Abstract
Vaccine induced protection against infection by HIV or highly pathogenic and virulent SIV-strains has been limited. Here, in a proof of concept study, we show that a novel vaccine approach significantly protects Rhesus macaques from mucosal infection by the highly pathogenic strain SIVmac251. We vaccinated 3 cohorts of 12 macaques each with live, irradiated vaccine cells secreting the modified ER chaperone gp96−Ig. Cohort 1 was vaccinated with cells secreting gp96SIVIg carrying SIV peptides. Cohort 2 in addition received recombinant envelope protein SIV-gp120. Cohort 3 was injected with cells secreting gp96-Ig (no SIV antigens) vaccines. Cohort 2 was protected from infection. After seven rectal challenges with highly pathogenic SIVmac251 the hazard ratio was 0.27 corresponding to a highly significant, 73% reduced risk of viral acquisition. The apparent success of the novel vaccine modality recommends further study.
Keywords: non-human primates, gp96-chaperone, vaccine, SIV, vaccine efficacy, cellular immunity, humoral immunity, mucosa, rectum
Introduction
Gp96 is a dominant ER chaperone and a danger associated molecular pattern (DAMP). In its chaperone function, gp96 in the ER receives all cellular peptides generated by the proteasome from endogenous proteins that are translocated by the TAP into the ER for subsequent selection and trimming for MHC I loading. When released from necrotic cells gp96 functions as DAMP serving as adjuvant to activate DC via TLR2 and TLR4 (1) and, by being endocytosed by CD91, as antigen-carrier for antigen cross presentation to CD8 T cells (2), (3, 4). By replacing gp96’s ER retention sequence with the hinge and Fc domain of IgG1we generated a secreted chaperone, gp96-Ig, which optimally cross primes antigen specific CD8 T cells at 10−15M peptide concentration (5, 6). Since gp96-Ig carries all peptides of a cell that will be selected in the recipient/vaccinee for MHC I loadingincluding transfected or infected antigens, it has the broadest, theoretically possible antigenic epitope-spectrum for cross-priming of CD8 T cells by any MHC I type. Gp96-activated DC in addition can take up antigenic proteins and, after processing, present their epitopes via MHC II thereby promoting antibody production by B cells. Gp96 thus is a powerful Th1 adjuvant for CTL priming and for stimulation of Th1 type antibodies that are of isotype IgG2a and IgG2b in mice (our own unpublished data).
Protection from HIV infection requires mucosal immunity. Comparison of gp96ovaIg vaccination in mice and of gp96SIVIg vaccines in macaques by the subcutaneous, intrarectal, intravaginal or intraperitoneal route demonstrated that i.p.-vaccination generates a stronger mucosal CTL response in mucosal IEL and LPL than ever reported (7, 8). The i.p route therefore was chosen here to determine protective efficiency against mucosal SIV challenge in a proof of principle study.
Materials and Methods
Animals and Vaccine cells
Indian-origin, outbred, young adult, male and female, specific pathogen-free (SPF) rhesus monkeys (Macaca mulatta36 animals) were housed and handled in accordance with the standards of the Association for the Assessment and Accreditation of Laboratory Animal Care International at Rockville, ABL (MD, USA). Groups were balanced for Mamu-A*01 (3 in each group), Mamu-B*08 (1 in each group), and for susceptible and resistant TRIM5α alleles. There were no Mamu-B*17+ animals. Gp96SIVIg-vaccine cells were generated by transfection of 293 cells with plasmids encoding gp96-Ig, SIVmac251 rev-tat-nef (rtn), Gag and gp160 as described previously (8). Macaques were injected intraperitoneally with 107 irradiated gp96SIVIg vaccine cells in HBSS that secrete 10µg/24h gp96SIVIg. In one group of macaques 100 µg recombinant SIVgp120 protein (ABL) was added to the vaccine cells. Mock controls received 293-gp96-Ig not transfected with SIV antigens.
