Abstract
Peptidases have important roles in controlling physiological signaling through their regulation of bioactive peptides. Understanding and controlling bioactive peptide regulation is of great biomedical interest and approaches that elucidate the interplay between peptidases and their substrates are vital for achieving this goal. Here, we highlight the utility of recent peptidomics approaches in identifying endogenous substrates of peptidases. These approaches reveal bioactive substrates and help characterize the biochemical functions of the enzyme. Most recently, peptidomics approaches have been applied to address the challenging question of identifying the peptidases responsible for regulating specific bioactive peptides. Since peptidases are of great biomedical interest, these approaches will begin to impact our ability to identify new drug targets that regulate important bioactive peptides.
Introduction
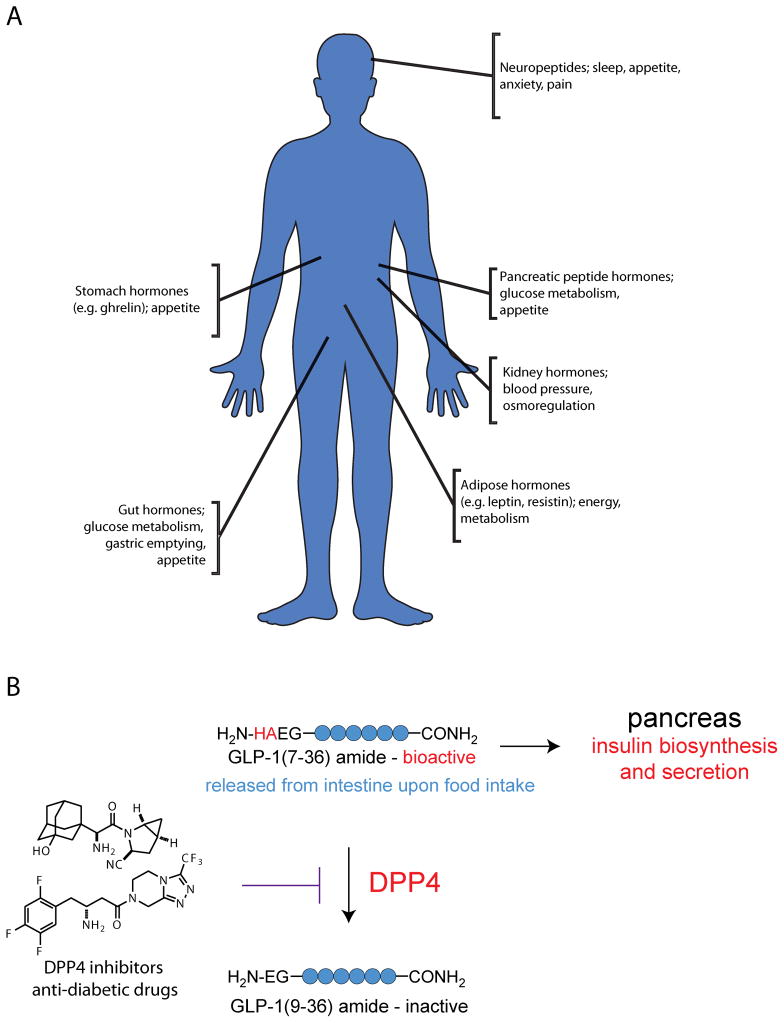
Bioactive peptides are central regulators of physiology, controlling a wide variety of important biological processes (Fig. 1A). A classic example is the bioactive peptide insulin’s role in glucose homeostasis1. Insulin lowers blood glucose levels and insulin dysregulation and resistance are both associated with diabetes mellitus. Other bioactive peptides have roles in processes such as pain sensation2–4, sleep regulation 5,6 and food intake7. Some can even control highly complex phenotypes, including emotional and social behaviors8. For instance, oxytocin, a peptide initially characterized as a regulator of uterine contractions and parturition9 has since been found to also influence maternal behavior10 and control feelings of fear and trust in humans11,12.
