Abstract
Induced pluripotent stem cells (iPSCs) have great potential for regenerative medicine as well as for basic and translational research. However, following the initial excitement over the enormous prospects of this technology, several reports uncovered serious concerns regarding its safety for clinical applications and reproducibility for laboratory applications such as disease modeling or drug screening. In particular, the genomic integrity of iPSCs is the focus of extensive research. Epigenetic remodeling, aberrant expression of reprogramming factors, clonal selection, and prolonged in vitro culture are potential pathways for acquiring genomic alterations. In this review, we will critically discuss current reprogramming technologies particularly in the context of genotoxicity, and the consequences of these alternations for the potential applications of reprogrammed cells. In addition, current strategies of genetic modification of iPSCs, as well as applicable suicide strategies to control the risk of iPSC-based therapies will be introduced.
Introduction
Induced pluripotent stem cells (iPSCs) have recently been derived from somatic cells by re-expressing a combination of transcription factors conferring pluripotency.1,2,3 iPSCs share many characteristics with embryonic stem cells (ESCs), including pluripotency, unlimited self-renewal, and the ability to generate chimeric animals following blastocyst injection.1 Moreover, iPSCs theoretically should be able to overcome a number of hurdles preventing clinical development of ESCs. For example, they can be generated and administered autologously, avoiding alloimmune rejection, and there are no ethical and fewer regulatory barriers to clinical development, with no need for human embryonic tissue for cell line generation. iPSCs have great potential for regenerative medicine as well as for facilitating study of developmental biology, investigating the pathophysiology of human diseases, and developing and testing new therapies, via “disease in a dish” modeling. However, genomic alterations in iPSCs related to the process of reprogramming, either vector-related, culture-related, or pluripotency induction-related; as well those resulting from genetic correction of diseased iPSCs, have raised concerns regarding the reliability of iPSC disease models in research and even more importantly for the safety of future clinical applications in regenerative medicine.
Genotoxicity is a term used to refer to heritable and potentially toxic or deleterious effects on a cell's genetic material. It can be self-perpetuating, via induction of genomic instability and further mutations, often culminating in overt transformation to malignancy. Genotoxic agents include radiation, chemical compounds, or integrating genetic elements such as retroviruses. Prolonged culture can select for genomic abnormalities in cultured cells.4 Genotoxicities span from gross karyotypic aberrations, such as changes in chromosomal number or translocations; subkaryotypic copy number variations (CNVs), including deletions, gains, or amplifications; to single base pair point mutations. These alterations can result in dysregulated or dysfunctional mRNA and thus proteins, leading to abnormal outcomes such as apoptosis, alterations in cell cycle, or in the most extreme case malignant transformation. For example, loss of heterozygosity of tumor suppressor genes can predispose to tumorigenesis.5
There has been concern regarding insertional genotoxicity since first murine and then human iPSCs were generated utilizing integrating retroviral vectors. Retrovirus-mediated insertional activation of adjacent proto-oncogenes in transduced hematopoietic cells resulted in leukemias in several pioneering gene therapy clinical trials6,7,8,9 and in nonhuman primate models.10 The development of nonintegrating reprogramming vectors has in part allayed these concerns, but many possible applications for reprogrammed cells in regenerative medicine will require permanent modification or correction of autologous cells with some sort of permanent gene transfer.
Several methods have been applied to detect alterations at different genomic scales. (Figure 1, for comprehensive reviews, see refs. 11,12) Traditional G-band karyotyping is well-established and widely available, and can detect gross chromosomal abnormalities. However, these karyotyping methods have low resolution, detecting only changes >3 Mb in size.12 Fluorescence in situ hybridization-based protocols, such as multiplex fluorescence in situ hybridization13 and spectral karyotyping14 can better resolve chromosomal translocations or detect specific recurrent aneuploidy by means of chromosome-specific probes. Molecular karyotyping via array-based comparative genomic hybridization (aCGH)15 or single-nucleotide polymorphism (SNP) arrays16,17,18 have higher resolution but poor sensitivity for detecting minor subclones compared with traditional karyotyping, and cannot be used to detect mosaicism because these assays read out the pooled hybridization of genomes from many cells.11 Sequencing-based methods19,20 have the highest resolution and good sensitivity for detecting both changes in single bases as well as subgenomic CNVs, but are currently costly and require specialized bioinformatics support. Many investigators have begun to combine these techniques to provide complementary information on genomic integrity. In this review, we will discuss the current knowledge regarding genotoxicity and genomic integrity of iPSCs, and summarize possible strategies going forward to minimize these risks. Table 1 lists the key studies regarding genotoxicity in pluripotent cells to be discussed in this review.
Figure 1.
Current methodologies used to assess genotoxicity in pluripotent stem cells (PSCs). The relative resolution of various methodologies used to detect genotoxicity in PSCs is shown. The sensitivity of each methodology depends on the number of cells or clones screened and whether automated high-throughput detection can be employed. *Can be used to detect genomic abnormalities that lead to expression changes. aCGH, array-based comparative genomic hybridization; FISH, fluorescence in situ hybridization; SKY, spectral karyotyping; SNP, single-nucleotide polymorphism.
Table 1. Recent studies on genotoxicity in induced pluripotent stem cells.

Genomic Stability in ESCs
Most commonly, ESCs are derived from surplus frozen-thawed in vitro fertilized (IVF) embryos. Chromosomal abnormalities are very common in human embryos, especially in IVF embryos, with a complex and as yet poorly understood spectrum of abnormalities found in more than half of cleavage-stage IVF embryos, including mosaic embryos containing both normal diploid and aneuploid cells and completely aneuploid embryos.21 In addition, Hardarson et al. showed that only half of surplus IVF embryos were chromosomally normal when cultured to the blastocyst stage in vitro.22 It is believed that embryos with chromosomal abnormalities fail to develop normally and eventually undergo apoptosis or fail to implant, explaining low human fecundity, with up to 70% of natural conceptions estimated to be lost before birth (for a review, see refs. 23,24). It is possible that chromosomally abnormal cells within the inner cell mass degrade or die, and cannot give rise to ESC lines, corresponding to low ESC establishment rate.25 But it is also possible that ESCs can be generated from abnormal cells unable to implant and form an embryo, thus not representing a normal human pluriopotent cell.
Overall ESCs seem to be better protected against genetic instability compared with somatic cells. For example, ESCs have high sensitivity to ultraviolet-related DNA damage and activate efficient DNA repair mechanisms.26 Moreover, they readily undergo apoptosis or differentiate following severe DNA damage. For example, the tumor suppressor TP53 gene becomes activated in response to DNA damage and causes cell cycle arrest or apoptosis of affected cells.5 The p53 protein has been reported to translocate to the nucleus after DNA damage signaling in ESCs, bind to the Nanog promoter, and cause downregulation of the Nanog gene and subsequent differentiation.27 The spontaneous mutation frequency in murine ESCs has been reported to be significantly lower than in mouse embryonic fibroblasts,28 perhaps because ESCs with mutations do not survive due to activation of the p53 pathway. In contrast, Hyka-Nouspikel et al. found that DNA damage induced by ultraviolet irradiation caused apoptosis of the vast majority of ESCs but a small population continued to proliferate and maintained a pluripotent phenotype.29 Seemingly, irradiated surviving cells displayed a higher rate of point mutations that potentially could be the initial step for acquiring even more dramatic karyotypical abnormalities known to arise in prolonged ESC cultures. Systematic studies are necessary to identify and subsequently reduce the risk of DNA-damaging conditions (e.g., oxidative stress due to non-physiological oxygen tension or environmental pollutants) during the in vitro culture.
