Abstract
Background
Abnormal gastrointestinal permeability has been linked to irritable bowel syndrome (IBS). The lactulose-to-mannitol ratio is traditionally used to assess small intestine permeability while sucralose and sucrose are used to assess colonic and gastric permeability respectively. We used a single 4-probe test solution to assess permeability throughout the gastrointestinal tract in IBS patients and healthy controls by measuring the recovery of the probes in urine after ingestion using a modified liquid chromatography mass spectrometry protocol.
Methods
Fasting participants (N = 59) drank a permeability test solution (100 ml: sucralose, sucrose, mannitol, and lactulose). Urine was collected over a 5-h period and kept frozen until analysis. Urinary sugar concentrations were measured using an liquid chromatography/triple quadruple mass spectrometer.
Results
Colonic permeability was significantly lower in IBS patients when compared to healthy controls (p = 0.011). Gastric and small intestinal permeability did not significantly differ between the groups.
Conclusions
The study demonstrates the clinical potential of this non-invasive method for assessing alterations in gastrointestinal permeability in patients with IBS.
Keywords: intestinal permeability, lactulose-to-mannitol ratio, sucralose, sucrose, mass spectrometry, multiple reaction monitoring
1. Introduction
Approximately 10–15% of adults in the U.S. suffer from irritable bowel syndrome (IBS), which yields an excess of eight billion dollars in medical costs annually (1–4). Various gastrointestinal disorders, including IBS, are associated with changes in gastrointestinal permeability (GIP) (5–9). It is thought that weakening of intercellular tight junctions results in a luminal antigen insult, which causes mucosal immune and inflammatory responses (5–9). Although more than 40 different conditions have been associated with increased GIP, the clinical implications of increased GIP remain poorly understood (10,11) and the diagnosis of gastrointestinal dysfunctions remains difficult (12).
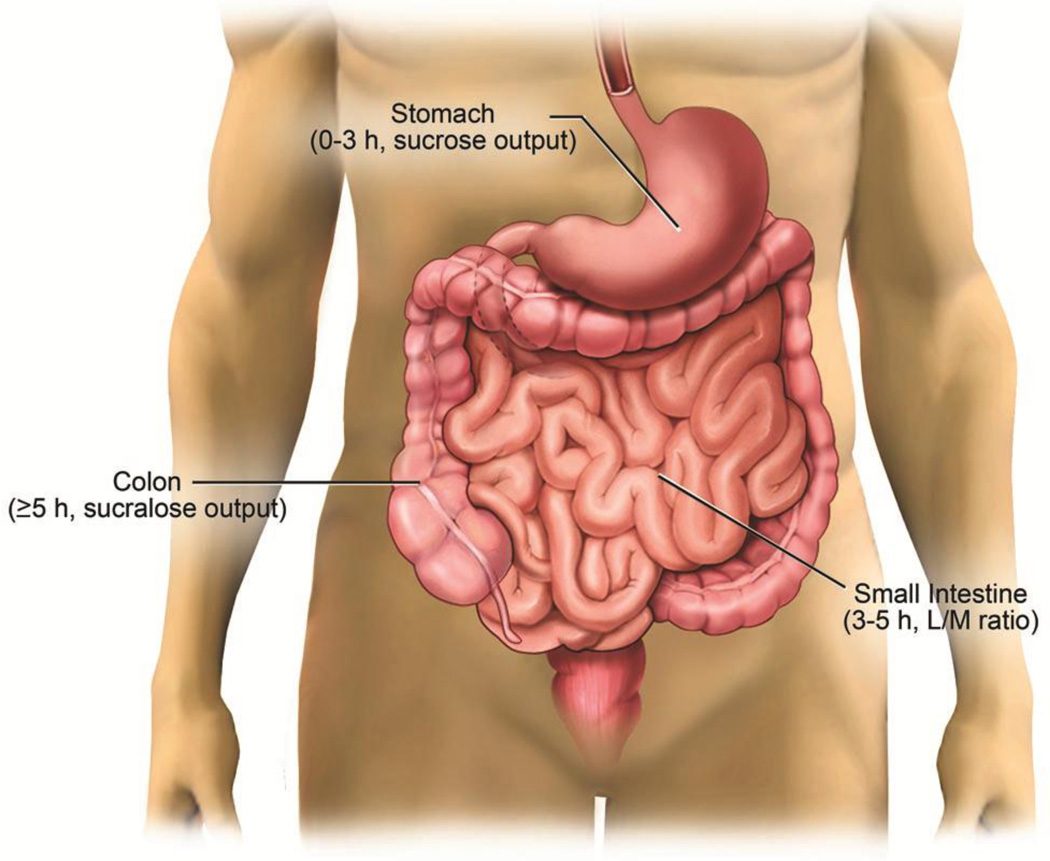
Numerous and highly sensitive methods for measuring GIP are available (e.g., Ussing chamber technique), however these techniques are often highly invasive and unsuitable for routine diagnostic purposes in a clinical setting. For that reason, GIP is commonly assessed by quantifying the excretion of orally delivered probe molecules (6,10,11,13) in urine. Different probes may be used to assess permeability at specific locations throughout the gastrointestinal (GI) tract. Typically solutions containing only 1 to 3 probes are used to assess one part of the gastrointestinal tract (14–16). For instance; sucrose is degraded in the first three h after ingesting the sugar solution shortly after leaving the stomach, and can be used to assess gastric permeability. The reliability