Abstract
Nitroxyl (HNO) has a unique, but varied, set of biological properties including beneficial effects on cardiac contractility and stimulation of glucose uptake by GLUT1. These biological effects are largely initiated by HNO’s reaction with cysteine residues of key proteins. The intracellular production of HNO has not yet been demonstrated, but the small molecule, hydroxylamine (HA), has been suggested as possible intracellular source. We examined the effects of this molecule on glucose uptake in L929 fibroblast cells. HA activates glucose uptake from 2 to 5-fold within two minutes. Prior treatment with thiol active compounds, such as iodoacetamide (IA), cinnamaldehyde (CA), or phenylarsine oxide (PAO) blocks HA activation of glucose uptake. Incubation of HA with the peroxidase inhibitor, sodium azide, also blocks the stimulatory effects of HA. This suggests that HA is oxidized to HNO by L929 fibroblast cells, which then reacts with cysteine residues to exert its stimulatory effects. The data suggest that GLUT1 is acutely activated in L929 cells by modification of cysteine residues, possibly the formation of a disulfide bond within GLUT1 itself.
Keywords: hydroxylamine, nitroxyl, glucose uptake, GLUT1, L929 cells
1. Introduction
There is increasing interest in the unique biological effects of nitroxyl (HNO), effects that can be distinguished from the effects of nitric oxide (NO) [1-7]. HNO reacts with several cardiac sarcoplasmic proteins involved in calcium release and uptake, and thereby, enhances cardiac contractility [8-11]. The observation that HNO also inhibits aldehyde dehydrogenase makes it an effective treatment for alcoholism [12, 13]. Many of the biological effects of HNO have been attributed to its propensity to react with cysteine residues. HNO can add to a single cysteine residue to form a N-hydroxysulfenamide, which can rearrange to produce a sulfinamide, or react with second cysteine residue to generate a disulfide bond [3]. The reaction of HNO with thiols accounts for its inhibitory effects on, glyceraldehyde-3-phosphate dehydrogenase [14, 15] and cysteine proteases such as papain [16] and cathepsin B [17]. More recently, we have shown that HNO rapidly activates GLUT1 in L929 fibroblast cells and this effect can be block prior treatment with thiol-reactive reagents, such as iodoacetamide and cinnamaldehyde [18].
There is an increasing list of reagents or cellular conditions, including azide [19, 20], methylene blue [21], osmotic stress [22, 23], C-peptide [24], glucose deprivation [25, 26] and HNO [18] that acutely activate GLUT1. Acute activation of GLUT1 does not involve a change in either the expression or the membrane concentration of the transporter [19]. While the mechanism for the acute activation of GLUT1 is not completely understood, there is mounting evidence that activation of GLUT1 in L929 fibroblast cells requires modification of key cysteine residues [18, 27, 28]. The data in these previously cited studies are consistent with the suggestion that GLUT1 is more active when it assembles into tetramers. These tetramers appear to be stabilized by the conformational changes that occur within GLUT1 after the formation of an internal disulfide bond [29-31].
It is not clear if the effects of HNO are simply pharmacological, or if they actually represent a cellular signaling system [3, 4, 7]. The substrate for the production of HNO is not known but several small molecules have been suggested, including hydroxylamine (HA) [5, 7, 32, 33]. To explore this further, we investigated the effects of HA on the transport activity of GLUT1 in L929 fibroblast cells (GLUT1 is the exclusive glucose transporter in this cell line [34]).
2. Materials and Methods
2.1 Chemicals
Angeli’s salt (AS) was a generous gift of Dr. John P. Toscano (Johns Hopkins University). Phenylarsine oxide (PAO), cinnamaldehyde (CA), iodoacetamide (IA), (HA), sodium azide (Az), 2-deoxy-D-glucose-[1,2-3H] (2DG) and D-mannitol-1-14C were purchased from the Sigma-Aldrich Chemical Company (St. Louis, MO, USA).
2.2 Cell culture
L929 mouse fibroblast cells were obtained from the American Type Culture Collection. To initiate each experiment, approximately 1.5 × 105 L929 fibroblast cells were plated into each well of a 24-well culture-treated plate in 1.0 mL of low glucose (5.5 mM) DMEM (Dulbecco’s Modified Eagle Medium) supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin. The cells were grown overnight at 37 °C in an incubator supplied with humidified room air with 5% CO2. Since the magnitude of stimulatory effects varies somewhat depending on the confluency of the cells, experiments were normally done with cells near confluency (3.2 × 105 cells/well for a 24-well plate).
