Abstract
Injection of oncolytic vesicular stomatitis virus (VSV) into established B16ova melanomas results in tumor regression, in large part by inducing innate immune reactivity against the viral infection, mediated by MyD88- and type III interferon (IFN)-, but not TLR-4-, signaling. We show here that intratumoral (IT) treatment with lipopolysaccharide (LPS), a TLR-4 agonist, significantly enhanced the local therapy induced by VSV by combining activation of different innate immune pathways. Therapy was further enhanced by co-recruiting a potent antitumor, adaptive T-cell response by using a VSV engineered to express the ovalbumin tumor-associated antigen ova, in combination with LPS. However, the combination of IT LPS with systemically delivered VSV resulted in rapid morbidity and mortality in the majority of mice. Decreasing the intravenous (IV) dose of VSV to levels at which toxicity was ameliorated did not enhance therapy compared with IT LPS alone. Toxicity of the systemic VSV + IT LPS regimen was associated with rapidly elevated levels of serum tumor necrosis factor-α (TNF-α) and interleukin (IL)-6, which neither systemic VSV, nor IT LPS, alone induced. These data show that therapy associated with direct IT injections of oncolytic viruses can be significantly enhanced by combination with agonists of innate immune activation pathways, which are not themselves activated by the virus alone. Importantly, they also highlight possible, unforeseen dangers of combination therapies in which an immunotherapy, even delivered locally at the tumor site, may systemically sensitize the patient to a cytokine shock-like response triggered by IV delivery of oncolytic virus.
Introduction
Vesicular stomatitis virus (VSV) was originally proposed as a candidate oncolytic virus due to its sensitivity to the antiviral effects of interferon (IFN). This sensitivity mediates an apparent replicative preference for tumor, as opposed to normal cells, as many malignant cells have defective IFN signaling pathways.1,2,3 Consistent with this, VSV is highly cytotoxic to transformed cells in vitro1,4 and extensive replication leading to tumor clearance has been shown in vivo.5,6 However, we and others, have demonstrated that the innate antiviral immune response against VSV plays a critical role in antitumor therapy in immunocompetent hosts.7,8,9 In particular, in our B16ova/C57BL/6 model, tumor regression following intratumoral (IT) injection of VSV is dependent on innate immune signaling through the myeloid differentiation primary response gene 88 (MyD88) adaptor protein, the type III IFN-Λ, CD8+ T cells, and natural killer cells, but does not require progressive viral replication.4,10,11,12
Despite reports that VSV can activate both TLR-4 and TLR-7 signaling,13,14 in our hands therapy of B16ova tumors was not diminished in TLR-4 or TLR-7 knockout mice.11 Therefore, we hypothesized that direct IT activation of a combination of innate immune signaling pathways, through both VSV-, and TLR-4–mediated mechanisms, would generate enhanced local antitumor clearance. In this respect, lipopolysaccharide (LPS), an immunogenic cell wall component of Gram negative bacteria, signals through TLR-4 via both MyD88-dependent MyD88-independent pathways resulting in expression of type-I IFNs and other proinflammatory cytokines. Intraperitoneal, as well as intravenous (IV), administration of LPS is associated with a fatal, sepsis-like syndrome in both mice and humans15,16,17,18,19,20 and would therefore, not be used clinically. However, with its known activities as a TLR-4–activating agent, we tested its activity in a proof-of-principle capacity to investigate whether combination with VSV would enhance immune-mediated viro-immunotherapy through the simultaneous activation of different innate immune-stimulating pathways.
We show here that the combination of VSV and LPS generates significantly enhanced therapy of B16ova tumors, compared with either agent alone, upon direct IT administration. Moreover, therapy was further enhanced by recruitment of an adaptive T-cell component, induced by expressing a tumor-associated antigen (TAA) from the VSV. When the virus was given IV along with IT LPS, however, severe toxicity was observed, associated with rapidly elevated levels of tumor necrosis factor-α (TNF-α) and interleukin (IL)-6. Potentially, fatal sensitization of mice to systemically delivered oncolytic virus by IT LPS highlights the dangers which might be associated in patients treated with combination therapies using systemic virus and either local, or systemically, acting immunotherapies. In addition, our results indicate the importance of careful screening for bacterial infections in patients to be treated systemically with oncolytic viruses.
Results
IT LPS treats established tumors
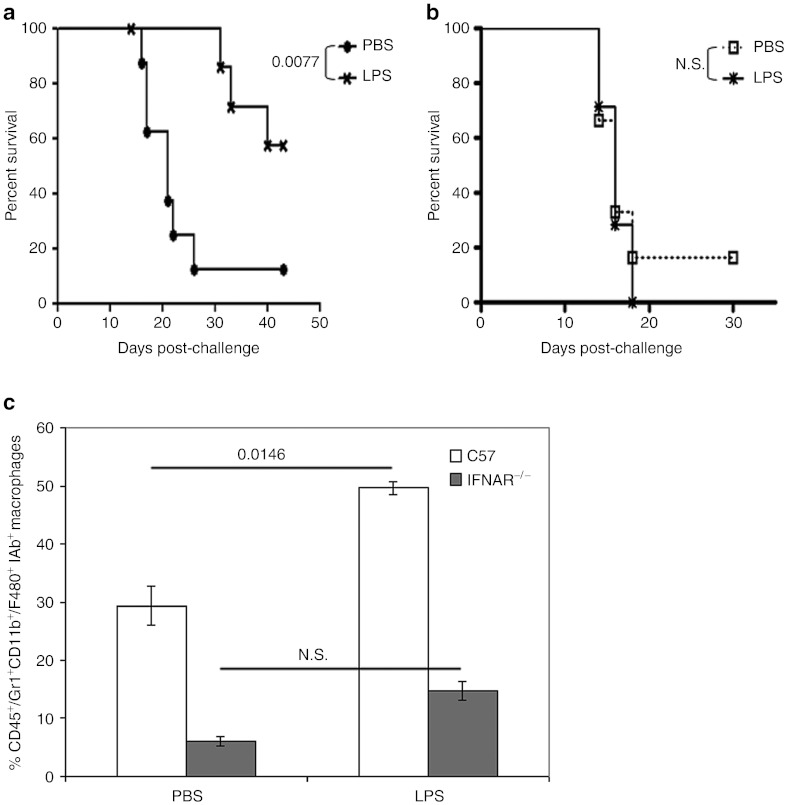
Similar to the innate immune-activating effects of VSV, IT LPS as a single agent both significantly enhanced survival compared with treatment with phosphate-buffered saline (PBS), and cured over 50% of mice bearing established subcutaneous B16ova tumors (Figure 1a). Therapy was lost in IFNAR−/− mice lacking type I IFN signaling (Figure 1b) indicating a predominant role for MyD88-independent, as opposed to MyD88-dependent, TLR-4 signaling21 in this therapy. Although IT LPS induced a significant increase in tumor-infiltrating Gr1+CD11b+F4/80+ macrophages, compared with PBS-treated mice, similar treatment of IFNAR−/− mice was not associated with increased macrophage infiltration (Figure 1c), suggesting that macrophage activation played a major role in the therapy. Consistent with this, tumor regressions were consistently associated with the development of scabs at the tumor site in LPS-treated mice. These normally resolved as the tumors regressed and no systemic toxicity was observed in these mice.
Figure 1.