Study design
Macaques were primed at week 0 with vaccine or mock cells alone without gp120-addition and boosted at week 6 and 25 adding gp120 to one group. Beginning at week 33 all monkeys were weekly challenged by up to 7 intrarectal instillations of 120 TCID50 highly pathogenic SIVmac251 swarm virus (not cloned) (NIH challenge stock, Dr. Nancy Miller, virus was propagated in macaque’s PBMCs) which generates 3–4 founder viruses in infected mock controls. Viral loads were determined weekly (NASBA, Bioqual Inc. Rockville, MD) and challenge discontinued when positive. Animals were euthanized at week 52. In a parallel study (P.I. Franchini, G) twenty four animals received 100µg gp120/alum or alum alone at week 12 and 24. All animals were challenged, with the same virus stock provided by Dr. N. Miller and at the same dose as described above at week 28. All animal studies were approved by the University of Miami Miller School of Medicine Institutional Animal Care and Use Committee (IACUC).
Tissue preparation, flow cytometry and SIV gp120 antibodies in serum
Mononuclear cells were isolated from blood, rectal tissue pre- or post-vaccination as described (8). SIV-specific cellular immune responses were assessed by multiparameter intracellular cytokine staining (ICS) assay. Humoral immune responses were measured by Env ELISA and ELISPOT for antibody secreting cells and flow cytometric analysis of the plasmablast frequency in the peripheral blood.
Statistical Analysis
Analyses of virological and immunological data were performed by Wilcoxon rank-sum tests and analysis of survival by log-rank tests. For these tests, p<0.05 was considered significant and two-tailed tests were performed. Hazard ratios (HR) calculated by the Gehan Wilcoxon test do not require a consistent hazard ratio but do require consistent higher risk for one group. HRs are also calculated by proportional hazards regression analysis with exact resolution of ties computed in SAS 9.2. Immunological correlates were evaluated using both, parametric and non-parametric correlation tests. Graphical analysis was performed using GraphPad Prism package (GraphPad).
Results and Discussion
Gp96SIVIg vaccines induce cellular and humoral immune responses
Some SIV vaccine concepts have shown post-infection virological control (9–11), other studies in humans (12) and macaque (13) vaccine studies reported significant protection against acquisition of SIV/HIV infection which appear to require specific cellular and humoral responses (14, 15).
293-gp96SIVIg cells were created by permanent transfection of HEK293 cells (not containing T antigen) with plasmids encoding gp96-Ig, SIV rev, nef tat (as fusion protein), gag and gp120 as described (8). I.p. injection of 293-gp96SIVIg generated extraordinary mucosal, rectal and vaginal frequencies of polyepitope specific MHC restricted CTL in LPL and IEL for SIV gag, tat, nef and gp120, secreting IFN-γ and IL-2 upon antigen stimulation (8). Here we determined the protective activity of the gp96SIVIg-vaccine strategy in 36 Indian-origin rhesus macaques (Macaca mulatta), divided into 3 groups of 12, balanced by gender, MHC type and TRIM5α expression. Group I received 293-gp9SIVIg to generate CTL; in group II SIVmac251 gp120-protein was added to generate CTL and antibody; group 3 was the control group receiving 293-gp96-Ig not containing SIV antigens (Fig. 1A). A protein only group, gp120/alum done in parallel (Franchini, unpublished) is included for comparison.
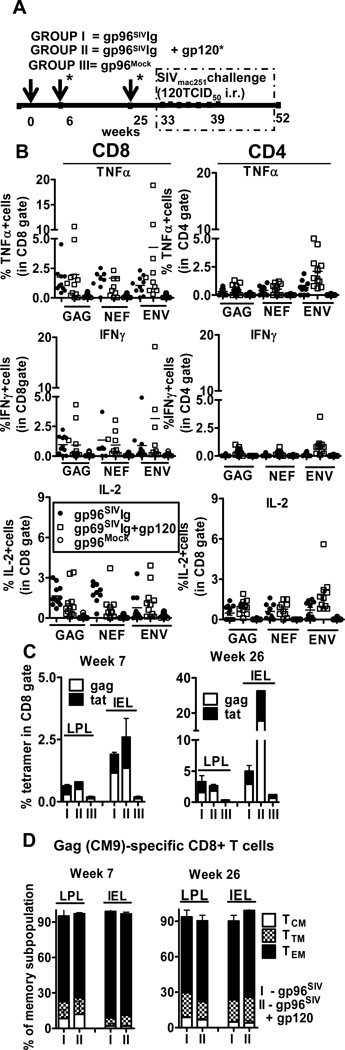
Figure 1. Gp96SIVIg+gp120 vaccines induce cellular immune responses.
