Abstract
Radiation-induced lung injury (RILI) presents a common and major obstacle in the radiotherapy of thoracic cancers. The aim of this study was to examine whether RILI could be alleviated by mesenchymal stem cells (MSCs) expressing soluble transforming growth factor-β (TGF-β) type II receptor via an adenovirus (Ad-sTβR). Here, we systemically administered male MSCs into female mice challenged with thoracic irradiation. The data showed that either MSCs or Ad-sTβR transduced MSCs (Ad-sTβR-MSCs) specifically migrated into radiation-injured lung. Ad-sTβR-MSCs obviously alleviated lung injury, as reflected by survival and histopathology data, as well as the assays of malondialdehyde (MDA), hydroxyproline, plasma cytokines, and the expression of connective tissue growth factor (CTGF) and α-smooth muscle actin (α-SMA). Furthermore, MSCs and Ad-sTβR-MSCs could adopt the characteristics of alveolar type II (ATII) cells. However, the MSCs levels in the lungs were relatively low to account for the noted therapeutic effects, suggesting the presence of other mechanisms. In vivo, MSCs-conditioned medium (MSCs CM) significantly attenuated RILI. In vitro, MSCs CM protected ATII cells against radiation-induced apoptosis and DNA damage, and modulated the inflammatory response, indicating the beneficial effects of MSCs are largely due to its paracrine activity. Our results provide a novel insight for RILI therapy that currently lack efficient treatments.
Introduction
Radiation therapy (RT) is an important treatment for thoracic cancers, but could damage the lungs due to the generation of reactive oxygen species and the subsequent inflammation and fibrosis.1 Radiation-induced lung injury (RILI) remains a common and major obstacle in the application of thoracic radiation, resulting in considerable morbidity and limiting the dose of radiation. Thus, alleviating RILI is critical to improve both tumor control and patient quality of life.1,2
RILI is a complex pathological process, resulting in an early radiation pneumonitis and late pulmonary fibrosis.3 One of the hallmarks of RILI is the induction and activation of various cytokines, chemokines, and growth factors,2,4 including transforming growth factor-β1 (TGF-β1).5,6 TGF-β1 is a multifunctional cytokine that modulates the infiltration of inflammatory cells, production of cytokines, proliferation of fibroblasts, deposition of collagen, and epithelial-mesenchymal transition.7 Elevation of plasma TGF-β1 levels during RT predicts RILI in patients with non-small cell lung cancer.8 In the present study, TGF-β1 was increased in the murine lungs upon irradiation (Figure 1a). TGF-β1 binds to the extracellular domain of TGF-β type II receptor (Ex-TβRII) and activates the downstream signal transduction (Figure 1b).9,10 Blocking the binding of TGF-β1 to Ex-TβRII, by means of administration of soluble TGF-β type II receptor (sTβR)9,10,11 or the anti-TGFβ1 antibody 1D11,12 has become an option for ameliorating RILI. Given the difficulties in achieving adequate delivery to the damaged lungs without unacceptable systemic effects, it is necessary to develop a more specific approach to deliver sTβR.
Figure 1.
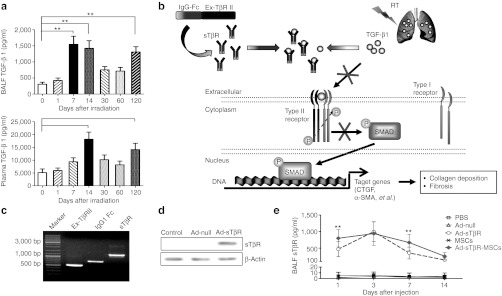
Radiation-induced lung injury (RILI) was associated with transforming growth factor-β1 (TGF-β1) expression. (a) The time course of active TGF-β1 levels in the bronchoalveolar lavage fluid (BALF) (upper panel) and plasma (lower panel) after thoracic irradiation, as measured by enzyme-linked immunosorbent assay (ELISA) (*P < 0.05, **P < 0.01, n = 6). (b) Schematic representation of action of sTβR. The active TGF-β1 initially binds to the extracellular domain of TGF-β type II receptor (Ex-TβRII), recruits and phosphorylates the type I receptor to form heteromeric complexes, thus triggering the subsequent activation of downstream signal transduction. sTβR competes with TβRII to bind with active TGF-β1, subsequently blocking the effects of TGF-β1, which plays a crucial role in collagen deposition and fibrosis. (c) Adenovirus construction. PCR products of Ex-TβRII, IgG1 Fc, and fusion gene sTβR were detected by agarose gel electrophoresis. (d) Western blot analysis of sTβR protein in 293 cells 48 hours after Ad-sTβR, Ad-null transduction. (e) The sTβR levels in BALF were assayed by ELISA after administrations of phosphate-buffered saline (PBS), Ad-null (1.25 × 108 PFU), Ad-sTβR (1.25 × 108 PFU), mesenchymal stem cells (MSCs) (5 × 105), and Ad-sTβR-MSCs (1.25 × 108 PFU, 5 × 105 cells) (*P < 0.05, **P < 0.01, Ad-sTβR-MSCs versus Ad-sTβR; n = 6).
Mesenchymal stem cells (MSCs) show significant potential for clinical utility, due to their convenient isolation and culture, low immunogenicity, regenerative and multiple differentiation abilities, and potent immunosuppressive effects.13 MSCs could home to the injured lungs and adopt the specific lung cell phenotypes,14 and alleviate lung injury induced by bleomycin, endotoxin, or hyperoxia.15,16,17,18,19,20 However, mechanisms for this protection are not restricted to the engraftment and differentiation of MSCs. Importantly, MSCs repair lung injury through secreting anti-inflammatory and reparative growth factors, and also cell-to-cell contacts.16,17,19,20 These properties make MSCs as a promising candidate for the treatment of RILI.
Recent studies have demonstrated that therapeutic genes modified MSCs could efficiently deliver target genes to the injured sites and enhance therapeutic effects.21,22,23,24,25 In the present study, we combined two therapeutic strategies, namely MSCs treatment and sTβR overexpression, and identified the efficacy of genetically modified MSCs on treating RILI in a mouse model.
Results
Lung radiation-increased TGF-β1 production
Radiation caused an elevation of TGF-β1 in the bronchoalveolar lavage fluid and plasma of C57BL/6 mice. There were two peaks in TGF-β1 in the bronchoalveolar lavage fluid: at 7–14 and 120 days after the irradiation (Figure 1a, upper panel), respectively. Plasma TGF-β1 also displayed two peaks (Figure 1a, lower panel).
