Abstract
Purpose
Nearly half of cancer metastases become clinically evident five or more years after primary tumor treatment; thus metastatic cells survived without emerging for extended periods. This dormancy has been explained by at least two countervailing scenarios: cellular quiescence and balanced proliferation; these entail dichotomous mechanistic etiologies. To examine the boundary parameters for balanced proliferation, we performed in silico modeling.
Experimental Design
To illuminate the balanced proliferation hypothesis, we explored the specific boundary probabilities under which proliferating micrometastases would remain dormant. A two-state Markov chain Monte Carlo model simulated micrometastatic proliferation and death according to stochastic survival probabilities. We varied these probabilities across 100 simulated patients each with 1,000 metastatic deposits and documented whether the micrometastases exceeded one million cells, died out, or remained dormant (survived 1,218 generations).
Results
The simulations revealed a narrow survival probability window (49.7 – 50.8 percent) that allowed for dormancy across a range of starting cell numbers, and even then for only a small fraction of micrometastases. The majority of micrometastases died out quickly even at survival probabilities that led to rapid emergence of a subset of micrometastases. Within dormant metastases, cell populations depended sensitively on small survival probability increments.
Conclusions
Metastatic dormancy as explained solely by balanced proliferation is bounded by very tight survival probabilities. Considering the far larger survival variability thought to attend fluxing microenvironments, it is more probable that these micrometastatic nodules undergo at least periods of quiescence rather than exclusively being controlled by balanced proliferation.
Keywords: Metastatic dormancy, balanced proliferation, Markov chain Monte Carlo, Branching process, Metastatic outgrowth
INTRODUCTION
The dormancy dilemma impacts millions of cancer patients worldwide. Certain epithelial carcinomas such as from breast or prostate tissues can recur years or decades after primary tumor ablation (1). This late recurrence afflicts nearly 50% of breast cancer patients that develop metastases; other carcinomas exhibit similar late recurrence rates (2). This recurrence is most problematic within metastatic organs such as the liver, lung, and brain where tumor resection may not be possible and chemotherapeutic agents appear to be ineffective in most cases (3, 4). For instance, aggressive treatment (e.g. adjuvant chemotherapy) of breast cancer after surgical removal of the evident primary tumor may reduce 10-year recurrence by less than one-third. Consequently, ostensibly cured cancer patients harbor unseen metastases for decades that eventually emerge into untreatable, lethal tumors. This long period between primary tumor treatment and metastatic appearance is referred to as metastatic latency though the tumor cell behavior during this period is unknown.
Metastatic latency allows disseminated tumor cells to avoid clinical detection and withstand aggressive and toxic neoadjuvant treatment (5). How the disseminated carcinoma cells maintained a long-term, clinically undetectable state in an ectopic site such as the liver is unknown (6). Although extravasation of carcinoma cells from the primary tumor is common, but most of these cells seem not to establish metastases (7, 8), it is likely that the pre-metastatic microenvironment contributes to whether carcinoma cells will seed, survive, and either proliferate to prompt tumor formation or exhibit a dormant phenotype.
Because in vitro and in vivo experimental systems are not sufficiently malleable to consistently and reliably recapitulate the metastatic latency phenotype, in silico models have evolved to explore the mechanisms of metastatic competency, dormancy and emergence (9–11). Many of these in silico models predominately simulate metastatic latency according to the theory of balanced proliferation among micrometastases of greater than one million cells. Therefore they introduce various biological factors such as angiogenesis, immune response, hypoxia, and growth factor availability and deduce through partial differential equations what impact these have on the ability for the micrometastases to emerge or maintain a dormant state. A major limitation to this approach is that little empirical biological data are available to make these models translatable to the in vivo response. Moreover, such studies often assume that latent metastases experience balanced proliferation, meaning additional biological data, even if available, would not resolve latency as being due to balanced proliferation, cellular quiescence or a combination of both.
Notwithstanding these limitations, in silico models are becoming increasingly important to offering insights in prioritizing the landscape of contributing metastatic factors. By using an Approximate Bayesian Computation model applied to empirical patient data Willis et al inferred that metastatic breast cancer late relapse evolved from just 1 to 6 micrometastases that escape from dormancy (12). Other approaches used the Gompertzian growth function to calculate periods of metastatic growth and growth arrest to fit clinical data as presented in the Munich Cancer Registry (13). These results predicted that metastatic seeding occurred before the clinical detectability of the primary tumors. Computational models have also been used to predict the metastatic response according to primary tumor removal and front line chemotherapy (14).
Biologically, the issue of metastatic dormancy has been approached as balanced proliferation and death, quiescence, or a combination of both. In arguing for balanced proliferation and death, investigators invoke various constraints, such as the ‘angiogenic switch’, prior to which the metastatic nodule is constrained to a certain mass in the absence of a vascular supply (2, 15). In other considerations, multiple feedback mechanisms are invoked to maintain a small, but cycling cell population (16). The cellular and/or molecular mechanisms behind tumor dormancy present with an absence of solid clinical data, though emerging evidence of phenotypic plasticity suggests some other possible routes to dormancy and may suggest quiescence of the disseminated tumor cells.