Type-I interferon signaling is critical for antitumor effects of intratumoral LPS. Survival (tumor <1.0 cm in any diameter) of (a) C57BL/6 or (b) IFNAR−/− mice (n = 8/group) bearing subcutaneous (s.c.) tumors following treatment with three intratumoral doses of LPS (200 µg/50 µl) or PBS (50 µl), starting on day 7 (days 7, 9, 11). (c) Tumors from either C57BL/6 (in a) or IFNAR−/− (in b) were harvested 1 day after the second injection (day 10) and analyzed for CD11b+F4/80+IAb+ macrophages by flow cytometry (n = 3/group). Macrophage percentages were determined relative to the total number of CD45+ cells in the tumor and are shown as mean ± SD. LPS, lipopolysaccharide; N.S., not significant; PBS, phosphate-buffered saline.
Combined TLR pathway activation enhances therapy
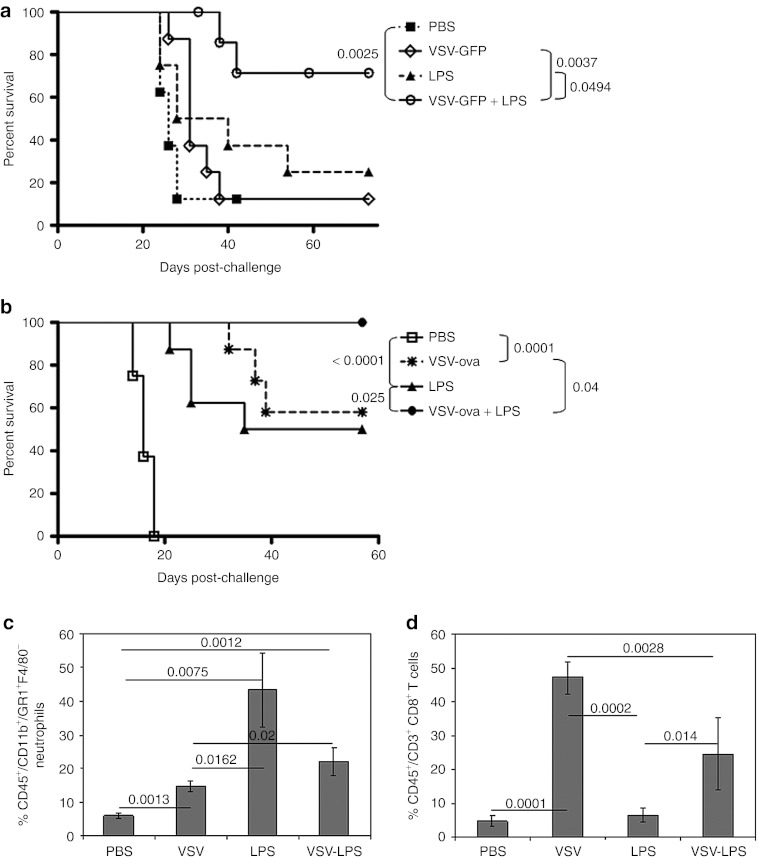
We sought to exploit a combination of innate immune activation pathways using VSV-mediated, MyD88-dependent but TLR-4–independent signaling,11 along with LPS-mediated, MyD88-independent, and TLR-4–dependent signaling (Figure 1). Treatment with IT VSV expressing green fluorescent protein (GFP) (VSV-GFP) followed by IT LPS significantly improved overall survival compared to treatment with VSV-GFP alone, LPS alone, or PBS (Figure 2a). Interestingly, IT LPS followed by IT VSV conferred no survival benefit compared with IT LPS alone (data not shown), suggesting that VSV may sensitize LPS-activated antitumor effectors (such as macrophages) (Figure 1c).
Figure 2.
Enhanced therapy and immune cell infiltration following combination intratumoral VSV-ova + LPS treatment. (a) Survival (tumor <1.0 cm in any diameter) of C57BL/6 mice (n = 8/group) bearing subcutaneous tumors following treatment with three intratumoral doses of VSV-GFP (5 × 108 PFU/50 µl), PBS (50 µl), and/or LPS (200 µg/50 µl) starting on day 7 (days 7, 9, 11). LPS (200 µg/50 µl), when given in combination with VSV-GFP, was given on day 8. (b) Survival (as in a) following treatment with three intratumoral doses of VSV-ova (5 × 108 PFU/50 µl), PBS (50 µl), and/or LPS (200 µg/50 µl) starting on day 7 (days 7, 9, 11). LPS (200 µg/50 µl), when given in combination with VSV-ova, was given on day 8. Tumors were harvested following a single round of treatment (n = 3/group) and analyzed for infiltrating (c) CD11b+Gr1+F4/80− neutrophils or (d) CD3+CD8+ T lymphocytes. Percentages were determined relative to the total number of CD45+ cells in the tumor and are shown as mean ± SD. GFP, green fluorescent protein; LPS, lipopolysaccharide; PBS, phosphate-buffered saline; ova, ovalbumin; PFU, plaque-forming unit; VSV, vesicular stomatitis virus.
We hypothesized that recruitment of additional adaptive, antitumor T-cell responses would further enhance the therapy induced by innate activation of the VSV/LPS combination. In this respect, we have previously shown that engineering VSV to express tumor-associated antigens leads to significantly improved priming of antitumor T-cell immune responses.12,22,23 Consistent with this hypothesis, IT treatment of B16ova tumors with both VSV-ova and LPS significantly improved survival over either treatment alone (P = 0.025 compared with LPS; P = 0.04 compared with VSV-ova) and cured 100% of the treated mice (Figure 2b), compared with only 60–70% of mice treated with VSV-GFP + LPS (Figure 2a).
IT LPS alone was associated with significantly higher levels of tumor-infiltrating CD11b+Gr1+F4/80− neutrophils compared to treatment with either PBS (P = 0.0075) or VSV-ova alone (P = 0.0162) (Figure 2c). Correspondingly, the combination of LPS with VSV-ova significantly increased the percentage of tumor-infiltrating neutrophils compared to treatment with VSV-ova alone (P = 0.02) and PBS (P = 0.0012) (Figure 2c). IT VSV-ova recruited significantly higher levels of CD3+CD8+ T cells into the tumor compared with either PBS (P = 0.0001) or LPS (P = 0.0002). Although the combination of VSV-ova + LPS did not recruit as high levels of infiltrating T cells compared with VSV-ova alone (P = 0.0028), it did significantly enhance T-cell accumulation compared with LPS treatment alone (P = 0.014) (Figure 2d).
