Abstract
In utero exposure to cigarette smoke has severe consequences for the developing fetus, including increased risk of birth complications and behavioral and learning disabilities later in life. Evidence from animal models suggests that the cognitive deficits may be a consequence of in utero nicotine exposure in the brain during critical developmental periods. However, maternal smoking exposes the fetus to not only nicotine but also a hypoxic intrauterine environment. Thus, both nicotine and hypoxia are capable of initiating cellular cascades, leading to long-term changes in synaptic patterning that have the potential to affect cognitive functions. The present study investigates the combined effect of in utero exposure to nicotine and hypoxia on neuronal and glial elements in the hippocampal CA1 field. Fetal guinea pigs were exposed in utero to normoxic or hypoxic conditions in the presence or absence of nicotine. Hypoxia increased the protein levels of matrix metalloproteinase-9 (MMP-9) and synaptophysin and decreased the neural density as measured by NeuN immunoreactivity (ir). Nicotine exposure had no effect on these neuronal parameters but dramatically increased the density of astrocytes immunopositive for glial fibrillary acidic protein (GFAP). Further investigation into the effects of in utero nicotine exposure revealed that both GFAP-ir and NeuN-ir in the CA1 field were significantly reduced in adulthood. Taken together, our data suggest that prenatal exposure to nicotine and hypoxia not only alters synaptic patterning acutely during fetal development, but that nicotine also has long-term consequences that are observed well into adulthood. Moreover, these effects most likely take place through distinct mechanisms.
Keywords: Neuronal insult, Synaptic Patterning, Matrix Metalloproteinase, Glial Fibrillary Acidic Protein, CNS Development, Smoking
INTRODUCTION
Maternal cigarette smoking during pregnancy is a significant, yet preventable, risk factor to the health and development of a fetus. Complications long associated with maternal smoking during pregnancy include low birth weight, a higher risk for Sudden Infant Death Syndrome, and increased tendency to develop behavioral and learning disabilities, including Attention Deficit and Hyperactivity Disorder (Gaither et al. 2009; Ginzel et al. 2007; Martin et al. 2008; Slotkin 1998; Suzuki et al. 2008; Winzer-Serhan 2008). Nicotine is a significant component of cigarette smoke that easily crosses the placental barrier, enters the fetal circulatory system, and spreads throughout the developing organs (Lambers and Clark 1996). In addition to nicotine, smoking mothers inhale significant amounts of carbon monoxide that interferes with the ability of their circulatory system to efficiently deliver oxygen to tissues resulting in a reduced oxygen (hypoxic) environment for the fetus (Rees et al. 2008).
Much research has been conducted to investigate the effects of nicotine on the developing fetal brain, (reviewed in (Ginzel et al. 2007; Slotkin 1998)), while many other studies have examined the developmental effects of hypoxia, usually with regards to preterm births (reviewed in (Barrett et al. 2007). Nicotine is capable of mimicking acetylcholine, interacting with nicotinic acetylcholine receptors (nAChRs) in both the heart and brain (Slotkin et al. 1999). High levels of nicotine exposure as a result of maternal smoking leads to increased stimulation of these receptors, aberrant neuronal maturation and increased expression of nAChR subunit mRNA (Slotkin 1998; Lv et al. 2008). This early disruption of nAChRs leads to long-term functional impairments, including an impaired ability to respond to stimuli and learn an auditory-cued task (Liang et al. 2006; Ginzel et al. 2007). In addition, preterm exposure to hypoxia can result in reduced catecholamine levels, decreased gray matter in the cerebral cortex and an increased risk of fetal death (Ginzel et al. 2007; Inder et al. 2005; Barrett et al. 2007).
Taken together, the above suggest that prenatal exposure to either nicotine or hypoxia has serious consequences for the developing fetus. However, actual cigarette smoking by pregnant mothers exposes the developing fetus to both nicotine and a hypoxic environment, making it important to examine the effects of both in combination. We have developed an experimental model that allows us to examine the effects of nicotine and hypoxia both individually and in combination. To more completely examine the effects of maternal cigarette smoking, pregnant guinea pigs were exposed to either (i) normoxic conditions with normal drinking water, (ii) normoxic conditions with nicotine in the drinking water, (iii) hypoxic conditions with normal drinking water, or (iv) hypoxic conditions with nicotine in the drinking water.
Previous work using this model has shown that maternal exposure to either hypoxia (Oh et al. 2008) or nicotine (Thompson et al. 2011) increases the expression and/or activity of MMP-2 and MMP-9 in the fetal heart. Matrix metalloproteinases (MMPs) are zinc-binding peptidases that function to remodel the extracellular matrix through cleavage (Zitka et al. 2010). Additionally, MMPs also play a role in the regulation of signaling events via cleavage of cell surface molecules (reviewed in (Klein and Bischoff 2011)). MMPs are present in both the developing and adult brain, and contribute to neurogenesis, stabilization of synapses and dendritic spines, as well as aiding and promoting synaptic remodeling (Candelario-Jalil et al. 2009; Ethell and Ethell 2007; Wojcik et al. 2009). In rats, maternal hypoxia late in gestation increases the activity of MMP-2 and MMP-9 in neonatal brains (Tong et al. 2010). However, it is not currently known whether in utero exposure to nicotine or nicotine plus hypoxia induces similar changes in MMPs in the fetal brain. Here, we explored this question and its potential consequences for synaptic patterning.
We focused our investigation on the hippocampus as it plays an important role in learning and memory (Squire 1992), and early damage to the hippocampus is likely responsible for some of the long-term cognitive deficits resulting from maternal smoking (Hallak et al. 2000). The hippocampus contains a large number of nAChRs, which have been shown to change in response to nicotine treatment and affect synaptic plasticity (Hernandez-Morales and Garcia-Colunga 2009; Placzek et al. 2009). Additionally, maternal hypoxia has been reported to cause significant damage localized to the hippocampus (Hallak et al. 2000).
The present study examines the combined effects of nicotine and hypoxia on both neuronal and glial elements in the hippocampus and assesses the long-term consequences with the aim of more completely understanding the mechanism by which maternal smoking affects fetal development.
MATERIALS AND METHODS
Justification of Species
All procedures and animal care were in accordance with the guidelines of the University of Maryland Institutional Animal Care and Use Committee and conform to the Guide for the Care and Use of Laboratory Animals published by US NIH Publication No. 85-23, 1996. In short gestation species such as the rat and mouse, which are commonly used systems for CNS development, the majority of mid to late stage events (growth surge) occur postnatally. In long-gestational animals, like the guinea pig and human, this growth spurt occurs in utero, beginning mid gestation and finishing, shortly after birth (Byrnes et al. 2003; Dobbing and Sands 1970). This pattern of brain development in the guinea pig more closely resembles that of the humans, including cerebral development, maturation of amino acid concentration and brain esterases, and myelination, making it an ideal system for modeling human CNS development (Lagnado and Hardy 1967; Oja et al. 1968; Slavin et al. 1997).
Fetal Studies
Female Duncan-Hartley guinea pigs were purchased from a commercial breeder (Harlan Sprague Dawley, Indianapolis, IN) and time-mated in our animal facility. Animals were housed in the animal facility and given free access to food and water. Typically, the gestational duration of these animals is 65 days. Starting on gestational day (GD) 52 until GD 62 the pregnant guinea pigs were administered either water or nicotine bitartrate dehydrate (NIC, 200ug/ml; Sigma, St. Louis, MO) in the drinking water. We chose this time point as (1) earlier exposures to nicotine increased the risk of spontaneous abortions and (2) this period corresponds to the prenatal brain growth spurt of the guinea pig developing brain. Also beginning on GD 52, half of the pregnant dams from each of the aforementioned groups were placed in a plexiglass chamber containing 10.5% O2 for 10 days (HPX) as previously described (Oh et al. 2008). This level of hypoxia is sufficient in reducing fetal body weight without inducing spontaneous abortion in the pregnant guinea pig. The other half were designated as normoxic controls and housed under normal room air (NMX; 21% O2). Each treatment group contained 4–7 pregnant females and yielded 4–7 pups per treatment group.
