Abstract
Rationale
Exposure to intrauterine inflammation impairs lung growth, but paradoxically protects the neonatal pulmonary vasculature from hyperoxic injury. The mechanisms mediating these contradictory effects are unknown.
Objective
To identify the role of NF-κB in mediating cytoprotective and pro-inflammatory responses to inflammation in the fetal pulmonary endothelium.
Methods and Results
In newborn rats exposed to intraamniotic lipopolysaccharide (LPS), we found increased expression of the NF-κB target gene manganese superoxide dismutase (MnSOD) in the pulmonary endothelium. Supporting these in vivo findings, LPS induced NF-κB activation and MnSOD expression in isolated fetal pulmonary arterial endothelial cells. Additionally, LPS exposure caused apoptosis, and suppressed cellular growth and induced P-selectin expression. LPS-induced NF-κB activation that proceeded through specific isoforms of the inhibitory protein IκB mediated these diverse responses; NF-κB signaling through IκBα degradation resulted in MnSOD upregulation and preserved cell growth, whereas NF-κB signaling through IκBβ degradation mediated apoptosis and P-selectin expression.
Conclusions
These findings suggest that selective inhibition of NF-κB activation that results from IκBβ degradation preserves the enhanced antioxidant defense and protects the developing pulmonary vascular endothelium from ongoing inflammatory injury.
Keywords: NF-κB, IκB, chorioamnionitis, pulmonary hypertension, bronchopulmonary dysplasia, endothelium
Introduction
Chorioamnionitis is an inflammatory response to infection of the placental membranes, with prevalence inversely proportional to gestational age at delivery.(1) Clinical chorioamnionitis significantly contributes to the development of bronchopulmonary dysplasia (BPD), a chronic lung disease of infancy,(1, 2) and is associated with pulmonary hypertension and severe neonatal hypoxic respiratory failure in the newborn.(3, 4) Those clinical findings link antenatal inflammation with endothelial dysfunction and injury in the developing lung, given that the endothelium critically regulates neonatal lung growth and the perinatal transition of pulmonary vasculature.(5–9) The link between antenatal inflammation and pulmonary endothelial injury has been demonstrated in experimental chorioamnionitis, which causes fetal pulmonary vascular remodeling, pulmonary hypertension in the fetus and newborn, as well as impaired lung vascular and alveolar growth in the neonate.(10–12) However, the underlying mechanisms that regulate the response of the developing pulmonary endothelium to antenatal inflammatory stress remain largely unknown.
Our recent study showed that exposure to either intraamniotic lipopolysaccharide (LPS) or postnatal hyperoxia impairs lung vascular growth.(13) Interestingly, intraamniotic LPS protects the neonatal pulmonary vasculature against hyperoxic injury. These findings indicate that intrauterine inflammatory stress induces both a cytoprotective response and disruptive effects on the growth of the developing pulmonary vasculature. However, the mechanisms underlying these paradoxical responses are unclear. Animal studies have reported that systemic or direct pulmonary exposure to LPS in neonatal or adult animals stimulates the expression of lung antioxidant enzymes, such as manganese superoxide dismutase (MnSOD), thereby increasing the tolerance to hyperoxic lung injury.(14–17) Moreover, LPS exposure induces MnSOD in cultured pulmonary vascular endothelial cells (PAEC) isolated from the fetus as well as the adult.(18, 19) However, whether intrauterine inflammatory stress induces MnSOD expression in pulmonary endothelium has not been studied. Moreover, the transcriptional mechanism through which LPS upregulates MnSOD expression in lung vascular endothelial cells is unknown.
The transcription factor NF-κB mediates cellular response to inflammatory stress. In quiescent cells, the IκB family of inhibitory proteins sequesters NF-κB dimers in the cytoplasm. The main cytoplasmic isoforms of IκB responsible for keeping NF-κB inactive are IκBα and IkBβ. Exposure to inflammatory stimuli results in the degradation of both IκBα and IκBβ, thereby releasing NF-κB dimers, allowing nuclear translocation and downstream gene expression.(20) In addition to the well-studied NF-κB activity in immune cells, LPS-induced NFκB activation has been reported in endothelial cells isolated from adult vasculature and human umbilical vein.(21, 22) Moreover, NF-κB activation in endothelial cells mediates the expression of cytokines, chemokines and adhesion molecules, which recruit circulating leukocytes into the inflamed tissue.(23) In fact, endothelial NF-κB activity has been shown responsible for inflammatory lung injury and systemic vascular dysfunction in adult animals with endotoxemia.(24, 25) Paradoxically, inflammatory stress-induced NF-κB activation also upregulates the transcription of cytoprotective genes, including antioxidant enzymes and anti-inflammatory mediators.(26, 27) However, the role of inflammatory stress-induced endothelial NF-κB activation in directing pro-inflammatory and cytoprotective responses in the developing pulmonary vasculature is unknown.
To determine the mechanisms underlying the protective effect of antenatal inflammation against subsequent hyperoxic injury in the developing pulmonary vasculature, we hypothesized that intraamniotic LPS enhances expression of MnSOD in fetal lung vascular endothelium. We further hypothesized that NF-κB activation regulates disparate responses of the fetal pulmonary vascular endothelium to inflammatory stress. We report that intraamniotic LPS exposure increases MnSOD protein expression in the pulmonary vascular endothelium of newborn rats. In addition, in fetal pulmonary vascular endothelial cells (PAEC), LPS exposure induces MnSOD expression but also causes apoptosis, suppresses cell growth and expression of vascular endothelial growth factor receptor 2 (VEGFR2), while inducing P-selectin expression. Moreover, we found that LPS-induced NF-κB activation mediates these diverse responses specifically through IκBα and IκBβ degradation; NF-κB signaling through IκBα degradation mediates MnSOD expression and is required to preserve cell growth and VEGFR2 expression, whereas NF-κB signaling through IκBβ degradation mediates apoptosis and P-selectin expression. Our findings demonstrate for the first time that, selective inhibition of IκBβ-mediated NF-κB activation maintains the inflammatory stress-induced upregulation of antioxidant enzyme and preserves cellular growth, as shown in fetal pulmonary vascular endothelial cells.
Materials and Methods
Animals and Intra-amniotic LPS administration
Fetal Sprague-Dawley rats were exposed to intra-amniotic injection of LPS (10 mcg in 50 µl normal saline, L5418, Sigma-Aldrich,) or saline (50 µl) at 20 days gestation and delivered via Cesarean section two days later as previously described.(13)
Whole lung homogenate and SOD expression and activity
Lung tissue harvested at birth was processed as previously described, and assessed for SOD1, MnSOD, and SOD3 expression via Western blot.(13) SOD activity was determined in total lung homogenates using an SOD assay kit-WST (Dojindo Molecular Technologies) as previously described.(28)
Immunohistochemical localization of pulmonary MnSOD
To assess MnSOD expression in pulmonary vascular endothelial cells, immunofluorescence double staining of MnSOD and von Willebrand factor (vWF) was performed on cryosections cut from OCT-embedded newborn rat lung blocks. The sections were blocked with 0.5% horse serum in 0.5% bovine serum albumin diluted with PBS for 1 h at room temperature, and then were incubated with anti-MnSOD antibody (1:250; Millipore #06-984) and anti-vWF antibody (1:50; Thermo Pierce MA1-82048) at 4°C overnight. On the following day, the sections were incubated with secondary antibodies at 1:250 (Alexa Fluor 594 donkey-anti-rabbit for MnSOD, and Alexa Fluor 488 donkey-anti-mouse for vWF; Molecular Probes) for 2h at room temperature. The sections were then mounted with 4',6-diamidino-2-phenylindole (DAPI; Vector Laboratories) and imaged with an Olympus IX71 fluorescence microscope (Olympus America, Center Valley, PA). To further examine the architecture of pulmonary vasculature that was shown in immunofluorescence staining, adjacent cryosections were stained with hematoxylin and eosin, and were imaged under light microscope.
Isolation and culture of fetal ovine pulmonary artery endothelial cells (PAECs)
Proximal fetal ovine PAECs were isolated as previously described,(29) and endothelial cell phenotype was confirmed by positive immunostaining for von Willebrand factor, endothelial nitric oxide synthase, vascular endothelial cadherin, and VEGFR2, positive uptake of acetylated LDL, and negative staining for desmin. Cells from passages 4–8 were used for all experiments, and cells from individual animals were kept separate throughout all experiments.
Cell Culture and LPS exposure
Fetal ovine PAEC cells were grown in DMEM supplemented with 10% FBS, 1% antibiotic/antimycotic (Invitrogen), and 1% nonessential amino acid solution (Sigma-Aldrich) and maintained at 37 °C in 5% CO2 and 95% room air. In all experiments, cells were seeded at 15,000 cells/cm2 in plastic culture dishes and allowed to adhere overnight prior to exposure. For LPS exposures, the medium was aspirated from the cells (~80% confluent) and fresh medium containing LPS (L6529, Sigma-Aldrich) added.
Treatment of PAECs with the NF-κB inhibitors parthenolide and BAY-7085
Fetal ovine PAECs were treated with inhibitors of the NF-κB pathway, BAY-7085 and parthenolide (Sigma-Aldrich). We chose to separately test two different inhibitors of NF-κB activation to help ensure that any effect observed was due to NF-κB inhibition rather than off-target effects. BAY-7085 was diluted in ethanol. Parthenolide was diluted in DMSO. PAECs were pretreated with either BAY-7085 or parthenolide for 1 hour prior to LPS exposure, and maintained in the media throughout the LPS exposure.
Preparation of Cell Lysate, Cytosolic and Nuclear Extractions
Cells were washed with ice-cold PBS, trypsinized, and pelleted. Whole cell lysate, nuclear and cytosolic fractions were obtained and protein content determined as previously described.(30)
Cell fractionation and immunoblot analysis
Cell lysate, cytosolic or nuclear extracts were electrophoresed (4–12% polyacylamide gel, Invitrogen). Proteins were transferred to an Immobilon membrane (Millipore) and blotted with anti-IκBα (sc-371), anti-IκBβ (sc-9130), anti-P-selectin (sc-6943), anti-SOD3 (sc-67809) or anti-lamin B (sc-6216) antibody (Santa Cruz Biotechnology); anti-c-Rel (4774), anti-VEGFR 2 (2479), anti-caspase-3 (9665) antibody (Cell Signaling); anti-p50 (ab7971), anti-SOD1 (ab13498) antibody (Abcam); anti-Mn-SOD (06-984), or anti-tubulin antibody (Millipore 05-829). Densitometric analysis was performed using ImageJ.