of this test relies on the notion that small intestine hydrolysis of sucrose is so fast and absolute that up take of sucrose can be deemed as a specific characteristic of gastric permeability. Small intestine permeability testing depends on the differential excretion between a small probe (e.g., mannitol, L-rhamnose) and a large probe (e.g., lactulose). Lactulose tomannitol (L/M) ratio is widely used to assess small intestine permeability between 3 and 5 h after consumption of test solution. In order to study colonic permeability, urine needs to be collected for at least 5 h and probes that do not undergo fermentation by colonic bacteria, such as artificial disaccharide sucralose or chromium-labeled ethylenediaminetetraacetic acid (51Cr-EDTA), are necessary (Fig. 1) (8,9,13,17,18).
Figure 1.
Standard sugar test solution is used to determine permeability throughout the GI tract. Gastric absorption of sucrose occurs between 0–3 h after solution ingestion. Lactulose is absorbed in the small intestine between 3–5 h and mannitol is used to standardize surface area. Colonic permeability is typically assessed by sucralose excretion ≥ 5 h.
Relatively few studies have used a four probe permeability solution to examine permeability at different points along the GI tract specifically in the diagnosis of IBS (19). The use of a single four probe permeability solution in charactering IBS and identifying specific GI regions of interest is not yet employed as a routine procedure but may prove to be a useful diagnostic approach. The current study describes a protocol for examining GI permeability in patients with IBS and a control group using a single 4-probe solution and a modified liquid chromatography mass-spectrometry assay to measure recovery of the probes in urine. The effective non-invasive assessment of GIP is an important diagnostic tool indigestive diseases, including IBS, and monitoring outcomes in response to treatment (10,11). It is therefore important to be able to more comprehensively characterize GIP along the whole GI tract in order to make more informed diagnoses and better identify appropriately targeted interventions.
1. Materials and Methods
2.1. Design and setting
The study population was composed of patients who fulfilled Rome III criteria (1) for IBS for >6 months and matched healthy controls. Participants with a history of organic GI disease (e.g., inflammatory bowel disease, celiac disease) or currently taking medications daily for GI symptoms (e.g., antispasmodics, laxatives) were excluded. Participants were recruited under a natural history protocol conducted at the National Institutes of Health (NIH) Hatfield Clinical Research Center (CRC).
2.2. Participants
The cohort of 59 participants (47.46% male, 59.32% Caucasian, 26.86 ± 6.98 y, 24.66 ± 4.88 mg/m2) included IBS patients (n = 20) and matched healthy controls (n = 39) (Table 1).
Table 1.
