Abstract
The halophilic Archaeon Halorubrum lacusprofundi, isolated from the perennially cold and hypersaline Deep Lake in Antarctica, was recently sequenced and compared to 12 Haloarchaea from temperate climates by comparative genomics. Amino acid substitutions for 604 H. lacusprofundi proteins belonging to conserved haloarchaeal orthologous groups (cHOGs) were determined and found to occur at 7.85% of positions invariant in proteins from mesophilic Haloarchaea. The following substitutions were observed most frequently: (a) glutamic acid with aspartic acid or alanine; (b) small polar residues with other small polar or non-polar amino acids; (c) small non-polar residues with other small non-polar residues; (d) aromatic residues, especially tryptophan, with other aromatic residues; and (e) some larger polar residues with other similar residues. Amino acid substitutions for a cold-active H. lacusprofundi β-galactosidase were then examined in the context of a homology modeled structure at residues invariant in homologous enzymes from mesophilic Haloarchaea. Similar substitutions were observed as in the genome-wide approach, with the surface accessible regions of β-galactosidase displaying reduced acidity and increased hydrophobicity, and internal regions displaying mainly subtle changes among smaller non-polar and polar residues. These findings are consistent with H. lacusprofundi proteins displaying amino acid substitutions that increase structural flexibility and protein function at low temperature. We discuss the likely mechanisms of protein adaptation to a cold, hypersaline environment on Earth, with possible relevance to life elsewhere.
Introduction
The surface of Earth is mostly covered by salty oceans, 90% of which are at temperatures of 5°C or lower. Many perennially cold environments are also hypersaline, with higher NaCl concentrations than seawater, especially in the Arctic and Antarctic regions, due to brine exclusion from sea ice and concentration by evaporitic processes. Extremophilic microbes are abundant in such extreme environments and are of growing interest from the perspective of basic biology and biotechnology [1]–[5]. Evidence for the existence of cold hypersaline brines elsewhere in our solar system has also been provided [6]–[8]. As a result, the mechanisms of survival of extremophiles in freezing conditions and saturating salinity are also garnering attention in the field of astrobiology [9].
Halorubrum lacusprofundi, a member of the ancient class of microorganisms in the Domain Archaea, is one of the few cold and salt-adapted species available in pure culture [10]. This microbe was isolated from Deep Lake, Antarctica where temperatures remain below 0°C for 8 months of the year. Due to the extremely high salinity of Deep Lake, reported as ∼ 3.5 M NaCl, the freezing point is greatly depressed and the lake remains liquid throughout the year, even when temperatures reach a minimum of −18°C during the winter. In the laboratory, H. lacusprofundi is a psychrotolerant microorganism capable of growth at sub-zero temperatures, while its optimum growth temperature is 30°C, lower than most other related haloarchaeal microorganisms (Table 1) [11], [12].
Table 1. Characteristics of sequenced Haloarchaea and the 604 cHOGs included in study.
| Haloarchaeon | Genome size (Mbp) | Growth temperature range (oC) | Average proteome MW | Average proteome pI | ||
| Halorubrum lacusprofundi | 3.7 | −2–42 | 32.27 | 4.39 | ||
| Halobacterium sp. NRC-1 | 2.6 | 15–50 | 31.06 | 4.59 | ||
| Halorhabdus utahensis | 3.1 | 17–55 | 31.96 | 4.40 | ||
| Haloferax volcanii | 4.0 | 20–49 | 31.63 | 4.55 | ||
| Halalkalicoccus jeotgali | 3.7 | 21–50 | 31.48 | 4.57 | ||
| Halogeometricum borinquense | 3.9 | 22–58 | 32.18 | 4.50 | ||
| Natronomonas pharaonis | 2.8 | 23–56 | 31.58 | 4.40 | ||
| Haloterrigena turkmenica | 5.4 | 23–57 | 32.43 | 4.33 | ||
| Haloquadratum walsbyi | 3.2 | 25–55 | 33.32 | 4.61 | ||
| Halopiger xanaduensis | 4.4 | 28–45 | 32.54 | 4.30 | ||
| Halomicrobium mukohataei | 3.3 | 35–52 | 31.89 | 4.39 | ||
| Natrialba magadii | 4.4 | 37–40* | 32.95 | 4.32 | ||
| Haloarcula marismortui | 4.3 | 40–50* | 32.08 | 4.40 | ||
NOTES: The proteome molecular weight (MW) and isoelectric point (pI) data are averages for the 604 protein families used in amino acid composition analysis. Growth temperature ranges are indicated, except those marked with *,where only the optimal growth temperatures were reported.
Recently, the genome of H. lacusprofundi was sequenced (http://www.ncbi.nlm.nih.gov/bioproject/18455), providing an opportunity to better understand its adaptation to a cold, hypersaline environment. The H. lacusprofundi genome is organized into chromosome I (2.7 Mbp), chromosome II (526 kbp), and a megaplasmid (431 kbp), and codes for ca. 3,560 proteins. Like most Haloarchaea, this organism has high GC-composition, with chromosome I having the highest (66.7% GC), and the two smaller replicons, lower GC content (57.1 and 54.9%, respectively) [13], [14]. Preliminary analysis of its genome sequence revealed 4 different csp genes, which are thought to be involved in the organisms’ cold response [15]. Recent comparative genomic analysis also identified 784 core haloarchaeal orthologous groups of proteins (cHOGs) conserved in H. lacusprofundi and 12 other Haloarchaea [16], [17]. These findings showed that the Haloarchaea constitute a well-defined and coherent phylogenetic group for comparative genomic studies.
An interesting region of the genome of H. lacusprofundi that has come under scrutiny is a gene cluster for carbohydrate utilization, including the gene for a glycoside hydrolase family 42 β-galactosidase enzyme named bga [18]. The bga gene was recently introduced into the genetically tractable haloarchaeon Halobacterium sp. NRC-1 and overexpressed under the control of a cold shock protein gene promoter [19]. After purification, the enzyme was found to be active over a wide range of temperatures, retaining a substantial fraction of its maximum activity even when temperatures were as low as −5°C. The cold-active β-galactosidase enzyme also exhibited extremely halophilic character, with maximal activity at 4 M concentrations of sodium or potassium chloride [19]. As a result, H. lacusprofundi β-galactosidase was found to constitute an excellent model enzyme for studying protein function at cold, hypersaline conditions.
Relatively few studies have thus far addressed the mechanisms of cold-adapted proteins in hypersaline conditions [1], [5], [20]–[22]. Such conditions increase the viscosity of the medium, decrease the solubility and flexibility of proteins, and reduce the speed of enzymatic reactions. Proteins active under hypersaline conditions are known to display high surface negative charges, which enhance binding of water and improve solubility [22]–[24]. Some cold active proteins have also been shown to display negative charges at the surface, and increase activity and improve folding at higher concentrations of salt [25], [26]. Therefore, adaptations in halophilic proteins may also increase conformational flexibility, allowing for “breathing” necessary for promoting catalysis at reduced temperatures. These findings suggest that synergistic mechanisms may be operating in high salt and low temperature conditions.
