hASC were cultured in control or angiogenic medium and for immunostaining of CD31+ differentiated cells forming highly branched networks (green) and nuclei staining with DAPI (blue). Bar = 100 µm. Representative fluorescence microscopy was used for the (b) CD31 network length measurement and (c) cell number counting, n = 13. *P ≤ 0.05 in control medium versus angiogenic medium. (d) Proangiogenic and pro-survival growth factors or (f) cytokines mRNA expression contents as well as (e) human protein secretion were measured in cultured hASC, n = 7–9. #P ≤ 0.05 in young versus old donors groups. DAPI, 4′,6-diamidino-2-phenylindole; hASC, human adipose-derived stromal cell; HGF, hepatocyte growth factor; IGF, insulin-like growth factor; IL, interleukin; ns, not significant; TGF-β, transforming growth factor-β TNF-α, tumor necrosis factor-α VEGF, vascular endothelial growth factor.
Aging decreased ROS generation by hASC
Aging is commonly associated with an increase in oxidative stress status.28,29 So, we investigated the ROS level in SVF and hASC from donors of different ages. As expected, we observed a 1.8-fold increase in ROS level in freshly isolated SVF cells with the age of donor (P < 0.05, data not shown). In contrast to SVF, ROS level was decreasing with aging when considering hASC (Figure 4a). To validate this surprising result, aconitase activity that is well known to be inhibited by ROS and so to indirectly reflect mitochondrial oxidative stress, was determined.30 We detected a consistent increase in aconitase activity of hASC from older donors (Figure 4b). To further explore the decrease in ROS production by hASC from older donors, we looked at antioxidant enzymes. Even though no change in the glutathione peroxidase and catalase mRNA expression with the age of donor was depicted (data not shown), a significant increase in their enzymatic activity was measured (Figure 4c). Our results suggested that aging was associated with a decrease in oxidative stress in hASC that was not reflected in crude SVF cell fraction. This effect seemed to be linked to an increase in antioxidant defences.
Figure 4.
Aging effect on oxidative stress status of hASC. The oxidative status represented by (a) ROS production and the (b) associated aconitase activity were assessed on hASC, n = 17. (c) Antioxidant defences represented by catalase and glutathione peroxidase activities were then determined on hASC. Results are expressed as degradation of H2O2 µmol/ml/mn for catalase and as oxidation of NADPH µmol/mn for glutathione peroxidase, normalized with proteins content, n = 4–6. #P ≤ 0.05 in young versus old donor groups. A.U., arbitrary unit; hASC, human adipose-derived stromal cell; ROS, reactive oxygen species.
A hypoxia preconditioning of hASC from older donors increased redox metabolism and paracrine secretion
Hypoxia preconditioning has been described to improve mouse ASC angiogenic potential.24 Surprisingly, exposure of hASC from 20–35 years old donors to 0.5% O2 for 24 hours lead to no significant change in ROS levels (Figure 5a) and was correlated with a strong increase in catalase activity compared with control (2.1-fold increase, Figure 5b). In contrast, hASC from older donors had a significant increase in ROS level after hypoxia preconditioning (1.8-fold increase, Figure 5a) with no change in catalase activity (Figure 5b). No change in glutathione peroxidase activity could be observed with a hypoxia preconditioning whatever the age of donors (Figure 5c). The increase in secreted VEGF was much higher in hypoxia-preconditioned hASC from the older donors than the younger ones (2.2- versus 1.4-fold rise, respectively; Figure 5d). A similar increase in the mRNA content of hypoxia-inducible genes coding for IL-8, IL-6, and VEGF was observed in hASC from both groups (data not shown). These results highlighted that short exposure of aged hASC to low oxygen pressure reversed the effect of aging by restoring ROS level. Moreover, our data indicated that hASC from younger donors had a strong ability to manage oxidative stress under hypoxia by developing antioxidant activities.
Figure 5.

Hypoxia-preconditioning effect on in vitro redox metabolism and paracrine activity of hASC. hASC from donors aged 20–35 years and over 50 years were cultured in 0.5% O2 for 24 hours before the measurement of (a) ROS production, (b) catalase, and (c) glutathione peroxidase activities as well as (d) VEGF secretion in the culture medium, n = 3–5. *P ≤ 0.05 and ***P ≤ 0.001 in normoxia versus hypoxia culture conditions and #P ≤ 0.05 in young versus old donor groups in hypoxia culture condition. hASC, human adipose-derived stromal cell; ns, not significant; ROS, reactive oxygen species; VEGF, vascular endothelial growth factor.
