Abstract
Concepts bind together the features commonly associated with objects and events to form networks in long-term semantic memory. These conceptual networks are the basis of human knowledge and underlie perception, imagination, and the ability to communicate about experiences and the contents of the environment. Although it is often assumed that this distributed semantic information is integrated in higher-level heteromodal association cortices, open questions remain about the role and anatomic basis of heteromodal representations in semantic memory. Here we used combined neuroimaging evidence from functional magnetic resonance imaging (fMRI) and diffusion tensor imaging (DTI) to characterize the cortical networks underlying concept representation. Using a lexical decision task, we examined the processing of concepts in four semantic categories that varied on their sensory-motor feature associations (sight, sound, manipulation, and abstract). We found that the angular gyrus was activated across all categories regardless of their modality-specific feature associations, consistent with a heteromodal account for the angular gyrus. Exploratory analyses suggested that categories with weighted sensory-motor features additionally recruited modality-specific association cortices. Furthermore, DTI tractography identified white matter tracts connecting these regions of modality-specific functional activation with the angular gyrus. These findings are consistent with a distributed semantic network that includes a heteromodal, integrative component in the angular gyrus in combination with sensory-motor feature representations in modality-specific association cortices.
Keywords: DTI, fMRI, language, semantic memory, sensory-motor, heteromodal
1. INTRODUCTION
Neural representations of meaning are fundamental to human cognition. Our long-term semantic memory—our knowledge for words, objects, people, and so on—is engaged by processes as elemental as object recognition and as elaborate as thought and language. Although the neural representation of semantic memory is not fully understood, many theories propose that concepts are grounded to some extent in the brain’s sensory and motor systems, and thus recruit networks of activation in modality-specific association cortices (Barsalou, 2008; Martin, 2007; Pulvermüller, 2005). These sensory-motor theories of semantic memory often assume that conceptual networks include heteromodal association regions where information converges (Barsalou, 2008; Damasio, 1989; Martin, 2007). However, within the most prominent sensory-motor frameworks, heteromodal regions have received comparatively little attention (Barsalou, 2008; Martin, 2007; Pulvermüller, 2005).
While it is well accepted that sensory and motor association areas are engaged when people imagine the features of objects and actions (e.g., imagining the sound of thunder activates auditory association cortex), a more controversial view is that these same sensory and motor areas also contribute to the basic understanding of objects and actions (e.g., knowing what thunder is requires the representation of sound information about thunder in auditory association cortex) (Barsalou, 2008; Martin, 2007; Pulvermüller, 2005). An alternative explanation is that such activation of sensory and motor association regions reflects post-conceptual processing that occurs after the meaning of the object or action has been accessed (Machery, 2007; Mahon and Caramazza, 2008).
Thus, an important challenge has been to examine the neural basis of concepts in a manner that minimizes post-conceptual processing effects. One approach has been to examine the neural basis of single word comprehension, a process that automatically activates conceptual representations and minimizes demands on mental imagery (Pulvermüller et al., 2005). A number of studies suggest that words with sensory and motor associations recruit modality-specific association cortices (e.g., the word “thunder” automatically recruits auditory association cortex) (Hauk et al., 2004; Kiefer et al., 2008; Raposo et al., 2009). However, these findings have not been consistently demonstrated, and there is disagreement over whether the relevant activations fall within regions of modality-specific association cortices (Bedny et al., 2008; Postle et al., 2008).
Building on theories that emphasize this sensory-motor perspective are theories that also include a heteromodal component in semantic memory. This approach emphasizes a critical role for heteromodal association cortices in integrating semantic information from across numerous sensory-motor feature modalities (Binder and Desai, 2011; Koenig and Grossman, 2007; Patterson et al., 2007). Indeed, several heteromodal association regions have been consistently implicated in semantic memory (Binder and Desai, 2011; Koenig and Grossman, 2007; Patterson et al., 2007; Reilly and Peelle, 2008). These cortical regions, centered on the angular gyrus in the parietal lobe and running the entire length of the temporal lobe through the middle temporal gyrus, are located at the convergence of numerous modality-specific processing pathways, and have undergone rapid evolutionary expansion in humans relative to monkeys (Hill et al., 2010; Orban et al., 2004; Sherwood et al., 2008). Anatomically, these regions differ from sensory and motor association cortices in that they tend to have larger and more complex dendritic fields (Elston et al., 2001; Jacobs et al., 2001), lower myelin content (Glasser and Van Essen, 2011), and, in studies of homologous regions in monkeys, lower neuron densities (Collins et al., 2010)—all of which may reflect an anatomic specialization for higher-level multimodal functions (Glasser and Van Essen, 2011; Jacobs et al., 2001). These regions are thus well suited to performing heteromodal semantic processes, such as integrating the broad range of information associated with a concept (Binder and Desai, 2011; Patterson et al., 2007) and acting as convergence zones in the feedforward and feedback activation of distributed semantic networks (Damasio, 1989). Such heteromodal processes are thought to underlie a number of semantic memory functions (Binder and Desai, 2011; Binder et al., 2009; Dove, 2011; McClelland and Rogers, 2003; Patterson et al., 2007). For example, they may support the ability to generalize across the specific instances of objects within a concept category (e.g., while not all cars look the same, our concept for car encompasses them all). They may also facilitate the efficient comprehension of natural language by processing heteromodal semantic content without activating the full conceptual network of each encountered word.
A well-known account of heteromodal semantic processing is the hub-and-spoke model of the anterior temporal lobe, in which the anterior temporal lobe functions as an integrative hub with reciprocal white matter connections (the spokes) to modality-specific association cortices (Patterson et al., 2007). This hypothesis was first put forward to account for the multimodal semantic impairment in the semantic variant of primary progressive aphasia (svPPA), also known as semantic dementia. Patients with this disease show a profound loss of semantic knowledge, including difficulty naming objects and people, and difficulty recognizing objects and their functions (Bonner et al., 2010; Hodges and Patterson, 2007; Lambon Ralph and Patterson, 2008). Because svPPA is most commonly associated with atrophy in anterior and ventral portions of temporal cortex, the anterior temporal lobe has been a major focus of investigations into concept representation (Patterson et al., 2007).
It is not necessarily the case, however, that semantic integration is confined to a single region (Damasio, 1989). Indeed there is considerable evidence that another region of heteromodal cortex, the angular gyrus, is essential to concept representation (Binder et al., 2009; Koenig and Grossman, 2007). The angular gyrus has long been considered a higher-level association area in the human brain (Geschwind, 1965), and is implicated in semantic processing in a range of tasks, from single word recognition (Binder et al., 2003) to sentence-level comprehension (Pallier et al., 2011). A recent meta-analysis identified the angular gyrus as the most common region of functional activation in semantic memory studies (Binder et al., 2009). Lesions and disease affecting this region are associated with a range of lexical-semantic impairments in stroke patients and in Alzheimer’s disease (Ardila et al., 2000; Benson, 1979; Cipolotti et al., 1991; Damasio, 1981; Dronkers et al., 2004; Grossman et al., 2003; Grossman et al., 1997; Kertesz et al., 1982; Rapcsak and Rubens, 1994). In addition, the angular gyrus appears to have the widespread white matter connectivity expected for a heteromodal integrative region: it is situated at the convergence of major white matter pathways connecting sensory and motor association areas as well as language and episodic memory regions of the frontal, parietal, and temporal lobes (Caspers et al., 2011; Turken and Dronkers, 2011; Uddin et al., 2010). Similarly, studies of the macaque homologue of the angular gyrus (area PG/7a) have shown that it receives converging inputs from nearly all modality-specific association cortices (Andersen et al., 1990; Cavada and Goldman-Rakic, 1989a, b; Jones and Powell, 1970; Leichnetz, 1980; Mesulam et al., 1977; Pandya and Seltzer, 1982; Petrides and Pandya, 1984; Selemon and Goldman-Rakic, 1988; Seltzer and Pandya, 1978, 1984, 1994).
Here we propose a cortical network of concept representation relying primarily on a heteromodal integrative region in the angular gyrus along with distributed feature representations in sensory and motor association regions. We predict that as a locus of integrated concept representations, the angular gyrus will be consistently activated during the comprehension of words regardless of their modality-specific associations. We also predict that words will activate sensory and motor cortices associated with their most salient semantic features. Finally, we predict that regions of sensory and motor feature representation will have white matter projections to heteromodal cortex in the angular gyrus.
We tested these predictions using an fMRI analysis of single word comprehension in combination with DTI tractography to assess white matter connectivity. Using words with differential feature weightings in four dimensions (sight, sound, manipulation, and abstract), we tested for evidence of heteromodal semantic processing across all word categories. We also looked for evidence of differential activation across categories in functionally defined sensory and motor cortical regions. Finally, we characterized the white matter connectivity between regions of heteromodal and modality-specific semantic activation.
2. METHODS
2.1 Participants
Twenty-two healthy young adults from the University of Pennsylvania community participated in the study. All participants were right-handed, native English speakers, and all were in good health with no history of neurological difficulty, as determined by a pre-experiment screening. We obtained informed consent from all participants according to a protocol approved by the University of Pennsylvania Institutional Review Board, and we paid subjects for their participation. We excluded one participant from the analysis because he fell asleep in the scanner and did not complete the experimental tasks, and another because of data corruption. We therefore report results for 20 participants (13 female) ranging in age from 18 to 37 years (M=23.5, SD=4.2) with a group mean of 15.7 years of education (SD=2.3). Three of these participants are not included in the functional localizer analyses (see below) owing to technical difficulties during the acquisition of the sequences.
2.2 Lexical decision task
2.2.1 Stimuli
The lexical decision experiment included 160 nouns divided into four categories: Sight words (e.g., pyramid; n=40), Sound words (e.g., thunder; n=40), Manipulation words (e.g., scissors; n=40), and Abstract words (e.g., essence; n=40). We obtained the stimuli from a set of 489 nouns probed in a norming study (n=22 young adults) on a scale from 0 to 6 for how strongly they were associated with each of the three modality-specific features: sight, sound, and manipulation. Sight words had significantly higher sight ratings (M=5.8, SD=0.1) than Sound words (M=3.3, SD=1.8), Manipulation words (M=5.3, SD=0.3), and Abstract words (M=0.4, SD=0.3; all p<0.0001). Sound words had significantly higher sound ratings (M=5.1, SD=0.4) than Manipulation words (M=1.1, SD=0.6), Sight words (M=0.4, SD=0.3), and Abstract words (M=0.3, SD=0.4; all p<0.0001). Manipulation words had significantly higher manipulation ratings (M=4.5, SD=0.3) than Sight words (M=1.0, SD=0.7), Sound words (M=1.0, SD=0.7), and Abstract words (M=0.2, SD=0.2; all p<0.0001). Foils were pronounceable pseudowords (n=120). All categories were matched on letter length [F(4,275)=1.21, p=0.31], syllable length [F(4,275)=0.69, p=0.60], and log HAL frequency norms [F(3,155)=2.23, p=0.09] (Lund and Burgess, 1996). All stimulus material are listed in Appendix I. Additional information on the properties of the stimuli are included in Appendix II.