Evaluation of nuclear NF-κB binding by EMSA
A 32P-labeled oligonucleotide with the consensus sequence for NF-κB (5’-AGTTGAGGGGACTTTCCCAGGC-3’) (Promega, Madison, WI) was used as a probe to evaluate NF-κB binding ability as described previously.(31) To identify nonspecific binding of nuclear proteins, competition reactions were performed by addition of either 50-fold excess of the non-radiolabeled NF-κB consensus sequence or 50-fold excess of non-radiolabeled mutated NF-κB consensus sequence (5’-AGTTGAGGCGACTTTCCCAGGC-3’) (Santa Cruz Biotechnology, Santa Cruz, CA) to the reaction mixtures prior to electrophoresis. In separate experiments, in order to identify the NF-κB subunit proteins in the binding complex, 2.5 µl of p50 or p65 antibodies (Calbiochem) was incubated with nuclear proteins for 1 hour at 37°C prior to addition of the radiolabeled probe.
Evaluation of caspase-3 activity
Caspase-3 activity was assessed as a measure of apoptosis as previously described.(30)
Growth assay
Fetal PAECs were plated at 1 × 105 cells/well into six-well plates and allowed to adhere overnight. The following day (day 0) the medium was changed to DMEM with 2.5% FBS plus LPS (0.1 to 100 ng/mL), with and without BAY 11-7085 (1 nmol/L) or parthenolide (0.1 µmol/L) pretreatment. These doses were chosen after dose-response experiments showed no affect of BAY 11-7085 or parthenolide on baseline growth after prolonged (3 day) exposure when compared to control (data not shown). Media was changed daily, and cell counts were performed on days 0 and 3 after removing cells with trypsin digestion. All conditions were run in triplicate. Growth studies with treatment were performed in DMEM with 2.5% FBS, based on previous studies that determined that this was the lowest serum concentration that supported fetal PAEC survival with some proliferation.(32)
Statistical Analysis
For comparison between treatment groups, the null hypothesis that no difference existed between treatment means was tested by ANOVA for multiple groups or t test for 2 groups (InStat). Statistical significance (p<0.05) between and within groups was determined by means of Bonferroni method of multiple comparisons.
Results
Antenatal exposure to LPS increases SOD activity and MnSOD expression
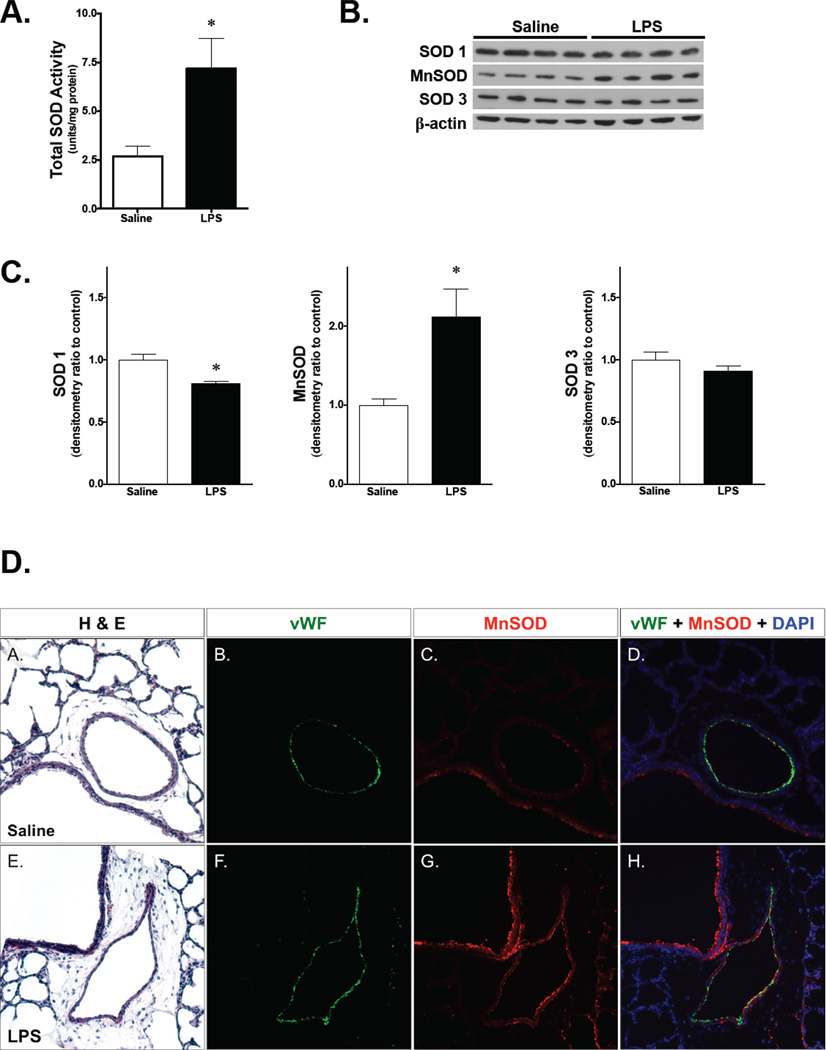
Our recent study demonstrated that exposure to intraamniotic LPS protects against hyperoxia-induced neonatal pulmonary vascular injury.(13) Since higher SOD activity attenuates hyperoxic injury,(14–17) the expression of pulmonary SOD in newborn mice following exposure to intraamniotic LPS was evaluated. Intraamniotic LPS resulted in a significant increase in total pulmonary SOD activity when compared to saline-injected controls on DOL 1 (Fig. 1A). On Western analysis, intraamniotic LPS increased the protein expression of MnSOD (SOD2), while it decreased SOD1 expression, and SOD3 did not change. (Fig. 1B and 1C). These data show that specific upregulation of MnSOD expression is responsible for the increased pulmonary SOD activity in newborn rats after exposure to antenatal LPS.
Figure 1. Exposure to intraamniotic LPS induces MnSOD expression and activity in newborn rat lung.
A) Total pulmonary SOD activity measured in whole lung homogenate from newborn rats exposed to intraamniotic LPS. Saline, intraamniotic saline injection; LPS, intraamniotic LPS injection. *, p<0.05 vs. saline control. Values are means ± SE, n=4 animals/group.
B) Representative Western blot showing SOD1, MnSOD, and SOD3 in whole lung homogenate from newborn rats following exposure to intraamniotic saline vs. LPS. β-actin is shown as a loading control.
C) Densitometric evaluation of SOD1, MnSOD, and SOD3 protein expression in whole lung homogenate from newborn rats exposed to intraamniotic saline vs. LPS. *, p<0.05 vs. saline control. Values are means ± SE, n=4 animals/group.
D) Representative immunofluorescence and H&E staining of newborn rat lungs following intraamniotic injection of saline (A–D) or LPS (E–H), all at 200X magnification. MnSOD was stained in red, vWF in green, and DAPI in blue. LPS, lipopolysaccharide; vWF, von Willebrand factor; MnSOD, manganese superoxide dismustase; DAPI, 4',6-diamidino-2-phenylindole.
The source of increased MnSOD expression in the newborn lung examined by immunofluorescence staining. In saline-exposed newborn rats, MnSOD was expressed almost exclusively in the bronchial epithelium (Fig. 1A–D). However, LPS-exposed newborn rats demonstrated increased intensity of MnSOD signal (Fig 1G, C vs. G), part of which was co-localized with vWF, a marker of endothelial cells (Fig 1H, D vs. H). H&E staining on the adjacent lung sections confirmed the architecture of pulmonary vasculature (Fig 1A, 1E), which was double positive for MnSOD and vWF as shown in immunofluoresecne staining of LPS-exposed rat lungs. These findings indicate that increased MnSOD expression in the pulmonary endothelium contributes to elevated MnSOD in the fetal lung in response to intra-amniotic LPS.
Increased MnSOD protein expression in Fetal PAEC exposed to LPS
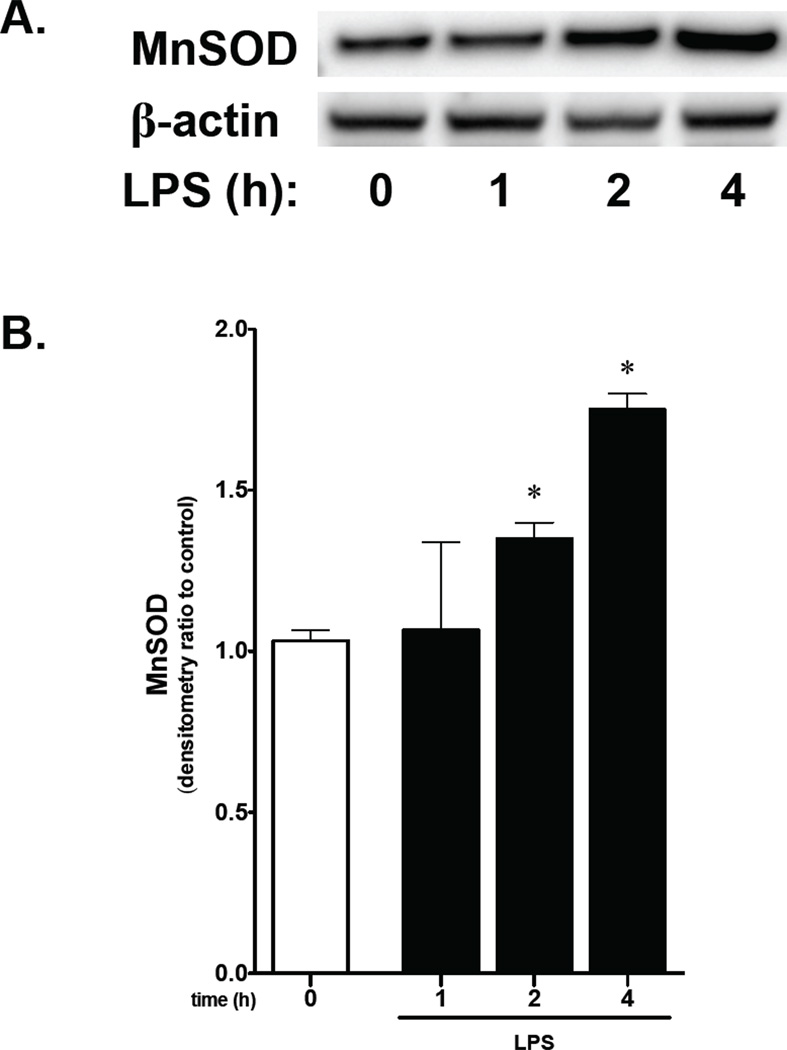
To further evaluate the in vivo observation of increased MnSOD expression in the developing pulmonary endothelium following intraamniotic LPS, we exposed fetal PAEC to LPS. Following exposure to LPS for 2 to 4 hours, protein levels of MnSOD increased significantly (Fig. 2A, 2B). These findings support the in vivo data showing that exposure to antenatal LPS results in increased MnSOD expression in the fetal pulmonary endothelium.