A hypoxia preconditioning of hASC from older donors improved in vivo angiogenesis
The effect of a hypoxia preconditioning on in vivo angiogenic capacities of hASC was assessed in the mouse model of ischemic hindlimb. Intramuscular transplantation of hASC treated by hypoxia preconditioning resulted in an increase in hindlimb conservation only with hASC from older donors (Supplementary Figure S2a). More remarkably an improvement in the ischemic limb tissue perfusion reflected by a 1.4-fold rise in Doppler score compared with control was observed in contrast to a 1.2-fold reduction from donors aged 20–35 years (Figure 6a and Supplementary Figure S2b). Thus hypoxia preconditioning seemed to trigger a positive effect only on hASC from older donors, which was further confirmed by an increase in vessel density (Figure 6b, Supplementary Figure S3) and in the rate of hASC engraftment (0.96 ± 0.22 versus 1.46 ± 0.11 for hASC >50 years old donors after normoxic and hypoxic culture, respectively, in comparison to 1.83 ± 0.24% for hASC from younger donors, Figure 6c). Green fluorescent protein expressing hASC could be identified in connective tissue surrounding muscular fibers or closely associated to vessels with no evidence of their integration to vessel wall (Figure 6d). Therefore, our results demonstrated that a hypoxia preconditioning restored angiogenic capacities of hASC from older donors more likely through an increase in redox metabolism and paracrine secretion, according to in vitro results and to a lack of in vivo evidence for their direct participation to vessel formation.
Figure 6.
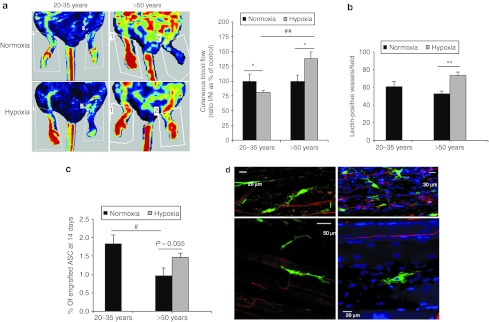
Hypoxia-preconditioning effect on in vivo angiogenic capacities of hASC. Transplantation of 1 × 106 hypoxia-preconditioned hASC was intramuscularly injected in the ischemic hindlimb, in comparison to control animals. (a) Representative photomicrographs of laser Doppler perfusion imaging and quantitative evaluation of cutaneous blood flow in preserved limb, 14 days after treatment were presented. Cutaneous blood flow is expressed as the percentage normalized to control of the ratio ischemic leg perfusion/nonischemic leg perfusion (I/NI), n = 3–7 independent experiments. *P ≤ 0.05 in normoxia versus hypoxia culture conditions and ##P ≤ 0.01 in young versus old donor groups treated animals. (b) Vessel density was assessed on tissue section after fluorescent lectin perfusion in animals and quantified. **P ≤ 0.01 in normoxia versus hypoxia. (c) The amount of hASC present in muscle tissue was calculated using quantitative PCR using human Alu-specific sequence detection. ##P ≤ 0.01 in young versus old donor groups. #P ≤ 0.05 in young versus old donor groups. (d) Imaging of GFP expressing hASC (green) within muscle tissue was performed using multiphoton microscopy, with lectin-positive vessels in red and DAPI-labeled nuclei in blue. DAPI, 4′,6-diamidino-2-phenylindole; GFP, green fluorescent protein; hASC, human adipose-derived stromal cell.
An antioxidant treatment reversed the effect of hypoxia preconditioning in hASC from older donors
To test whether the effect of hypoxic preconditioning on hASC from older donors could be attributed to the ROS generation, hASC incubated in hypoxic chamber were simultaneously treated with N-acetylcysteine (NAC), an antioxidant. As expected such treatment caused a strong decrease in hypoxia-induced ROS generation (1.5-fold decrease in hypoxic NAC-treated hASC versus hypoxic hASC, Figure 7a). It should be noted that we carefully selected a concentration of NAC having no significant effect per se on basal ROS production in normoxic hASC (Supplementary Figure S4). NAC efficiently counteracted VEGF protein upregulation induced by hypoxia preconditioning (Figure 7b). Moreover, intramuscular transplantation of hASC treated simultaneously by hypoxia preconditioning and NAC, resulted in a decrease in tissue perfusion in the ischemic limb as revealed by a 1.4-fold rise in Doppler score compared with only hypoxic preconditioning of hASC (Figure 7c,d). Our results demonstrated that the hypoxia-preconditioning effects on hASC capacities were mediated at least in part by an increase in ROS generation.
Figure 7.
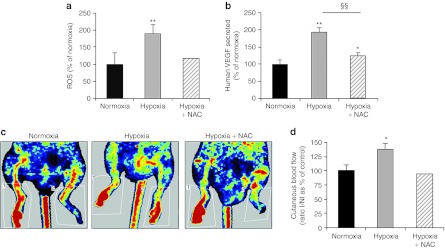
Reversal effect of antioxidant treatment on capacities of hypoxic-preconditioned hASC from older donors. hASC from donors over 50 years old were simultaneously incubated at 0.5% O2 and treated with NAC. After 24 hours, (a) ROS production and (b) VEGF secretion were assessed. Transplantation of 1 × 106 hypoxia-preconditioned hASC treated or not with NAC were intramuscularly injected in the ischemic hindlimb. (c) Representative photomicrographs of laser Doppler perfusion imaging and the (d) quantitative evaluation of cutaneous blood flow in preserved limb, 14 days after treatment are presented. Cutaneous blood flow is expressed as the percentage normalized to control of the ratio ischemic leg perfusion/nonischemic leg perfusion (I/NI), n = 1–5 independent experiments. *P ≤ 0.05 and **P ≤ 0.01 in normoxia versus hypoxia or hypoxia + NAC culture conditions. §§P ≤ 0.01 in hypoxia-preconditioning culture condition, in the presence versus the absence of NAC. hASC, human adipose-derived stromal cell; NAC, N-acetylcysteine; ROS, reactive oxygen species; VEGF, vascular endothelial growth factor.