Sample characteristics for the whole cohort as well as IBS and control groups. IBS and control groups did not differ significantly in acute phase markers.
| Variable | Overall (N = 59) |
IBS (n = 20) |
Controls (n = 39) |
|---|---|---|---|
| Gender- n (%) | |||
| Male | 28 (47.46) | 8 (40.00) | 20 (51.28) |
| Female | 31 (52.54) | 12 (60.00) | 19 (48.72) |
| Race- n (%) | |||
| Asian | 9 (15.25) | 2 (10.00) | 7 (17.95) |
| African American | 11 (18.64) | 4 (20.00) | 7 (17.95) |
| Caucasian | 35 (59.32) | 13 (65.00) | 22 (56.41) |
| Other/Mixed | 4 (6.78) | 1 (5.00) | 3 (7.69) |
| Age (mean ± SD) | 26.86 ± 6.98 | 25.65 ± 6.88 | 27.49 ± 7.04 |
| Range (Yr) | (14.00 – 45.00) | (14.00 – 44.00) | (15.00 – 45.00) |
| BMI (mean ± SD) | 24.65 ± 4.80 | 24.45 ± 3.81 | 24.76 ± 5.28 |
| Range (kg/m2) | (18.65 – 43.22) | (20.19 – 35.07) | (18.65 – 43.22) |
| Alb (mean ± SD) | 4.09 ± 0.37 | 4.09 ± 0.46 | 4.10 ± 0.31 |
| Range (g/dl) | (3.40 – 4.90) | (3.40 – 4.90) | (3.50 – 4.90) |
| CRP (mean ± SD) | 1.99 ± 3.04 | 2.54 ± 3.90 | 1.71 ± 2.51 |
| Range (mg/l) | (0.04 – 17.50) | (0.04 – 17.50) | (0.04 –13.60) |
IBS = Irritable Bowel Syndrome, BMI = body mass index, Alb = albumin, CRP = C-reactive protein.
2.3. Gastrointestinal permeability solution
After an overnight fast, participants drank the permeability test solution (100 ml solution containing sucrose [10 g/dl], lactulose [5 g/dl], mannitol [1 g/dl], and sucralose [0.1g/dl]). The total osmolarity of the solution is 501 mM. Subjects remained fasting and drank water ad libitum to insure adequate hydration and urine flow. All urine excreted was collected during the 5-hour interval into a plastic container on ice with no additives. Samples were vortexed and aliquoted into 3 ml tubes and then kept frozen at −80°C until analyzed.
2.4. Mass spectrometry
Urine sugar concentrations were measured using an Agilent 1200 HPLC system consisting of a pump, degasser, auto sampler, and column heater coupled to an Agilent 6460 Triple quadruple mass spectrometer equipped with an electrospray ionization (ESI) source using Agilent Jet Stream Technology (Agilent Technologies, Santa Clara, CA) tuned for unit mass resolution. Fragment or voltage and collision energy for each sugar was selected by Agilent Mass Hunter Optimizer (Agilent). A 6-point calibration curve in mass spectrometry grade water (Millipore Synergy filtered, Billerica, MA) was prepared for sucrose and sucralose (100, 50, 10, 5, 1, and 0 µg/ml) as well as lactulose (400, 200, 40, 20, 4, and 0 µg/ml) and a 5 point calibration curve for mannitol (200, 40, 20, 4, and 0 µg/ml). Samples were prepared by adding 100 µl of methanol containing raffinose (40 µg/ml) (Sigma-Aldrich, St Louis, MO) as internal standard to 100 µl of urine or calibrator. Samples were then vortex mixed for 30 sec before centrifugation for 10 min at 13,000 RPM. For the measurement of sucrose, sucralose, and lactulose, 100 µl of supernatant was diluted with 900 µl water/methanol (50% v/v). For the measurement of mannitol, 10 µl of supernatant was diluted with 990 µl of water methanol (50% v/v). Samples (20 µl) were injected onto a ZORBAX Carbohydrate Analysis Column, 4.6 mm ID × 150 mm (5 µm) (Agilent). Chromatographic separation was achieved by isocratic elution of sugars with mobile phase A (water) set at 25% and mobile phase B (methanol spiked) set at 75%, flow rate was 1.0 ml/min and the total analysis time was 10 min. The column temperature was set at 30°C. Samples were introduced into the mass spectrometer operated in negative ionization multiple reaction monitoring (MRM) mode.