2.3 General experimental design
To initiate an experiment, the media from cells in 24-well plates were removed and then incubated in 0.4 mL of fresh treatment media consisting of either low-glucose DMEM alone (0% FBS) (basal) or low-glucose DMEM plus the chemical of interest (see figure legends). Cells were maintained at 37 °C for the times indicated. In all experiments using AS, the solid compound was quickly dissolved in media at room temperature immediately distributed to the cells in the 24-well plate (process took about 30 seconds). All other reagents were added to the media or 2DG uptake solution from 100-200x aqueous (sodium azide, IA, HA), or ethanol (CA) or DMSO (PAO) stock solutions. Ethanol and DMSO at the concentrations added have no effect on glucose uptake [27, 28].
2.4 Glucose uptake assay
Glucose uptake was measured using the radiolabeled glucose analog 2-deoxyglucose (2DG) as previously described [35]. Briefly, the media was replaced with 0.2 mL of glucose-free HEPES buffer (140 mM NaCl, 5 mM KCl, 20 mM HEPES/Na pH=7.4, 2.5 mM MgSO4, 1 mM CaCl2, 2 mM NaPyruvate, 1 mM mannitol) supplemented with 1.0 mM (0.3 μCi/mL) 2-DG (1,2-3H) and 1.0 mM (0.02 μCi/mL) mannitol (1-14C). Uptake media was supplemented with additional compounds, such as AS or HA, as indicated in the figure legends. After a 10-minute incubation, cells were washed twice with cold glucose-free HEPES. The cells were lysed in 0.5 mL lysis buffer (10 mM Tris pH=7.4, 150 mM NaCl, 5 mM EDTA, 1.0% triton X-100, 0.4% SDS) and the 3H-2 DG and 14C-mannitol were measured using scintillation spectrometry. Mannitol is not normally taken up by cells, therefore the inclusion of 14C-mannitol in the uptake media is an extracellular marker and it allows us to account for any surface binding, to monitor potential toxic effects of the experimental treatments that would compromise the cell membrane, and to account for excess radioactivity that might remain after the washes.
2.5 Statistical analysis
Experimental conditions were repeated in triplicate or quadruplicate and glucose uptake was measured and reported as nmol/10 min/well ±standard error. Statistical significance was determined by either ANOVA followed by a post-hoc Dunnett test or a two-tailed t-test. Statistical significance is reported at P< 0.01 or P<0.05. Experiments were repeated several times and results from representative experiments are reported. Alternatively, several experiments were combined and normalized to glucose uptake under control conditions.
3. Results
3.1 Hydroxylamine activates glucose uptake in a dose dependent manner
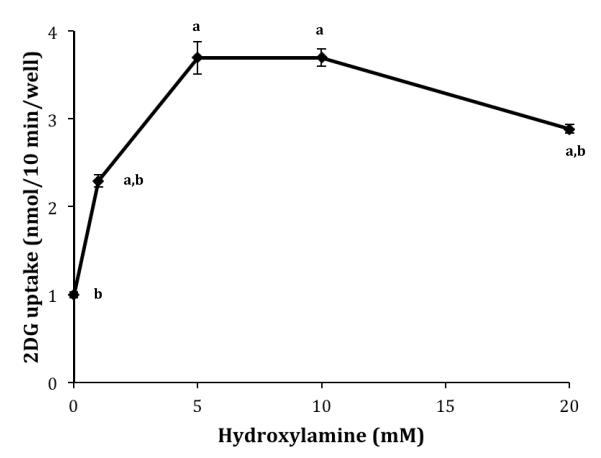
HNO, supplied via AS, activates glucose uptake in L929 cells within minutes. However, a cellular source of HNO has not been identified. It has been suggested that HA can be oxidized to produce HNO [7]. Therefore, we were interested in determining the effects of HA on glucose uptake. The dose dependent effects of HA on glucose uptake were measured by varying the concentration of HA in the uptake media from 0-20 mM, the same effective concentration range as HNO [18]. As seen in Figure 1, 1.0 mM HA significantly activated glucose uptake (2.3-fold) and both 5.0 and 10.0 mM HA were maximally stimulating (3.7-fold). At 20.0 mM HA glucose uptake was slightly, but significantly reduced from the maximum effect. This is virtually identical to the results observed for HNO [18]. HA did not produce any visible toxic effects at any of the concentrations tested, as assessed by cell morphology and cell attachment.
Figure 1.
Dose dependent effects of HA on 2DG uptake. Ten-minute 2DG uptakes were measured in the presence of HA concentrations ranging from 0-20 mM. Data are means ± S.E. of four wells from a representative experiment. aSignificantly different than 0 mM exposure to AS at P<0.01 and bsignificantly different than maximum effect at 5.0 mM HA at P<0.05.