2.2.2 Experimental design
Each trial was composed of two 3000 ms events. In the first event, a blank white screen was presented for 2500 ms followed by a fixation crosshair for 500 ms. In the second event, a pronounceable letter string was presented and participants responded by button press to indicate whether the letter string was a real word or not. Real word and pseudoword stimuli were randomly ordered.
2.3 Functional localizers
2.3.1 Stimuli
We administered functional localizers to identify visual, auditory and hand-motor cortical regions. We designed the stimuli for these tasks to have minimal semantic content and elicit strong, modality-specific sensory processing. In the visual localizer, participants viewed complex, unnameable blobs (composites of distorted, geometric images; n=25). In the auditory localizer, participants listened to complex, unnameable sounds (composites of distorted environmental sounds; n=25). In the hand-motor localizer, participants performed simple hand movements (learned before the imaging session) that were associated with simple visual symbols (+, o, ~, *).
2.3.2 Experimental design
We administered the functional localizers in separate scanning blocks following the lexical decision experiment. In the visual and auditory localizers, each trial was composed of two 3000 ms events. In the first event, a blank white screen was presented for 2500 ms followed by a fixation event for 500 ms (a crosshair in the visual localizer and a beep in the auditory localizer). In the second event, a stimulus item was presented for 3000 ms. During the stimulus presentation, participants performed a one-back task by pressing a button if the same stimulus item had been presented in the previous trial (which occurred in 20% of trials).
For the hand-motor localizer, participants underwent two training sessions outside of the scanner in which they over-learned four hand movements with associated symbols. Participants performed the following hand movements when the corresponding symbol appeared on the screen: 1) make a fist when “o” appears; 2) spread your fingers apart when “+” appears; 3) twist your wrist when “~” appears; and 4) pinch your fingers together when “*” appears. There were separate blocks for right and left hand movements. All movements within a block were for the same hand. We instructed participants to perform the hand movements below their elbows without moving the upper halves of their arms in order to minimize head movement in the scanner. The training session included 172 practice movements. Participants were able to perform the task with ease by the end of the training session. In the scanner, each block (one per hand) included 26 trials of 2 movements each. Each trial was a 3000 ms event that included presentation of the following: a symbol (1000 ms), a blank white screen (500 ms), a second symbol (1000 ms), and a blank white screen (500 ms). In a baseline condition, participants named the symbols as they appeared on the screen: 1) ”circle” for “o”; 2) “cross” for “+”; 3) “tilde” for “~”; and 4) “asterisk” for “*”. We instructed participants to whisper the names with minimal jaw movement in order to prevent head movement in the scanner.
We administered practice sessions for all tasks (lexical decision and localizers) before entering the scanner to familiarize participants with the tasks and ensure that all instructions were understood. Practice stimulus items were not re-presented in the scanner for any of the tasks. We presented instructions again before the start of each task in the scanner. In a questionnaire administered after the scan, we asked participants if they had trouble performing or remembering the instructions for any of the tasks; none reported problems.
For all tasks, we used E-Prime 1.0 (Psychology Software Tools, Inc., Pittsburgh, PA) to present stimuli and record responses. Across all tasks, a quarter of the events were 3000 ms null events, which produced a stochastic design (Henson, 2007).
2.4 Functional imaging acquisition and analysis
We scanned participants on a Siemens 3.0T Trio scanner, beginning each session with acquisition of a high-resolution T1-weighted structural image using an MPRAGE protocol (TR=3000ms, TE=3ms, flip angle=15°, 1 mm slice thickness, 192 × 256 matrix, resolution=.9766 × .9766 × 1 mm). We collected 863 blood oxygenation level-dependent (BOLD) fMRI images across 8 blocks, each with fat saturation, 3 mm isotropic voxels, a flip angle of 90°, TR=3 s, TEeff=30 ms, and a 64 × 64 matrix.
We performed image processing and statistical analyses with SPM5 (Wellcome Trust Centre for Neuroimaging, London, UK). To remove low-frequency drifts, we applied a high-pass filter with a cutoff period of 128 s. Autocorrelations were removed with a first-order autoregressive model. Functional images were realigned to the first image (Friston et al., 1995), coregistered with the structural image (Ashburner and Friston, 1997), normalized to standard Montreal Neurological Institute (MNI) space using unified segmentation with resampling of images into isotropic 2 mm voxels (Ashburner and Friston, 2005), and spatially smoothed using an 8 mm full-width at half-maximum (FWHM) isotropic Gaussian kernel. We then individually modeled each participant’s data. We used a general linear model to calculate parameter estimates for each variable for each subject and to perform linear contrasts for comparisons of interest. We included additional regressors to account for scanning block effects. In order to make inferences across participants, we entered the parameter estimates into a second-level random effects analysis.
Regions of interest (ROIs) for the angular gyrus and middle temporal pole were created using anatomic labels (Tzourio-Mazoyer et al., 2002) from the WFU Pick Atlas integrated into SPM5 (Maldjian et al., 2003). We maximized signal strength in the temporal pole ROI by constraining it to voxels that had a signal of at least 80% of the global signal for each participant (Devlin et al., 2000), keeping 22% of the voxels and resulting in an ROI that was 1,312 mm3 in size. For whole-brain analyses of the lexical decision task, we used a cluster-defining threshold of p<0.005 uncorrected, and performed a cluster-level correction for family-wise error across the whole brain at p<0.05 based on cluster extent using random field theory (Worsley et al., 1992).
The button-press task resulted in significant clusters of activation along motor cortex and the lateral sulcus. These motor-related responses were consistent across conditions and not of interest. For display purposes, we have not shown these responses in the whole-brain analysis in Figure 3. We removed the activation relating to the button-press response by removing the prominent left primary motor cortex cluster and any cluster whose mean activity was significantly correlated with activation in left primary motor cortex. This removed the left primary motor cortex activation and an adjacent cluster strongly correlated with the motor cortex activation (p<0.001) deep in the lateral sulcus. These results are shown in Supplementary Figure 1.
Figure 3.
Heteromodal whole-brain analyses showing greater activity for words compared to pseudowords, as well as overlap of activation across categories (center).
In order to explore activation differences across concrete word categories, we performed ROI analyses within sensory and motor association cortices. We created a sensory-motor ROI by taking the peak activation clusters in each hemisphere from all three functional localizers and combining them into a single mask. The visual localizer was contrasted with the auditory localizer as a baseline, and vice-versa. The motor localizer was contrasted with the symbol-naming task as a baseline. All contrasts used a cluster-defining threshold of p<0.001 uncorrected, and were cluster-level corrected for family-wise error across the whole brain at p<0.05 using cluster extent.
2.5 Tractography acquisition and analysis
Diffusion tensor imaging data were available for 15 of the fMRI participants, collected in separate scanning sessions within one year of the fMRI scan (median difference between scan dates was 26 days). We acquired diffusion-weighted images with a single-shot, spin-echo, echo planar imaging sequence (FOV=245 mm; matrix size=128 × 128; number of slices=57; voxel size=2.2 mm isotropic; TR=6700 ms; TE=85 ms; fat saturation), including 30 volumes with diffusion weighting (b=1000 s/mm2) along 30 non-collinear directions and one volume without diffusion weighting (b=0 s/mm2).
We processed the diffusion-weighted images with Advanced Normalization Tools (ANTS, http://www.picsl.upenn.edu/ANTS/) (Avants et al., 2008) and Camino (Cook et al., 2006) using the associated PipeDream analysis framework (http://sourceforge.net/projects/neuropipedream/). To remove motion and distortion artifacts, we performed affine co-registration of each diffusion-weighted image to the unweighted (b=0) image. Diffusion tensors were computed using a linear least squares algorithm (Salvador et al., 2005) implemented in Camino.
We created a study-specific T1 template from the T1-weighted images of the participants using ANTS. We first corrected intensity inhomogeneity in the T1 images with the N4ITK algorithm (Tustison et al., 2010). We then constructed a preliminary template by averaging the images, rigidly realigning each image to the average, and then re-averaging over 5 iterations. To further refine the template, we registered each image to the preliminary template with a nonlinear deformation algorithm and re-averaged over 5 iterations. This registration used the highly accurate (Klein et al., 2009), symmetric diffeomorphic algorithm for high-dimensional normalization in ANTS.
Using this study-specific template, we registered each participant’s T1 image to the template space via the symmetric diffeomorphic procedure in ANTS. We then registered the fractional anisotropy (FA) images for each participant to their T1 image, and then to the template. Tensors were reoriented using the preservation of principal directions algorithm (Alexander et al., 2001).
We created functionally defined ROIs from the fMRI study to use as seed regions for the tractography analysis. To create the angular gyrus ROI, we took the region of overlapping activation (at least three overlapping clusters) from the contrasts of each word condition relative to pseudowords (see Figure 3). We created ROIs in sensory and motor areas by taking the activation clusters from contrasts of the modality-weighted word conditions (see Figure 4). These ROIs were registered to the template space. All ROIs extended into white matter with the exception of the activation cluster for Sight words. Because fiber tracking in gray matter is unreliable, we dilated the Sight word activation cluster by 3 mm to ensure that it extended into white matter.
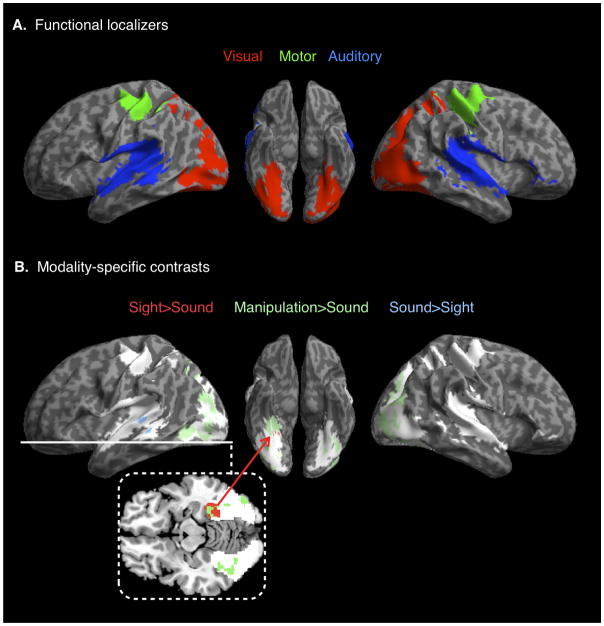
Figure 4.