Ad-sTβR-MSCs remained the characteristics of plain MSCs
The cDNA sequences that encode extracellular domain of TβRII (Ex-TβRII) and IgG1 Fc fragment were fused by overlap PCR to generate the fusion gene sTβR (Figure 1c). A recombinant adenoviral vector expressing the transgene sTβR (Ad-sTβR) was constructed, and the adenoviral vector containing no transgene (Ad-null) was used as a control. The expression of the fusion protein sTβR was evident after Ad-sTβR transduction in 293 cells (Figure 1d). sTβR concentrations in bronchoalveolar lavage fluid were obviously elevated following administration of Ad-sTβR or Ad-sTβR-MSCs, and were very low or barely detectable following administration of phosphate-buffered saline (PBS), Ad-null, or MSCs (Figure 1e).
The isolated male MSCs could be induced to differentiate into osteocytes, adipocytes, and chondrocytes (Figure 2a). Similar to typical MSCs, the MSCs derived in our experiments were positive for Sca-1, CD44, and CD29 and were negative for CD31, CD45, CD11b, and CD117 (Figure 2b), as revealed by fluorescence-activated cell sorting.
Figure 2.
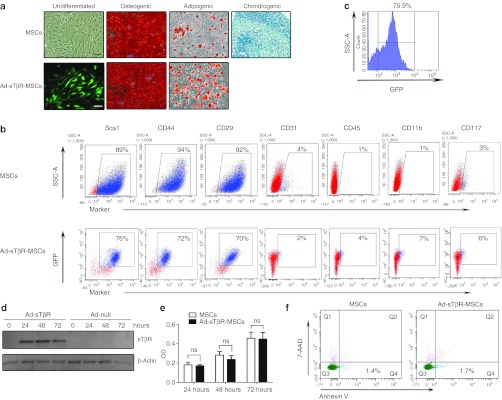
Characterization of mesenchymal stem cells (MSCs) and Ad-sTβR-MSCs. (a) The differentiation of MSCs and Ad-sTβR-MSCs into osteocytes, adipocytes, and chondrocytes. Bar = 100 µm. (b) Fluorescence-activated cell sorting (FACS) analysis of the cell surface markers. (c) FACS analysis of the GFP-positive cells in MSCs transduced with adenovirus. (d) Western blot analysis of sTβR protein in MSCs transduced with adenovirus for 0 hour, 24 hours, 48 hours, and 72 hours. (e) The proliferative abilities of MSCs and Ad-sTβR-MSCs were determined by MTT assay (ns, not significant; n = 3). (f) The apoptosis of MSCs transduced with or without Ad-sTβR for 48 hours was detected by FACS, Annexin V+/7-AAD– cells represented early stage apoptotic cells.
The transduction condition in MSCs was optimized. A moderate multiplicity of infection of 250 produced 75.9% transduction of MSCs on day 2 (Figure 2c) and without apparent effects on cell morphology (data not shown), and was used in the further experiments. Western blot analysis revealed the presence of sTβR protein in MSCs transduced with Ad-sTβR (Figure 2d).
Transduction of Ad-sTβR did not affect cell proliferation (Figure 2e), apoptosis (Figure 2f), differentiation ability and surface markers of MSCs, with the only exception of chondrogenic differentiation (Figure 2a, b). TGF-β was essential in the differentiation of MSCs into chondrocytes, and Ad-sTβR blocked the effect of TGF-β and therefore affected the chondrogenic differentiation of MSCs.
MSCs migrated toward the lungs injured by radiation
To learn whether MSCs could specifically home to injured tissues, we quantified the rate of male MSCs in the lungs and other tissues of female mice 30 days after the irradiation (Figure 3a). Standard curves of Y6 (representing male MSCs DNA) and GAPDH (representing total mouse DNA), generated by diluting male genomic DNA into female genomic DNA, were used as reference controls (Supplementary Figure S1). Higher percentage of male MSCs was detected in the lungs as compared to in the hearts (0.10% versus 0.02%), and even lower in other tissues (Figure 3b).
Figure 3.

The homing capacity of mesenchymal stem cells (MSCs) and Ad-sTβR-MSCs. (a) Time-line for the experiment in vivo. Phosphate-buffered saline (PBS), MSCs (5 × 105), and Ad-sTβR-MSCs (1.25 × 108 PFU, 5 × 105 cells) were injected into female mice immediately and 14 days after radiation, and detections were carried out on day 30 after radiation. (b) Quantification of MSCs rates in different tissues of irradiated mice with MSCs treatment by real-time PCR analysis. The MSCs levels in the lungs were served as control (*P < 0.05, **P < 0.01, n = 5–6). (c) Quantification of MSCs and Ad-sTβR-MSCs in lungs by real-time PCR analysis. The mice without radiation were injected with MSCs or Ad-sTβR-MSCs, and served as controls (ns, not significant; *P < 0.05, **P < 0.01, n = 5-6). (d) The migration of MSCs and Ad-sTβR-MSCs toward DMEM, uninjured lungs and the lungs from mice 30 days after radiation therapy (RT) (RT 30d lung), as determined by transwell assays (#P < 0.05, ##P < 0.01, RT 30d lung versus uninjured lung in each group; ns, not significant; n = 5–6). (e) SDF-1α levels in bronchoalveolar lavage fluid (BALF) and plasma were assayed by enzyme-linked immunosorbent assay (ELISA) 30 days after radiation (*P < 0.05, **P < 0.01, n = 5–6). (f) Quantification of MSCs and Ad-sTβR-MSCs levels in lungs 30 days after irradiation with administration of AMD3100 (200 µg in 250 µl PBS per dose, three times per week) (ns, not significant; n = 5-6). (g) The migration of MSCs and Ad-sTβR-MSCs in the presences of SDF-1α (100 ng/ml) or plus AMD3100 (100 µg/ml) were determined by transwell assays. (ns, not significant; *P < 0.05, **P < 0.01, n = 5–6).
Only few MSCs were detected in the lungs of nonirradiated mice receiving MSCs and Ad-sTβR-MSCs. The irradiation induced 20- and 15-fold increases of MSCs in the lungs in mice receiving MSCs and Ad-sTβR-MSCs, respectively (Figure 3c). In ex vivo experiments, MSCs migrated toward the lungs collected on day 30 after RT (RT 30d lung) (Figure 3d). Transduction of Ad-sTβR did not alter the homing and migration capacities of MSCs (Figure 3c, d).