3.2 Time course of HA-activation and recovery of glucose uptake
HA-activation occurs within the ten-minute time frame required for the measurement of glucose uptake. To explore this more carefully, glucose uptake was measure in the presence of 5.0 mM HA for 0, 1.5, 3, 5, 8, 10, 15, or 20 minutes and the subsequent uptakes were expressed per minute. The zero time exposure to HA was the uptake per minute for a 20-minute exposure to the uptake solution without HA present. Previous work has shown that 2DG uptake is linear for 30 minutes even under activating conditions (data not shown). As seen in Figure 2, the maximum rate of uptake (4.7-fold higher) occurs within the first 1.5 minutes of exposure to HA.
Figure 2.
Time course for AS-activation of 2DG uptake. 2DG uptakes in the presence of 5.0 mM AS were measured for 2, 3, 5, 8, 10, 15, and 20 minutes and expressed as the average nmol 2DG/minute ± S.E from four wells of a representative experiment. The uptake at zero minutes exposed to AS represents the per-minute uptake for a 20-minute uptake for cells not exposed to HA. aSignificantly different than zero time exposure to AS and bsignificantly different than maximum effect at 1.5 minute exposure to HA at P<0.01.
To measure the recovery from the effects of HA, L929 cells were exposed to HA for 10 minutes, followed by incubation in fresh DMEM media (5.5 mM glucose) without HA and uptakes were measured either immediately or after 5, 25 or 50 minutes. As shown in Figure 3, about 60% of the activation recovers within 5 minutes, with 40% of the activity maintained for 25 minutes and dropping to control levels after 50 minutes.
Figure 3.
Effect of recovery time on HA-activated 2DG uptake. L929 fibroblast cells were incubated at 37 °C for 10 minutes in DMEM media (5.5 mM glucose) supplemented with 5.0 mM HA. Ten-minute 2DG uptakes were then measured immediately or media were replaced with HA-free media and uptakes were measured after 5, 25, or 50 minutes. Data are expressed as a percentage of full activation (0 minutes recovery) ± S.E. from four wells of a representative experiment. aSignificantly different than basal uptake (0% activation) and bsignificantly different than 100% activation at P<0.01.
3.3 Activating effect of HA is blocked by iodoacetamide
Pretreating L929 cells with the thiol-active compound, iodoacetamide (IA) blocked the activating effects of AS (HNO) [18]. We were curious to determine if IA could also block the effects of HA. L929 cells were exposed to 0.75 mM IA for 20 minutes and glucose uptake was measured in the presence and absence of HA. As seen in Figure 4, IA treatment alone had no effect on glucose uptake, but it completely blocked the activating effects of HA, suggesting HA is exerting its effects via an interaction with cysteine residues.
Figure 4.
Effect of iodoacetamide on HA-activated 2DG uptake. L929 fibroblast cells were treated for 20 minutes at 37 °C in DMEM media (5.5 mM glucose) alone or supplemented with 0.75 mM iodoacetamide. Ten-minute 2DG uptakes were then measured in the presence and absence of 5 mM HA. (Con) represent control cells not treated with iodoacetamide or HA, (IA) represent cells exposed to iodoacetamide during the 20-minute treatment, (HA) represent cells exposed to hydroxylamine during the 10-minute uptake, (IA+HA) represent cells pre-treated with iodoacetamide and exposed to HA during the uptake phase. Data are means ± S.E. from four wells of a representative experiment. *Significantly elevated from control at P<0.01.
3.4 Cinnamaldehye and phenylarsine oxide also block the activating effects of HA
Both cinnamaldehyde (CA) and phenylarsine oxide (PAO) react with thiols and have been shown to be acute activators of glucose uptake in L929 cells [27, 28]. We would predict that if HA is exerting its effects via a reaction with a thiol, the prior treatment and activation by CA and PAO should block additional activation by HA. L929 cells were treated with either 2.0 mM CA or 50.0 μM PAO for 30 minutes and glucose uptakes were measured in the presence and absence of 5.0 mM HA. As seen in the Figure 5 and previously reported [27, 28], both CA and PAO acutely activated glucose uptake (2.3 and 2-7-fold respectively). However, HA was more effective and in this experiment we observed a robust 5-fold activation of glucose uptake. Prior treatment and activation of glucose uptake by CA and PAO blocked a full activation by HA.
Figure 5.
Effects of CA and PAO on HA-activated 2DG uptake. L929 fibroblast cells were incubated at 37 °C for 20 minutes in DMEM media (5.5 mM glucose) alone (Con) or supplemented with either 2.0 mM cinnamaldehyde (CA) or 50 μM phenylarsine oxide (PAO). Ten-minute 2DG uptakes were then measured in the presence and absence of 5.0 mM hydroxylamine (HA). Data are expressed as a percentage of full activation (0 minutes recovery) ± S.E. from three wells of a representative experiment. aSignificantly different than basal uptake control (Con) and bsignificantly different than HA-activation control (HA) at P<0.01.