Modality-specific activations in sensory and motor regions. (A) Activations from functional localizers for visual, auditory, and hand-motor cortices. These were combined into a single, sensory-motor mask. (B) Modality-specific activations for Sight (red), Sound (blue), and Manipulation (green) word categories, constrained to the sensory-motor mask (shown in white) in an exploratory analysis (p<0.05 voxelwise uncorrected, cluster peak at p<0.005 uncorrected).
Using Camino, we performed streamline tractography analyses on the average of the DT images from all participants. Because nearly all of the functionally defined ROIs were in the left hemisphere, we restricted the tractography analysis to this hemisphere. This required warping the Sight word activation ROI to the left hemisphere. We seeded streamlines in voxels with an FA>0.2, and performed fiber tracking using the fiber assignment by continuous tracking (FACT) algorithm (Mori et al., 1999). The tracking procedure terminated if a voxel was reached with an FA<0.1 or if the streamline trajectory changed by more than 45 degrees in a successive step. The procedure identified fiber tracts connecting pairs of functional ROIs by keeping only streamlines that intersected both ROIs. We compared these tracts with an anatomically labeled DTI white matter atlas (Oishi et al., 2008) to identify their anatomic location and to ensure that they were biologically plausible.
3. RESULTS
3.1 Behavioral results
Accuracy and reaction times for the lexical decision task are shown in Figure 1. Accuracy was near perfect (>98%) for all word conditions and, thus, was not further analyzed. A repeated measures ANOVA of reaction times for all conditions showed a significant effect of condition [F(4, 76)=38.0, p<0.001, ηp2=0.67]. This effect was driven by the significantly longer reaction time for pseudowords (M=972.0 ms, SD=192.4 ms) relative to all other word categories (all p<0.001, corrected for multiple comparisons) and a longer reaction time for Abstract words compared to Sight words (p<0.001, corrected for multiple comparisons). There were no other differences in reaction time across conditions.
Figure 1.
Behavioral performance on the lexical decision task. (A) Accuracy was near ceiling for all categories. (B) Reaction times were consistently rapid for all categories. Bars represent means with standard errors.
3.2 Functional imaging results
3.2.1 Heteromodal effects
We first tested the hypotheses that the angular gyrus and the middle temporal pole in the anterior temporal lobe function as heteromodal regions in semantic processing using anatomic ROIs (Tzourio-Mazoyer et al., 2002). In contrast to modality-specific regions that are differentially recruited by sensory and motor features, we predicted that these heteromodal regions would be activated by all semantic categories. We therefore looked for evidence that these regions were activated across all word categories during the lexical decision task by contrasting each of the four real word categories with the pseudoword baseline, as shown in Figure 2. A repeated measures ANOVA showed a significant effect of region [F(1,19)=5.82, p<0.05], with the angular gyrus showing a stronger response than the middle temporal pole in the anterior temporal lobe. In post-hoc t-tests, we found that individual word categories significantly activated the angular gyrus (all comparisons: t(19)>3.5, p<.01, corrected for 4 multiple comparisons), but that only the Abstract word category activated the middle temporal pole (t(19)=4.28, p<.005, corrected for 4 multiple comparisons). A repeated measures ANOVA showed no effect of word category within the angular gyrus [F(3, 57)=2.0, p=.13], consistent with the hypothesis that the angular gyrus is a heteromodal region contributing to all semantic categories.
Figure 2.
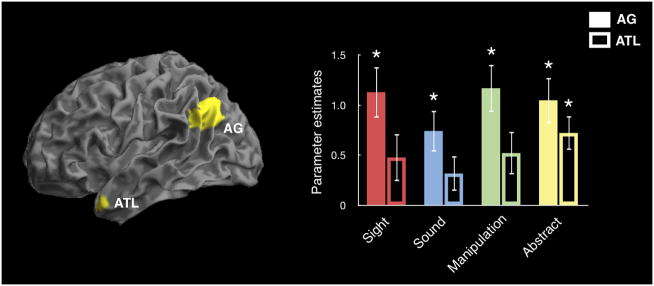
Region of interest (ROI) analyses for heteromodal regions implicated in semantic memory: the angular gyrus (AG) and the middle temporal pole in the anterior temporal lobe (ATL). Parameter estimates were extracted from both ROIs for the activation of each word category relative to the pseudoword baseline. * = parameter estimates differed significantly from zero.
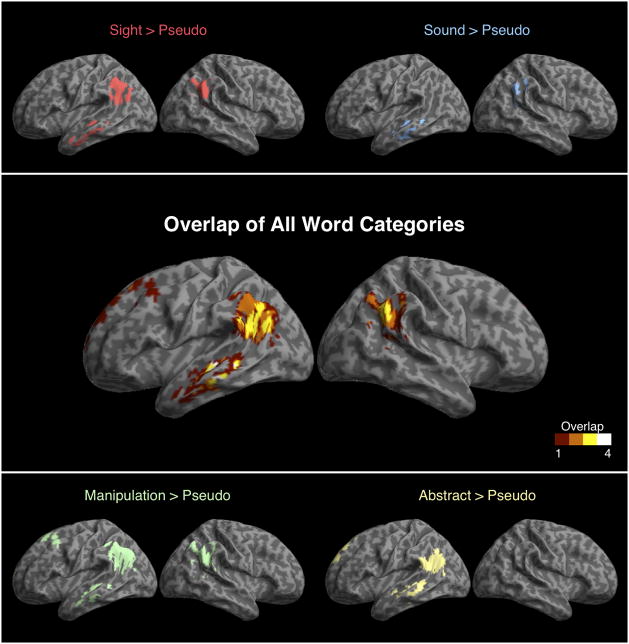
We next performed whole-brain analyses of the same contrasts to look for regions that showed a high degree of overlap across categories, as shown in Figure 3 and listed in Table 1. All word categories showed significant effects in left and/or right angular gyrus and left lateral temporal cortex, consistent with the expected pattern of activation for a heteromodal semantic region. We additionally found that, across participants, the degree of activation in the angular gyrus for words relative pseudowords correlated with the average reaction time for responses to words (Pearson r=−.45, p<.05). However, this activation did not correlate with reaction times to pseudowords (Pearson r=−.07, p=.4). There was also no correlation between activity in the angular gyrus for pseudowords and reaction times to pseudowords (Pearson r=.18, p=.5). This suggests a link between angular gyrus activation and concept representation.
TABLE 1.
fMRI RESULTS
| Heteromodal effects | Peak voxel (MNI)
|
Region | Z | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Sight > Pseudo | −44 | −22 | 58 | L precentral gyrus | 5.94 |
| 16 | −56 | −12 | R cerebellum | 5.10 | |
| −32 | −26 | −14 | L fusiform gyrus | 4.83 | |
| −46 | −20 | 18 | L lateral sulcus | 4.58 | |
| −48 | −58 | 40 | L angular gyrus | 4.13 | |
| 52 | −52 | 32 | R angular gyrus | 3.78 | |
| Sound > Pseudo | −44 | −22 | 56 | L precentral gyrus | 5.49 |
| −54 | −30 | −16 | L middle temporal gyrus | 5.40 | |
| −42 | −20 | 22 | L lateral sulcus | 5.38 | |
| 20 | −48 | −24 | R cerebellum | 4.46 | |
| 52 | −42 | 38 | R angular gyrus | 4.29 | |
| Manipulation > Pseudo | 14 | −54 | −16 | R cerebellum | 4.01 |
| −38 | −20 | 50 | L precentral gyrus | 3.98 | |
| −44 | −18 | 18 | L lateral sulcus | 3.88 | |
| −22 | 16 | 52 | L superior/middle frontal gyrus | 6.27 | |
| −44 | −36 | −4 | L middle temporal gyrus | 5.02 | |
| −42 | −66 | 24 | L angular gyrus | 4.96 | |
| 6 | 62 | 6 | L and R superior frontal gyrus | 4.23 | |
| 50 | −44 | 24 | R angular gyrus | 3.35 | |
| Abstract > Pseudo | −40 | −20 | 52 | L precentral gyrus | 5.50 |
| −42 | −18 | 14 | L lateral sulcus | 5.46 | |
| 10 | −62 | −14 | R cerebellum | 5.18 | |
| −46 | −58 | 18 | L angular gyrus | 4.71 | |
| −4 | 52 | 44 | L superior frontal gyrus | 4.70 | |
| −8 | −24 | 44 | L cingulate gyrus | 4.38 | |
| Modality-specific effects | |||||
| Sight > Sound | 38 | −42 | −10 | R fusiform/lingual gyrus | 2.95 |
| Manipulation > Sound | −40 | −64 | −6 | L fusiform gyrus | 3.22 |
| −40 | −80 | 26 | L intraparietal sulcus | 2.90 | |
| 32 | −46 | −16 | R fusiform gyrus | 2.85 | |
| 34 | −70 | 40 | R intraparietal sulcus | 2.83 | |
| Sound > Sight | −68 | −32 | 4 | L superior/middle temporal gyri | 2.65 |
3.2.2 Modality-specific effects
Sensory-motor theories of semantic memory predict that words with sensory and motor feature associations will have semantic representations in the corresponding modality-specific association cortices. This suggests differential effects across the sensory and motor word categories. We conducted analyses constrained to a localizer-defined sensory-motor mask that encompassed the modality-specific cortices for which we had hypothesized differential semantic effects. The sensory-motor mask was composed of regions that showed significant effects in our sensory-motor localizer tasks, which included visual, auditory, and hand-motor localizers as shown in Figure 4A. We explored these predictions by performing contrasts across the concrete word categories. Sight words and Manipulation words were each contrasted with the Sound words, which have weaker visual and manipulation feature associations, to examine the cortical activation associated with sight and manipulation feature processing. The inverse contrast of Sound words relative to Sight words was used to examine the cortical activation associated with auditory feature processing.
Initial assessment of these comparisons at the same threshold as the heteromodal analysis (voxelwise p<.005, cluster-extent corrected within the search volume) did not reveal significant modality-specific effects for any of the comparisons. Given the previous findings implicating modality-specific association cortices in semantic processing (Barsalou, 2008; Martin, 2007; Pulvermüller, 2005), and our a priori hypothesis for these regions, we conducted follow-up, exploratory analyses within the same localizer-constrained search volume. For these analyses we used a voxelwise threshold of p<0.05 uncorrected, retaining only clusters whose peak voxel reached p<0.005 uncorrected.