ESC cultures are not homogenous. Although the majority of ESCs have been found to be karyotypically normal,30,31 frequent non-random structural and numerical aberrations have been observed after prolonged culture. Recurrent abnormalities include gains of chromosomes 12, 17, and X, and duplications of 20q11.21. These genomic regions contain genes that are involved in cell growth or survival.32,33,34,35,36,37 O'Neill et al. compared epigenetic control in mouse inner cell mass and ESCs, and found subtle differences in histone modification on Nanog and lineage-specific genes between the two cell types.38 These changes are possibly resulted from a culture adaptation process in the ESCs. Baker et al. linked karyotypic abnormalities found in ESC cultures to tumorigenic events leading to germ cell tumors.33 In addition to karyotypic changes, recurrent CNVs, including amplification at 20q11.21, have been reported using aCGH and SNP array techniques.35,37 In a sequential study examining early and late passage ESCs, 8/9 lines at late passage showed one or more genomic aberrations commonly found in cancer cells including gain of chromosome 17q and amplification of MYC.39
Several studies showed that “culture adapted” or karyotypically abnormal human ESCs were more likely to form less differentiated teratocarcinoma-like tumors in immunocompromised mice.40,41,42,43 Werbowetski-Ogilvie et al. subcloned morphologically distinct ESCs from the widely used human ESC line, H9 (WAO9).43 Although standard karyotyping did not reveal any abnormalities, the subclones possessed subkaryotypic abnormalities identified by aCGH. The lines expressed pluripotency markers at high levels and showed features of neoplastic transformation, including a high-proliferative capacity and a nine- to 20-fold increase in the frequency of tumor-initiating cells. Later, the clones were used to successfully screen for anticancer drugs, underlining their neoplastic characteristics.44
The mechanism of genomic instability in ESCs after prolonged in vitro culture is not well understood. Passaging via dissociating enzymes is believed to generate more abnormalities than passaging by mechanical disruption.32 But, it is not clear whether the higher alteration rate is due to the reagents themselves or whether manual splitting is superior because it allows selection of more normal appearing ESCs by the investigator. Oxygen tension, medium conditions, feeder cells, and cryopreservation procedures could also contribute to genomic instability (for a review, see ref. 45).
First Generation iPSCs Generated Using Viruses For Transgene Delivery
Initially, integrating viral vectors were used to overexpress the transcription factors necessary for reprogramming of somatic cells.2,3 This method is robust, by far the most efficient, and reproducible among hundreds of labs. However, vector-associated insertional mutagenesis via activation or disruption of genes involved in proliferation, differentiation or apoptosis is a significant concern (for comprehensive reviews, see refs. 46,47). Because of the low efficiency of reprogramming even utilizing integrating vectors, it has been speculated that insertional mutagenesis may be required for successful reprogramming via activation of pluripotency or growth-impacting genes.47 However, studies mapping retroviral48,49 and lentiviral50 insertion sites did not reveal recurrent integration sites shared among different iPSC clones, or insertions clustering within genes in certain pathways. Furthermore, mRNA expression analysis did not reveal any pattern of gene dysregulation adjacent to insertion sites.50
In order to completely avoid issues related to insertional mutagenesis, nonintegrating methods using recombinant proteins,51,52 mRNA,53,54 microRNA,55,56 or nonintegrating viruses such as adenovirus57 and Sendai virus58 have been applied to reprogramming. In addition, the piggyBac transposon system allows seamless removal of integrated reprogramming vector sequences.59 However, these methods are generally much less efficient for iPSC generation compared with lentiviruses or retroviruses, and/or are expensive, labor intensive, and in some cases, difficult to reproduce in other labs. An episomal vector system60 has been drawing attention for its robustness and reproducibility but still harbors the theoretical risk of integration into host chromosomes at a very low frequency. Standard-integrating lentiviruses can be constructed with loxP sites, permitting excision of the reprogramming transgenes and proviral regulatory elements via expression of cre-recombinase following iPSC generation, leaving only short and theoretically relatively inert inactive DNA sequences behind.61 The DNA “tag” left behind in the reprogrammed cells and their progeny can be used for later identification of the cells in vivo. Modern PCR or sequencing technologies can also allow reliable identification of proviral insertion sites and consequently the selection of “safe harbor” iPSCs, based on vector localization to areas of the genome thought to be inert in terms of activating or inactivating genes or regulatory elements.62
In addition to insertional mutagenesis, the reactivation or incomplete inactivation of reprogramming transgenes when utilizing integrating vectors is a concern. One hallmark of pluripotency is the silencing of the pluripotency transgenes expressed from retroviral or lentiviral vectors and reactivation of the endogenous pluripotency factors. However, there is some possibility of reactivation of the transgenes in the pluripotent state or in differentiated cells. Indeed, Okita et al. reported that reactivation of Myc, a well-known oncogene, contributed to tumor formation in chimeric mice derived from blastocyst-injected iPSCs.63 Tong et al. also demonstrated that expression of transgenic Myc was statistically higher in tumorous tissues than in normal tissues among iPSC mice, indicating that the overexpression of transgenic Myc may contribute to tumor formation in F0 iPSC mice.64 In order to reduce the risk of reactivated transgenes, polycistronic vector systems with a cre-excisable loxP cassette as described above permit excision of the reprogramming factors once iPSCs are generated.65,66,67
Genomic Instability in iPSCs
Chromosomal analyses
Analogous to cultured ESCs, the occurrence of karyotypic aberrations has been observed in iPSCs. Mayshar et al. examined 17 human ESC and 46 human iPSC lines through the analysis of gene expression data.68 The human iPSCs showed a similar frequency of chromosome 12 duplications compared with human ESCs. However, unlike human ESCs, no aberration involving chromosome 17 was detected in human iPSCs. Another large scale study conducted by Taapken et al. analyzing 40 human ESCs and 219 human iPSC lines using G-banding found that the incidence of genomic abnormalities was not different between the cell types.69 The most common recurrent abnormality observed in both cell types was trisomy 12, followed by trisomy 8. However, trisomy 8 occurred more frequently in iPSCs than ESCs, whereas trisomy 17 was again only observed in ESCs.
Subchromosomal analyses
Because only a small number of reprogramming factor-targeted somatic cells are finally converted to a fully pluripotent state in primary reprogramming experiments, it has been speculated that clonal selection either on the starting somatic cell level or during the epigenetic remodeling plays a role in iPSC generation.70 Data from “secondary” reprogramming experiments suggest rather a stochastic nature of reprogramming; implying that all targeted cells can eventually be converted to iPSCs.71,72 However, these studies could also not exclude a possible selection bias that occurred during “primary” iPSC derivation.
Therefore, high-resolution methods such as CNV analysis, using CGH and/or SNP arrays73,74,75 and even whole genome sequencing approaches76,77,78 have been applied to iPSCs to address this question. Hussein et al. found significantly more CNVs in early passage human iPSC lines relative to human ESCs and fibroblasts.73 Martins-Taylor et al. reported recurrent CNVs in human iPSC lines derived from different somatic cells using fluorescence in situ hybridization and aCGH.75 In this experiment, recurrent iPSC-specific CNVs (1q31.3 and 17q21.1), and common CNVs in both iPSCs and ESCs (20q11.21 and 2p11.2) were found. Laurent et al. examined large numbers of cell lines (186 human pluripotent and 119 nonpluripotent samples) by high-resolution SNP genotyping.74 They found that human ESC and iPSC lines had a higher frequency of CNVs compared to nonpluripotent cells and that some regions with recurrent changes were in close proximity to known pluripotent genes. Human iPSC lines showed more deletions of tumor suppressor genes right after reprogramming and an increase in duplications of tumor-promoting genes after prolonged passaging.
Gore et al. performed exome sequencing of human iPSCs and their parental fibroblasts.79 A total of 124 mutations were found in 22 otherwise karyotypically normal human iPSC lines, with an average of five protein-coding point mutations in the regions sampled per each line. The majority of mutations were nonsynonymous and enriched in genes which have been associated with different malignancies. Interestingly, the ratio of nonsynonymous/synonymous mutations found in this study was very similar to the results recently reported in cancer genome-sequencing projects,80,81,82 arguing for selection pressure.83
In marked contrast, Quinlan et al. performed whole genome sequencing of three murine iPSC lines derived in the same experiment and found only one or two de novo mutations per iPSC line.78 Moreover, no activation of endogenous retroelements, common surrogates for genomic instability, was detected. Similarly, Cheng et al. examined three human iPSC lines derived from two different types of parental cells (CD34-positive cells and marrow stromal cells) from one healthy donor by episomal vectors.77 They identified 6–12 single-nucleotide variants within coding regions in each iPSC line and half of the single-nucleotide variants showed synonymous changes. No residual vector sequences could be detected, and there were no CNVs.
