Abstract
Objective
The authors examined the relationship between cannabis use and the course of illness in schizophrenia over 10 years following first psychiatric hospitalization.
Method
We assessed 229 patients with a schizophrenia-spectrum disorder five times: during the first admission, and 6 months, 2 years, 4 years, and 10 years later. Ratings of cannabis use and psychiatric symptoms (psychotic, negative, disorganized, and depressive) were made at each assessment.
Results
The lifetime rate of cannabis use was 66.2%, and survival analysis revealed that this usage was associated with an earlier onset of psychosis. The rates of current use ranged from 10% to 18% across assessments. Cannabis status was moderately stable, with concordance between waves ranging rtet = 0.48 – 0.78. Mixed-effects logistic regression revealed that changes in cannabis use were associated with changes in psychotic symptoms over time even after gender, age, socio-economic status, other drug use, antipsychotic medication use, and other symptoms were controlled. Structural equation modeling indicated that the association with psychotic symptoms was bi-directional.
Conclusions
Cannabis use is associated with an adverse course of psychotic symptoms in schizophrenia, and vice versa, even after taking into account other clinical, substance, and demographic variables. The specificity of this relationship suggests that clinical interventions to reduce cannabis use may be best targeted at individuals with prominent psychotic symptoms.
INTRODUCTION
Elevated rates of cannabis use have repeatedly been observed among individuals with schizophrenia. Specifically, a recent review of 53 treatment studies found that the average 12-month prevalence of cannabis use was 29.2% among patients with psychosis (1), as compared with 4.0% in the general U.S. population (2). Furthermore, prospective studies indicate that cannabis use is associated with a twofold increase in odds of developing schizophrenia and related disorders (3–6), and retrospective studies have suggested that cannabis use may hasten the onset of schizophrenia (7, 8). These findings are typically interpreted as evidence that cannabis plays an etiologic role in psychosis, although the precise mechanism remains unknown (9). One review of the existing evidence concluded that cannabis is unlikely to cause permanent neurological changes associated with schizophrenia, although the transient neurochemical effects of this substance may be particularly adverse among individuals with a genetic vulnerability to psychosis (10).
While the role of cannabis exposure in the onset of psychosis has received much attention, there have been relatively few systematic studies of the impact of continued cannabis use on the course of schizophrenia. It was found that some individuals with schizophrenia report using cannabis to relieve symptoms or medication side effects (11). Also, some cross-sectional studies reported that cannabis use is associated with less severe negative and disorganized symptoms (12).
On the other hand, other cross-sectional data indicate that cannabis users have more severe psychotic symptoms than non-users (13, 14). Furthermore, a recent review (15) of thirteen longitudinal studies concluded that cannabis use and misuse are associated with increased rates of relapse, but that the links with specific symptoms were less consistent. For example, two longitudinal studies of cannabis abuse in schizophrenia yielded conflicting findings: one reported an association with severity of thought disorder but not psychotic or negative symptoms (16) and the other reported an association with severity of negative symptoms but not disorganized/psychotic symptoms (17). Zammit and colleagues (15) noted that these inconsistencies across studies can be explained in part by methodological limitations, such as small sample sizes and neglecting to control for initial symptom severity and other confounders.
Despite these mixed findings, there is emerging evidence from two recent studies that patients with psychotic disorders may experience symptom exacerbation even from mild cannabis exposure. For example, Grech and colleagues (18) found that individuals with recent onset psychosis who consistently used cannabis had more severe psychotic/disorganized symptoms than non-users even after adjustment for age, gender, and ethnicity. Examining symptom severity globally rather than within specific domains, Degenhardt and colleagues (19) found a small but significant linear relationship between number of days using cannabis in the past month and severity of subsequent psychiatric symptoms across a one year follow-up, even after controlling for demographic variables and initial symptom severity. Together, these studies suggest that even minor cannabis use may be associated with worse outcomes. However, it remains unclear what aspects of schizophrenia course are affected.
In addition to schizophrenia symptoms, there is evidence to suggest that cannabis use is associated with depression and level of functioning. A link between heavy cannabis use and depression has been observed in the general population (20). This has not been well studied in schizophrenia although one report found no relationship between cannabis use and changes in depressive symptoms (19). Furthermore, some studies suggested that cannabis use is more likely to occur among better functioning patients (13, 19) despite its associations with greater symptom severity. For instance, a cross-sectional study found schizophrenia patients with comorbid cannabis abuse to have better premorbid adjustment (21).
In sum, there is accumulating evidence that cannabis use may worsen the course of schizophrenia, but it is still unclear what is driving this effect, as many previous studies did not control for initial symptom severity and examined general illness severity rather than specific symptoms. The goal of the current study is to address these limitations by identifying specific characteristics of schizophrenia associated with cannabis use during the 10 years following first admission for psychosis and to investigate the direction of these associations. The primary strengths of the current study are a relatively large sample size, comprehensive assessment of clinical course, and a long follow-up with multiple assessment points.