In order to address the adaptation of halophilic proteins to cold temperatures in more detail, we compared predicted proteins of the cold adapted halophilic species, H. lacusprofundi, to orthologous proteins conserved in mesophilic Haloarchaea. To provide structural context and interpret the significance of observed amino acid substitutions in genome-wide analysis, we utilized a model structure of the cold-active β-galactosidase of H. lacusprofundi. The bioinformatic results in this investigation reveal some key strategies in the design of H. lacusprofundi proteins, likely to be relevant for protein function in cold, hypersaline environments.
Results
Selection and Alignment of Orthologous Haloarchaeal Protein Sequences
Thirteen completely sequenced genomes of Haloarchaea available in NCBI were used for comparative genomic analysis, including 12 mesophilic species and the cold-adapted H. lacusprofundi (see Materials and Methods). The mesophilic species displayed growth ranges between ca. 20 and 50°C while H. lacusprofundi also grew at temperatures below 20°C down to −2°C (Table 1). Previously, we used best reciprocal blast analysis and protein clustering of these haloarchaeal predicted proteomes to establish 784 core haloarchaeal orthologous groups, or cHOGs, conserved in all of the Haloarchaea [17]. We selected 604 of these cHOG protein families that were unique in each of the 13 Haloarchaea for further analysis (Table S1).
Preliminary analysis of the 604 cHOGs confirmed that the proteins are very similar in molecular weight (MW) and isoelectric point (pI) (Table 1). Protein MW averages for sequenced Haloarchaea ranged from 31.06–33.32, and 32.27 for H. lacusprofundi, while the protein pI averages ranged from 4.30–4.61, and 4.39 for H. lacusprofundi. In order to identify amino acid residues different in H. lacusprofundi proteins compared to the mesophilic orthologs, the 12 mesophilic haloarchaeal protein sequences for each of the 604 selected protein families were first aligned to each other to generate multiple sequence alignments [27]. Profiles of the mesophilic proteins were constructed for each protein family and then aligned with the orthologous H. lacusprofundi proteins, and the identity of residues varying in the cold-adapted species that were invariant in the mesophilic sequences (5,541 residues out of 70,589 total) were extracted. The specific amino acid substitutions observed between the mesophilic profiles and the H. lacusprofundi orthologs were tallied and plotted, illustrating the differences in amino acid composition in the cold-adapted species (Figure 1, Table 2, and Table S2) [27].
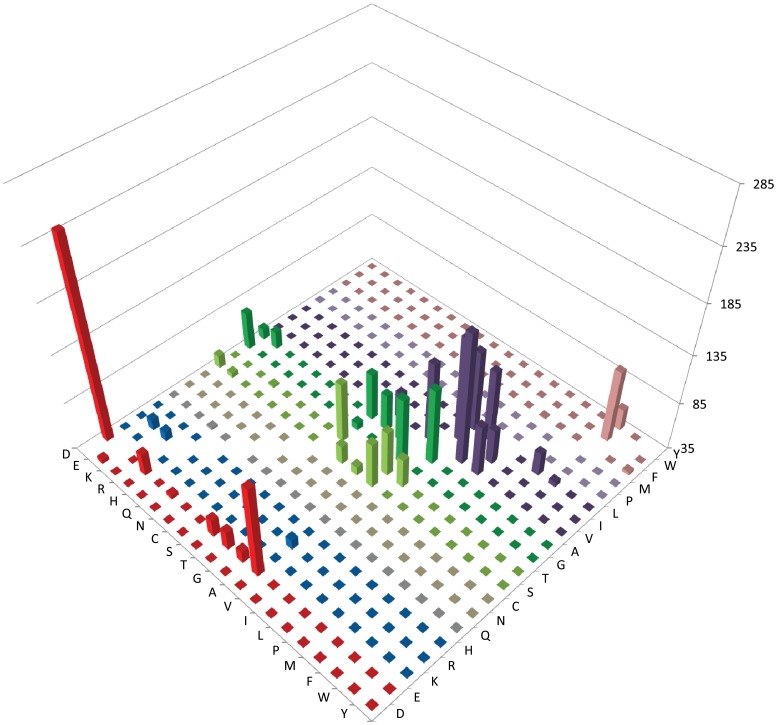
Figure 1. Amino acid substitution matrix of selected core haloarchaeal orthologous proteins for invariant residues in mesophilic versus cold-adapted H. lacusprofundi.
Amino acids conserved in 604 protein families in 12 mesophilic sequences are indicated on the right-axis, H. lacusprofundi amino acids are on left-axis, and the number of amino acid substitutions on the vertical axis, with the floor placed at 35 amino acids, to emphasize higher peaks.
Table 2. Selected amino acid substitutions between mesophilic invariant residues in 604 cHOGs and H. lacusprofundi orthologs.
| Mesophilic preference | H. lacusprofundi preference | *Number of substitutions | *Percent of substitutions |
| D | E | 39 | 0.70 |
| E | D | 241 | 4.35 |
| E | A | 131 | 2.36 |
| E | R | 58 | 1.05 |
| E | T | 54 | 0.97 |
| E | S | 532 | 0.96 |
| E | G | 47 | 0.85 |
| E | Q | 39 | 0.70 |
| R | E | 46 | 0.83 |
| R | A | 45 | 0.81 |
| R | K | 45 | 0.81 |
| Q | R | 37 | 0.67 |
| S | A | 82 | 1.48 |
| S | T | 54 | 0.97 |
| S | D | 48 | 0.87 |
| S | G | 44 | 0.79 |
| S | E | 40 | 0.72 |
| T | S | 97 | 1.75 |
| T | A | 82 | 1.48 |
| T | V | 65 | 1.17 |
| T | R | 37 | 0.67 |
| G | A | 104 | 1.88 |
| G | D | 74 | 1.34 |
| G | S | 42 | 0.76 |
| A | V | 114 | 2.06 |
| A | S | 84 | 1.52 |
| A | G | 81 | 1.46 |
| A | T | 73 | 1.32 |
| A | E | 53 | 0.96 |
| A | D | 46 | 0.83 |
| V | I | 171 | 3.09 |
| V | A | 118 | 2.13 |
| V | L | 88 | 1.59 |
| V | T | 63 | 1.14 |
| I | V | 144 | 2.60 |
| I | L | 71 | 1.28 |
| L | V | 119 | 2.15 |
| L | I | 110 | 1.99 |
| L | M | 59 | 1.06 |
| L | F | 40 | 0.72 |
| P | A | 60 | 1.08 |
| F | Y | 38 | 0.69 |
| W | F | 110 | 1.99 |
| Y | F | 57 | 1.03 |
A total of 5,541 invariant residues in mesophiles were substituted in H. lacusprofundi and those amino acid substitutions with more than 35 (0.67%) cases are shown.