Figure 1.
Peptides control a wide range of important biological processes. A) Bioactive peptides are found in many organs and control diverse physiological processes. B) DPP4 regulates GLP-1 levels. GLP-1(7–36) amide is released from the gut in response to food intake, and stimulates biosynthesis and secretion of insulin. DPP4 inactivates this species by removing the N-terminal dipeptide, resulting in the inactive species GLP-1(9–36) amide. By inhibiting DPP4, recently developed diabetes drugs increase levels of GLP-1 and insulin, thus affording better control of blood glucose levels.
Given this wide range of biology regulated by bioactive peptides, there is great potential for developing therapeutics targeting some of these peptides, or the enzymes that produce or degrade them. A few notable treatments have already been developed, a recent example being the development and approval of inhibitors of dipeptidyl peptidase 4 (DPP4) as a treatment for diabetes13 (Fig 1B). These inhibitors act by preventing DPP4 from degrading its substrate, the incretin glucagon-like peptide 1 (GLP-1), which normally stimulates insulin biosynthesis and secretion. Thus, treatment with these inhibitors increases GLP-1 and insulin levels, resulting in lower blood glucose levels. Given the important role of peptidases in regulating bioactive peptide levels and the demonstrated medical utility of targeting peptidases to regulate bioactive peptide levels, it is of great interest to characterize the role different peptidases play in the regulation of specific bioactive peptides.
The human genome codes for well over 500 peptidases and proteases14, and though some are well characterized, there are many examples of proteases whose in vivo functions are still largely unknown. Yet other proteases have suspected biological roles, but the molecular pathways through which they achieve this function remain unknown15. Peptidase activity has important roles in several phases of the peptide lifecycle, including the production, activation, inactivation and degradation of bioactive peptides,16,17 thus regulating levels of the active species through several avenues. Although some of these peptide-peptidase pairings are known, there are still a vast number of bioactive peptides whose regulation by peptidases is not well characterized and peptidases whose endogenous substrates are incompletely mapped.
Since existing in vitro approaches were not ideal for discovering physiologically-relevant interactions, new methods for characterizing endogenous peptidase-substrate interactions were clearly necessary. Novel peptidomics approaches were developed which allowed global assessments of peptide levels and easy identification of even slightly differing peptide species, such as those that may result from a cleavage event. With these advantages, peptidomics has become a powerful tool both for characterizing the full set of endogenous substrates regulated by a given peptidase and also for identifying the peptidase responsible for regulating levels of a given bioactive peptide species in vivo, as will be described in the following.
PEPTIDOMICS FOR SUBSTRATE DISCOVERY
Identifying the natural substrates of a peptidase is one of the most challenging biochemical problems known. In vitro, a peptidase may cleave many substrates that it will never encounter in a cell or tissue. As a result, traditional biochemical approaches for determining endogenous substrates for peptidases were often unreliable. For instance, although DPP4 shows a marked preference for cleaving after proline residues in vitro18, one of its main substrates, GLP-1, through which DPP4 controls insulin levels, has an alanine residue in this position. Discrepancies like these can make it hard or impossible to infer endogenous substrates from substrate specificity information gleaned using traditional approaches.