Discussion
Up to now, little information is available on the relevance of adipose tissue as a source of autologous stem/progenitor cell in elderly patients, a population most likely targeted to benefit from this novel source of therapeutic cells. In this study, we demonstrated for the first time that the age of donor impaired therapeutic angiogenic capacities of hASC in mice ischemic limb, however, such impairment can be reversed by hypoxia preconditioning. Although, no change in hASC number, phenotype, and proliferation after one step expansion in full clinical grade like conditions was observed with aging, several mechanisms to explaining the adverse effects of aging can be proposed from our in vitro experiments, all related to a decrease in (i) hASC ability to differentiate in endothelial cells, (ii) secretion of proangiogenic and pro-survival factors such as VEGF, and (iii) oxidative stress. We also revealed that hypoxic preconditioning increases redox metabolism and paracrine secretion and improves in vivo angiogenic capacities of hASC from older donors. Finally, we clearly identified ROS generation as a key signal that modulates angiogenic capacities of hASC during hypoxia.
In order to highlight mechanisms that may account for the putative deleterious consequences of aging, we analyzed intrinsic properties of adipose tissue cells and obtained no modification in number, phenotype, and growth rate with aging. Similar conclusions were obtained by Zhu et al. that reported no significant difference in the rate of proliferation after cell passage from donors aged over 50 years compared with younger ones.21 However, our data are not consistent with studies on hASC showing that the percent of CFU-F is decreased with age of donor22,31 and the availability of CD45−, CD34+, and CD133+ ASC from visceral adipose tissue is impaired in aged donors with variable degrees of cardiovascular risk.23 Such discrepancy can first be explained by the location of adipose tissue used to isolate ASC as it is demonstrated that visceral and subcutaneous fat pads present functional, metabolic, and cell population differences.32 Second, divergent results could originate from the number of passages of cultured ASC. Indeed, in our study we used primary culture of hASC and it is well known that long-term culture emphasizes the aging process, with an increase in senescence and reduction in proliferation rate.33 Comparatively BM-MSC displayed marked differences such as a decrease in CFU-F number and proliferation rate from older donors,8,34,35 while frequency of senescence of MSC and ASC seems to be in similar range. However, no direct comparison of both cell types should strictly be performed as long-term culture with higher doubling population time is required to produce equivalent amount of MSC in comparison to ASC that may introduce critical intrinsic differences in cells.
ASC participate in tissue regeneration through their angiogenic capacities that can also be affected during aging. A single in vitro study demonstrated that aging impairs functional angiogenic capacities of hASC in matrigel tube formation assay and acetylated-low-density lipoprotein captation.23 In the present work, we also observe that aging decreases hASC ability to differentiate into endothelial-like cells. Similarly, ASC isolated from 12-month-old mice compared with 3-month-old mice showed impaired proliferation and disturbance in the expression of several secreted proteins associated with a decrease of their in vitro angiogenic properties.24 Since it has been assumed that paracrine mechanisms strongly contribute to revascularization of ischemic hindlimb by murine ASC, we investigated the effect of aging on paracrine activity of hASC. For the first time, our results show a decrease in several proangiogenic and antiapoptotic growth factors and cytokines expression with aging. These results are consistent with data showing reduced endothelial alignment of human umbilical vein endothelial cells in the presence of ASC-conditioned medium from aged versus young mice.24 Thus, a decrease in promoting endothelial differentiation and paracrine activity can explain the adverse effects of aging on hASC neovascularization capacities.
As several reports demonstrate that aging is commonly associated with oxidative stress,28,29 it was reasonable to postulate that oxidative stress status in hASC could be involved in the changes occurring with aging. Indeed, according to previous studies that reported an increase of oxidative stress in BM-MSC with aging,8 we observed an increase in mitochondrial ROS production in crude and freshly isolated SVF cells with age of donor. Surprisingly, we observed that aging decreases mitochondrial ROS production when considering the hASC subpopulation. This result is linked to an increase in antioxidant defences in hASC from old donors, which can be in apparent contradiction with the common hypothesis of aging-induced oxidative stress. In fact, this discrepancy could be explained by the installation of an adaptive metabolism in ASC subpopulation in reaction to the sustained environmental oxidative stress taking place within the tissue during aging that is revealed here in freshly isolated SVF.