(A) Schematics of the vaccination and challenge protocol. Details of vaccine composition and testing have been described in methods and . (B) Polyepitope specific rectal lamina propria CD8+ and CD4+ T cells secrete TNFα, IFNγ and IL-2 upon SIV-specific peptide stimulation. SIV-specific CD8 T cell responses at week 26 were detected using pools of 15-meric peptides overlapping by 11 amino acids covering the entire Gag, Nef, and Env proteins by multiparameter ICS assay. Intracellular staining for TNFα, IFNγ and IL-2 was performed on freshly isolated rectal lamina propria mononuclear cells from rectal pinch biopsies stimulated for 5 h with overlapping SIV peptides in the presence of monensin and brefeldin A. After gating on live, CD3+ CD8+ or CD3+CD4+T cells, frequency of cytokine positive cells was determined. (C) Vaccination induces gag- and tat-specific CD8+ T cells in lamina propria and intraepithelial compartment of rectal mucosa. Pinch biopsies from the rectal mucosa at week 7 and week 26 (5 days after 2nd and 3rd vaccination) were analyzed. SIV-specific CD8 T cells were detected by Mamu-A*01/Gag181–189 CM9 (CTPYDINQM; Gag-CM9) and Tat 28–35 SL8 (TTPESANL; Tat-SL8) tetramer staining. After gating on the CD8+ population, the percentage of tetramer-positive cells was determined. (D) Phenotype analysis of CD8+ SIV-gag+ T cells in lamina propria and intraepithelial compartment. The markers CD28 and CD95 define the central memory (TCM), transitional memory (TTM) and effector memory (TEM) among rhesus macaque T cells. TCM, TTM and TEM cells expressing CD28+CD95+, CD28+CD95− and CD28−CD95− phenotypes, respectively.
Vaccination was in week 0, 6 and 25, the immune response was determined in weeks 7 and 26. Potent MHC restricted CTL in IEL and LPL secreting multiple cytokines were generated in group I and II but not in controls (Fig. 1B–C) (16, 17). The gp96SIVIg vaccine in Rhesus macaques resulted in the preferential development of TEM in the lamina propria and epithelial layer (Fig. 1D) in agreement with our previous findings (7). SIV specific CD4 responses were also detected in gut lamina propria. Importantly, we observed an increase in the frequency of envelope specific CD4 responses (Fig 1B, open squares) only in the animals vaccinated with gp96SIV-Ig+gp120 indicating that MHC II presentation of gp120-derived peptides by DC required addition of gp120-protein (Fig. 1B).