In summary, it still remains unclear whether reprogramming itself is a mutagenic process and moreover what the biological consequences of the described abnormalities might be. The studies published to date reported conflicting data, suggesting that in this nascent field, lab to lab variability in culture conditions or passaging procedures could potentially account for the inconsistent results. It appears that iPSCs and possibly also ESCs are more heterogeneous than initially hypothesized, and that current characterization strategies are not sufficient to identify a “standard” iPSC. Each line may already contain multiple subclones, and with passaging may acquire new changes. For both clinical and “disease in a dish” applications, it will be important for the field to agree upon a standardized approach to characterization, both initially and with prolonged culture. In addition, epigenetic or functional instability of iPSCs are also of concern, and should be carefully evaluated along with genotoxicity.84,85,86 Before a consensus can be reached, additional studies reflecting different reprogramming strategies, parental cell sources, and cell culture conditions are necessary to assess the risk and impact of genomic and epigenetic changes during the reprogramming process.
Potential Mechanisms of Genotoxicity in iPSCs
Selection of pre-existing mutations
The ontogeny and chronology of genomic alterations in iPSC is an important issue. It is possible that parental target cells carrying mutations are selected and expanded during reprogramming (preferentially or at random). Indeed, Gore et al. found that at least half of the mutations in human iPSCs could be detected at low frequency in the starting parental fibroblast population.79 Young et al. detected five shared genetic variants in coding regions in four independent murine iPSC clones generated in one reprogramming experiment.76 Two of the variants were also found in the parental mouse embryonic fibroblast cells. The authors concluded that pre-existing variability in target cells accounts for most of the genetic abnormalities in iPSCs. However, two other independent reprogramming experiments presented in the same study did not reveal any shared variants. In a similar approach, Ji et al. examined human foreskin fibroblast-derived iPSCs at two different passages (p6, p12) and their parental cells by whole exome sequencing.87 Based on previously published average cell division rates for fibroblast and iPSCs, the authors estimated that 19% of the mutations were present in the parental fibroblasts and another 7% were acquired during the passaging of the iPSCs. However, in the proposed model the majority of mutations would occur during reprogramming or during early passages.
Reprogramming-associated genotoxicity
As described above, ESCs have multiple pathways involved in maintaining genomic integrity. Human iPSCs also showed highly similar DNA damage responses after γ-irradiation when compared with ESCs.88 In contrast, Fan et al. reported a lower apoptosis rate after γ-irradiation measured by 7AAD/annexin-V staining in one iPSC line when compared with H9 cells.89 If iPSCs are not completely reprogrammed to an embryonic state, it seems possible that in some clones the DNA damage response pathways are not activated to the same degree as in ESCs. Luo et al. detected microsatellite instability, often associated with impaired mismatch repair or excise repair mechanisms, in one of two investigated karyotypically normal iPSCs.90 Although both studies operated with a limited sample number, the experimental data suggest that the DNA damage response could be considered as an important criterion for pluripotency. It also can be speculated that the continuous process of epigenetic remodeling leaves a window where the DNA damage response of the targeted cell still resemble somatic characteristics eventually allowing for selection of cells with genomic aberrations. Indeed, the p53-mediated DNA damage response has been implicated as a roadblock to reprogramming, and ablation of TP53 has been shown to increase reprogramming efficiency.91,92,93 However, removal of this checkpoint is worrisome even it increases efficiency, because resulting iPSCs may harbor increased genetic damage. The ectopic overexpression of the reprogramming transcription factors in the differentiated target cells before they turn on genomic protection pathways may predispose to oncogenic mutations, and these mutations may themselves increase proliferation and result in clonal dominance and expansion specifically of abnormal iPSCs. Pasi et al. proposed that Myc expression in murine iPSCs might contribute to genomic instability through DNA replicative stress.94 However, other groups observed no clear effect of Myc on the frequency of CNVs or protein-coding point mutations in human iPSCs.73,79
To date, available data suggests that the reprogramming method itself, whether integrating or nonintegrating, does not impact on reprogramming-associated genotoxicity. Recurrent genomic abnormalities were detected in iPSCs derived from episomal vectors69,79 as well as mRNA methods.79 Gore et al. compared three integrating methods (four-factor retroviral, four-factor lentiviral, and three-factor retroviral) and two nonintegrating methods (episomal vector and mRNA delivery) and did not observe significant differences in the frequency of protein-coding point mutations between iPSCs generated utilizing these diverse methods.79 Two additional studies also found no higher incidence of aneuploidy in human iPSCs generated with integrating viral vectors compared with lines derived with nonintegrating methods.68,69
In vitro culture-associated genotoxicity
As reported for ESCs, extended in vitro culture may predispose to genotoxic events. Indeed, Mayshar et al. observed rapid selection for cells with trisomy 12 during in vitro culture (p45: normal, p58: mosaic, p63: full trisomy).68 However, Hussein et al. demonstrated significantly more CNVs present in early passage human iPSCs than intermediate passage cells, potentially due to a selective procedure against mutated cells during culture.73 Chin et al. also demonstrated that late passage human iPSCs cluster more closely with human ESCs in the context of gene expression.95 The impact of in vitro culture on genomic stability of PSCs warrants careful evaluation of ESC or iPSC karyotype close in time to their experimental use, and more importantly potential clinical application. More systematic studies on culture conditions are very important to identify and potentially eliminate factors negatively affecting genomic integrity.
Genotoxicity Associated with Genetic Modification of Pscs
Many desired clinical applications for iPSCs or their differentiated progeny would require genetic modification or correction of these cells. In the case of a patient with a known disease-causing mutation, autologous iPSCs would be generated from skin fibroblasts or another somatic cell source, followed by genetic correction or augmentation before expansion and then differentiation to the desired cell type of interest before transplantation. Proof of principle was initially demonstrated in a murine model of sickle cell disease,96 rapidly followed by demonstration that cells derived from iPSCs could contribute to tissues in vivo and correct various disease models, at least in rodents.97,98,99,100 Traditional gene therapy approaches targeting somatic cells have been generally limited to gene augmentation via expression of a normal gene product from an integrating or nonintegrating viral vector, since homologous recombination (HR) or other gene correction approaches are inefficient, and selection of self-renewing or highly proliferative individual-corrected clones was not feasible from transduced primary somatic cells. The ability to genetically modify and then continuously select and expand appropriately corrected autologous self-renewing iPSCs via HR or gene targeting greatly extends the potential of genetic therapies. It has also been argued that the use of traditional semi-random integrating vectors such as HIV-based lentiviruses to correct iPSCs can be made safer by screening individual-transduced iPSCs for integration sites, and selecting only those with vector integration sites in “safe harbor” genomic loci for transplantation.62
The major drawback to traditional HR approaches for correction of disease-causing mutations is extremely low efficiency. However, optimized protocols allowing for successful HR-mediated correction of iPSCs following plasmid nucleofection have been reported, including correction of the Huntington's disease locus in iPSCs generated from Huntington's disease patient fibroblasts, but required extensive screening.101 More efficient approaches have been sought. Zinc-finger nuclease (ZFN) technology, utilizing a designer locus-specific dimer of zinc-finger DNA-binding domains and a Fok1 endonuclease inducing double strand breakage at the targeted region, was developed almost a decade ago, and has been rapidly applied to genetic correction or modification of iPSCs (for a review, see ref. 102). ZFN-mediated targeting increases efficiency very significantly over plasmid-based HR, and has been reported to correct a number of mutations in iPSCs, including the PIG-A gene defect causing paroxysmal hemoglobinuria in human patient,103 gp91phox deficiency from a patient with X-linked chronic granulomatous disease,104 and the α-1 antitrypsin defect in iPSCs from deficient patients, with demonstration that hepatocytes differentiated from these cells could engraft and function in vivo in a xenograft model.105 A second approach to more efficient gene correction utilizes “transcription activator-like effector nucleases (TALENs)” that are generated by fusing the TAL effector DNA-binding domain and a DNA cleavage domain. In some studies, TALENs are more efficient with a higher specificity and less off-target effects as compared with ZFNs106,107,108 and are being increasingly utilized to correct PSCs.106
However, a potential limitation of nuclease-mediated gene correction approaches is off-target DNA breaks induced at related sequences elsewhere in the genome, which may cause unpredictable genotoxic effects.102 Hockemeyer et al. reported efficient gene targeting via ZFNs in human ESCs and Southern blot analysis did not reveal any off-target integrations.109 They did not detect ZFN-mediated double-stranded breaks or error-prone repair elsewhere in the genome. However, two recent papers utilized high-throughput sensitive deep-sequencing approaches and reported quite extensive off-target cleavage sites for ZFNs, raising questions regarding specificity.110,111 TALENs may be more specific than ZFNs, however, similar highly sensitive assays for off-target double-stranded breaks using TALENs have not been reported.