Genome-wide Analysis of Amino Acid Substitutions
The great majority of amino acid residues invariant in the selected 604 protein families in 12 mesophilic Haloarchaea were also conserved in H. lacusprofundi, ranging from 86.0–97.4% and averaging 92.2% conservation (Table S2). Of the 7.85% amino acid substitutions identified in H. lacusprofundi sequences, several amino acids were substituted more frequently than expected based on composition, especially glutamic acid (E), tryptophan (W), serine (S), isoleucine (I), and threonine (T) (Table S2). Some amino acids were substituted less frequently than expected, including aspartic acid (D), cysteine (C), glycine (G), phenylalanine (F), lysine (K), and proline (P). When the ratios of specific amino acid substitutions in H. lacusprofundi proteins to proteins of mesophilic Haloarchaea were calculated, increases in D and decreases in E and W were most clearly apparent (data not shown).
Most of the amino acid substitutions observed in H. lacusprofundi proteins resulted in subtle changes in size, charge, or hydrophobicity. When amino acid substitutions were displayed using a matrix, 2,706 substitutions out of 5,541 total or 48.8% were found to occur between highly similar amino acid residues (Figure 1 and Table S2). E was frequently replaced by D or alanine (A) (Table 2 and Table S2). Polar residues, T and S, and the small residues, G and A, were substituted with similar polar or non-polar amino acids. Non-polar residues, valine (V), I, and leucine (L), were usually substituted with one of the same three non-polar residues. Aromatic amino acids, especially W, were replaced with other aromatic residues. Several other amino acids were also replaced with other similar amino acids.
Frequently Observed Amino Acid Substitutions in H. lacusprofundi Proteins
The amino acid substitutions that were frequently observed in the cold-adapted H. lacusprofundi species at invariant positions in the mesophilic proteins were analyzed by the total numbers and percent of substitutions, and percent of substitutions weighted by abundance of specific amino acids (Table 2, Figures 1, 2, 3, and Table S2). The substitution frequencies were very similar using all methods and were classified into the following categories:
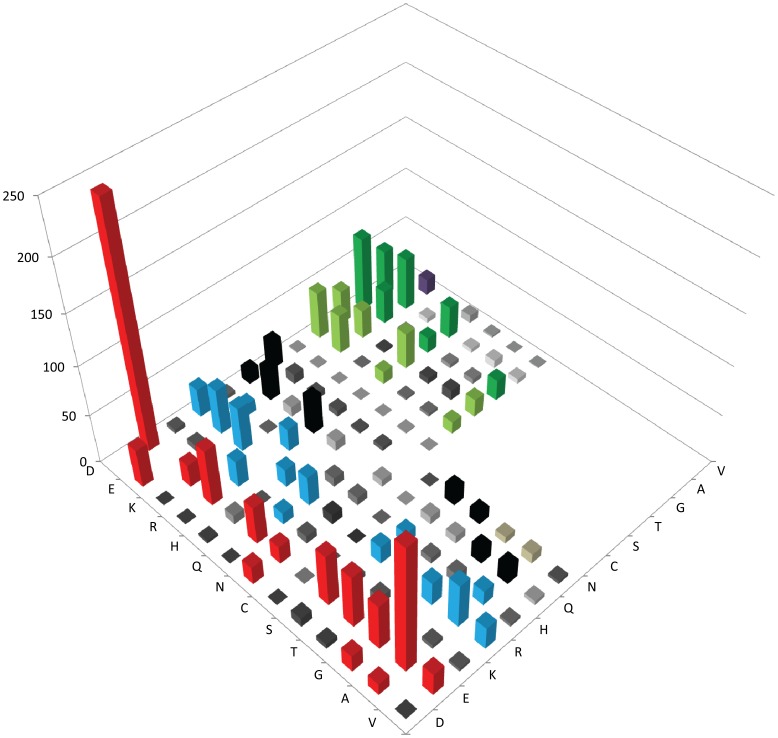
Figure 2. Amino acid substitution matrix of selected core haloarchaeal orthologous proteins for invariant negatively and positively charged and polar and small non-polar residues in mesophilic versus corresponding cold-adapted H. lacusprofundi proteins.
Amino acids conserved in 604 protein families in 12 mesophilic sequences are indicated on the right-axis, H. lacusprofundi amino acids are on left-axis, and the number of amino acid substitutions on the vertical axis, with higher peaks (>10) colored for emphasis.
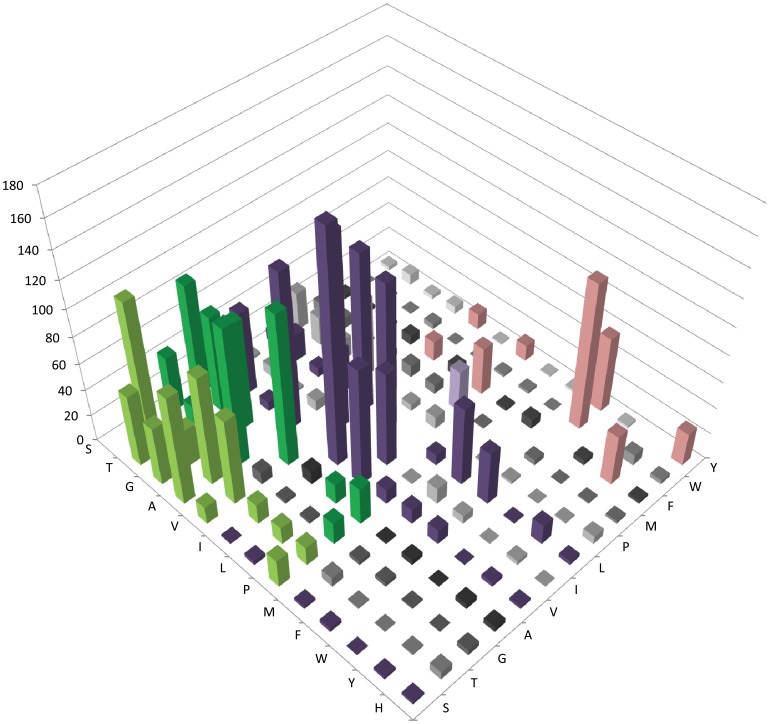
Figure 3. Amino acid substitution matrix of selected core haloarchaeal orthologous proteins for invariant non-polar residues in mesophilic versus cold-adapted H. lacusprofundi proteins.
Amino acids conserved in 604 protein families in 12 mesophilic sequences are shown on the right-axis, H. lacusprofundi amino acids are on left-axis, and the number of amino acid substitutions on the vertical axis, with higher peaks (>10) colored for emphasis.
(i) Substitutions of acidic, basic, and long polar residues
Overall, E was substituted most frequently among all of the amino acids (13.43% of total substitutions) (Figure 2 and Table S2). A large proportion of these were substituted with D (4.35%) and A (2.36%), which differ in presence or absence of the carboxylic acid group in the side chain. At lower frequencies, E was also substituted by the basic amino acid, arginine (R) (1.05%), the long polar amino acid, glutamine (Q) (0.70%), or the small polar amino acids, S, T, and G (0.85–0.97%). D was substituted at a fraction of the rate of E (only 1.89% of total substitutions), most frequently with E (0.70%).