ROS production has been shown to play major and positive roles in blood vessel growth36,37 and we demonstrated, at least in mice, that such signals can greatly improve the angiogenic potential of mice ASC.38 It has also been shown that exposure to hypoxia induces ROS production which in turn triggers angiogenesis and MSC survival.39,40,41 Results in mice showed that ASC exhibits a higher in vitro angiogenic potential after hypoxic culture.19,24,42 Surprisingly, our data indicates that hypoxia preconditioning of hASC from donors aged 20–35 years did not trigger improvement in their angiogenic capacities in ischemic hindlimb model. This is consistent with the lack of change in ROS level that can be explained by the increase in catalase activity, an antioxidant defence, detected after the hypoxia-preconditioning treatment. Taken together, this result seems to suggest that hASC from 20–35 years old donors has a strong ability to counteract oxidative stress by developing antioxidant activity in response to low pressure oxygen exposure for 24 hours. In contrast, our results clearly show that hypoxia preconditioning improved in vivo angiogenic capacities of hASC from donors over 50 years old. This effect was correlated with an increase in ROS production and can be reversed by antioxidant molecule during preconditioning, suggesting the key role of ROS in the hypoxia effects consistent with our previous work in mice.38 Interestingly, there is no change in antioxidant defences developed after hypoxic preconditioning of ASC from older donors which produce already high basal activity, suggesting that these cells do not have the ability to respond to an additional oxidative stress. Thus hypoxic stress in hASC from older donors generates sufficient levels of ROS in hASC to recover a high therapeutic benefit in ischemic limb. Such effect seems independent of direct participation of hASC to the formation of new vessels through differentiation into endothelial cells. Indeed, our results with green fluorescent protein-ASC indicated no evidence of their integration to vessel wall. Moreover, in vitro data showed that although no significant change in cell number and phenotype were observed after a hypoxia preconditioning, there is a decrease in the CD31-positive cellular network of aged hASC cultured in angiogenic medium (Supplementary Figure S5). Finally, hypoxia effects were significantly reduced with the addition of antioxidant molecule during the preconditioning suggesting a key role of ROS. This is consistent with our previous study on mice ASC treated with antimycin, a potent ROS generator,38 and data that recently reporting the pivotal role of ROS generation in the hypoxia-induced proliferation and migration of human MSC from adipose tissue and BM.25,43
In conclusion, the present work demonstrated for the first time that the increasing age of the donor impaired angiogenic capacities of hASC in ischemic model and that hypoxic preconditioning reversed the adverse effect of aging. This highlights the importance of ROS production to enhance hASC therapeutic potential in ischemic tissue.
Materials and Methods
Adipose tissue cell isolation and culture of hASC. Subcutaneous adipose tissue was obtained from donors aged 20–35 years (mean ± SD: 29 ± 5 years old) and over 50 years (61 ± 7 years old) undergoing elective abdominal dermolipectomy in no obese patients (body mass index <26). No objection certificates were obtained according to the bioethic law no. 2004-800 of 6 August 2004. Briefly, hASC were isolated as previously described44 and in Supplementary Materials and Methods, by enzymatic digestion using 2 mg/ml collagenase A (Roche Diagnostics, Indianapolis, IN) and centrifugation to obtain SVF cells that were seeded at 4,000 cell/cm2 in hASC culture medium based on Good Manufacturing Practice: α-MEM medium (Invitrogen, Carlsbad, CA) supplemented with 2% human plasma (EFS Midi-Pyrénées, Toulouse, France), 100 µg/ml streptomycin, 100 U/ml penicillin, 25 µg/ml amphotericin (Invitrogen), and 10 U/ml heparin Choay (Sigma-Aldrich, St Louis, MO). Adherent cells were cultured for 10–14 days at 37 °C in a humidified atmosphere of 5% CO2 until they reached confluence.
CFU-F assay. To evaluate the frequency of mesenchymal-like progenitors in the human SVF, the CFU-F assay was performed as described in the Supplementary Materials and Methods.44
Cell phenotyping. Freshly isolated SVF cells and hASC were labeled with anti-CD90-FITC, CD73-PE, CD34-PerCP, CD45-APC-Cy7 (BD Biosciences, San Jose, CA), and CD105-APC (eBiosciences, San Diego, CA) and analyzed on a fluorescent-activated cell sorter (FACS Canto II; Becton Dickinson, Mountain View, CA). Data acquisition and analysis were performed using FACS Diva software (Becton Dickinson, Mountain View, CA).
Cell growth. SVF were plated at 4,000 cell/cm2 and cultured in the hASC culture medium. Everyday, the cell number recovered after trypsin step was determined with a cell coulter (Becton coulter).