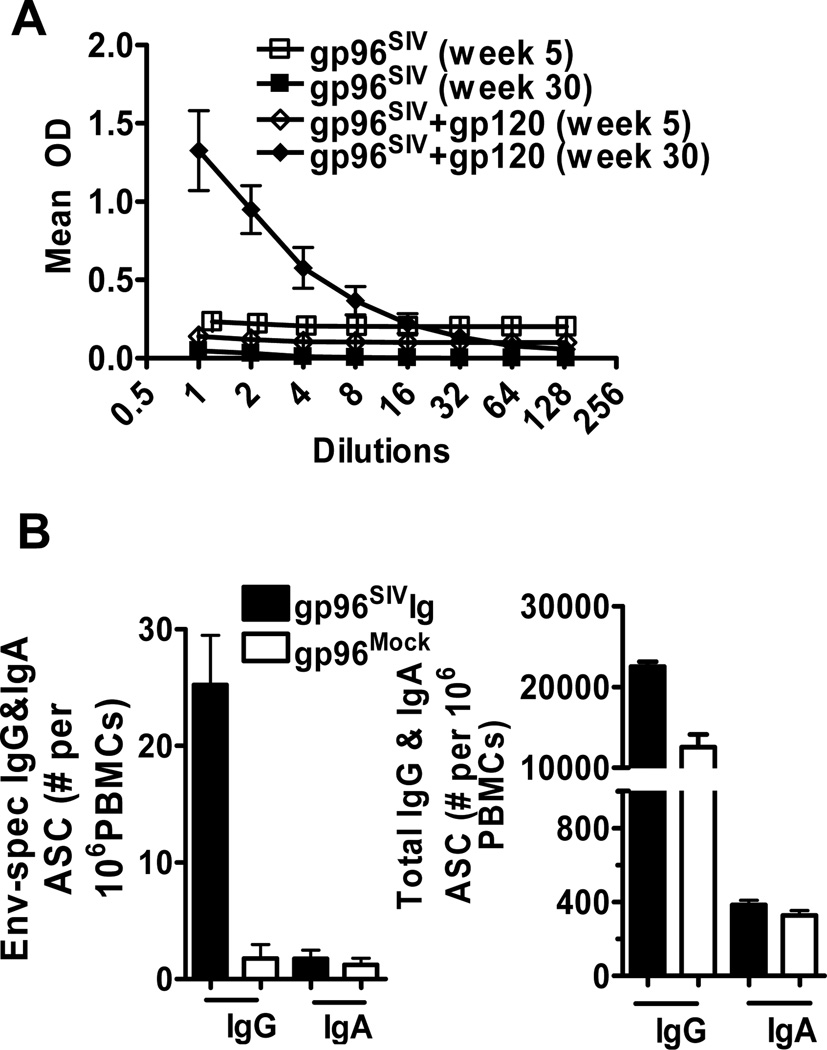
Elevated humoral immune responses were found only in group II as measured by ELISA for gp120-specific IgG and IgA antibodies (Fig. 2A) (18), by ELIspot assay for gp120-specific antibody secreting cells (Fig. 2B) (19) and by multi-parameter staining for plasmablasts (not shown) (20).
Figure 2. Gp96SIVIg+gp120 vaccines induce humoral immune responses.
(A) SIVmac251 gp120 ELISA at week 5 and 30 (B) SIVmac251 gp120 specific and total antibody secreting cells at week 26 was determined by ELISPOT. Error bars represent standard error of the mean (SEM)
Protective efficacy of the gp96SIVIg vaccines
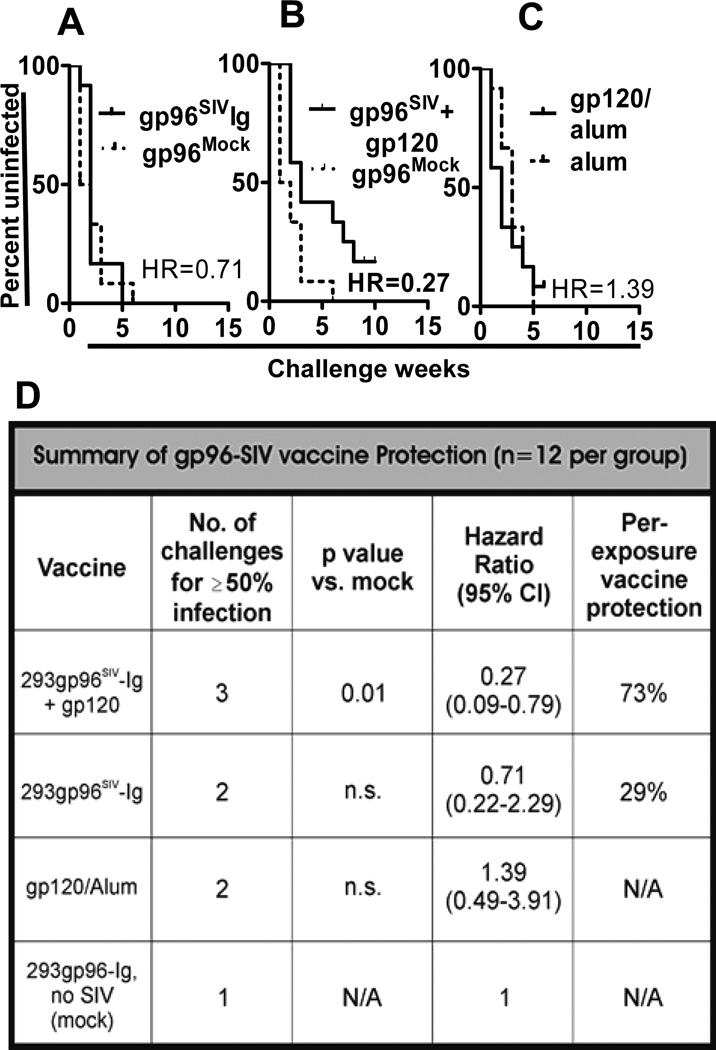
To evaluate the protective power of the immune response induced by gp96SIVIg vaccines, all 36 macaques were challenged starting at week 33 (8 weeks after the last vaccination) with up to seven weekly intrarectal instillations of SIVmac251 swarm virus, 120 TCID50 (NIH stock provided by Dr. Miller). Challenge of individual macaques was discontinued when they had positive virus titers in blood, assessed 5 days after each challenge. Intrarectal inoculation of 120TCID50 SIVmac251 generates 3–4 founder viruses in control, unvaccinated monkeys (unpublished Franchini et al.). Gp96SIVIg + gp120 vaccination (group II) induced statistically significant (p=0.01) protection against SIV acquisition. After 7 rectal challenges the hazard ratio was HR=0.27, 95% confidence interval CI(95) 0.09 to 0.79 calculated with the Graphpad/Prism statistics package, or HR=0.32, CI(95) 0.13 to 0.8 computed with exact resolution of ties in SAS 9.2 (Fig. 3A) corresponding to a vaccine efficacy of VE=73% or 68% (VE=100×[1-HR]). Protection was completely unaffected by the presence of TRIM5α or restrictive MHC alleles (not shown) confirming a previous report (21). In contrast, 50% of mock-control macaques (group III) became infected after the first challenge, compared with only 8.3% of the gp96SIVIg group I and 0% of the combined vaccine group II; for 50% infection, macaques in group I required two challenges, whereas those in group II required three challenges (Fig. 3D).