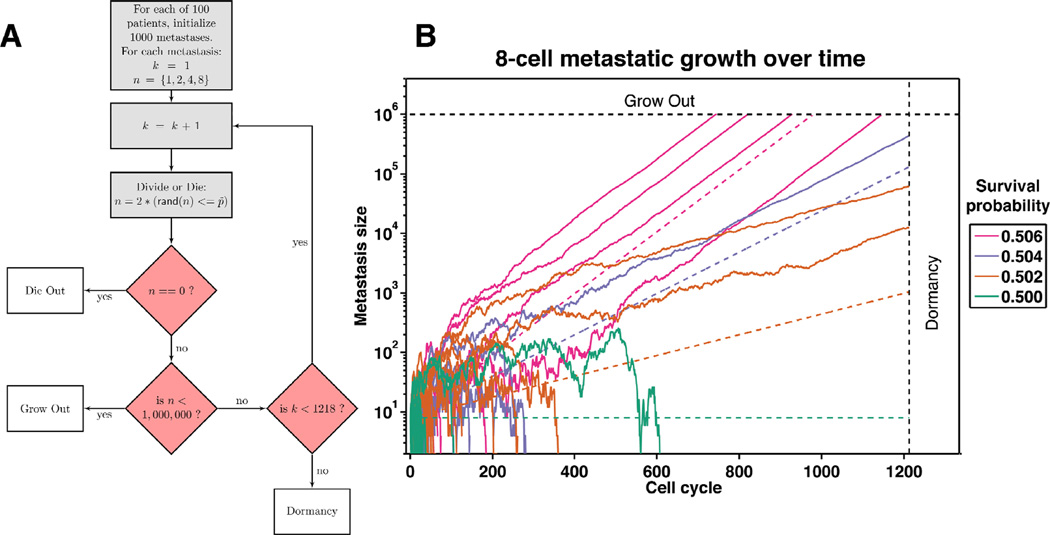
Thus, the situation remains unsettled as to which behavior predominates during these latent periods. To take an unbiased approach, we modeled metastatic growth dynamics by invoking a two state, Markov chain Monte Carlo (MCMC) simulation where only cell-survival probability and starting metastasis populations were varied. As depicted in Figure 1, the MCMC method assigns a survival probability for each cell in each metastasis during each replication cycle. Cells will then either proliferate or die as statistically influenced by the survival probabilities allowing for metastases to exhibit a range of outcomes not predetermined. If metastatic dormancy can emerge across a wide spectrum of survival probabilities then balanced proliferation may be the dominant contributor. However, if the latent phenotype via balanced proliferation is only manifest within a narrow probability window we propose that cellular quiescence would likely participate nontrivially in protracted metastatic survival.
Figure 1.
Metastatic cell fates are determined via a Markov chain Monte Carlo method developed in the Matlab programming environment. Panel A depicts the simulation’s progression starting with 1,000 metastases initialized for each of the 100 patients. Within each simulation, there exists a starting cell number denoted by n (1, 2, 4, or 8) across survival probabilities ranging from 30 to 70 percent. Starting with the first cell cycle (denoted by k) a random number is generated and compared to the survival probability. If n is equal to zero, the metastasis dies out, and the next metastasis simulation (up to 1000) begins. If n reaches 1 million, the metastasis is assumed to grow out to be clinically evident. If k reaches 1,218 while n is greater than zero, the metastasis goes dormant. Panel B traces the fate of 100 individual metastases across four survival probabilities, demonstrating the variability in metastatic progression. Once metastases reach approximately 1,000 cells the growth rate equals the expected growth rate as depicted by the dotted lines.
METHODS
Markov Chain Monte Carlo (MCMC) Model Overview (Figure 1)
Markov chain Monte Carlo simulation is a computational method that samples from a probability distribution in order to assign system outcomes commensurate with the underlying distribution (17). This model is described as a branching process whereby each cell in cell cycle k will randomly give rise to zero or two cells in the k + 1 cell cycle. We applied MCMC simulation as a means to model the fate of extravasated circulating tumor cells into the metastatic site. We developed two Markov chain Monte Carlo approaches to assign cell fates. The first, a fixed probability model, assigned static survival probabilities to every cell in each metastatic nodule; the probability of survival for each individual cell was not dependent on the other cells in that metastatic nodule.
The second approach incorporated a stochastic element where the survival probability for any given cell was sampled randomly between +/− 10 percentage points around the specified mean survival probability for the group. We refer to this variable probability as p~ where appropriate to distinguish it from the assigned probability p. All results herein were developed from the second, stochastic model, diagrammed in Figure 1A. Both formulations, however, are examples of branching processes, which allow for convenient analytic expressions for some aspects of tumor behavior. In our two-state MCMC system, every cell in each metastasis could either divide or die per cell cycle, thus mimicking the balanced proliferation phenotype. Notably, cells were individually assigned a new survival probability at each cycle with the stochastically assigned probability adjustment within 10 percentage points above or below the initially assigned survival probability. For example, simulations at a fixed 60 percent survival probability will allow for random fluctuations between 50 and 70 percent survival. Although these survival probabilities coalesced to the expected means, this additional percentage point adjustment further imitated the dynamic survival conditions of the metastatic niche.
These simulated micrometastases could devolve to zero cells (died out), exceed one million cells (clinical metastasis) or exceed 1,218 cycles with fewer than one million cells (dormant). Our data represent one hundred compiled patient trials (100,000 micrometastases) for each combination of cell survival rate and starting cell number. For the progressive clinical metastases, we capture the range of cycles at which this occurred. For the dormant metastases we document the mean cell numbers at the time of dormancy. Although this model does not achieve pseudo-equilibrium given all simulated metastases terminate under one of 3 conditions, dormancy is nonetheless modeled by means of achieving neither the zero or 1 million cell boundary before reaching 1,218 cycles; though it is likely that should there be no cycle limit, the cell boundaries will be reached.