We next examined the roles of SDF-1α/CXCR4 axis, a signaling pathway associated with the active recruitment of stem cells,13,26 in the homing of MSCs to irradiated lungs. On day 30 after radiation, the SDF-1α levels in bronchoalveolar lavage fluid and plasma were significantly increased (Figure 3e). The number of MSCs in irradiated lungs was reduced by cotreatment with the CXCR4 antagonist AMD3100 (Figure 3f). In vitro experiments, SDF-1α induced marked migration of MSCs and Ad-sTβR-MSCs; cotreatment with AMD3100 blocked the migration induced by SDF-1α (Figure 3g). Furthermore, transduction of Ad-sTβR did not affect the migration of MSCs both in vivo and in vitro (Figure 3f, g). These data suggested that the increased MSCs in the lungs reflected active radiation-induced homing of MSCs rather than physical entrapment of circulating MSCs.
Ad-sTβR-MSCs attenuated early lung injury
Exudation of inflammatory cells in the alveolar septa was apparent on days 30 after irradiation (Figure 4b). Such a change decreased over time in the mice treated with either MSCs or Ad-sTβR-MSCs. Treatment with Ad-sTβR-MSCs seemed superior to MSCs for decreasing the alveolar thickness (a measure of the lung damage) (Figure 4c), but the difference was not statistically significant.
Figure 4.
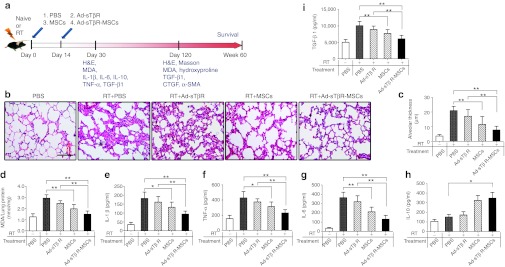
Protective effects of Ad-sTβR-MSCs on radiation-induced lung injury (RILI) 30 days after radiation. (a) Schematic representation of the therapeutic potential of Ad-sTβR-MSCs on RILI. (b) Histological assessment of Ad-sTβR-MSCs treatment on RILI, representative photomicrographs of hematoxylin and eosin (H&E) staining. Bar = 50 µm. (c) The radiation-induced lung damage was determined by measuring the alveolar thickness (*P < 0.05, **P < 0.01, n = 5). (d) Malondialdehyde (MDA) levels in lung homogenates (*P < 0.05, **P < 0.01, n = 5). (e–i) The concentrations of (e) plasma IL-1β, (f) tumor necrosis factor-α (TNF-α), (g) IL-6, (h) IL-10, and active (i) TGF-β1 were determined by enzyme-linked immunosorbent assay (ELISA) (*P < 0.05, **P < 0.01, n = 5). MSCs, mesenchymal stem cells.
Compared with the RT+PBS group, MSCs and Ad-sTβR-MSCs treatments decreased malondialdehyde (MDA) concentrations (a measure of the oxidative stress) in the lungs by 33% and 49%, respectively (Figure 4d).
The irradiation induced obvious increases in representative proinflammatory and profibrotic cytokines in plasma, including interleukin-1β (IL-1β), tumor necrosis factor-α, IL-6, and active TGF-β1. Treatment with either MSCs or Ad-sTβR-MSCs significantly decreased plasma concentrations of these cytokines (Figure 4e–g, i). Ad-sTβR-MSCs increased plasma levels of the anti-inflammatory cytokine IL-10 (Figure 4h). Compared with treatment with MSCs alone, Ad-sTβR-MSCs tended to be more potent in reducing the proinflammatory, profibrotic cytokines and increasing the anti-inflammatory cytokine, but the differences did not reach statistical significance.
Ad-sTβR-MSCs improved survival and lung fibrosis
No mice receiving irradiation plus PBS survived to 60 weeks (the period of observation). The survival rate at 60 weeks after the irradiation in mice receiving MSCs and Ad-sTβR-MSCs was 40% and 80%, respectively. Ad-sTβR-MSCs seemed to be superior to MSCs alone, but the difference was not statistically significant (Figure 5a).
Figure 5.
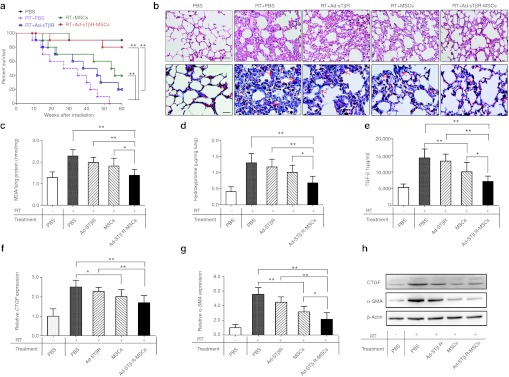
Improvements of survival and lung fibrosis by Ad-sTβR-MSCs treatment. (a) Effects of Ad-sTβR-MSCs on long-term survival (*P < 0.05, **P < 0.01, n = 10). (b) Hematoxylin and eosin (H&E) (upper panel) and Masson's trichrome staining (lower panel) of lung sections, examples of focal fibrotic lesions (arrows) are marked. Bar = 50 µm. (c) Malondialdehyde (MDA) levels, (d) hydroxyproline content in lung homogenates, and active (e) transforming growth factor-β1 (TGF-β1) concentrations in plasma (*P < 0.05, **P < 0.01, n = 5–8). (f–g) The mRNA and (h) protein levels of connective tissue growth factor (CTGF) and α-smooth muscle actin (α-SMA) in lungs were analyzed by quantitative reverse transcription-PCR (RT-PCR) and western blot assays (*P < 0.05, **P < 0.01, n = 5–8). MSCs, mesenchymal stem cells.
On day 120 after the irradiation, the lung injury was apparent in the RT+PBS group (Figure 5b, upper panel). The histopathological changes included thickening of alveolar septa, infiltration of inflammatory cells and interstitial hyperplasia. MSCs treatment, particularly Ad-sTβR-MSCs, attenuated such changes. Ad-sTβR-MSCs treatment was more effective than MSCs alone in attenuating the radiation-induced elevation of MDA levels (Figure 5c).