Figure 2. LPS exposure increases MnSOD protein expression in fetal PAEC in vitro.
A) Representative Western blot showing MnSOD in whole cell lysate from fetal PAEC exposed to LPS (10 ng/mL, 0–4h) with β-actin as loading control.
B) Densitometric evaluation of cytosolic MnSOD in fetal PAECs exposed to LPS. *, p<0.05 vs. unexposed control. Values are means ± SE of three independent experiments.
LPS induces NF-κB activity in Fetal PAEC
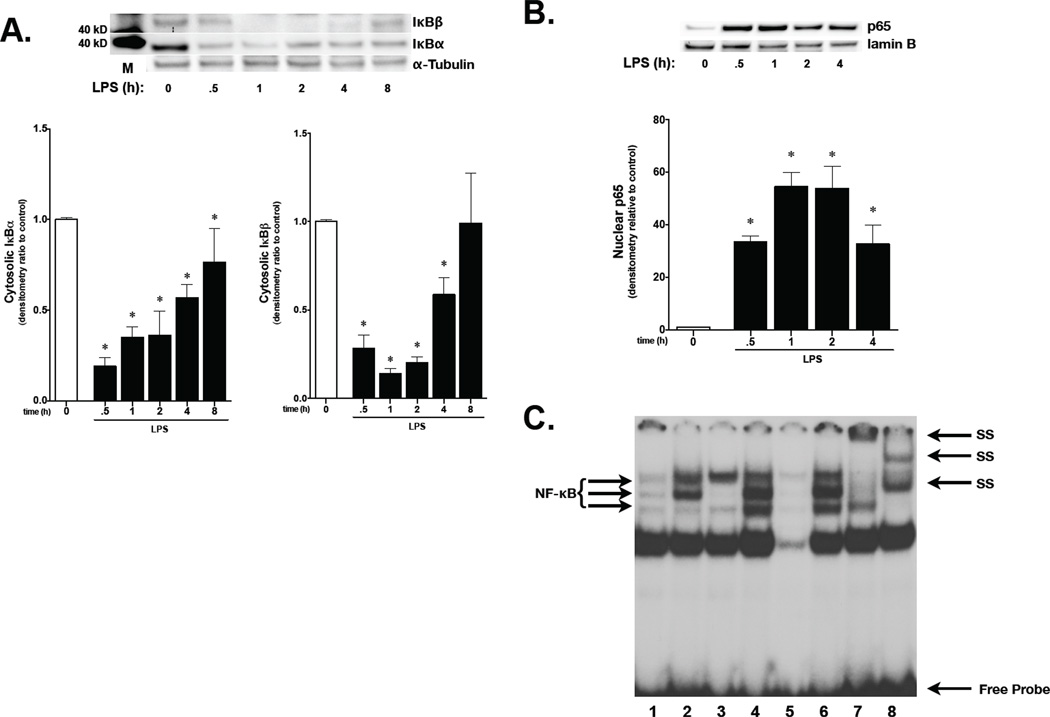
As LPS is known to induce NF-κB activity, and cytokines induce MnSOD expression via NF-κB activation,(33, 34) NF-κB activity was assessed in fetal PAEC. Cytoplasmic IκBα and IκBβ degradation occurred within 30 minutes of exposure to LPS (10 ng/ml, Fig. 3A). Densitometric evaluation of IκB levels following LPS confirmed significant decreases of IκB levels starting at 30 minutes of exposure and continuing through 8 hours for IκBα and 4 hours for IκBβ (Fig. 3A).
Figure 3. LPS induces NF-κB activation in fetal PAEC.
A) Representative Western blot showing IκBβ and IκBα in cytosolic extracts from fetal PAEC exposed to LPS (1 or 10 ng/mL), with tubulin as loading control. Densitometric evaluation is provided. C, control; h, hours; *, p <0.05 vs. control. Values are means ± SE of four independent experiments for each group.
B) Representative Western blot showing p65 in nuclear extracts from fetal PAEC exposed to LPS (10 ng/mL, 0–4h), with Lamin B as loading control. Densitometric evaluation is provided. C, control; h, hours; *, p <0.05 vs. control. Values are means ± SE of four independent experiments for each group.
C) Representative EMSA of nuclear extracts from fetal PAEC exposed to LPS (10 ng/mL, 1h and 18h). Bands representing NF-κB consensus sequence binding, nonspecific binding, free probe, and super shift (SS) bands are labeled. Lane 1, control, 1h; Lane 2, LPS 10 ng/mL, 1h; Lane 3, control, 18h; Lane 4, LPS 10 ng/mL, 18h; Lane 5, cold, 50-fold excess of unlabeled oligonucleotide added to 18h sample, LPS 10 ng/mL; Lane 6, mutant, 50-fold excess of mutated oligonucleotide added to 18h sample, LPS 10 ng/mL; Lane 7, p65 supershift, LPS 10 ng/mL; Lane 8, p50 supershift, LPS 10 ng/mL.
To confirm that degradation of IκB was associated with nuclear translocation of NF-κB subunits, nuclear extracts were subjected to Western blot and probed for the NF-κB subunit p65. Corresponding with the timing of IκB degradation, nuclear p65 increased significantly after 30 minutes of exposure to LPS, and remained elevated through 4 hours of exposure (Fig. 3B). Furthermore, EMSA revealed increased NF-κB DNA binding at 1 hours of exposure to LPS, which was persistent through 18 hours of exposure (Fig. 3C, lanes 2 and 4 respectively). Supershift analysis confimed presence of the NF-κB subunits p65 (lane 7) and p50 (lane 8). These results confirm that LPS induces IκB degradation, subsequent NF-κB nuclear translocation and DNA binding in fetal PAEC.
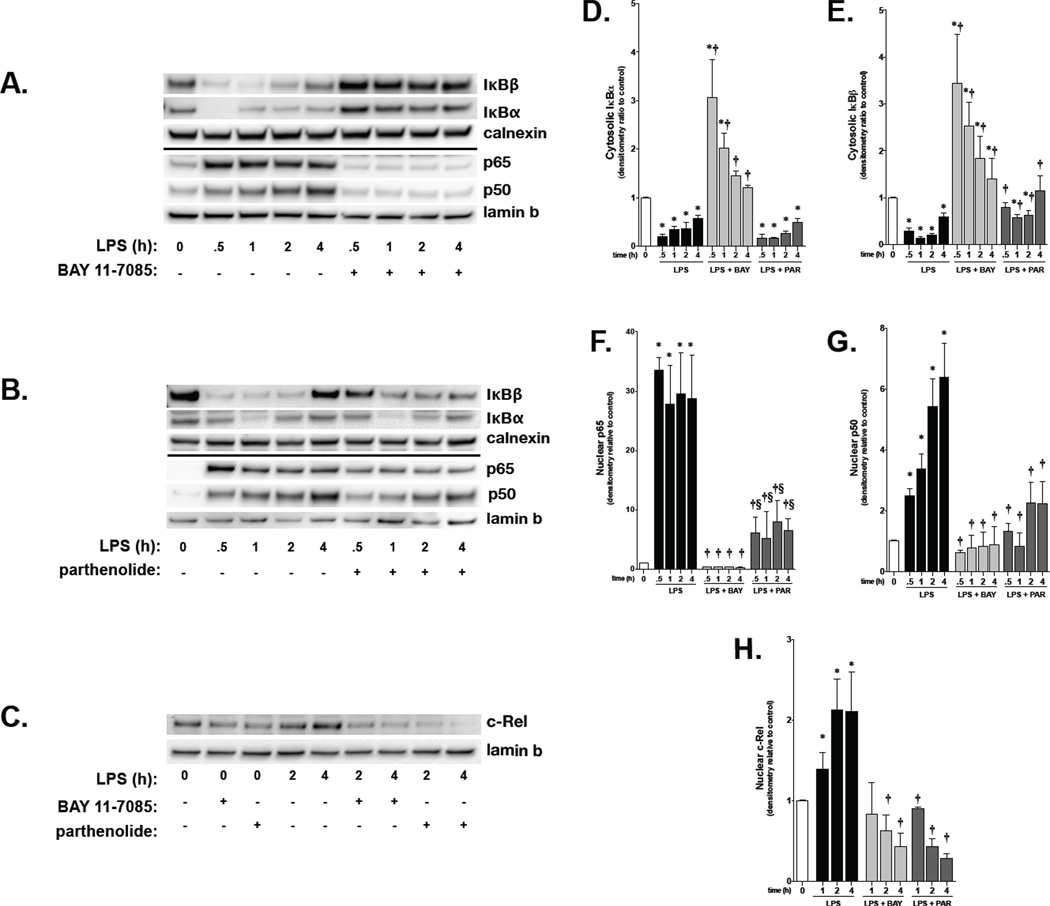
NF-κB inhibitors BAY 11-7082 and parthenolide have differential effects on IκB Mediated NF-κB activation in fetal PAEC
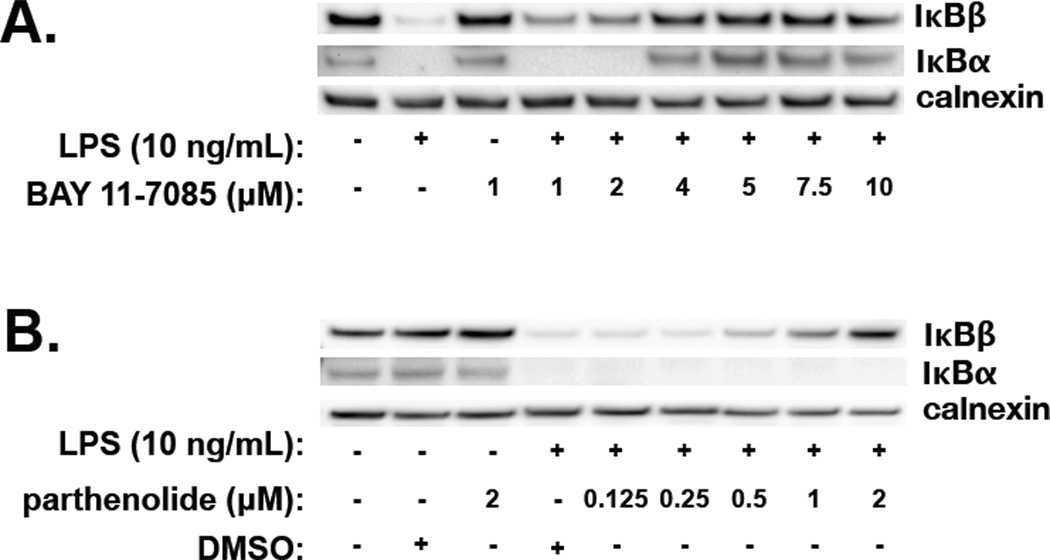
Having demonstrated that LPS-induced NF-κB activity occurs in fetal PAEC, the effect of NF-κB inhibitors was assessed. Parthenolide and BAY 11-7085, both IKK inhibitors, were used. Fetal PAEC were pretreated with either BAY 11-7085 or parthenolide for 1 hour prior to LPS (10 ng/ml, 1 hour) exposure. BAY 11-7085 completely inhibited IκBα and IκBβ degradation at a dose of 4 µmol/L (Fig. 4A). In contrast, parthenolide did not inhibit IκBα degradation at any dose, while IκBβ degradation was inhibited in a dose-dependent fashion starting at 0.5 µmol/L (Fig. 4B). Doses of parthenolide above 2 µmol/L were not assessed by Western analysis as visible cytotoxicity was noted prohibiting adequate protein collection. Based on these experiments, doses of BAY 11-7085 (4 µmol/L) and parthenolide (2 µmol/L) were used for all further experiments.