Senescence-associated β-galactosidase assay. Confluent hASC were incubated at 37 °C with fresh senescence-associated β-gal stain solution: 1 mg/ml of 5-bromo-4-chloro-3-indolyl b-D-galactosidase (X-gal), 40 mmol/l citric acid sodium phosphate pH 6, 5 mmol/l potassium ferrocyanide, 5 mmol/l potassium ferricyanide, 150 mmol/l NaCl, 2 mmol/l MgCl2. The perinuclear blue color is proportional to β-galactosidase activity and indicative of senescent cells. Cells stained were scored under an optical microscope.
Mouse model of ischemic hindlimb. Adult athymic nude mice (Harlan, Gannat, France) were housed in pathogen-free animal facilities (IFR150). All experimental procedures were done in compliance with French Ministry of Agriculture regulations (animal facility registration no.: MP/01/14/03/11) for animal experimentation. After anesthesia by isoflurane inhalation, a ligature was placed on the right femoral artery as previously described.13 Five hours after occlusion, 1 × 106 hASC were administrated by intramuscular injection in three different sites (gastrocnemius, gracilis, and quadriceps muscles, 20 µl per injection) of the ischemic leg. For each hASC sample, 6–8 mice were injected with cells (treated group) and compared with 6–8 mice (control group) undergoing similar procedures and receiving 0.9% NaCl. Independent experiments were repeated several times with distinct hASC samples (and detailed in legend to the figures). Vascular function analysis was assessed by ischemic limb conservation evaluated in percent of control, by laser Doppler imaging and limb perfusion expressed as a ratio of right ischemic to left nonischemic leg and by capillary density estimated after vessel labeling with fluorescent lectin as detailed in the Supplementary Materials and Methods. Quantification of hASC in mice muscle was performed by PCR analysis using the human Alu-specific sequence as described in Supplementary Materials and Methods.
Endothelial differentiation. Endothelial differentiation was performed as previously described.44 hASC were plated at 100,000 cell/cm2 on gelatin-coated wells and cultured for 10 days either in hASC culture medium, i.e., control medium, or control medium supplemented with VEGF (10 ng/ml; Sigma-Aldrich), i.e., angiogenic medium. Endothelial differentiation was assessed by CD31 immunostaining observed with a fluorescence microscope and analyzed with the Elements AR 3.0 image analyzer software (Nikon, Champigny sur Marne, France).
Determination of intracellular ROS generation. Intracellular ROS generation was assessed using 6-carboxy-2′,7′-dichlorodihydrofluorescein diacetate, diacetoxymethylester (H2-DCFDA) as previously described.45 Freshly isolated SVF cells or hASC were incubated in culture medium with H2-DCFDA (2 µm) for 30 minutes at 37 °C. Cells were washed in phosphate-buffered saline and analyzed on a fluorescent-activated cell sorter (FACS Canto II).
Enzymatic activities measurement. Aconitase enzymatic activity was measured using a Bioxytech aconitase-340 assay kit (Oxis Research, Foster City, CA). For catalase activity, hASC homogenates were suspended in 90 mmol/l potassium phosphate buffer (pH 7.2) with hydrogen peroxide 0.03%. Catalase activity was determined by measuring decomposition of H2O2 at 240 nm. Glutathione peroxidase activity was measured in hASC homogenates incubated with 45 mmol/l Tris-Azide buffer, 3 mmol/l reduced glutathione, 0.1 mU/ml glutathione reductase, 17 µmol/l NADPH, and 0.6 mmol/l X of t-butylhydroperoxide as substrate. The NADPH absorbance was measured at 340 nm. One enzymatic unit of glutathione peroxidase activity corresponds to the oxidation of 1 mmol of NADPH/minute. Results were normalized to protein content.
Real-time PCR. Total RNAs were extracted from hASC using RNeasy Mini Kit (QIAGEN, Valencia, CA). About 1 µg was reverse transcribed for single-stranded cDNA using High Capacity cDNA Reverse Transcription kit (Applied Biosystems, Foster City, CA); 20 ng of cDNA were analyzed by real-time PCR using Power SYBRgreen master mix with the primers for VEGF, HGF, IL6, TNF-α, p16INK4A, PUM (0.3 µmol/l final) or using Taqman Universal PCR Master Mix with primers and probe TaqMan Gene Expression Assay for IGF1, TGF-β2, IL8, CXCL1 (Applied Biosystems). All primer sequences and PCR conditions are detailed in Supplementary Materials and Methods and Supplementary Table S1.
Determination of VEGF protein levels. Human VEGF, HGF, IGF-1, and TGF-β2 levels were measured using a RayBio Elisa Kit (RayBiotech, Clinisciences, Nanterre, France) according to manufacturer's instructions. Assays were performed on the collected supernatants of hASC treated 24 hours under normoxic (21% O2) or hypoxic (O.5% O2) atmosphere.
Hypoxia-preconditioning in vitro. Confluent hASC were placed in hypoxic incubator Invivo2 500 (RUSKINN Technology, Bridgend, UK) at 0.5% O2. Antioxidant NAC (0.5 mmol/l) was added simultaneously. After 24 hours, supernatant and cells were recovered for analyses.