Fibrosis in the lungs was assessed by Masson's trichrome staining and hydroxyproline detection. Radiation-induced marked collagen deposition, such changes were attenuated by MSCs treatment, and more so by Ad-sTβR-MSCs treatment (Figure 5b, lower panel). Treatment with Ad-sTβR-MSCs, but not MSCs alone, also significantly reduced the radiation-increased hydroxyproline (Figure 5d).
Active TGF-β1 in plasma was elevated after radiation. This elevation was attenuated by MSCs alone, and more so by Ad-sTβR-MSCs (Figure 5e). Connective tissue growth factor (CTGF) and α-smooth muscle actin (α-SMA) are important downstream molecules in the TGF-β1 pathway, and participate in the process of fibrosis.27 Irradiation increased the relative mRNA and protein levels of CTGF and α-SMA in the lungs (Figure 5f–h). Such an effect was significantly inhibited by MSCs alone or Ad-sTβR-MSCs.
MSCs adopted lung cell phenotypes in radiation-injured lungs
To determine whether male MSCs assumed lung cell phenotypes in recipient female mice, alveolar type II (ATII), endothelial cells and myofibroblasts in the lungs of mice receiving RT, MSCs, RT+MSCs, or RT+Ad-sTβR-MSCs were isolated by fluorescence-activated cell sorting with antibodies against proSP-C, CD31, and α-SMA, respectively.18,28 In mice receiving MSCs alone, the ratios of Y chromosome-derived cells (representing MSCs) were 16% and 11% in ATII cells on days 30 and 120 after the irradiation, respectively (Figure 6a). MSCs accounted for <2% endothelial cells and 1% myofibroblasts (Figure 6b, c). Such percentages were similar in the lungs of mice receiving RT+Ad-sTβR-MSCs (Figure 6a–c).
Figure 6.
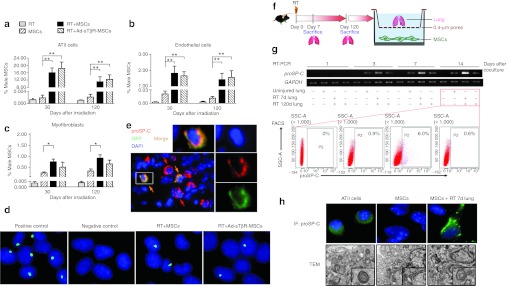
Mesenchymal stem cells (MSCs) adopted alveolar type II (ATII) cells phenotype. (a–c) Quantitative real-time PCR analysis of the male MSCs rates in isolated lung (a) ATII cells, (b) endothelial cells, and (c) myofibroblasts from mice receiving radiation therapy (RT), MSCs, RT+MSCs, or RT+Ad-sTβR-MSCs (*P < 0.05, **P < 0.01, n = 5). (d) Y chromosome FISH assay in ATII cells from male mice (positive control), control female mice (negative control) and female mice of RT+MSCs or RT+Ad-sTβR-MSCs group, magnification ×1,000. (e) Immunofluorescence (IF) of frozen lung sections from RT+Ad-sTβR-MSCs group on days 30 by using de-convolution microscopy. Nuclear staining (DAPI, blue), Ad-sTβR-MSCs (GFP, green), and the ATII cells (proSP-C, red), magnification ×400. (f) Schematic representation of coculture assay ex vivo. MSCs were cocultured with irradiated lung tissue. (g) The proSP-C mRNA expression was detected by RT-PCR in MSCs cocultured with the uninjured lung, RT 7d lung and RT 120d lung (upper panel). The proSP-C positive cells were counted by fluorescence-activated cell sorting (FACS) 14 days after coculture (lower panel). (h) The proSP-C expression and lamellar bodies in MSCs cocultured with RT 7d lung for 14 days were detected by IF (upper panel) and transmission electron microscopy (TEM) (lower panel), respectively. IF, magnification ×1,000. TEM, magnification ×12,000.
The results were confirmed by in situ Y chromosome FISH analysis (green signal for hybridization) in isolated ATII cells (Figure 6d). The green signals were detected in a few cells from female mice receiving MSCs, either naive or transduced with Ad-sTβR previously.
We then costained lung sections for proSP-C (a marker of ATII cells29) and GFP (a marker for Ad-sTβR-MSCs). Deconvolution microscopy revealed proSP-C colocalization with GFP-labeled MSCs (Figure 6e).
MSCs adopted features of ATII cells when cocultured with injured lungs
MSCs could adopt immunophenotypic characteristics of ATII cells when cocultured with oxygen-damaged lungs.19 By using a coculture system (Figure 6f), we found that when cocultured with the lungs collected on day 7 after RT (RT 7d lung), MSCs expressed proSP-C mRNA in a time-dependent manner (Figure 6g, upper panel). However, MSCs expressed low levels of proSP-C when cocultured with the uninjured lungs or the lungs collected on day 120 after RT (RT 120d lung). On day 14 after the coculture, proSP-C positive cells accounted for 6% in MSCs cocultured with RT 7d lung, and <1% in other groups (Figure 6g, lower panel).
Immunofluorescence assay revealed the expression of proSP-C protein in MSCs cocultured with RT 7d lung for 14 days, and freshly isolated ATII cells were used a reference for proSP-C expression (Figure 6h, upper panel). Lamellar bodies are secretary organelles found in ATII cells.17 Transmission electron microscopy visualized lamellar bodies in MSCs cocultured with RT 7d lung for 14 days (Figure 6h, lower panel).
MSCs CM protected ATII cells against radiation injury and modulated the inflammatory response in vitro
The low rate of MSCs in injured lungs seemed insufficient to explain the therapeutic benefit. Increasing evidence suggests that the therapeutic benefit of MSCs is mediated by a paracrine mechanism, in which MSCs could secret some anti-inflammatory and reparative molecules. We therefore explored the potential effects of MSCs-conditioned medium (MSCs CM) on protecting against injury of ATII cells and modulating the inflammatory response.
Murine ATII cells were isolated and subjected to 14Gy radiation, and then incubated in DMEM, MSCs CM, or cocultured with MSCs for 24–48 hours (Figure 7a). Both MSCs and MSCs CM prevented radiation-induced ATII cells apoptosis (Figure 7b, c) and DNA damage (Figure 7d, e).
Figure 7.