Among basic residues, R substitutions were the most frequently observed, partly due to its high abundance (6.82% of total substitutions), while the less abundant K and histidine (H) residues were less frequently substituted (1.61 and 2.44% of total substitutions, respectively) (Figure 2 and Table S2). R was substituted most frequently with either K, E, or A (∼0.8% each of total substitutions) and less frequently with T, Q, D, or L (0.51–0.61% total substitutions range). Both K and H were substituted most frequently with R (less than 0.5% of total substitutions in both cases). Among amino acids with long polar side chains, asparagine (N) and Q were also less abundant than R, and substitutions were observed less frequently (2.09% and 3.25% of total substitutions, respectively), generally with similar size amino acids (Figure 2 and Table S2). Q was substituted most frequently with R or D (0.67% and 0.60%, respectively), while N was substituted most frequently with D (0.51%).
(ii) Substitutions of small polar residues
In most cases, polar residues T and S, and the smallest residues, G and A, were substituted with similar polar or non-polar amino acids, primarily S, T, G, A, or V and occasionally acidic residues (Figure 3 and Table S2). For S and T, which were substituted 6.21% and 8.36% of total frequency, respectively, the most common substitutions were with each other or A (0.97–1.75%), a similar size non-polar residue. Other less frequent substitutions for S were with D, E, or G (0.72–0.87%). For T, most substitutions were with the same three amino acids, R or V (0.51–1.17%).
For the small amino acids, G and A, the frequency of substitutions was considerably higher for the latter (10.40% of total) than the former (6.52% of total), while their abundance was similar. A was most frequently substituted by V, S, G, and T (1.32–2.06%), which are more similar in size and polarity, and less frequently with E, D, R, and P (0.51–0.96%), which are less similar. G was substituted most frequently by A or D (1.34–1.88%) and less frequently with S or E (0.61–0.76%).
(iii) Substitutions of small non-polar residues
The three non-polar residues, V, I, and L, were frequently substituted with the same non-polar residues, or in some cases A, methionine (M), or other similar residues (Figure 3 and Table S2). However, V and L were more abundant and more frequently substituted (9.37% and 8.46% of total) compared to I (4.76%). V was most frequently substituted by I, A, L, or T (1.14–3.09%). L was most frequently substituted with V or I (2.15% and 1.99%, respectively), and less frequently with M, F, or A (0.61–1.06%). I was most frequently substituted with V or L (2.60% and 1.28%, respectively).
(iv) Substitutions of aromatic and other residues
While W was one of the least abundant amino acids, it was one of the most frequently substituted, particularly with another large aromatic residue, F (1.99% of total and 11.07% of specific substitutions) (Figure 3 and Table S2). The two aromatic residues, F and tyrosine (Y), were somewhat more abundant than W, and were frequently replaced, usually with each other (0.69–1.03%), or with L (0.65% for F).
For the remaining residues, M was most commonly substituted with L (0.56%) and P was substituted most frequently with A, D, or S (0.52–1.08%) (Figure 3 and Table S2). C was the least abundant amino acid and the least likely to be substituted (0.47% of total substitutions), primarily with A or S.
Analysis of the Cold-active β-galactosidase from H. lacusprofundi
The H. lacusprofundi genome harbors a gene coding for a glycosyl hydrolase family 42 β-galactosidase with homologs in four mesophilic Haloarchaea (H. lucentense SB1, H. volcanii, H. turkmenica, and H. xanaduensis) [18], [19], [28]. The proteins are very similar in MW (74.46–78.06), pI (4.18–4.28), and sequence (>60% identity). We aligned these five β-galactosidase protein sequences to first determine amino acid differences between mesophilic Haloarchaea and H. lacusprofundi homologs. The cold-active β-galactosidase from H. lacusprofundi deviated at 29 out of 321 invariant amino acid positions (9.03%) in mesophilic Haloarchaea, which is similar to the substitution frequency observed in comparative genomic analysis (7.85%) (Table 3). Of the 29 amino acid substitutions, 25 were distinct (with four substitutions occurring twice) and 18 (72%) occurred at high frequency (≥0.67%) in genome-wide analysis. Conversely, of the 44 highest frequency substitutions observed in genome-wide analysis, 16 substitutions (36%) were observed in β-galactosidase. These results show a good correlation between amino acid substitutions in β-galactosidase compared with those found in the 604 cHOGs analyzed by comparative genomics.
Table 3. Amino acid substitutions between invariant residues in family 42 β-galactosidases from mesophilic Haloarchaea and cold-active H. lacusprofundi β-galactosidase.
| Mesophile amino acid | H. lacusprofundi amino acid | Residue number | MW change | Hydrophobicity change* | Surface (%) |
| D | S | 189 | −28 | 50 | 15 |
| D | S | 651 | −28 | 50 | 20 |
| D | N | 251 | −1 | 27 | 10 |
| D | P | 235 | −18 | 9 | 25 |
| E | Q | 284 | −1 | 21 | <5(4) |
| E | S | 451 | −42 | 26 | 25 |
| H | F | 404 | 10 | 92 | <5(3) |
| Q | A | 23 | −57 | 51 | 5 |
| S | A | 263 | −16 | 46 | <5(0) |
| S | C | 324 | 16 | 54 | 15 |
| T | R | 19 | 55 | −27 | 45 |
| T | N | 180 | 13 | −41 | <5(0) |
| T | A | 181 | −30 | 28 | 45 |
| T | A | 460 | −30 | 28 | <5(1) |
| G | R | 643 | 99 | −14 | 40 |
| A | G | 328 | −14 | −41 | <5(4) |
| A | T | 383 | 30 | −28 | <5(2) |
| A | S | 384 | 16 | −46 | 10 |
| V | L | 348 | 14 | 21 | <5(2) |
| V | I | 476 | 14 | 23 | 25 |
| V | A | 604 | −28 | −35 | 5 |
| I | V | 443 | −14 | −23 | <5(1) |
| L | Q | 427 | 15 | −107 | 5 |
| L | I | 299 | 0 | 2 | 5 |
| L | V | 434 | −14 | −21 | <5(3) |
| L | V | 482 | −14 | −21 | 5 |
| L | T | 635 | −12 | −84 | <5(1) |
| L | F | 484 | 34 | 3 | <5(2) |
| L | F | 387 | 34 | 3 | 30 |
Hydrophobicity was calculated based on ref. [66].