Figure 4. The NF-κB inhibitors BAY-7085 and Parthenolide have differential effects on LPS-induced IκBα and IκBβ degradation in fetal PAEC.
A) Representative Western blot showing IκBβ and IκBα in whole cell lysates from fetal PAEC pretreated with BAY-7085 (0–10 µmol/L, 1h) prior to LPS exposure (10 ng/mL, 1h). Calnexin is shown as a loading control.
B) Representative Western blot showing IκBβ and IκBα protein in whole cell lysate from fetal PAEC pretreated with parthenolide (0–2 µmol/L, 1h), DMSO vehicle, or control, and prior to LPS exposure (10 ng/mL, 1h). Calnexin is shown as a loading control.
Having identified these doses, the time course of LPS-induced NF-κB activation following BAY 11-7085 and parthenolide pretreatment was determined. In fetal PAEC pretreated with BAY 11-7085, degradation of both IκBα and IκBβ was prevented through 4 hours of exposure to LPS (Fig. 5A, 5D and 5E). Prevention of IκB degradation by BAY 11-7085 was associated with complete inhibition of p65, p50 and c-Rel nuclear translocation in LPS-exposed PAEC (Fig 5A, 5C, 5F, 5G and 5H). These data suggest that pretreating with BAY 11-7085 completely prevents NF-κB activation. In contrast to BAY 11-7085, parthenolide did not affect IκBα degradation but selectively attenuated LPS-induced IκBβ degradation (Fig. 5B, 5D and 5E). This effect of parthenolide resulted in attenuated p65 and p50 nuclear translocation (Fig. 5B, 5F and 5G) and complete inhibition of c-Rel nuclear translocation in LPS-exposed PAEC (Fig. 5C and 5H). Of note, cells pretreated with parthenolide prior to LPS demonstrated significantly less p65 and p50 nuclear translocation when compared to LPS treatment alone (Fig. 5B, 5F and 5G). Furthermore, p65 nuclear translocation was significantly higher in cells pretreated with parthenolide when compared to BAY 11-7085 pretreatment prior to exposure to LPS at all time points (Fig. 5F). Based on previous reports that c-Rel containing NF-κB dimers bind preferentially to IκBβ, (35–41) our data suggests that parthenolide specifically inhibits LPS-induced c-Rel nuclear translocation through preventing IκBβ degradation. Accordingly, the presence of LPS-induced p65 and p50 nuclear translocation likely represents intact NF-κB signaling through IκBα degradation.
Figure 5. The NF-κB inhibitors BAY-7085 and Parthenolide demonstrate differential effects on LPS-induced NF-κB activation in fetal PAEC.
A) Representative Western blot showing IκBβ and IκBα protein in cytosolic extracts, and p65 and p50 protein in nuclear extracts, in fetal PAEC pretreated with BAY-7085 (4 µmol/L, 1h) prior to LPS exposure (10 ng/ml). Calnexin and Lamin B are shown as cytosolic and nuclear loading controls, respectively.
B) Representative Western blot showing IκBβ and IκBα protein in cytosolic extracts, and p65 and p50 protein in nuclear extracts, in fetal PAEC pretreated with parthenolide (2 µmol/L, 1h) prior to LPS exposure (10 ng/ml). Calnexin and Lamin B are shown as cytosolic and nuclear loading controls, respectively.
C) Representative Western blot showing c-Rel protein in nuclear extracts, in fetal PAEC pretreated with parthenolide (2 µmol/L, 1h) or BAY-7085 (4 µmol/L, 1h) prior to LPS exposure (10 ng/ml). Lamin B is shown as nuclear loading control.
D) Densitometric evaluation of cytosolic IκBα in fetal PAECs pretreated with NF-κB inhibitors and exposed to LPS. *, p<0.05 vs. unexposed control; † p<0.05 vs. time matched LPS exposed. Values are means ± SE of five independent experiments for each group.
E) Densitometric evaluation of cytosolic IκBβ in PAEC pretreated with NF-κB inhibitors and exposed to LPS. *, p<0.05 vs. unexposed control; †, p<0.05 vs. time matched LPS exposed. Values are means ± SE of five independent experiments for each group.
F) Densitometric evaluation of nuclear p65 in PAEC pretreated with NF-κB inhibitors and exposed to LPS. *, p<0.05 vs. unexposed conrol; †, p<0.05 vs. time-matched LPS-exposed; §, p<0.05 vs. time-matched BAY + LPS-exposed. Values are means ± SE of five independent experiments for each group.
G) Densitometric evaluation of nuclear p50 in PAEC pretreated with NF-κB inhibitors and exposed to LPS. *, p<0.05 vs. unexposed conrol; †, p<0.05 vs. time-matched LPS-exposed; §, p<0.05 vs. time-matched BAY + LPS-exposed. Values are means ± SE of five independent experiments for each group.
H) Densitometric evaluation of nuclear c-Rel in PAEC pretreated with NF-κB inhibitors and exposed to LPS. *, p<0.05 vs. unexposed conrol; †, p<0.05 vs. time-matched LPS-exposed. Values are means ± SE of four independent experiments for each group.
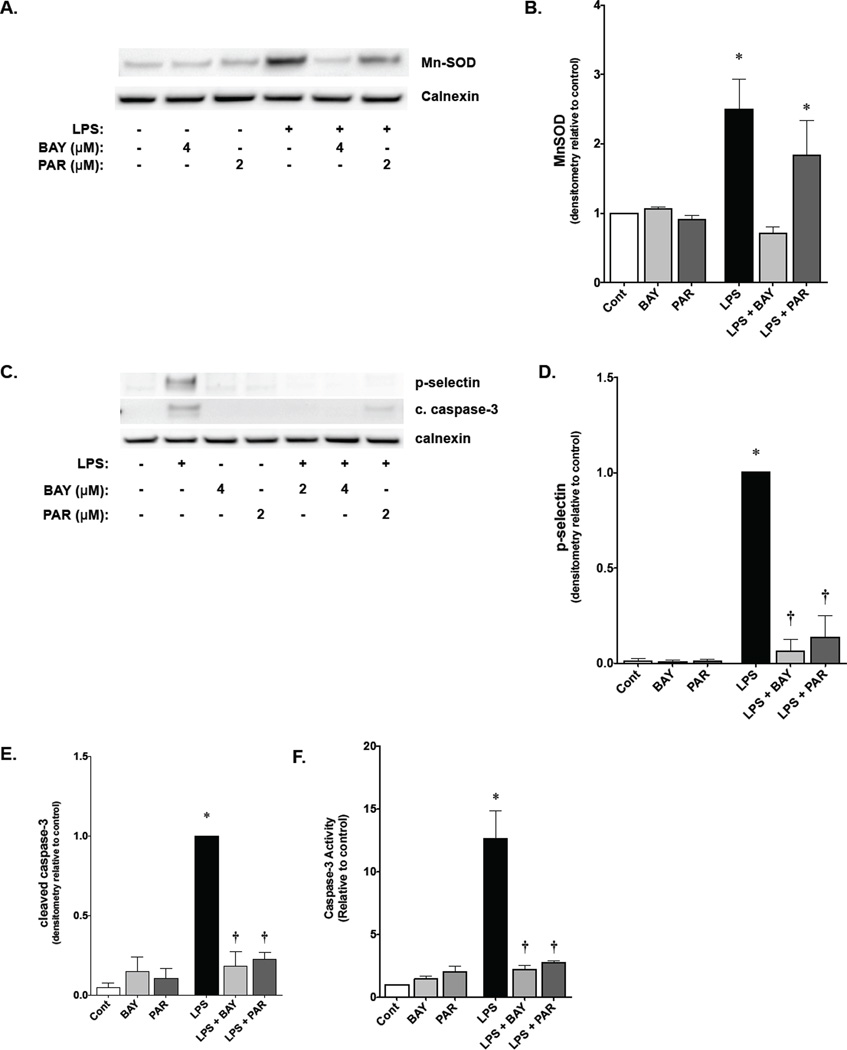
NF-κB inhibitors BAY 11-7085 and parthenolide have differential effects on NF-κB-mediated MnSOD expression in fetal PAEC
Given the in vitro data demonstrating that LPS exposure induced MnSOD activity and protein expression in the fetal lung, the effect of NF-κB blockade on MnSOD expression in fetal PAEC was assessed. A significant increase in MnSOD expression was seen in fetal PAEC exposed to LPS (Fig. 6A, 6B). Pretreatment with BAY 11-7085 completely abrogated this upregulation (Fig. 6A, 6B). In contrast, pretreatment with parthenolide did not affect in LPS-induced MnSOD expression (Fig. 6A and 6B). These results suggest that LPS-induced NF-κB activity that proceeds through IκBα is responsible for MnSOD expression.