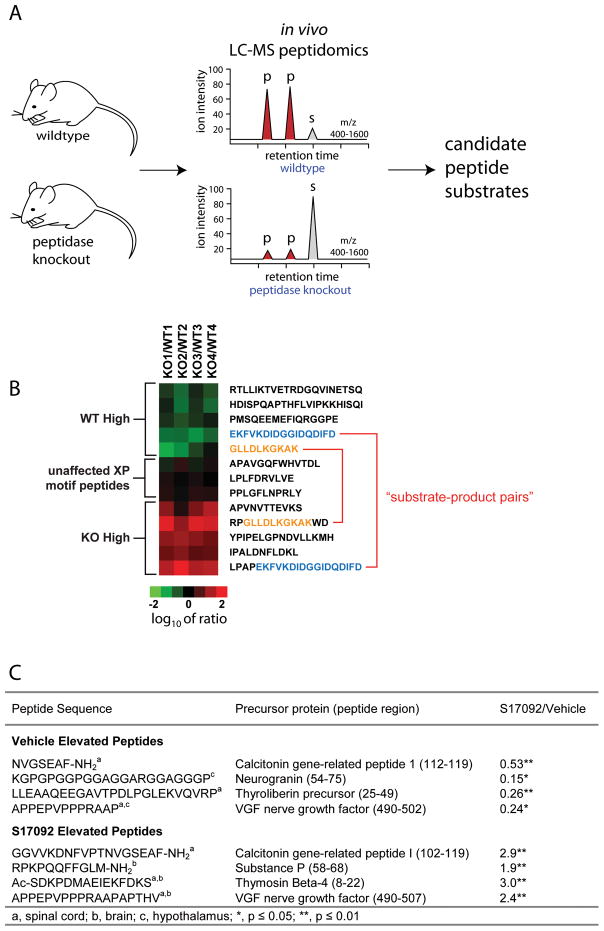
The new peptidomics approaches developed in the past few years seek to address some of these issues by providing a more general strategy for identifying substrates regulated by a peptidase in a biological context. The general approach is based on either chemically inhibiting or genetically knocking out a given peptidase and assessing the global changes in the peptidome following this perturbation. In the absence of a given peptidase activity, substrates of this enzyme will begin to accumulate and the levels of the peptidase’s normal products will be reduced relative to a sample with enzyme activity, as no substrate is being turned over in the absence of this enzyme (Fig. 2A). Mass spectrometry is the enabling tool that allows a global comparison of peptide levels between the two samples, facilitating the identification of peptides elevated in each sample and hence likely to be substrates or products of the peptidase.
Figure 2.
Peptide profiling reveals endogenous substrates of peptidases. A) In the peptide profiling technique, mass spectrometry is used for a global comparison of peptide levels in samples with and without enzyme activity. In the sample without enzyme activity, there should be no substrate turnover, resulting in substrate accumulation and lower levels of the peptidase product, relative to a sample with normal enzyme activity. Either chemical inhibitors or genetic knockouts can be used to achieve the removal of enzyme activity. B) Applying this approach to DPP4, a number of previously unknown endogenous substrates of this enzyme were identified. For some of these substrates, the corresponding product could also be seen elevated in the sample containing enzyme activity. C) Inhibiting POP using the specific chemical inhibitor S17092 and application of the peptide profiling approach resulted in the identification of several new substrates of this enzyme, including some bioactive peptides (substance P). The profiling further revealed that not all internal prolines are processed by POP, as previously believed and that POP has a length preference, as the CGRP sequence identified as a substrate was no longer cleaved efficiently when part of the complete CGRP sequence.
One of the earliest LC-MS peptide profiling approaches to determine peptidase substrates was the characterization of the peptidome of mice lacking carboxypeptidase E (CPE)19,20. CPE is one of the main enzymes involved in the processing of bioactive peptides and is responsible for removing C-terminal basic residues from peptide precursors to produce the finished peptide species. Thus, peptides changing in response to CPE ablation are likely substrates or products of this enzyme and most probably also bioactive peptides. In a study of the pituitary of Cpefat/fat mice, 72 peptides were observed and the majority were present at lower levels in the Cpefat/fat mice than in the wild-type mice, indicating a broad role for CPE in peptide processing20. A subsequent study of the prefrontal cortex of Cpefat/fat mice identified 32 changing peptides, including seven novel neuropeptides19. These authors elected to use a labeled approach for quantifying differences in peptide levels between samples. In such approaches, the abundance of one labeled form of the peptide is compared to the other labeled form and the relative abundance of the peptide in the two different sample groups can thus be determined21.