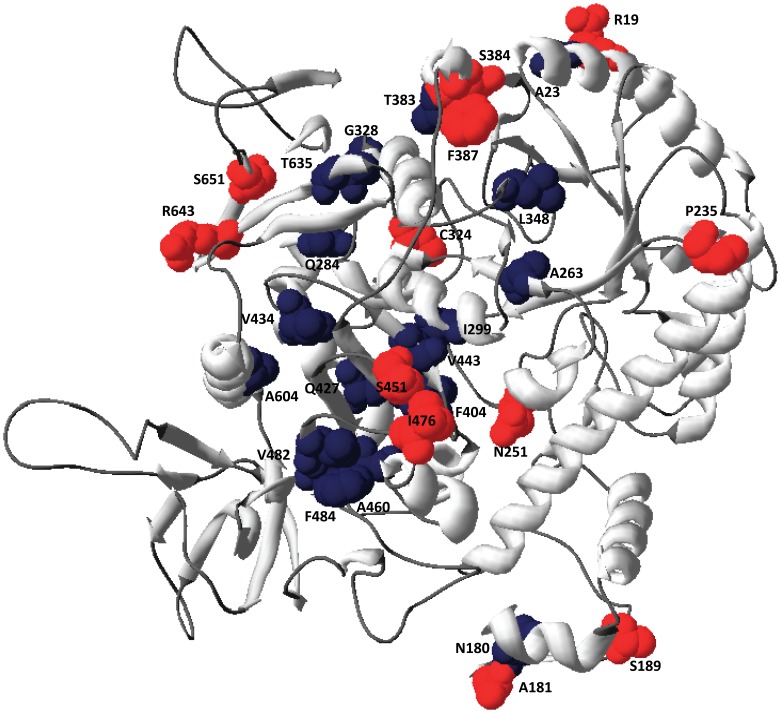
Amino acid substitutions in the H. lacusprofundi β-galactosidase that were at positions invariant in the mesophile enzymes mapped to the model structure of the enzyme using DeepView (Table 3 and Figure 4). Seventeen amino acid residues that were ≤5% solvent-exposed were classified as buried, and twelve residues that were ≥10% solvent-exposed were classified as surface [29]. Five acidic residues (D and E), mapping to the surface of mesophilic proteins in the modeled structure were substituted either with polar residues (S, Q, or N), or P, increasing hydrophobicity and decreasing negative charges at the protein surface (Figure 4). Four other substitutions observed at the protein surface also increased hydrophobicity, specifically S with C, T with A, V with I, and L with F. Only three substitutions resulting in surface residues with decreased hydrophobicity were found, A with S, and T or G with R, the latter two of which mapped near the amino- or carboxy-termini of the protein, respectively.
Figure 4. Model of H. lacusprofundi β-galactosidase highlighting differences with mesophilic Haloarchaea.
The protein backbone is colored gray, substitutions of surface residues are shown colored red, and substitutions of internal residues are shown colored dark blue. The protein structure was illustrated using Swiss-PDBViewer [65].
Amino acid substitutions observed in internal regions of the H. lacusprofundi β-galactosidase were primarily in non-polar or small polar residues, usually with very similar residues (Table 3 and Figure 4). L was the most common amino acid to be substituted internally (6 residues), with V (X 2), I, Q, T, or F. A was substituted with G or T, V was substituted with L or A, while I was substituted with V. Q and T were substituted with A or N, and H was substituted with F. A single E residue with intermediate solvent exposure (4%) was substituted with Q. Overall, most substitutions in internal residues resulted in relatively small alterations in molecular weight and/or hydrophobicity.
Discussion
We have explored the general amino acid sequence characteristics of over 600 core haloarchaeal orthologous protein families (cHOGs) and determined which invariant residues in mesophilic Haloarchaea were substituted in the cold-adapted H. lacusprofundi haloarchaeon. Interestingly, of the amino acid residues substituted in H. lacusprofundi (5,541/70,589 invariant or 7.85%), about half of the changes (2,706/5,541 or 48.8%) resulted in small alterations in molecular weight and/or polarity, e.g. in acidic amino acids like E, polar ones like T and S, non-polar residues like V and L, and aromatic amino acids, especially W. We also determined amino acid substitutions in the context of the modeled structure of H. lacusprofundi β-galactosidase at positions invariant in mesophilic homologs (29/321 or 9.03%). We again found substitutions primarily of non-polar and polar amino acids with similar residues, e.g. L with V, and T with A, in interior regions, and of charged amino acids with residues with increased hydrophobicity, e.g. D and E with S, at the surface. These approaches together confirmed that certain types of amino acid substitutions are commonly found in H. lacusprofundi proteins and are likely to be responsible for their adaptation to cold temperatures in a hypersaline environment.
Our findings are generally consistent with previous studies that have addressed amino acid composition in protein adaptation to low temperatures, such as the genome sequence analysis of the cold-growing bacterium Colwellia psychrerythraea, which found a lower proportion of charged amino acids in surface composition [30]. Another study that analyzed the sequence of nearly 400 cold-adapted proteins, also reported similar differences at the protein surface, consistent with alterations in amino acid contacts with the solvent [31]. Changes at the surface resulted in greater entropic effects rather than specific effects like diminished numbers of salt bridges. Other reports on temperature-dependent activity of proteins pointed to decreasing numbers of hydrogen bonds, bound ions, and salt bridges at the surface [32]. In our genome-wide analysis, more than a third of the observed substitutions in the H. lacusprofundi β-galactosidase were in surface residues, primarily at D and E, and the overwhelming majority of changes decreased charge and/or increased protein hydrophobicity at its surface. The amino acid changes increasing hydrophobicity at the enzyme surface are expected to diminish the network of hydrogen bonds around the protein, increasing structural flexibility necessary for maintaining catalytic activity in colder low-density water [33], [34]. The few observed changes increasing charges at the surface occurred primarily near the amino- and carboxy-termini of β-galactosidase, regions known to usually have greater solvent exposure and less rigid structure [35].
Previous studies have reported increases in small amino acids and decreases in the volume and mass per residue in proteins active in colder temperatures [36], [37]. Such changes may also result in decreasing hydrophobicity within the core of the enzyme [38], [39]. Consistent with these studies, in our comparative genome analysis, many of the substitutions identified in H. lacusprofundi proteins also decreased the size of the amino acid side chain, e.g. E, V, or T with A, I with V, and W with F, respectively. Similar results were also obtained for H. lacusprofundi β-galactosidase, where about one-half of internal substitutions resulted in smaller amino acid residues, while the other half resulted in larger residues in the cold-adapted protein. Both kinds of substitutions likely produce small perturbations in packing within internal regions of the protein structure, leading to an increase in protein flexibility and greater activity at lower temperatures.
Changes in the overall protein charge and content of acidic and basic amino acids, as well as P, have previously been reported in cold-active proteins [31], [32], [40]. For example, analysis of the complete genome sequence of Psychrobacter arcticus, a cold-growing bacterium from Siberian permafrost, showed the reduced use of P and R, in addition to acidic amino acids, in a significant portion of the predicted proteome [41]. In our genome-wide analysis, slight reduction in the content of P and R was also found in H. lacusprofundi proteins, while W content was significantly (13.2%) decreased, likely reflecting the trend toward smaller, more hydrophobic amino acids internally (Table S2). For acidic residues in H. lacusprofundi proteins, D content increased while E content decreased genome-wide, resulting in no net change in protein charge. The overall pIs and molecular weights for H. lacusprofundi proteins were not significantly altered compared to mesophilic Haloarchaea.