The transitions of the precursor to product ion were monitored with a dwell time of 200 ms for each substance. The MRM transitions monitored, acquisition time, and standard curve regression coefficients for each sugar probe are reported in Table 2. Sugar peaks were identified and measured using Agilent Mass Hunter Workstation software. All mass spectrometry analyses were performed by a blinded operator. Limit of quantitation was defined as a signal-to-noise ratio of >20:1. In this study all of the analysis exceeded this signal to noise ratio. Sucrose and sucralose were linear up to 100 µg/ml, lactulose was linear up to 400 µg/ml and mannitol was linear up to 200 µg/ml. Linearity was defined by a correlation coefficient of >0.99. All samples that exceeded the linear limit were analyzed in dilution. Matrix effects were not formally evaluated in this study; this is a limitation that should be addressed in further follow up studies. However, in order to minimize potential matrix effects, an effective minimum dilution of 1 to 20 was used for all samples analyzed.
Table 2.
Liquid Chromatography Tandem Mass Spectrometry, MRM transitions for each sugar probe, acquisition time and calibration curve regression coefficients.
| Probe | MRM Transitions | Acquisition Time (min) |
Points used (N) |
R2 | |
|---|---|---|---|---|---|
|
Precursor Ion (Q1) |
Product Ion (Q3) |
||||
| Sucralose | 395.0 | 359.0 | 1.99 | 6 | 0.999 |
| Sucrose | 341.1 341.1 |
179.0 119.0 |
2.29 | 6 | 0.995 |
| Mannitol | 181.1 181.1 |
119.0 101.1 |
2.41 | 5 | 0.998 |
| Lactulose | 341.1 | 161.1 | 2.64 | 6 | 0.998 |
MRM = multiple reaction monitoring. Raffinose was used as internal standard in each run.
2.5. Data and statistical analysis
Raw mass spectrometry (MS) data were converted to: Sucrose or sucralose output (%) = [(urine concentration from MS × total urine volume excreted) / probe input] × 100. L/M Ratio: Fractional excretion (FE) of lactulose and mannitol = (urine concentration from MS × total urine volume excreted) / probe input. L/M Ratio = FE lactulose / FE mannitol. Independent sample t-test and unpaired t-tests were used with SPSS 15.0 (Chicago, IL) and GraphPad Prism 5 (La Jolla, CA). Statistical significance was set a priori top ≤ 0.05.
2. Results
Table 1 gives a detailed summary of the demographic composition of the sample as well as the ranges of biometric and clinical markers. IBS patients (n = 20) and healthy controls (n = 39) did not differ significantly in the biometric measures age (p = NS) and body mass index (BMI, p = NS), or in the acute phase clinical indictors albumin (p = NS) and C-reactive protein (CRP, p = NS).
3.1. Gastrointestinal permeability
We found that this particular adaptation of the classic (13,18,20–22) gastrointestinal permeability methods, using an Agilent 1200 HPLC with a ZORBAX Carbohydrate Analysis Column separated sugar probes chromatographically. The coupling to an LC-MS/MS operated in the negative ionization MRM mode along with the use of raffinose as internal control allowed for accurate quantification (Table 2).