METHODS
Participants
Data were obtained from the 229 individuals with DSM-IV research consensus diagnoses of schizophrenia, schizoaffective disorder, or schizophreniform disorder participating in the Suffolk County Mental Health Project (22). A total of 675 participants were recruited from the inpatient units of the 12 psychiatric facilities in Suffolk County between 1989 and 1995. The inclusion criteria were ages 15–60, first admission either concurrent or during the previous six months, clinical evidence of psychosis, ability to understand the assessment procedures in English, and capacity to provide written informed consent. Following the baseline assessment (72% response rate), face-to-face interviews were performed 6 months and 2, 4, and 10 years later. The interviews included the Structured Clinical Interview for DSM-III-R and, later, DSM-IV (SCID; 23) and were administered by trained master’s-level mental health professionals. Baseline interviews assessed current and lifetime conditions, whereas follow-up interviews included current and interval ratings. The 229 respondents who are the focus of this report received a longitudinal consensus DSM-IV diagnosis of a schizophrenia-spectrum disorder after the 2-year follow-up assessment (24). These diagnoses were formulated by a team of 4 or more psychiatrists using all available information from interviews, medical records, and significant others.
The procedures for obtaining informed consent were approved annually by the Committees on Research Involving Human Subjects at Stony Brook University and by the Institutional Review Boards of all hospitals where respondents were recruited. After complete description of the study to the subjects, written informed consent was obtained, and for participants ages 15–17, written consent of parents was also required.
The cross-sectional analyses of cannabis use were based on all available respondents at a given time point. As noted, there were 229 participants at baseline. At 10 years, 162 provided complete data, 25 provided partial, 10 refused, 19 were untraceable, and 13 died. The 162 with complete information were similar to the remaining 67 participants on all variables in this study (p > 0.10).
Measures
Cannabis use and age at first use were assessed as part of the Substance Use Disorders module of the SCID and items modeled on the National Household Survey on Drug Abuse interview (25). Participants were asked about their cannabis use in the past 30 days at baseline and at 10 years, and in the prior six months at the 6-month, 2-year, and 4-year follow-ups. Cannabis use was dichotomized (using at least once in the specified time frame vs. no use during that time frame). Frequency of use and percent with lifetime DSM-III-R abuse/dependence determined by the SCID are also examined in relation to use. The lifetime data were collected before publication of the DSM-IV, but diagnostic criteria for substance use disorders in DSM-III-R and DSM-IV are similar.
Symptoms of psychosis in the month preceding each interview were measured with the Scale for the Assessment of Positive Symptoms (SAPS; 26) and the Scale for the Assessment of Negative Symptoms (SANS; 27, 28). The SANS taps affective blunting, alogia, avolition, anhedonia/asociality, and inattention, whereas the SAPS measures psychotic symptoms (hallucinations and delusions) and disorganized symptoms (bizarre behavior and thought disorder). Factor analysis indicated that in this sample the SANS can be best scored as a single overall index and the SAPS as two subscales: psychotic and disorganized (for details, see 29). Age of onset of psychosis was based on a timeline for each psychotic symptom assessed during the SCID combined with information from medical records and informants (30).
Symptoms of depression in the month preceding interview were assessed with the SCID depression module, administered without skip-outs. The nine DSM-IV criterion symptoms were rated as: 1 = not present, 2 = questionable, and 3 = definite. Depression was operationalized as a composite of these nine criterion symptoms.
Illness severity was operationalized as interviewers’ (baseline) and psychiatrists’ (follow-up) consensus Global Assessment of Functioning (GAF) rating for the worst week in the past month.
Five background variables were included: sex, age at baseline, parental socioeconomic status (of parent with the higher occupational level), presence/absence of other drug use (stimulants, cocaine, etc. assessed in parallel with cannabis use), and presence/absence of past month antipsychotic medication use.
Data Analysis
Proportional hazards regression was used to examine the association between first cannabis use (modeled as a time-varying covariate) and the onset of first psychotic symptom, with adjustment for gender, age cohort, and SES at baseline. Stability of cannabis status was evaluated with tetrachoric correlations. Cross-sectional comparisons of users and non-users were made with multiple linear regression. Longitudinal correlates of cannabis use (no/yes) were examined using a mixed-effects logistic regression model (31). These within-person analyses tested which variables were associated with starting or stopping of cannabis use for a person over time. The independent variables included time-varying clinical covariates (SAPS, SANS, depression, other drug use, and antipsychotic medications) and demographic characteristics (age, sex, and SES). GAF was analyzed separately. These models had a fixed term for time (measured in years) and a random intercept term, which allows the estimation of a separate trajectory for each individual. All variables except time were standardized with respect to their grand means and standard deviations (across all subjects and waves) to facilitate interpretation. In this analysis, the random effects covariance structure was specified as an unstructured covariance matrix, as it imposed the fewest constraints and provided a better fit than all other covariance matrices.