For β-galactosidase, as in the genome-wide analysis, a slight increase was observed in the content of D for the H. lacusprofundi enzyme, while the content of E was slightly reduced, without any significant change in overall charge. Interestingly, however, four D and one E residues conserved in the mesophilic enzymes were found to be substituted to uncharged residues of smaller size at the surface of the cold-adapted enzyme. These observations indicate that there are two countervailing forces at work in adaptation of this enzyme to cold temperatures at high salt concentrations. While surface negative charges are critical for protein function in high salinity, activity at cold temperatures likel requires limiting the number of negative charges at the surface in order to achieve the desired surface hydrophobicity [5]. As a result, the design of such novel salt and cold adapted proteins must involve careful selection of surface residues, a prediction which may be tested by genetic engineering of H. lacusprofundi proteins, and comparing results to investigations on other halophilic and cold-active β-galactosidases (e.g. [28], [42]).
Several mutagenesis studies have explored the importance of specific amino acid residues on protein function in cold temperature or high salinity. For example, when E residues on the protein surface were mutated in a halophilic, relatively salt insensitive TATA-binding protein, it was converted into non-halophilic, salt sensitive variants [43]. The investigation pointed to the importance of surface negative charges for the uptake of cations and discharge of water accompanying macromolecular complex formation at high salinity. When the cold adapted isocitrate lyase from C. psychrerythraea was mutagenized, an internal A residue was found to be critical for activity at cold temperatures [44]. In our analysis, surface and internal hydrophobic residues were similarly predicted to be important in the function of the cold and salt adapted β-galactosidase. The availability of the H. lacusprofundi enzyme expression system in Halobacterium sp. NRC-1 provides an ideal platform for testing the roles of those specific amino acid residues by mutagenesis in the future [19].
The adaptive mechanisms of proteins in cold brine are of significant interest, since most of the habitable environments on Earth are cold and salty. Genome-wide comparisons of proteins from H. lacusprofundi, a cold-adapted member of the ancient class of microorganisms in the Domain Archaea, to other mesophilic Haloarchaea growing at moderate temperatures have led to insights into polyextremophilic protein function. This study has provided a detailed catalog of the amino acid changes in proteins from the cold adapted organism in otherwise invariant residues in mesophiles. The cold-adapted proteins from H. lacusprofundi have more hydrophobic residues on the surface and subtle changes to many non-polar and polar residues buried in the interior, based on the structural model of a cold-active β-galactosidase enzyme. Amino acid substitutions observed in the cold adapted protein are consistent with small perturbations providing greater flexibility at colder temperatures. The general relevance of these results to cold-active proteins is underscored by similar findings in other microorganisms isolated from perennially cold systems, such as the Arctic and Antarctic oceans, Greenland glaciers, etc. [3], [45]–[47].
Since the Antarctic Deep Lake is one of the coldest and most extreme environments from which microbes have been cultured, the unusual and unique properties of H. lacusprofundi are of relevance to astrobiology [9]. Such Antarctic environments may be analogs of regions of Earth’s sister planet, Mars, where the potential for biological activity is of intense interest. Images from the Mars Reconnaissance Orbiter has shown evidence for seasonal emergence of liquid flows down steep rocky cliffs in summer, findings consistent with briny liquid water emerging from underground reservoirs on Mars [6]. Recent photographs from the Curiosity Rover have also suggested that running surface water was once prevalent on ancient Mars [48], although the temperature and salinity characteristics during that time are not yet known. On Europa, the entire surface is presently covered by frozen water-ice, and liquid water beneath the surface may be generated by dissipation of tidal forces [7], [8]. Additional studies of model polyextremophilic enzymes and cultured microbes from extreme environments on Earth are likely to provide further insights into how life may be able to cope with similar challenging conditions on other worlds.
Materials and Methods
Selection of Haloarchaeal Proteins
Predicted proteins were selected from the genome sequences of 13 Haloarchaea, 12 of which are mesophiles and 1 is a cold-adapted species, H. lacusprofundi (Table 1). We targeted proteins belonging to conserved haloarchaeal orthologous groups, or cHOGs, conserved in all 13 sequenced haloarchaeal genomes. Of the 784 cHOGs, 604 were found as a single copy in each genome, and did not contain any paralogs (Table S1). The sequences of proteins in the 604 non-paralogous clusters were obtained from NCBI for 13 Haloarchaea: Halobacterium sp. NRC-1 ATCC 700922 [49], Haloarcula marismortui ATCC 43049 [50], Natronomonas pharaonis DSM 2160 [51], Haloquadratum walsbyi DSM 16790 [52], Halorubrum lacusprofundi ATCC 49239 (http://www.ncbi.nlm.nih.gov/bioproject/18455), Halogeometricum borinquense DSM 11551 [53], Halomicrobium mukohataei DSM 12286 [54], Halorhabdus utahensis DSM 12940 [55], Haloferax volcanii DS2 ATCC 29605 [56], Haloterrigena turkmenica DSM 5511 [57], Natrialba magadii ATCC 43099 [58], Halalkalicoccus jeotgali B3 [59], and Halopiger xanaduensis SH-6 [60].
Amino Acid Composition Analysis
In-house Perl scripts were used to facilitate bioinformatic analyses using the EMBOSS suite of programs [27]. Each of the 604 non-paralogous cHOG protein sequences were extracted and used to calculate molecular weights and pIs. Amino acid sequences of each cHOG from the 12 mesophilic Haloarchaea were aligned with progressive, pairwise alignments and the Blosum62 matrix [61], [62]. Consensus sequences were created for each position with 100% identity and compared to the protein sequence of the H. lacusprofundi ortholog for each cHOG. The resulting pairwise alignments containing the mesophilic identity and H. lacusprofundi sequence were used to identify and tabulate (a) positions where residues are conserved in the 12 mesophilic organisms and differ in H. lacusprofundi, and (b) positions where residues are conserved in all 13 sequences.
β-galactosidase Protein Sequence Analysis
The amino acid sequence of cold-active β-galactosidase from H. lacusprofundi was aligned to mesophilic β-galactosidases from four Haloarchaea, Haloferax lucentense (H. alicantei), H. volcanii, H. xanaduensis, and H. turkmenica as described above. Invariant residues in the mesophilic haloarchaeal proteins which varied in H. lacusprofundi were tabulated [63]. The structure of H. lacusprofundi β-galactosidase was modeled and illustrated using the crystal structure of β-galactosidase from Thermus thermophilus A4 with the Swiss-PDBViewer [64], [65]. Solvent accessibility was calculated, and buried and surface residues defined as having accessibilities ≤5% and ≥10%, respectively [29].
Supporting Information
Table of 604 orthologous proteins from 12 mesophilic Haloarchaea and H. lacusprofundi .