Assumptions
We simulated patients in groups of one hundred person cohorts across a matrix of cell survival probabilities (0.3 to 0.7, given as percentages) and number of initially extravasated cells. For each patient we modeled one thousand carcinoma deposits to the liver at the time of primary tumor resection. The probability of an outgrowth is based on individual micrometastasis and not on patient load of total micrometastases. Each deposit started with one, two, four, or eight cells (later at 1000 and 4000 cells for boundary validation) so as to separate initial extravasation and seeding from subsequent metastatic behavior. We also assumed a one-and-one-half to three-day cell cycle so that 1,218 cell cycles represented five to ten person years (dormancy) at a cycle time of 1.5–3 days per mitosis. Micrometastases that grew beyond one million cells were classified as approaching clinically evident and were not simulated further. Finally, we assumed that the initial one thousand micrometastatic deposits did not give rise to additional deposits and were free to either die out, grow out to greater than one million cells, or become dormant. Representative traces of micrometastatic-tumor growth for four survival probabilities (50.0, 50.2, 50.4, and 50.6) are shown in Figure 1B.
RESULTS
Survival after metastatic extravasation is rate limiting
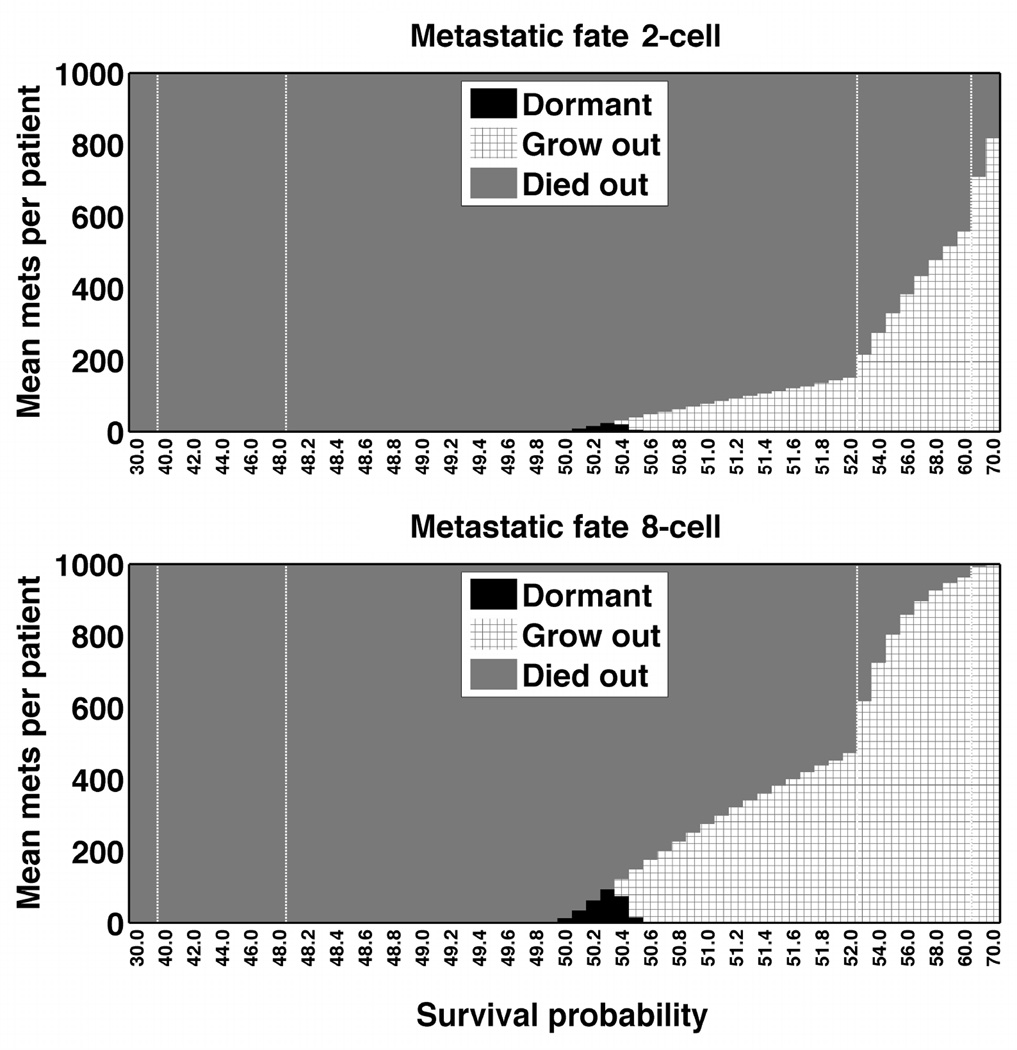
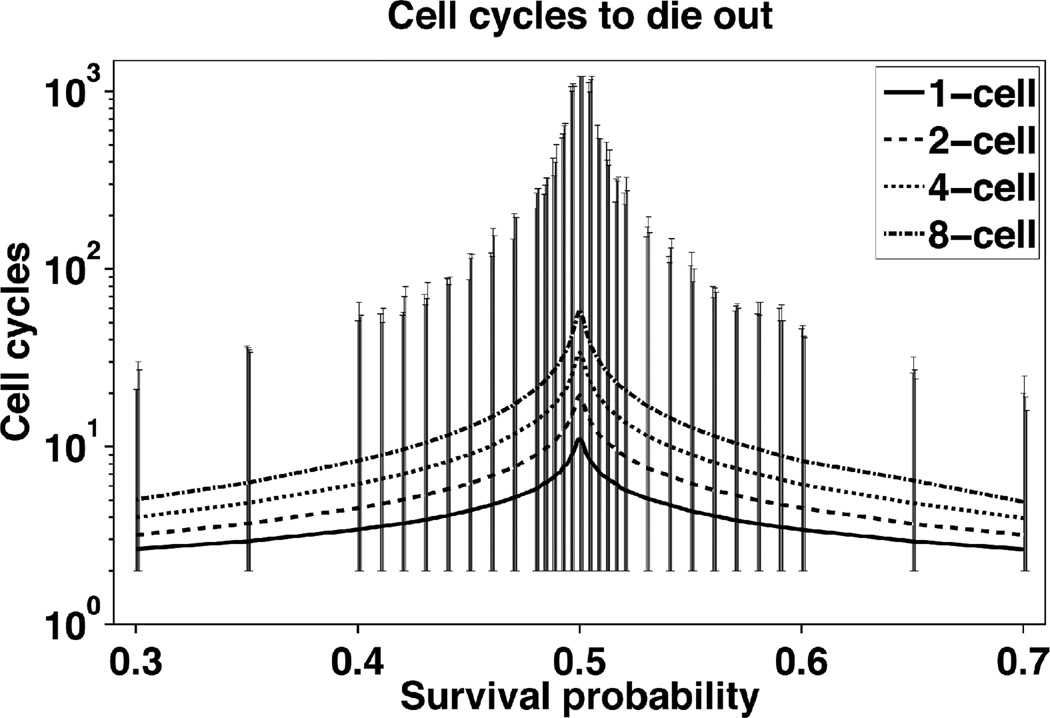
The metastatic fate across all simulated patient trials for 1, 2, 4, and 8 cell starting metastases revealed that the majority of metastases died out until the survival probability exceeded 60% (Figure 2). Sampled time courses of metastatic tumor growth illustrate this phenomenon, even for probabilities above 50% (Figure 1B). Eight-cell starting metastases conferred a grow-out advantage particularly above the 60% survival probability whereas one-cell starting metastases still exhibited metastatic abatement in that range. Of those metastases that die out, they do so quickly in fewer than 60 cycles (Figure 3). Dormant metastases represented a small fraction and were tightly bounded by a small survival probability.