All methods to genetically correct iPSCs, whether standard HR or targeting via ZFNs or TALENs, expose the cells to a number of stresses that could increase the risk of genotoxicity, in addition to off-target nuclease damage or integration of targeting cassettes. For instance, electroporation or nucleofection followed by drug selection may potentiate DNA damage. Studies have been performed to evaluate genomic instability of ESCs and/or iPSCs after genetic modification. Ruby et al. performed karyotype analysis on three gene-targeted human ESC clones and observed that 15% of the cells from one clone carried trisomies 12 and 17.112 Interestingly, however, with passaging, the abnormal trisomic subclones disappeared. Abnormal karyotypes were also reported following utilization of a bacterial artificial chromosome -based HR system to generate a fluorescent NANOG reporter system in human ESCs, with gain of chromosomes 12, 17 or 20 in a subset of targeted clones.113 Song et al. detected abnormal karyotypes in a small fraction (10–20%) of TP53−/− human ESCs after bacterial artificial chromosome targeting.114
Functional Relevance of Genomic Integrity in Pscs
Genomic aberrations can lead to overexpression of oncogenes or deficiency of tumor suppressor genes, increasing the risk for tumor formation following transplantation of iPSC-derived cells. Given the inherent risk of tumor formation due to residual undifferentiated iPSCs, any additional risk due to genotoxic events may be difficult to detect or attribute. Thus far there is little data regarding the degree of risk associated with alterations in genomic integrity in pluripotent cells, and whether the changes detected to date impact on pluripotency, differentiation potential (cell fate, function), and/or tumorigenicity. Recently, the correlation between copy number and levels of gene expression has been studied in PSCs. Mayshar et al. demonstrated that NANOG and GDF3 were significantly overexpressed in cell lines exhibiting trisomy 12.68 The nonsynonymous mutations identified by whole exome sequencing of iPSCs by Gore et al. were over-represented in genes mutated or linked to causative effects in cancers.79 F0 mice generated from iPSCs via either blastocyst injection or tetraploid complementation had enhanced tumor incidence,63,64 however, whether this outcome resulted from genetic changes already present in the iPSCs or from abnormal epigenetic gene regulation in iPSC-derived tissues is unknown. Werbowetski-Ogilvie et al. identified and characterized functional disability of genotypically abnormal human ESCs during in vitro culture.43 When neural derivatives of human PSCs carrying the chromosome 1q defect were implanted into the brains of nude rats, they failed to integrate and expand, supporting the notion that genomic instability can limit their clinical potential in addition to increasing tumor risk.115 On the other hand, a recent study indicated that iPSCs can become immunogenic during generation. Zhao et al. demonstrated that karyotypically normal murine iPSCs can be rejected in a T-cell–dependent manner in the syngeneic setting.116 Although the exact mechanism for immune rejection of these cells is unknown, and this result has to be validated in other models, it is at least conceivable that mutations arising during iPSC generation could result in the production of immunogenic gene products.
Future Directions For Minimizing the Genotoxicity Risk in ipscs
Standardization of genetic evaluation of iPSCs
As PSC cultures are phenotypically heterogeneous and not all cells to be utilized therapeutically can conceivably be tested in real time before utilization, it will be challenging to design and standardize a meaningful and feasible strategy to screen for genotoxicity. To overcome clonal and subclonal heterogeneity, highly sensitive methods must be used on relatively large cell samples. As described earlier, combinations of complementary techniques may provide the most meaningful information,45 and the most relevant approaches may differ when screening newly derived iPSC clones for further expansion and utilization, versus genetically corrected iPSC clones, versus cells being prepared for a master cell bank versus an actual clinical product following expansion and differentiation. It is safe to say at present that translational investigators and regulatory agencies are going to be challenged to design approaches for genotoxicity testing that will not completely preclude early stage well-designed phase і clinical trials. Moving into clinical trials or relevant large animal models will eventually be the only way to discover whether any of the mutations detected actually correlate with adverse outcomes. New laboratory data appears continuously in this fast moving area and there is an understandable desire to translate the technology into clinical applications. However, the lack of standardized iPSC derivation protocols, differentiation procedures, and consequently uncertain safety assessments of PSCs for therapeutic purposes call for a timely and close collaboration between researchers and regulators, and will likely result in iterative evolution of what is required to initiate each new clinical trial.
Preclinical models for evaluating safety and efficacy of iPSC-based therapy
To date, most experimental data on the safety and efficacy of human iPSC-derived cells is limited to xenotransplant models, specifically immunocompromised mice. However, the profoundly immunodeficient xenotransplant setting does not reflect most aspects of clinical reality, and there are numerous physiological differences between humans and mice that limit the predictive value of this model. It is impossible to study the immune response to iPSCs and their progeny in these models, and homing and tissue integration may be difficult to model when the transplanted cells and the environment are from disparate species. The rhesus macaque (Macaca mulatta) is developmentally and physiologically closely related to humans, and practically, most cytokines, antibodies, and other reagents cross-react, making translation to clinical trials more straightforward.117 Rhesus ESCs are well characterized and have begun to be studied in tissue repair models.118,119 Moreover, rhesus iPSCs resemble, unlike the leukemia inhibitory factor-dependent murine counterparts, human iPSCs in terms of derivation procedures and culture conditions.120 In the field of hematopoietic gene therapy, nonhuman primate models have proven their value in the assessment of genotoxic events due to insertional mutagenesis of viral vectors.121 We believe that preclinical development of a nonhuman primate model will provide very relevant information for moving iPSC-derived therapies forward into clinical applications.120,122 However, like clinical trials, large animal models require a complex and expensive infrastructure. Hence to reduce the number of animal studies needed in the future and move forward into clinical trials, the parallel development of validated and high-throughput in vitro models analogous to approaches applied to detect genotoxicity in the field of gene therapy is desirable.123
Suicide gene strategy in iPSC-based therapy
Given the discussed uncertainties in assessing the risk of genomic alterations in the context of iPSC-based therapies it would be beneficial to have an “exit strategy” if necessary. Introducing a suicide gene under either a constitutive or a pluripotent-specific promoter in iPSCs has been proposed to reduce tumor risk by providing a tool to eliminate vector-expressing cells both before and after transplantation.46 In the context of adoptive T-cell therapies for hematological malignancies, this concept has already been successfully translated into clinical practice.124,125 Recently, Zhong et al. introduced a suicide gene, either yeast cytosine deaminase or inducible caspase-9, into nonhuman primate iPSCs.126 They were able to effectively eliminate teratoma-initiating iPSCs in vitro and in vivo in immunodeficient mice via activation of the suicide process. This system was able to restrict tumor growth after tumor formation had already occurred in vivo. Although this system will require further optimization for its specificity, this strategy may increase the feasibility and safety of iPSC-based therapy.