VSV-ova + LPS enhances both general and specific T-cell activation
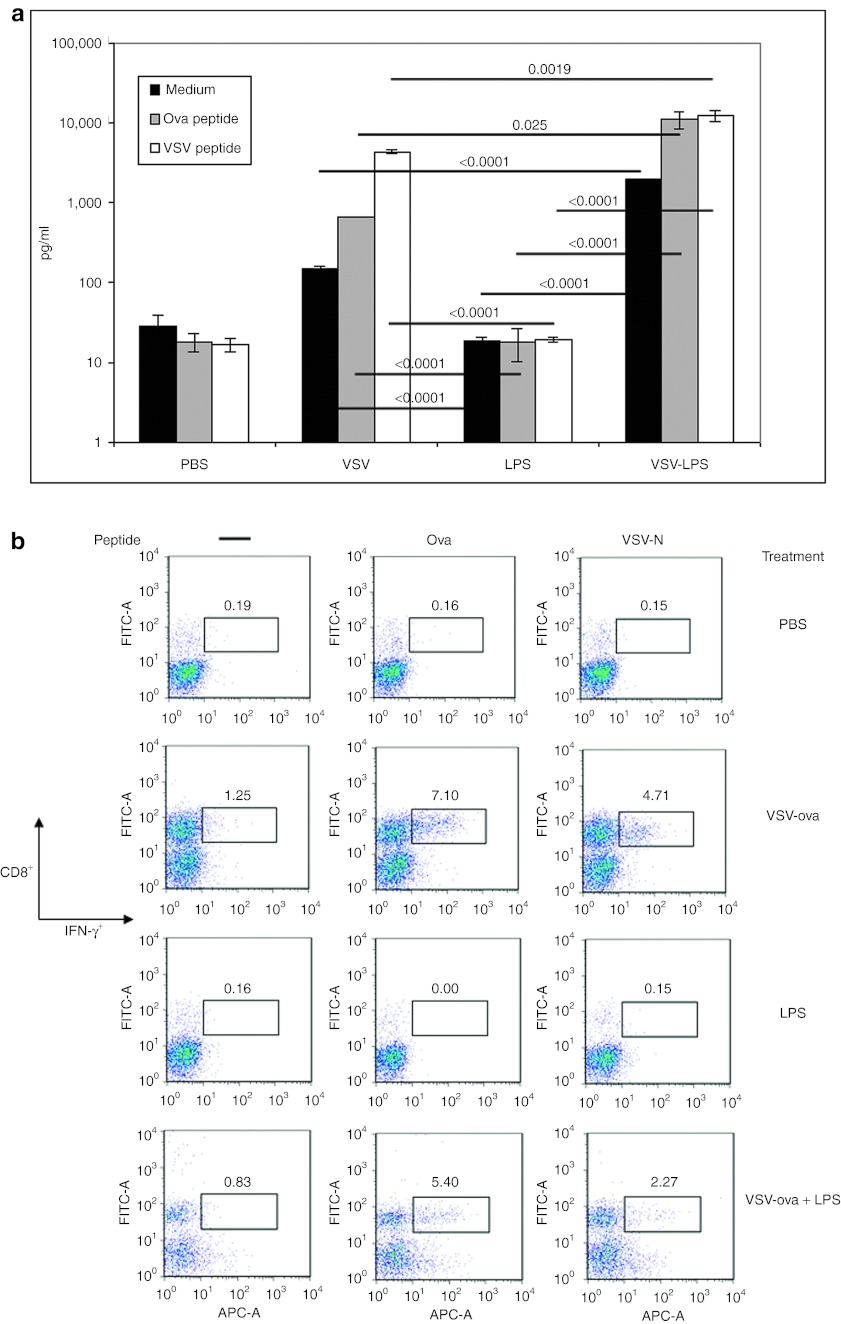
IT VSV-ova alone induced a nonspecific T-cell activation in the tumor-draining lymph nodes, consistent with our previous observations4 (Figure 3a). IT VSV-ova also significantly increased both VSV-specific and ova-specific T-cell responses compared to treatment with PBS. In contrast, IT LPS activated neither nonspecific, nor VSV, nor ova-specific, T-cell responses (Figure 3a). Treatment with the combination of VSV-ova + LPS, however, significantly enhanced all three types of T-cell responses compared with either alone (P ≤ 0.025 for all comparisons) (Figure 3a).
Figure 3.
Shift in generalized and antigen-specific T-cell activation in mice treated with VSV-ova + LPS. (a,b) C57BL/6 mice bearing 7-day subcutaneous tumors (n = 8/group) were administered intratumorally VSV-ova (5 × 108 PFU/50 µl), PBS (50 µl), and/or LPS (200 µg/50 µl). LPS (200 µg/50 µl), when given in combination with VSV-ova, was given on day 8. (a) Tumor-draining lymph nodes and (b) tumors were harvested on day 14 (n = 3/group). Samples were pulsed with either no peptide (medium), ova or VSV-specific peptides for 48 hours (in a) or 1 hour (in b) with T-cell activation being assessed by IFN-γ ELISA of cell-free supernates (in a) or intracellular IFN-γ staining (in b). Data are shown as mean ± SD where appropriate. ELISA, enzyme-linked immunosorbent assay; IFN, interferon; LPS, lipopolysaccharide; PBS, phosphate-buffered saline; ova, ovalbumin; PFU, plaque-forming unit; VSV, vesicular stomatitis virus.
Treatment with VSV-ova also resulted in infiltration of the tumor with both tumor (ova)-specific, as well as viral (VSV)-specific, CD8+ T cells, which were not induced by LPS alone (Figure 3b). With combination VSV-ova + LPS treatment, significantly greater IT CD8+ T-cell infiltration with tumor-specific T cells was generated compared with LPS alone (although this was less than that with VSV-ova alone) and the addition of LPS to VSV-ova treatment reduced the extent of the infiltration with virus-specific CD8+ T cells (Figure 3b). These data indicate that the combination VSV-ova + LPS treatment allows for retention of both the generalized T-cell activation, as well as the TAA, ova-specific T-cell activation that is characterized by treatment with VSV-ova alone, while adding a significant adaptive, TAA-specific component to tumor therapy that is not provided by LPS treatment alone.
IV VSV combined with IT LPS results in rapid toxicity
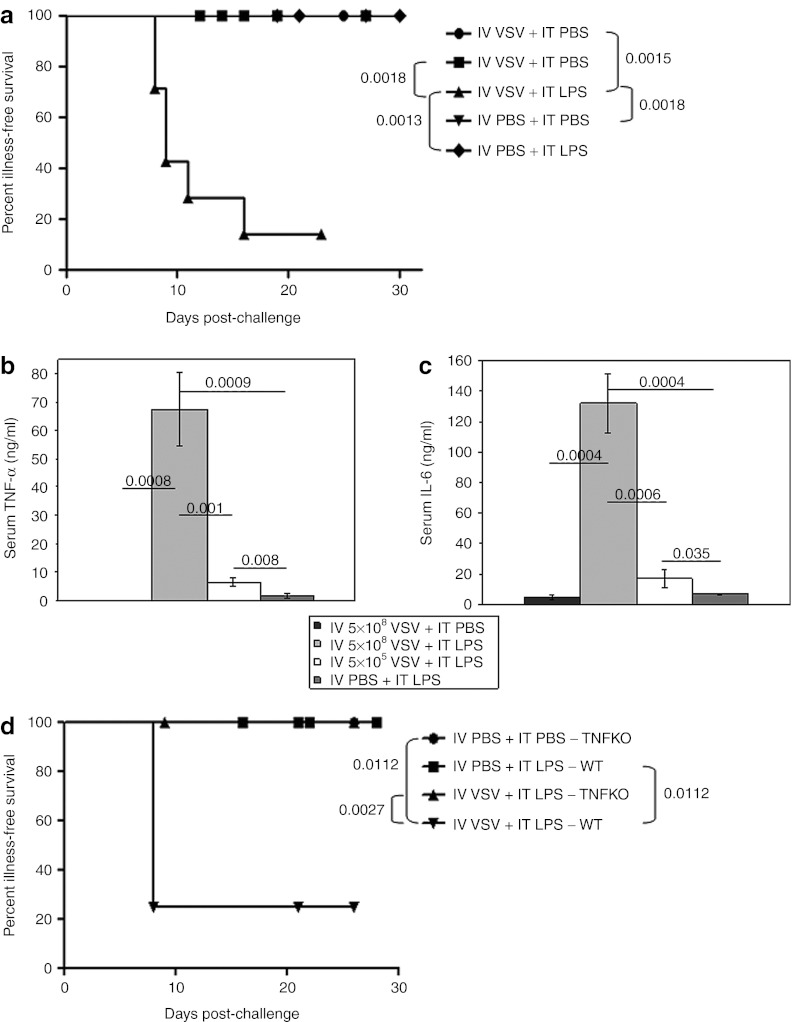
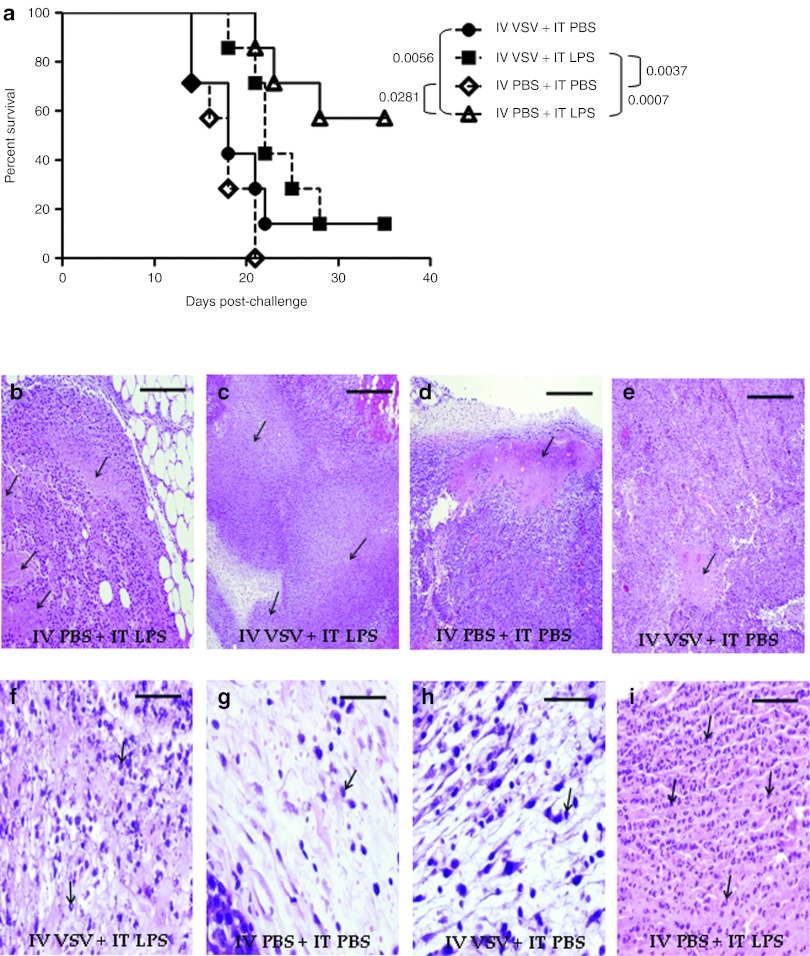
We investigated whether one, or both, components of this improved treatment regimen could be supplied systemically. During these studies, we observed that combination treatment with IV VSV (5 × 108 plaque-forming units (PFU)/dose) followed 6 hours later by IT LPS resulted in rapid mortality (Figure 4a). Neither treatment alone induced these effects. Moreover, toxicity was not prevented by using an attenuated VSV (VSV-Δ51) in which a mutant matrix protein increases the transport of type-I IFN mRNA molecules to the cytoplasm, thereby decreasing the virus' toxicity profile.5 Equivalent levels of toxicity were observed when IV VSV was administered either before, or after, IT LPS. Consistent with the rapid onset toxicity being associated with a virus-mediated cytokine storm24 and LPS-induced septic shock, very high levels of both TNF-α and IL-6 were present in the serum following treatment with IV VSV + IT LPS, which were significantly greater than induced by either IV VSV (P = 0.0008 for TNF-α and P = 0.0004 for IL-6), or LPS (P = 0.0009 for TNF-α and P = 0.0004 for IL-6), alone (Figure 4b,c). A reduction in the dose of IV VSV to a level in which no similar toxicity was observed, correlated with a loss of serum TNF-α and IL-6 (Figure 4b,c). This VSV-induced hyperresponsive state was, however, short-lived because increasing the interval between administration of IV VSV and IT LPS from 6 to 24 hours completely ameliorated overt signs of toxicity, but was also not associated with antitumor therapy. In addition, toxicity was completely lost in mice lacking TNF (TNFKO mice) following treatment with IV VSV + IT LPS (Figure 4d), confirming the role of TNF-α in the toxicity of the combination treatment.