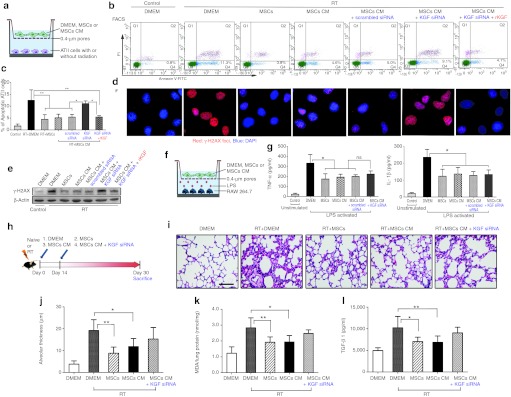
Protective effects of mesenchymal stem cells (MSCs)-conditioned medium (CM) in vitro and in vivo. (a) Schematic representation of in vitro incubation/coculture assay of radiation-injured alveolar type II (ATII) cells with DMEM, MSCs, or MSCs-conditioned medium (MSCs CM). (b) Fluorescence-activated cell sorting (FACS) analysis of the apoptotic irradiated-ATII cells, when incubated/cocultured for 48 hours with DMEM, MSCs, MSCs CM, MSCs CM in the presence of keratinocyte growth factor (KGF) siRNA or plus recombinant KGF (rKGF). (c) The statistic results of FACS (*P < 0.05, **P < 0.01, n = 5). (d) The DNA damage was assayed by γ-H2AX staining for irradiated ATII cells incubated/cocultured for 24 hours with DMEM, MSCs, MSCs CM, MSCs CM in the presence of KGF siRNA or plus rKGF. Magnification ×1,000. (e) Western blot analysis of γ-H2AX. (f) Schematic representation of in vitro incubation/coculture assay of lipopolysaccharide (LPS)-stimulated macrophage RAW264.7 with of DMEM, MSCs, or MSCs CM. (g) Enzyme-linked immunosorbent assay (ELISA) analysis of the tumor necrosis factor-α (TNF-α) and IL-1β concentrations in the medium of LPS-activated RAW264.7 incubated/cocultured for 24 hours with DMEM, MSCs, MSCs CM, or KGF siRNA-pretreated MSCs CM (*P < 0.05, **P < 0.01, n = 5). (h) Schematic representation of in vivo effects of MSCs, MSCs CM, or KGF siRNA-pretreated MSCs CM on radiation-induced lung injury (RILI) 30 days after irradiation. (i) Representative hematoxylin and eosin (H&E) stained lung sections from five experimental groups. Bar = 50 µm. (j) The radiation-induced lung damage was semiquantified by measuring the alveolar thickness (*P < 0.05, **P < 0.01, n = 5). (k) Malondialdehyde (MDA) levels in the lung homogenates were assayed (*P < 0.05, **P < 0.01, n = 5). (l) Enzyme-linked immunosorbent assay (ELISA) analysis of the plasma active transforming growth factor-β1 (TGF-β1) (*P < 0.05, **P < 0.01, n = 5).
Keratinocyte growth factor (KGF) is a critical mediator for repairing the lung epithelial cells upon damage.23 MSCs are reported to secrete some reparative molecules, including KGF.30 In our experiments, pretreatment of MSCs with KGF siRNA abolished the protective effects of MSCs CM on injured ATII cells (Figure 7b–e and Supplementary Figure S2). Such protective effects were partially restored by adding recombinant KGF (rKGF) to the KGF siRNA-pretreated MSCs CM.
MSCs modulate the inflammatory response.16,31 Activated macrophages RAW264.7 were incubated/cocultured with DMEM, MSCs, or MSCs CM for 24 hours (Figure 7f). Both MSCs and MSCs CM inhibited the secretion of proinflammatory cytokines from activated RAW264.7, including tumor necrosis factor-α and IL-1β (Figure 7g). Addition of KGF siRNA did not prevent the effects, suggesting the presence of other soluble factors that could mediate the effects of MSCs on inflammatory response.
MSCs CM protected against RILI in vivo
Mice exposed to thoracic irradiation were injected intravenously on days 0 and 14 with MSCs, MSCs CM, or MSCs CM pretreated with KGF siRNA (Figure 7h). Thirty days later, the injury was estimated by lung histopathology, MDA levels in the lungs and active TGF-β1 in the plasma (Figure 7i–l). The thickness of alveolar septa (Figure 7i, j), lung MDA (Figure 7k) and plasma TGF-β1 levels (Figure 7l) significantly decreased in mice treated with either MSCs or MSCs CM. KGF siRNA seemed to attenuate the protective effects of MSCs CM, but the difference did not reach statistical significance.
Evaluation of possible tumorigenicity of MSCs
MSCs may transform to malignant cells.32,33 We examined the potential adverse effects of MSCs or Ad-sTβR-MSCs in female mice receiving whole thoracic irradiation (n = 15/group). The mice were examined every day and observed up to 24 months. Pathological examination of the lung, liver, spleen, brain, kidney, heart, and ovary immediately prior to imminent death revealed no gross or microscopic tumors in any subject (data not shown). Four out of the 15 mice (40%) receiving RT+Ad-sTβR-MSCs were still alive at the end of the experiment (24 months; Supplementary Figure S3).
Discussion
Stem cell-based gene delivery could achieve selective expression in the target tissue, improve the efficacy of gene therapy and reduce therapeutic toxicity.34 The results of the present study have demonstrated the homing and therapeutic efficacy of implanted Ad-sTβR-MSCs in a mouse model of RILI. More strikingly, MSCs repair the lung injury via adopting ATII cells characteristics and largely through a paracrine mechanism.
The irradiation results in a 20-fold increase in male MSCs levels in the injured lungs. MSCs are able to selectively migrate to the injured lungs. The increased number of MSCs in irradiated lungs may be partially explained by the increase in vascular permeability induced by radiation, which in theory should increase the passage of MSCs through the lung capillaries. However, a recent report has shown that few MSCs could be found in perivascular sites after radiation and suggested that increased vascular permeability is not the dominant reason for the homing of MSCs to the irradiated sites.35 Previous studies have demonstrated that some cytokines and their receptors (e.g., SDF-1/CXCR4, MCP-1/CCR2, VEGF/VEGFR, PDGF-BB/PDGFR-β) are responsible for the active homing of MSCs to injured sites. The injured cells secrete various cytokines, which in turn increase the expression of these chemokine and/or their receptors on MSCs, eventually facilitating the migration of MSCs to the injured sites.26,34,35,36 Our data suggest that the SDF-1α/CXCR4 axis could facilitate the MSCs homing to the injured lungs upon irradiation and that the homing of MSCs is an active process rather than just passive trapping. The involvement of other potential cytokines/receptors axes needs further study.