On GD 62 (near term), pregnant mothers were anesthetized with ketamine (80 mg/ kg) and xylazine (1 mg/kg) and given lidocaine subcutaneously along the midline of the abdomen. An abdominal incision was made and fetuses removed via hysterectomy. Fetal blood was obtained via cardiac puncture from anesthetized fetuses via a 25-gauge syringe needle and a 1 ml tuberculin syringe, and fetal serum cotinine levels were measured (Cotinine Direct ELISA, Bio-Quant, San Diego, CA) as an index of fetal nicotine distribution following maternal ingestion. Brains were harvested and flash frozen or submersion fixed for 3 days in 4% paraformaldehyde and 2.5% acrolein in phosphate buffered saline (PBS). At the time of harvesting, brain weights were recorded. To insure a proper fixation of the hippocampal regions, the brain was blocked into 2–3mm slabs that contained the hippocampus. Following fixation, the brains were sucrose embedded for 3 days with 30% sucrose in PBS. After sucrose embedding, the brains were frozen on dry ice and stored at −80°C until processed for immunocytochemistry. On a technical note, the animals were not transcardially perfused with fixative because the peripheral organs including the heart were harvested for a separate study (Thompson et al. 2011).
Adult Studies
In the experiment to address programming effects of prenatal exposure to nicotine the dams were allowed to deliver naturally and the pups raised in normal control conditions until postnatal day 90. On that day, animals (n=4 for vehicle, n=5 nicotine) were anesthetized with ketamine (80 mg/ kg) and xylazine (1 mg/kg) and transcardially perfused with 0.9% saline followed by 4% paraformaldehyde and 2.5% acrolein in PBS. The brains were removed, post-fixed overnight in the paraformaldehyde/acrolein fixative. Following post-fixation the brains were sucrose embedded for 3 days with 30% sucrose in PBS. After sucrose embedding, the brains were frozen on dry ice and stored at −80°C until processed for immunocytochemistry.
Western Blots
Animals were treated as described above (n=5 per treatment group); the brains were collected and flash frozen in a dry ice-cooled bath of 2-methylbutane (Sigma) and stored at −80°C until processed. The brains were sectioned in the coronal plane into 350μm slabs with a cryostat and the CA1 region was micropunched according to the Palkovits micropunch procedure (Palkovits M 1988). Micropunches were homogenized via sonication in buffer containing 1% NP-40 (Sigma), 0.5% sodium deoxycholate (Sigma), 0.1% SDS (Quality Biological, Inc, Gaithersburg, MD), 150 mM NaCl, and 50 mM Tris HCl (pH8). At the time of homogenization, a protease inhibitor cocktail (0.1 mg/ 100 mL of aprotinin, leupeptin and pepstatin; 1 mm phenylmethylsulfonyl fluoride) was added. Protein concentration was determined using a bicinchoninic assay kit (Pierce, Rockford, IL). Protein (15μg) was loaded into a 10% Tris-glycine SDS-PAGE gel (Invitrogen, Carlsbad, CA). Electrophoresis and blotting was performed using a polyvinyl difluoride membrane (Invitrogen). The membranes were washed in 20 mM Tris-buffered saline solution with 0.05% Tween 20 (T-TBS). For MMP-9 (rabbit polyclonal antibody Chemicon International Inc, Temecula, CA), membranes were blocked in 5% powdered milk in T-TBS for 1 h at room temperature and washed three times in T-TBS before being incubated in primary antibody solution (T-TBS, 1:2,000) overnight at 4°C. For synaptophysin (mouse monoclonal, BD Biosciences, San Jose, CA), membranes were blocked overnight in 5% milk in T-TBS, washed three times in T-TBS, and incubated in primary antibody solution (T-TBS, 1:30,000) for 2 h at room temperature. After incubating in the primary antibody, all membranes were incubated in the appropriate IgG secondary antibody solution (anti-mouse or anti-rabbit, Cell Signaling Technology, Danvers, MA, T-TBS, 1:2000) for 1 h at room temperature. The Phototype-HRP chemiluminescent system (Cell Signaling Technology, Danvers, MA) was used for detection of the protein recognized by the antisera. The blots were exposed to Hyperfilm-ECL (Kodak, Rochester, NY) for varying exposure times. The films were then scanned into a computer at 1200 dpi, and the scanned images were analyzed using ImageJ freeware (http://rsbweb.nih.gov/ij/). The optical densities were measured for each individual band. In our preliminary analysis it was determined that hypoxic treatment dramatically changed the protein levels of GAPDH (data not shown), a housekeeping gene commonly used for normalizing gel-loading inconsistencies. As an alternative to GAPDH, we used Ponceau S staining of the membrane, which allows for accurate quantification of the amount of total protein bound to a membrane (Klein et al. 1995). Membranes were stained with Ponceau S solution (0.5% Ponceau in 1% glacial acetic acid made in dH2O) to standardize for any errors in sample loading as previously described (Holder and Mong 2010; Olesen and Auger 2005). The grayscale density of the prominent Ponceau S stained band at approximately 45kDa was quantified with NIH Image software as described above. The data are reported as a ratio of the immunoreactive band density to Ponceau S staining intensity at 45kDa.
Immunocytochemistry and Data Analysis
The brains were sectioned (50 μm) in the coronal plane with a cryostat and stored in a cryoprotectant solution of ethylene glycol/glucose in phosphate buffered saline. After sectioning, the tissue was divided into cohorts consisting of anatomically match sections from all treatment groups. Each cohort was processed in the same tray for either NeuN (n=6–7/group) or GFAP (n=4–6/group) immunoreactivity according to standard laboratory procedure. Briefly, sections were rinsed of cryoprotectant in 0.1 M phosphate-buffered saline (PBS), reacted with 1% sodium borohydride, and then reacted with 30% hydrogen peroxide. After primary and secondary antibody incubation, tissue was incubated with an avidin-biotin complex and visualized with nickel-enhanced 3, 3'-diaminobenzidine tetrahydrochloride (DAB; Polysciences Inc, Warrington, PA). NeuN antibody (mouse monoclonal, Chemicon, Temecula, CA) was used at a 1:600,000 dilution and the GFAP antibody (rabbit polyclonal, Sigma, St. Louis, MO) was used at a 1:500,000 dilution. The appropriate secondary antibody was used at a 1:800 dilution (biotinylated anti-mouse and anti rabbit, Vector Laboratories, Burlingame, CA). The tissue was then mounted on gelatin-coated slides and coverslipped.
A single examiner blinded to the treatment conditions performed densitometry of the immunoreactive stains using a Nikon Eclipse E600 microscope connected to a cool snap CCD camera. Images were captured with the CCD camera at 20× and analyzed using ImageJ freeware (http://rsbweb.nih.gov/ij/). The sections analyzed for NeuN-ir and GFAP-ir were from one-in-three series (adjacent sections were separated by 150 μm) and contained the CA1 field of the hippocampus and granular retrosplenial cortex (RSg) that corresponded anatomically to Plates 42 and 43 in an adolescent guinea pig atlas (Luparello 1967). Three brain sections per animals were chosen. The right and left sides of each brain region were measured and averaged to represent a single measurement for each section.
To assess NeuN immunoreactive density, background levels were normalized across all captured images via the `Subtract Background' IMAGEJ macro. Next, the `Threshold' tool was used to highlight the NeuN positive cells (as represented in the Figure 3 inset) and the mean optical density was measured. For the CA1 fields, to ensure that only the pyramidal cell layer was being measured, an eight-point box (rectangular selection tool) was placed over the highlighted pyramidal layer so that at least 6 of the 8 points reached the edge of the highlighted layer. Three measurements of the mean optical density were taken along the length of the CA1 field and averaged. For the RSg, a 400μm × 300μm box was drawn and the mean optical density was measured.
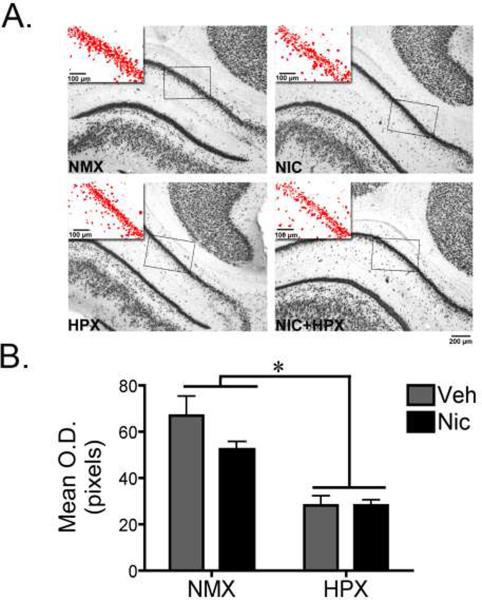
Figure 3. Prenatal hypoxia significantly decreased NeuN immunoreactivity in the fetal hippocampus.