3.5 Azide inhibits the activating effects of HA
It has been shown that hydroxylamine can be oxidized to HNO by peroxidases and other hemeproteins [32]. If the activation of glucose uptake by HA requires its conversion to HNO, peroxidase inhibitors should block HA’s effects. To test this, glucose uptake was measured in the presence of both HA and sodium azide, a potent peroxidase inhibitor. The results are shown in Figure 6. Sodium azide alone activated glucose uptake 1.5-fold, but it completely blocked the activating effects of HA. Somewhat surprisingly, sodium azide also blocked the activating effects of AS. The magnitude of the response to HA and AS were both somewhat lower in this experiment, but they were equal to each other in magnitude. None of the conditions utilized in this experiment appeared to affect cell viability as monitored by changes in cell morphology or cell attachment.
Figure 6.
Effects of Azide on HA- and AS-activated 2DG uptake. 2DG uptakes were measured without any additions (Con), or with the addition of 5.0 mM sodium azide (Az), 5.0 mM hydroxylamine (HA), 5.0 mM Angeli’s salt (AS), 5.0 mM HA plus 5.0 mM azide (HA+Az), or 5.0 mM AS plus 5.0 mM azide (HA+Az). Data from 12-20 samples were normalized to untreated cells (Con) and are expressed as a fraction of control ± S.E.. *Significantly different than basal uptake (Con) at P<0.01.
Discussion
The establishment of nitric oxide (NO) as a cellular signaling molecule has spurred research into the biological effects of other nitrogen oxide species. As a result, it has now been shown that nitroxyl (HNO), a one-electron reduced analog of NO, has a unique set of biological effects that distinguishes it from NO [1-7]. In particular, HNO has been shown to be efficacious in the treatment of alcoholism [12] and has been suggested as a possible therapeutic agent for the treatment of heart failure [36]. Much of the biological effects of HNO have been ascribed to its reaction with the thiol side chains of cysteine residues. The nucleophilic addition of a thiol to HNO produces N-hydroxysulfenamide, which can either rearrange to form a sulfinamide or react with a second vicinal cysteine residue to produce a disulfide and release hydroxylamine [2, 3].
Recently it has been shown that HNO, supplied by the decomposition of AS, acutely activates the glucose uptake activity of GLUT1 [18]. The activating effects of HNO were blocked by either the prior incubation of AS with thiols or a prior treatment of the cells with thiol reactive compounds, such as iodoacetamide or cinnamaldehyde. The data supported a mechanism of GLUT1 activation that was initially suggested by Curruthers based on his work on the GLUT1 protein from erythrocytes. Curruthers demonstrated that the formation of a disulfide bond between Cys 347 and Cys 421 within GLUT1 stabilizes a GLUT1 tetramer, which was a more active form of the transporter [29-31]. They also demonstrated that iodoacetamide can react with Cys 421. In addition, studies on phospholamban, as well as computatational studies, suggest that HNO can partition to hydrophobic regions where formation of a disulfide bond is the preferred product [3, 37, 38]. This strengthens the suggestion that HNO can react with the membrane bound GLUT1 to form a disulfide bond.
In order to establish HNO as a distinct cellular signaling system, research needs to identify an endogenous source of HNO and provide clarity on how specificity is achieved [2, 3]. HA has been suggested as possible small molecule precursor to HNO [7, 32, 33]. In particular, it has been demonstrated that HA can be oxidize by horseradish peroxidase and a number of other hemeproteins to produce HNO [32]. The data presented here indicate that HA activates glucose uptake in a manner that is virtually identical to the action of HNO, as administered via AS [18]. The most straightforward explanation of the data presented in this study is that HA is oxidized to HNO by a cellular enzyme, which then exerts its effects via HNO’s action on cysteine residues. This conclusion is based on five observations. First, both AS and HA have nearly identical maximum effects at 5.0 mM (Figure 1, and [18], and Figure 6). Second, both reagents have an initial burst of activation (1-2 minutes) followed by a reduction in glucose uptake to a steady state value (Figures 2 and [18]). Third, the activating effects of both reagents show a similar biphasic recovery with about 60% of the activity lost in the first 5-10 minutes (Figure 3 and [18]). Fourth, the actions of both compounds are blocked by prior treatment of the cells with iodoacetamide, which reacts with free thiols (Figure 4 and [18]) or by pretreatment with the thiol active compounds, CA and PAO, which themselves are activating (Figure 5 and [18]). And finally, sodium azide, a known inhibitor of peroxidases and hemeproteins, completely blocks both AS-induced and HA-induced activation of glucose uptake (see Figure 6). This is consistent with HA being converted to HNO by an enzyme present in L929 fibroblast cells. While these data are consistent with the oxidation of HA to HNO, this needs to be confirmed by the direct detection of HNO. However, these experiments are very difficult due to the reactivity of HNO and its tendency to dimerize, and then dehydrate to nitrous oxide. There are detection assays that that involve chemically trapping HNO, but these techniques have not been fully tested in whole cell experiments [39].