Figure 4.
High-dose, systemic VSV, when combined with local LPS treatment, results in acute toxicity. (a) Illness-free survival of C57BL/6 mice (mice not showing any signs of physical distress) bearing subcutaneous tumors after treatment with intravenous (IV) VSV-Δ51 (5 × 108 PFU/100 µl) or PBS (100 µl) followed by intratumoral (IT) PBS (50 µl) or LPS (200 µg/50 µl) 6 hours later (n = 7/group). Injections started on day 7 and continued every other day for a total of four (days 7, 9, 11, 13). (b,c) Mice (as in a) were treated with IV VSV-Δ51 (5 × 108 PFU/100 µl or 5 × 105 PFU/100 µl) or PBS (100 µl) followed by IT PBS (50 µl) or LPS (200 µg/50 µl) 6 hours later (n = 7/group). Cardiac terminal bleeds were done 1.5 hours after a single combination treatment. Serum (b) TNF-α or (c) IL-6 levels were determined by ELISA and are shown as mean ± SD. (d) Illness-free survival (as in a) of tumor-bearing C57BL/6 or TNFKO mice after a single treatment with IV VSV-Δ51 (5 × 108 PFU/100 µl) or PBS (100 µl) followed by IT PBS (50 µl) or LPS (200 µg/50 µl) 6 hours later (LPS only groups: n = 5/group; VSV + LPS groups: n = 8/group). ELISA, enzyme-linked immunosorbent assay; IL, interleukin; KO, knockout; LPS, lipopolysaccharide; PBS, phosphate-buffered saline; PFU, plaque-forming unit; TNF-α, tumor necrosis factor-α VSV, vesicular stomatitis virus; WT, wild-type.
Combining IT LPS with systemic, mid-dose VSV does not enhance antitumor therapy
Since toxicity of the combination IV VSV + IT LPS treatment could be abrogated by lowering the dose of VSV, we investigated whether a non-toxic regimen could be therapeutically effective against B16ova tumors. The highest, non-toxic dose of VSV (5 × 106 PFU/dose) which could be used in combination with IT LPS had no substantial effect on the survival of tumor-bearing mice and was consistently less effective than IT LPS alone (Figure 5a). Although there was no significant difference in the levels of necrosis observed in tumors treated with either IT LPS (Figure 5b) or non-toxic IV VSV + IT LPS (Figure 5c) both of which were significantly greater than levels in control-treated tumors (Figure 5d,e), treatment with non-toxic IV VSV + IT LPS (Figure 5f) significantly reduced the level of tumor-infiltrating immune cells compared with IT LPS alone (Figure 5i). Both IT LPS and non-toxic IV VSV + IT LPS tumors were significantly more infiltrated than control-treated tumors (Figure 5g,h).
Figure 5.
Systemic VSV combined with LPS does not improve local, LPS-only therapy or increase immune cell infiltration. (a) Survival (tumor <1.0 cm in any diameter) of C57BL/6 mice (n = 7/group) bearing subcutaneous tumors after treatment with intravenous (IV) VSV-Δ51 (5 × 106 PFU/100 µl) or PBS (100 µl) followed by intratumoral (IT) PBS (50 µl) or LPS (200 µg/50 µl) 6 hours later. Injections started on day 7 and continued every other day for a total of four (days 7, 9, 11, 13). (b–i) H&E stained tumors harvested 1 day after the last treatment (day 14). Arrows identify (b–e) representative areas of necrosis (×4 magnification) or (f–i) immune cell infiltration (×40 magnification). Bar equals 10 mm for all images. H&E, hematoxylin and eosin; LPS, lipopolysaccharide; PBS, phosphate-buffered saline; VSV, vesicular stomatitis virus.