Figure 2.
Metastatic fate for 2-cell (top) and 8-cell (bottom) starting metastases followed identical trends with the majority of metastases dying out. The metastatic fate is coded with black designating the metastases that remained dormant, grey the ones that died out, and patterned those that grew out. X-axis represents survival probability from 30 to 70 percent with vertical bars representing incremental probability rate changes. Shown are stochastic models; the fixed survival percentages exhibited similar fates (data not shown).
Figure 3.
Micrometastases that die do so quickly when survival probability is low or high. As p increases mets are, on average, surviving longer, even though most still die. The symmetric, decreasing curves capture results from the increased likelihood of cell survival, meaning that those that do die out must converge to that fate quickly. Vertical bars indicate cell survival maxima and minima for non-surviving metastases. Only near the dormancy survival ranges do micrometastases have marked increases in cell cycles to die out, but still well below 1,218 cycles.
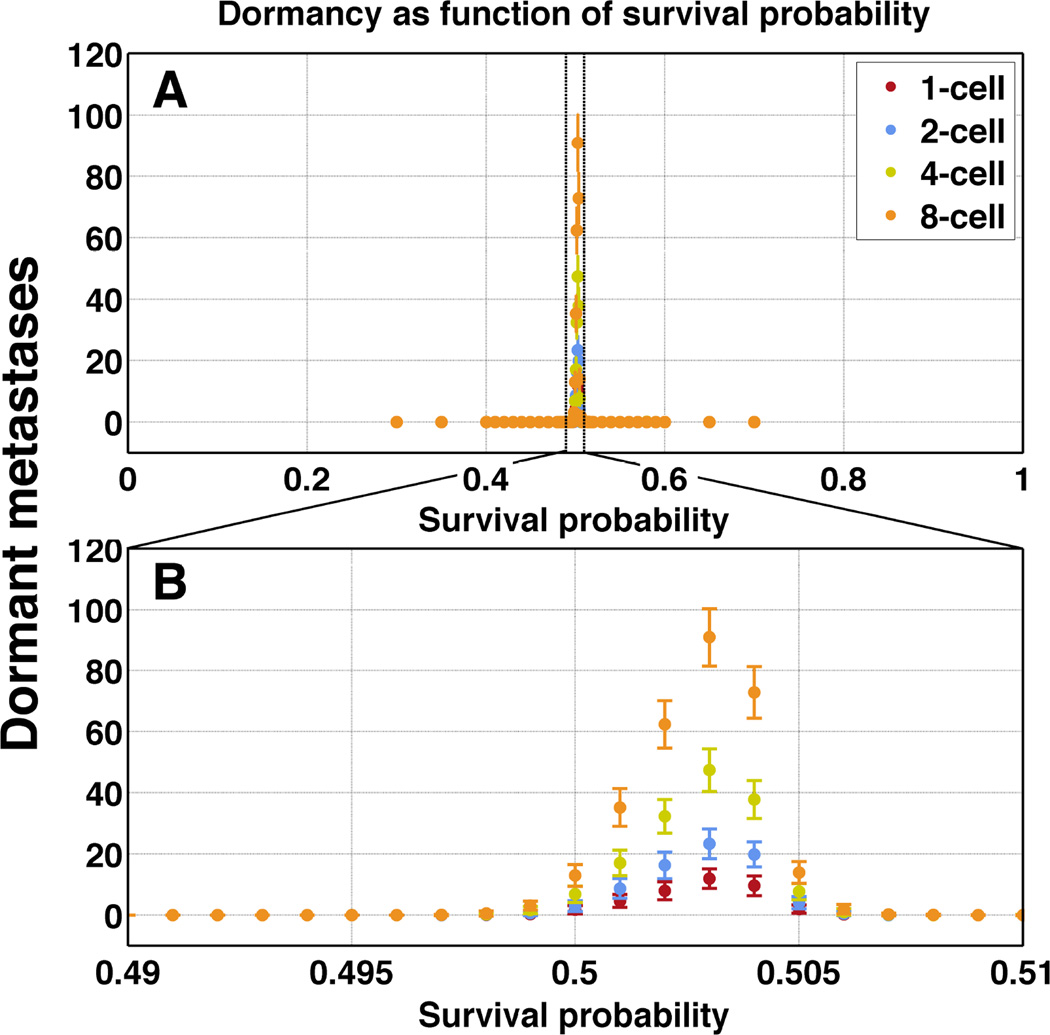
Dormant metastases arise only for survival probabilities proximal to 50 percent
Metastatic dormancy was a rare outcome that manifested only between 49.7 and 50.8 percent survival probability (Figure 4, top panel). Starting with one versus eight cells in the micrometastases did not impact this survival probability range. However, the mean number of dormant metastases increased as the starting cell number increased, up to a maximum of 80 mean dormant metastases per patient (still less than 10 percent) for eight-cell simulations (Figure 4, bottom panel). In a small series of 1000- or 4000-cell micrometastases the boundary percentages were within the same narrow range.