Results from these exploratory analyses are shown in Figure 4B and listed in Table 1. The Sight word condition activated a visual association region in the ventral temporal area, including fusiform, parahippocampal, and lingual gyri, which may support visual feature representation. The Manipulation word condition recruited dorsal stream visual association regions of parietal-occipital cortex, which may have a role in representing the visuomotor features of concepts with hand-motor associations. The Manipulation words also activated a visual association region in fusiform gyrus, similar to that for Sight words. The Sound words activated an auditory association region on posterior superior temporal and middle temporal gyri, which may support auditory feature representation.
These contrasts were all constrained to the same, combined sensory-motor mask, but the effect for each word category fell within regions related to the specific modality of that category’s weighted feature, keeping in mind that the Manipulation words activated a dorsal visual region and not a motor region. This suggests that words recruit, to some extent, concept representations in sensory and motor association cortices, even during lower-level lexical processing in which mental imagery is unlikely to be a driving force.
3.3 Tractography results
The fMRI results showed a pattern of activity in angular gyrus consistent with heteromodal semantic processing. We thus set out to test the anatomic prediction that this region of heteromodal activation would have white matter connections with regions of modality-specific feature representation, consistent with it being an integrative semantic region. We used DTI tractography to test this hypothesis and the alternative hypothesis that regions of modality-specific feature representation would have direct anatomic connections with one another but not with the angular gyrus.
We performed a streamline tractography analysis to identify tracts connecting the area of overlapping activation in the angular gyrus with activation clusters in modality-specific association cortices for the Sight, Sound, and Manipulation word categories. This DTI analysis revealed tracts connecting angular gyrus with modality-specific activation clusters for each word category, as shown in Figure 5. Comparing these results with a DTI white matter atlas (Oishi et al., 2008), we found that the tracts connecting the angular gyrus with activations in the temporal lobe for Sight, Sound, and Manipulation words corresponded to portions of the arcuate fasciculus, while tracts connecting the angular gyrus with the parietal lobe activation for Manipulation words corresponded to a portion of the superior longitudinal fasciculus. Performing the same tractography analysis and looking for direct connections between the modality-specific activation clusters failed to yield any tracts. Together, these DTI results are consistent with the hypothesis that the angular gyrus has direct anatomic connections to areas associated with sensory and motor feature processing in modality-specific association cortices.
Figure 5.
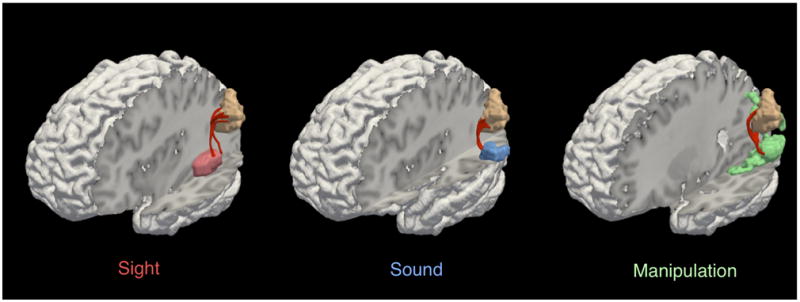
Tractography of angular gyrus and sensory-motor regions. Tracts are shown connecting the angular gyrus activation cluster (tan) with activation clusters in sensory-motor regions for Sight (red; left panel), Sound (blue; middle panel), and Manipulation (green; right panel) words.
4. DISCUSSION
An ongoing challenge in cognitive neuroscience is determining the degree to which heteromodal and modality-specific association cortices contribute to long-term memory representations. In the current study, using both fMRI and DTI, we evaluated the possibility that the angular gyrus functions as a heteromodal integrative region, binding together distributed semantic information from modality-specific association cortices. This hypothesis was supported by the observation that (1) the angular gyrus is consistently activated by concepts with differing modality-specific associations and (2) the angular gyrus has white matter connectivity to regions of modality-specific feature representation. These findings suggest that concepts rely on distributed semantic networks that involve a heteromodal component in the angular gyrus and modality-specific feature representations in sensory and motor areas. We discuss these findings in detail below.
4.1 The contribution of heteromodal association cortex to concept representation
Heteromodal association regions are hypothesized to integrate semantic information from across numerous modality-specific inputs. The angular gyrus and the anterior temporal lobe are two regions that have been implicated in heteromodal semantic processing, based on both patient data and functional neuroimaging (Binder and Desai, 2011; Koenig and Grossman, 2007; Patterson et al., 2007). As previously noted, the anatomic connectivity and cytoarchitecture of the angular gyrus and its homologue in the macaque suggest a heteromodal integrative function for this region. Thus we hypothesized that the angular gyrus would perform a heteromodal function in the representation of word meaning and be activated by words regardless of the modality of their semantic feature associations. This heteromodal account builds on sensory-motor theories that emphasize the role of modality-specific association cortices in concept representation (Barsalou, 2008; Martin, 2007; Pulvermüller, 2005).
We tested the heteromodal theory of concept representation for both the angular gyrus and the middle temporal pole in the anterior temporal lobe using ROI analyses of our fMRI data. We found that relative to pseudowords, all word categories activated the left angular gyrus, but that only Abstract words activated the left middle temporal pole. Left angular gyrus activation was also observed in whole-brain analyses of the same contrasts. These demonstrated overlapping activation across modality-specific categories in heteromodal cortex, including the angular gyrus and left mid-lateral temporal lobe. These results support the hypothesis that the angular gyrus performs a heteromodal function in concept representation for all semantic categories.
Although our results implicate the angular gyrus in concept representation, they are less clear on the role of the anterior temporal lobe. First, the fMRI signal in the most anterior regions of the temporal lobe is poor, owing to the susceptibility artifact caused by the nearby sinuses (Devlin et al., 2000). Though the significant activation for Abstract words in the middle temporal pole ROI suggests sufficient signal detection, we cannot rule out the possibility that susceptibility artifact contributed to null findings for some of the categories. Second, the anterior temporal lobe hypothesis is largely motivated by the study of patients with svPPA, but given the anatomic distribution of disease in svPPA, the best location at which to test the contribution of the anterior temporal lobe remains unclear. Although many studies have focused on the importance of temporal polar regions in the semantic deficit of these patients (Patterson et al., 2007), a recent investigation associated their impairment for object knowledge with atrophy in fusiform gyrus and considered this to be a critical semantic region (Mion et al., 2010). As discussed below, the fusiform gyrus was activated in the present study during lexicality judgments of words weighted for sight features and may be particularly important for the representations of visual information. Considering these points, our findings with respect to the anterior temporal lobe hypothesis should be interpreted with caution.
In addition to the angular gyrus, we also observed consistent activation across word categories in lateral temporal cortex along the middle temporal gyrus. Homologues of these lateral temporal regions in the macaque brain contain heteromodal association cortex that is contiguous with the heteromodal cortex of the inferior parietal lobe (Hyvärinen, 1982; Seltzer and Pandya, 1978). In humans, the lateral temporal lobe has widespread white matter connectivity (Turken and Dronkers, 2011) and has been implicated in language comprehension in functional neuroimaging studies (Binder et al., 2009), in studies of stroke patients (Dronkers et al., 2004; Kemmerer et al., In press), and in studies of patients with svPPA (Peelle et al., 2008). This evidence is consistent with the lateral temporal lobe having a role in semantic memory. It is unclear, however, how much overlap in function there is between lateral temporal cortex and the angular gyrus. Although these regions appear to be highly correlated in resting state activation (Buckner et al., 2009; Thomas Yeo et al., 2011), there are also many examples of lexical-semantic tasks for which these two regions are differentially recruited (Price, 2010).
Our fMRI results with respect to the angular gyrus are consistent with previous findings associating lesions of the angular gyrus with various lexical-semantic impairments, including anomia, transcortical sensory aphasia, and impaired sentence comprehension (Ardila et al., 2000; Benson, 1979; Cipolotti et al., 1991; Damasio, 1981; Dronkers et al., 2004; Kertesz et al., 1982; Rapcsak and Rubens, 1994). These results fit well with findings in Alzheimer’s disease, associating loss of tissue and function in the angular gyrus with impaired semantic memory (Desgranges et al., 1998; Grossman et al., 2003; Grossman et al., 1997). While more studies are needed to adequately characterize the precise anatomy of these impairments, the evidence is consistent with the hypothesis that the angular gyrus is a heteromodal region involved in higher-level semantic processes.
Further work is needed to more specifically characterize the role of heteromodal regions in semantic memory. It will be important to assess other semantic processes that heteromodal regions might support beyond binding together distributed sensory-motor information. Studies could examine whether these regions support the ability to generalize across the specific instances of a concept, whether they facilitate efficient comprehension by accessing heteromodal content without activating a full semantic network, and whether they support inferences about object concepts that are not apparent from their sensory-motor features. Furthermore, sub-regions within the angular gyrus may be differentially connected to other regions of the brain and may thus support functionally specific heteromodal processes (Seghier, 2012). Though our findings show activation across much of the angular gyrus during word processing, it will be important in future studies to pull apart the different roles that sub-regions of the angular gyrus may have in semantic memory.
4.2 Conceptual vs. lexical representations
One possibility is that activation in the angular gyrus for real words relative to pseudowords is driven purely by lexical information. For several reasons, however, we think that angular gyrus activation in this study is unlikely to be driven by lexical processing alone. First, the pseudowords in the baseline condition have many of the features of real word forms without the semantic associations, but do not similarly recruit the angular gyrus. Second, angular gyrus activation in this study was bilateral, which contrasts with the left lateralized activation that would have been expected of a narrowly lexical effect (Friederici, 2011; Price, 2010). Finally, the angular gyrus has also been implicated in semantic processing in picture-based tasks (Vuilleumier et al., 2002) and across both picture and lexical tasks (von Stein et al., 1999). These observations suggest that the angular gyrus does not process purely lexical information but rather serves a broadly integrative function that includes the sensory-motor and abstract features associated with words and objects. Nonetheless, further work is needed to address the precise relationship between lexical and conceptual processing in the angular gyrus.