Clinically useful TLR-activating agents do not mimic efficacy of LPS
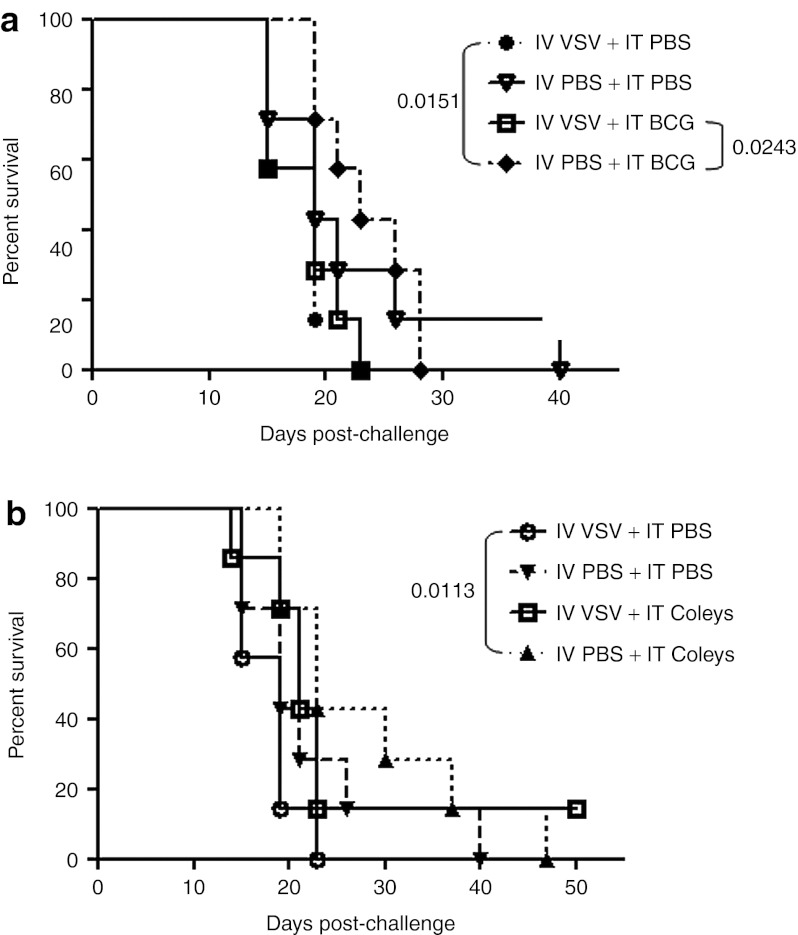
Our ultimate goal was to translate our proof-of-principle studies with LPS into clinically useful protocols of combination VSV/TLR-activating antitumor therapies. To this end, we tested two nonspecific immune adjuvants in combination with IV VSV. Although IT treatment with both bacillus Calmette–Guérin (BCG)25,26 (Figure 6a), and Coley's toxin27,28,29 (Figure 6b), gave significant improvements over control-treated mice (P = 0.0151 for BCG; P = 0.0113 for Coley's toxin) in the experiments of Figure 6, these effects were consistently less impressive than with IT LPS alone (Figures 1 and 2) and significance was not achieved in all experiments. Similar to the results with LPS, single-agent IT therapy was not improved by IV VSV before IT BCG or Coley's toxin. In addition, no systemic toxicity was observed with the combination of IV VSV either at low (5 × 106 PFU/dose) or high (5 × 108 PFU/dose) dose of IV virus followed by IT BCG or Coley's toxin treatment.
Figure 6.
Neither efficacy nor toxicity observed following treatment with BCG or Coley's toxin in combination with systemic VSV. (a) Survival (tumor <1.0 cm in any diameter) of C57BL/6 mice (n = 7/group) bearing subcutaneous tumors after treatment with intravenous (IV) VSV-Δ51 (5 × 106 PFU/100 µl) or PBS (100 µl) followed by intratumoral (IT) PBS (50 µl) or BCG (1 mg/50 µl) 6 hours later. Injections started on day 7 and continued every other day for a total of four (days 7, 9, 11, 13). (b) Survival (as in a) after treatment with IV VSV-Δ51 (5 × 106 PFU/100 µl) or PBS (100 µl) followed by IT PBS (50 µl) or Coley's toxin (final dilution of 0.05 Coley's toxin in 50 µl PBS) 6 hours later. Injections started on day 7 and continued every other day for a total of four (days 7, 9, 11, 13). BCG, bacillus Calmette–Guérin; PBS, phosphate-buffered saline; PFU, plaque-forming unit; VSV, vesicular stomatitis virus.
Discussion
We show here that it is possible to improve the antitumor efficacy of IT VSV by combining it with the nonspecific, immune activator LPS. These results are significant as they show that oncolytic virotherapy can be enhanced with agents which augment, rather than suppress, the innate immune reactivity at the tumor site and open the way for novel, combination clinical protocols with nonspecific immune adjuvants. We have previously demonstrated that the antitumor efficacy of IT VSV in the B16ova/C57BL/6 model is mediated primarily by innate immune antiviral responses dependent upon MyD88 signaling, host effector natural killer and T cells as well as type III IFN, leading to the killing of both infected and uninfected, bystander, tumor cells.4,7,11,12 Therefore, we hypothesized that combining IT VSV with an agent that stimulates innate immune signaling pathways separate from those triggered by VSV would enhance the overall potency of IT VSV-mediated tumor clearance. Since loss of TLR-4 signaling had no effect on efficacy of IT VSV,11 as a proof-of-principle study, we tested the combination of VSV with the TLR-4 agonist LPS.13,21,30 Here, we show that IT LPS has significant antitumor activity as a single agent (Figure 1a), a therapy which was associated with type-I IFN signaling (Figure 1b) and extensive recruitment of IT macrophages (Figure 1c) and neutrophils (Figure 2c).
These data suggested that VSV-mediated, MyD88-dependent but TLR-4–independent signaling11 would act cooperatively, or even synergistically, with LPS-mediated, TLR-4–dependent and type-I IFN-mediated, MyD88-independent immune activation.21,31 Consistent with this hypothesis, therapy generated by local delivery of VSV was significantly enhanced by combining IT virus with IT LPS (Figure 2a). In addition, by expressing a defined TAA from the VSV, we were able to further enhance the combination therapy (Figure 2b) by activating, and recruiting, adaptive antitumor effectors (anti-ova CD8+ T cells) to the tumor site (Figure 3), along with the potent innate effectors activated by both VSV and LPS (Figure 2c,d). In this latter respect, different patterns of tumor infiltration were induced by each treatment alone–for example, neutrophil-high, CD8+ T cell-low for LPS and neutrophil-low, T cell-high for VSV (Figure 2c,d). Although the therapeutically successful combination altered these profiles relative to either single treatment alone, the resultant pattern of tumor infiltration retained what we believe to be the best features of each treatment individually—namely a neutrophil-intermediate (lower than LPS, but higher than VSV alone) (Figure 2c) and a CD8+ T cell-intermediate pattern (Figures 2d and 3). It seems likely that it was this ability of the combination treatment to mediate an overall recruitment of immune effectors of different classes simultaneously, relative to either treatment alone, which resulted in improved tumor clearance and survival.