For variables that covaried with changes in cannabis use over time, we also evaluated the direction of the association using structural equation modeling (SEM). We specified a cross-lagged model, in which each follow-up variable was predicted jointly by cannabis use and symptomatology from the preceding wave (Figure 1). Thus, the model assumes contributions of symptoms to future cannabis use above and beyond past cannabis use, and vice versa. We constrained equivalent paths to be equal, namely, paths from the symptom variable to cannabis use at each wave and from cannabis use to the symptom variable at each wave. The change in chi-square from the unconstrained to the constrained model was non-significant (p=.85). The model also included cannabis exposure prior to baseline, which predicted both baseline variables. SEM was performed using Mplus version 5.1. In evaluating the model, we considered two fit indices, the Comparative Fit Index (CFI) and the root-mean-square error of approximation (RMSEA). Conventional rule-of-thumb guidelines suggest that CFI values of ≥ 0.90 indicate an adequate fit, and values of ≥ 0.95 indicate an excellent fit; RMSEA values of ≤ 0.10 indicate an adequate fit, and values of ≤ 0.06 indicate an excellent fit (32).
Figure 1.
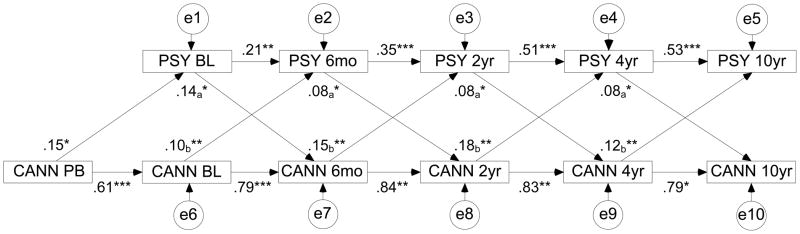
Associations between cannabis use and psychotic symptoms. PSY = level of psychotic symptoms (numbers indicate time of the assessment); CANN = cannabis status (user or non-user); PB = pre-baseline; e = disturbances on all variables. Paths constrained to be equal have the same subscripts. *p<0.05, **p< 0.01, ***p<0.001
Missing data were addressed in SEM using the full information maximum likelihood (FIML) method (33). FIML estimates models from all available data, thus minimizing attrition-related biases (34). An analogous approach was employed in mixed-effects logistic regression, so that data from each participant was included in the analysis. Thus, the longitudinal analyses were based on 880 observations.
RESULTS
Descriptive Characteristics
The characteristics of the sample and comparison of baseline users and non-users are presented in Table 1. At baseline, the lifetime use rate was 66.2%. Sixty-four lifetime users (43.0%) met DSM-III-R criteria for abuse or dependence. Current cannabis users were younger at first admission and had an earlier age of onset. Although the differences in gender and SES were non-significant, cannabis use was more common among males and participants from blue collar families.
Table 1.
Demographic characteristics of the sample and their associations with current (past 30 days) cannabis use at baseline
| Demographic Variables | Full Sample (N=229) | Cannabis users at baseline (N=23) | Cannabis non-users at baseline (N=202) | Comparison | |||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | OR (95% CI) | |
|
| |||||||
| Gender | |||||||
| Male | 149 | 65.1 | 18 | 78.3 | 128 | 63.4 | 2.08 (.74–5.84) |
| Female | 80 | 34.9 | 5 | 21.7 | 74 | 36.6 | -- |
| SES of family of origin | |||||||
| Low (semi/unskilled) | 81 | 35.4 | 7 | 30.4 | 74 | 36.6 | 1.40 (0.39–4.99) |
| Medium (skilled) | 83 | 36.2 | 12 | 52.2 | 69 | 34.2 | 2.57 (0.79–8.34) |
| High (white collar) | 65 | 28.4 | 4 | 17.4 | 59 | 29.2 | -- |
| Lifetime cannabis use, prior to baseline | 149 | 66.2 | 23 | 100.0 | 123 | 61.8 | N/A |
|
| |||||||
| M | SD | M | SD | M | SD | t (df) | |
|
| |||||||
| Age at baseline | 28.02 | 8.55 | 23.65 | 6.37 | 28.47 | 8.69 | 2.58 (223) * |
| Age of onset, psychosis | 25.60 | 7.76 | 22.61 | 6.21 | 25.99 | 7.91 | 1.98 (212) * |
Note: At baseline, four individuals did not provide clear information about cannabis use during the past 30 days. Dashes indicate reference category.
p<0.05
Consistent with prior studies, survival analysis showed that among users, the risk of psychosis onset in any given year following exposure to cannabis doubled compared to same-age non-users (HR=1.97, 95% CI=1.48–2.62, p<.001). Inspection of patterns of cannabis use among age cohorts revealed that cannabis use was more prevalent and started at an earlier age among participants who were younger than 35 at baseline (data not shown). This cutoff is consistent with the normalization of cannabis use in the 1960s–1970s (35). After adjusting for age cohort (baseline age < 35 vs. 35+), gender and SES, the association of cannabis use with age of onset of psychosis remained significant (HR=1.34, 95% CI=1.01–1.77, p<0.05).