The small 1.5-fold stimulation of glucose uptake by exposure to azide during the 10-minute measurement of glucose uptake (Figure 6) is in line with previous studies that investigated the effects of azide as a cellular respiration inhibitor. Exposure of a variety of cells to azide for 20-30 minutes activates glucose uptake 3- to 5-fold [18-20]. Interestingly in this study, while azide alone is slightly activating, it completely inhibits the effects of HA suggesting that azide is also inhibiting an enzyme responsible for the oxidation of HA to HNO. We did not initially expect that azide would also inhibit the effects of AS since this compound releases HNO directly. In fact, data from a previous study had indicated that exposure of cells to azide activated glucose uptake and the subsequent exposure to AS further activated uptake [18]. However, in that study the exposure to azide and AS was sequential. The inhibitory effects of azide reported in this study occurs when the cells are exposed simultaneously to azide and AS. The inhibition of AS activation suggests that HNO may not reacting directly with GLUT1 (or a protein responsible for activating GLUT1), but rather it may be oxidizing any available vicinal thiols to release HA, which can then be reoxidized to HNO. The eventual modification of GLUT1 may be dependent on this HNO-HA redox cycling. This HNO-HA redox cycle has been suggested as a possible signaling mechanism [7]. The millimolar concentration of HA required to activate glucose uptake is significantly higher than the expected intracellular concentration of HA and higher than the micromolar concentrations required for other reported physiological effects of HA [40]. However, it is reasonable to expect that an exogenous source of HA or AS would not be able to target specific proteins, especially in a cellular environment that has high concentrations of proteins and thiols. Therefore, these results also do not preclude the possibility that cells could target specific proteins by a local or focused production of HNO from HA within the cell.
We are suggesting that the activating effects of HA are mediated by its oxidation to HNO, which then reacts with key cysteine residues, likely within GLUT1 itself, to generate a more active form of the transporter. However, there are a couple of other possibilities to consider. First, it is known that HNO alters calcium uptake and release in cardiac cells [8, 9] and that calcium can alter glucose uptake rates [41, 42]. However, it seems unlikely that calcium is involved in this HA-effect since previous work has shown that neither altering calcium concentrations in the media nor treatment with the calcium channel blocker, dantrolene, alters glucose uptake in L929 cells [43]. Second, there are plausible pathways for the conversion of HA to NO [7] and it has been previously shown that sodium nitroprusside, a NO donor, acutely activates glucose uptake [43]. This mechanism also seems unlikely since the NO effect required a much longer activation time (30-45 minutes compared to 1.5 minutes), and its effect was only about half the effect of HA. However, future experiments should confirm this by investigating the effects of HA in the presence of either a NO scavenger and/or an inhibitor of guanylyl cyclase.
5. Conclusions
HA acutely activates the transport activity of GLUT1 in L929 fibroblast cells in a manner that is nearly identical to the effects of HNO. The data suggest that HA can be converted to HNO, which activates glucose uptake by its reactions with cysteine residues. Results are consistent with a proposed mechanism for GLUT1 activation that involves the formation of a disulfide bond within GLUT1, or reaction with a yet not known protein that regulates GLUT1 activity.
Research Highlights.