(A) Representative photomicrographs of NeuN immunoreactivity (ir) in the CA1 region of the hippocampus from fetal guinea pigs exposed in utero to nicotine (n=6), hypoxia (n=6), nicotine and hypoxia (n=6), or normoxic conditions (n=7). The inset represents the area quantified with the threshold tool in ImageJ. (B) Quantification of NeuN-ir in the CA1 region of the hippocampus. Data represent bilateral optical density measurements in three sections that were averaged to yield a single value per animal. A two-way ANOVA revealed a significant main effect of hypoxia (F1,21=35.5, p<0.001). Tukey Kramer post-hoc analysis revealed that hypoxia with and without nicotine significantly decreased NeuN-ir in the CA1 region of the hippocampus compared to normoxic with or without nicotine (*p<0.05). All values are mean optical density in pixels ± SEM. NMX, normoxic; NIC, nicotine; HPX, hypoxic, O.D., optical density.
Changes in astrocytic surface area were assessed via GFAP immunoreactive density. Similar to the NeuN quantification above, background was normalized. Next a 400μm × 300μm box was placed either at the apex of the CA1 field (see Figure 4A) or the RSg (see Figure 5A) and the mean optical density measured. Of note, the placement of the CA1 measurement box was specifically selected to encompass stratum (str.) oriens and str. radiatum. GFAP-ir as expected was absent from the pyramidal layer (see Figure 4), but present in the str. oriens and str. radiatum, which lie directly above and below the pyramidal neuron cell body layer, respectively. These layers contain the basal (oriens) and apical (radiatum) dendrites from the pyramidal neurons as well as inhibitory cells and fiber tracts (The Hippocampus Book).
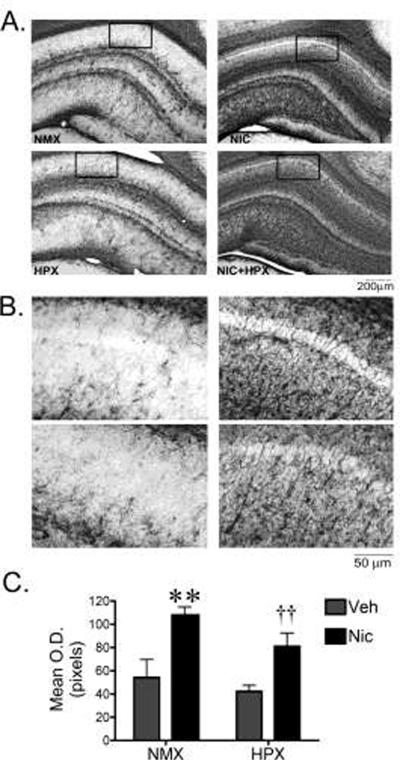
Figure 4. Prenatal nicotine in the presence and absence of hypoxia significantly increased GFAP immunoreactivity in the fetal hippocampus.
(A,B) Representative photomicrographs of GFAP immunoreactivity (ir) in the CA1 region of the hippocampus from fetal guinea pigs exposed in utero to nicotine (n=4), hypoxia (n=6), nicotine and hypoxia (n=5), or normoxic (n=5) conditions. The boxed field in (A) is representative of the area quantified. (B) Higher magnification photomicrographs of the CA1 region of the hippocampus used for quantification. (C) Quantification of GFAP-ir in the CA1 region of the hippocampus. Data represent bilateral optical density measurements in three sections that were averaged to yield a single value per animal. Two-way ANOVA revealed a significant main effect of nicotine (F1,19=19.2, p<0.001). Post-hoc analysis revealed that treatment with nicotine either alone or in conjunction with hypoxia increased the GFAP-ir density compared to hypoxic and normoxic conditions (**nicotine vs. normoxic or hypoxic groups, p<0.0002; †† nicotine plus hypoxia vs. normoxic or hypoxic groups, p<0.0005).
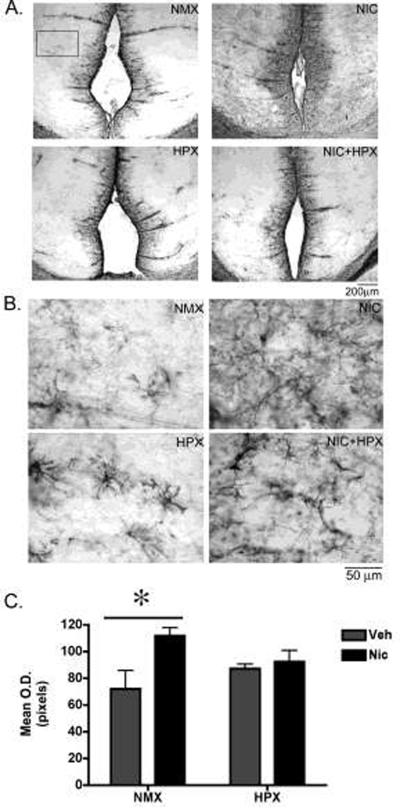
Figure 5. Prenatal nicotine but not nicotine plus hypoxia significantly increased GFAP immunoreactivity in the fetal retrosplenial (RSg) cortex.
(A,B) Representative photomicrographs of GFAP immunoreactivity (ir) in the retrosplenial cortex from fetal guinea pigs exposed in utero to nicotine (n=4), hypoxia (n=6), nicotine and hypoxia (n=5), or normoxic (n=5) conditions. The boxed field in (A) is representative of the area quantified. (B) Higher magnification photomicrographs of the RSg cortex used for quantification. (C) Quantification of GFAP-ir in the RSg cortex. Data represent bilateral optical density measurements in three sections that were averaged to yield a single value per animal. Two-way ANOVA revealed a significant main effect of nicotine (F1,19=6.6, p<0.02). Post-hoc analysis revealed that prenatal nicotine alone increased GFAP-ir in the RSg cortex compared to normoxic controls (Tukey Kramer post-hoc analysis p<0.05). All values are mean optical density in pixels ± SEM. NMX, normoxic; NIC, nicotine; HPX, hypoxic, O.D., optical density.
Statistical Analysis
Results are expressed as means ± SEM. The distribution of data did not deviate significantly from normality. Western blot data was analyzed with two-way ANOVA followed by post-hoc tests where appropriate. Adult immunocytochemistry data was analyzed with a two-tailed unpaired Student's t-test. Significance levels for all experiments were set at p=0.05. Statistical analyses were performed with the Statistica 10 software package (StatSoft, Inc., Tulsa, OK).
RESULTS
General observations
The average nicotine intake was 20.0±2.4 mg/kg/d, which is well within the range of 7–60mg/kg/d reported to increase plasma nicotine to levels achieved by habitual smokers (Benowitz and Gourlay 1997). The average fetal serum continine levels in nicotine exposed animals were 85+/− 5ng/mL and continine was undetectable in untreated fetuses. These levels are within the range measured in moderate, habitual smokers (Benowitz and Gourlay 1997; Jauniaux et al. 1999) indicating sufficient nicotine exposure of the fetus. The brains from the hypoxic and hypoxic plus nicotine exposed groups weighed approximately 8% less than the normoxic controls but this did not reach statistical significance (normoxic, 2.5 ± 0.07 g; nicotine, 2.510 ± 0.05 g, hypoxic, 2.386 ± 0.04 g and hypoxic plus nicotine 2.331 ± 0.08g; one-way ANOVA F(3,55)=2.57; p=0.064; Data are mean ± SEM).
Prenatal hypoxia alone increased MMP-9 protein levels in the CA1 hippocampal sub-field
In the guinea pig fetal heart, prenatal hypoxia increases protein levels of MMP- 9 and activity of MMP-2 (Oh et al. 2008) while prenatal nicotine increases protein levels of the active form of MMP-2 (Thompson et al. 2011). Since MMP-9 is expressed in the developing hippocampus, we tested whether in utero exposure to hypoxia and/or nicotine increased MMP-9 protein levels in the CA1 region of the fetal hippocampus. A two-way ANOVA of both the active and proform of MMP-9, with atmosphere (hypoxic or normoxic conditions) and drug (nicotine or vehicle) as factors, revealed a significant interaction between prenatal nicotine and hypoxia (Figure 1A proform: F(1,16)=8.83, p<0.01; Figure 1B active: F(1,16)=10.36, p<0.01). Compared to all other treatment groups, hypoxia significantly increased protein levels of the 92 kDa proform of MMP-9 by approximately 200% (Tukey Kramer post-hoc analysis p<0.002). The proform is activated when cleaved by extracellular proteinases to an 86 kDa protein (Okada et al. 1992). Hypoxia also increased protein levels of the active form compared to all other treatment groups (Tukey Kramer post-hoc analysis p<0.05). Interestingly, when exposed in utero to the combination of hypoxia and nicotine the protein levels of both the pro and active form of MMP-9 were not significantly different than that of the controls (Figure 1), suggesting that the addition of nicotine blocks the increased expression of MMP-9. Nicotine alone had no effect on MMP-9 protein levels.