A related point is that there is some debate over the degree to which semantic information is engaged during word reading and word recognition tasks, with some cognitive models arguing that semantic information is not necessary for successful word reading (Coltheart et al., 1993; but see Woollams et al., 2007). This raises the question of whether a lexical decision task is well suited for addressing questions about semantic representation. We believe that there are in fact two orthogonal questions in this debate: (1) a question of the necessity of semantic information in word recognition and (2) a question of the automaticity of semantic information in word recognition. From this perspective, even if lexical information alone were sufficient for performing a lexical decision task, this would not rule out the possibility that semantic information is automatically engaged during the task as well. For the purposes of our study, the question of automaticity is of primary importance given that we relate the semantic features of words to fMRI activation in word recognition. We argue that the basic semantic information associated with a word is automatically activated during the lexical decision task, keeping in mind that more extensive semantic networks can be engaged during tasks that require more in-depth semantic processing (Evans et al., 2012; Reilly and Peelle, 2008). This is in line with how many other investigators characterize both lexical decision and passive reading tasks (Barsalou, 2008; Binder et al., 2009; Kiefer and Pulvermüller, 2011; Martin, 2007; Vigliocco et al., 2009; Wang et al., 2010), and this idea has widespread empirical support, with evidence showing, for example, that both reading and lexical decision result in rapid activation of cortical semantic networks (Kiefer et al., 2008; Pulvermüller et al., 2001) and can produce semantic priming effects (Myung et al., 2006; Wheatley et al., 2005). Hence, semantic activation is an automatic and fundamental consequence of word reading, and it is safe to assume that the lexical decision task in our study automatically engaged semantic representations.
4.3 The contribution of modality-specific association cortices to concept representation
According to the heteromodal model, concepts are represented in semantic networks that include a heteromodal integrative region and some contribution of feature representations in sensory and motor areas. Therefore, we predicted that in addition to the angular gyrus, concepts would activate a network of features in modality-specific association cortices. While many studies have previously demonstrated that the active retrieval of sensory and motor feature information recruits sensory and motor areas (Barsalou, 2008; Martin, 2007), fewer studies have investigated the role of sensory and motor areas in semantic memory using tasks that implicitly elicit the conceptual representations associated with words (Kiefer et al., 2008).
In this study, we used a straightforward lexical decision task with minimal demands on semantic retrieval to examine the role of feature associations from three different modalities (sight, sound, and manipulation) in concept representation. In an exploratory analysis, we found suggestive evidence for activation in modality-specific association cortices for each modality-weighted category. Sight words recruited a region of visual association cortex in right fusiform gyrus. Manipulation words recruited visual association regions in parietal and occipital cortices associated with dorsal stream processing of visuomotor information, and additionally recruited visual association regions in both left and right fusiform gyrus. Sound words recruited an auditory association region on left posterior superior temporal and middle temporal gyri. Although others have found strong semantic processing effects in modality-specific association regions (Barsalou, 2008; Martin, 2007), these effects were subtle in the present study and were only observed in an exploratory analysis at an uncorrected statistical threshold. Nonetheless, the effects we observed were anatomically specific for each feature modality, bearing in mind that the Manipulation words activated a dorsal visual region and not a motor region. Furthermore, in a separate study using the same task we found converging evidence that at least for the auditory modality, atrophy of modality-specific association cortex is related to a modality-specific semantic impairment (Bonner and Grossman, 2012).
The weak activation in modality-specific regions that we observed in this study may be related to the depth of semantic processing elicited by word comprehension. It has been suggested that basic word comprehension is largely supported by schematic representations in heteromodal association cortices along with a more variable contribution of feature representations in sensory and motor regions (Binder and Desai, 2011; Reilly and Peelle, 2008). By this account, one would expect a lexical decision task to recruit strong activation in heteromodal association cortex and weaker activation in sensory and motor regions. This is indeed consistent with the findings in our study.
We did not see activation for the Manipulation word category within the region of the motor localizer. This is somewhat inconsistent with previous work that implicates motor cortex in the semantic representation of action verbs (Grossman et al., 2008; Hauk et al., 2004). However, considering that our study looked at nouns rather than verbs, our findings may be more closely related to investigations of manipulable object concepts rather than action verb concepts (Chao et al., 1999; Grossman et al., 2002). As summarized in a recent meta-analysis (Binder et al., 2009), numerous studies suggest that manipulable object concepts rely on a visual association area around the temporal-occipital-parietal junction and another region around the supramarginal gyrus associated with motor planning of hand-object interactions and stroke-induced limb apraxia (Binkofski and Buxbaum, In press). We compared our findings to those summarized in the meta-analysis (Binder et al., 2009). We took the coordinates for both activation clusters for manipulable artifacts in the meta-analysis (Figure 5 in Binder et al., 2009) and created 8 mm spherical ROIs at these coordinates. Extracting data from these ROIs, we found a significant effect for Manipulation words relative to Sound words in the region of the temporal-occipital-parietal junction (t(19) = 2.8, one-tailed p = 0.006; MNI coordinate = [−44, −63, 2]). However in the supramarginal gyrus we found the opposite effect, with less activation for Manipulation words relative to Sounds words (t(19) = 2.09, p = 0.051; MNI coordinate = [−52, −39, 28]). Altogether, our results are more consistent with a representation weighted in the visuomotor system rather than the motor or somatosensory systems for nouns with manipulation features. We note however that we might have observed greater activation in the motor or somatosensory systems if our task had elicited deeper semantic processing.
We found activation of visual association regions in the right hemisphere for both the Sight and Manipulation conditions. Although lexical processing is typically associated with the left hemisphere, several previous fMRI investigations have found evidence suggesting that semantic processing may be bilateral and, particularly, that concrete word semantics may recruit regions in the right hemisphere (Binder et al., 2005; Sabsevitz et al., 2005). The role of the right hemisphere in word knowledge has also been demonstrated in studies of patients with svPPA, in which impaired knowledge of concrete words correlates with cortical atrophy (Bonner et al., 2009) and hypometabolism (Mion et al., 2010) in right fusiform gyrus, and in studies of stroke patients, where right fusiform gyrus lesions have been associated with impaired knowledge of concrete concepts (Vandenbulcke et al., 2006). In this context, it is worth noting that both Sight and Manipulation words activated bilateral angular gyrus in whole-brain analyses, whereas Abstract words activated only left angular gyrus. This is consistent with the hypothesis that concrete words have more bilateral semantic representations than Abstract words (Binder et al., 2005). Such an effect is predicted by dual coding theory (Paivio, 1991), whereby abstract concepts rely primarily on verbal-based representations in the left hemisphere, and concrete concepts additionally rely on bilateral sensory-based representations.
These exploratory findings suggest that in addition to heteromodal association cortex, object concepts recruit to some extent sensory and motor association areas. This is consistent with a model of semantic memory in which concept representations rely on distributed networks centered on heteromodal integrative regions, with the support of modality-weighted feature representations in sensory and motor association cortices.
4.4 White matter connectivity of heteromodal association cortex
A heteromodal integrative region should have reciprocal white matter connections with modality-specific association cortices. The angular gyrus is situated at the convergence of major white matter pathways connecting sensory and motor association areas as well as language and episodic memory regions of the frontal, parietal, and temporal lobes (Caspers et al., 2011; Turken and Dronkers, 2011; Uddin et al., 2010). Previous DTI studies have demonstrated the widespread connectivity of the angular gyrus and the mapping of connectivity patterns onto anatomically defined subregions of inferior parietal cortex (Caspers et al., 2011; Turken and Dronkers, 2011; Uddin et al., 2010). In our study, by comparison, we used DTI to evaluate the connectivity of a functionally defined semantic network. This focused analysis allowed us to test the predictions of both the heteromodal theory and the primarily sensory-motor theory of concept representation using cortical seed regions associated with semantic processing in our fMRI study.
We identified white matter tracts connecting the angular gyrus with three different regions of modality-specific association cortex implicated in the representation of modality-weighted semantic features. This included tracts running through the arcuate fasciculus connecting the angular gyrus to visual association regions in ventral temporal cortex and auditory association regions in the superior temporal lobe, as well as tracts running through the superior longitudinal fasciculus connecting the angular gyrus with visuomotor association regions in parietal cortex.
We additionally tested the hypothesis that the areas of modality-specific feature processing would have direct connections with one another, as would be expected from a strictly sensory-motor account in which concepts rely completely on modality-specific association regions and do not recruit heteromodal cortex. This analysis failed to yield tracts directly connecting these modality-specific activation clusters. These negative findings should be interpreted cautiously because there may be less robust anatomic projections between modality-specific association cortices that are not detectable in our DTI analysis. These DTI findings are nevertheless consistent with previous connectivity studies in both humans and macaques (Caspers et al., 2011), and provide evidence for anatomic connections between the angular gyrus and regions of modality-specific semantic representation.
4.5 Conclusions
Our results are consistent with a model of semantic memory in which the angular gyrus functions as a heteromodal integrative region with white matter connections to modality-specific association cortices. In a lexical decision task, the angular gyrus was implicated in the processing of all words regardless of sensory-motor feature associations, consistent with the expected activation of a heteromodal semantic region. We also found some suggestion that words with modality-weighted feature associations recruited sensory-motor areas in the corresponding modality. DTI tractography demonstrated evidence of white matter connections between the angular gyrus and the sensory-motor areas associated with modality-specific feature representation. Thus, the neural representation of a concept appears to rely on a distributed network that includes a heteromodal region in the angular gyrus in combination with feature representations in sensory and motor association cortices.
Supplementary Material
Heteromodal whole-brain analyses showing overlap of activation across categories. These are the same results as shown in Figure 3, but with activation clusters shown along left motor cortex that relate to the button-press response. We identified activations that correlated with activity in primary motor cortex. Parameter estimates were extracted from spheres (4 mm radius) around the peak voxel of each cluster for the activation of words relative pseudowords. We found that across participants activity in left primary motor cortex (excluding one outlier whose motor cortex activation was greater than three standard deviations from the mean) strongly correlated with activity in an adjacent cluster deep in the lateral sulcus (Pearson r=.7, p<.001) but did not correlate with activity in the angular gyrus or the lateral temporal lobe (all Pearson r<.35, p>.15).
We use fMRI and DTI of a lexical decision task to examine semantic memory networks
We examine heteromodal and modality-specific components of semantic memory
All word categories recruit heteromodal association cortex of the angular gyrus
Exploratory analyses suggest that modality-weighted categories recruit distinct sensory-motor association regions
DTI shows heteromodal convergence of white matter connectivity in the angular gyrus
Acknowledgments
This research was supported by the National Institutes of Health AG015116, AG017586, NS044266, AG032953, NS053488, AG038490, and the Wyncote Foundation. We are grateful to Delani Gunawardena and the radiographers at the Hospital of the University of Pennsylvania for their assistance with data collection, and to our volunteers for their participation.