Figure 3. Protective efficacy of the gp96SIVIg vaccines.
Kaplan Meier plots of (A) number of challenges required for acquisition of infection in vaccine group I (gp96SIV) and (B) group II (gp96SIV+gp120) vs group III (gp96Mock) and (C) in animals that received only gp120 protein and alum. (D) Statistical analyses include the number of challenges required for 50% infection, hazard ratios (HR) with 95% confidence intervals (CI) and per-exposure vaccine efficacy in each group.
We observed that some animals had very high plasma virus titers on the day of first testing post challenge. For those with virus loads exceeding 106RNA copies/ml plasma, 8 in group I, 5 in group II and 2 in group III, it is possible that the animal had already been infected one week earlier but with as yet undetectable virus in blood. Rescoring data under this conservative assumption also gave results indicating significant protection in group II (HR=0.31; CI(95) 0.1 to 0.95 p=0.041, Mantel Cox) (Supplementary Figure S1C).
Gp96SIVIg alone (group I) did not provide significant protection (Fig. 3B). Likewise, adjuvanted gp120 alone is not protective (Franchini unpublished and Fig. 3C). Although infection did occur in most macaques vaccinated with the combined vaccine (group II) (Fig. 3B), viral acquisition required significantly more challenges than in the other groups (compare Fig. 3B, A), indicating a substantial degree of immunity. Gp120 protein in the vaccine cocktail was essential for the generation of antibody, antibody secreting cells (ASC) and plasmablasts in blood (Fig. 2A, B).
Infected macaques showed peak plasma virus loads on day 14 following infection (Supplementary Figure S1B) and then a relatively stable state of virus replication. Macaques vaccinated with gp96SIV-Ig+gp120 had a 1 log reduction of the mean peak virus load compared with mock controls at week 3 (p=0.048, Wilcoxon rank-sum test). Overall, however, vaccinated groups did not show significant virological control once infected (Supplementary Figure S1A).