(DOC)
Genome-wide tally and percent of amino acid substitutions between invariant mesophilic haloarchaeal proteins (vertical) and H. lacusprofundi (horizontal) for 604 selected cHOGs.
(DOC)
Acknowledgments
We thank Satyajit L DasSarma for valuable assistance with systems administration, database management, and Perl scripts.
Funding Statement
This work was supported by the National Aeronautics and Space Administration grant 396 NNX10AP47G. RK was partially supported by an ASM International Fellowship for Asia. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Feller G, Gerday C (2003) Psychrophilic enzymes: hot topics in cold adaptation. Nat Rev Microbiol 1: 200–208. [DOI] [PubMed] [Google Scholar]
- 2. Cavicchioli R (2006) Cold-adapted archaea. Nat Rev Microbiol 4: 331–343. [DOI] [PubMed] [Google Scholar]
- 3. Rodrigues DF, Tiedje JM (2008) Coping with our cold planet. Appl Environ Microbiol 74: 1677–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cavicchioli R, Charlton T, Ertan H, Mohd Omar S, Siddiqui KS, et al. (2011) Biotechnological uses of enzymes from psychrophiles. Microb Biotechnol 4: 449–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Karan R, Capes MD, DasSarma S (2012) Function and biotechnology of extremophilic enzymes in low water activity. Aquatic Biosystems 8: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McEwen AS, Ojha L, Dundas CM, Mattson SS, Byrne S, et al. (2011) Seasonal flows on warm Martian slopes. Science 333: 740–743. [DOI] [PubMed] [Google Scholar]
- 7. Khurana KK, Kivelson MG, Russell CT (2002) Searching for liquid water in Europa by using surface observatories. Astrobiol 2: 93–103. [DOI] [PubMed] [Google Scholar]
- 8. Marion GM, Fritsen CH, Eicken H, Payne MC (2003) The search for life on Europa: limiting environmental factors, potential habitats, and Earth analogues. Astrobiol 3: 785–811. [DOI] [PubMed] [Google Scholar]
- 9. DasSarma S (2006) Extreme halophiles are models for astrobiology. Microbe 1: 120–126. [Google Scholar]
- 10. Franzmann PD, Stackebrandt E, Sanderson K, Volkman JK, Cameron DE, et al. (1988) Halobacterium lacusprofundi sp. nov., a halophilic bacterium isolated from Deep Lake, Antarctica. Syst Appl Microbiol 11: 20–27. [Google Scholar]
- 11. Bowman JP, McCammon SA, Rea SM, McMeekin TA (2000) The microbial composition of three limnologically disparate hypersaline Antarctic lakes. FEMS Microbiol Lett 183: 81–88. [DOI] [PubMed] [Google Scholar]
- 12. Reid IN, Sparks WB, Lubow S, McGrath M, Livio M, et al. (2006) Terrestrial models for extraterrestrial life: methanogens and halophiles at Martian temperatures. Int J Astrobio 5: 89–97. [Google Scholar]
- 13.DasSarma S, Capes M, DasSarma P (2008) Haloarchaeal megaplasmids. In: Schwartz E, editor. Microbial Megaplasmids. Berlin: Springer-Verlag. 3–30.
- 14. DasSarma SL, Capes MD, DasSarma P, DasSarma S (2010) HaloWeb: the haloarchaeal genomes database. Saline Systems 6: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Giaquinto L, Curmi PM, Siddiqui KS, Poljak A, DeLong E, et al. (2007) Structure and function of cold shock proteins in archaea. J Bacteriol 189 5738–5748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Capes MD, Coker JA, Gessler R, Grinblat-Huse V, DasSarma SL, et al. (2011) The information transfer system of halophilic archaea. Plasmid 65: 77–101. [DOI] [PubMed] [Google Scholar]
- 17. Capes MD, DasSarma P, DasSarma S (2012) The core and unique proteins of haloarchaea. BMC Genomics 13: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Anderson I, Scheuner C, Göker M, Mavromatis K, Hooper SD, et al. (2011) Novel insights into the diversity of catabolic metabolism from ten haloarchaeal genomes. PLoS ONE 6: e20237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Karan R, Capes MD, DasSarma P, DasSarma S (2013) Cloning, overexpression, purification, and characterization of a polyextremophilic β-galactosidase from the Antarctic haloarchaeaon Halorubrum lacusprofundi . BMC Biotechnol 13: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fields PA (2001) Protein function at thermal extremes: balancing stability and flexibility. Comp Biochem Physiol Part A Mol Integr Physiol 129: 417–431. [DOI] [PubMed] [Google Scholar]
- 21. Siddiqui KS, Cavicchioli R (2006) Cold-adapted enzymes. Annu Rev Biochem 75: 403–433. [DOI] [PubMed] [Google Scholar]
- 22. Rasmussen BF, Stock AM, Ringe D, Petsko GA (1992) Crystalline ribonuclease A loses function below the dynamical transition at 220 K. Nature 357: 423–424. [DOI] [PubMed] [Google Scholar]
- 23. Dym O, Mevarech M, Sussman JL (1995) Structural features that stabilize halophilic malate dehydrogenase from an archaebacterium. Science 267: 1344–1346. [DOI] [PubMed] [Google Scholar]
- 24. Britton KL, Baker PJ, Fisher M, Ruzheinikov S, Gilmour DJ, et al. (2006) Analysis of protein solvent interactions in glucose dehydrogenase from the extreme halophile Haloferax mediterranei . Proc Natl Acad Sci USA 103: 4846–4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Srimathi S, Jayaraman G, Feller G, Danielsson B, Narayanan PR (2007) Intrinsic halotolerance of the psychrophilic α-amylase from Pseudoalteromonas haloplanktis . Extremophiles 11: 505–515. [DOI] [PubMed] [Google Scholar]
- 26. Altermark B, Helland R, Moe E, Willassen NP, Smalås AO (2008) Structural adaptation of endonuclease I from the cold-adapted and halophilic bacterium Vibrio salmonicida . Acta Crystallogr D Biol Crystallogr 64: 368–376. [DOI] [PubMed] [Google Scholar]
- 27. Rice P, Longden I, Bleasby A (2000) EMBOSS: the European Molecular Biology Open Software Suite. Trends Genet 16: 276–277. [DOI] [PubMed] [Google Scholar]
- 28. Holmes ML, Dyall-Smith ML (2000) Sequence and expression of a halobacterial β-galactosidase gene. Mol Microbiol 36: 114–122. [DOI] [PubMed] [Google Scholar]
- 29. Miller S, Lesk AM, Janin J, Chothia C (1987) The accessible surface area and stability of oligomeric proteins. Nature 328: 834–836. [DOI] [PubMed] [Google Scholar]
- 30. Methé BA, Nelson KE, Deming JW, Momen B, Melamud E, et al. (2005) The psychrophilic lifestyle as revealed by the genome sequence of Colwellia psychrerythraea 34H through genomic and proteomic analyses. Proc Natl Acad Sci U S A 102: 10913–10918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sælensminde G, Halskau Ø Jr, Jonassen I (2009) Amino acid contacts in proteins adapted to different temperatures: hydrophobic interactions and surface charges play a key role. Extremophiles 13: 11–20. [DOI] [PubMed] [Google Scholar]