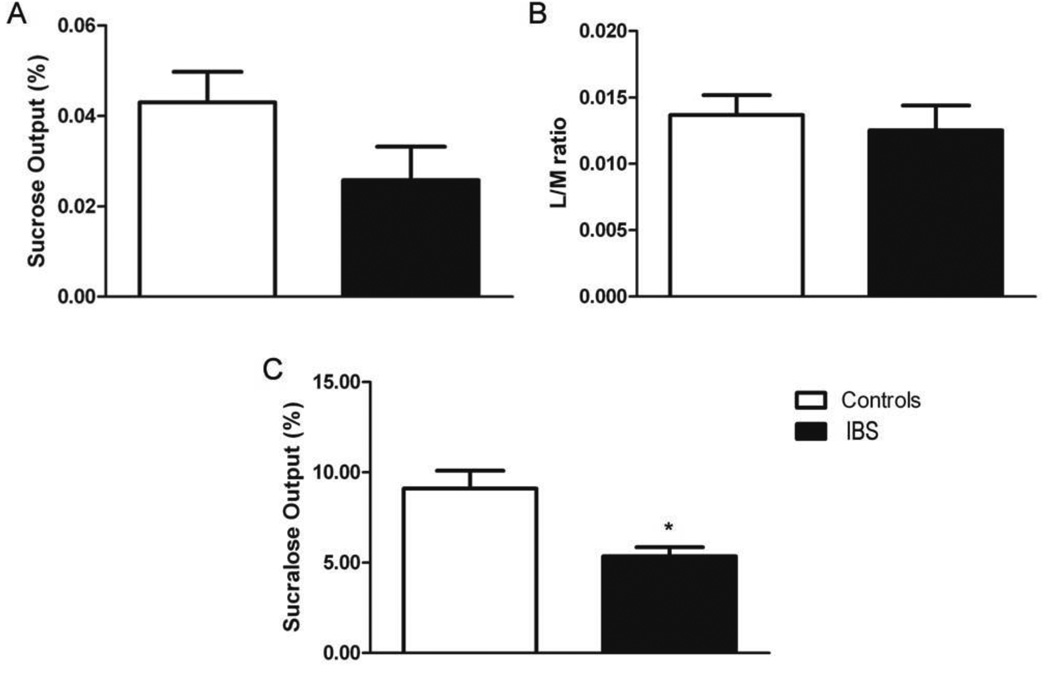
The mean (± 1 SD) of sucrose recovery, lactulose to mannitol ratio (L/M) and sucralose recovery for the whole cohort, IBS group and control group as well as p value for difference between the control and IBS group is indicated in Table 3. Figures 2A to C illustrate differences in sucrose (2A) and sucralose (2C) recovery and the L/M ratio (2B) between IBS and control groups, characterizing differences in intestinal permeability at different points in the gastrointestinal tract.
Table 3.
Relative probe recovery results characterizing gastrointestinal permeability (GIP) of IBS patients vs. healthy controls at various points along the GI tract. IBS patients exhibited significantly elevated colonic permeability compared to healthy controls.
| Location | GIP probe | Overall (N = 59) |
IBS (n = 20) |
Controls (n = 39) |
P value |
|---|---|---|---|---|---|
| Stomach | Sucrose (%) | 0.037 ± 0.040 | 0.026 ± 0.033 | 0.043 ± 0.042 | NS |
| Small Intestine | L/M ratio | 0.013 ± 0.009 | 0.012 ± 0.008 | 0.014 ± 0.009 | NS |
| Colon | Sucralose (%) | 7.837 ± 5.427 | 5.357 ± 2.213 | 9.109 ± 6.132 | 0.011* |
Data expressed as mean ± one standard deviation. L/M = lactulose-to-mannitol ratio.
p < 0.05
Figure 2.
Gastrointestinal permeability of IBS patients vs. healthy controls. (A) Percentage sucrose output (gastric); (B) L/M ratio (small intestine) and (C) Percentage sucralose output (colon). IBS patients exhibited elevated colonic permeability as evidenced by a significant decrease in the recovery of sucralose compared to healthy controls.* p = 0.011.
The percentage sucrose output indicated that gastric permeability was not significantly different (p = 0.118) between the IBS group (0.026±0.040%) and healthy controls (0.043±0.042%) (Figs. 2A and Table 3). The ratio of lactulose-to-mannitol excretion commonly used to determine small intestine permeability also showed no significant difference (p = 0.45) between the IBS group (0.012±0.008, and healthy controls (0.014±0.009) (Figs. 2B and Table 3). In contrast colonic permeability, as assessed by the percent of sucralose output, was significantly decreased in the IBS group (5.357±2.213%) compared to healthy controls (9.109±6.132, p = 0.011) (Figure 2C and Table 3).