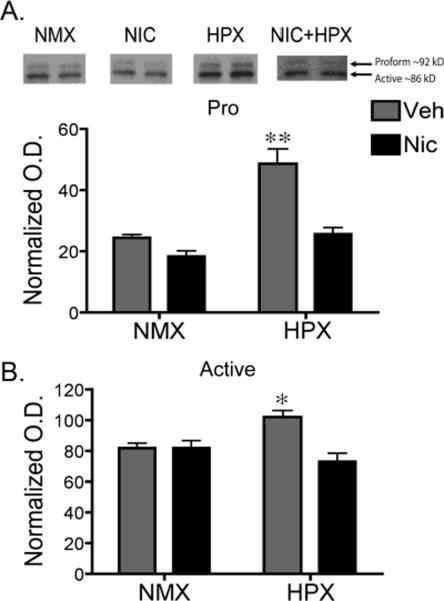
Figure 1. Prenatal hypoxia significantly increased protein levels of both the pro- and active forms of MMP-9 in the fetal hippocampus.
Representative immunoblots of the proform (A) and active (B) MMP-9 protein taken from micropunched tissue of the hippocampal CA1 region of fetal guinea pigs exposed in utero to nicotine (n=5), hypoxia (n=5), nicotine and hypoxia (n=5), or normoxic (n=5) conditions (top panel, each lane is a representative example from individual animals). For quantification, MMP-9 densities were normalized by taking the ratio of the optical density of the MMP-9 immunoreactive band (either proform or active) to that of the optical density of the Ponceau S stained band at approximately 45kDa. Two-way ANOVA revealed a significant interaction between nicotine and hypoxia in both the pro- (F1,16=8.83, p<0.01) and active (F1,16=10.36, p<0.01) form of MMP-9. Tukey-Kramer post hoc analysis revealed that hypoxia alone induced a significant increase in both the pro (**p<0.002) and active (*p<0.05) form of MMP-9 compared to all other treatment groups. All values are the normal optical density mean ± SEM. NMX, normoxic; NIC, nicotine; HPX, hypoxic, O.D., optical density.
Prenatal hypoxia alone increased synaptophysin protein levels in the CA1 hippocampal subfield
MMP-9 has been implicated in changes in synaptic patterning and remodeling in the hippocampus (Ethell and Ethell 2007). Thus, the increase in fetal hippocampus MMP-9 protein expression in response to prenatal hypoxia suggests that synaptic patterning may also be altered. Here, we tested whether in utero exposure to hypoxia and/or nicotine increased expression of synaptophysin, a presynaptic terminal marker, in the CA1 region of the fetal hippocampus. A two-way ANOVA with atmosphere (hypoxic or normoxic conditions) and drug (nicotine or vehicle) as factors revealed a significant interaction between nicotine exposure and hypoxia (Figure 2; F(1,16)=5.58, p<0.03). Hypoxia significantly increased synaptophysin protein levels by approximately 38% compared to all other groups (Tukey Kramer post-hoc analysis p<0.05). Similar to the MMP-9 protein levels, synaptophysin protein levels in the group exposed to the combination of hypoxia plus nicotine were not significantly different from controls. Nicotine alone had no effect.
Figure 2. Prenatal hypoxia significantly increased synaptophysin protein levels compared to normal controls in the fetal hippocampus.
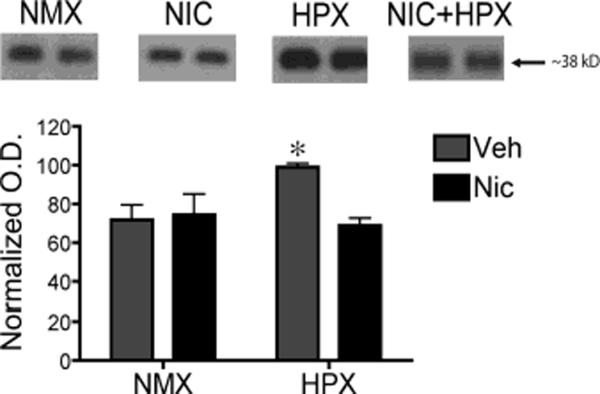
Representative immunoblots of synaptophysin protein taken from micropunched tissue of the hippocampal CA1 region of fetal guinea pigs exposed in utero to nicotine (n=5), hypoxia (n=5), nicotine and hypoxia (n=5), or normoxic (n=5) conditions (top panel). For quantification, synaptophysin density was normalized by taking the ratio of the optical density of the immunoreactive band to that of the optical density of the Ponceau S stained band at approximately 45kDa. Two-way ANOVA revealed a significant interaction between nicotine and hypoxia in synaptophysin protein levels (F1,16=5.58, p<0.03). Tukey-Kramer post hoc analysis revealed that prenatal hypoxia alone induced a significant increase in synaptophysin compared to all other treatment groups (*p<0.05). All values are the normal optical density mean ± SEM. NMX, normoxic; NIC, nicotine; HPX, hypoxic, O.D., optical density.
Prenatal hypoxia alone decreased the density of NeuN-immunoreactive neurons
Using NeuN immunoreactivity (ir) as a neuron specific marker, we examined whether neuronal density in the CA1 region of the hippocampus was affected by in utero exposure to hypoxia and/or nicotine. There was a significant main effect of hypoxia on NeuN-ir (Figure 3, F(1,21)=35.5, p<0.001). Prenatal hypoxia in the presence or absence of prenatal nicotine significantly reduced NeuN-ir compared to normal controls (Tukey Kramer post-hoc analysis, p<0.05). Analysis of the RSg cortex revealed no significant difference between treatment groups (normoxic, 13.6 ± 1.5 O.D.; nicotine, 14.7 ± 1.2 O.D., hypoxic, 14.6 ± 1.0 O.D. and hypoxic plus nicotine 14.5 ± 1.0 O.D.; Data are the mean ± SEM, Two-way ANOVA F(1,21)=0.1924, p=0.74) suggesting the effects of prenatal hypoxia and nicotine on NeuN-ir are specific to the hippocampus.
Prenatal nicotine but not hypoxia increased the density of GFAP-immunoreactive glia
Prenatal exposure to neurotoxins and/or neuronal insult often results in reactive gliosis that is measured as increased GFAP-ir (O'Callaghan 1993). Additionally, reactive astrocytes express MMP-2 and -9 following neuronal insults induced by mechanical means (Kyrkanides et al. 2001; Muir et al. 2002; Hsu et al. 2008; Yin et al. 2006) or ischemia (Lee et al. 2004; Ranasinghe et al. 2009; Rivera et al. 2002; Rosenberg et al. 2001). Thus, we assessed whether hypoxia and/or nicotine exposure induced a reactive response in astrocytes that could be correlated with the hypoxia-induced increase in MMP-9 protein levels in the developing hippocampus. There was a significant main effect of nicotine on GFAP-ir density (Figure 4, F(1,19)=19.2, p<0.001). Prenatal nicotine in the presence or absence of prenatal hypoxia significantly increased GFAP-ir density compared to normal control and prenatal hypoxia groups (Tukey Kramer post-hoc analysis, p<0.0005). In the RSg cortex, there was a significant main effect of nicotine (Figure 5, F(1,19)=6.6, p<0.02). Post-hoc analysis revealed that prenatal nicotine alone increased GFAP-ir in the RSg cortex compared to normoxic controls (Tukey Kramer post-hoc analysis p<0.05). The GFAP-ir density in the hypoxia-exposed groups with or without nicotine was increased compared to the normoxic controls, but did not reach statistical significance. These findings suggest that astrocytes the developing RSg cortex may be more sensitive to fetal nicotine exposure than to changes in the oxygen availability.