Figure 4.
Dormancy only manifests between 49.7 to 50.8 percent survival probability. Mean number of dormant metastases per 1000 initial nodules (ordinate) increases as starting cell number increases, though they remain bounded by the same restraints in terms of survival probabilities (abscissa). Panel B is an enlarged view of the narrow probability window indicated in panel A.
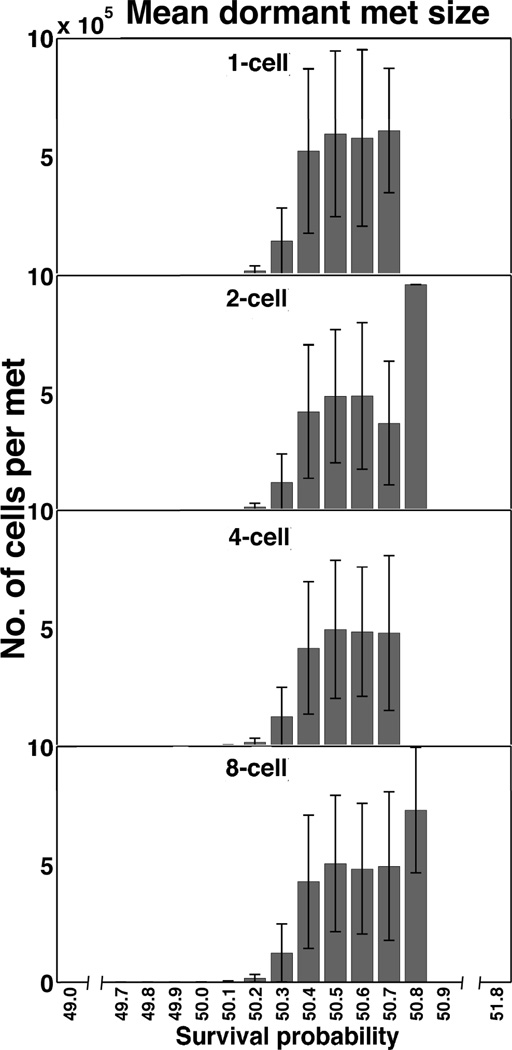
Dormant metastases exhibited a large range in cell numbers
Although dormancy was tightly bounded proximal to the 50 percent survival probability, a dramatic variance of the mean dormant metastatic size was highly sensitive to small changes in survival probability (Figure 5). Half of the survival range (between 49.7 and 50.2 percent) yielded dormant metastases comprised of fewer than a few thousand cells. Interestingly, between 50.3 and 50.7 percent survival probabilities the populations peaked at around 500,000 cells. Not until the 50.8 percent survival probability did the dormant metastases (a single metastasis for 2-cell initialization, 3 metastases for the 8-cell case) approach the outgrowth condition – reaching nearly one million cells.
Figure 5.
Dormant metastases exhibit a wide range of cell numbers per metastasis. The number of cells in each dormant nodule (ordinate) spanned from hundreds to nearly one million cells, with similar results between 1-cell to 8-cell starting metastases across the survival percentages. This range is highly sensitive to small changes in survival probability.
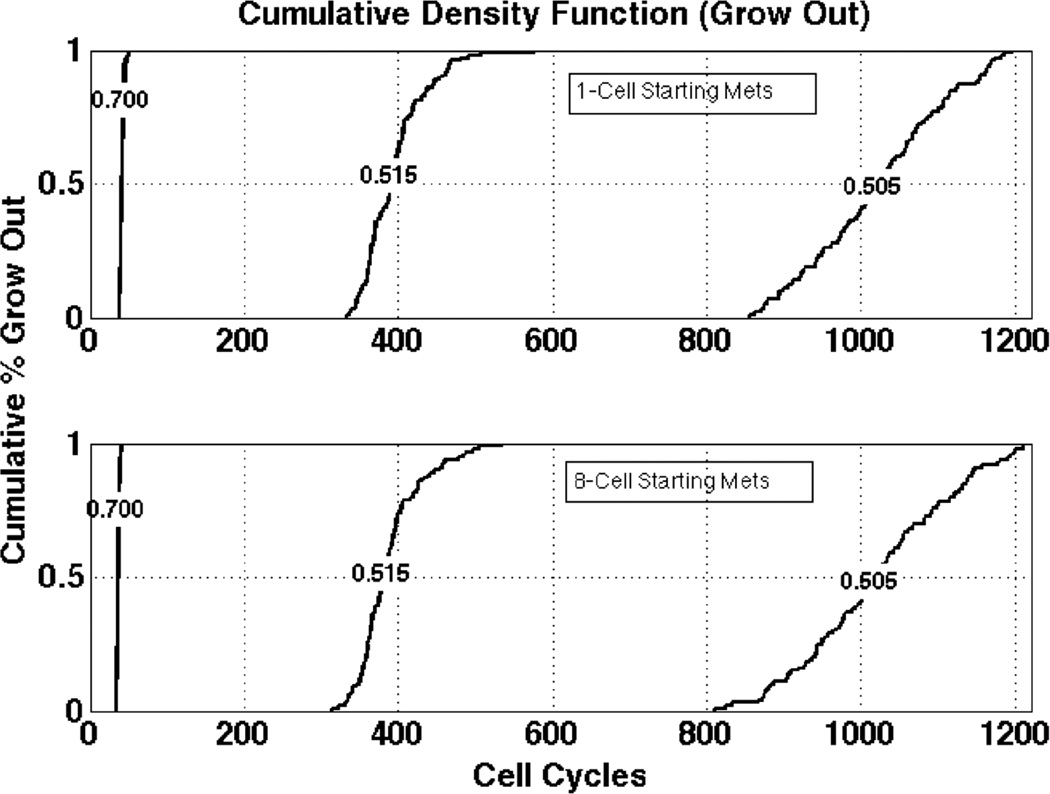
Metastatic outgrowth was highly sensitive to small changes to survival probability
The cumulative density function in Figure 6 compares 1-cell versus 8-cell starting metastases to the outgrowth condition. At the 70 percent survival probability, all of the metastases that grew out did so in less than 50 cycles. In contrast, half the metastases grew out in approximately 400 cycles for the 51.5 percent survival probability. Interestingly, at just above the 50 percent survival probability, a one-percentage point increase in survival probability resulted in the required number of cycles for the surviving metastases to achieve outgrowth being reduced by two-thirds.