More recently, label-free methods have been developed to characterize two members of the prolyl peptidase family. Label-free methods forego the use of isotope labeling and instead compare relative ion intensities between two samples to obtain information regarding the relative abundance of peptides between samples. In applying this approach to DPP422, mice with genetically ablated DPP4 expression (KO) were compared to mice with normal DPP4 expression (WT), whereas for prolyl oligopeptidase (POP)23, mice treated with the POP inhibitor S17092 were compared with vehicle-treated animals. Whereas genetic knockouts have advantages in their specific and complete removal of enzyme activity, inhibitors can be beneficial in this approach in that their rapid effect largely avoids the compensatory effects that can sometimes be seen in knockout animal models. Which approach is chosen will largely depend on the availability of specific inhibitors that are active in mice or of appropriate knockout mice.
When profiling POP and DPP4, many new substrates of both enzymes were discovered (Fig. 2B, 2C) and subsequently validated in vitro with purified recombinant enzyme. For DPP4, an additional pre-fractionation step was shown to greatly increase sensitivity and peptidome coverage, allowing detection of ten-fold more DPP4 substrates than in the initial profiling experiments24. In addition to a new appreciation for the range of substrates that these enzymes process, the results from these profiling experiments also led to new biological insights. In the case of DPP4, the peptidomics studies led to a further elucidation of DPP4s role in the renal catabolism of proline-containing peptides.
In the case of POP, the substrates observed suggested that POP prefers shorter peptide substrates as a cleavage site found in a short peptide is completely uncut by POP when it is placed into a longer peptide. Furthermore, Tenorio-Laranga and colleagues have also carried out several peptide profiling experiments on POP25,26 using a 2D iTRAQ method and uncovered an extensive list of peptides regulated by POP upon in vivo inhibition, as well as made the interesting observation that, based on the observed peptide changes, POP may indirectly control cleavage at dibasic sites such as those used during peptide processing. As can be seen from these examples, peptide profiling consistently reveals a number of endogenous substrates of a peptidase, which would not have been possible using the types of simple peptide substrates typically utilized for in vitro evaluations of peptidase selectivity.
PEPTIDOMICS: THE MISSING LINK: CONNECTING BIOACTIVE PEPTIDES TO PEPTIDASES
While peptide profiling, as described in the section above, provides a unique opportunity for learning about the full range of substrates and biology regulated by a given peptidase, the reverse question is also of great interest: For a given bioactive peptide, how does one determine the enzyme(s) responsible for regulating its levels in a biological system? Occasionally, scientists have been able to find the enzyme responsible for the degradation of a bioactive peptide, such as in the case of DPP4 and GLP-1, where previous knowledge about the substrate specificity of DPP4 allowed hypotheses about which other bioactive peptides might also be regulated by this enzyme. It was thus possible to screen possible peptide substrates against DPP4 and observe which peptides were in fact degraded. While the biomedically important regulation of GLP-1 by DPP4 was discovered in this manner27, the technique is not generally applicable to any given peptide, since the enzyme degrading it could be unknown, not thoroughly characterized, or have an in vitro substrate specificity that differs significantly from its endogenous substrates.
Another frequently used technique to find the enzyme that cleaves a given bioactive peptide is to purify a peptide-degrading activity from tissue lysate, to identify the fraction of the lysate with the highest specific activity. However, problems can arise with this technique if the endogenous processing pathway differs significantly from that observed in lysates. For instance, in the case of the peptide hormone called peptide histidine isoleucine (PHI), it was demonstrated that while the endogenous processing pathway exclusively involves C-terminal processing, in lysates both C- and N-terminal processing activity is observed28. This example demonstrates how crucial it is to know which fragments are biologically relevant. Peptidomics offers novel advantages in addressing this problem, which has been made use of in the design a new peptidomics-based strategy for elucidating the enzymes responsible for the processing of bioactive peptides in vivo.