Figure 6. Effects of BAY-7085 and Parthenolide on NF-κB target gene expression and apoptosis in fetal PAEC.
A) Representative Western blot showing MnSOD protein in whole cell lysates from fetal PAEC pretreated with BAY-7085 (4 µmol/L, 1h) or parthenolide (2 µmol/L, 1h) prior to LPS exposure (10 ng/mL, 6h). Calnexin is shown as a loading control.
B) Densitometric evaluation of MnSOD in fetal PAEC pretreated with NF-κB inhibitors (1h) and exposed to LPS (10 ng/mL, 6h). *, p<0.05 vs. control. Values are means ± SE of four independent experiments for each group.
C) Representative Western blot showing P-selectin and cleaved caspase-3 protein in whole cell lysates from fetal PAEC pretreated with BAY-7085 (2 or 4 µmol/L, 1h) or parthenolide (2 µmol/L, 1h) prior to LPS exposure (10 ng/mL, 6h). Calnexin is shown as a loading control.
D) Densitometric evaluation of P-selectin in whole cell lysate from fetal PAEC pretreated with NF-κB inhibitors and exposed to LPS (10 ng/mL, 6h). *, p<0.05 vs. control; †, p<0.05 vs. time-matched LPS-exposed. Values are means ± SE of four independent experiments for each group.
E) Densitometric evaluation of cleaved caspase-3 in whole cell lysate from fetal PAEC pretreated with NF-κB inhibitors and exposed to LPS (10ng/mL, 6h). *, p<0.05 vs. control; †, p<0.05 vs. time-matched LPS-exposed. Values are means ± SE of four independent experiments for each group.
F) Caspase-3 activity of fetal PAEC pretreated with BAY-7085 or parthenolide followed by exposure to LPS. Cont, unexposed; BAY, 4 µmol/L BAY-7085; PAR, 2 µmol/L parthenolide; LPS, 10 ng/mL LPS; LPS + BAY, 10 ng/mL LPS, 4 µmol/L BAY-7085; LPS + PAR, 10 ng/mL LPS, 2 µmol/L parthenolide. *, p< 0.05.
NF-κB inhibitors BAY 11-7085 and parthenolide prevent NF-κB-mediated target pro-inflammatory gene expression and apoptosis in fetal PAEC
Having demonstrated a differential effect of BAY 11-7085 and parthenolide on NF-κB signaling, the expression of pro-inflammatory NF-κB regulated genes and apoptosis was assessed. Both parthenolide and BAY 11-7085 pretreatment prior to LPS exposure prevented expression of P-selectin (Fig 6C, 6D). Additionally, both BAY 11-7085 and parthenolide pretreatment prevented LPS-induced apoptosis in fetal PAEC as assessed by caspase-3 cleavage by Western blot (Fig. 6C, 6E). The results on Western blot were corroborated by caspase-3 activity assay (Fig. 6F). These results suggest that preventing NF-κB activity through IκBβ attenuates inflammation and apoptosis following exposure to LPS. Thus, the pro-inflammatory component of LPS-induced NF-κB activation is attenuated by preventing IκBβ degradation, while the protective, acute phase response of MnSOD expression, is preserved by allowing NF-κB signaling to proceed through IκBα.
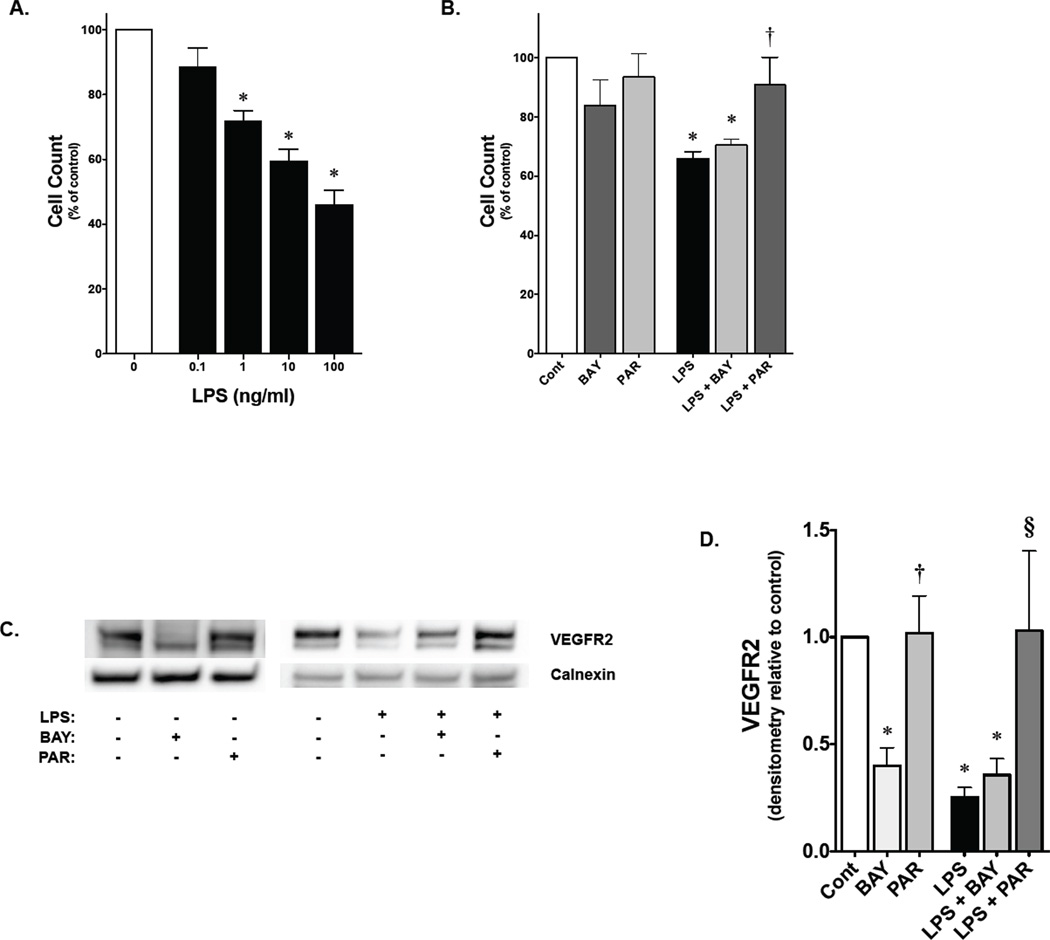
Parthenolide, but not BAY 11-7085, attenuates LPS inhibition of PAEC growth and impaired VEGFR2 expression
Having noted that LPS-induced apoptosis was inhibited by both BAY 11-7085 and parthenolide, the effect on cell growth was assessed. Fetal PAEC demonstrated a dose-dependent decrease in cell number through 3 days of LPS exposure (Fig. 7A). The effect of NF-κB inhibition on LPS-induced decrease in cell growth was assessed after BAY 11-7085 and parthenolide pretreatment. Parthenolide pretreatment abrogated the LPS-induced decrease in cell number (Fig. 7B) that was associated with preserved VEGFR2 expression (Fig. 7C, 7D). In contrast, although BAY 11-7085 was able to inhibit apoptosis, pretreatment was not able to prevent the LPS-induced decrease in cell growth (Fig. 7B), and was associated with decreased VEGFR2 expression (Fig. 7C, 7D).
Figure 7. Parthenolide attenuates LPS-induced inhibition of fetal PAEC proliferation.
A) Fetal PAEC cell number expressed as percent of control following exposure to LPS (range, 0.1–100 ng/ml) for 3 days. *, p<0.05 vs. unexposed control. Values are means ± SE of three independent experiments for each group
B) Fetal PAEC cell number expressed as percent of control following exposure to either BAY 11-7085 (1 nmol/L), parthenolide (0.1 µmol/L), LPS (1 ng/ml) or combination for 3 days. *, p<0.05 vs. control; †, p<0.05 vs. LPS and LPS+BAY exposed. Values are means ± SE of three independent experiments for each group.
C) Representative Western blot showing VEGFR2 protein in whole cell lysates from fetal PAEC pretreated with BAY-7085 (4 µmol/L, 1h) or parthenolide (2 µmol/L, 1h) prior to LPS exposure (10 ng/mL, 6h). Calnexin is shown as a loading control.
D) Densitometric evaluation of VEGFR2 in fetal PAEC pretreated with NF-κB inhibitors (1h) and exposed to LPS (10 ng/mL, 6h). *, p<0.05 vs. control. †, p<0.05 vs. BAY-7085 pre-treatment; §, p<0.05 vs. time matched LPS and BAY+LPS exposed. Values are means ± SE of three independent experiments for each group.
Discussion
This study highlights several novel findings. First, we found that intraamniotic LPS exposure during late gestation increases MnSOD expression in pulmonary vascular endothelium of newborn rats. Furthermore, we showed that up-regulation of MnSOD in fetal pulmonary vascular endothelial cells in response to LPS is dependent upon NF-κB activation. Third, we demonstrated the differential roles of IκBα and IκBβ in regulating downstream effects of LPS-induced NF-κB activity on fetal PAEC: NF-κB activation through IκBα degradation mediates LPS-upregulated MnSOD, whereas NF-κB signaling through IκBβ degradation mediates LPS-induced apoptosis and P-selectin expression. Moreover, we found that IκBα degradation is required for protecting growth of fetal PAEC against LPS, likely through preservation of VEGFR2 expression and induction of MnSOD expression. These findings collectively support our hypothesis that endothelial NF-κB activation regulates disparate responses of fetal pulmonary artery endothelium toward inflammatory stress.