Conclusions
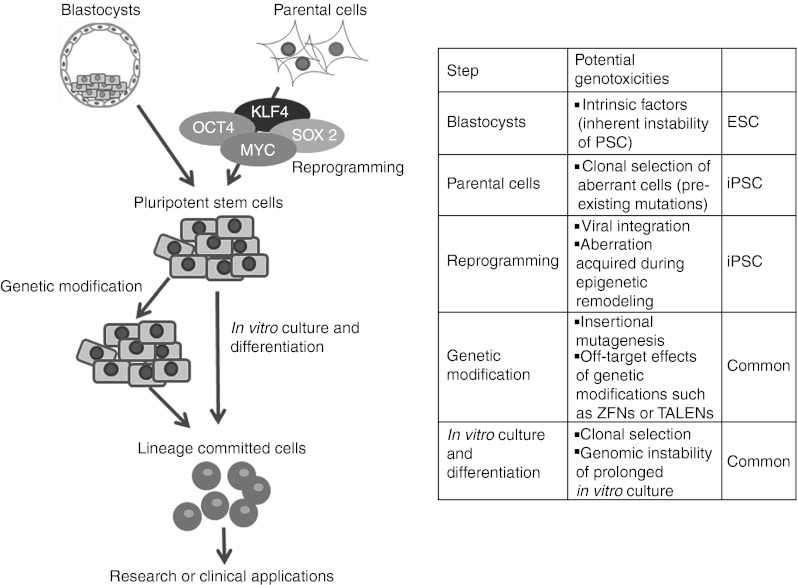
Testing for genotoxicity in iPSCs must undergo a long journey in vitro and in animal models before clinical applications can be safely contemplated. The processes of initial culture adaptation of parental cells, reprogramming, in vitro expansion, differentiation, and/or gene modification procedures may all contribute to later risks as summarized in this review (Figure 2). Therefore, regular and rational monitoring will be essential, but the field is evolving so rapidly that at present the best and most efficient approach to monitor is not yet clear and requires further translational investigation, particularly in relevant preclinical animal models.
Figure 2.
Genotoxic events implicated at each step of pluripotent stem cell (PSC) derivation and propagation. Genotoxicity in PSCs could be inherited from genomic abnormalities present in cultured embryos (in case of ESCs) or parental cells (iPSCs). Reprogramming involves global epigenetic remodeling and might affect genomic integrity of cells in the intermediate state as they move towards pluripotency. Prolonged in vitro culture and/or gene modification procedures are potential risk factors for acquiring genomic alterations in PSCs and their progeny. ESC, embryonic stem cell; iPSC, induced PSC; TALEN, transcription activator-like effector nuclease; ZFN, zinc-finger nuclease.
Acknowledgments
This work was supported by the Divisions of Intramural Research at the National Heart, Lung, and Blood Institute, and the National Center for Regenerative Medicine at the National Institutes of Health. The authors declared no conflict of interest.
References
- Takahashi K., and, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K.et al. (2007Induction of pluripotent stem cells from adult human fibroblasts by defined factors Cell 131861–872. [DOI] [PubMed] [Google Scholar]
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S.et al. (2007Induced pluripotent stem cell lines derived from human somatic cells Science 3181917–1920. [DOI] [PubMed] [Google Scholar]
- Ben-David U, Mayshar Y., and, Benvenisty N. Large-scale analysis reveals acquisition of lineage-specific chromosomal aberrations in human adult stem cells. Cell Stem Cell. 2011;9:97–102. doi: 10.1016/j.stem.2011.06.013. [DOI] [PubMed] [Google Scholar]
- Livingstone LR, White A, Sprouse J, Livanos E, Jacks T., and, Tlsty TD. Altered cell cycle arrest and gene amplification potential accompany loss of wild-type p53. Cell. 1992;70:923–935. doi: 10.1016/0092-8674(92)90243-6. [DOI] [PubMed] [Google Scholar]
- Hacein-Bey-Abina S, Von Kalle C, Schmidt M, McCormack MP, Wulffraat N, Leboulch P.et al. (2003LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1 Science 302415–419. [DOI] [PubMed] [Google Scholar]
- Ott MG, Schmidt M, Schwarzwaelder K, Stein S, Siler U, Koehl U.et al. (2006Correction of X-linked chronic granulomatous disease by gene therapy, augmented by insertional activation of MDS1-EVI1, PRDM16 or SETBP1 Nat Med 12401–409. [DOI] [PubMed] [Google Scholar]
- Boztug K, Schmidt M, Schwarzer A, Banerjee PP, Díez IA, Dewey RA.et al. (2010Stem-cell gene therapy for the Wiskott-Aldrich syndrome N Engl J Med 3631918–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe SJ, Mansour MR, Schwarzwaelder K, Bartholomae C, Hubank M, Kempski H.et al. (2008Insertional mutagenesis combined with acquired somatic mutations causes leukemogenesis following gene therapy of SCID-X1 patients J Clin Invest 1183143–3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seggewiss R, Pittaluga S, Adler RL, Guenaga FJ, Ferguson C, Pilz IH.et al. (2006Acute myeloid leukemia is associated with retroviral gene transfer to hematopoietic progenitor cells in a rhesus macaque Blood 1073865–3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkan C, Coe BP., and, Eichler EE. Genome structural variation discovery and genotyping. Nat Rev Genet. 2011;12:363–376. doi: 10.1038/nrg2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feuk L, Carson AR., and, Scherer SW. Structural variation in the human genome. Nat Rev Genet. 2006;7:85–97. doi: 10.1038/nrg1767. [DOI] [PubMed] [Google Scholar]
- Speicher MR, Gwyn Ballard S., and, Ward DC. Karyotyping human chromosomes by combinatorial multi-fluor FISH. Nat Genet. 1996;12:368–375. doi: 10.1038/ng0496-368. [DOI] [PubMed] [Google Scholar]
- Schröck E, du Manoir S, Veldman T, Schoell B, Wienberg J, Ferguson-Smith MA.et al. (1996Multicolor spectral karyotyping of human chromosomes Science 273494–497. [DOI] [PubMed] [Google Scholar]
- Iafrate AJ, Feuk L, Rivera MN, Listewnik ML, Donahoe PK, Qi Y.et al. (2004Detection of large-scale variation in the human genome Nat Genet 36949–951. [DOI] [PubMed] [Google Scholar]
- Conrad DF, Andrews TD, Carter NP, Hurles ME., and, Pritchard JK. A high-resolution survey of deletion polymorphism in the human genome. Nat Genet. 2006;38:75–81. doi: 10.1038/ng1697. [DOI] [PubMed] [Google Scholar]
- Hinds DA, Kloek AP, Jen M, Chen X., and, Frazer KA. Common deletions and SNPs are in linkage disequilibrium in the human genome. Nat Genet. 2006;38:82–85. doi: 10.1038/ng1695. [DOI] [PubMed] [Google Scholar]
- McCarroll SA, Hadnott TN, Perry GH, Sabeti PC, Zody MC, Barrett JC.et al. (2006Common deletion polymorphisms in the human genome Nat Genet 3886–92. [DOI] [PubMed] [Google Scholar]
- Korbel JO, Urban AE, Affourtit JP, Godwin B, Grubert F, Simons JF.et al. (2007Paired-end mapping reveals extensive structural variation in the human genome Science 318420–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuzun E, Sharp AJ, Bailey JA, Kaul R, Morrison VA, Pertz LM.et al. (2005Fine-scale structural variation of the human genome Nat Genet 37727–732. [DOI] [PubMed] [Google Scholar]
- Vanneste E, Voet T, Le Caignec C, Ampe M, Konings P, Melotte C.et al. (2009Chromosome instability is common in human cleavage-stage embryos Nat Med 15577–583. [DOI] [PubMed] [Google Scholar]
- Hardarson T, Caisander G, Sjögren A, Hanson C, Hamberger L., and, Lundin K. A morphological and chromosomal study of blastocysts developing from morphologically suboptimal human pre-embryos compared with control blastocysts. Hum Reprod. 2003;18:399–407. doi: 10.1093/humrep/deg092. [DOI] [PubMed] [Google Scholar]
- Santos MA, Kuijk EW., and, Macklon NS. The impact of ovarian stimulation for IVF on the developing embryo. Reproduction. 2010;139:23–34. doi: 10.1530/REP-09-0187. [DOI] [PubMed] [Google Scholar]
- Macklon NS, Geraedts JP., and, Fauser BC. Conception to ongoing pregnancy: the 'black box' of early pregnancy loss. Hum Reprod Update. 2002;8:333–343. doi: 10.1093/humupd/8.4.333. [DOI] [PubMed] [Google Scholar]
- Cowan CA, Klimanskaya I, McMahon J, Atienza J, Witmyer J, Zucker JP.et al. (2004Derivation of embryonic stem-cell lines from human blastocysts N Engl J Med 3501353–1356. [DOI] [PubMed] [Google Scholar]
- Maynard S, Swistowska AM, Lee JW, Liu Y, Liu ST, Da Cruz AB.et al. (2008Human embryonic stem cells have enhanced repair of multiple forms of DNA damage Stem Cells 262266–2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T, Chao C, Saito S, Mazur SJ, Murphy ME, Appella E.et al. (2005p53 induces differentiation of mouse embryonic stem cells by suppressing Nanog expression Nat Cell Biol 7165–171. [DOI] [PubMed] [Google Scholar]
- Cervantes RB, Stringer JR, Shao C, Tischfield JA., and, Stambrook PJ. Embryonic stem cells and somatic cells differ in mutation frequency and type. Proc Natl Acad Sci USA. 2002;99:3586–3590. doi: 10.1073/pnas.062527199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyka-Nouspikel N, Desmarais J, Gokhale PJ, Jones M, Meuth M, Andrews PW.et al. (2012Deficient DNA damage response and cell cycle checkpoints lead to accumulation of point mutations in human embryonic stem cells Stem Cells 301901–1910. [DOI] [PubMed] [Google Scholar]