3. Discussion
We found that colonic permeability was significantly decreased in IBS patients compared to healthy controls. Some studies have found that colonic permeability remains unaltered even in very extreme conditions, such as ulcerative colitis (22) and anti-cancer therapy (23), while other studies have reported increased colonic permeability in both IBS and IBD patients (7,8,17,23,24). Few studies have demonstrated a decrease in permeability at the colonic level similar to that which we report here. These reports of decreased colonic permeability however appear to be due to hormonal (e.g., estradiol) or diet effects (25,26) or are associated with diseases such as HIV (27). Our result is therefore unexpected as participants were screened for organic disease; this suggests that the observed decrease in colonic permeability is a potential marker of IBS in our study population. Approximately half of the patients in our cohort suffer from diarrhea (55%). It is conceivable that the accelerated transit throughout the GI tract directly affects the absorption of sucralose or that a net increase in colonic fluid as a result of secretion of fluids by crypt cells, due to diarrhea, dilutes sucralose in the lumen. Gastric permeability was not altered in the IBS patients when compared to controls. This was expected because organic diseases (e.g., ulcers, inflammatory bowel disease), which are the main causes for altered gastric permeability (Marshal), were exclusionary. Interestingly, lactulose-to-mannitol (L/M) ratio was not significantly different between IBS patients and healthy controls. Although, several studies reported elevated L/M ratio in IBS patients (e.g., diarrhea predominant IBS) (14,29), other studies found that, like in our study, small intestine permeability was not altered in IBS patients (9,30).
Our results demonstrate the effectiveness of using a 4-probe GI permeability solution in identifying differences in GI permeability among IBS patients and healthy controls. This further demonstrates the potential of this approach in the diagnosis of IBS and in identifying relevant GI regions where normal mucosal permeability may be dysregulated and could be specifically targeted for treatment. Urine was not tested at defined intervals during the 5 hour collection period but recovery of all 4 probes were simultaneously quantified in a single sample representing all urine accumulated over the 5-h period using a modified HPLC-MS/MS assay. Although this approach sacrifices some resolution in charactering permeability along the GI tract this strategy appears robust in recovering differences in GI permeability in our patient population and may represent cost effective and expedient first-step diagnostic procedure.
This study is not without limitations. The current mass-spectrometry method includes only one internal standard, raffinose; ideally the method should be further optimized with an appropriate deuterated internal standard for each probe. Moreover, determination of sugar concentrations in serum could also be used to corroborate urine findings (30–32). Serum sugar quantification also offers an acceptable alternative to urine that can be used in the pediatric setting because it reduces the collection time from 5 h to 90 min (30). Despite these limitations, this protocol may represent a clinically meaningful and non-invasive method for assessment of gastrointestinal permeability alterations along the whole gastrointestinal tract in patients with IBS.
Highlights.
Recovery of sugar probes accurately measured using a HLPC-mass spectrometry assay
Raffinose was used as an internal standard
IBS patients exhibited decreased colonic permeability compared to healthy controls
Acknowledgments
The authors thank the study participants, NIH Clinical Center and NIDDK Metabolic Program of Care (Dr. Kong Chen). We also thank Dr. Robert Shulman for the formulation of the test solution and Dr. Margaret Heitkemper for her mentorship. This study was funded by NINR’s Intramural Research Training Awards (Del Valle-Pinero, Fourie, and Patel) and 1ZIANR000018-03 award (PI: Henderson).