The microenvironment influences the in vivo fate of MSCs. In bleomycin-induced lung injury, early (but not late) injection of MSCs ameliorates inflammation and collagen deposition.17 MSCs administrated immediately after radiation differentiate into functional lung cells, while MSCs administrated 2 months after radiation mainly differentiate into myofibroblasts.28 Our in vivo and ex vivo studies reveal that exposure the MSCs early after the injury could facilitate the MSCs to acquire the ATII cell characteristics.
A recent study has shown that MSCs could reduce the mortality rate of mice with RILI.37 No oncology progression and significant adverse changes are observed when MSCs are tested in a clinical trial enrolling of 11 patients with RILI.37 ATII cells have been thought to be the stem cells of alveolar type I cells,29 and thereby are the main target cells in RILI. In our study, MSCs could acquire the characteristics of ATII cells in vivo. Ex vivo experiments show that MSCs express proSP-C and lamellar bodies when cocultured with injured lungs. These findings are compatible with previous reports that MSCs or bone marrows cells could acquire the ATII cell markers.18,19,28,38 Specific mechanisms may include differentiation of MSCs into ATII cells, fusion of MSCs with resident ATII cells or a combination of both. Cellular fusion is not evident in our ex vivo coculture experiments, but we could not rule this possibility in vivo, as previous described.17,18 Bone marrow cells are reported to contribute to the lung epithelium independent of cell infusion.39 The precise mechanisms on how MSCs adopt the phenotype of ATII cells remain unclear and need further study.
There are contrasting reports concerning the engraftment and differentiation of MSCs in the lungs. Systemic or intratracheal administration of MSCs may lead to engraftment and/or differentiation into the lung epithelium.17,18,19,28,40 Other investigators report that bone marrows cells are unable to engraft into lung epithelium.41,42 This discrepancy may reflect the differences in experiment design, and more specifically, different cell isolation and enrichment, animal strain, lung injury model, time course to transplant and the methods to evaluate engraftment.19,43
In our study, only 0.1% lung cells are derived from transplanted MSCs. Obviously, such finding could not fully support the noted protective effects, suggesting other factors are involved. Accumulating evidence shows that the beneficial effects of MSCs are due to their capacity to secret paracrine factors that repair injured cells and modulate inflammatory responses.14,44 MSCs inhibit the lipopolysaccharide-induced lung inflammatory response independent of lung epithelium replacement.15,16 MSCs protect lungs against bleomycin-induced injury by reducing two fundamental proinflammatory cytokines tumor necrosis factor-α and IL-1β in a paracrine way.31 MSCs CM prevents O2-induced ATII cell apoptosis and hyperoxia-induced lung injury.19,20 Our results further support the notion that MSCs CM could attenuate RILI in vivo and protect ATII cells against radiation-induced injury in vitro, as well as modulate the inflammatory responses in vitro.
IL-1RA,31 IL-10,16 PGE2,45 TSG-6,46 KGF,30 G-CSF, and GM-CSF18 are implicated in the therapeutic effects of MSCs. KGF is a critical factor that mediates the repair of injured-lung epithelial cells, and MSCs are reported to repair epithelial cell damage via secreting KGF.23,30 The abrogation of the protective effects of MSCs CM against ATII cells by a KGF siRNA in the present study indicates the crucial roles of KGF. However, KGF siRNA pretreatment does not significantly abrogate the anti-inflammatory effects and lung protection of MSCs CM, indicating the presence of other important molecules.
Fibrosis after lung irradiation is a perplexing process involving activation of various proinflammatory and profibrotic cytokines produced by damaged alveolar epithelial cells, endothelial cells and activated interstitial cells.2 Strategies that alleviate the initial lung cell damage and acute inflammation could prevent the ensuing lung fibrosis. MSCs are reported to alleviate the lung inflammation and fibrosis in many experimental models.17,18,28 In the present study, MSCs alone could significantly decrease some markers of fibrosis (TGF-β1, CTGF, and α-SMA), but do not significantly decrease the hydroxyproline content, a direct index reflecting the fibrosis in the lungs, suggesting the limited effects of stem cell therapy on fibrosis.
At least three factors contribute to the benefits of Ad-sTβR-MSCs on RILI in our study. First, MSCs deliver sTβR to injured sites, and thus effectively inhibit the TGF-β signaling pathway. Second, MSCs acquire the ATII cell phenotypes upon irradiation despite the paucity of this finding. Third, MSCs protect against injury of ATII cells and modulate the inflammatory response in a paracrine way.
Long-term in vitro culture of MSCs could lead to malignant transformation, even generating sarcoma in the recipient lungs.32,33 To minimize unexpected transformation, MSCs from passage five are used in our experiments. No sarcoma or other tumor type is observed in a time period up to 24 months.
In conclusion, our experiments reveal that MSCs selectively target injured lungs and allow higher treatment efficiency of the delivered gene therapy. MSCs, either plain or genetically modified to carry the sTβR gene could repair the lung injury via adopting the ATII cell phenotype and through a paracrine manner. The combined strategy is promising in the treatment of RILI.
Materials and Methods
All are available in the Supplementary Materials and Methods.
SUPPLEMENTARY MATERIAL Figure S1. Negative linear relationship between threshold cycle (Ct) of PCR amplification and the logarithm of male DNA dilution ratios in female DNA standards. Figure S2. Expression and secretion of KGF by MSCs transfected with KGF siRNA for 24 hours. Figure S3. Long-term observation of mice treated with MSCs and Ad-sTβR-MSCs. Materials and Methods.
Acknowledgments
This work was supported by National Natural Science Fund of China (30870734, 81172131, 81101698), “The 12th 5-year plan” for Technology Platform Construction of Innovative Drug Research and Development of China (2011ZX09302-001), and China Postdoctoral Science Foundation (20110491720). The authors declared no conflict of interest.
Supplementary Material
Negative linear relationship between threshold cycle (Ct) of PCR amplification and the logarithm of male DNA dilution ratios in female DNA standards.
Expression and secretion of KGF by MSCs transfected with KGF siRNA for 24 hours.
Long-term observation of mice treated with MSCs and Ad-sTβR-MSCs.