APPENDIX I. STIMULUS MATERIALS
| Sight | Sound | Manipulation | Abstract | Pseudoword | ||
|---|---|---|---|---|---|---|
| ant | airplane | axe | affliction | aspatome | kasimator | proomtrance |
| apple | alarm | ball | apathy | bamble | kellatine | prootch |
| beaver | ambulance | boomerang | apology | blar | klairge | punastan |
| blueberry | applause | broom | dilemma | blummitch | korf | rackel |
| brick | band | brush | doctrine | bonkle | kose | remmud |
| broccoli | belch | calculator | domain | boovel | krub | rendug |
| butterfly | bomb | camera | enigma | borb | lagric | rettle |
| cactus | carol | chisel | envy | borber | lammet | rolk |
| carrot | chime | chopsticks | epic | bronge | larkiston | ruggle |
| chimney | choir | computer | essence | buntle | lavid | sappage |
| cloud | commotion | crayon | fate | canchy | lonk | scrab |
| corn | concert | crowbar | fee | cantroole | lunketeer | shabed |
| crown | cricket | doorknob | gist | cheldo | lunnet | shermoffer |
| daffodil | dynamite | drumstick | grudge | chutlent | lurble | sistrem |
| diamond | explosion | eraser | hazard | dassipo | maculee | skidget |
| flamingo | fireworks | floss | heresy | diffle | mafer | slurt |
| goldfish | gunshot | fork | hindrance | dinkatoo | marlorn | splook |
| grass | heartbeat | hairbrush | hint | dolan | marskel | spreellain |
| icicle | hiccup | handle | honor | dorzle | meavey | starp |
| lemon | infant | key | internship | dranby | miffle | taltrow |
| lobster | laughter | knife | luck | eldapree | minulas | tarpec |
| moon | lullaby | lever | mercy | enkuar | mitter | tasinow |
| mountain | melody | lighter | myth | favy | moffle | teeble |
| mushroom | noise | needle | origin | fep | morance | tekkel |
| newspaper | opera | pencil | paradox | fickulation | mulvatose | tohlo |
| octopus | orchestra | pitchfork | perjury | fimmel | mundle | torrac |
| peach | rattlesnake | pliers | precursor | flandor | nagmet | troodle |
| penguin | riot | razor | protocol | flern | natchitar | tunnery |
| pineapple | rocket | scalpel | proxy | fordent | newper | ulch |
| pumpkin | rooster | scissors | saga | frannitch | ortoma | vell |
| pyramid | ruckus | screwdriver | silence | frapple | papple | vetta |
| rose | singer | slingshot | skill | frinkle | permi | wengrel |
| sky | siren | spoon | solution | gennery | pernasan | wimerson |
| snail | song | stapler | soul | gruzzle | petchelok | worpel |
| spider | storm | sword | strife | guppet | phoog | wroon |
| star | symphony | syringe | synopsis | gutchup | pishton | wuffle |
| sun | thunder | tool | theme | hoffle | pitchel | wump |
| tree | train | toothbrush | upkeep | jaster | plig | yalter |
| turtle | uproar | utensil | weekend | julg | porlent | zelk |
| zebra | waterfall | wrench | zeal | jun | pranny | zummelton |
APPENDIX II. STIMULUS CHARACTERISTICS
Summary of linguistic features of the stimuli. Values are means with standard deviations. Frequency norms (Lund and Burgess, 1996) were obtained from the English Lexicon Project website (Balota et al., 2007). Familiarity ratings were obtained in a previous study (Bonner and Grossman, 2012). Imageability ratings were obtained from the MRC psycholinguistic database (Coltheart, 1981) and were available for 62% of the items. Norms for orthographic neighborhood, unigram, bigram, and trigram frequencies were obtained from the CELEX database (Baayen et al., 1995).
| Stimulus characteristics | Sight | Sound | Manipulation | Abstract | Pseudoword |
|---|---|---|---|---|---|
| Letter length | 6.1 (1.8) | 6.6 (1.8) | 6.6 (2.0) | 6.0 (1.8) | 6.4 (1.6) |
| Syllable length | 1.9 (0.80) | 2.1 (0.73) | 1.9 (0.74) | 2.1 (0.9) | 2.0 (0.7) |
| Lexical frequency (HAL log frequency) | 8.1 (1.7) | 8.0 (1.5) | 7.5 (2.0) | 8.5 (1.4) | NA |
| Familiarity (0 to 6) | 5.7 (0.2) | 5.5 (0.3) | 5.5 (0.4) | 5.0 (0.5) | NA |
| Imageability (100 to 700) | 605 (26) | 585 (31) | 581 (42) | 355 (61) | NA |
| Orthographic neighborhood frequency (per million) | 120.7 (678.8) | 15.1 (31.6) | 24.8 (85.3) | 49.9 (235.6) | 6.9 (22.1) |
| Unigram frequency (per million) | 248407 (55426) | 259720 (51138) | 251315 (42541) | 258448 (55845) | 247544 (49863) |
| Bigram frequency (per million) | 17682 (8378) | 21463 (9950) | 19365 (8189) | 21975 (13888) | 17833 (7668) |
| Trigram frequency (per million) | 1535 (1241) | 2353 (2875) | 2256 (2239) | 2597 (4858) | 1274 (1192) |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander DC, Pierpaoli C, Basser PJ, Gee JC. Spatial transformations of diffusion tensor magnetic resonance images. Medical Imaging, IEEE Transactions on. 2001;20:1131–1139. doi: 10.1109/42.963816. [DOI] [PubMed] [Google Scholar]
- Andersen RA, Asanuma C, Essick G, Siegel RM. Corticocortical connections of anatomically and physiologically defined subdivisions within the inferior parietal lobule. The Journal of Comparative Neurology. 1990;296:65–113. doi: 10.1002/cne.902960106. [DOI] [PubMed] [Google Scholar]
- Ardila A, Concha M, Rosselli M. Angular gyrus syndrome revisited: Acalculia, finger agnosia, right-left disorientation and semantic aphasia. Aphasiology. 2000;14:743–754. [Google Scholar]
- Ashburner J, Friston K. Multimodal Image Coregistration and Partitioning--A Unified Framework. Neuroimage. 1997;6:209–217. doi: 10.1006/nimg.1997.0290. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Avants BB, Epstein CL, Grossman M, Gee JC. Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Medical Image Analysis. 2008;12:26–41. doi: 10.1016/j.media.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baayen R, Piepenbrock R, Gulikers L. The CELEX lexical database (version release 2)[CD-ROM] Philadelphia, PA: Linguistic Data Consortium, University of Pennsylvania; 1995. [Google Scholar]
- Balota D, Yap M, Hutchison K, Cortese M, Kessler B, Loftis B, Neely J, Nelson D, Simpson G, Treiman R. The English Lexicon Project. Behavior Research Methods. 2007;39:445–459. doi: 10.3758/bf03193014. [DOI] [PubMed] [Google Scholar]
- Barsalou LW. Grounded cognition. Annual Review of Psychology. 2008;59:617–645. doi: 10.1146/annurev.psych.59.103006.093639. [DOI] [PubMed] [Google Scholar]
- Bedny M, Caramazza A, Grossman E, Pascual-Leone A, Saxe R. Concepts Are More than Percepts: The Case of Action Verbs. The Journal of Neuroscience. 2008;28:11347–11353. doi: 10.1523/JNEUROSCI.3039-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson DF. Aphasia, alexia and agraphia. Churchill Livingstone; New York: 1979. [Google Scholar]
- Binder J, Desai R. The neurobiology of semantic memory. Trends in Cognitive Sciences. 2011;15:527–536. doi: 10.1016/j.tics.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder J, Desai R, Graves W, Conant L. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cerebral Cortex. 2009;19:2767–2796. doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder J, McKiernan KA, Parsons ME, Westbury CF, Possing ET, Kaufman JN, Buchanan L. Neural correlates of lexical access during visual word recognition. Journal of Cognitive Neuroscience. 2003;15:372–393. doi: 10.1162/089892903321593108. [DOI] [PubMed] [Google Scholar]
- Binder JR, Westbury CF, McKiernan KA, Possing ET, Medler DA. Distinct brain systems for processing concrete and abstract concepts. Journal of Cognitive Neuroscience. 2005;17:905–917. doi: 10.1162/0898929054021102. [DOI] [PubMed] [Google Scholar]
- Binkofski F, Buxbaum LJ. Two action systems in the human brain. Brain and Language. doi: 10.1016/j.bandl.2012.07.007. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner MF, Ash S, Grossman M. The new classification of primary progressive aphasia into semantic, logopenic, or nonfluent/agrammatic variants. Current Neurology and Neuroscience Reports. 2010;10:484–490. doi: 10.1007/s11910-010-0140-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner MF, Grossman M. Gray matter density of auditory association cortex relates to knowledge of sound concepts in primary progressive aphasia. The Journal of Neuroscience. 2012;32:7986–7991. doi: 10.1523/JNEUROSCI.6241-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner MF, Vesely L, Price C, Anderson C, Richmond L, Farag C, Avants B, Grossman M. Reversal of the concreteness effect in semantic dementia. Cognitive Neuropsychology. 2009;26:568–579. doi: 10.1080/02643290903512305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Sepulcre J, Talukdar T, Krienen FM, Liu H, Hedden T, Andrews-Hanna JR, Sperling RA, Johnson KA. Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer’s disease. The Journal of Neuroscience. 2009;29:1860–1873. doi: 10.1523/JNEUROSCI.5062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspers S, Eickhoff SB, Rick T, von Kapri A, Kuhlen T, Huang R, Shah NJ, Zilles K. Probabilistic fibre tract analysis of cytoarchitectonically defined human inferior parietal lobule areas reveals similarities to macaques. Neuroimage. 2011;58:362–380. doi: 10.1016/j.neuroimage.2011.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavada C, Goldman-Rakic PS. Posterior parietal cortex in rhesus monkey: I. Parcellation of areas based on distinctive limbic and sensory corticocortical connections. Journal of Comparative Neurology. 1989a;287:393–421. doi: 10.1002/cne.902870402. [DOI] [PubMed] [Google Scholar]
- Cavada C, Goldman-Rakic PS. Posterior parietal cortex in rhesus monkey: II. Evidence for segregated corticocortical networks linking sensory and limbic areas with the frontal lobe. Journal of Comparative Neurology. 1989b;287:422–445. doi: 10.1002/cne.902870403. [DOI] [PubMed] [Google Scholar]
- Chao LL, Haxby JV, Martin A. Attribute-based neural substrates in temporal cortex for perceiving and knowing about objects. Nature Neuroscience. 1999;2:913–919. doi: 10.1038/13217. [DOI] [PubMed] [Google Scholar]
- Cipolotti L, Butterworth B, Denes G. A specific deifict for numbers in a case of dense calculia. Brain. 1991;114:2619–2637. doi: 10.1093/brain/114.6.2619. [DOI] [PubMed] [Google Scholar]