Prevalence and Stability of Cannabis Use
At baseline and 10 years, the one-month rates of use were 10%, while the 6-month rates obtained during the other follow-up intervals ranged 13–18% (Table 2). The average frequency of use was 9.0 days per month (1.3 joints per day).
Table 2.
Stability and prevalence of cannabis use (users versus non-users): tetrachoric correlations
| Baseline (N=225) | Month 6 (N=184) | Year 2 (N=166) | Year 4 (N=143) | Year 10 (N=162) | |
|---|---|---|---|---|---|
| Stability | --- | ||||
| Baseline | |||||
| 6-month | 0.70 | --- | |||
| 2-year | 0.51 | 0.73 | --- | ||
| 4-year | 0.48 | 0.75 | 0.74 | --- | |
| 10-year | 0.66 | 0.52 | 0.45 | 0.78 | --- |
|
| |||||
| Prevalence | |||||
| Users, N (%) | 23 (10.2) | 26 (14.1) | 30 (18.1) | 19 (13.3) | 16 (9.9) |
| Non-users, N (%) | 202 (89.8) | 158 (85.9) | 136 (81.9) | 124 (86.7) | 146 (90.1) |
Note: Cannabis status was assessed for the past 30 days at Baseline and at 10 years, and it was assessed for the past six months at the 6-month, 2-year, and 4-year points.
Cannabis status showed substantial stability over the 10 years, with correlations among waves ranging from 0.48 to 0.78 (Table 2). Although patterns of cannabis use tended to persist, a fair number of individuals stopped or started over the 10 years. In fact, of the 62 individuals who were using at any of the waves, only 7 used cannabis at each available time point. Six of them had a history of cannabis abuse or dependence. We also compared users and non-users with regard to the presence/absence of antipsychotic medication use but found no differences at any of the five assessment points (all ps>0.05).
Cross-sectional Comparisons of Cannabis Use and Symptom Severity
Cannabis users had elevated levels of psychotic symptoms at 4 of the 5 time points, with an average effect size of β=0.19 (unadjusted model; Table 3). The only other significant difference was greater depression severity in users at year 2. After adjustment for covariates, the difference in psychotic symptoms was significant at 3 time points (2 after Bonferroni correction); the average effect size remained β=0.19. For participants using at least 3 times/month, the adjusted effects were similar (the average effect for psychotic symptoms β=0.18, 3 associations with psychosis significant, one after Bonferroni correction).
Table 3.
Differences in symptoms and illness severity: cross-sectional comparisons
| Dependent Variables | Non-User | User | Unadjusted Modela | Adjusted Modelb | ||
|---|---|---|---|---|---|---|
| M | SD | M | SD | β | β | |
| GAF, lowest | ||||||
| Baseline | 26.98 | 7.98 | 28.73 | 9.08 | 0.07 | 0.04 |
| 6-month | 41.43 | 12.95 | 36.92 | 13.46 | −0.12 | −0.09 |
| 2-year | 39.43 | 11.78 | 39.07 | 12.28 | −0.01 | −0.01 |
| 4-year | 40.11 | 11.04 | 40.37 | 16.95 | 0.01 | 0.11 |
| 10-year | 40.68 | 12.30 | 40.57 | 15.64 | −0.00 | 0.06 |
| SAPS, psychotic | ||||||
| Baseline | 13.05 | 10.01 | 18.91 | 13.93 | 0.17* | 0.20** |
| 6-month | 4.11 | 7.17 | 8.65 | 9.11 | 0.21** | 0.23*** |
| 2-year | 4.29 | 6.62 | 6.77 | 8.82 | 0.13 | 0.15 |
| 4-year | 3.85 | 7.00 | 7.79 | 7.81 | 0.19* | 0.11 |
| 10-year | 5.60 | 8.00 | 14.54 | 16.40 | 0.27*** | 0.26*** |
| SAPS, disorganized | ||||||
| Baseline | 7.39 | 7.02 | 5.78 | 4.99 | −0.07 | −0.08 |
| 6-month | 2.73 | 4.39 | 3.62 | 4.38 | 0.07 | 0.08 |
| 2-year | 2.80 | 4.09 | 2.40 | 3.13 | −0.04 | 0.01 |
| 4-year | 3.11 | 4.69 | 4.05 | 4.61 | 0.07 | 0.06 |
| 10-year | 4.18 | 5.94 | 5.23 | 5.28 | 0.05 | 0.03 |
| SANS | ||||||
| Baseline | 23.64 | 13.54 | 26.91 | 15.13 | 0.07 | 0.03 |
| 6-month | 23.89 | 14.69 | 23.15 | 16.11 | −0.02 | −0.04 |
| 2-year | 23.07 | 13.06 | 22.03 | 11.60 | −0.03 | −0.06 |
| 4-year | 22.47 | 13.80 | 18.05 | 12.60 | −0.11 | −0.12 |
| 10-year | 22.58 | 14.88 | 23.08 | 16.68 | 0.01 | −0.04 |