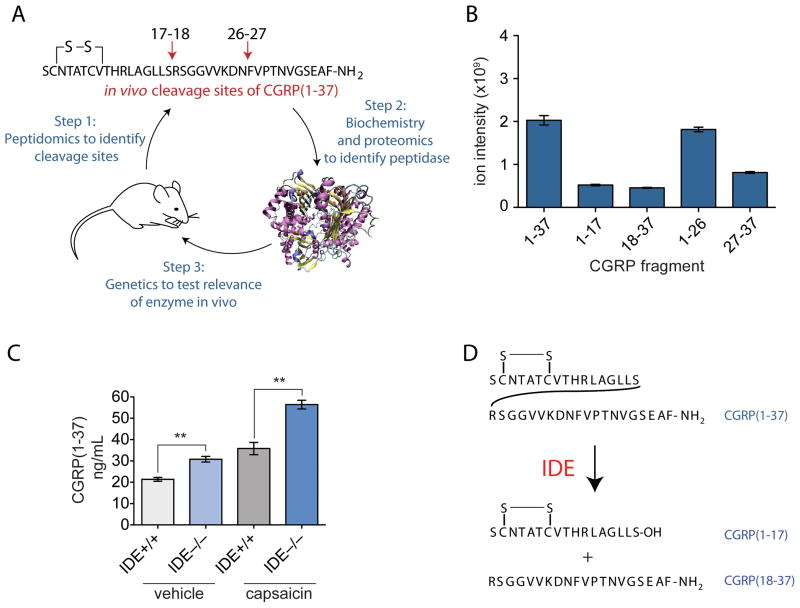
In this strategy, which was first applied to the bioactive peptide calcitonin gene-related peptide (CGRP)29, a vasodilator30 and pain mediator4, the first step is identifying the fragments produced endogenously by peptidomics (Fig 3A). These peptide fragments can then be used to identify the likely endogenous cleavage sites. In the case of CGRP, for example, the four main fragments identified were CRGP1–17, CGRP18–37, CRGP1–26 and CGRP27–37, indicating that the endogenous CGRP cleavage sites are between residues 17–18 and 26–27 of the CGRP sequence. By offering an unbiased view of the endogenous peptides, peptidomics offers an easy solution to the vexing problem of trying to identify the endogenous cleavage sites of a peptide.
Figure 3.
A new peptidomics technique reveals the proteolytic processing pathway and enzyme responsible for inactivating CGRP. A) The technique involved three main steps. Initially, peptidomics is used to identify the biologically relevant fragments of a given bioactive peptide. In step 2, this crucial information about the proteolytic processing pathway is used to design an assay used for purifying the enzyme responsible for this cleaving activity. Proteomics is used to identify candidate enzymes in the most active purified fractions, and these are then recombinantly expressed to identify the enzyme(s) with the desired, biologically relevant, cleaving activity(B). In the final step, the identified enzyme is tested for its ability to regulate levels of the bioactive peptide in vivo(C). Here, the enzyme is either chemically inhibited or genetically knocked out, and levels of the intact bioactive peptide species compared between samples, the expectation being that in the sample without enzyme activity, the bioactive peptide will be degraded less and so be present at higher levels. In the case of CGRP, where IDE had been identified as the candidate enzyme capable of performing both of the two biologically relevant cleavages, it was in fact observed that CGRP levels were significantly higher in the IDE knockout mouse. Additionally, capsaicin, which leads to CGRP release into the bloodstream, was administered to the mice to test IDEs ability to regulate CGRP under different physiological conditions and it was found that IDE still regulated CGRP levels under these conditions, with significantly higher levels of CGRP observed in the IDE knockout mice than in their wildtype littermates. Using this novel approach, an entirely new model for CGRP regulation has been established, with IDE as a key regulator of this peptide’s levels in vivo(D).
Using the production of CGRP1–17 and CGRP18–37 as a bioassay, insulin-degrading enzyme (IDE) was purified from tissue lysates and identified as a candidate CGRP-degrading enzyme. Interestingly, while the bioassay was designed to identify peptidases that cleave between amino acids 17 and 18, in vitro assays demonstrated that IDE is able to cleave CGRP at 17–18 and 26–27 (Fig 3B), which improves the likelihood that the correct enzyme was identified.