- Collins CE, Airey DC, Young NA, Leitch DB, Kaas JH. Neuron densities vary across and within cortical areas in primates. Proceedings of the National Academy of Sciences. 2010;107:15927–15932. doi: 10.1073/pnas.1010356107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coltheart M. The MRC Psycholinguistic database Quarterly. Journal of Experimental Psychology. 1981;33:497–505. [Google Scholar]
- Coltheart M, Curtis B, Atkins P, Haller M. Models of reading aloud: Dual-route and parallel-distributed-processing approaches. Psychological Review. 1993;100:589–608. [Google Scholar]
- Cook P, Bai Y, Nedjati-Gilani S, Seunarine K, Hall M, Parker G, Alexander D. Camino: open-source diffusion-MRI reconstruction and processing. International Society for Magnetic Resonance in Medicine. 2006;14:2759. [Google Scholar]
- Damasio A. Time-locked multiregional retroactivation: A systems level proposal for the neural substrates of recall and recognition. Cognition. 1989;33:25–62. doi: 10.1016/0010-0277(89)90005-x. [DOI] [PubMed] [Google Scholar]
- Damasio H. Cerebral localization of the aphasias. In: Sarno MT, editor. Acquired aphasia. Academic Press; Orlando: 1981. pp. 27–50. [Google Scholar]
- Desgranges B, Baron JC, de la Sayette V, Petit-Taboue MC, Benali K, Landeau B, Lechevalier B, Eustache F. The neural substrates of memory systems impairment in Alzheimer’s disease: A PET study of resting brain glucose utilization. Brain. 1998;121:611–631. doi: 10.1093/brain/121.4.611. [DOI] [PubMed] [Google Scholar]
- Devlin JT, Russell RP, Davis MH, Price CJ, Wilson J, Moss HE, Matthews PM, Tyler LK. Susceptibility-Induced Loss of Signal: Comparing PET and fMRI on a Semantic Task. Neuroimage. 2000;11:589–600. doi: 10.1006/nimg.2000.0595. [DOI] [PubMed] [Google Scholar]
- Dove G. On the need for embodied and dis-embodied cognition. Frontiers in Psychology. 2011:1. doi: 10.3389/fpsyg.2010.00242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dronkers NF, Wilkins DP, Van Valin RD, Jr, Redfern BB, Jaeger JJ. Lesion analysis of the brain areas involved in language comprehension. Cognition. 2004;92:145–177. doi: 10.1016/j.cognition.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Elston GN, Benavides-Piccione R, DeFelipe J. The pyramidal cell in cognition: a comparative study in human and monkey. The Journal of Neuroscience. 2001;21:RC163. doi: 10.1523/JNEUROSCI.21-17-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans G, Lambon Ralph M, Woollams A. What’s in a word? A parametric study of semantic influences on visual word recognition. Psychonomic Bulletin & Review. 2012;19:325–331. doi: 10.3758/s13423-011-0213-7. [DOI] [PubMed] [Google Scholar]
- Friederici A. The brain basis of language processing: from structure to function. Physiological Reviews. 2011;91:1357–1392. doi: 10.1152/physrev.00006.2011. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Ashburner J, Frith CD, Poline JB, Heather JD, Frackowiak RSJ. Spatial registration and normalization of images. Human Brain Mapping. 1995;3:165–189. [Google Scholar]
- Geschwind N. Disconnexion syndromes in animals and man. Brain. 1965;88:237–297. doi: 10.1093/brain/88.2.237. [DOI] [PubMed] [Google Scholar]
- Glasser MF, Van Essen DC. Mapping human cortical areas in vivo based on myelin content as revealed by T1- and T2-weighted MRI. The Journal of Neuroscience. 2011;31:11597–11616. doi: 10.1523/JNEUROSCI.2180-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman M, Anderson C, Khan A, Avants B, Elman L, McCluskey L. Impaired action knowledge in amyotrophic lateral sclerosis. Neurology. 2008;71:1396–1401. doi: 10.1212/01.wnl.0000319701.50168.8c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman M, Koenig P, DeVita C, Glosser G, Alsop D, Detre J, Gee J. The neural basis for category-specific knowledge: An fMRI study. Neuroimage. 2002;15:936–948. doi: 10.1006/nimg.2001.1028. [DOI] [PubMed] [Google Scholar]
- Grossman M, Koenig P, Glosser G, DeVita C, Moore P, Rhee J, Detre J, Alsop D, Gee J. Neural basis for semantic memory difficulty in Alzheimer’s disease: An fMRI study. Brain. 2003;126:292–311. doi: 10.1093/brain/awg027. [DOI] [PubMed] [Google Scholar]
- Grossman M, White-Devine T, Payer F, Onishi K, D’Esposito M, Robinson KM, Alavi A. Constraints on the cerebral basis for semantic processing from neuroimaging studies of Alzheimer’s disease. Journal of Neurology, Neurosurgery, and Psychiatry. 1997;63:152–158. doi: 10.1136/jnnp.63.2.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauk O, Johnsrude I, Pulvermüller F. Somatotopic representation of action words in the motor and premotor cortex. Neuron. 2004;41:301–307. doi: 10.1016/s0896-6273(03)00838-9. [DOI] [PubMed] [Google Scholar]
- Henson R. Efficient experimental design for fMRI. Statistical parametric mapping. The analysis of functional brain images. 2007:193–210. [Google Scholar]
- Hill J, Inder T, Neil J, Dierker D, Harwell J, Van Essen D. Similar patterns of cortical expansion during human development and evolution. Proceedings of the National Academy of Sciences. 2010;107:13135–13140. doi: 10.1073/pnas.1001229107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges J, Patterson K. Semantic dementia: a unique clinicopathological syndrome. Lancet Neurology. 2007;6:1004–1014. doi: 10.1016/S1474-4422(07)70266-1. [DOI] [PubMed] [Google Scholar]
- Hyvärinen J. Posterior parietal lobe of the primate brain. Physiological Reviews. 1982;62:1060–1129. doi: 10.1152/physrev.1982.62.3.1060. [DOI] [PubMed] [Google Scholar]
- Jacobs B, Schall M, Prather M, Kapler E, Driscoll L, Baca S, Jacobs J, Ford K, Wainwright M, Treml M. Regional dendritic and spine variation in human cerebral cortex: a quantitative golgi study. Cerebral Cortex. 2001;11:558–571. doi: 10.1093/cercor/11.6.558. [DOI] [PubMed] [Google Scholar]
- Jones EG, Powell TPS. An anatomical study of converging sensory pathways within the cerebral cortex of the monkey. Brain. 1970;93:793–820. doi: 10.1093/brain/93.4.793. [DOI] [PubMed] [Google Scholar]
- Kemmerer D, Rudrauf D, Manzel K, Tranel D. Behavioral patterns and lesion sites associated with impaired processing of lexical and conceptual knowledge of actions. Cortex. doi: 10.1016/j.cortex.2010.11.001. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kertesz A, Sheppard A, MacKenzie R. Localization in Transcortical Sensory Aphasia. Archives of Neurology. 1982;39:475–478. doi: 10.1001/archneur.1982.00510200017002. [DOI] [PubMed] [Google Scholar]
- Kiefer M, Pulvermüller F. Conceptual representations in mind and brain: Theoretical developments, current evidence and future directions. Cortex. 2011 doi: 10.1016/j.cortex.2011.04.006. [DOI] [PubMed] [Google Scholar]
- Kiefer M, Sim EJJ, Herrnberger B, Grothe J, Hoenig K. The sound of concepts: four markers for a link between auditory and conceptual brain systems. The Journal of Neuroscience. 2008;28:12224–12230. doi: 10.1523/JNEUROSCI.3579-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein A, Andersson J, Ardekani BA, Ashburner J, Avants B, Chiang MC, Christensen GE, Collins DL, Gee J, Hellier P, Song JH, Jenkinson M, Lepage C, Rueckert D, Thompson P, Vercauteren T, Woods RP, Mann JJ, Parsey RV. Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. Neuroimage. 2009;46:786–802. doi: 10.1016/j.neuroimage.2008.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig P, Grossman M. Process and content in semantic memory. In: Hart JJ, Kraut MA, editors. Neural basis of semantic memory. Cambridge University Press; Cambridge: 2007. pp. 247–264. [Google Scholar]
- Lambon Ralph MA, Patterson K. Generalization and differentiation in semantic memory. Annals of the New York Academy of Sciences. 2008;1124:61–76. doi: 10.1196/annals.1440.006. [DOI] [PubMed] [Google Scholar]
- Leichnetz GR. An intrahemispheric columnar projection between two cortical multisensory convergence areas (inferior parietal lobule and prefrontal cortex): an anterograde study in macaque using HRP gel. Neuroscience Letters. 1980;18:119–124. doi: 10.1016/0304-3940(80)90313-4. [DOI] [PubMed] [Google Scholar]
- Lund K, Burgess C. Producing high-dimensional semantic spaces from lexical co-occurrence. Behavior Research Methods, Instruments, & Computers. 1996;28:203–208. [Google Scholar]
- Machery E. Concept empiricism: A methodological critique. Cognition. 2007;104:19–46. doi: 10.1016/j.cognition.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Mahon BZ, Caramazza A. A critical look at the embodied cognition hypothesis and a new proposal for grounding conceptual content. Journal of Physiology, Paris. 2008;102:59–70. doi: 10.1016/j.jphysparis.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Martin A. The representation of object concepts in the brain. Annual Review of Psychology. 2007;58:25–45. doi: 10.1146/annurev.psych.57.102904.190143. [DOI] [PubMed] [Google Scholar]
- McClelland JL, Rogers TT. The parallel distributed processing approach to semantic cognition. Nature Reviews Neuroscience. 2003;4:310–322. doi: 10.1038/nrn1076. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Van Hoesen GW, Pandya DN, Geschwind N. Limbic and sensory connections of the inferior parietal lobule (area PG) in the rhesus monkey: A study with a new method for horseradish peroxidase histochemistry. Brain Research. 1977;136:393–414. doi: 10.1016/0006-8993(77)90066-x. [DOI] [PubMed] [Google Scholar]
- Mion M, Patterson K, Acosta-Cabronero J, Pengas G, Izquierdo-Garcia D, Hong YT, Fryer TD, Williams GB, Hodges JR, Nestor PJ. What the left and right anterior fusiform gyri tell us about semantic memory. Brain. 2010;133:3256–3268. doi: 10.1093/brain/awq272. [DOI] [PubMed] [Google Scholar]