REFERENCES
- Graves PR, Siddiqui F, Anscher MS., and, Movsas B. Radiation pulmonary toxicity: from mechanisms to management. Semin Radiat Oncol. 2010;20:201–207. doi: 10.1016/j.semradonc.2010.01.010. [DOI] [PubMed] [Google Scholar]
- Tsoutsou PG., and, Koukourakis MI. Radiation pneumonitis and fibrosis: mechanisms underlying its pathogenesis and implications for future research. Int J Radiat Oncol Biol Phys. 2006;66:1281–1293. doi: 10.1016/j.ijrobp.2006.08.058. [DOI] [PubMed] [Google Scholar]
- Cappuccini F, Eldh T, Bruder D, Gereke M, Jastrow H, Schulze-Osthoff K.et al. (2011New insights into the molecular pathology of radiation-induced pneumopathy Radiother Oncol 10186–92. [DOI] [PubMed] [Google Scholar]
- Zhang M, Qian J, Xing X, Kong FM, Zhao L, Chen M.et al. (2008Inhibition of the tumor necrosis factor-alpha pathway is radioprotective for the lung Clin Cancer Res 141868–1876. [DOI] [PubMed] [Google Scholar]
- Yuan X, Liao Z, Liu Z, Wang LE, Tucker SL, Mao L.et al. (2009Single nucleotide polymorphism at rs1982073:T869C of the TGFbeta 1 gene is associated with the risk of radiation pneumonitis in patients with non-small-cell lung cancer treated with definitive radiotherapy J Clin Oncol 273370–3378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue J, Gan L, Li X, Li J, Qi G, Wu Y.et al. (2010Effects of lysophosphatidic acid and its receptors LPA1/3 on radiation pneumonitis Oncol Rep 241515–1520. [DOI] [PubMed] [Google Scholar]
- Ikushima H., and, Miyazono K. TGFbeta signalling: a complex web in cancer progression. Nat Rev Cancer. 2010;10:415–424. doi: 10.1038/nrc2853. [DOI] [PubMed] [Google Scholar]
- Zhao L, Wang L, Ji W, Wang X, Zhu X, Hayman JA.et al. (2009Elevation of plasma TGF-beta1 during radiation therapy predicts radiation-induced lung toxicity in patients with non-small-cell lung cancer: a combined analysis from Beijing and Michigan Int J Radiat Oncol Biol Phys 741385–1390. [DOI] [PubMed] [Google Scholar]
- Nishioka A, Ogawa Y, Mima T, Jin YJ, Sonobe H, Kariya S.et al. (2004Histopathologic amelioration of fibroproliferative change in rat irradiated lung using soluble transforming growth factor-beta (TGF-beta) receptor mediated by adenoviral vector Int J Radiat Oncol Biol Phys 581235–1241. [DOI] [PubMed] [Google Scholar]
- Rabbani ZN, Anscher MS, Zhang X, Chen L, Samulski TV, Li CY.et al. (2003Soluble TGFbeta type II receptor gene therapy ameliorates acute radiation-induced pulmonary injury in rats Int J Radiat Oncol Biol Phys 57563–572. [DOI] [PubMed] [Google Scholar]
- Haiping Z, Takayama K, Uchino J, Harada A, Adachi Y, Kura S.et al. (2006Prevention of radiation-induced pneumonitis by recombinant adenovirus-mediated transferring of soluble TGF-beta type II receptor gene Cancer Gene Ther 13864–872. [DOI] [PubMed] [Google Scholar]
- Anscher MS, Thrasher B, Rabbani Z, Teicher B., and, Vujaskovic Z. Antitransforming growth factor-beta antibody 1D11 ameliorates normal tissue damage caused by high-dose radiation. Int J Radiat Oncol Biol Phys. 2006;65:876–881. doi: 10.1016/j.ijrobp.2006.02.051. [DOI] [PubMed] [Google Scholar]
- Karp JM., and, Leng Teo GS. Mesenchymal stem cell homing: the devil is in the details. Cell Stem Cell. 2009;4:206–216. doi: 10.1016/j.stem.2009.02.001. [DOI] [PubMed] [Google Scholar]
- D'Agostino B, Sullo N, Siniscalco D, De Angelis A., and, Rossi F. Mesenchymal stem cell therapy for the treatment of chronic obstructive pulmonary disease. Expert Opin Biol Ther. 2010;10:681–687. doi: 10.1517/14712591003610614. [DOI] [PubMed] [Google Scholar]
- Xu J, Woods CR, Mora AL, Joodi R, Brigham KL, Iyer S.et al. (2007Prevention of endotoxin-induced systemic response by bone marrow-derived mesenchymal stem cells in mice Am J Physiol Lung Cell Mol Physiol 293L131–L141. [DOI] [PubMed] [Google Scholar]
- Gupta N, Su X, Popov B, Lee JW, Serikov V., and, Matthay MA. Intrapulmonary delivery of bone marrow-derived mesenchymal stem cells improves survival and attenuates endotoxin-induced acute lung injury in mice. J Immunol. 2007;179:1855–1863. doi: 10.4049/jimmunol.179.3.1855. [DOI] [PubMed] [Google Scholar]
- Ortiz LA, Gambelli F, McBride C, Gaupp D, Baddoo M, Kaminski N.et al. (2003Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects Proc Natl Acad Sci USA 1008407–8411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas M, Xu J, Woods CR, Mora AL, Spears W, Roman J.et al. (2005Bone marrow-derived mesenchymal stem cells in repair of the injured lung Am J Respir Cell Mol Biol 33145–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Haaften T, Byrne R, Bonnet S, Rochefort GY, Akabutu J, Bouchentouf M.et al. (2009Airway delivery of mesenchymal stem cells prevents arrested alveolar growth in neonatal lung injury in rats Am J Respir Crit Care Med 1801131–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslam M, Baveja R, Liang OD, Fernandez-Gonzalez A, Lee C, Mitsialis SA.et al. (2009Bone marrow stromal cells attenuate lung injury in a murine model of neonatal chronic lung disease Am J Respir Crit Care Med 1801122–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnecchi M, He H, Liang OD, Melo LG, Morello F, Mu H.et al. (2005Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells Nat Med 11367–368. [DOI] [PubMed] [Google Scholar]