| Depression | ||||||
| Baseline | 16.20 | 4.38 | 16.74 | 4.92 | 0.04 | 0.02 |
| 6-month | 12.98 | 3.70 | 13.15 | 3.95 | 0.02 | −0.02 |
| 2-year | 12.38 | 3.73 | 14.00 | 3.80 | 0.17* | 0.12 |
| 4-year | 10.72 | 3.95 | 11.32 | 3.82 | 0.05 | −0.03 |
| 10-year | 11.90 | 4.14 | 12.00 | 3.65 | 0.01 | 0.02 |
p<0.05,
p<0.01,
p<0.002 (Bonferroni correction for 25 comparisons)
Linear regression including cannabis status as the only predictor.
Multiple regression including age, gender, SES, cannabis status, other drug status, and antipsychotic medication as predictors (Beta coefficient indicates effect of cannabis status).
Note: The N’s for users and non-users at each timepoint are located at the bottom of Table 2.
Longitudinal Associations of Cannabis Use and Symptom Severity
The within-person analyses revealed that changes in cannabis status were linked with changes in psychotic symptoms (p=0.012) even after adjusting for the covariates and other symptom domains (Table 4). Thus, an increase in psychotic symptoms was associated with greater likelihood of using cannabis; conversely, a decrease in psychotic symptoms was associated with lower likelihood of use. An inverse association with disorganized symptoms (p = .049) was also observed but did not survive Bonferroni correction. No other effects were significant.
Table 4.
Correlates of cannabis status over time: mixed-effects logistic regression analysis
| aOR | 95% CI | |
|---|---|---|
| Overall illness severity (GAF)a | 1.05 | 0.72–1.53 |
| Symptom severityb | ||
| SAPS psychotic | 1.64** | 1.12–2.43 |
| SAPS disorganized | 0.63* | 0.40–1.00 |
| SANS | 1.51 | 0.98–2.32 |
| Depression | 0.77 | 0.52–1.15 |
p < 0.05,
p < 0.0125 (Bonferroni correction for 4 comparisons)
Adjusted for age, sex, SES, other drug use, antipsychotic medication use, and time.
Adjusted for age, sex, SES, other drug use, antipsychotic medication use, time, and each symptom scale SAPS = Scale for the Assessment of Positive Symptoms.
SANS = Scale for the Assessment of Negative Symptoms.
We examined the direction of the identified longitudinal correlation between cannabis status and psychotic symptoms using SEM. A cross-lagged model that included the five assessment waves as well as pre-baseline cannabis exposure (Figure 1) yielded excellent fit to the data (CFI=0.95, RMSEA=0.05). All paths in the model were significant. Effects of cannabis use on later psychotic symptoms (average β=0.11) and of psychotic symptoms on later cannabis use (average β=0.10) were comparable and statistically significant, indicating a bi-directional relationship. Thus, lower severity of psychotic symptoms predicted cessation of cannabis use, whereas higher severity was associated with increased likelihood of use at the next assessment. Conversely, cannabis use predicted an increase in severity of psychotic symptoms.
DISCUSSION
Two-thirds of the present sample had a lifetime history of cannabis use prior to first hospitalization, and survival analysis confirmed the association of this use with an earlier age of onset of psychosis. Moreover, exposure to cannabis before baseline predicted more severe psychotic symptoms at baseline. Across the 10-year follow-up, rates of current cannabis use ranged from 10–18%, and users were found to have more severe psychotic symptoms at 2 of the 5 assessment points. These differences in psychotic symptom severity are unlikely to be due to medication use, which was controlled in the multivariate analyses and did not differ between cannabis users and non-users at any assessment. In addition, patterns of cannabis use and severity of psychotic symptoms were found to covary over time, and mathematical modeling revealed this relationship to be bi-directional. In other words, changes in cannabis use were predictive of changes in psychotic symptoms and vice versa.