Figure 6.
Cycles to outgrowth between 1 and 8 cell metastases. The cumulative fraction (ordinate) of micrometastases that grow out as a function of survival probability leads to fewer cycles (abscissa) with even a 1-percentage point increase in cell survival reducing outgrowth time by nearly two thirds.
DISCUSSION
Research into metastatic latency has rapidly evolved over the past decade given the high prevalence of this condition and the lack of effective clinical interventions. Unfortunately, this phenomenon is difficult to recapitulate across in vitro and in vivo experimental systems. Moreover, it is not certain whether metastatic latency results from small deposits of quiescent carcinoma cells that had recently extravasated into the metastatic site or whether small- to mid-sized micrometastases maintain clinically undetectable sizes based on balanced proliferation and cell death (or a combination of explanations). This distinction is critical as therapeutic approaches would be different for either condition: that of targeting proliferating or quiescent cells.
Because of the metastatic latency prevalence, patients are confronted with the dilemma of undergoing extensive and toxic systemic treatments for putative metastases based on the characteristics of the primary nodule and statistical considerations of similar patients that experienced metastatic latency. While the ability to predict which patients will suffer metastatic recurrence has improved (18), the current approach to these dreaded sequelae only reduces the rate by a third, while evoking untoward toxicity including hair loss, neurological impairments, opportunistic infections, bleeding, and death. As most of the therapy is ineffective, we need to characterize the status of the disseminated tumor cells prior to their frank metastatic emergence so as to more effectively target them. What is not known is whether these few surviving cells exist in cellular quiescence or undergo balanced proliferation to engage the dormant phenomenon. This distinction has immense implications, as predominantly only cycling cells are susceptible to chemotherapy, and is therefore the focus of this paper.
Given these limitations, many in silico models have been developed that may help prioritize or frame the metastatic etiology (19, 20). For example, Michor et al computed the probability of metastatic dissemination from primary tumors based on oncogenic mutations in RAS, ERBB2/NEU or MYC (21). Implementing a stochastic Moran process the authors simulated a single genetic mutation that gave rise to neutral, advantageous or disadvantageous metastatic potential. These simulations computed the temporal accumulation of metastases as a function of primary tumor size. Further model development eventually may help clinicians validate whether to treat more aggressively with adjuvant therapy. Another study implemented a Voronoi tessellation approach to model the impact heterogeneous or homogenous extracellular matrix (ECM) stiffness had on metastatic and primary tumor growth kinetics (9). Whereas non-rigid, homogenous ECM gave rise to isotropic tumors and rigid homogenous ECM invoked anisotropic morphologies, a heterogeneous ECM stiffness exhibited both properties, but in a predicable manner. Consequently this modeling if matured may assist clinicians in determining which metastatic tumor-adjacent tissue (in critical metastatic organs such as the brain) may likely harbor disseminated tumor cells – leading to either more or less aggressive tissue resection. Although these models have promise to improve clinical treatments there is a dearth of modeling within the very early stages of metastatic extravasation.
The model presented here invokes explicit representation of each cell and leverages repeated computed trials to sample the distributions of die out, grow out, and dormancy phenotypes exactly. However, analytic formulations called branching processes can also be applied to derive convenient predictions for this type of growth (22). These models exploit the fact that daughter cells are independent from one another and thus behave as if individual metastases (branching processes) themselves, lending to a compact, recursive formulation: hn(t) = [fn(t)]N. Here, h(t) is the 'probability generating function' (pgf) at cell-cycle n, and is computed as the product of N identical pgf's (for N starting cells in the micrometastatic tumor) (23). The pgf allows us to quickly compute the expected cell population after n cell cycles , the expected variance , and the probability of eventual extinction . In all expressions, the inputs are the number of starting cells, N, the survival probability, p, and the number of cell cycles, n, just as in our stochastic simulation model. The recursive formula above cannot yield the exact population distribution for any large n (23), such as our cell cycle limit of 1,218. That is, while we can predict dying out and survival, we cannot exactly predict dormancy with the model, for which simulations are required.