Hydroxylamine activates glucose uptake in L929 fibroblast cells within minutes
Actions of hydroxylamine are virtually identical to actions of nitroxyl
Activation by hydroxylamine is block by thiol reactive compounds
Azide, a peroxidase inhibitor, blocks activating effects of hydroxylamine
Acknowledgements
This research was supported by a NIH R15 grant (DK08193-1A1). Special thanks to John P. Toscano for the gift of the AS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Kemp-Harper BK. Nitroxyl (HNO): a novel redox signaling molecule. Antioxid Redox Signal. 2011;14:1609–1613. doi: 10.1089/ars.2011.3937. [DOI] [PubMed] [Google Scholar]
- [2].Fukuto JM, Bianco CL, Chavez TA. Nitroxyl (HNO) signaling. Free Radic Biol Med. 2009;47:1318–1324. doi: 10.1016/j.freeradbiomed.2009.06.014. [DOI] [PubMed] [Google Scholar]
- [3].Fukuto JM, Carrington SJ. HNO signaling mechanisms. Antioxid Redox Signal. 2011;14:1649–1657. doi: 10.1089/ars.2010.3855. [DOI] [PubMed] [Google Scholar]
- [4].Irvine JC, Ritchie RH, Favaloro JL, Andrews KL, Widdop RE, Kemp-Harper BK. Nitroxyl (HNO): the Cinderella of the nitric oxide story. Trends Pharmacol Sci. 2008;29:601–608. doi: 10.1016/j.tips.2008.08.005. [DOI] [PubMed] [Google Scholar]
- [5].Switzer CH, Flores-Santana W, Mancardi D, Donzelli S, Basudhar D, Ridnour LA, Miranda KM, Fukuto JM, Paolocci N, Wink DA. The emergence of nitroxyl (HNO) as a pharmacological agent. Biochim Biophys Acta. 2009;1787:835–840. doi: 10.1016/j.bbabio.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Paolocci N, Jackson MI, Lopez BE, Miranda K, Tocchetti CG, Wink DA, Hobbs AJ, Fukuto JM. The pharmacology of nitroxyl (HNO) and its therapeutic potential: not just the Janus face of NO. Pharmacol Ther. 2007;113:442–458. doi: 10.1016/j.pharmthera.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Reisz JA, Bechtold E, King SB. Oxidative heme protein-mediated nitroxyl (HNO) generation. Dalton Trans. 2010;39:5203–5212. doi: 10.1039/c000980f. [DOI] [PubMed] [Google Scholar]
- [8].Cheong E, Tumbev V, Abramson J, Salama G, Stoyanovsky DA. Nitroxyl triggers Ca2+ release from skeletal and cardiac sarcoplasmic reticulum by oxidizing ryanodine receptors. Cell Calcium. 2005;37:87–96. doi: 10.1016/j.ceca.2004.07.001. [DOI] [PubMed] [Google Scholar]
- [9].Tocchetti CG, Wang W, Froehlich JP, Huke S, Aon MA, Wilson GM, Di Benedetto G, O’Rourke B, Gao WD, Wink DA, Toscano JP, Zaccolo M, Bers DM, Valdivia HH, Cheng H, Kass DA, Paolocci N. Nitroxyl improves cellular heart function by directly enhancing cardiac sarcoplasmic reticulum Ca2+ cycling. Circ Res. 2007;100:96–104. doi: 10.1161/01.RES.0000253904.53601.c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Froehlich JP, Mahaney JE, Keceli G, Pavlos CM, Goldstein R, Redwood AJ, Sumbilla C, Lee DI, Tocchetti CG, Kass DA, Paolocci N, Toscano JP. Phospholamban thiols play a central role in activation of the cardiac muscle sarcoplasmic reticulum calcium pump by nitroxyl. Biochemistry. 2008;47:13150–13152. doi: 10.1021/bi801925p. [DOI] [PubMed] [Google Scholar]
- [11].Paolocci N, Saavedra WF, Miranda KM, Martignani C, Isoda T, Hare JM, Espey MG, Fukuto JM, Feelisch M, Wink DA, Kass DA. Nitroxyl anion exerts redox-sensitive positive cardiac inotropy in vivo by calcitonin gene-related peptide signaling. Proc Natl Acad Sci U S A. 2001;98:10463–10468. doi: 10.1073/pnas.181191198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].DeMaster EG, Redfern B, Nagasawa HT. Mechanisms of inhibition of aldehyde dehydrogenase by nitroxyl, the active metabolite of the alcohol deterrent agent cyanamide. Biochem Pharmacol. 1998;55:2007–2015. doi: 10.1016/s0006-2952(98)00080-x. [DOI] [PubMed] [Google Scholar]
- [13].Shoeman DW, Shirota FN, DeMaster EG, Nagasawa HT. Reaction of nitroxyl, an aldehyde dehydrogenase inhibitor, with N-acetyl-L-cysteine. Alcohol. 2000;20:55–59. doi: 10.1016/s0741-8329(99)00056-7. [DOI] [PubMed] [Google Scholar]