These results demonstrate that cannabis use after onset of schizophrenia is associated with more severe psychotic symptoms over a 10-year follow-up, the longest reported assessment period to date. This study builds upon a prior report that cannabis exposure is most strongly associated with psychotic symptoms (18) as well as a briefer longitudinal study that predicted changes in general psychiatric symptoms from cannabis use over the course of a year (19).
The current findings also shed new light on two important issues. First, the link between cannabis use and psychotic symptoms was apparent even after controlling for negative, disorganized, and depressive symptoms, as well as other drug use, antipsychotic medication use, and demographic variables. Moreover, these other symptoms as well as overall illness severity were not significantly associated with use. Disorganized symptoms showed only a marginally significant inverse effect. Thus, we cannot rule out the possibility that some individuals used cannabis to obtain relief from disorganized symptoms (11, 12), but in the current sample a more robust association was found with psychotic symptoms. This suggests that individuals with schizophrenia who use cannabis are not more severely ill overall, but suffer specifically from more severe psychotic symptoms. Second, this relationship with psychotic symptoms was bidirectional: cannabis exposure predicted severity of psychosis, and individuals with more severe psychotic symptoms were more likely to use cannabis in the future. This is consistent with findings from two recent reports, one conducted in a non-clinical sample (36) and the other focused on relapse among individuals with recent-onset psychosis over a 6-month follow-up (37).
These findings are especially noteworthy when viewed in the context of several limitations. First, the lag between assessments increased over time to reduce participant burden, which required additional modeling in longitudinal analyses. Second, the timeframe of cannabis use variables changed during the course of the study, affecting the prevalence rates and making it difficult to fully align timeframes of cannabis use and symptoms. Both issues are important caveats, but we did not find evidence of their affecting the longitudinal associations. Third, the number of users at each assessment was relatively small, although the prevalence rates are consistent with previous studies (1, 7, 8). Fourth, cannabis exposure was not verified with toxicology screens. Importantly, these limitations made significant results less likely, and our findings are more notable because of that.
The clinical relevance of our findings is underscored by the fact that cannabis use is a potentially modifiable behavior, and successful cessation may lead to appreciable reduction in severity of psychotic symptoms (19). Although our naturalistic study did not find differences in rates of cannabis use by treatment with antipsychotic medications, clinical studies have shown that antipsychotics may facilitate cannabis cessation in patients by reducing the severity of psychotic symptoms. In fact, there is some evidence that clozapine may be particularly effective for patients with schizophrenia and comorbid substance abuse (38). Indeed, it has been suggested that the integration of psychiatric and substance abuse treatments into a single approach for dually diagnosed individuals may produce better results than treating the two issues separately (39). This remains to be demonstrated empirically, however, as a recent review of treatment for patients with dual diagnoses concluded that the efficacy of integrated treatment is uncertain (40).
In conclusion, the current study demonstrates that exposure to cannabis among patients with schizophrenia is associated with an adverse course of psychotic symptoms, and vice versa. This bi-directional relationship between cannabis use and psychotic symptoms was observed after taking into account other clinical and demographic variables, and may have implications for future studies of both behavioral and biological treatment interventions. As our understanding of the biology of cannabis use and psychosis continues to improve, it may be possible to identify mechanisms by which exposure to cannabis may influence psychosis, and these mechanisms may become future targets for intervention.
Acknowledgments
FUNDING
National Institute of Health (MH-44801 to E.J.B.)
The authors thank the mental health professionals in Suffolk County, the mental health project psychiatrists and staff, and most of all, the study participants and their families and friends.