- Buzzard JJ, Gough NM, Crook JM., and, Colman A. Karyotype of human ES cells during extended culture. Nat Biotechnol. 2004;22:381–2; author reply 382. doi: 10.1038/nbt0404-381. [DOI] [PubMed] [Google Scholar]
- Rosler ES, Fisk GJ, Ares X, Irving J, Miura T, Rao MS.et al. (2004Long-term culture of human embryonic stem cells in feeder-free conditions Dev Dyn 229259–274. [DOI] [PubMed] [Google Scholar]
- Mitalipova MM, Rao RR, Hoyer DM, Johnson JA, Meisner LF, Jones KL.et al. (2005Preserving the genetic integrity of human embryonic stem cells Nat Biotechnol 2319–20. [DOI] [PubMed] [Google Scholar]
- Baker DE, Harrison NJ, Maltby E, Smith K, Moore HD, Shaw PJ.et al. (2007Adaptation to culture of human embryonic stem cells and oncogenesis in vivo Nat Biotechnol 25207–215. [DOI] [PubMed] [Google Scholar]
- Draper JS, Smith K, Gokhale P, Moore HD, Maltby E, Johnson J.et al. (2004Recurrent gain of chromosomes 17q and 12 in cultured human embryonic stem cells Nat Biotechnol 2253–54. [DOI] [PubMed] [Google Scholar]
- Spits C, Mateizel I, Geens M, Mertzanidou A, Staessen C, Vandeskelde Y.et al. (2008Recurrent chromosomal abnormalities in human embryonic stem cells Nat Biotechnol 261361–1363. [DOI] [PubMed] [Google Scholar]
- Hanson C., and, Caisander G. Human embryonic stem cells and chromosome stability. APMIS. 2005;113:751–755. doi: 10.1111/j.1600-0463.2005.apm_305.x. [DOI] [PubMed] [Google Scholar]
- Lefort N, Feyeux M, Bas C, Féraud O, Bennaceur-Griscelli A, Tachdjian G.et al. (2008Human embryonic stem cells reveal recurrent genomic instability at 20q11.21 Nat Biotechnol 261364–1366. [DOI] [PubMed] [Google Scholar]
- O'Neill LP, VerMilyea MD., and, Turner BM. Epigenetic characterization of the early embryo with a chromatin immunoprecipitation protocol applicable to small cell populations. Nat Genet. 2006;38:835–841. doi: 10.1038/ng1820. [DOI] [PubMed] [Google Scholar]
- Maitra A, Arking DE, Shivapurkar N, Ikeda M, Stastny V, Kassauei K.et al. (2005Genomic alterations in cultured human embryonic stem cells Nat Genet 371099–1103. [DOI] [PubMed] [Google Scholar]
- Plaia TW, Josephson R, Liu Y, Zeng X, Ording C, Toumadje A.et al. (2006Characterization of a new NIH-registered variant human embryonic stem cell line, BG01V: a tool for human embryonic stem cell research Stem Cells 24531–546. [DOI] [PubMed] [Google Scholar]
- Herszfeld D, Wolvetang E, Langton-Bunker E, Chung TL, Filipczyk AA, Houssami S.et al. (2006CD30 is a survival factor and a biomarker for transformed human pluripotent stem cells Nat Biotechnol 24351–357. [DOI] [PubMed] [Google Scholar]
- Yang S, Lin G, Tan YQ, Zhou D, Deng LY, Cheng DH.et al. (2008Tumor progression of culture-adapted human embryonic stem cells during long-term culture Genes Chromosomes Cancer 47665–679. [DOI] [PubMed] [Google Scholar]
- Werbowetski-Ogilvie TE, Bossé M, Stewart M, Schnerch A, Ramos-Mejia V, Rouleau A.et al. (2009Characterization of human embryonic stem cells with features of neoplastic progression Nat Biotechnol 2791–97. [DOI] [PubMed] [Google Scholar]
- Sachlos E, Risueño RM, Laronde S, Shapovalova Z, Lee JH, Russell J.et al. (2012Identification of drugs including a dopamine receptor antagonist that selectively target cancer stem cells Cell 1491284–1297. [DOI] [PubMed] [Google Scholar]
- Lefort N, Perrier AL, Laâbi Y, Varela C., and, Peschanski M. Human embryonic stem cells and genomic instability. Regen Med. 2009;4:899–909. doi: 10.2217/rme.09.63. [DOI] [PubMed] [Google Scholar]
- Wu C., and, Dunbar CE. Stem cell gene therapy: the risks of insertional mutagenesis and approaches to minimize genotoxicity. Front Med. 2011;5:356–371. doi: 10.1007/s11684-011-0159-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochedlinger K., and, Plath K. Epigenetic reprogramming and induced pluripotency. Development. 2009;136:509–523. doi: 10.1242/dev.020867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varas F, Stadtfeld M, de Andres-Aguayo L, Maherali N, di Tullio A, Pantano L.et al. (2009Fibroblast-derived induced pluripotent stem cells show no common retroviral vector insertions Stem Cells 27300–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoi T, Yae K, Nakagawa M, Ichisaka T, Okita K, Takahashi K.et al. (2008Generation of pluripotent stem cells from adult mouse liver and stomach cells Science 321699–702. [DOI] [PubMed] [Google Scholar]
- Winkler T, Cantilena A, Métais JY, Xu X, Nguyen AD, Borate B.et al. (2010No evidence for clonal selection due to lentiviral integration sites in human induced pluripotent stem cells Stem Cells 28687–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Wu S, Joo JY, Zhu S, Han DW, Lin T.et al. (2009Generation of induced pluripotent stem cells using recombinant proteins Cell Stem Cell 4381–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bru T, Clarke C, McGrew MJ, Sang HM, Wilmut I., and, Blow JJ. Rapid induction of pluripotency genes after exposure of human somatic cells to mouse ES cell extracts. Exp Cell Res. 2008;314:2634–2642. doi: 10.1016/j.yexcr.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakubov E, Rechavi G, Rozenblatt S., and, Givol D. Reprogramming of human fibroblasts to pluripotent stem cells using mRNA of four transcription factors. Biochem Biophys Res Commun. 2010;394:189–193. doi: 10.1016/j.bbrc.2010.02.150. [DOI] [PubMed] [Google Scholar]
- Warren L, Manos PD, Ahfeldt T, Loh YH, Li H, Lau F.et al. (2010Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA Cell Stem Cell 7618–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anokye-Danso F, Trivedi CM, Juhr D, Gupta M, Cui Z, Tian Y.et al. (2011Highly efficient miRNA-mediated reprogramming of mouse and human somatic cells to pluripotency Cell Stem Cell 8376–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SL, Chang DC, Lin CH, Ying SY, Leu D., and, Wu DT. Regulation of somatic cell reprogramming through inducible mir-302 expression. Nucleic Acids Res. 2011;39:1054–1065. doi: 10.1093/nar/gkq850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W., and, Freed CR. Adenoviral gene delivery can reprogram human fibroblasts to induced pluripotent stem cells. Stem Cells. 2009;27:2667–2674. doi: 10.1002/stem.201. [DOI] [PubMed] [Google Scholar]
- Fusaki N, Ban H, Nishiyama A, Saeki K., and, Hasegawa M. Efficient induction of transgene-free human pluripotent stem cells using a vector based on Sendai virus, an RNA virus that does not integrate into the host genome. Proc Jpn Acad, Ser B, Phys Biol Sci. 2009;85:348–362. doi: 10.2183/pjab.85.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woltjen K, Michael IP, Mohseni P, Desai R, Mileikovsky M, Hämäläinen R.et al. (2009piggyBac transposition reprograms fibroblasts to induced pluripotent stem cells Nature 458766–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Hu K, Smuga-Otto K, Tian S, Stewart R, Slukvin II.et al. (2009Human induced pluripotent stem cells free of vector and transgene sequences Science 324797–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers A, Jean JC, Sommer CA, Omari A, Ford CC, Mills JA.et al. (2010Generation of transgene-free lung disease-specific human induced pluripotent stem cells using a single excisable lentiviral stem cell cassette Stem Cells 281728–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papapetrou EP, Lee G, Malani N, Setty M, Riviere I, Tirunagari LM.et al. (2011Genomic safe harbors permit high ß-globin transgene expression in thalassemia induced pluripotent stem cells Nat Biotechnol 2973–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okita K, Ichisaka T., and, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–317. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- Tong M, Lv Z, Liu L, Zhu H, Zheng QY, Zhao XY.et al. (2011Mice generated from tetraploid complementation competent iPS cells show similar developmental features as those from ES cells but are prone to tumorigenesis Cell Res 211634–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papapetrou EP., and, Sadelain M. Generation of transgene-free human induced pluripotent stem cells with an excisable single polycistronic vector. Nat Protoc. 2011;6:1251–1273. doi: 10.1038/nprot.2011.374. [DOI] [PubMed] [Google Scholar]