Changes induced by in utero exposure to nicotine persisted into adulthood
In the normal developing brain, postnatal changes in astrocyte morphology in response to stimuli such as gonadal hormones persist into adulthood (Mong and Blutstein 2006). The observed robust astrocytic changes in response to prenatal nicotine exposure prompted us to explore the potential long-term consequences of these changes to the cytoarchitecture of the adult hippocampus. In adult guinea pigs, the density of both NeuN-ir (Figure 6A, two-tailed student's t-test, t(7) = 2.826, p<0.05) and GFAP-ir (Figure 6B, two-tailed student's t-test, t(6) = 3.371, p<0.05) were decreased in animals treated in utero with nicotine compared to controls.
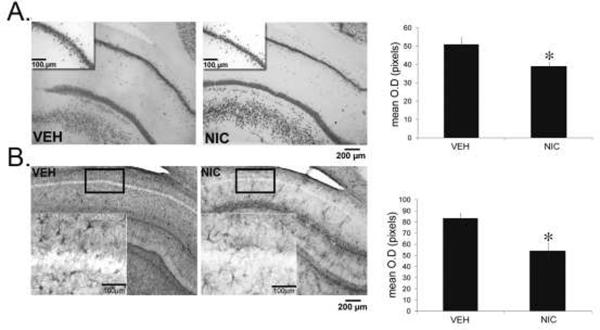
Figure 6. Prenatal nicotine decreased NeuN and GFAP immunoreactivity in the adult hippocampus.
Representative photomicrographs and quantification of the density of NeuN-ir (A) and GFAP-ir (B) in the CA1 region of the hippocampus from adult guinea pigs exposed in utero to nicotine (n=5) or vehicle (n=4). The inset represents the areas quantified. The densities of GFAP- and NeuN- ir were significantly decreased in nicotine-exposed animals. *p<0.05 compared to vehicle. All values are mean optical density in pixels ± SEM. VEH, vehicle; NIC, nicotine.
DISCUSSION
Maternal cigarette smoking exposes the developing fetus to multiple neurotoxic agents including nicotine and a hypoxic environment, resulting in significant consequences for the pregnancy as well as the developing fetus. Using the fetal guinea pig, we assessed the effects of nicotine and hypoxia alone and in combination on the development of the hippocampus. We found that nicotine and hypoxia alter synaptic patterning through different mechanisms and this has long-term consequences for the adult brain.
Hypoxia induces changes in the expression of proteins involved in synaptic pattering
In the nascent CNS, MMPs, especially MMP-2 and -9, are involved in a myriad of cellular processes contributing to the normal development of the nervous system that include but are not limited to axonal guidance, synaptogenesis, and synaptic plasticity (Ethell and Ethell 2007). Moreover, evidence from adult and neonatal studies is emerging to suggest that MMPs mediate remodeling of the neuronal substrate following a neuronal insult (Yong 2005). Recently, Ranasinge and colleagues report that in the rat brain (GD 18 to PN 3), under normal developmental conditions, MMP-2 activity in the cortex is predominant over MMP-9. However, significant increases in MMP-9 expression and activity are detected in the cortex and hippocampus 6 to 24 hours following a hypoxic insult in neonatal rats (PN 3) while MMP-2 remains unchanged (Ranasinghe et al. 2009). In the present study, maternal exposure to a chronic hypoxic environment increased protein levels of the pro- and active form of MMP-9 in the fetal hippocampus. It is not clear whether increases in MMP-9 expression represent beneficial or detrimental changes for the developing neural substrates following a hypoxic insult.
In adult studies of the rodent hippocampus, MMP-3 and MMP-9 are involved in fiber sprouting and synaptic remodeling following an excitotoxic response to kainite acid (Szklarczyk et al. 2002; Zhang et al. 1998). Additionally, in vitro studies suggest that MMP-9 is active in hippocampal synapse formation and synaptic plasticity by influencing dendritic spine development and synapse stabilization (Bilousova et al. 2006). Here, we report a coincident increase in synaptophysin, a major synaptic vesicle protein associated with synapse formation. Thus, it is tempting to speculate that under hypoxic conditions, activated MMP-9 may be restructuring synapses in the developing hippocampus. Future work will investigate whether the two increases are causally related.
Alternatively, the increased expression of MMP-9 may be contributing to the observed loss of CA1 pyramidal neurons. It is well established that fetal hypoxia consequently leads to neuronal death in sensitive brain regions that include the CA1 region of the hippocampus, cerebellar Purkinje cells and the cerebral cortex (Rees et al. 2008). Evidence from a MMP-9 genetic knockout model suggests that an increased activation of MMP-9 following an acute ischemic insult during neonatal development (PN 7) contributes to neuronal loss in the hippocampus and cortex (Svedin et al. 2007). However, it is not known whether chronic fetal exposure as used in the present study would result in a similar activation of MMP-9 and subsequent neuronal loss.
Nevertheless, it is of note that potential increases in synapse formation as suggested by increased synaptophysin protein levels may represent a compensatory response to cellular damage and neuronal loss by increasing synaptic connections between existing neurons. Curiously, nicotine treatment in the presence of hypoxia appeared to have blocked the hypoxia induced increase in MMP-9 and synaptophysin protein while having no effect on neuronal cell loss. These results raise the possibility of a neuroprotective effect of nicotine against hypoxia. In a gerbil model of global ischemia, nicotine pretreatment attenuated cognitive impairment and cell death (Nanri et al. 1998). In rat primary cortical cultures nicotine was protective against acute hypoxic insult through a nicotinic acetylcholinergic dependent mechanism (Hejmadi et al. 2003). Nicotine is also protective in hippocampal slices against oxygen glucose deprivation (Rosa et al. 2006). While our present observation is difficult to interpret without further investigation, it does suggest that MMP-9 is involved in both synaptic formation and neuronal loss, and raises the possibility of a neuroprotective effect of nicotine in the fetal guinea pig hippocampus.
Nicotine, not hypoxia increased GFAP
An early response to neuronal injury is the proliferation of GFAP immunopositive astrocytes, referred to as reactive astrocytes. It is well established that acute ischemic-hypoxia induces reactive astrocytes (O'Callaghan 1993; Pekny and Pekna 2004). However less is understood about the effects of chronic maternal hypoxia on fetal brain development. To our knowledge only one other published study in the rat has investigated the influence of maternal hypoxia on GFAP positive astrocytes. In this study, a small but significant increase in GFAP positive cells was observed in the periventicular white matter in adult offspring exposed to maternal hypoxia during gestation (Wang et al. 2010). In the present study, we did not detect a significant increase in GFAP positive astrocytes in the hippocampus or cortex in fetuses exposed to maternal hypoxia. Thus, when compared to the results from acute ischemic-hypoxic studies it is likely that astrocytes may respond differently to a chronic insult from maternal hypoxia.
Conversely, nicotine exposed animals showed a robust increase in GFAP positive astrocytes compared to normoxic controls. It is not clear why we see such a robust response to nicotine by the fetal astrocytes. One possible explanation is that this increase represents a neurotoxic response to nicotine. In support of this possibility, we observe an approximate 25% decrease in the density of NeuN positive neurons that did not reach statistical significance in the fetal brain of the nicotine exposed group compared to controls. While the mechanism of cell loss was not determined, these data are suggestive of a neurotoxic effect of nicotine. In fact, in the brains of adult offspring exposed to nicotine during gestation, NeuN density is significantly reduced; suggesting that neuronal loss continued after nicotine exposure has ceased.
Alternatively, the increase in GFAP-ir may represent a premature differentiation or maturation of hippocampal astrocytes as a result of the activation of nAChR (Slotkin 2004). The intermediate filaments of astrocytes are composed of GFAP and vimentin, a less cell-specific filament protein predominant in radial and immature glia (Bignami et al. 1972). As development progresses, radial glia are transformed into stellate process-bearing astrocytes that is characterized by an accumulation of GFAP (Culican et al. 1990; Schmechel and Rakic 1979). This is a hallmark of astrocyte maturation (Eng 1985). GFAP is co-localized with the alpha 7 subunit of the nAChR throughout the hippocampus and cortex (Teaktong et al. 2004) suggesting that astrocytes are able to respond directly to nicotine. Thus, the potential activation of these receptors could have triggered morphological and cellular changes that are reflected in the increased GFAP-ir in the nicotine treated animals.