- [14].Lopez BE, Rodriguez CE, Pribadi M, Cook NM, Shinyashiki M, Fukuto JM. Inhibition of yeast glycolysis by nitroxyl (HNO): mechanism of HNO toxicity and implications to HNO biology. Arch Biochem Biophys. 2005;442:140–148. doi: 10.1016/j.abb.2005.07.012. [DOI] [PubMed] [Google Scholar]
- [15].Lopez BE, Wink DA, Fukuto JM. The inhibition of glyceraldehyde-3-phosphate dehydrogenase by nitroxyl (HNO) Arch Biochem Biophys. 2007;465:430–436. doi: 10.1016/j.abb.2007.06.017. [DOI] [PubMed] [Google Scholar]
- [16].Vaananen AJ, Salmenpera P, Hukkanen M, Miranda KM, Harjula A, Rauhala P, Kankuri E. Persistent susceptibility of cathepsin B to irreversible inhibition by nitroxyl (HNO) in the presence of endogenous nitric oxide. Free Radic Biol Med. 2008;45:749–755. doi: 10.1016/j.freeradbiomed.2008.05.025. [DOI] [PubMed] [Google Scholar]
- [17].Vaananen AJ, Kankuri E, Rauhala P. Nitric oxide-related species-induced protein oxidation: reversible, irreversible, and protective effects on enzyme function of papain. Free Radic Biol Med. 2005;38:1102–1111. doi: 10.1016/j.freeradbiomed.2005.01.007. [DOI] [PubMed] [Google Scholar]
- [18].Salie MJ, Oram DS, Kuipers DP, Scripture JP, Chenge J, MacDonald GJ, Louters LL. Nitroxyl (HNO) acutely activates the glucose uptake activity of GLUT1. Biochimie. 2012;94:864–869. doi: 10.1016/j.biochi.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Shetty M, Loeb JN, Vikstrom K, Ismail-Beigi F. Rapid activation of GLUT-1 glucose transporter following inhibition of oxidative phosphorylation in clone 9 cells. J Biol Chem. 1993;268:17225–17232. [PubMed] [Google Scholar]
- [20].Rubin D, Ismail-Beigi F. Distribution of Glut1 in detergent-resistant membranes (DRMs) and non-DRM domains: effect of treatment with azide. Am J Physiol Cell Physiol. 2003;285:C377–383. doi: 10.1152/ajpcell.00060.2003. [DOI] [PubMed] [Google Scholar]
- [21].Louters LL, Dyste SG, Frieswyk D, Tenharmsel A, Vander Kooy TO, Walters L, Whalen T. Methylene blue stimulates 2-deoxyglucose uptake in L929 fibroblast cells. Life Sci. 2006;78:586–591. doi: 10.1016/j.lfs.2005.05.082. [DOI] [PubMed] [Google Scholar]
- [22].Barnes K, Ingram JC, Porras OH, Barros LF, Hudson ER, Fryer LG, Foufelle F, Carling D, Hardie DG, Baldwin SA. Activation of GLUT1 by metabolic and osmotic stress: potential involvement of AMP-activated protein kinase (AMPK) J Cell Sci. 2002;115:2433–2442. doi: 10.1242/jcs.115.11.2433. [DOI] [PubMed] [Google Scholar]
- [23].Barros LF, Barnes K, Ingram JC, Castro J, Porras OH, Baldwin SA. Hyperosmotic shock induces both activation and translocation of glucose transporters in mammalian cells. Pflugers Arch. 2001;442:614–621. doi: 10.1007/s004240100577. [DOI] [PubMed] [Google Scholar]
- [24].Meyer JA, Froelich JM, Reid GE, Karunarathne WK, Spence DM. Metal-activated C-peptide facilitates glucose clearance and the release of a nitric oxide stimulus via the GLUT1 transporter. Diabetologia. 2008;51:175–182. doi: 10.1007/s00125-007-0853-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kumar A, Xiao YP, Laipis PJ, Fletcher BS, Frost SC. Glucose deprivation enhances targeting of GLUT1 to lipid rafts in 3T3-L1 adipocytes. Am J Physiol Endocrinol Metab. 2004;286:E568–576. doi: 10.1152/ajpendo.00372.2003. [DOI] [PubMed] [Google Scholar]
- [26].Roelofs B, Tidball A, Lindborg AE, TenHarmsel A, Vander Kooy TO, Louters LL. Acute activation of glucose uptake by glucose deprivation in L929 fibroblast cells. Biochimie. 2006;88:1941–1946. doi: 10.1016/j.biochi.2006.08.004. [DOI] [PubMed] [Google Scholar]
- [27].Scott J, Opejin A, Tidball A, Stehouwer N, Rekman J, Louters LL. Dual action of phenylarsine oxide on the glucose transport activity of GLUT1. Chemico-biological interactions. 2009;182:199–203. doi: 10.1016/j.cbi.2009.08.008. [DOI] [PubMed] [Google Scholar]