- Sommer CA, Sommer AG, Longmire TA, Christodoulou C, Thomas DD, Gostissa M.et al. (2010Excision of reprogramming transgenes improves the differentiation potential of iPS cells generated with a single excisable vector Stem Cells 2864–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soldner F, Hockemeyer D, Beard C, Gao Q, Bell GW, Cook EG.et al. (2009Parkinson's disease patient-derived induced pluripotent stem cells free of viral reprogramming factors Cell 136964–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayshar Y, Ben-David U, Lavon N, Biancotti JC, Yakir B, Clark AT.et al. (2010Identification and classification of chromosomal aberrations in human induced pluripotent stem cells Cell Stem Cell 7521–531. [DOI] [PubMed] [Google Scholar]
- Taapken SM, Nisler BS, Newton MA, Sampsell-Barron TL, Leonhard KA, McIntire EM.et al. (2011Karotypic abnormalities in human induced pluripotent stem cells and embryonic stem cells Nat Biotechnol 29313–314. [DOI] [PubMed] [Google Scholar]
- Yamanaka S. Elite and stochastic models for induced pluripotent stem cell generation. Nature. 2009;460:49–52. doi: 10.1038/nature08180. [DOI] [PubMed] [Google Scholar]
- Wernig M, Lengner CJ, Hanna J, Lodato MA, Steine E, Foreman R.et al. (2008A drug-inducible transgenic system for direct reprogramming of multiple somatic cell types Nat Biotechnol 26916–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maherali N, Ahfeldt T, Rigamonti A, Utikal J, Cowan C., and, Hochedlinger K. A high-efficiency system for the generation and study of human induced pluripotent stem cells. Cell Stem Cell. 2008;3:340–345. doi: 10.1016/j.stem.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussein SM, Batada NN, Vuoristo S, Ching RW, Autio R, Närvä E.et al. (2011Copy number variation and selection during reprogramming to pluripotency Nature 47158–62. [DOI] [PubMed] [Google Scholar]
- Laurent LC, Ulitsky I, Slavin I, Tran H, Schork A, Morey R.et al. (2011Dynamic changes in the copy number of pluripotency and cell proliferation genes in human ESCs and iPSCs during reprogramming and time in culture Cell Stem Cell 8106–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins-Taylor K, Nisler BS, Taapken SM, Compton T, Crandall L, Montgomery KD.et al. (2011Recurrent copy number variations in human induced pluripotent stem cells Nat Biotechnol 29488–491. [DOI] [PubMed] [Google Scholar]
- Young MA, Larson DE, Sun CW, George DR, Ding L, Miller CA.et al. (2012Background mutations in parental cells account for most of the genetic heterogeneity of induced pluripotent stem cells Cell Stem Cell 10570–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L, Hansen NF, Zhao L, Du Y, Zou C, Donovan FX.et al. (2012Low incidence of DNA sequence variation in human induced pluripotent stem cells generated by nonintegrating plasmid expression Cell Stem Cell 10337–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan AR, Boland MJ, Leibowitz ML, Shumilina S, Pehrson SM, Baldwin KK.et al. (2011Genome sequencing of mouse induced pluripotent stem cells reveals retroelement stability and infrequent DNA rearrangement during reprogramming Cell Stem Cell 9366–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore A, Li Z, Fung HL, Young JE, Agarwal S, Antosiewicz-Bourget J.et al. (2011Somatic coding mutations in human induced pluripotent stem cells Nature 47163–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L, Ellis MJ, Li S, Larson DE, Chen K, Wallis JW.et al. (2010Genome remodelling in a basal-like breast cancer metastasis and xenograft Nature 464999–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W, Jiang Z, Liu J, Haverty PM, Guan Y, Stinson J.et al. (2010The mutation spectrum revealed by paired genome sequences from a lung cancer patient Nature 465473–477. [DOI] [PubMed] [Google Scholar]
- Pleasance ED, Cheetham RK, Stephens PJ, McBride DJ, Humphray SJ, Greenman CD.et al. (2010A comprehensive catalogue of somatic mutations from a human cancer genome Nature 463191–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenman C, Stephens P, Smith R, Dalgliesh GL, Hunter C, Bignell G.et al. (2007Patterns of somatic mutation in human cancer genomes Nature 446153–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi A, Park IH, Wen B, Murakami P, Aryee MJ, Irizarry R.et al. (2009Differential methylation of tissue- and cancer-specific CpG island shores distinguishes human induced pluripotent stem cells, embryonic stem cells and fibroblasts Nat Genet 411350–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishino K, Toyoda M, Yamazaki-Inoue M, Fukawatase Y, Chikazawa E, Sakaguchi H.et al. (2011DNA methylation dynamics in human induced pluripotent stem cells over time PLoS Genet 7e1002085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wissing S, Muñoz-Lopez M, Macia A, Yang Z, Montano M, Collins W.et al. (2012Reprogramming somatic cells into iPS cells activates LINE-1 retroelement mobility Hum Mol Genet 21208–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji J, Ng SH, Sharma V, Neculai D, Hussein S, Sam M.et al. (2012Elevated coding mutation rate during the reprogramming of human somatic cells into induced pluripotent stem cells Stem Cells 30435–440. [DOI] [PubMed] [Google Scholar]
- Momcilovic O, Knobloch L, Fornsaglio J, Varum S, Easley C., and, Schatten G. DNA damage responses in human induced pluripotent stem cells and embryonic stem cells. PLoS ONE. 2010;5:e13410. doi: 10.1371/journal.pone.0013410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Robert C, Jang YY, Liu H, Sharkis S, Baylin SB.et al. (2011Human induced pluripotent cells resemble embryonic stem cells demonstrating enhanced levels of DNA repair and efficacy of nonhomologous end-joining Mutat Res 7138–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo LZ, Gopalakrishna-Pillai S, Nay SL, Park SW, Bates SE, Zeng X.et al. (2012DNA repair in human pluripotent stem cells is distinct from that in non-pluripotent human cells PLoS ONE 7e30541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utikal J, Polo JM, Stadtfeld M, Maherali N, Kulalert W, Walsh RM.et al. (2009Immortalization eliminates a roadblock during cellular reprogramming into iPS cells Nature 4601145–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi L, Lu C, Hu W, Sun Y., and, Levine AJ. Multiple Roles of p53-Related Pathways in Somatic Cell Reprogramming and Stem Cell Differentiation. Cancer Res. 2012;72:5635–5645. doi: 10.1158/0008-5472.CAN-12-1451. [DOI] [PubMed] [Google Scholar]
- Marión RM, Strati K, Li H, Murga M, Blanco R, Ortega S.et al. (2009A p53-mediated DNA damage response limits reprogramming to ensure iPS cell genomic integrity Nature 4601149–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasi CE, Dereli-öz A, Negrini S, Friedli M, Fragola G, Lombardo A.et al. (2011Genomic instability in induced stem cells Cell Death Differ 18745–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin MH, Mason MJ, Xie W, Volinia S, Singer M, Peterson C.et al. (2009Induced pluripotent stem cells and embryonic stem cells are distinguished by gene expression signatures Cell Stem Cell 5111–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna J, Wernig M, Markoulaki S, Sun CW, Meissner A, Cassady JP.et al. (2007Treatment of sickle cell anemia mouse model with iPS cells generated from autologous skin Science 3181920–1923. [DOI] [PubMed] [Google Scholar]