Metastatic lesions are visible through imaging modalities such as MRI when they approach 0.1 cubic centimeter. A tumor of this small size actually consists of approximately 100 million cells (24). Therefore, disseminated tumor cell deposits of less than this cannot be detected without invasive biopsy and subsequent analyses such as immunohistochemistry. Because metastatic latency can be explained by two very different mechanisms – that of cellular quiescence or proliferation offset by cell death (or a combination of both), we sought to model the second hypothesis and determine what survival probability range would give rise to the dormant phenotype. We simulated 100 patients each with 1000 metastatic deposits (1, 2, 4, and 8-cell starting sizes) across stochastic survival probabilities discontinuous between 30 and 70 percent. The modeling revealed a very narrow survival probability range between 49.7 and 50.8 percent that gave rise to dormant metastases. The reason why this dormancy probability window is so small is based upon how sensitive simulated metastases are to small changes in survival probability. If the probability of survival is less than 50 percent, the metastases all die out with the exception of the few dormant clusters because the probability favors the boundary condition of dying out. Only a rare escape from this tendency will therefore evoke a dormant metastasis. Once the probability of survival exceeds 50.8 percent, the grow-out condition of reaching 1 million cells begins to dominate. Thus, we conclude that it is unlikely that metastatic dormancy is governed predominantly by internally balanced proliferation and cell death. As shown in Figure 3, it may seem unexpected that micrometastases will die out even at survival probabilities exceeding 70 percent. The reason for this phenomenon is closely related to the number of starting cells in the metastases. It is difficult for a simulated metastasis to live through its first few generations especially when it has two starting cells; because when a cell dies the metastasis evolves closer to the boundary of dying out. As Figure 2 demonstrates, the chance of a metastasis dying out is reduced when there are eight starting cells versus two starting cells. Of the metastases that die out, they do so in a short time (Figure 3) as this relates to the fact that the metastases start near the null boundary and thus are easy to involute early before attaining a greater cell number. An often suggested model involves micrometastases growing and then being constrained at a subclinical size by the need for external supports; this is the basis of the postulated ‘angiogenic switch’ (25). However, even here the constraints must be tightly controlled; in an initial series of examining metastases of 1000 or 4000 starting cells, the range for staying dormant remained constrained similarly to the smaller micrometastases at between 49.5% and 51% survival probabilities; if the survival percentage drops below this, the metastatic nodules quickly die out (data not shown). Thus, even this tumor extrinsic constraint would need to be tightly controlled or frequently vacillate between outgrowth and involution. In the spirit of Occam’s razor, we propose that extended periods of cellular quiescence most likely accounts for this dormant period.
While there are numerous models for dormancy these suffer from significant differences in the animal hosts, such as life-span and time-scale to development, and a lack of information about the micrometastatic state in actual cancer patients. This approach does not account for the richness of interactions and signals that may constrain or support micrometastatic nodules. For instance, it is possible that an ‘angiogenic switch’ is required for outgrowth once these nodules reach a certain size. However, the experimental data are lacking currently to build models incorporating such inputs. Rather, such models would presuppose these constraints and be built to account for the limitations and interventions (26). Herein, we chose an a priori unbiased calculation to simply define the boundary conditions of metastatic outgrowth regardless of the actual biological and physiological networks.
The value of mathematical models lies in their ability to suggest avenues for investigation. Recent findings of tumor cells in circulation or bone marrow despite no evidence of metastases may provide some insights into the behavior of the early metastatic cells. Interestingly, ongoing proliferation is evident only in few of these cells (27) consistent with quiescence of these cells. If these cells were truly quiescent then therapies targeted to killing growing cells would not be effective and may even cause outgrowth if the dormant microenvironment is perturbed. Still such findings are only suggestive, and highlight the need to examine human micrometastases with innovative technologies to determine the actual proliferative rates in these hidden metastases. As our currently available chemotherapy mainly attacks cycling cells, the mode of quiescence would make this treatment less effective and necessitate new approaches to the problem of metastatic dormancy.
STATEMENT OF TRANSLATIONAL RELEVANCE.
Dormancy of metastases constitutes an ominous unknown for cancer patients – should there be adjuvant treatment or not. The call for therapy and selection of type of therapy should rationally depend on the biological properties of metastatic cells. If the cells in these clinically silent micrometastases are in a state of balanced proliferation and death, then agents targeting cycling cells are appropriate. However, if the cells are quiescent, then other approaches, or none, are appropriate. As clinical data are scarce, and experimental systems are not established to study this directly, we used an unbiased in silico model to identify the boundary condition probabilities that would allow for micrometastases to remain dormant for 5–10 years even while undergoing regular cell cycling. The resultant tight one percentage point range suggests that the metastatic cells likely undergo at least periods of quiescence. This would suggest that for longer-term dormancy, novel approaches need to be developed.
ACKNOWLEDGEMENTS
We thank members of the Wells and Bahar laboratories for helpful discussions.
GRANT SUPPORT
This work was supported by grants from the VA Merit Award program and NIH (UH2TR000496) to A. Wells.
Abbreviations
- ECM
Extracellular Matrix
- MCMC
Markov chain Monte Carlo
Footnotes
The authors have no conflicts of interest in regards to this work.