References
- 1.Green B, Young R, Kavanagh D. Cannabis use and misuse prevalence among people with psychosis. Br J Psychiatry. 2005;187:306–13. doi: 10.1192/bjp.187.4.306. [DOI] [PubMed] [Google Scholar]
- 2.Compton WM, Grant BF, Colliver JD, Glantz MD, Stinson FS. Prevalence of marijuana use disorders in the United States: 1991–1992 and 2001–2002. JAMA. 2004;291:2114–21. doi: 10.1001/jama.291.17.2114. [DOI] [PubMed] [Google Scholar]
- 3.Arseneault L, Cannon M, Witton J, Murray RM. Causal association between cannabis and psychosis: examination of the evidence. Br J Psychiatry. 2004;184:110–7. doi: 10.1192/bjp.184.2.110. [DOI] [PubMed] [Google Scholar]
- 4.Smit F, Bolier L, Cuijpers P. Cannabis use and the risk of later schizophrenia: a review. Addiction. 2004;99:425–30. doi: 10.1111/j.1360-0443.2004.00683.x. [DOI] [PubMed] [Google Scholar]
- 5.Hall W, Degenhardt L. Cannabis use and the risk of developing a psychotic disorder. World Psychiatry. 2008;7:68. doi: 10.1002/j.2051-5545.2008.tb00158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hall W, Degenhardt L. Cannabis use and psychosis: a review of clinical and epidemiological evidence. Aust N Z J Psychiatry. 2000;34:26–34. doi: 10.1046/j.1440-1614.2000.00685.x. [DOI] [PubMed] [Google Scholar]
- 7.Hambrecht M, Hafner H. Cannabis, vulnerability, and the onset of schizophrenia: an epidemiological perspective. Aust N Z J Psychiatry. 2000;34:468–75. doi: 10.1080/j.1440-1614.2000.00736.x. [DOI] [PubMed] [Google Scholar]
- 8.Veen ND, Selten JP, van der Tweel I, Feller WG, Hoek HW, Kahn RS. Cannabis use and age at onset of schizophrenia. Am J Psychiatry. 2004;161:501–6. doi: 10.1176/appi.ajp.161.3.501. [DOI] [PubMed] [Google Scholar]
- 9.Degenhardt L, Hall W, Lynskey M. Testing hypotheses about the relationship between cannabis use and psychosis. Drug Alcohol Depend. 2003;71:37–48. doi: 10.1016/s0376-8716(03)00064-4. [DOI] [PubMed] [Google Scholar]
- 10.DeLisi LE. The effect of cannabis on the brain: can it cause brain anomalies that lead to increased risk for schizophrenia? Curr Opin Psychiatry. 2008;21:140. doi: 10.1097/YCO.0b013e3282f51266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dixon L, Haas G, Weiden P, Sweeney J, Frances A. Acute effects of drug abuse in schizophrenic patients: clinical observations and patients’ self-reports. Schizophr Bull. 1990;16:69. doi: 10.1093/schbul/16.1.69. [DOI] [PubMed] [Google Scholar]
- 12.Bersani G, Orlandi V, Kotzalidis GD, Pancheri P. Cannabis and schizophrenia: impact on onset, course, psychopathology and outcomes. Eur Arch Psychiatry Clin Neurosci. 2002;252:86–92. doi: 10.1007/s00406-002-0366-5. [DOI] [PubMed] [Google Scholar]
- 13.Peralta V, Cuesta MJ. Influence of cannabis abuse on schizophrenic psychopathology. Acta Psychiatr Scand. 1992;85:127–30. doi: 10.1111/j.1600-0447.1992.tb01456.x. [DOI] [PubMed] [Google Scholar]
- 14.Negrete JC, Knapp WP, Douglas DE, Smith WB. Cannabis affects the severity of schizophrenic symptoms: results of a clinical survey. Psychol Med. 1986;16:515–20. doi: 10.1017/s0033291700010278. [DOI] [PubMed] [Google Scholar]
- 15.Zammit S, Moore TH, Lingford-Hughes A, Barnes TR, Jones PB, Burke M, Lewis G. Effects of cannabis use on outcomes of psychotic disorders: systematic review. Br J Psychiatry. 2008;193:357–63. doi: 10.1192/bjp.bp.107.046375. [DOI] [PubMed] [Google Scholar]
- 16.Caspari D. Cannabis and schizophrenia: results of a follow-up study. Eur Arch Psychiatry Clin Neurosci. 1999;249:45–9. doi: 10.1007/s004060050064. [DOI] [PubMed] [Google Scholar]
- 17.Arias Horcajadas F, Sánchez Romero S, Padín Calo JJ. Influencia del consumo de drogas en las manifestaciones clínicas de la esquizofrenia [Relevance of drug use in clinical manifestations of schizophrenia] Actas Esp Psiquiatr. 2002;30:65–73. [PubMed] [Google Scholar]
- 18.Grech A, Van Os J, Jones PB, Lewis SW, Murray RM. Cannabis use and outcome of recent onset psychosis. Eur Psychiatry. 2005;20:349–53. doi: 10.1016/j.eurpsy.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 19.Degenhardt L, Tennant C, Gilmour S, Schofield D, Nash L, Hall W, McKay D. The temporal dynamics of relationships between cannabis, psychosis and depression among young adults with psychotic disorders: findings from a 10-month prospective study. Psychol Med. 2007;37:927–34. doi: 10.1017/S0033291707009956. [DOI] [PubMed] [Google Scholar]