This is the first study reporting that LPS activates NF-κB in fetal vascular endothelial cells. The pulmonary vascular endothelium is not only a prime target of inflammatory insults,(42) but also play a pivotal role in orchestrating vascular and alveolar growth in in the developing lung.(5–8) The present study reports that LPS-induced NF-κB activation mediates multiple downstream cellular responses. First, we demonstrate that LPS-induced NF-κB activation upregulates MnSOD, which builds on previous work showing that NF-κB is involved in the induction of MnSOD by pro-inflammatory stimuli.(33, 34) Second, NF-κB signaling provides a transcriptional mechanism to explain the previously reported apoptotic response to LPS in fetal PAEC.(18) Third, the present study reports that LPS-activated NF-κB in fetal PAEC mediates the induction of P-selectin, which has been found significantly contributing to inflammatory lung injury in endotoxinemia.(43) The ability of LPS-induced NF-κB activation to regulate the expression of both cytoprotective and pro-inflammatory genes in fetal PAEC, as demonstrated in the present study, uncovers the transcriptional regulation that links the otherwise seemingly contradictory findings in our previous work.(13) We believe this mechanism contributes to the finding that intraamniotic LPS exposure increases the resistance of pulmonary vasculature to neonatal hyperoxic injury but paradoxically impairs lung vascular and alveolar growth in neonatal rats raised in room air.(13)
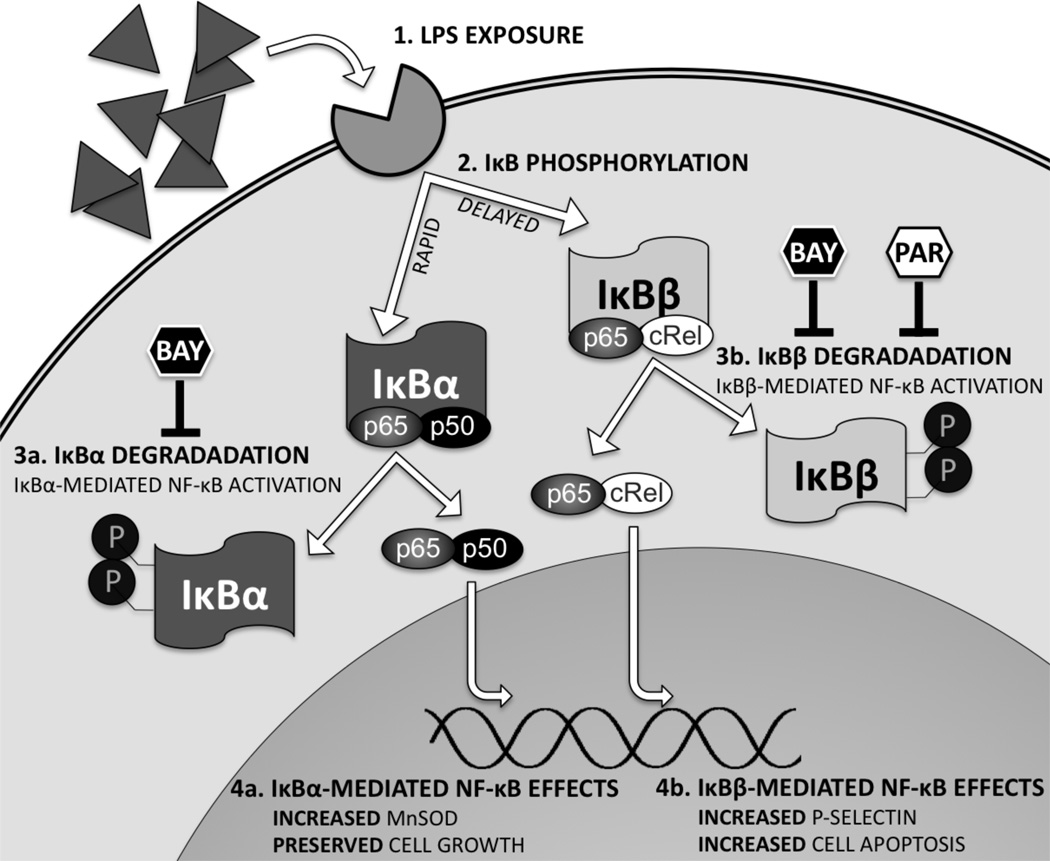
This is the first study reporting that IκBα and IκBβ differentially regulate NF-κB-mediated target gene expression in endothelial cells in response to inflammatory stress (Fig. 8). Both IκBα and IκBβ are traditionally viewed as negative regulators that inhibit NF-κB nuclear translocation and activation. Only recently has it been recognized that IκBβ is a co-activator for NF-κB-mediated transcription of inflammatory cytokines.(44, 45) However, the role of IκBβ in regulating endothelial inflammatory response has not been reported. By using fetal pulmonary vascular endothelial cells, we demonstrates that IκBα and IκBβ play differential roles in regulating the downstream gene targets in response to inflammatory stress. As shown in this study, complete NF-κB inhibition achieved through inhibiting both IκBα and IκBβ degradation abolishes LPS-induced MnSOD upregulation, while preventing apoptosis and P-selectin expression in fetal PAEC. In contrast, selective inhibition of IκBβ degradation preserves MnSOD upregulation, prevents P-selectin upregulation and protects endothelial cell growth. These findings highlight that NF-κB activation mediated through IκBβ degradation plays a pro-injury role, which is distinct from the cytoprotective role of IκBα in integrating inflammatory response of the developing pulmonary vascular endothelium.
Figure 8. Summary of NF-κB Signaling Effects in the Fetal Pulmonary Endothelium.
The fetal pulmonary endothlium are exposed to LPS, triggering Toll-like receptor 4 (TLR4) binding(1). This leads to activation of the IKK complex, and subsequent phosphorylation of IκBα in a rapid-phase response, then IκBβ in a delayed-phase response(2). Phosphorylation targets IκBα for ubiquitination and subsequent proteosomal degradation, allowing dissociation of the NF-κB complex (p65-p50), resulting in nuclear translocation, and DNA binding(3a). With prolonged exposure to LPS, IKK phosphorylation of IκBβ leads to ubiquitination and subsequent proteosomal degradation, releasing IκBβ-bound NF-κB dimers (p65-cRel), resulting in nuclear translocation, and DNA binding (3b). IκBα-mediated NF-κB activation results transcription of genes including MnSOD, and other gene and protein targets work to preserve proliferation(4a). IκBβ-mediated NF-κB activation results in transcription of genes including P-selectin, and other targets that induce apoptosis(4b). BAY-7085 blocks this process by preventing degradation of both IκB proteins. In contrast, parthenolide blocks this process by selective inhibition of IκBβ degradation.
Mechanisms underlying the differential roles of IκBα and IκBβ in regulating fetal lung endothelial response to inflammatory stress may reflect the known differences between IκBα and IκBβ. The penultimate step of NF-κB nuclear translocation and DNA binding converges on the inhibitory proteins IκBα and IκBβ.(20) Although both IκBα and IκBβ inhibit NF-κB activation, signaling that proceeds through these proteins has unique implications on target gene expression. As IκBα and IκBβ preferentially bind unique NF-κB dimer combinations, and each dimer combination has different affinity for DNA sequences, specific genes are targeted.(35, 36) Additionally, following degradation, newly synthesized IκBα enters the nucleus and removes DNA-bound NF-κB complexes through its nuclear export sequence, thereby terminating the activity of NF-κB dimers on transcribing the target genes.(20) In contrast, IκBβ has no nuclear export sequence, and once in the nucleus facilitates and stabilizes DNA binding of NF-κB dimers, resulting in expression of specific pro-inflammatory target genes.(38–40, 44, 45) This has important implications on the expression of NF-κB target genes, as the duration of NF-κB nuclear localization plays a role in determining which target genes are expressed, including pro-inflammatory chemokines.(41, 46) Thus, the effect of specifically inhibiting LPS-induced IκBβ degradation in fetal pulmonary endothelial cells may be due to inhibiting the nuclear translocation of specific NF-κB dimers, or simply from truncating the duration and blunting NF-κB activity. By utilizing knockout or transgenic mice with controlled expression of IκBα and IκBβ, respectively, future animal studies will determine whether selective inhibition of endothelial IκBβ degradation during inflammatory stress may attenuate fetal lung injury from antenatal inflammation while preserving normal lung development.
The anti-inflammatory properties of the sesquiterpene lactone parthenolide have been attributed to its effects on NF-κB. Reported mechanisms of action include inhibition of IKK activity or p65 DNA binding.(47) Previous studies have demonstrated that parthenolide can inhibit LPS-induced NF-κB activation in vitro and in vivo.(48–54) However, in these studies, the effect of parthenolide on LPS-induced IκB degradation was not assessed. Conflicting data exists on whether parthenolide inhibits LPS-induced IκBα degradation, while the effect on IκBβ degradation has never been reported. Parthenolide inhibits LPS-induced IκBα degradation and NF-κB activation in RAW 264.7, peripheral blood mononuclear, and vascular smooth muscle cells.(55–58) In contrast to these findings, others have found parthenolide can prevent LPS-induced NF-κB activation despite only transient or absent inhibition of IκBα degradation.(59, 60) Our data shows conclusively that parthenolide inhibits IκBβ degradation, NF-κB nuclear translocation and NF-κB target gene expression in fetal pulmonary endothelial cells. Thus, the differences between our findings and those previously reported are most likely stimulus-dependent, dose-dependent, cell-type specific, and developmentally regulated in cells and tissues being studied. The discovery of differential effects of BAY-7085 and parthenolide on the IκB isoforms was unexpected, but we believe the data is in concert with previous findings regarding the roles of IκBα and IκBβ in mediating LPS-induced NF-κB activation. Additionally, it must be acknowledged that the use of pharmacologic inhibitors in this study raises the question of “off-target” effects on various signaling pathways and the cell-type specificity of these effects. For example, parthenolide can affect MAP kinase signaling stimulated by LPS,(61, 62) and ERK and AP-1 activity stimulated by TNF-α and IL-1β.(63) Thus, the interpretation of our data must be tempered by the reality that there may be yet undiscovered affects of parthenolide and BAY-7085 on intracellular signaling, and their effects on specific types of cells not completely understood. However, these findings suggest that there may be therapeutic advantages to selectively targeting the pro-inflammatory consequences of NF-κB activation that occur with IκBβ degradation.
Our studies are limited in that we used only pharmacologic agents to inhibit inflammatory stress-induced NF-κB activity in the fetal PAECs. We chose to use pharmacologic inhibition, rather than transient overexpression or silencing of the IκB isoforms for multiple reasons. First, over expression of either IκBα or IκBβ would lead to nearly complete inhibition of LPS-induced NF-κB activity due to the cellular abundance of inhibitory protein. Moreover, silencing expression of either IκBα or IκBβ alters both constitutive and induced NF-κB activity.(64)
In conclusion, the present study demonstrates that LPS-induced NF-κB activation mediates both cytoprotective and disruptive responses in fetal pulmonary vascular endothelial cells. LPS-induced NF-κB activity achieves this differential transcriptional regulation through specific isoforms of IκB proteins; NF-κB signaling via IκBα degradation upregulates MnSOD and preserves cell growth, whereas NF-κB activation through IκBβ degradation induces apoptosis and P-selectin expression. The disparate responses mediated by NF-κB signaling in fetal PAEC parallel the antioxidant and injurious effects of experimental chorioamnionitis on the neonatal pulmonary vasculature. We speculate that selective inhibition of endothelial NF-κB activation that results from IκBβ degradation may prevent endothelial dysfunction and preserve the enhanced antioxidant defense in the developing pulmonary vasculature exposed to intrauterine inflammatory stress.