REFERENCES
- 1.Naumov GN, MacDonald IC, Weinmeister PM, Kerkvliet N, Nadkarni KV, Wilson SM, et al. Persistence of solitary mammary carcinoma cells in a secondary site: a possible contributor to dormancy. Cancer Res. 2002;62:2162–2168. [PubMed] [Google Scholar]
- 2.Aguirre-Ghiso JA. Models, mechanisms and clinical evidence for cancer dormancy. Nat Rev Cancer. 2007;7:834–846. doi: 10.1038/nrc2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fisher B, Anderson S, Bryant J, Margolese RG, Deutsch M, Fisher ER, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347:1233–1241. doi: 10.1056/NEJMoa022152. [DOI] [PubMed] [Google Scholar]
- 4.Townson JL, Chambers AF. Dormancy of solitary metastatic cells. Cell Cycle. 2006;5:1744–1750. doi: 10.4161/cc.5.16.2864. [DOI] [PubMed] [Google Scholar]
- 5.Aguirre-Ghiso JA. The problem of cancer dormancy: understanding the basic mechanisms and identifying therapeutic opportunities. Cell Cycle. 2006;5:1740–1743. doi: 10.4161/cc.5.16.3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wells A, Yates C, Shepard CR. E-cadherin as an indicator of mesenchymal to epithelial reverting transitions during the metastatic seeding of disseminated carcinomas. Clin Exp Metastasis. 2008;25:621–628. doi: 10.1007/s10585-008-9167-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luzzi KJ, MacDonald IC, Schmidt EE, Kerkvliet N, Morris VL, Chambers AF, et al. Multistep nature of metastatic inefficiency: dormancy of solitary cells after successful extravasation and limited survival of early micrometastases. Am J Pathol. 1998;153:865–873. doi: 10.1016/S0002-9440(10)65628-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mocellin S, Hoon D, Ambrosi A, Nitti D, Rossi CR. The prognostic value of circulating tumor cells in patients with melanoma: a systematic review and meta-analysis. Clin Cancer Res. 2006;12:4605–4613. doi: 10.1158/1078-0432.CCR-06-0823. [DOI] [PubMed] [Google Scholar]
- 9.Jiao Y, Torquato S. Emergent behaviors from a cellular automaton model for invasive tumor growth in heterogeneous microenvironments. PLoS Comput Biol. 2011;7:e1002314. doi: 10.1371/journal.pcbi.1002314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Antal T, Krapivsky PL. Outbreak size distributions in epidermics with multiple stages. Journal of Statistical Mechanics. 2012;2012:p07018. [Google Scholar]
- 11.LaPorta CAM, Zapperi S, Sethna JP. Senescent cells in growing tumors: population dynamics and cancer stem cells. PLoS Computational Biology. 2012;8:e1002316. doi: 10.1371/journal.pcbi.1002316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Willis L, Alarcón T, Elia G, Jones JL, Wright NA, Tomlinson IP, et al. Breast cancer dormancy can be maintained by small numbers of micrometastases. Cancer Res. 2010;70:4310–4317. doi: 10.1158/0008-5472.CAN-09-3144. [DOI] [PubMed] [Google Scholar]
- 13.Haustein V, Schumacher U. A dynamic model for tumour growth and metastasis formation. J Clin Bioinforma. 2012;2:11. doi: 10.1186/2043-9113-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haeno H, Michor F. The evolution of tumor metastases during clonal expansion. J Theor Biol. 2010;263:30–44. doi: 10.1016/j.jtbi.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Naumov GN, Akslen LA, Folkman J. Role of angiogenesis in human tumor dormancy: animal models of the angiogenic switch. Cell Cycle. 2006;5:1779–1787. doi: 10.4161/cc.5.16.3018. [DOI] [PubMed] [Google Scholar]
- 16.Marshall JC, Collins JW, Nakayama J, Horak CE, Liewehr DJ, Steinberg SM, et al. Effect of Inhibition of the Lysophosphatidic Acid Receptor 1 on Metastasis and Metastatic Dormancy in Breast Cancer. J Natl Cancer Inst. 2012 doi: 10.1093/jnci/djs319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dodds MG, Vicini P. Assessing convergence of Markov chain Monte Carlo simulations in hierarchical Bayesian models for population pharmacokinetics. Ann Biomed Eng. 2004;32:1300–1313. doi: 10.1114/b:abme.0000039363.94089.08. [DOI] [PubMed] [Google Scholar]
- 18.Kamiya N, Suzuki H, Endo T, Yano M, Naoi M, Nishimi D, et al. Clinical usefulness of bone markers in prostate cancer with bone metastasis. Int J Urol. 2012 doi: 10.1111/j.1442-2042.2012.03098.x. [DOI] [PubMed] [Google Scholar]
- 19.Berman JJ, Moore GW. The role of cell death in the growth of preneoplastic lesions: a Monte Carlo simulation model. Cell Proliferation. 1992;25:549–557. doi: 10.1111/j.1365-2184.1992.tb01459.x. [DOI] [PubMed] [Google Scholar]
- 20.Ycart B. Fluctuation analysis with cell deaths. arXiv preprint arXiv. 2012 e1207.4375. [Google Scholar]
- 21.Michor F, Nowak MA, Iwasa Y. Stochastic dynamics of metastasis formation. J Theor Biol. 2006;240:521–530. doi: 10.1016/j.jtbi.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 22.Watson HW, Galton F. On the probability of the extinction of families. The Journal of the Anthropological Institute of Great Britain and Ireland. 1875;4:138–144. [Google Scholar]
- 23.Harris T. The theory of branching processes. United States Air Force Project RAND. 1964. pp. 1–248. [Google Scholar]
- 24.Klein CA, Holzel D. Systemic cancer progression and tumor dormancy: mathematical models meet single cell genomics. Cell Cycle. 2006;5:1788–1798. doi: 10.4161/cc.5.16.3097. [DOI] [PubMed] [Google Scholar]
- 25.Naumov GN, Folkman J, Straume O, Akslen LA. Tumor-vascular interactions and tumor dormancy. APMIS. 2008;116:569–585. doi: 10.1111/j.1600-0463.2008.01213.x. [DOI] [PubMed] [Google Scholar]
- 26.Divoli A, Mendonca EA, Evans JA, Rzhetsky A. Conflicting biomedical assumptions for mathematical modeling: the case of cancer metastasis. PLoS Comput Biol. 2011;7:e1002132. doi: 10.1371/journal.pcbi.1002132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bednarz-Knoll N, Alix-Panabieres C, Pantel K. Clinical relevance and biology of circulating tumor cells. Breast Cancer Res. 2011;13:228. doi: 10.1186/bcr2940. [DOI] [PMC free article] [PubMed] [Google Scholar]