- 20.Degenhardt L, Hall W, Lynskey M. Exploring the association between cannabis use and depression. Addiction. 2003;98:1493–504. doi: 10.1046/j.1360-0443.2003.00437.x. [DOI] [PubMed] [Google Scholar]
- 21.Arndt S, Tyrrell G, Flaum M, Andreasen NC. Comorbidity of substance abuse and schizophrenia: the role of pre-morbid adjustment. Psychol Med. 1992;22:379–88. doi: 10.1017/s0033291700030324. [DOI] [PubMed] [Google Scholar]
- 22.Bromet EJ, Schwartz JE, Fennig S, Geller L, Jandorf L, Kovasznay B, Lavelle J, Miller A, Pato C, Ram R, Rich C. The epidemiology of psychosis: the Suffolk County Mental Health Project. Schizophr Bull. 1992;18:243–55. doi: 10.1093/schbul/18.2.243. [DOI] [PubMed] [Google Scholar]
- 23.First MB, Spitzer RL, Gibbon M, Williams JBM. Structured clinical interview diagnostic for DSM-IV axis I disorders-clinician version. New York: Biometrics Research Department of New York State Psychiatric Institute; 1997. [Google Scholar]
- 24.Schwartz JE, Fennig S, Tanenberg-Karant M, Carlson G, Craig T, Galambos N, Lavelle J, Bromet EJ. Congruence of Diagnoses 2 Years After a First-Admission Diagnosis of Psychosis. Arch Gen Psychiatry. 2000;57:593–600. doi: 10.1001/archpsyc.57.6.593. [DOI] [PubMed] [Google Scholar]
- 25.Clayton RR, Voss HL, LoSciuto L, Martin SS, Skinner WF, Robbins C, Santos RL. National Institute on Drug Abuse (DHHS Publication No ADM 88–1586) Washington, DC: US Government Printing Office; 1988. National household survey on drug abuse: main findings 1985. [Google Scholar]
- 26.Andreasen NC. The Scale for the Assessment of Positive Symptoms (SAPS) Iowa City: The University of Iowa; 1984. [Google Scholar]
- 27.Andreasen NC. The Scale for the Assessment of Negative Symptoms (SANS) Iowa City: The University of Iowa; 1983. [Google Scholar]
- 28.Grube BS, Bilder RM, Goldman RS. Meta-analysis of symptom factors in schizophrenia. Schizophr Res. 1998;31:113–20. doi: 10.1016/s0920-9964(98)00011-5. [DOI] [PubMed] [Google Scholar]
- 29.Kotov R, Guey LT, Bromet EJ, Schwartz JE. Smoking in Schizophrenia: Diagnostic Specificity, Symptom Correlates, and Illness Severity. Schizophr Bull. 2010;36:173–81. doi: 10.1093/schbul/sbn066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Craig TJ, Bromet EJ, Fennig S, Tanenberg-Karant M, Lavelle J, Galambos N. Is There an Association Between Duration of Untreated Psychosis and 24-Month Clinical Outcome in a First-Admission Series? Am J Psychiatry. 2000;157:60. doi: 10.1176/ajp.157.1.60. [DOI] [PubMed] [Google Scholar]
- 31.Verbeke G, Molenberghs G. Linear mixed models for longitudinal data. New York: Springer-Verlag; 2000. [Google Scholar]
- 32.Hu L, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling. 1999;6:1–55. [Google Scholar]
- 33.Schafer JL, Graham JW. Missing data: our view of the state of the art. Psychol Methods. 2002;7:147–77. [PubMed] [Google Scholar]
- 34.McArdle JJ, Hamagami F. Modeling incomplete longitudinal and cross-sectional data using latent growth structural models. Exp Aging Res. 1992;18:145–66. doi: 10.1080/03610739208253917. [DOI] [PubMed] [Google Scholar]
- 35.Miller JD. National survey on drug abuse: main findings 1982. Washington, DC: US Government Printing Office; 1983. [Google Scholar]
- 36.Ferdinand RF, Sondeijker F, van der Ende J, Selten JP, Huizink A, Verhulst FC. Cannabis use predicts future psychotic symptoms, and vice versa. Addiction. 2005;100:612–8. doi: 10.1111/j.1360-0443.2005.01070.x. [DOI] [PubMed] [Google Scholar]
- 37.Hides L, Dawe S, Kavanagh DJ, Young RM. Psychotic symptom and cannabis relapse in recent-onset psychosis. Prospective study. Br J Psychiatry. 2006;189:137–43. doi: 10.1192/bjp.bp.105.014308. [DOI] [PubMed] [Google Scholar]
- 38.Noordsy DL, Green AI. Pharmacotherapy for schizophrenia and co-occurring substance use disorders. Curr Psychiatry Rep. 2003;5:340–6. doi: 10.1007/s11920-003-0066-5. [DOI] [PubMed] [Google Scholar]
- 39.Drake RE, Essock SM, Shaner A, Carey KB, Minkoff K, Kola L. Implementing dual diagnosis services for consumers with severe mental illness. Psychiatr Serv. 2001;52:469–76. doi: 10.1176/appi.ps.52.4.469. [DOI] [PubMed] [Google Scholar]
- 40.Tiet QQ, Mausbach B. Treatments for patients with dual diagnosis: a review. Alcohol Clin Exp Res. 2007;31:513–36. doi: 10.1111/j.1530-0277.2007.00336.x. [DOI] [PubMed] [Google Scholar]