The sequence dependency of these therapeutic effects (VSV followed by LPS, rather than vice versa) suggests that VSV sensitizes immune cells within the tumor microenvironment, which then become hyperactivated by the subsequent presence of LPS. By having both agents focused within the tumor, we believe that the subsequent cytokine storm induced from the tumor-infiltrating immune effectors, which respond to the combination, leads to potent killing of both infected, and bystander, tumor cells.
Therefore, our data indicate that the direct antitumor efficacy of IT delivery of an oncolytic virus could be significantly enhanced through combination with an additional activator of innate immune effector mechanisms. These results are significant for developing new clinical protocols in which oncolytic viruses could be combined locally with clinically approved immune stimulators. In addition, they suggest that innate immune stimulation, as opposed to suppression, may also be therapeutically effective. Importantly, we did not observe any increased levels of VSV replication in tumors treated with both VSV and LPS (data not shown), indicating that the enhanced therapy was not associated with better oncolysis as a result of increased viral replication. The therapeutic effects resulting from the balance between viral replication, the antiviral innate response, and the resultant antitumor response will differ between viruses, models, and species. Therefore, therapeutic strategies aimed at suppressing the IT, antiviral innate immune response, in order to boost levels of oncolytic viral replication, should be carefully monitored to ensure that they do not lead to decreased therapy as a result of diminishing the antitumor effects induced by the antiviral innate response—and vice versa.
Because our eventual goal is systemic delivery of oncolytic viruses, we also investigated whether the same improved therapeutic effects could be achieved following IV delivery of VSV along with local delivery of LPS into the tumor. In contrast to therapeutically effective, non-toxic IT combination therapy, IV VSV followed by IT LPS induced an oftentimes lethal, cytokine-induced toxicity (Figure 4a), associated with very rapid elevations in serum TNF-α and IL-6 (Figure 4b,c). In this scenario, systemic virus appeared to sensitize host immune responder cells for subsequent reactivity to LPS which, presumably, escaped from the local site of tumor injection. Our data are, therefore, consistent with a model in which high levels of systemic VSV lead to a VSV-mediated sensitization of circulating innate immune effector cells. Subsequent leakage of LPS from the IT-injected tumors then further activates these sensitized effectors to release cytokines, such as TNF-α and IL-6, leading to the severe cytokine storm toxicity that we observed in the systemic tissues (Figure 4) but was diverted away from the tumor (Figure 5b–i). Importantly, toxicity was also observed in mice treated with IT LPS followed by high-dose IV VSV.
At lower levels of VSV-mediated immune cell sensitization, subsequent activation by LPS escaping from the injected tumor was not sufficient for systemic toxicity—but neither was it able to mediate local tumor clearance by immune effectors still resident in the tumor (Figure 5a). Only by having high IT levels of VSV, to recruit and sensitize infiltrating immune effectors, could the subsequent LPS-activated cytokine storm be highly focused on the tumor site to result in significant antitumor therapy (Figure 2). We were unable to demonstrate any significant tumor control using systemic VSV with IT administration of agents, such as BCG or Coley's, which could be clinically useful. This, combined with the fact that treatment with IV VSV + IT LPS is closely associated with potent toxicity, suggests that this type of combination therapy will be highly problematic to pursue into the clinic. This conclusion should not, however, in any way diminish enthusiasm for pursuing either treatment (systemic oncolytic virotherapy or TLR-targeted immunotherapy) alone.
We believe that the data presented here is potentially very important in highlighting the possible adverse events which may result from systemic delivery of oncolytic viruses in clinical trials. IV delivery of an oncolytic virus to a patient with a pre-existing, or newly acquired, bacterial infection,32 for instance, may lead to sensitization and subsequent systemic cytokine storm-related toxicity similar to that observed in our murine model. In addition, it is possible that certain combination treatment protocols may lead to the systemic release of bacterial products, such as LPS, or other innate immune activators, during, or shortly after, virus administration. For example, whole body radiation therapy can lead to the translocation of LPS-containing microbes out of the gut.33,34 Similar effects may also be induced by nonspecific immunotherapies, chemotherapy, or even antibiotic treatment. Precisely to test this, we investigated whether similar toxicities would be induced in our preclinical model using two nonspecific immunotherapies, which have been used in patients (in contrast to LPS). Neither BCG, nor Coley's toxin, showed the potency of LPS either in their local IT antitumor therapy (Figure 6) or in their systemic toxicity-inducing profiles in combination with a prior dose of IV VSV. As such, the induction of the severe toxicities primed by IV viruses will be difficult to predict and will undoubtedly depend upon the nature, timing, and route of administration of the immune stimulus with which they are used in combination.
It is relatively unlikely that the circulation of (rather short-lived) systemic oncolytic viruses would coincide with a subsequent boost of a systemic immune activation event through the presence of infection or even therapeutic agents. However, detrimental synergy between concurrent viral and bacterial infections in both animal models and humans, have been amply demonstrated.15,17,18,35,36,37,38 Therefore, with an increasing repertoire of combination therapies between oncolytic viruses and chemotherapeutics, biologics, radiotherapy or immunotherapies,39,40,41 our results show that careful combination dose escalation phase I trials will be needed to optimize such combination therapies.
Materials and Methods
Cell line. Murine B16ova melanoma cells (H2-Kb) were derived from B16 cells transduced with a cDNA encoding the chicken ovalbumin gene.42 Cell lines were grown in Dulbecco's Modified Eagle's Medium (Life Technologies, Carlsbad, CA) supplemented with 10% (vol/vol) fetal calf serum (Life Technologies), L-glutamine (Life Technologies), and 5 mg/ml G418 (Mediatech, Manassas, VA) to select for retention of the ova gene. All cell lines were routinely monitored and found to be free of Mycoplasma infection.
Mice. C57BL/6 (Thy 1.2+) and TNFKO mice (C57BL/6 background) were purchased from The Jackson Laboratory (Bar Harbor, ME) at 6–8 weeks of age. IFNAR−/− mice, previously backcrossed onto the C57BL/6 background, were obtained as a kind gift from Dr Roberto Cattaneo, Mayo Clinic (Rochester, MN).
Viruses. VSV-GFP and VSV-ova (Indiana serotype) were generated by cloning the cDNA for GFP or chicken ovalbumin, respectively, into the plasmid pVSV-XN2 as described previously.43 VSV-Δ51, methionine deletion in the viral matrix protein (residue 51), was a kind gift from Dr John Bell, Ottawa Hospital Research Institute (Ottawa, Ontario, Canada) and has also been described previously.5 VSV bulk amplification was performed by infecting BHK-21 cells (multiplicity of infection = 0.01) for 24 hours. Filtered supernatants were harvested and subjected to two rounds of 10% sucrose (10% wt/vol) in 1× PBS (Mediatech, Herndon, VA) cushion centrifugation at 27,000 rpm for 1 hour at 4 °C. The pelleted virus was resuspended in 1× PBS, aliquoted and stored at −80 °C. Viral titers were measured by standard plaque assay on BHK-21 cells.12,43
In vivo studies. All procedures were approved by the Mayo Foundation Institutional Animal Care and Use Committee. To establish subcutaneous tumors, 5 × 105 B16ova tumor cells in 100 µl of PBS were injected into the flanks of C57BL/6 mice (day 0). IT virus, LPS or PBS administration (50 µl) began on day 7 and continued every other day for a total of one (day 7) or three (days 7, 9, 11) injections, based on the investigative goals of the study. LPS (Sigma-Aldrich, St Louis, MO), when given in combination with VSV, was given on day 8. For semi-systemic combination experiments, IV VSV or PBS (100 µl) was administered starting on day 7 followed by IT LPS, BCG (TICE BCG; Organon Teknika, Durham, NC), Coley's toxin (MBVax Bioscience, Ancaster, Ontario, Canada) or PBS (50 µl) 6 hours later. Combination treatment was given either once (day 7) or for a total of four regimens (days 7, 9, 11, 13). Mice were examined daily for overall health and tumor sizes were measured three times weekly using calipers. Tumor volume was calculated as 0.52 × width2 × length.44 Mice were euthanized at any sign of distress or when tumor size was ~1.0 × 1.0 cm in two perpendicular directions.