Abbreviations
- Alb
albumin
- 51Cr-EDTA
chromium-labeled ethylenediaminetetraacetic acid
- FE
fractional excretion
- GIP
gastrointestinal permeability
- IBD
inflammatory bowel disease
- IBS
irritable bowel syndrome
- L/M
ratio lactulose-to-mannitol ratio
- TJ
tight junction
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Drossman DA, Corazziari E, Delvaux M, Spiller RC. Rome III: The functional gastrointestinal disorders. McLean, VA: Degnon Associates, Inc.; 2006. [Google Scholar]
- 2.Drossman DA, Dumitrascu DL. Rome III: New standard for functional gastrointestinal disorders. J Gastrointestin Liver Dis. 2006;15:237–241. [PubMed] [Google Scholar]
- 3.Saito YA, Schoenfeld P, Locke GR., 3rd The epidemiology of irritable bowel syndrome in North America: A systematic review. Am J Gastroenterol. 2002;97:1910–1915. doi: 10.1111/j.1572-0241.2002.05913.x. [DOI] [PubMed] [Google Scholar]
- 4.Thompson WG, Longstreth GF, Drossman DA, et al. Functional bowel disorders and functional abdominal pain. Gut. 1999;45(Suppl 2) doi: 10.1136/gut.45.2008.ii43. II43-1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bertiaux-Vandaele N, Youmba SB, Belmonte L, et al. The expression and the cellular distribution of the tight junction proteins are altered in irritable bowel syndrome patients with differences according to the disease subtype. Am J Gastroenterol. 2011;106:2165–2173. doi: 10.1038/ajg.2011.257. [DOI] [PubMed] [Google Scholar]
- 6.Camilleri M, Gorman H. Intestinal permeability and irritable bowel syndrome. Neurogastroenterol Motil. 2007;19:545–552. doi: 10.1111/j.1365-2982.2007.00925.x. [DOI] [PubMed] [Google Scholar]
- 7.Gecse K, Roka R, Sera T, et al. Leaky gut in patients with diarrhea-predominant irritable bowel syndrome and inactive ulcerative colitis. Digestion. 2011;85:40–46. doi: 10.1159/000333083. [DOI] [PubMed] [Google Scholar]
- 8.Rao AS, Camilleri M, Eckert DJ, et al. Urine sugars for in vivo gut permeability: validation and comparisons in irritable bowel syndrome-diarrhea and controls. Am J Physiol Gastrointest Liver Physiol. 2011;301:G919–G928. doi: 10.1152/ajpgi.00168.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tibble JA, Sigthorsson G, Foster R, et al. Use of surrogate markers of inflammation and Rome criteria to distinguish organic from nonorganic intestinal disease. Gastroenterology. 2002;123:450–460. doi: 10.1053/gast.2002.34755. [DOI] [PubMed] [Google Scholar]
- 10.Bjarnason I, Cederborg A, Akvist A, Smale S. Intestinal barrier function. In: Zabielski RGP, Westrom B, editors. Biology of the intestine in growing animals, Vol. 2002. pp. 657–693. [Google Scholar]
- 11.Bjarnason I, MacPherson A, Hollander D. Intestinal permeability: an overview. Gastroenterology. 1995;108:1566–1581. doi: 10.1016/0016-5085(95)90708-4. [DOI] [PubMed] [Google Scholar]
- 12.Nakamura RM, Matsutani M, Barry M. Advances in clinical laboratory tests for inflammatory bowel disease. Clin Chim Acta. 2003;335:9–20. doi: 10.1016/s0009-8981(03)00286-9. [DOI] [PubMed] [Google Scholar]
- 13.Fleming SC, Kapembwa MS, Laker MF, et al. Rapid and simultaneous determination of lactulose and mannitol in urine, by HPLC with pulsedamperometric detection, for use in studies of intestinal permeability. Clin Chem. 1190;36:797–799. [PubMed] [Google Scholar]
- 14.Spiller RC, Jenkins D, Thornley JP, et al. Increased rectal mucosal enteroendocrine cells, t lymphocytes, and increased gut permeability following acute Campylobacter enteritis and in post-dysenteric irritable bowel syndrome. Gut. 2000;47:804–811. doi: 10.1136/gut.47.6.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marshall JK, Thabane M, Garg AX, et al. Intestinal permeability in patients with irritable bowel syndrome after a waterborne outbreak of acute gastroenteritis in Walkteron, Ontario. Aliment Pharmacol Ther. 2004;20:1317–1322. doi: 10.1111/j.1365-2036.2004.02284.x. [DOI] [PubMed] [Google Scholar]