Correlates of protection against acquisition of infection with the gp96SIVIg vaccines
Our data show that vaccination with gp96SIVIg alone is not protective even though it provides for potent antigen cross-presentation of SIV antigens generating CD8 CTL and little or no antibody (Fig 3A). Likewise, gp120-protein vaccination alone does not provide protection although it generates antibody and little CTL (Fig. 3C). Since the combined vaccine provides significant protection (Fig. 3B) and generates both CTL and antibody it is inescapable the both cellular and humoral immunity is required for protection. The data indicate that gp96-Ig serves as MHC II adjuvant for gp120 (Fig 1B). Since immunization takes place in a gp96-Ig created Th1 environment, antibody responses are likely to be polarized to IgG3 and IgG1. Isotyping of the antibody response in a protected macaque (Supplementary Figure S2D) showed predominant IgG3 and IgG1 isotypes.
Analysis of the correlation of protection with a mixed CTL/antibody response (Supplementary Figure S2A–C) is likely to reveal the effector component that limits the degree of protection. In this case it appears that antibody is limiting relative to CTL activity.
The primary goal of current efforts in HIV/SIV is to find a modality of vaccination that provides immunity to protect from infection by subsequent viral challenge. This goal has been elusive.
In this first test of the novel modality of cell secreted gp96-Ig-vaccination we have achieved a degree of significant protection in a highly pathogenic SIV-model that has not been seen in any study previously and is matched by only one recent report (22) using traditional vaccines.
Our next challenge is to improve the degree of protection to near 100%. The correlative analysis suggests that the CTL response to our vaccine is necessary and sufficient while the antibody response limits the degree of protection. This result provides a clear path for further development.
We are using the i.p. route because it gives the highest degree of mucosal CD8 CTL-based immunity compared to other routes (7) and therefore is the best basis for proof of concept studies. Upon achieving full protection of macaques, the final challenge will be to adapt the methodology to routes of vaccination that achieve comparable mucosal immunity and protection but are more suitable for human use. The applicability of our results using i.p. immunization to vaccine efficacy using clinically relevant routes of administration remains to be determined."
Supplementary Material
Acknowlegments
We thank B. Felber and G. Pavlakis for providing plasmid DNA; Nancy Miller for providing the SIVmac251 virus stock; NIH Nonhuman Primate Reagent Resource for macaque’s IgG isotype reagents. James L. Phillips from Flow Cytometry Core Facility, Sylvester Cancer Center University of Miami for expert help; J. Treece, D.Weiss, MG. Ferrari and P. Markham for animal husbandry and care; E. Lee for the quantitative analysis of viral RNA.
The work was carried out with support from the National Institute of Health NIAID R33 AI 073234 to ERP and Intramural Research Program of the NIH to GF, National Cancer Institute, Center for Cancer Research and support from the Alliance for Cancer Gene Therapy (ACGT), New York, to ERP
References
- 1.Vabulas RM, Braedel S, Hilf N, Singh-Jasuja H, Herter S, Ahmad-Nejad P, Kirschning CJ, Da Costa C, Rammensee HG, Wagner H, Schild H. The endoplasmic reticulum-resident heat shock protein Gp96 activates dendritic cells via the Toll-like receptor 2/4 pathway. J Biol Chem. 2002;277:20847–20853. doi: 10.1074/jbc.M200425200. [DOI] [PubMed] [Google Scholar]
- 2.Binder RJ, Han DK, Srivastava PK. CD91: a receptor for heat shock protein gp96. Nature immunology. 2000;1:151–155. doi: 10.1038/77835. [DOI] [PubMed] [Google Scholar]