Early NIC exposure results in changes to the adult offspring
Long-standing evidence has established nicotine as a neurotoxin in the developing CNS (Ginzel et al. 2007; Slotkin 2004, 2008; Roy et al. 2002). Moreover, these studies clearly demonstrate that fetal nicotine exposure has long-term consequences for the brains of adolescent and adult offspring. While the majority of studies have focused on the persistent changes to neurons since it more directly underlies behavioral changes, recent work has begun to address the contribution of the maternal nicotine-induced changes in glial cells and specifically astrocytes in the offspring (Abdel-Rahman et al. 2005; Abdel-Rahman et al. 2004; Abou-Donia et al. 2006; Roy et al. 2002). The potential activation of astrocytic nicotinic acetylcholine receptors in the fetal brain raises the possibility that perturbations to the prenatal environment may trigger signaling cascades ultimately resulting in changes to the adult offspring. Indeed, we have observed that when nicotine exposed guinea pigs were allowed to reach adulthood, there were significant decreases in both astrocytic surface area and neuronal density. These data are consistent with human literature in which chronic nicotine exposure results in a significant decrease in GFAP-ir in the hippocampus and a decrease in the colocalization of the alpha 7-receptor subunit with GFAP-ir (Teaktong et al. 2004). Interestingly, the changes in neuronal density are consistent with changes we observed in the fetal guinea pig, but the changes in the astrocytic surface area are reversed. In the fetal hippocampus nicotine increases GFAP-ir compared to controls, while in the adult hippocampus animals exposed in utero to nicotine have decreased GFAP-ir compared to controls. One interpretation of these results is that the increased GFAP-ir in the fetal hippocampus is indicative of a reactive gliosis and that this early life trauma may impair normal astrocyte maturation, leading to decreased GFAP-ir astrocytes in adulthood. In rat models, adolescent rats that were exposed throughout gestation (GD 4–20) showed an increase in GFAP-ir in the CA1 hippocampal subfields on PN 30 and 60. This increase in GFAP-ir was accompanied by a decrease in CA1 pyramidal neurons and these were correlated with changes in behavioral deficits (Abdel-Rahman et al. 2005). This suggests that the timing of nicotine exposure may be critical in determining the fate of the adult brain or that there are distinct mechanisms by which neonatal nicotine exposure affects neurons and glial cells. Nonetheless, our study provides further evidence that nicotine-induced changes during brain development have lasting detrimental effects on brain structure and potentially behavior.
Overall, the present study utilized a more complete model of maternal smoking and presents evidence that a prolonged exposure during fetal development to nicotine and hypoxia alters synaptic patterning and glial morphology, although probably through different mechanisms. Additionally, this work emphasizes the vulnerability of the fetal brain to nicotine exposure, which we have shown leads to permanent alterations in the adult brain. Future work will further elucidate the different pathways through which nicotine and hypoxia exert their effects and determine potential points of convergence.
REFERENCES
- Abdel-Rahman A, Dechkovskaia AM, Mehta-Simmons H, Sutton JM, Guan X, Khan WA, Abou-Donia MB. Maternal exposure to nicotine and chlorpyrifos, alone and in combination, leads to persistently elevated expression of glial fibrillary acidic protein in the cerebellum of the offspring in late puberty. Archives of toxicology. 2004;78(8):467–476. doi: 10.1007/s00204-004-0560-5. doi:10.1007/s00204-004-0560-5. [DOI] [PubMed] [Google Scholar]
- Abdel-Rahman A, Dechkovskaia AM, Sutton JM, Chen WC, Guan X, Khan WA, Abou-Donia MB. Maternal exposure of rats to nicotine via infusion during gestation produces neurobehavioral deficits and elevated expression of glial fibrillary acidic protein in the cerebellum and CA1 subfield in the offspring at puberty. Toxicology. 2005;209(3):245–261. doi: 10.1016/j.tox.2004.12.037. doi:10.1016/j.tox.2004.12.037. [DOI] [PubMed] [Google Scholar]
- Abou-Donia MB, Khan WA, Dechkovskaia AM, Goldstein LB, Bullman SL, Abdel-Rahman A. In utero exposure to nicotine and chlorpyrifos alone, and in combination produces persistent sensorimotor deficits and Purkinje neuron loss in the cerebellum of adult offspring rats. Archives of toxicology. 2006;80(9):620–631. doi: 10.1007/s00204-006-0077-1. doi:10.1007/s00204-006-0077-1. [DOI] [PubMed] [Google Scholar]
- Barrett RD, Bennet L, Davidson J, Dean JM, George S, Emerald BS, Gunn AJ. Destruction and reconstruction: hypoxia and the developing brain. Birth defects research Part C, Embryo today : reviews. 2007;81(3):163–176. doi: 10.1002/bdrc.20095. doi:10.1002/bdrc.20095. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Gourlay SG. Cardiovascular toxicity of nicotine: implications for nicotine replacement therapy. Journal of the American College of Cardiology. 1997;29(7):1422–1431. doi: 10.1016/s0735-1097(97)00079-x. [DOI] [PubMed] [Google Scholar]
- Bignami A, Eng LF, Dahl D, Uyeda CT. Localization of the glial fibrillary acidic protein in astrocytes by immunofluorescence. Brain research. 1972;43(2):429–435. doi: 10.1016/0006-8993(72)90398-8. [DOI] [PubMed] [Google Scholar]
- Bilousova TV, Rusakov DA, Ethell DW, Ethell IM. Matrix metalloproteinase-7 disrupts dendritic spines in hippocampal neurons through NMDA receptor activation. Journal of neurochemistry. 2006;97(1):44–56. doi: 10.1111/j.1471-4159.2006.03701.x. doi:10.1111/j.1471-4159.2006.03701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrnes ML, Reynolds JN, Brien JF. Brain growth spurt-prenatal ethanol exposure and the guinea pig hippocampal glutamate signaling system. Neurotoxicology and teratology. 2003;25(3):303–310. doi: 10.1016/s0892-0362(02)00354-9. [DOI] [PubMed] [Google Scholar]
- Candelario-Jalil E, Yang Y, Rosenberg GA. Diverse roles of matrix metalloproteinases and tissue inhibitors of metalloproteinases in neuroinflammation and cerebral ischemia. Neuroscience. 2009;158(3):983–994. doi: 10.1016/j.neuroscience.2008.06.025. doi:10.1016/j.neuroscience.2008.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culican SM, Baumrind NL, Yamamoto M, Pearlman AL. Cortical radial glia: identification in tissue culture and evidence for their transformation to astrocytes. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1990;10(2):684–692. doi: 10.1523/JNEUROSCI.10-02-00684.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbing J, Sands J. Growth and development of the brain and spinal cord of the guinea pig. Brain research. 1970;17(1):115–123. doi: 10.1016/0006-8993(70)90311-2. [DOI] [PubMed] [Google Scholar]
- Eng LF. Glial fibrillary acidic protein (GFAP): the major protein of glial intermediate filaments in differentiated astrocytes. Journal of neuroimmunology. 1985;8(4–6):203–214. doi: 10.1016/s0165-5728(85)80063-1. [DOI] [PubMed] [Google Scholar]
- Ethell IM, Ethell DW. Matrix metalloproteinases in brain development and remodeling: synaptic functions and targets. Journal of neuroscience research. 2007;85(13):2813–2823. doi: 10.1002/jnr.21273. doi:10.1002/jnr.21273. [DOI] [PubMed] [Google Scholar]
- Gaither KH, Brunner Huber LR, Thompson ME, Huet-Hudson YM. Does the use of nicotine replacement therapy during pregnancy affect pregnancy outcomes? Maternal and child health journal. 2009;13(4):497–504. doi: 10.1007/s10995-008-0361-1. doi:10.1007/s10995-008-0361-1. [DOI] [PubMed] [Google Scholar]