- Mei SH, McCarter SD, Deng Y, Parker CH, Liles WC., and, Stewart DJ. Prevention of LPS-induced acute lung injury in mice by mesenchymal stem cells overexpressing angiopoietin 1. PLoS Med. 2007;4:e269. doi: 10.1371/journal.pmed.0040269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilar S, Scotton CJ, McNulty K, Nye E, Stamp G, Laurent G.et al. (2009Bone marrow stem cells expressing keratinocyte growth factor via an inducible lentivirus protects against bleomycin-induced pulmonary fibrosis PLoS ONE 4e8013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Moon HH, Kim HA, Hwang KC, Lee M., and, Choi D. Hypoxia-inducible vascular endothelial growth factor-engineered mesenchymal stem cells prevent myocardial ischemic injury. Mol Ther. 2011;19:741–750. doi: 10.1038/mt.2010.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang OD, Mitsialis SA, Chang MS, Vergadi E, Lee C, Aslam M.et al. (2011Mesenchymal stromal cells expressing heme oxygenase-1 reverse pulmonary hypertension Stem Cells 2999–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Mora A, Shim H, Stecenko A, Brigham KL., and, Rojas M. Role of the SDF-1/CXCR4 axis in the pathogenesis of lung injury and fibrosis. Am J Respir Cell Mol Biol. 2007;37:291–299. doi: 10.1165/rcmb.2006-0187OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn TA. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J Clin Invest. 2007;117:524–529. doi: 10.1172/JCI31487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan X, Liu Y, Han Q, Jia M, Liao L, Qi M.et al. (2007Injured microenvironment directly guides the differentiation of engrafted Flk-1(+) mesenchymal stem cell in lung Exp Hematol 351466–1475. [DOI] [PubMed] [Google Scholar]
- Kim CF, Jackson EL, Woolfenden AE, Lawrence S, Babar I, Vogel S.et al. (2005Identification of bronchioalveolar stem cells in normal lung and lung cancer Cell 121823–835. [DOI] [PubMed] [Google Scholar]
- Lee JW, Fang X, Gupta N, Serikov V., and, Matthay MA. Allogeneic human mesenchymal stem cells for treatment of E. coli endotoxin-induced acute lung injury in the ex vivo perfused human lung. Proc Natl Acad Sci USA. 2009;106:16357–16362. doi: 10.1073/pnas.0907996106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz LA, Dutreil M, Fattman C, Pandey AC, Torres G, Go K.et al. (2007Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury Proc Natl Acad Sci USA 10411002–11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilar S, Nye E, Chan J, Loebinger M, Spencer-Dene B, Fisk N.et al. (2007Murine but not human mesenchymal stem cells generate osteosarcoma-like lesions in the lung Stem Cells 251586–1594. [DOI] [PubMed] [Google Scholar]
- Tolar J, Nauta AJ, Osborn MJ, Panoskaltsis Mortari A, McElmurry RT, Bell S.et al. (2007Sarcoma derived from cultured mesenchymal stem cells Stem Cells 25371–379. [DOI] [PubMed] [Google Scholar]
- Sasportas LS, Kasmieh R, Wakimoto H, Hingtgen S, van de Water JA, Mohapatra G.et al. (2009Assessment of therapeutic efficacy and fate of engineered human mesenchymal stem cells for cancer therapy Proc Natl Acad Sci USA 1064822–4827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klopp AH, Spaeth EL, Dembinski JL, Woodward WA, Munshi A, Meyn RE.et al. (2007Tumor irradiation increases the recruitment of circulating mesenchymal stem cells into the tumor microenvironment Cancer Res 6711687–11695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielske SP, Livant DL., and, Lawrence TS. Radiation increases invasion of gene-modified mesenchymal stem cells into tumors. Int J Radiat Oncol Biol Phys. 2009;75:843–853. doi: 10.1016/j.ijrobp.2008.06.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kursova LV, Konoplyannikov AG, Pasov VV, Ivanova IN, Poluektova MV., and, Konoplyannikova OA. Possibilities for the use of autologous mesenchymal stem cells in the therapy of radiation-induced lung injuries. Bull Exp Biol Med. 2009;147:542–546. doi: 10.1007/s10517-009-0538-7. [DOI] [PubMed] [Google Scholar]
- Sugahara K, Tokumine J, Teruya K., and, Oshiro T. Alveolar epithelial cells: differentiation and lung injury. Respirology. 2006;11 Suppl:S28–S31. doi: 10.1111/j.1440-1843.2006.00804.x. [DOI] [PubMed] [Google Scholar]
- Harris RG, Herzog EL, Bruscia EM, Grove JE, Van Arnam JS., and, Krause DS. Lack of a fusion requirement for development of bone marrow-derived epithelia. Science. 2004;305:90–93. doi: 10.1126/science.1098925. [DOI] [PubMed] [Google Scholar]
- Krause DS, Theise ND, Collector MI, Henegariu O, Hwang S, Gardner R.et al. (2001Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell Cell 105369–377. [DOI] [PubMed] [Google Scholar]
- Chang JC, Summer R, Sun X, Fitzsimmons K., and, Fine A. Evidence that bone marrow cells do not contribute to the alveolar epithelium. Am J Respir Cell Mol Biol. 2005;33:335–342. doi: 10.1165/rcmb.2005-0129OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotton DN, Fabian AJ., and, Mulligan RC. Failure of bone marrow to reconstitute lung epithelium. Am J Respir Cell Mol Biol. 2005;33:328–334. doi: 10.1165/rcmb.2005-0175RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aliotta JM, Sanchez-Guijo FM, Dooner GJ, Johnson KW, Dooner MS, Greer KA.et al. (2007Alteration of marrow cell gene expression, protein production, and engraftment into lung by lung-derived microvesicles: a novel mechanism for phenotype modulation Stem Cells 252245–2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss DJ, Kolls JK, Ortiz LA, Panoskaltsis-Mortari A., and, Prockop DJ. Stem cells and cell therapies in lung biology and lung diseases. Proc Am Thorac Soc. 2008;5:637–667. doi: 10.1513/pats.200804-037DW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Németh K, Leelahavanichkul A, Yuen PS, Mayer B, Parmelee A, Doi K.et al. (2009Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production Nat Med 1542–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RH, Pulin AA, Seo MJ, Kota DJ, Ylostalo J, Larson BL.et al. (2009Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6 Cell Stem Cell 554–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Negative linear relationship between threshold cycle (Ct) of PCR amplification and the logarithm of male DNA dilution ratios in female DNA standards.
Expression and secretion of KGF by MSCs transfected with KGF siRNA for 24 hours.
Long-term observation of mice treated with MSCs and Ad-sTβR-MSCs.