- 3.Arnold D, Faath S, Rammensee H, Schild H. Cross-priming of minor histocompatibility antigen-specific cytotoxic T cells upon immunization with the heat shock protein gp96. J Exp Med. 1995;182:885–889. doi: 10.1084/jem.182.3.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singh-Jasuja H, Scherer HU, Hilf N, Arnold-Schild D, Rammensee HG, Toes RE, Schild H. The heat shock protein gp96 induces maturation of dendritic cells and down-regulation of its receptor. European journal of immunology. 2000;30:2211–2215. doi: 10.1002/1521-4141(2000)30:8<2211::AID-IMMU2211>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 5.Yamazaki K, Nguyen T, Podack ER. Cutting edge: tumor secreted heat shock-fusion protein elicits CD8 cells for rejection. J Immunol. 1999;163:5178–5182. [PubMed] [Google Scholar]
- 6.Oizumi S, Strbo N, Pahwa S, Deyev V, Podack ER. Molecular and cellular requirements for enhanced antigen cross-presentation to CD8 cytotoxic T lymphocytes. J Immunol. 2007;179:2310–2317. doi: 10.4049/jimmunol.179.4.2310. [DOI] [PubMed] [Google Scholar]
- 7.Strbo N, Pahwa S, Kolber MA, Gonzalez L, Fisher E, Podack ER. Cell-secreted Gp96-Ig-peptide complexes induce lamina propria and intraepithelial CD8+ cytotoxic T lymphocytes in the intestinal mucosa. Mucosal immunology. 2010;3:182–192. doi: 10.1038/mi.2009.127. [DOI] [PubMed] [Google Scholar]
- 8.Strbo N, Vaccari M, Pahwa S, Kolber MA, Fisher E, Gonzalez L, Doster MN, Hryniewicz A, Felber BK, Pavlakis GN, Franchini G, Podack ER. Gp96 SIV Ig immunization induces potent polyepitope specific, multifunctional memory responses in rectal and vaginal mucosa. Vaccine. 2011;29:2619–2625. doi: 10.1016/j.vaccine.2011.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu J, O'Brien KL, Lynch DM, Simmons NL, La Porte A, Riggs AM, Abbink P, Coffey RT, Grandpre LE, Seaman MS, Landucci G, Forthal DN, Montefiori DC, Carville A, Mansfield KG, Havenga MJ, Pau MG, Goudsmit J, Barouch DH. Immune control of an SIV challenge by a T-cell-based vaccine in rhesus monkeys. Nature. 2009;457:87–91. doi: 10.1038/nature07469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Letvin NL, Rao SS, Montefiori DC, Seaman MS, Sun Y, Lim SY, Yeh WW, Asmal M, Gelman RS, Shen L, Whitney JB, Seoighe C, Lacerda M, Keating S, Norris PJ, Hudgens MG, Gilbert PB, Buzby AP, Mach LV, Zhang J, Balachandran H, Shaw GM, Schmidt SD, Todd JP, Dodson A, Mascola JR, Nabel GJ. Immune and Genetic Correlates of Vaccine Protection Against Mucosal Infection by SIV in Monkeys. Science translational medicine. 3 doi: 10.1126/scitranslmed.3002351. 81ra36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hansen SG, Ford JC, Lewis MS, Ventura AB, Hughes CM, Coyne-Johnson L, Whizin N, Oswald K, Shoemaker R, Swanson T, Legasse AW, Chiuchiolo MJ, Parks CL, Axthelm MK, Nelson JA, Jarvis MA, Piatak M, Jr, Lifson JD, Picker LJ. Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature. 473:523–527. doi: 10.1038/nature10003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, Premsri N, Namwat C, de Souza M, Adams E, Benenson M, Gurunathan S, Tartaglia J, McNeil JG, Francis DP, Stablein D, Birx DL, Chunsuttiwat S, Khamboonruang C, Thongcharoen P, Robb ML, Michael NL, Kunasol P, Kim JH. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. The New England journal of medicine. 2009.;361:2209–2220. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 13.Barouch DH, O'Brien KL, Simmons NL, King SL, Abbink P, Maxfield LF, Sun YH, La Porte A, Riggs AM, Lynch DM, Clark SL, Backus K, Perry JR, Seaman MS, Carville A, Mansfield KG, Szinger JJ, Fischer W, Muldoon M, Korber B. Mosaic HIV-1 vaccines expand the breadth and depth of cellular immune responses in rhesus monkeys. Nature medicine. 16:319–323. doi: 10.1038/nm.