- [28].Plaisier C, Cok A, Scott J, Opejin A, Bushhouse KT, Salie MJ, Louters LL. Effects of cinnamaldehyde on the glucose transport activity of GLUT1. Biochimie. 2011;93:339–344. doi: 10.1016/j.biochi.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Hebert DN, Carruthers A. Glucose transporter oligomeric structure determines transporter function. Reversible redox-dependent interconversions of tetrameric and dimeric GLUT1. J Biol Chem. 1992;267:23829–23838. [PubMed] [Google Scholar]
- [30].Zottola RJ, Cloherty EK, Coderre PE, Hansen A, Hebert DN, Carruthers A. Glucose transporter function is controlled by transporter oligomeric structure. A single, intramolecular disulfide promotes GLUT1 tetramerization. Biochemistry. 1995;34:9734–9747. doi: 10.1021/bi00030a011. [DOI] [PubMed] [Google Scholar]
- [31].Pessino A, Hebert DN, Woon CW, Harrison SA, Clancy BM, Buxton JM, Carruthers A, Czech MP. Evidence that functional erythrocyte-type glucose transporters are oligomers. J Biol Chem. 1991;266:20213–20217. [PubMed] [Google Scholar]
- [32].Donzelli S, Espey MG, Flores-Santana W, Switzer CH, Yeh GC, Huang J, Stuehr DJ, King SB, Miranda KM, Wink DA. Generation of nitroxyl by heme protein-mediated peroxidation of hydroxylamine but not N-hydroxy-L-arginine. Free Radic Biol Med. 2008;45:578–584. doi: 10.1016/j.freeradbiomed.2008.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Donzelli S, Espey MG, Thomas DD, Mancardi D, Tocchetti CG, Ridnour LA, Paolocci N, King SB, Miranda KM, Lazzarino G, Fukuto JM, Wink DA. Discriminating formation of HNO from other reactive nitrogen oxide species. Free Radic Biol Med. 2006;40:1056–1066. doi: 10.1016/j.freeradbiomed.2005.10.058. [DOI] [PubMed] [Google Scholar]
- [34].Liong E, Kong SK, Au KK, Li JY, Xu GY, Lee YL, Kwok TT, Choy YM, Lee CY, Fung KP. Inhibition of glucose uptake and suppression of glucose transporter 1 mRNA expression in L929 cells by tumour necrosis factor-alpha. Life Sci. 1999;65:PL215–220. doi: 10.1016/s0024-3205(99)00408-7. [DOI] [PubMed] [Google Scholar]
- [35].Van Dyke DA, Walters L, Frieswyk D, Kokmeyer D, Louters LL. Acute effects of troglitazone and nitric oxide on glucose uptake in L929 fibroblast cells. Life Sci. 2003;72:2321–2327. doi: 10.1016/s0024-3205(03)00119-x. [DOI] [PubMed] [Google Scholar]
- [36].Feelisch M. Nitroxyl gets to the heart of the matter. Proc Natl Acad Sci U S A. 2003;100:4978–4980. doi: 10.1073/pnas.1031571100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Karim CB, Stamm JD, Karim J, Jones LR, Thomas DD. Cysteine reactivity and oligomeric structures of phospholamban and its mutants. Biochemistry. 1998;37:12074–12081. doi: 10.1021/bi980642n. [DOI] [PubMed] [Google Scholar]
- [38].Sherman MP, Grither WR, McCulla RD. Computational investigation of the reaction mechanisms of nitroxyl and thiols. J Org Chem. 2010;75:4014–4024. doi: 10.1021/jo100172t. [DOI] [PubMed] [Google Scholar]
- [39].Reisz JA, Zink CN, King SB. Rapid and selective nitroxyl (HNO) trapping by phosphines: kinetics and new aqueous ligations for HNO detection and quantitation. J Am Chem Soc. 2011;133:11675–11685. doi: 10.1021/ja203652z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Huang Y. Hydroxylamine-induced relaxation inhibited by K+ channel blockers in rat aortic rings. Eur J Pharmacol. 1998;349:53–60. doi: 10.1016/s0014-2999(98)00178-2. [DOI] [PubMed] [Google Scholar]
- [41].Rockl KS, Witczak CA, Goodyear LJ. Signaling mechanisms in skeletal muscle: acute responses and chronic adaptations to exercise. IUBMB Life. 2008;60:145–153. doi: 10.1002/iub.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Klip A, Schertzer JD, Bilan PJ, Thong F, Antonescu C. Regulation of glucose transporter 4 traffic by energy deprivation from mitochondrial compromise. Acta Physiol (Oxf) 2009;196:27–35. doi: 10.1111/j.1748-1716.2009.01974.x. [DOI] [PubMed] [Google Scholar]
- [43].Van Dyke DA, Walters L, Frieswyk D, Kokmeyer D, Louters LL. Acute effects of troglitazone and nitric oxide on glucose uptake in L929 fibroblast cells. Life Sci. 2003;72:2321–2327. doi: 10.1016/s0024-3205(03)00119-x. [DOI] [PubMed] [Google Scholar]