In vitro T-cell activation and ELISA for IFN-γ secretion. Tumor-draining lymph nodes were excised from euthanized mice. Single-cell suspensions were prepared by crushing tissues through a 100 µm filter and red blood cells were removed by incubation in ACK buffer (distilled H2O containing 0.15 mol/l NH4Cl, 10 mmol/l KHCO3, and 0.1 mmol/l EDTA adjusted to pH 7.2–7.4) for 2 minutes. Cells were resuspended at 1 × 106 cells/ml in Iscove's Modified Dulbecco's Medium (Gibco, Grand Island, NY) + 5% fetal bovine serum + 1% Pen-Strep + 40 µmol/l 2-ME and pulsed with ova-specific (5 µg/ml), VSV-N specific peptide (5 µg/ml) or medium for 48 hours. Supernatants were then collected and tested for IFN-γ production by enzyme-linked immunosorbent assay (ELISA) as directed in the manufacturer's instructions (Mouse IFN-γ ELISA Kit, OptEIA; BD Biosciences, San Diego, CA). The synthetic H-2Kb-restricted peptides ova257-264: SIINFEKL, VSV-N52-59: RGYVYQGL were synthesized at the Mayo Foundation Core Facility (Rochester, MN).
Flow cytometry and IFN-γ intracellular staining. Tumors were excised from euthanized mice and dissociated in vitro to achieve single-cell suspensions. Red blood cells were lysed with ACK lysis buffer for 2 minutes as described up above. Remaining cells from each tissue were washed and resuspended in PBS wash buffer containing 0.1% bovine serum albumin, and incubated with directly conjugated primary antibodies for 30 minutes at 4 °C. Cells were then washed and resuspended in 500 µl PBS containing 4% formaldehyde. For intracellular IFN-γ staining, tumor cell suspensions were incubated for 4 hours in the presence of peptides of interest (5 µg/ml) and Golgi Plug reagent (BD Biosciences). Following incubation, the samples were permeabilized and stained using the Cytofix/Cytoperm kit from BD Biosciences, according to the manufacturer's instructions. Extracellular and intracellular staining samples were analyzed by flow cytometry and data were analyzed using Flowjo software (Flowjo, Ashland, OR).
Serum collection and assessment of TNF-α and IL-6 levels. VSV-Δ51 (5 × 108 PFU/100 µl) or PBS (100 µl) was injected IV into tumor-bearing, C57BL/6 mice on day 7. Six hours after VSV administration, mice were treated with IT LPS (50 µl) or PBS (50 µl). Animals were euthanized and cardiac bleeds were done 1.5 hours after LPS treatment. Serum was collected and levels of TNF-α and IL-6 were determined by ELISA according to manufacturer's instructions (Mouse TNF-α and IL-6 ELISA Kits, OptEIA; BD Biosciences).
Histopathology. Tumors were fixed in 10% formalin in PBS (wt/vol), paraffin-embedded and sectioned. Hematoxylin and eosin-stained sections were prepared for analysis of tissue destruction and gross immune infiltration. Sections were analyzed by an independent pathologist who was blinded to the experimental design.
Statistics. Survival data from the animal studies were analyzed by the log-rank test using GraphPad Prism 5 (GraphPad Software, La Jolla, CA). Two-sample, unequal variance Student's t-test analysis was applied for in vitro data. Statistical significance was determined at the level of P < 0.05.
Acknowledgments
This work was supported by the Richard M Schulze Family Foundation, the Mayo Foundation, and by National Institutes of Health grants CA107082, CA130878, and CA132734. The authors declared no conflict of interest.
REFERENCES
- Stojdl DF, Lichty B, Knowles S, Marius R, Atkins H, Sonenberg N.et al. (2000Exploiting tumor-specific defects in the interferon pathway with a previously unknown oncolytic virus Nat Med 6821–825. [DOI] [PubMed] [Google Scholar]
- Colamonici OR, Domanski P, Platanias LC., and, Diaz MO. Correlation between interferon (IFN) alpha resistance and deletion of the IFN alpha/beta genes in acute leukemia cell lines suggests selection against the IFN system. Blood. 1992;80:744–749. [PubMed] [Google Scholar]
- Grandér D., and, Einhorn S. Interferon and malignant disease–how does it work and why doesn't it always. Acta Oncol. 1998;37:331–338. doi: 10.1080/028418698430548. [DOI] [PubMed] [Google Scholar]
- Galivo F, Diaz RM, Wongthida P, Thompson J, Kottke T, Barber G.et al. (2010Single-cycle viral gene expression, rather than progressive replication and oncolysis, is required for VSV therapy of B16 melanoma Gene Ther 17158–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stojdl DF, Lichty BD, tenOever BR, Paterson JM, Power AT, Knowles S.et al. (2003VSV strains with defects in their ability to shutdown innate immunity are potent systemic anti-cancer agents Cancer Cell 4263–275. [DOI] [PubMed] [Google Scholar]
- Balachandran S., and, Barber GN. Vesicular stomatitis virus therapy of tumors. IUBMB Life. 2000;50:135–138. doi: 10.1080/713803696. [DOI] [PubMed] [Google Scholar]
- Wongthida P, Diaz RM, Galivo F, Kottke T, Thompson J, Pulido J.et al. (2010Type III IFN interleukin-28 mediates the antitumor efficacy of oncolytic virus VSV in immune-competent mouse models of cancer Cancer Res 704539–4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitbach CJ, Paterson JM, Lemay CG, Falls TJ, McGuire A, Parato KA.et al. (2007Targeted inflammation during oncolytic virus therapy severely compromises tumor blood flow Mol Ther 151686–1693. [DOI] [PubMed] [Google Scholar]
- Bridle BW, Stephenson KB, Boudreau JE, Koshy S, Kazdhan N, Pullenayegum E.et al. (2010Potentiating cancer immunotherapy using an oncolytic virus Mol Ther 181430–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galivo F, Diaz RM, Thanarajasingam U, Jevremovic D, Wongthida P, Thompson J.et al. (2010Interference of CD40L-mediated Tumor Immunotherapy by Oncolytic VSV Human Gene Ther 21439–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wongthida P, Diaz RM, Galivo F, Kottke T, Thompson J, Melcher A.et al. (2011VSV oncolytic virotherapy in the B16 model depends upon intact MyD88 signaling Mol Ther 19150–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz RM, Galivo F, Kottke T, Wongthida P, Qiao J, Thompson J.et al. (2007Oncolytic immunovirotherapy for melanoma using vesicular stomatitis virus Cancer Res 672840–2848. [DOI] [PubMed] [Google Scholar]
- Georgel P, Jiang Z, Kunz S, Janssen E, Mols J, Hoebe K.et al. (2007Vesicular stomatitis virus glycoprotein G activates a specific antiviral Toll-like receptor 4-dependent pathway Virology 362304–313. [DOI] [PubMed] [Google Scholar]
- Lund JM, Alexopoulou L, Sato A, Karow M, Adams NC, Gale NW.et al. (2004Recognition of single-stranded RNA viruses by Toll-like receptor 7 Proc Natl Acad Sci USA 1015598–5603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doughty L, Nguyen K, Durbin J., and, Biron C. A role for IFN-alpha beta in virus infection-induced sensitization to endotoxin. J Immunol. 2001;166:2658–2664. doi: 10.4049/jimmunol.166.4.2658. [DOI] [PubMed] [Google Scholar]