- Ginzel KH, Maritz GS, Marks DF, Neuberger M, Pauly JR, Polito JR, Schulte-Hermann R, Slotkin TA. Critical review: nicotine for the fetus, the infant and the adolescent? Journal of health psychology. 2007;12(2):215–224. doi: 10.1177/1359105307074240. doi:10.1177/1359105307074240. [DOI] [PubMed] [Google Scholar]
- Hallak M, Hotra JW, Kupsky WJ. Magnesium sulfate protection of fetal rat brain from severe maternal hypoxia. Obstetrics and gynecology. 2000;96(1):124–128. doi: 10.1016/s0029-7844(00)00844-9. [DOI] [PubMed] [Google Scholar]
- Hejmadi MV, Dajas-Bailador F, Barns SM, Jones B, Wonnacott S. Neuroprotection by nicotine against hypoxia-induced apoptosis in cortical cultures involves activation of multiple nicotinic acetylcholine receptor subtypes. Molecular and cellular neurosciences. 2003;24(3):779–786. doi: 10.1016/s1044-7431(03)00244-6. [DOI] [PubMed] [Google Scholar]
- Hernandez-Morales M, Garcia-Colunga J. Effects of nicotine on K+ currents and nicotinic receptors in astrocytes of the hippocampal CA1 region. Neuropharmacology. 2009;56(6–7):975–983. doi: 10.1016/j.neuropharm.2009.01.024. doi:10.1016/j.neuropharm.2009.01.024. [DOI] [PubMed] [Google Scholar]
- The Hippocampus Book. Oxford University Press; Oxford: [Google Scholar]
- Holder MK, Mong JA. Methamphetamine enhances paced mating behaviors and neuroplasticity in the medial amygdala of female rats. Hormones and behavior. 2010;58(3):519–525. doi: 10.1016/j.yhbeh.2010.04.006. doi:10.1016/j.yhbeh.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu JY, Bourguignon LY, Adams CM, Peyrollier K, Zhang H, Fandel T, Cun CL, Werb Z, Noble-Haeusslein LJ. Matrix metalloproteinase-9 facilitates glial scar formation in the injured spinal cord. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28(50):13467–13477. doi: 10.1523/JNEUROSCI.2287-08.2008. doi:10.1523/JNEUROSCI.2287-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inder TE, Warfield SK, Wang H, Huppi PS, Volpe JJ. Abnormal cerebral structure is present at term in premature infants. Pediatrics. 2005;115(2):286–294. doi: 10.1542/peds.2004-0326. doi:10.1542/peds.2004-0326. [DOI] [PubMed] [Google Scholar]
- Jauniaux E, Gulbis B, Acharya G, Thiry P, Rodeck C. Maternal tobacco exposure and cotinine levels in fetal fluids in the first half of pregnancy. Obstetrics and gynecology. 1999;93(1):25–29. doi: 10.1016/s0029-7844(98)00318-4. [DOI] [PubMed] [Google Scholar]
- Klein D, Kern RM, Sokol RZ. A method for quantification and correction of proteins after transfer to immobilization membranes. Biochemistry and molecular biology international. 1995;36(1):59–66. [PubMed] [Google Scholar]
- Klein T, Bischoff R. Physiology and pathophysiology of matrix metalloproteases. Amino acids. 2011;41(2):271–290. doi: 10.1007/s00726-010-0689-x. doi:10.1007/s00726-010-0689-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyrkanides S, O'Banion MK, Whiteley PE, Daeschner JC, Olschowka JA. Enhanced glial activation and expression of specific CNS inflammation-related molecules in aged versus young rats following cortical stab injury. Journal of neuroimmunology. 2001;119(2):269–277. doi: 10.1016/s0165-5728(01)00404-0. [DOI] [PubMed] [Google Scholar]
- Lagnado JR, Hardy M. Brain esterases during development. Nature. 1967;214(5094):1207–1210. doi: 10.1038/2141207a0. [DOI] [PubMed] [Google Scholar]
- Lambers DS, Clark KE. The maternal and fetal physiologic effects of nicotine. Seminars in perinatology. 1996;20(2):115–126. doi: 10.1016/s0146-0005(96)80079-6. [DOI] [PubMed] [Google Scholar]
- Lee JE, Kim YJ, Kim JY, Lee WT, Yenari MA, Giffard RG. The 70 kDa heat shock protein suppresses matrix metalloproteinases in astrocytes. Neuroreport. 2004;15(3):499–502. doi: 10.1097/00001756-200403010-00023. [DOI] [PubMed] [Google Scholar]
- Liang K, Poytress BS, Chen Y, Leslie FM, Weinberger NM, Metherate R. Neonatal nicotine exposure impairs nicotinic enhancement of central auditory processing and auditory learning in adult rats. The European journal of neuroscience. 2006;24(3):857–866. doi: 10.1111/j.1460-9568.2006.04945.x. doi:10.1111/j.1460-9568.2006.04945.x. [DOI] [PubMed] [Google Scholar]
- Luparello TJ. Stereotaxic Atlas of the Forebrain of the Guinea Pig. Williams and Wilkins; Baltimore: 1967. [Google Scholar]
- Lv J, Mao C, Zhu L, Zhang H, Pengpeng H, Xu F, Liu Y, Zhang L, Xu Z. The effect of prenatal nicotine on expression of nicotine receptor subunits in the fetal brain. Neurotoxicology. 2008;29(4):722–726. doi: 10.1016/j.neuro.2008.04.015. doi:10.1016/j.neuro.2008.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JA, Kung HC, Mathews TJ, Hoyert DL, Strobino DM, Guyer B, Sutton SR. Annual summary of vital statistics: 2006. Pediatrics. 2008;121(4):788–801. doi: 10.1542/peds.2007-3753. doi:10.1542/peds.2007-3753. [DOI] [PubMed] [Google Scholar]
- Mong JA, Blutstein T. Estradiol modulation of astrocytic form and function: implications for hormonal control of synaptic communication. Neuroscience. 2006;138(3):967–975. doi: 10.1016/j.neuroscience.2005.10.017. [DOI] [PubMed] [Google Scholar]
- Muir EM, Adcock KH, Morgenstern DA, Clayton R, von Stillfried N, Rhodes K, Ellis C, Fawcett JW, Rogers JH. Matrix metalloproteases and their inhibitors are produced by overlapping populations of activated astrocytes. Brain research Molecular brain research. 2002;100(1–2):103–117. doi: 10.1016/s0169-328x(02)00132-8. [DOI] [PubMed] [Google Scholar]
- Nanri M, Yamamoto J, Miyake H, Watanabe H. Protective effect of GTS-21, a novel nicotinic receptor agonist, on delayed neuronal death induced by ischemia in gerbils. Japanese journal of pharmacology. 1998;76(1):23–29. doi: 10.1254/jjp.76.23. [DOI] [PubMed] [Google Scholar]
- O'Callaghan JP. Quantitative features of reactive gliosis following toxicant-induced damage of the CNS. Annals of the New York Academy of Sciences. 1993;679:195–210. doi: 10.1111/j.1749-6632.1993.tb18299.x. [DOI] [PubMed] [Google Scholar]
- Oh C, Dong Y, Liu H, Thompson LP. Intrauterine hypoxia upregulates proinflammatory cytokines and matrix metalloproteinases in fetal guinea pig hearts. American journal of obstetrics and gynecology. 2008;199(1):78, e71–76. doi: 10.1016/j.ajog.2007.12.004. doi:10.1016/j.ajog.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Oja SS, Uusitalo AJ, Vahvelainen ML, Piha RS. Changes in cerebral and hepatic amino acids in the rat and guinea pig during development. Brain research. 1968;11(3):655–661. doi: 10.1016/0006-8993(68)90153-4. [DOI] [PubMed] [Google Scholar]
- Okada Y, Shinmei M, Tanaka O, Naka K, Kimura A, Nakanishi I, Bayliss MT, Iwata K, Nagase H. Localization of matrix metalloproteinase 3 (stromelysin) in osteoarthritic cartilage and synovium. Laboratory investigation; a journal of technical methods and pathology. 1992;66(6):680–690. [PubMed] [Google Scholar]
- Olesen KM, Auger AP. Sex differences in Fos protein expression in the neonatal rat brain. Journal of neuroendocrinology. 2005;17(4):255–261. doi: 10.1111/j.1365-2826.2005.01302.x. doi:10.1111/j.1365-2826.2005.01302.x. [DOI] [PubMed] [Google Scholar]
- Palkovits M BM. Maps and Guide to Microdissection of the Rat Brain. Elseveir; New York, NY: 1988. [Google Scholar]
- Pekny M, Pekna M. Astrocyte intermediate filaments in CNS pathologies and regeneration. The Journal of pathology. 2004;204(4):428–437. doi: 10.1002/path.1645. doi:10.1002/path.1645. [DOI] [PubMed] [Google Scholar]
- Placzek AN, Zhang TA, Dani JA. Nicotinic mechanisms influencing synaptic plasticity in the hippocampus. Acta pharmacologica Sinica. 2009;30(6):752–760. doi: 10.1038/aps.2009.39. doi:10.1038/aps.2009.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranasinghe HS, Williams CE, Christophidis LJ, Mitchell MD, Fraser M, Scheepens A. Proteolytic activity during cortical development is distinct from that involved in hypoxic ischemic injury. Neuroscience. 2009;158(2):732–744. doi: 10.1016/j.neuroscience.2008.07.069. doi:10.1016/j.neuroscience.2008.07.069. [DOI] [PubMed] [Google Scholar]