Statistical analysis. Quantitative results were expressed as means ± SEM. For results represented in %, each independent experiment was expressed in % of the internal control in the experiment and means ± SEM of independent experiment were then calculated. The SEM of the 100% histogram was calculated using raw data to represent the variation between independent experiments. Student's t-tests were used for statistical analysis using Prism 4 software (GraphPad, San Diego, CA). Significance was defined as *P ≤ 0.05, **P ≤ 0.01, and ***P ≤ 0.001.
SUPPLEMENTARY MATERIAL Figure S1. Effect of aging on hASC senescence. Figure S2. Effect of aging and hypoxic preconditioning of hASC on in vivo angiogenic. Figure S3. Effect of hASC on vessel density. Figure S4. Effect of NAC on basal ROS production. Figure S5. Effect of hypoxia preconditioning on in vitro endothelial differentiation of hASC from donors >50 years old. Table S1. Primers used for qPCR. Materials and Methods.
Acknowledgments
We are grateful to l'Institut des Technologies Avancées en sciences du Vivant (ITAV) UMS 3039 and especially to Jacques Rouquette for welcoming us to perform light microscopy imaging. We also thank Marion Combes, Christophe Gruissard, Pascale Guillou, and Mélanie Gadelorge for their excellent technical assistance. We acknowledge the Zootechnie facility, IFR150 especially Corinne Evra for great help with the animals care and the quantitative transcriptomique facility of IFR150 and Genotoul. This work was supported by the Région Midi-Pyrénées subvention n° 10051267 and by the Fondation pour la Recherche Médicale (FRM) JF/GP/LC081117 program Vieillissement Cardiovasculaire Normal et Pathologique. S. De B. (DCV20070409252) and A.C. (DEA20100619639) were financially supported by FRM. The authors declared no conflict of interest.
Supplementary Material
Effect of aging on hASC senescence.
Effect of aging and hypoxic preconditioning of hASC on in vivo angiogenic.
Effect of hASC on vessel density.
Effect of NAC on basal ROS production.
Effect of hypoxia preconditioning on in vitro endothelial differentiation of hASC from donors >50 years old.
Primers used for qPCR.
REFERENCES
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD.et al. (1999Multilineage potential of adult human mesenchymal stem cells Science 284143–147. [DOI] [PubMed] [Google Scholar]
- Blair A, Baker CL, Pamphilon DH., and, Judson PA. Ex vivo expansion of megakaryocyte progenitor cells from normal bone marrow and peripheral blood and from patients with haematological malignancies. Br J Haematol. 2002;116:912–919. doi: 10.1046/j.0007-1048.2002.03354.x. [DOI] [PubMed] [Google Scholar]
- Hegner B, Weber M, Dragun D., and, Schulze-Lohoff E. Differential regulation of smooth muscle markers in human bone marrow-derived mesenchymal stem cells. J Hypertens. 2005;23:1191–1202. doi: 10.1097/01.hjh.0000170382.31085.5d. [DOI] [PubMed] [Google Scholar]
- Hoenig MR, Bianchi C, Rosenzweig A., and, Sellke FW. Decreased vascular repair and neovascularization with ageing: mechanisms and clinical relevance with an emphasis on hypoxia-inducible factor-1. Curr Mol Med. 2008;8:754–767. doi: 10.2174/156652408786733685. [DOI] [PubMed] [Google Scholar]
- Dzau VJ, Gnecchi M, Pachori AS, Morello F., and, Melo LG. Therapeutic potential of endothelial progenitor cells in cardiovascular diseases. Hypertension. 2005;46:7–18. doi: 10.1161/01.HYP.0000168923.92885.f7. [DOI] [PubMed] [Google Scholar]
- Torella D, Rota M, Nurzynska D, Musso E, Monsen A, Shiraishi I.et al. (2004Cardiac stem cell and myocyte aging, heart failure, and insulin-like growth factor-1 overexpression Circ Res 94514–524. [DOI] [PubMed] [Google Scholar]
- Sohal RS., and, Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996;273:59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolzing A, Jones E, McGonagle D., and, Scutt A. Age-related changes in human bone marrow-derived mesenchymal stem cells: consequences for cell therapies. Mech Ageing Dev. 2008;129:163–173. doi: 10.1016/j.mad.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Sethe S, Scutt A., and, Stolzing A. Aging of mesenchymal stem cells. Ageing Res Rev. 2006;5:91–116. doi: 10.1016/j.arr.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Bujak M, Kweon HJ, Chatila K, Li N, Taffet G., and, Frangogiannis NG. Aging-related defects are associated with adverse cardiac remodeling in a mouse model of reperfused myocardial infarction. J Am Coll Cardiol. 2008;51:1384–1392. doi: 10.1016/j.jacc.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H.et al. (2002Human adipose tissue is a source of multipotent stem cells Mol Biol Cell 134279–4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimble JM, Katz AJ., and, Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007;100:1249–1260. doi: 10.1161/01.RES.0000265074.83288.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planat-Benard V, Silvestre JS, Cousin B, André M, Nibbelink M, Tamarat R.et al. (2004Plasticity of human adipose lineage cells toward endothelial cells: physiological and therapeutic perspectives Circulation 109656–663. [DOI] [PubMed] [Google Scholar]