The final step in the strategy is to confirm that the peptidase identified is indeed the main enzyme responsible for regulating levels of the bioactive peptide in vivo. This can be done in cell culture, or preferably in an animal model, either a mouse model lacking the enzyme of interest, if one is available, as it was for IDE, or using a specific inhibitor of the enzyme. If the enzyme identified is indeed responsible for a major step in the degradation of a peptide, levels of the intact bioactive peptide should be elevated upon removal of the enzyme activity. Indeed, when comparing levels of full-length CGRP in IDE knockout mice to those in their wild-type littermates, a significant increase in CGRP levels was observed in the knockout mice, confirming a role for IDE in regulating physiological levels of CGRP.
Thus, this peptidomics approach allowed us to go from a given peptide whose manner of proteolytic processing was unknown, to identifying the enzyme responsible for regulating its levels in vivo. A powerful approach, there is great potential for applying this method to other bioactive peptides whose function and regulation are unknown and of interest. In particular, given the challenges related to peptides as therapeutics, if a bioactive peptide regulates medically interesting biology, it could be highly valuable to learn how to control levels of the peptide by regulating the activity of the enzymes producing or degrading it.
CONCLUSION
Peptides regulate a wide range of important biological processes and it is therefore of great biomedical interest to understand how peptide levels are regulated, in particular through the proteolytic events that produce and degrade the active species. Recently developed mass spectrometry-based peptidomics methods have proved to be empowering techniques that have provided novel opportunities for the study of the endogenous pathways for peptide regulation by peptidases. Peptidomics methods are also constantly improving31,32, and advances in technology and techniques should further enable these studies. Here, we have first described how peptide profiling has provided a method for elucidating the endogenous substrates of any given peptidase, by comparing the peptidomes of samples with and without enzyme activity. The possibilities this technique has opened will surely be of great use when evaluating the feasibility of inhibiting peptidases for therapeutic purposes, by giving a more comprehensive picture of the peptides and biology regulated by the enzyme of interest. The converse approach, namely the identification of the peptidase responsible for the proteolytic regulation of a given bioactive peptide, allows the discovery and characterization of the proteolytic regulatory pathways which control the levels of these important peptides. Given the wide range of biology controlled by such bioactive peptides, a method for elucidating their regulation should find broad application when investigating the possibility for regulating bioactive peptide levels in vivo through directly inhibiting the enzymes which produce or degrade them.
Highlights.
Peptidases control physiological signaling by regulating bioactive peptides.
Understanding and controlling bioactive peptide regulation is of biomedical interest.
Here, we describe peptidomics approaches to identify substrates of peptidases.
Conversely, peptidomics can also identify peptidases that regulate specific peptides.