- Nelson TJ, Martinez-Fernandez A, Yamada S, Perez-Terzic C, Ikeda Y., and, Terzic A. Repair of acute myocardial infarction by human stemness factors induced pluripotent stem cells. Circulation. 2009;120:408–416. doi: 10.1161/CIRCULATIONAHA.109.865154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernig M, Zhao JP, Pruszak J, Hedlund E, Fu D, Soldner F.et al. (2008Neurons derived from reprogrammed fibroblasts functionally integrate into the fetal brain and improve symptoms of rats with Parkinson's disease Proc Natl Acad Sci USA 1055856–5861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D, Alipio Z, Fink LM, Adcock DM, Yang J, Ward DC.et al. (2009Phenotypic correction of murine hemophilia A using an iPS cell-based therapy Proc Natl Acad Sci USA 106808–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian Q, Zhang Y, Zhang J, Zhang HK, Wu X, Zhang Y.et al. (2010Functional mesenchymal stem cells derived from human induced pluripotent stem cells attenuate limb ischemia in mice Circulation 1211113–1123. [DOI] [PubMed] [Google Scholar]
- An MC, Zhang N, Scott G, Montoro D, Wittkop T, Mooney S.et al. (2012Genetic correction of Huntington's disease phenotypes in induced pluripotent stem cells Cell Stem Cell 11253–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin J., and, Lako M. Concise review: putting a finger on stem cell biology: zinc finger nuclease-driven targeted genetic editing in human pluripotent stem cells. Stem Cells. 2011;29:1021–1033. doi: 10.1002/stem.658. [DOI] [PubMed] [Google Scholar]
- Zou J, Maeder ML, Mali P, Pruett-Miller SM, Thibodeau-Beganny S, Chou BK.et al. (2009Gene targeting of a disease-related gene in human induced pluripotent stem and embryonic stem cells Cell Stem Cell 597–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou J, Sweeney CL, Chou BK, Choi U, Pan J, Wang H.et al. (2011Oxidase-deficient neutrophils from X-linked chronic granulomatous disease iPS cells: functional correction by zinc finger nuclease-mediated safe harbor targeting Blood 1175561–5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusa K, Rashid ST, Strick-Marchand H, Varela I, Liu PQ, Paschon DE.et al. (2011Targeted gene correction of a1-antitrypsin deficiency in induced pluripotent stem cells Nature 478391–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockemeyer D, Wang H, Kiani S, Lai CS, Gao Q, Cassady JP.et al. (2011Genetic engineering of human pluripotent cells using TALE nucleases Nat Biotechnol 29731–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlem TJ, Hoshijima K, Jurynec MJ, Gunther D, Starker CG, Locke AS.et al. (2012Simple methods for generating and detecting locus-specific mutations induced with TALENs in the zebrafish genome PLoS Genet 8e1002861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mussolino C, Morbitzer R, Lütge F, Dannemann N, Lahaye T., and, Cathomen T. A novel TALE nuclease scaffold enables high genome editing activity in combination with low toxicity. Nucleic Acids Res. 2011;39:9283–9293. doi: 10.1093/nar/gkr597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockemeyer D, Soldner F, Beard C, Gao Q, Mitalipova M, DeKelver RC.et al. (2009Efficient targeting of expressed and silent genes in human ESCs and iPSCs using zinc-finger nucleases Nat Biotechnol 27851–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattanayak V, Ramirez CL, Joung JK., and, Liu DR. Revealing off-target cleavage specificities of zinc-finger nucleases by in vitro selection. Nat Methods. 2011;8:765–770. doi: 10.1038/nmeth.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel R, Lombardo A, Arens A, Miller JC, Genovese P, Kaeppel C.et al. (2011An unbiased genome-wide analysis of zinc-finger nuclease specificity Nat Biotechnol 29816–823. [DOI] [PubMed] [Google Scholar]
- Ruby KM., and, Zheng B. Gene targeting in a HUES line of human embryonic stem cells via electroporation. Stem Cells. 2009;27:1496–1506. doi: 10.1002/stem.73. [DOI] [PubMed] [Google Scholar]
- Fischer Y, Ganic E, Ameri J, Xian X, Johannesson M., and, Semb H. NANOG reporter cell lines generated by gene targeting in human embryonic stem cells. PLoS ONE. 2010;5:e12533. doi: 10.1371/journal.pone.0012533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H, Chung SK., and, Xu Y. Modeling disease in human ESCs using an efficient BAC-based homologous recombination system. Cell Stem Cell. 2010;6:80–89. doi: 10.1016/j.stem.2009.11.016. [DOI] [PubMed] [Google Scholar]
- Varela C, Denis JA, Polentes J, Feyeux M, Aubert S, Champon B.et al. (2012Recurrent genomic instability of chromosome 1q in neural derivatives of human embryonic stem cells J Clin Invest 122569–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao T, Zhang ZN, Rong Z., and, Xu Y. Immunogenicity of induced pluripotent stem cells. Nature. 2011;474:212–215. doi: 10.1038/nature10135. [DOI] [PubMed] [Google Scholar]
- Donahue RE., and, Dunbar CE. Update on the use of nonhuman primate models for preclinical testing of gene therapy approaches targeting hematopoietic cells. Hum Gene Ther. 2001;12:607–617. doi: 10.1089/104303401300057289. [DOI] [PubMed] [Google Scholar]
- Blin G, Nury D, Stefanovic S, Neri T, Guillevic O, Brinon B.et al. (2010A purified population of multipotent cardiovascular progenitors derived from primate pluripotent stem cells engrafts in postmyocardial infarcted nonhuman primates J Clin Invest 1201125–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson JA, Kalishman J, Golos TG, Durning M, Harris CP, Becker RA.et al. (1995Isolation of a primate embryonic stem cell line Proc Natl Acad Sci USA 927844–7848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Zhu F, Yong J, Zhang P, Hou P, Li H.et al. (2008Generation of induced pluripotent stem cells from adult rhesus monkey fibroblasts Cell Stem Cell 3587–590. [DOI] [PubMed] [Google Scholar]
- Calmels B, Ferguson C, Laukkanen MO, Adler R, Faulhaber M, Kim HJ.et al. (2005Recurrent retroviral vector integration at the Mds1/Evi1 locus in nonhuman primate hematopoietic cells Blood 1062530–2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong B, Trobridge GD, Zhang X, Watts KL, Ramakrishnan A, Wohlfahrt M.et al. (2011Efficient generation of nonhuman primate induced pluripotent stem cells Stem Cells Dev 20795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modlich U, Bohne J, Schmidt M, von Kalle C, Knöss S, Schambach A.et al. (2006Cell-culture assays reveal the importance of retroviral vector design for insertional genotoxicity Blood 1082545–2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Stasi A, Tey SK, Dotti G, Fujita Y, Kennedy-Nasser A, Martinez C.et al. (2011Inducible apoptosis as a safety switch for adoptive cell therapy N Engl J Med 3651673–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonini C, Ferrari G, Verzeletti S, Servida P, Zappone E, Ruggieri L.et al. (1997HSV-TK gene transfer into donor lymphocytes for control of allogeneic graft-versus-leukemia Science 2761719–1724. [DOI] [PubMed] [Google Scholar]
- Zhong B, Watts KL, Gori JL, Wohlfahrt ME, Enssle J, Adair JE.et al. (2011Safeguarding nonhuman primate iPS cells with suicide genes Mol Ther 191667–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-David U., and, Benvenisty N. High prevalence of evolutionarily conserved and species-specific genomic aberrations in mouse pluripotent stem cells. Stem Cells. 2012;30:612–622. doi: 10.1002/stem.1057. [DOI] [PubMed] [Google Scholar]