- Miranville A, Heeschen C, Sengenès C, Curat CA, Busse R., and, Bouloumié A. Improvement of postnatal neovascularization by human adipose tissue-derived stem cells. Circulation. 2004;110:349–355. doi: 10.1161/01.CIR.0000135466.16823.D0. [DOI] [PubMed] [Google Scholar]
- Rehman J, Traktuev D, Li J, Merfeld-Clauss S, Temm-Grove CJ, Bovenkerk JE.et al. (2004Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells Circulation 1091292–1298. [DOI] [PubMed] [Google Scholar]
- Kim Y, Kim H, Cho H, Bae Y, Suh K., and, Jung J. Direct comparison of human mesenchymal stem cells derived from adipose tissues and bone marrow in mediating neovascularization in response to vascular ischemia. Cell Physiol Biochem. 2007;20:867–876. doi: 10.1159/000110447. [DOI] [PubMed] [Google Scholar]
- Djian P, Roncari AK., and, Hollenberg CH. Influence of anatomic site and age on the replication and differentiation of rat adipocyte precursors in culture. J Clin Invest. 1983;72:1200–1208. doi: 10.1172/JCI111075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkland JL, Hollenberg CH., and, Gillon WS. Age, anatomic site, and the replication and differentiation of adipocyte precursors. Am J Physiol. 1990;258 2 Pt 1:C206–C210. doi: 10.1152/ajpcell.1990.258.2.C206. [DOI] [PubMed] [Google Scholar]
- El-Ftesi S, Chang EI, Longaker MT., and, Gurtner GC. Aging and diabetes impair the neovascular potential of adipose-derived stromal cells. Plast Reconstr Surg. 2009;123:475–485. doi: 10.1097/PRS.0b013e3181954d08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schipper BM, Marra KG, Zhang W, Donnenberg AD., and, Rubin JP. Regional anatomic and age effects on cell function of human adipose-derived stem cells. Ann Plast Surg. 2008;60:538–544. doi: 10.1097/SAP.0b013e3181723bbe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu M, Kohan E, Bradley J, Hedrick M, Benhaim P., and, Zuk P. The effect of age on osteogenic, adipogenic and proliferative potential of female adipose-derived stem cells. J Tissue Eng Regen Med. 2009;3:290–301. doi: 10.1002/term.165. [DOI] [PubMed] [Google Scholar]
- de Girolamo L, Lopa S, Arrigoni E, Sartori MF, Baruffaldi Preis FW., and, Brini AT. Human adipose-derived stem cells isolated from young and elderly women: their differentiation potential and scaffold interaction during in vitro osteoblastic differentiation. Cytotherapy. 2009;11:793–803. doi: 10.3109/14653240903079393. [DOI] [PubMed] [Google Scholar]
- Madonna R, Renna FV, Cellini C, Cotellese R, Picardi N, Francomano F.et al. (2011Age-dependent impairment of number and angiogenic potential of adipose tissue-derived progenitor cells Eur J Clin Invest 41126–133. [DOI] [PubMed] [Google Scholar]
- Efimenko A, Starostina E, Kalinina N., and, Stolzing A. Angiogenic properties of aged adipose derived mesenchymal stem cells after hypoxic conditioning. J Transl Med. 2011;9:10. doi: 10.1186/1479-5876-9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Park SH, Park SG, Choi JS, Xia Y., and, Sung JH. The pivotal role of reactive oxygen species generation in the hypoxia-induced stimulation of adipose-derived stem cells. Stem Cells Dev. 2011;20:1753–1761. doi: 10.1089/scd.2010.0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen JG, Frøbert O, Pilgaard L, Kastrup J, Simonsen U, Zachar V.et al. (2011Prolonged hypoxic culture and trypsinization increase the pro-angiogenic potential of human adipose tissue-derived stem cells Cytotherapy 13318–328. [DOI] [PubMed] [Google Scholar]
- Shibata KR, Aoyama T, Shima Y, Fukiage K, Otsuka S, Furu M.et al. (2007Expression of the p16INK4A gene is associated closely with senescence of human mesenchymal stem cells and is potentially silenced by DNA methylation during in vitro expansion Stem Cells 252371–2382. [DOI] [PubMed] [Google Scholar]