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haynes BF, Gilbert PB, McElrath MJ, Zolla-Pazner S, Tomaras GD, Alam SM, Evans DT, Montefiori DC, Karnasuta C, Sutthent R, Liao HX, DeVico AL, Lewis GK, Williams C, Pinter A, Fong Y, Janes H, DeCamp A, Huang Y, Rao M, Billings E, Karasavvas N, Robb ML, Ngauy V, de Souza MS, Paris R, Ferrari G, Bailer RT, Soderberg KA, Andrews C, Berman PW, Frahm N, De Rosa SC, Alpert MD, Yates NL, Shen X, Koup RA, Pitisuttithum P, Kaewkungwal J, Nitayaphan S, Rerks-Ngarm S, Michael NL, Kim JH. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. The New England journal of medicine. 366:1275–1286. doi: 10.1056/NEJMoa1113425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McMichael AJ, Haynes BF. Lessons learned from HIV-1 vaccine trials: new priorities and directions. Nature immunology. 13:423–427. doi: 10.1038/ni.2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hansen SG, Vieville C, Whizin N, Coyne-Johnson L, Siess DC, Drummond DD, Legasse AW, Axthelm MK, Oswald K, Trubey CM, Piatak M, Jr, Lifson JD, Nelson JA, Jarvis MA, Picker LJ. Effector memory T cell responses are associated with protection of rhesus monkeys from mucosal simian immunodeficiency virus challenge. Nature medicine. 2009;15:293–299. doi: 10.1038/nm.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vaccari M, Boasso A, Ma ZM, Cecchinato V, Venzon D, Doster MN, Tsai WP, Shearer GM, Fuchs D, Felber BK, Pavlakis GN, Miller CJ, Franchini G. CD4+ T-cell loss and delayed expression of modulators of immune responses at mucosal sites of vaccinated macaques following SIV(mac251) infection. Mucosal immunology. 2008;1:497–507. doi: 10.1038/mi.2008.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brocca-Cofano E, McKinnon K, Demberg T, Venzon D, Hidajat R, Xiao P, Daltabuit-Test M, Patterson LJ, Robert-Guroff M. Vaccine-elicited SIV and HIV envelope-specific IgA and IgG memory B cells in rhesus macaque peripheral blood correlate with functional antibody responses and reduced viremia. Vaccine. 2011;29:3310–3319. doi: 10.1016/j.vaccine.2011.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Florese RH, Demberg T, Xiao P, Kuller L, Larsen K, Summers LE, Venzon D, Cafaro A, Ensoli B, Robert-Guroff M. Contribution of nonneutralizing vaccine-elicited antibody activities to improved protective efficacy in rhesus macaques immunized with Tat/Env compared with multigenic vaccines. J Immunol. 2009;182:3718–3727. doi: 10.4049/jimmunol.0803115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wrammert J, Smith K, Miller J, Langley WA, Kokko K, Larsen C, Zheng NY, Mays I, Garman L, Helms C, James J, Air GM, Capra JD, Ahmed R, Wilson PC. Rapid cloning of high-affinity human monoclonal antibodies against influenza virus. Nature. 2008;453:667–671. doi: 10.1038/nature06890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fenizia C, Keele BF, Nichols D, Cornara S, Binello N, Vaccari M, Pegu P, Robert-Guroff M, Ma ZM, Miller CJ, Venzon D, Hirsch V, Franchini G. TRIM5alpha does not affect simian immunodeficiency virus SIV(mac251) replication in vaccinated or unvaccinated Indian rhesus macaques following intrarectal challenge exposure. J Virol. 2011;85:12399–12409. doi: 10.1128/JVI.05707-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barouch DH, Liu J, Li H, Maxfield LF, Abbink P, Lynch DM, Iampietro MJ, Sanmiguel A, Seaman MS, Ferrari G, Forthal DN, Ourmanov I, Hirsch VM, Carville A, Mansfield KG, Stablein D, Pau MG, Schuitemaker H, Sadoff JC, Billings EM, Rao M, Robb ML, Kim JH, Marovich MA, Goudsmit J, Michael NL. Vaccine protection against acquisition of neutralization-resistant SIV challenges in rhesus monkeys. Nature. 2012;482:89–93. doi: 10.1038/nature10766. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.