- Rees S, Harding R, Walker D. An adverse intrauterine environment: implications for injury and altered development of the brain. International journal of developmental neuroscience : the official journal of the International Society for Developmental Neuroscience. 2008;26(1):3–11. doi: 10.1016/j.ijdevneu.2007.08.020. doi:10.1016/j.ijdevneu.2007.08.020. [DOI] [PubMed] [Google Scholar]
- Rivera S, Ogier C, Jourquin J, Timsit S, Szklarczyk AW, Miller K, Gearing AJ, Kaczmarek L, Khrestchatisky M. Gelatinase B and TIMP-1 are regulated in a cell- and time-dependent manner in association with neuronal death and glial reactivity after global forebrain ischemia. The European journal of neuroscience. 2002;15(1):19–32. doi: 10.1046/j.0953-816x.2001.01838.x. [DOI] [PubMed] [Google Scholar]
- Rosa AO, Egea J, Gandia L, Lopez MG, Garcia AG. Neuroprotection by nicotine in hippocampal slices subjected to oxygen-glucose deprivation: involvement of the alpha7 nAChR subtype. Journal of molecular neuroscience : MN. 2006;30(1–2):61–62. doi: 10.1385/JMN:30:1:61. doi:10.1385/JMN:30:1:61. [DOI] [PubMed] [Google Scholar]
- Rosenberg GA, Cunningham LA, Wallace J, Alexander S, Estrada EY, Grossetete M, Razhagi A, Miller K, Gearing A. Immunohistochemistry of matrix metalloproteinases in reperfusion injury to rat brain: activation of MMP-9 linked to stromelysin-1 and microglia in cell cultures. Brain research. 2001;893(1–2):104–112. doi: 10.1016/s0006-8993(00)03294-7. [DOI] [PubMed] [Google Scholar]
- Roy TS, Seidler FJ, Slotkin TA. Prenatal nicotine exposure evokes alterations of cell structure in hippocampus and somatosensory cortex. The Journal of pharmacology and experimental therapeutics. 2002;300(1):124–133. doi: 10.1124/jpet.300.1.124. [DOI] [PubMed] [Google Scholar]
- Schmechel DE, Rakic P. A Golgi study of radial glial cells in developing monkey telencephalon: morphogenesis and transformation into astrocytes. Anatomy and embryology. 1979;156(2):115–152. doi: 10.1007/BF00300010. [DOI] [PubMed] [Google Scholar]
- Slavin AJ, Johns TG, Orian JM, Bernard CC. Regulation of myelin oligodendrocyte glycoprotein in different species throughout development. Developmental neuroscience. 1997;19(1):69–78. doi: 10.1159/000111187. [DOI] [PubMed] [Google Scholar]
- Slotkin TA. Fetal nicotine or cocaine exposure: which one is worse? The Journal of pharmacology and experimental therapeutics. 1998;285(3):931–945. [PubMed] [Google Scholar]
- Slotkin TA. Cholinergic systems in brain development and disruption by neurotoxicants: nicotine, environmental tobacco smoke, organophosphates. Toxicology and applied pharmacology. 2004;198(2):132–151. doi: 10.1016/j.taap.2003.06.001. doi:10.1016/j.taap.2003.06.001. [DOI] [PubMed] [Google Scholar]
- Slotkin TA. If nicotine is a developmental neurotoxicant in animal studies, dare we recommend nicotine replacement therapy in pregnant women and adolescents? Neurotoxicology and teratology. 2008;30(1):1–19. doi: 10.1016/j.ntt.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Epps TA, Stenger ML, Sawyer KJ, Seidler FJ. Cholinergic receptors in heart and brainstem of rats exposed to nicotine during development: implications for hypoxia tolerance and perinatal mortality. Brain research Developmental brain research. 1999;113(1–2):1–12. doi: 10.1016/s0165-3806(98)00173-4. [DOI] [PubMed] [Google Scholar]
- Squire LR. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychological review. 1992;99(2):195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Tanaka T, Kondo N, Minai J, Sato M, Yamagata Z. Is maternal smoking during early pregnancy a risk factor for all low birth weight infants? Journal of epidemiology / Japan Epidemiological Association. 2008;18(3):89–96. doi: 10.2188/jea.JE2007415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svedin P, Hagberg H, Savman K, Zhu C, Mallard C. Matrix metalloproteinase-9 gene knock-out protects the immature brain after cerebral hypoxia-ischemia. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27(7):1511–1518. doi: 10.1523/JNEUROSCI.4391-06.2007. doi:10.1523/JNEUROSCI.4391-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szklarczyk A, Lapinska J, Rylski M, McKay RD, Kaczmarek L. Matrix metalloproteinase-9 undergoes expression and activation during dendritic remodeling in adult hippocampus. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22(3):920–930. doi: 10.1523/JNEUROSCI.22-03-00920.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teaktong T, Graham AJ, Johnson M, Court JA, Perry EK. Selective changes in nicotinic acetylcholine receptor subtypes related to tobacco smoking: an immunohistochemical study. Neuropathology and applied neurobiology. 2004;30(3):243–254. doi: 10.1046/j.0305-1846.2003.00528.x. doi:10.1046/j.0305-1846.2003.00528.x. [DOI] [PubMed] [Google Scholar]
- Thompson LP, Liu H, Evans L, Mong JA. Prenatal nicotine increases matrix metalloproteinase 2 (MMP-2) expression in fetal guinea pig hearts. Reprod Sci. 2011;18(11):1103–1110. doi: 10.1177/1933719111404605. doi:10.1177/1933719111404605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong W, Chen W, Ostrowski RP, Ma Q, Souvenir R, Zhang L, Zhang JH, Tang J. Maternal hypoxia increases the activity of MMPs and decreases the expression of TIMPs in the brain of neonatal rats. Developmental neurobiology. 2010;70(3):182–194. doi: 10.1002/dneu.20770. doi:10.1002/dneu.20770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Cai R, Lv G, Huang Z, Wang Z. Hypoxia during pregnancy in rats leads to the changes of the cerebral white matter in adult offspring. Biochemical and biophysical research communications. 2010;396(2):445–450. doi: 10.1016/j.bbrc.2010.04.114. doi:10.1016/j.bbrc.2010.04.114. [DOI] [PubMed] [Google Scholar]
- Winzer-Serhan UH. Long-term consequences of maternal smoking and developmental chronic nicotine exposure. Frontiers in bioscience : a journal and virtual library. 2008;13:636–649. doi: 10.2741/2708. [DOI] [PubMed] [Google Scholar]
- Wojcik L, Sawicka A, Rivera S, Zalewska T. Neurogenesis in gerbil hippocampus following brain ischemia: focus on the involvement of metalloproteinases. Acta neurobiologiae experimentalis. 2009;69(1):52–61. doi: 10.55782/ane-2009-1729. [DOI] [PubMed] [Google Scholar]
- Yin KJ, Cirrito JR, Yan P, Hu X, Xiao Q, Pan X, Bateman R, Song H, Hsu FF, Turk J, Xu J, Hsu CY, Mills JC, Holtzman DM, Lee JM. Matrix metalloproteinases expressed by astrocytes mediate extracellular amyloid-beta peptide catabolism. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26(43):10939–10948. doi: 10.1523/JNEUROSCI.2085-06.2006. doi:10.1523/JNEUROSCI.2085-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong VW. Metalloproteinases: mediators of pathology and regeneration in the CNS. Nature reviews Neuroscience. 2005;6(12):931–944. doi: 10.1038/nrn1807. doi:10.1038/nrn1807. [DOI] [PubMed] [Google Scholar]
- Zhang JW, Deb S, Gottschall PE. Regional and differential expression of gelatinases in rat brain after systemic kainic acid or bicuculline administration. The European journal of neuroscience. 1998;10(11):3358–3368. doi: 10.1046/j.1460-9568.1998.00347.x. [DOI] [PubMed] [Google Scholar]
- Zitka O, Kukacka J, Krizkova S, Huska D, Adam V, Masarik M, Prusa R, Kizek R. Matrix metalloproteinases. Current medicinal chemistry. 2010;17(31):3751–3768. doi: 10.2174/092986710793213724. [DOI] [PubMed] [Google Scholar]