Acknowledgments
We owe thanks to Jason Gien, MD and Vivek Balasubramaniam, MD, for their help with the endothelial cell isolation and assays.
Sources of Funding
This work was supported by The Children’s Hospital Colorado Research Institute Research Scholar Award (to JRT), National Institutes of Health Grants K08 HL098562 (to CJW) and T32 HL07670 and R01 HL68702 (to SHA).
References
- 1.Speer CP. Chorioamnionitis: Important Risk Factor or Innocent Bystander for Neonatal Outcome? Neonatology. 2010;99:177–187. doi: 10.1159/000320170. [DOI] [PubMed] [Google Scholar]
- 2.Hartling L, Liang Y, Lacaze-Masmonteil T. Chorioamnionitis as a risk factor for bronchopulmonary dysplasia: a systematic review and meta-analysis. Archives of Disease in Childhood - Fetal and Neonatal Edition. 2012;97:F8–F17. doi: 10.1136/adc.2010.210187. [DOI] [PubMed] [Google Scholar]
- 3.Woldesenbet M, Perlman JM. Histologic chorioamnionitis: an occult marker of severe pulmonary hypertension in the term newborn. J Perinatol. 2005;25:189–192. doi: 10.1038/sj.jp.7211240. [DOI] [PubMed] [Google Scholar]
- 4.Woldesenbet M, Rosenfeld CR, Ramilo O, Johnson-Welch S, Perlman JM. Severe neonatal hypoxic respiratory failure correlates with histological chorioamnionitis and raised concentrations of interleukin 6 (IL6), IL8 and C-reactive protein. Arch Dis Child Fetal Neonatal Ed. 2008;93:F413–F417. doi: 10.1136/adc.2007.124503. [DOI] [PubMed] [Google Scholar]
- 5.Jakkula M, Le Cras TD, Gebb S, Hirth KP, Tuder RM, Voelkel NF, Abman SH. Inhibition of angiogenesis decreases alveolarization in the developing rat lung. Am J Physiol Lung Cell Mol Physiol. 2000;279:L600–L607. doi: 10.1152/ajplung.2000.279.3.L600. [DOI] [PubMed] [Google Scholar]
- 6.Abman SH. Bronchopulmonary dysplasia: "a vascular hypothesis". Am J Respir Crit Care Med. 2001;164:1755–1756. doi: 10.1164/ajrccm.164.10.2109111c. [DOI] [PubMed] [Google Scholar]
- 7.Stenmark KR, Abman SH. Lung vascular development: implications for the pathogenesis of bronchopulmonary dysplasia. Annu Rev Physiol. 2005;67:623–661. doi: 10.1146/annurev.physiol.67.040403.102229. [DOI] [PubMed] [Google Scholar]
- 8.Thébaud B, Abman SH. Bronchopulmonary dysplasia: where have all the vessels gone? Roles of angiogenic growth factors in chronic lung disease. Am J Respir Crit Care Med. 2007;175:978–985. doi: 10.1164/rccm.200611-1660PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abman SH. Impaired vascular endothelial growth factor signaling in the pathogenesis of neonatal pulmonary vascular disease. Adv Exp Med Biol. 2010;661:323–335. doi: 10.1007/978-1-60761-500-2_21. [DOI] [PubMed] [Google Scholar]
- 10.Moss TJ, Newnham JP, Willett KE, Kramer BW, Jobe AH, Ikegami M. Early gestational intra-amniotic endotoxin: lung function, surfactant, and morphometry. Am J Respir Crit Care Med. 2002;165:805–811. doi: 10.1164/ajrccm.165.6.2108053. [DOI] [PubMed] [Google Scholar]
- 11.Kallapur SG, Bachurski CJ, Le Cras TD, Joshi SN, Ikegami M, Jobe AH. Vascular changes after intra-amniotic endotoxin in preterm lamb lungs. American journal of physiology Lung cellular and molecular physiology. 2004;287:L1178–L1185. doi: 10.1152/ajplung.00049.2004. [DOI] [PubMed] [Google Scholar]
- 12.Polglase GR, Hooper SB, Gill AW, Allison BJ, Crossley KJ, Moss TJM, Nitsos I, Pillow JJ, Kluckow M. Intrauterine inflammation causes pulmonary hypertension and cardiovascular sequelae in preterm lambs. Journal of applied physiology (Bethesda, Md : 1985) 2010;108:1757–1765. doi: 10.1152/japplphysiol.01336.2009. [DOI] [PubMed] [Google Scholar]
- 13.Tang JR, Seedorf GJ, Muehlethaler V, Walker DL, Markham NE, Balasubramaniam V, Abman SH. Moderate postnatal hyperoxia accelerates lung growth and attenuates pulmonary hypertension in infant rats after exposure to intra-amniotic endotoxin. Am J Physiol Lung Cell Mol Physiol. 2010;299:L735–L748. doi: 10.1152/ajplung.00153.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frank L, Yam J, Roberts RJ. The role of endotoxin in protection of adult rats from oxygen-induced lung toxicity. Journal of Clinical Investigation. 1978;61:269–275. doi: 10.1172/JCI108936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang G, Berg JT, White JE, Lumb PD, Lee CY, Tsan MF. Protection against oxygen toxicity by tracheal insufflation of endotoxin: role of Mn SOD and alveolar macrophages. Am J Physiol. 1994;266:L38–L45. doi: 10.1152/ajplung.1994.266.1.L38. [DOI] [PubMed] [Google Scholar]
- 16.White CW, Lewis-Molock Y, Suzuki K, Taniguchi N, Shimizu H, Nguyen DD, Mason RJ. Effects of cytokines and endotoxin on lung manganese superoxide dismutase expression and immunohistochemical distribution. Chest. 1994;105:85S–86S. doi: 10.1016/s0012-3692(15)42688-1. [DOI] [PubMed] [Google Scholar]
- 17.Shim J, Chang Y, Park W. Intratracheal administration of endotoxin attenuates hyperoxia-induced lung injury in neonatal rats. Yonsei Med J. 2008;49:144–150. doi: 10.3349/ymj.2008.49.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sampath V, Radish AC, Eis AL, Broniowska K, Hogg N, Konduri GG. Attenuation of lipopolysaccharide-induced oxidative stress and apoptosis in fetal pulmonary artery endothelial cells by hypoxia. Free radical biology & medicine. 2009;46:663–671. doi: 10.1016/j.freeradbiomed.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shiki Y, Meyrick BO, Brigham KL, Burr IM. Endotoxin increases superoxide dismutase in cultured bovine pulmonary endothelial cells. The American journal of physiology. 1987;252:C436–C440. doi: 10.1152/ajpcell.1987.252.4.C436. [DOI] [PubMed] [Google Scholar]
- 20.Oeckinghaus A, Ghosh S. The NF-B Family of Transcription Factors and Its Regulation. Cold Spring Harbor Perspectives in Biology. 2009;1 doi: 10.1101/cshperspect.a000034. a000034-a000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wei AC, Fan J, Jones JJ, Hamilton JE, Li YH, Marshall JC, Rotstein OD. Delayed treatment with diethyl maleate prevents E-selectin expression in human endothelial cells. Surgery. 1999;126:286–292. [PubMed] [Google Scholar]
- 22.Chan EL, Haudek SB, Giroir BP, Murphy JT. Human coronary endothelial cell activation by endotoxin is characterized by NF-kappa B activation and TNF-alpha synthesis. Shock. 2001;16:349–354. doi: 10.1097/00024382-200116050-00005. [DOI] [PubMed] [Google Scholar]
- 23.Fan J, Ye RD, Malik AB. Transcriptional mechanisms of acute lung injury. American journal of physiology. Lung cellular and molecular physiology. 2001;281:L1037–L1050. doi: 10.1152/ajplung.2001.281.5.L1037. [DOI] [PubMed] [Google Scholar]
- 24.Ding J, Song D, Ye X, Liu SF. A pivotal role of endothelial-specific NF-kappaB signaling in the pathogenesis of septic shock and septic vascular dysfunction. J Immunol. 2009;183:4031–4038. doi: 10.4049/jimmunol.0900105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ye X, Ding J, Zhou X, Chen G, Liu SF. Divergent roles of endothelial NF-kappaB in multiple organ injury and bacterial clearance in mouse models of sepsis. J Exp Med. 2008;205:1303–1315. doi: 10.1084/jem.20071393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ben-Neriah Y, Karin M. Inflammation meets cancer, with NF-kappaB as the matchmaker. Nat Immunol. 2011;12:715–723. doi: 10.1038/ni.2060. [DOI] [PubMed] [Google Scholar]
- 27.Morgan MJ, Liu Z-g. Crosstalk of reactive oxygen species and NF-κB signaling. Cell research. 2011;21:103–115. doi: 10.1038/cr.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hartney T, Birari R, Venkataraman S, Villegas L, Martinez M, Black SM, Stenmark KR, Nozik-Grayck E. Xanthine oxidase-derived ROS upregulate Egr-1 via ERK1/2 in PA smooth muscle cells; model to test impact of extracellular ROS in chronic hypoxia. PLoS One. 2011;6:e27531. doi: 10.1371/journal.pone.0027531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Konduri GG, Ou J, Shi Y, Pritchard KA., Jr Decreased association of HSP90 impairs endothelial nitric oxide synthase in fetal lambs with persistent pulmonary hypertension. American journal of physiology. Heart and circulatory physiology. 2003;285:H204–H211. doi: 10.1152/ajpheart.00837.2002. [DOI] [PubMed] [Google Scholar]
- 30.Wright CJ, Agboke F, Muthu M, Michaelis KA, Mundy MA, La P, Yang G, Dennery PA. Nuclear factor-kappaB (NF-kappaB) inhibitory protein IkappaBbeta determines apoptotic cell death following exposure to oxidative stress. J Biol Chem. 2012;287:6230–6239. doi: 10.1074/jbc.M111.318246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang G, Madan A, Dennery PA. Maturational differences in hyperoxic AP-1 activation in rat lung. Am J Physiol Lung Cell Mol Physiol. 2000;278:L393–L398. doi: 10.1152/ajplung.2000.278.2.L393. [DOI] [PubMed] [Google Scholar]