Thus, peptidomics is an important new approach for identifying novel drug targets.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414:799–806. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- 2.De Felipe C, et al. Altered nociception, analgesia and aggression in mice lacking the receptor for substance P. Nature. 1998;392:394–397. doi: 10.1038/32904. [DOI] [PubMed] [Google Scholar]
- 3.Lin CCJ, et al. An antinociceptive role for substance P in acid-induced chronic muscle pain. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:E76–E83. doi: 10.1073/pnas.1108903108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Villalon CM, Olesen J. The role of CGRP in the pathophysiology of migraine and efficacy of CGRP receptor antagonists as acute antimigraine drugs. Pharmacol Ther. 2009;124:309–323. doi: 10.1016/j.pharmthera.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 5.Szentirmai E, Hajdu I, Obal F, Krueger JM. Ghrelin-induced sleep responses in ad libitum fed and food-restricted rats. Brain Res. 2006;1088:131–140. doi: 10.1016/j.brainres.2006.02.072. [DOI] [PubMed] [Google Scholar]
- 6.Ohno K, Sakurai T. Orexin neuronal circuitry: Role in the regulation of sleep and wakefulness. Front Neuroendocrinol. 2008;29:70–87. doi: 10.1016/j.yfrne.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 7.Cummings DE, Overduin J. Gastrointestinal regulation of food intake. J Clin Invest. 2007;117:13–23. doi: 10.1172/jci30227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.MacDonald K, MacDonald TM. The Peptide That Binds: A Systematic Review of Oxytocin and its Prosocial Effects in Humans. Harv Rev Psychiatr. 2010;18:1–21. doi: 10.3109/10673220903523615. [DOI] [PubMed] [Google Scholar]
- 9.Zeeman GG, KhanDawood FS, Dawood MY. Oxytocin and its receptor in pregnancy and parturition: Current concepts and clinical implications. Obstet Gynecol. 1997;89:873–883. doi: 10.1016/s0029-7844(97)00056-2. [DOI] [PubMed] [Google Scholar]
- 10.Takayanagi Y, et al. Pervasive social deficits, but normal parturition, in oxytocin receptor-deficient mice. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:16096–16101. doi: 10.1073/pnas.0505312102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature. 2005;435:673–676. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- 12.Baumgartner T, Heinrichs M, Vonlanthen A, Fischbacher U, Fehr E. Oxytocin shapes the neural circuitry of trust and trust adaptation in humans. Neuron. 2008;58:639–650. doi: 10.1016/j.neuron.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 13.Doupis J, Veves A. DPP4 Inhibitors: a new approach in diabetes treatment. Adv Ther. 2008;25:627–643. doi: 10.1007/s12325-008-0076-1. [DOI] [PubMed] [Google Scholar]
- 14.Overall CM, Blobel CP. In search of partners: linking extracellular proteases to substrates. Nat Rev Mol Cell Biol. 2007;8:245–257. doi: 10.1038/nrm2120. [DOI] [PubMed] [Google Scholar]
- 15.Martens K, et al. PREPL: a putative novel oligopeptidase propelled into the limelight. Biol Chem. 2006;387:879–883. doi: 10.1515/bc.2006.111. [DOI] [PubMed] [Google Scholar]
- 16.Fricker LD. Neuropeptide-processing enzymes: Applications for drug discovery. Aaps Journal. 2005;7 doi: 10.1208/aapsj070244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hook V, et al. Annual Review of Pharmacology and Toxicology. 48 :393–423. doi: 10.1146/annurev.pharmtox.48.113006.094812. (Annual Reviews, 2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leiting B, et al. Catalytic properties and inhibition of proline-specific dipeptidyl peptidases II, IV and VII. Biochem J. 2003;371:525–532. doi: 10.1042/bj20021643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lim J, et al. Altered neuropeptide processing in prefrontal cortex of Cpe(fat/fat) mice: implications for neuropeptide discovery. J Neurochem. 2006;96:1169–1181. doi: 10.1111/j.1471-4159.2005.03614.x. [DOI] [PubMed] [Google Scholar]
- 20.Che FY, Biswas R, Fricker LD. Relative quantitation of peptides in wild-type and Cpe(fat/fat) mouse pituitary using stable isotopic tags and mass spectrometry. J Mass Spectrom. 2005;40:227–237. doi: 10.1002/jms.742. [DOI] [PubMed] [Google Scholar]