- Mori S, Crain BJ, Chacko VP, Van Zijl PCM. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Annals of Neurology. 1999;45:265–269. doi: 10.1002/1531-8249(199902)45:2<265::aid-ana21>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Myung J-y, Blumstein SE, Sedivy JC. Playing on the typewriter, typing on the piano: manipulation knowledge of objects. Cognition. 2006;98:223–243. doi: 10.1016/j.cognition.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Oishi K, Zilles K, Amunts K, Faria A, Jiang H, Li X, Akhter K, Hua K, Woods R, Toga AW, Pike GB, Rosa-Neto P, Evans A, Zhang J, Huang H, Miller MI, van Zijl PC, Mazziotta J, Mori S. Human brain white matter atlas: identification and assignment of common anatomical structures in superficial white matter. Neuroimage. 2008;43:447–457. doi: 10.1016/j.neuroimage.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orban GA, Van Essen D, Vanduffel W. Comparative mapping of higher visual areas in monkeys and humans. Trends in Cognitive Sciences. 2004;8:315–324. doi: 10.1016/j.tics.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Paivio A. Dual coding theory: Retrospect and current status. Canadian Journal of Psychology. 1991;45:255–287. [Google Scholar]
- Pallier C, Devauchelle AD, Dehaene S. Cortical representation of the constituent structure of sentences. Proceedings of the National Academy of Sciences. 2011;108:2522–2527. doi: 10.1073/pnas.1018711108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandya DN, Seltzer B. Intrinsic connections and architectonics of posterior parietal cortex in the rhesus monkey. The Journal of Comparative Neurology. 1982;204:196–210. doi: 10.1002/cne.902040208. [DOI] [PubMed] [Google Scholar]
- Patterson K, Nestor P, Rogers T. Where do you know what you know? The representation of semantic knowledge in the human brain. Nature Reviews Neuroscience. 2007;8:976–987. doi: 10.1038/nrn2277. [DOI] [PubMed] [Google Scholar]
- Peelle JE, Troiani V, Gee J, Moore P, McMillan C, Vesely L, Grossman M. Sentence comprehension and voxel-based morphometry in progressive nonfluent aphasia, semantic dementia, and nonaphasic frontotemporal dementia. Journal of Neurolinguistics. 2008;21:418–432. doi: 10.1016/j.jneuroling.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Projections to the frontal cortex from the posterior parietal region in the rhesus monkey. The Journal of Comparative Neurology. 1984;228:105–116. doi: 10.1002/cne.902280110. [DOI] [PubMed] [Google Scholar]
- Postle N, McMahon KL, Ashton R, Meredith M, de Zubicaray GI. Action word meaning representations in cytoarchitectonically defined primary and premotor cortices. Neuroimage. 2008;43:634–644. doi: 10.1016/j.neuroimage.2008.08.006. [DOI] [PubMed] [Google Scholar]
- Price CJ. The anatomy of language: a review of 100 fMRI studies published in 2009. Annals of the New York Academy of Sciences. 2010;1191:62–88. doi: 10.1111/j.1749-6632.2010.05444.x. [DOI] [PubMed] [Google Scholar]
- Pulvermüller F. Brain mechanisms linking language and action. Nature Reviews Neuroscience. 2005;6:576–582. doi: 10.1038/nrn1706. [DOI] [PubMed] [Google Scholar]
- Pulvermüller F, Härle M, Hummel F. Walking or Talking?: Behavioral and Neurophysiological Correlates of Action Verb Processing. Brain and Language. 2001;78:143–168. doi: 10.1006/brln.2000.2390. [DOI] [PubMed] [Google Scholar]
- Pulvermüller F, Shtyrov Y, Ilmoniemi RJ. Brain signatures of meaning access in action word recognition. Journal of Cognitive Neuroscience. 2005;17:884–892. doi: 10.1162/0898929054021111. [DOI] [PubMed] [Google Scholar]
- Rapcsak SZ, Rubens AB. Localization of lesions in transcortical aphasia. In: Kertesz A, editor. Localization and neuroimaging in neuropsychology. Academic Press; San Diego: 1994. pp. 297–329. [Google Scholar]
- Raposo A, Moss HE, Stamatakis EA, Tyler LK. Modulation of motor and premotor cortices by actions, action words and action sentences. Neuropsychologia. 2009;47:388–396. doi: 10.1016/j.neuropsychologia.2008.09.017. [DOI] [PubMed] [Google Scholar]
- Reilly J, Peelle JE. Effects of semantic impairment on language processing in semantic dementia. Seminars in Speech and Language. 2008;29:032–043. doi: 10.1055/s-2008-1061623. [DOI] [PubMed] [Google Scholar]
- Sabsevitz DS, Medler DA, Seidenberg M, Binder JR. Modulation of the semantic system by word imageability. Neuroimage. 2005;27:188–200. doi: 10.1016/j.neuroimage.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Salvador R, Peña A, Menon DK, Carpenter TA, Pickard JD, Bullmore ET. Formal characterization and extension of the linearized diffusion tensor model. Human Brain Mapping. 2005;24:144–155. doi: 10.1002/hbm.20076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seghier ML. The angular gyrus: Multiple functions and multiple subdivisions. Neuroscientist. 2012 doi: 10.1177/1073858412440596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selemon LD, Goldman-Rakic PS. Common cortical and subcortical targets of the dorsolateral prefrontal and posterior parietal cortices in the rhesus monkey: evidence for a distributed neural network subserving spatially guided behavior. Journal of Neuroscience. 1988;8:4049–4068. doi: 10.1523/JNEUROSCI.08-11-04049.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seltzer B, Pandya DN. Afferent cortical connections and architectonics of the superior temporal sulcus and surrounding cortex in the rhesus monkey. Brain Research. 1978;149:1–24. doi: 10.1016/0006-8993(78)90584-x. [DOI] [PubMed] [Google Scholar]
- Seltzer B, Pandya DN. Further observations on parieto-temporal connections in the rhesus monkey. Experimental Brain Research. 1984;55:301–312. doi: 10.1007/BF00237280. [DOI] [PubMed] [Google Scholar]
- Seltzer B, Pandya DN. Parietal, temporal, and occipital projections to cortex of the superior temporal sulcus in the rhesus monkey: a retrograde tracer study. Journal of Comparative Neurology. 1994;343:445–463. doi: 10.1002/cne.903430308. [DOI] [PubMed] [Google Scholar]
- Sherwood CC, Subiaul F, Zawidzki TW. A natural history of the human mind: tracing evolutionary changes in brain and cognition. Journal of Anatomy. 2008;212:426–454. doi: 10.1111/j.1469-7580.2008.00868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, Roffman JL, Smoller JW, Zöllei L, Polimeni JR, Fischl B, Liu H, Buckner RL. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. Journal of Neurophysiology. 2011;106:1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turken AU, Dronkers NF. The neural architecture of the language comprehension network: converging evidence from lesion and connectivity analyses. Frontiers in Systems Neuroscience. 2011:5. doi: 10.3389/fnsys.2011.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tustison NJ, Avants BB, Cook PA, Yuanjie Z, Egan A, Yushkevich PA, Gee JC. N4ITK: Improved N3 Bias Correction. Medical Imaging, IEEE Transactions on. 2010;29:1310–1320. doi: 10.1109/TMI.2010.2046908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated Anatomical Labeling of Activations in SPM Using a Macroscopic Anatomical Parcellation of the MNI MRI Single-Subject Brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Supekar K, Amin H, Rykhlevskaia E, Nguyen DA, Greicius MD, Menon V. Dissociable Connectivity within Human Angular Gyrus and Intraparietal Sulcus: Evidence from Functional and Structural Connectivity. Cerebral Cortex. 2010;20:2636–2646. doi: 10.1093/cercor/bhq011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbulcke M, Peeters R, Fannes K, Vandenberghe R. Knowledge of visual attributes in the right hemisphere. Nature Neuroscience. 2006;9:964–970. doi: 10.1038/nn1721. [DOI] [PubMed] [Google Scholar]
- Vigliocco G, Meteyard L, Andrews M, Kousta S. Toward a theory of semantic representation. Language and Cognition. 2009:219. [Google Scholar]
- von Stein A, Rappelsberger P, Sarnthein J, Petsche H. Synchronization between temporal and parietal cortex during multimodal object processing in man. Cerebral Cortex. 1999;9:137–150. doi: 10.1093/cercor/9.2.137. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Henson RN, Driver J, Dolan RJ. Multiple levels of visual object constancy revealed by event-related fMRI of repetition priming. Nature Neuroscience. 2002;5:491–499. doi: 10.1038/nn839. [DOI] [PubMed] [Google Scholar]
- Wang J, Conder JA, Blitzer DN, Shinkareva SV. Neural representation of abstract and concrete concepts: a meta-analysis of neuroimaging studies. Human Brain Mapping. 2010;31:1459–1468. doi: 10.1002/hbm.20950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheatley T, Weisberg J, Beauchamp MS, Martin A. Automatic priming of semantically related words reduces activity in the fusiform gyrus. Journal of Cognitive Neuroscience. 2005;17:1871–1885. doi: 10.1162/089892905775008689. [DOI] [PubMed] [Google Scholar]
- Woollams AM, Ralph MAL, Plaut DC, Patterson K. SD-squared: On the association between semantic dementia and surface dyslexia. Psychological Review. 2007;114:316–339. doi: 10.1037/0033-295X.114.2.316. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Evans AC, Marrett S, Neelin P. A three-dimensional statistical analysis for CBF activation studies in human brain. Journal of Cerebral Blood Flow and Metabolism. 1992;12:900–918. doi: 10.1038/jcbfm.1992.127. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Heteromodal whole-brain analyses showing overlap of activation across categories. These are the same results as shown in Figure 3, but with activation clusters shown along left motor cortex that relate to the button-press response. We identified activations that correlated with activity in primary motor cortex. Parameter estimates were extracted from spheres (4 mm radius) around the peak voxel of each cluster for the activation of words relative pseudowords. We found that across participants activity in left primary motor cortex (excluding one outlier whose motor cortex activation was greater than three standard deviations from the mean) strongly correlated with activity in an adjacent cluster deep in the lateral sulcus (Pearson r=.7, p<.001) but did not correlate with activity in the angular gyrus or the lateral temporal lobe (all Pearson r<.35, p>.15).