- 32.Gien J, Seedorf GJ, Balasubramaniam V, Markham N, Abman SH. Intrauterine pulmonary hypertension impairs angiogenesis in vitro: role of vascular endothelial growth factor nitric oxide signaling. Am J Respir Crit Care Med. 2007;176:1146–1153. doi: 10.1164/rccm.200705-750OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kiningham KK, Xu Y, Daosukho C, Popova B, St Clair DK. Nuclear factor kappaB-dependent mechanisms coordinate the synergistic effect of PMA and cytokines on the induction of superoxide dismutase 2. The Biochemical journal. 2001;353:147–156. [PMC free article] [PubMed] [Google Scholar]
- 34.St Clair DK, Porntadavity S, Xu Y, Kiningham K. Transcription regulation of human manganese superoxide dismutase gene. Methods in enzymology. 2002;349:306–312. doi: 10.1016/s0076-6879(02)49345-7. [DOI] [PubMed] [Google Scholar]
- 35.Thompson J, Phillips R, Erdjument-Bromage H, Tempst P, Ghosh S. I kappa B-beta regulates the persistent response in a biphasic activation of NF-kappa B. Cell. 1995;80:573–582. doi: 10.1016/0092-8674(95)90511-1. [DOI] [PubMed] [Google Scholar]
- 36.Sen R, Smale ST. Selectivity of the NF-{kappa}B response. Cold Spring Harbor Perspectives in Biology. 2010;2 doi: 10.1101/cshperspect.a000257. a000257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tam WF, Sen R. IkappaB family members function by different mechanisms. J Biol Chem. 2001;276:7701–7704. doi: 10.1074/jbc.C000916200. [DOI] [PubMed] [Google Scholar]
- 38.Suyang H, Phillips R, Douglas I, Ghosh S. Role of unphosphorylated, newly synthesized I kappa B beta in persistent activation of NF-kappa B. Mol Cell Biol. 1996;16:5444–5449. doi: 10.1128/mcb.16.10.5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tran K, Merika M, Thanos D. Distinct functional properties of IkappaB alpha and IkappaB beta. Mol Cell Biol. 1997;17:5386–5399. doi: 10.1128/mcb.17.9.5386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Malek S, Huang D, Huxford T, Ghosh S, Ghosh G. X-ray crystal structure of an IkappaBbeta x NF-kappaB p65 homodimer complex. J Biol Chem. 2003;278:23094–23100. doi: 10.1074/jbc.M301022200. [DOI] [PubMed] [Google Scholar]
- 41.Hoffmann A, Levchenko A, Scott ML, Baltimore D. The IkappaB-NF-kappaB signaling module: temporal control and selective gene activation. Science. 2002;298:1241–1245. doi: 10.1126/science.1071914. [DOI] [PubMed] [Google Scholar]
- 42.Meyrick BO, Ryan US, Brigham KL. Direct effects of E coli endotoxin on structure and permeability of pulmonary endothelial monolayers and the endothelial layer of intimal explants. The American journal of pathology. 1986;122:140–151. [PMC free article] [PubMed] [Google Scholar]
- 43.Ohnishi M, Imanishi N, Tojo SJ. Protective effect of anti-P-selectin monoclonal antibody in lipopolysaccharide-induced lung hemorrhage. Inflammation. 1999;23:461–469. doi: 10.1023/a:1021917110651. [DOI] [PubMed] [Google Scholar]
- 44.Rao P, Hayden MS, Long M, Scott ML, West AP, Zhang D, Oeckinghaus A, Lynch C, Hoffmann A, Baltimore D, Ghosh S. IkappaBbeta acts to inhibit and activate gene expression during the inflammatory response. Nature. 2010;466:1115–1119. doi: 10.1038/nature09283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scheibel M, Klein B, Merkle H, Schulz M, Fritsch R, Greten FR, Arkan MC, Schneider G, Schmid RM. IkappaBbeta is an essential co-activator for LPS-induced IL-1beta transcription in vivo. J Exp Med. 2010;207:2621–2630. doi: 10.1084/jem.20100864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Han SJ, Ko HM, Choi JH, Seo KH, Lee HS, Choi EK, Choi IW, Lee HK, Im SY. Molecular mechanisms for lipopolysaccharide-induced biphasic activation of nuclear factor-kappa B (NF-kappa B) The Journal of biological chemistry. 2002;277:44715–44721. doi: 10.1074/jbc.M202524200. [DOI] [PubMed] [Google Scholar]
- 47.Gilmore TD, Herscovitch M. Inhibitors of NF-kappaB signaling: 785 and counting. Oncogene. 2006;25:6887–6899. doi: 10.1038/sj.onc.1209982. [DOI] [PubMed] [Google Scholar]
- 48.Li X, Cui X, Li Y, Fitz Y, Hsu L, Eichacker PQ. Parthenolide has limited effects on nuclear factor-kappa beta increases and worsens survival in lipopolysaccharide-challenged C57BL/6J mice. Cytokine. 2006;33:299–308. doi: 10.1016/j.cyto.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 49.Magni P, Ruscica M, Dozio E, Rizzi E, Beretta G, Maffei Facino R. Parthenolide inhibits the LPS-induced secretion of IL-6 and TNF-alpha and NF-kappaB nuclear translocation in BV-2 microglia. Phytother Res. 2012;26:1405–1409. doi: 10.1002/ptr.3732. [DOI] [PubMed] [Google Scholar]
- 50.Park SJ, Shin HJ, Youn HS. Parthenolide inhibits TRIF-dependent signaling pathway of Toll-like receptors in RAW264.7 macrophages. Mol Cells. 2011;31:261–265. doi: 10.1007/s10059-011-0032-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sheehan M, Wong HR, Hake PW, Zingarelli B. Parthenolide improves systemic hemodynamics and decreases tissue leukosequestration in rats with polymicrobial sepsis. Crit Care Med. 2003;31:2263–2270. doi: 10.1097/01.CCM.0000085186.14867.F7. [DOI] [PubMed] [Google Scholar]
- 52.Pakala SB, Reddy SD, Bui-Nguyen TM, Rangparia SS, Bommana A, Kumar R. MTA1 coregulator regulates LPS response via MyD88-dependent signaling. J Biol Chem. 2010;285:32787–32792. doi: 10.1074/jbc.M110.151340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.De Silva D, Mitchell MD, Keelan JA. Inhibition of choriodecidual cytokine production and inflammatory gene expression by selective I-kappaB kinase (IKK) inhibitors. Br J Pharmacol. 2010;160:1808–1822. doi: 10.1111/j.1476-5381.2010.00839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kang BY, Chung SW, Kim TS. Inhibition of interleukin-12 production in lipopolysaccharide-activated mouse macrophages by parthenolide, a predominant sesquiterpene lactone in Tanacetum parthenium: involvement of nuclear factor-kappaB. Immunol Lett. 2001;77:159–163. doi: 10.1016/s0165-2478(01)00211-5. [DOI] [PubMed] [Google Scholar]
- 55.Yip KH, Zheng MH, Feng HT, Steer JH, Joyce DA, Xu J. Sesquiterpene lactone parthenolide blocks lipopolysaccharide-induced osteolysis through the suppression of NF-kappaB activity. J Bone Miner Res. 2004;19:1905–1916. doi: 10.1359/JBMR.040919. [DOI] [PubMed] [Google Scholar]
- 56.Uchi H, Arrighi JF, Aubry JP, Furue M, Hauser C. The sesquiterpene lactone parthenolide inhibits LPS- but not TNF-alpha-induced maturation of human monocyte-derived dendritic cells by inhibition of the p38 mitogen-activated protein kinase pathway. J Allergy Clin Immunol. 2002;110:269–276. doi: 10.1067/mai.2002.126381. [DOI] [PubMed] [Google Scholar]
- 57.Wong HR, Menendez IY. Sesquiterpene lactones inhibit inducible nitric oxide synthase gene expression in cultured rat aortic smooth muscle cells. Biochem Biophys Res Commun. 1999;262:375–380. doi: 10.1006/bbrc.1999.1207. [DOI] [PubMed] [Google Scholar]
- 58.Lopez-Franco O, Hernandez-Vargas P, Ortiz-Munoz G, Sanjuan G, Suzuki Y, Ortega L, Blanco J, Egido J, Gomez-Guerrero C. Parthenolide modulates the NF-kappaB-mediated inflammatory responses in experimental atherosclerosis. Arterioscler Thromb Vasc Biol. 2006;26:1864–1870. doi: 10.1161/01.ATV.0000229659.94020.53. [DOI] [PubMed] [Google Scholar]
- 59.Saadane A, Masters S, DiDonato J, Li J, Berger M. Parthenolide inhibits IkappaB kinase, NF-kappaB activation, and inflammatory response in cystic fibrosis cells and mice. Am J Respir Cell Mol Biol. 2007;36:728–736. doi: 10.1165/rcmb.2006-0323OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sheehan M, Wong HR, Hake PW, Malhotra V, O'Connor M, Zingarelli B. Parthenolide, an inhibitor of the nuclear factor-kappaB pathway, ameliorates cardiovascular derangement and outcome in endotoxic shock in rodents. Mol Pharmacol. 2002;61:953–963. doi: 10.1124/mol.61.5.953. [DOI] [PubMed] [Google Scholar]
- 61.Fiebich BL, Lieb K, Engels S, Heinrich M. Inhibition of LPS-induced p42/44 MAP kinase activation and iNOS/NO synthesis by parthenolide in rat primary microglial cells. J Neuroimmunol. 2002;132:18–24. doi: 10.1016/s0165-5728(02)00279-5. [DOI] [PubMed] [Google Scholar]
- 62.Hwang D, Fischer NH, Jang BC, Tak H, Kim JK, Lee W. Inhibition of the expression of inducible cyclooxygenase and proinflammatory cytokines by sesquiterpene lactones in macrophages correlates with the inhibition of MAP kinases. Biochem Biophys Res Commun. 1996;226:810–818. doi: 10.1006/bbrc.1996.1433. [DOI] [PubMed] [Google Scholar]
- 63.Saadane A, Eastman J, Berger M, Bonfield TL. Parthenolide inhibits ERK and AP-1 which are dysregulated and contribute to excessive IL-8 expression and secretion in cystic fibrosis cells. J Inflamm (Lond) 2011;8:26. doi: 10.1186/1476-9255-8-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tergaonkar V, Correa R, Ikawa M, Verma I. Distinct roles of IkappaB proteins in regulating constitutive NF-kappaB activity. Nat Cell Biol. 2005;7:921–923. doi: 10.1038/ncb1296. [DOI] [PubMed] [Google Scholar]