- 21••.Wardman J, Fricker LD. Proprotein Convertases. In: Mbikay M, Seidah NG, editors. Methods in Molecular Biology. 768 . Humana Press Inc; 999 Riverview Dr, Ste 208, Totowa, Nj 07512-1165 USA: 2011. pp. 307–323. This article gives a detailed procedure for how to carry out the authors’ labeled peptidomics approach for substrate discovery. It provides a useful introduction and starting point for carrying out a peptide profiling experiment. [Google Scholar]
- 22.Tagore DM, et al. Peptidase substrates via global peptide profiling. Nat Chem Biol. 2009;5:23–25. doi: 10.1038/nchembio.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nolte WM, Tagore DM, Lane WS, Saghatelian A. Peptidomics of Prolyl Endopeptidase in the Central Nervous System. Biochemistry. 2009;48:11971–11981. doi: 10.1021/bi901637c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tinoco AD, Tagore DM, Saghatelian A. Expanding the Dipeptidyl Peptidase 4-Regulated Peptidome via an Optimized Peptidomics Platform. Journal of the American Chemical Society. 2010;132:3819–3830. doi: 10.1021/ja909524e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25•.Tenorio-Laranga J, Mannisto PT, Storvik M, Van der Veken P, Garcia-Horsman JA. Four day inhibition of prolyl oligopeptidase causes significant changes in the peptidome of rat brain, liver and kidney. Biochimie. 2012;94:1849–1859. doi: 10.1016/j.biochi.2012.04.005. In this study, the authors use long-term inhibition with small molecule to perform peptide profiling to identify substrates of POP. Unexpectedly, they find that POP, through an indirect pathway, regulates the cleavage of dibasic residues through an indirect pathway. This illustrates how peptide profiling approaches often lead to insights beyond the identification of direct substrates of an enzyme. [DOI] [PubMed] [Google Scholar]
- 26.Tenorio-Laranga J, Valero ML, Mannisto PT, del Pino MS, Garcia-Horsman JA. Combination of snap freezing, differential pH two-dimensional reverse-phase high-performance liquid chromatography, and iTRAQ technology for the peptidomic analysis of the effect of prolyl oligopeptidase inhibition in the rat brain. Anal Biochem. 2009;393:80–87. doi: 10.1016/j.ab.2009.06.019. [DOI] [PubMed] [Google Scholar]
- 27.Mentlein R, Gallwitz B, Schmidt WE. DIPEPTIDYLPEPTIDASE-IV HYDROLYZES GASTRIC-INHIBITORY POLYPEPTIDE, GLUCAGON-LIKE PEPTIDE-1(7–36)AMIDE, PEPTIDE HISTIDINE METHIONINE AND IS RESPONSIBLE FOR THEIR DEGRADATION IN HUMAN SERUM. Eur J Biochem. 1993;214:829–835. doi: 10.1111/j.1432-1033.1993.tb17986.x. [DOI] [PubMed] [Google Scholar]
- 28.Tinoco AD, et al. A Peptidomics Strategy To Elucidate the Proteolytic Pathways That Inactivate Peptide Hormones. Biochemistry. 2011;50:2213–2222. doi: 10.1021/bi2000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29••.Kim YG, Lone AM, Nolte WM, Saghatelian A. Peptidomics approach to elucidate the proteolytic regulation of bioactive peptides. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:8523–8527. doi: 10.1073/pnas.1203195109. This article describes the first use of a novel peptidomics technique to identify the peptidases responsible for regulating levels of a given bioactive peptide in vivo. In it, the authors identify IDE as the main CGRP-regulating enzyme. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smillie SJ, Brain SD. Calcitonin gene-related peptide (CGRP) and its role in hypertension. Neuropeptides. 2011;45:93–104. doi: 10.1016/j.npep.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 31••.Neupert S, Rubakhin SS, Sweedler JV. Targeted Single-Cell Microchemical Analysis: MS-Based Peptidomics of Individual Paraformaldehyde-Fixed and Immunolabeled Neurons. Chemistry & biology. 2012;19:1010–1019. doi: 10.1016/j.chembiol.2012.05.023. A method is described for the preparation of cells for single-cell mass spectrometry analysis, enabling analysis of the endogenous peptides in isolated individual cells. By allowing studies of the peptidomes in specific individual cells, the technique permits peptidomics studies to take into account and investigate the heterogeneity of cell populations. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yin P, et al. Peptidomic Analyses of Mouse Astrocytic Cell Lines and Rat Primary Cultured Astrocytes. J Proteome Res. 2012;11:3965–3973. doi: 10.1021/pr201066t. [DOI] [PMC free article] [PubMed] [Google Scholar]