- Sohal RS. Oxidative stress hypothesis of aging. Free Radic Biol Med. 2002;33:573–574. doi: 10.1016/s0891-5849(02)00885-7. [DOI] [PubMed] [Google Scholar]
- Sohal RS, Mockett RJ., and, Orr WC. Mechanisms of aging: an appraisal of the oxidative stress hypothesis. Free Radic Biol Med. 2002;33:575–586. doi: 10.1016/s0891-5849(02)00886-9. [DOI] [PubMed] [Google Scholar]
- Sipos I, Tretter L., and, Adam-Vizi V. Quantitative relationship between inhibition of respiratory complexes and formation of reactive oxygen species in isolated nerve terminals. J Neurochem. 2003;84:112–118. doi: 10.1046/j.1471-4159.2003.01513.x. [DOI] [PubMed] [Google Scholar]
- Alt EU, Senst C, Murthy SN, Slakey DP, Dupin CL, Chaffin AE.et al. (2012Aging alters tissue resident mesenchymal stem cell properties Stem Cell Res 8215–225. [DOI] [PubMed] [Google Scholar]
- Ibrahim MM. Subcutaneous and visceral adipose tissue: structural and functional differences. Obes Rev. 2010;11:11–18. doi: 10.1111/j.1467-789X.2009.00623.x. [DOI] [PubMed] [Google Scholar]
- Izadpanah R, Kaushal D, Kriedt C, Tsien F, Patel B, Dufour J.et al. (2008Long-term in vitro expansion alters the biology of adult mesenchymal stem cells Cancer Res 684229–4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roobrouck VD, Ulloa-Montoya F., and, Verfaillie CM. Self-renewal and differentiation capacity of young and aged stem cells. Exp Cell Res. 2008;314:1937–1944. doi: 10.1016/j.yexcr.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Kretlow JD, Jin YQ, Liu W, Zhang WJ, Hong TH, Zhou G.et al. (2008Donor age and cell passage affects differentiation potential of murine bone marrow-derived stem cells BMC Cell Biol 960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maulik N. Redox signaling of angiogenesis. Antioxid Redox Signal. 2002;4:805–815. doi: 10.1089/152308602760598963. [DOI] [PubMed] [Google Scholar]
- Maulik N., and, Das DK. Redox signaling in vascular angiogenesis. Free Radic Biol Med. 2002;33:1047–1060. doi: 10.1016/s0891-5849(02)01005-5. [DOI] [PubMed] [Google Scholar]
- Carrière A, Ebrahimian TG, Dehez S, Augé N, Joffre C, André M.et al. (2009Preconditioning by mitochondrial reactive oxygen species improves the proangiogenic potential of adipose-derived cells-based therapy Arterioscler Thromb Vasc Biol 291093–1099. [DOI] [PubMed] [Google Scholar]
- Wang JA, Chen TL, Jiang J, Shi H, Gui C, Luo RH.et al. (2008Hypoxic preconditioning attenuates hypoxia/reoxygenation-induced apoptosis in mesenchymal stem cells Acta Pharmacol Sin 2974–82. [DOI] [PubMed] [Google Scholar]
- Rosová I, Dao M, Capoccia B, Link D., and, Nolta JA. Hypoxic preconditioning results in increased motility and improved therapeutic potential of human mesenchymal stem cells. Stem Cells. 2008;26:2173–2182. doi: 10.1634/stemcells.2007-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JH, Zhang N., and, Wang JA. Improved anti-apoptotic and anti-remodeling potency of bone marrow mesenchymal stem cells by anoxic pre-conditioning in diabetic cardiomyopathy. J Endocrinol Invest. 2008;31:103–110. doi: 10.1007/BF03345575. [DOI] [PubMed] [Google Scholar]
- Thangarajah H, Vial IN, Chang E, El-Ftesi S, Januszyk M, Chang EI.et al. (2009IFATS collection: Adipose stromal cells adopt a proangiogenic phenotype under the influence of hypoxia Stem Cells 27266–274. [DOI] [PubMed] [Google Scholar]
- Busletta C, Novo E, Valfrè Di Bonzo L, Povero D, Paternostro C, Ievolella M.et al. (2011Dissection of the biphasic nature of hypoxia-induced motogenic action in bone marrow-derived human mesenchymal stem cells Stem Cells 29952–963. [DOI] [PubMed] [Google Scholar]
- Dromard C, Bourin P, André M, De Barros S, Casteilla L., and, Planat-Benard V. Human adipose derived stroma/stem cells grow in serum-free medium as floating spheres. Exp Cell Res. 2011;317:770–780. doi: 10.1016/j.yexcr.2011.01.001. [DOI] [PubMed] [Google Scholar]
- Carrière A, Carmona MC, Fernandez Y, Rigoulet M, Wenger RH, Pénicaud L.et al. (2004Mitochondrial reactive oxygen species control the transcription factor CHOP-10/GADD153 and adipocyte differentiation: a mechanism for hypoxia-dependent effect J Biol Chem 27940462–40469. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Effect of aging on hASC senescence.
Effect of aging and hypoxic preconditioning of hASC on in vivo angiogenic.
Effect of hASC on vessel density.
Effect of NAC on basal ROS production.
Effect of hypoxia preconditioning on in vitro endothelial differentiation of hASC from donors >50 years old.
Primers used for qPCR.