- Nansen A, Christensen JP, Marker O., and, Thomsen AR. Sensitization to lipopolysaccharide in mice with asymptomatic viral infection: role of T cell-dependent production of interferon-gamma. J Infect Dis. 1997;176:151–157. doi: 10.1086/514017. [DOI] [PubMed] [Google Scholar]
- Nguyen KB., and, Biron CA. Synergism for cytokine-mediated disease during concurrent endotoxin and viral challenges: roles for NK and T cell IFN-gamma production. J Immunol. 1999;162:5238–5246. [PubMed] [Google Scholar]
- Nansen A., and, Randrup Thomsen A. Viral infection causes rapid sensitization to lipopolysaccharide: central role of IFN-alpha beta. J Immunol. 2001;166:982–988. doi: 10.4049/jimmunol.166.2.982. [DOI] [PubMed] [Google Scholar]
- Glauser MP, Zanetti G, Baumgartner JD., and, Cohen J. Septic shock: pathogenesis. Lancet. 1991;338:732–736. doi: 10.1016/0140-6736(91)91452-z. [DOI] [PubMed] [Google Scholar]
- Sriskandan S., and, Altmann DM. The immunology of sepsis. J Pathol. 2008;214:211–223. doi: 10.1002/path.2274. [DOI] [PubMed] [Google Scholar]
- Kawai T., and, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- Wongthida P, Diaz RM, Pulido C, Rommelfanger D, Galivo F, Kaluza K.et al. (2011Activating systemic T-cell immunity against self tumor antigens to support oncolytic virotherapy with vesicular stomatitis virus Hum Gene Ther 221343–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kottke T, Errington F, Pulido J, Galivo F, Thompson J, Wongthida P.et al. (2011Broad antigenic coverage induced by viral cDNA library-based vaccination cures established tumors Nature Med 2011854–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krüttgen A., and, Rose-John S. Interleukin-6 in sepsis and capillary leakage syndrome. J Interferon Cytokine Res. 2012;32:60–65. doi: 10.1089/jir.2011.0062. [DOI] [PubMed] [Google Scholar]
- Sylvester RJ. Bacillus Calmette-Guérin treatment of non-muscle invasive bladder cancer. Int J Urol. 2011;18:113–120. doi: 10.1111/j.1442-2042.2010.02678.x. [DOI] [PubMed] [Google Scholar]
- Luo Y., and, Knudson MJ. Mycobacterium bovis bacillus Calmette-Guérin-induced macrophage cytotoxicity against bladder cancer cells. Clin Dev Immunol. 2010;2010:357591. doi: 10.1155/2010/357591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoption Cann SA, van Netten JP., and, van Netten C. Dr William Coley and tumour regression: a place in history or in the future. Postgrad Med J. 2003;79:672–680. [PMC free article] [PubMed] [Google Scholar]
- McCarthy EF. The toxins of William B. Coley and the treatment of bone and soft-tissue sarcomas. Iowa Orthop J. 2006;26:154–158. [PMC free article] [PubMed] [Google Scholar]
- Decker WK., and, Safdar A. Bioimmunoadjuvants for the treatment of neoplastic and infectious disease: Coley's legacy revisited. Cytokine Growth Factor Rev. 2009;20:271–281. doi: 10.1016/j.cytogfr.2009.07.004. [DOI] [PubMed] [Google Scholar]
- Kumar H, Kawai T., and, Akira S. Pathogen recognition by the innate immune system. Int Rev Immunol. 2011;30:16–34. doi: 10.3109/08830185.2010.529976. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Sato S, Hemmi H, Uematsu S, Hoshino K, Kaisho T.et al. (2003TRAM is specifically involved in the Toll-like receptor 4-mediated MyD88-independent signaling pathway Nat Immunol 41144–1150. [DOI] [PubMed] [Google Scholar]
- Vähä-Koskela MJ, Heikkilä JE., and, Hinkkanen AE. Oncolytic viruses in cancer therapy. Cancer Lett. 2007;254:178–216. doi: 10.1016/j.canlet.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulos CM, Wrzesinski C, Kaiser A, Hinrichs CS, Chieppa M, Cassard L.et al. (2007Microbial translocation augments the function of adoptively transferred self/tumor-specific CD8+ T cells via TLR4 signaling J Clin Invest 1172197–2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulos CM, Kaiser A, Wrzesinski C, Hinrichs CS, Cassard L, Boni A.et al. (2007Toll-like receptors in tumor immunotherapy Clin Cancer Res 1318 Pt 15280–5289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang WJ, Sarawar S, Nguyen P, Daly K, Rehg JE, Doherty PC.et al. (1996Lethal synergism between influenza infection and staphylococcal enterotoxin B in mice J Immunol 1575049–5060. [PubMed] [Google Scholar]
- Diavatopoulos DA, Short KR, Price JT, Wilksch JJ, Brown LE, Briles DE.et al. (2010Influenza A virus facilitates Streptococcus pneumoniae transmission and disease FASEB J 241789–1798. [DOI] [PubMed] [Google Scholar]
- Jansen AG, Sanders EA, VAN DER Ende A, VAN Loon AM, Hoes AW., and, Hak E. Invasive pneumococcal and meningococcal disease: association with influenza virus and respiratory syncytial virus activity. Epidemiol Infect. 2008;136:1448–1454. doi: 10.1017/S0950268807000271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallen AJ, Brunkard J, Moore Z, Budge P, Arnold KE, Fosheim G.et al. (2009Staphylococcus aureus community-acquired pneumonia during the 2006 to 2007 influenza season Ann Emerg Med 53358–365. [DOI] [PubMed] [Google Scholar]
- Ottolino-Perry K, Diallo JS, Lichty BD, Bell JC., and, McCart JA. Intelligent design: combination therapy with oncolytic viruses. Mol Ther. 2010;18:251–263. doi: 10.1038/mt.2009.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lolkema MP, Arkenau HT, Harrington K, Roxburgh P, Morrison R, Roulstone V.et al. (2011A phase I study of the combination of intravenous reovirus type 3 Dearing and gemcitabine in patients with advanced cancer Clin Cancer Res 17581–588. [DOI] [PubMed] [Google Scholar]
- Harrington KJ, Karapanagiotou EM, Roulstone V, Twigger KR, White CL, Vidal L.et al. (2010Two-stage phase I dose-escalation study of intratumoral reovirus type 3 dearing and palliative radiotherapy in patients with advanced cancers Clin Cancer Res 163067–3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linardakis E, Bateman A, Phan V, Ahmed A, Gough M, Olivier K.et al. (2002Enhancing the efficacy of a weak allogeneic melanoma vaccine by viral fusogenic membrane glycoprotein-mediated tumor cell-tumor cell fusion Cancer Res 625495–5504. [PubMed] [Google Scholar]
- Fernandez M, Porosnicu M, Markovic D., and, Barber GN. Genetically engineered vesicular stomatitis virus in gene therapy: application for treatment of malignant disease. J Virol. 2002;76:895–904. doi: 10.1128/JVI.76.2.895-904.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm T, Folkman J, Browder T., and, O'Reilly MS. Antiangiogenic therapy of experimental cancer does not induce acquired drug resistance. Nature. 1997;390:404–407. doi: 10.1038/37126. [DOI] [PubMed] [Google Scholar]