- 16.Zhou Q, Souba WW, Croce CM, Verne GN. MicroRNA-29a regulates intestinal membrane permeability in patients with irritable bowel syndrome. Gut. 2010;59:775–784. doi: 10.1136/gut.2009.181834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haas V, Buning C, Buhner S, et al. Clinical relevance of measuring colonic permeability. Eur J Clin Invest. 2009;39:139–144. doi: 10.1111/j.1365-2362.2008.02075.x. [DOI] [PubMed] [Google Scholar]
- 18.van Wijck K, van Eijk HM, Buurman WA, et al. Analytical approach to a multi-sugar whole gut permeability assay. J Chromatogr B Analyt Technol Biomed Life Sci. 2011;879:2794–2801. doi: 10.1016/j.jchromb.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 19.Shulman RJ, Eakin MN, Czyzewski DI, Jarrett M, Ou CN. Increased gastrointestinal permeability and gut inflammation in children with functional abdominal pain and irritable bowel syndrome. J Pediatr. 153:646–650. doi: 10.1016/j.jpeds.2008.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Willems D, Cadranel S, Jacobs W. Measurement of urinary sugars by HPLC in the estimation of intestinal permeability: Evaluation in pediatric clinical practice. Clin Chem. 1993;39:888–890. [PubMed] [Google Scholar]
- 21.Lostia AM, Lionetto L, Principessa L, et al. A liquid chromatography/mass spectrometry method for the evaluation of intestinal permeability. Clin Biochem. 2008;41:887–892. doi: 10.1016/j.clinbiochem.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 22.Buning C, Geissler N, Prager M, et al. Increased small intestinal permeability in ulcerative colitis: rather genetic than environmental and a risk factor for extensive disease? Inflamm Bowel Dis. 2012 doi: 10.1002/ibd.22909. [DOI] [PubMed] [Google Scholar]
- 23.Berstad A, Arslan G, Folvik G. Relationship between intestinal permeability and calprotectin concentration in gut lavage fluid. Scand J Gastroenterol. 2000;35:64–69. doi: 10.1080/003655200750024551. [DOI] [PubMed] [Google Scholar]
- 24.Braniste V, Leveque M, Buisson-Brenac C, et al. Oestradiol decreases colonic permeability through oestrogen receptor beta mediated up-regulation of occludin and junctional adhesion molecule-a in epithelial cells. J Physiol. 2009;587:3317–3328. doi: 10.1113/jphysiol.2009.169300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schepens MA, Rijnierse A, Schonewille AJ, et al. Dietary calcium decreases but short-chain fructo-oligosaccharides increase colonic permeability in rats. Br J Nutr. 2010;104:1780–1786. doi: 10.1017/S0007114510002990. [DOI] [PubMed] [Google Scholar]
- 26.Obinna FC, Cook G, Beale T, et al. Comparative assessment of small intestinal and colonic permeability in HIV infected homosexual men. AIDS. 1995;9:1009–1016. doi: 10.1097/00002030-199509000-00005. [DOI] [PubMed] [Google Scholar]
- 27.Zakostelska Z, Kverka M, Klimesova K, et al. Lysate of probiotic Lactobacillus casei dn-114 001 ameliorates colitis by strengthening the gut barrier function and changing the gut microenvironment. PLoS One. 2011;6:e27961. doi: 10.1371/journal.pone.0027961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dainese R, Galliani EA, De Lazzari F, et al. Discrepancies between reported food intolerance and sensitization test findings in irritable bowel syndrome patients. Am J Gastroenterol. 1999;94:1892–1897. doi: 10.1111/j.1572-0241.1999.01226.x. [DOI] [PubMed] [Google Scholar]
- 30.Fleming SC, Duncan A, Russell RI, Laker MF. Measurement of sugar probes in serum: an alternative to urine measurement in intestinal permeability testing. Clin Chem. 1996;42:445–448. [PubMed] [Google Scholar]
- 31.Katouzian F, Sblattero D, Not T, Tommasini A, Giusto E, Meiacco D, Stebel M, Marzari R, Fasano A, Ventura A. Dual sugar gut-permeability testing on blood drop on animal models. Clin Chim Acta. 2005;352(1–2):191–197. doi: 10.1016/j.cccn.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 32.Seimiya M, Osawa S, Hisae N, Shishido T, Yamaguchi T, Nomura F. A sensitive enzymatic assay for the determination of sucrose in serum and urine. Clin Chim Acta. 2004;343(1–2):195–199. doi: 10.1016/j.cccn.2004.01.016. [DOI] [PubMed] [Google Scholar]