- 32. Feller G (2010) Protein stability and enzyme activity at extreme biological temperatures. J Phys Condens Matter 22: 323101–323116. [DOI] [PubMed] [Google Scholar]
- 33. Fernández A, Scheraga HA (2003) Insufficiently dehydrated hydrogen bonds as determinants of protein interactions. Proc Natl Acad Sci USA 100: 113–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chaplin M (2006) Do we underestimate the importance of water in cell biology? Nat Rev Mol Cell Biol 7: 861–866. [DOI] [PubMed] [Google Scholar]
- 35. Jacob E, Unger R (2007) A tale of two tails: why are terminal residues of proteins exposed? Bioinformatics 23: e225–230. [DOI] [PubMed] [Google Scholar]
- 36. Metpally RRP, Reddy BVB (2009) Comparative proteome analysis of psychrophilic versus mesophilic bacterial species: Insights into the molecular basis of cold adaptation of proteins. BMC Genomics 10: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. De Vendittis E, Castellano I, Cotugno R, Ruocco MR, Raimo G, et al. (2008) Adaptation of model proteins from cold to hot environments involves continuous and small adjustments of average parameters related to amino acid composition. J Theor Biol 250: 156–171. [DOI] [PubMed] [Google Scholar]
- 38. Russell RJ, Gerike U, Danson MJ, Hough DW, Taylor GL (1998) Structural adaptations of the cold-active citrate synthase from an Antarctic bacterium. Structure 6: 351–361. [DOI] [PubMed] [Google Scholar]
- 39.Zartler ER, Jenney FE Jr, Terrell M, Eidsness MK, Adams MWW, et al. (2001) Structural basis for thermostability in aporubredoxins from Pyrococcus furiosus and Clostridium pasteurianum. Biochemistry 40: 7279–7290.251658240 [DOI] [PubMed]
- 40. Grzymski JJ, Carter BJ, DeLong EF, Feldman RA, Ghadiri A, et al. (2006) Comparative genomics of DNA fragments from six Antarctic marine planktonic bacteria. Appl Environ Microbiol 72: 1532–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ayala-del-Río HL, Chain PS, Grzymski JJ, Ponder MA, Ivanova N, et al. (2010) The genome sequence of Psychrobacter arcticus 273–4, a psychroactive Siberian permafrost bacterium, reveals mechanisms for adaptation to low-temperature growth. Appl Environ Microbiol 76: 2304–2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shumway MV, Sheridan PP (2012) Site-directed mutagenesis of a family 42 β-galactosidase from an antarctic bacterium. Int J Biochem Mol Biol 3: 209–218. [PMC free article] [PubMed] [Google Scholar]
- 43. Bergqvist S, Williams MA, O'Brien R, Ladbury JE (2003) Halophilic adaptation of protein-DNA interactions. Biochem Soc Trans. 31: 677–680. [DOI] [PubMed] [Google Scholar]
- 44. Sato Y, Watanabe S, Yamaoka N, Takada Y (2008) Gene cloning of cold-adapted isocitrate lyase from a psychrophilic bacterium, Colwellia psychrerythraea, and analysis of amino acid residues involved in cold adaptation of this enzyme. Extremophiles 12: 107–117. [DOI] [PubMed] [Google Scholar]
- 45. Mikucki JA, Pearson A, Johnston DT, Turchyn AV, Farquhar J, et al. (2009) A contemporary microbially maintained subglacial ferrous "ocean". Science 324: 397–400. [DOI] [PubMed] [Google Scholar]
- 46. Lanoil B, Skidmore M, Priscu JC, Han S, Foo W, et al. (2009) Bacteria beneath the West Antarctic ice sheet. Environ Microbiol 11: 609–615. [DOI] [PubMed] [Google Scholar]
- 47.Murray AE, Kenig F, Fritsen CH, McKay CP, Cawley KM, et al.. (2012) Microbial life at −13°C in the brine of an ice-sealed Antarctic lake. Proc Natl Acad Sci U S A doi: 10.1073/pnas.1208607109. [DOI] [PMC free article] [PubMed]
- 48.Kaufman M (2012) Mars Rover finds ancient streambed proof of flowing water – a first. National Geographic Website. Available: http://news.nationalgeographic.com/news/2012/09/120927-nasa-mars-science-laboratory-curiosity-rover-water-life-jpl/. Accessed 2013 Feb 9.
- 49. Ng WV, Kennedy SP, Mahairas GG, Berquist B, Pan M, et al. (2000) Genome sequence of Halobacterium species NRC-1. Proc Natl Acad Sci U S A 97: 12176–12181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Baliga NS, Bonneau R, Facciotti MT, Pan M, Glusman G, et al. (2004) Genome sequence of Haloarcula marismortui: a halophilic archaeon from the Dead Sea. Genome Res 14: 2221–2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Falb M, Pfeiffer F, Palm P, Rodewald K, Hickmann V, et al. (2005) Living with two extremes: conclusions from the genome sequence of Natronomonas pharaonis . Genome Res 15: 1336–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bolhuis H, Palm P, Wende A, Falb M, Rampp M, et al. (2006) The genome of the square archaeon Haloquadratum walsbyi: life at the limits of water activity. BMC Genomics 7: 169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Malfatti S, Tindall BJ, Schneider S, Fähnrich R, Lapidus A, et al. (2009) Complete genome sequence of Halogeometricum borinquense type strain (PR3T). Stand Genomic Sci 1: 150–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tindall BJ, Schneider S, Lapidus A, Copeland A, Rio TGD, et al. (2009) Complete genome sequence of Halomicrobium mukohataei type strain (arg-2T). Stand Genomic Sci 1: 270–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bakke P, Carney N, DeLoache W, Gearing M, Ingvorsen K, et al. (2009) Evaluation of three automated genome annotations for Halorhabdus utahensis . PLoS ONE 4: e6291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hartman AL, Norais C, Badger JH, Delmas S, Haldenby S, et al. (2010) The complete genome sequence of Haloferax volcanii DS2, a model archaeon. PLoS ONE 5: e9605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Saunders E, Tindall BJ, Fähnrich R, Lapidus A, Copeland A, et al. (2010) Complete genome sequence of Haloterrigena turkmenica type strain (4kT). Stand Genomic Sci 2: 107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Siddaramappa S, Challacombe JF, DeCastro RE, Pfeiffer F, Sastre DE, et al. (2012) A comparative genomics perspective on the genetic content of the alkaliphilic haloarchaeon Natrialba magadii ATCC 43099T. BMC Genomics 13: 165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Roh SW, Nam YD, Nam SH, Choi SH, Park HS, et al. (2010) Complete genome sequence of Halalkalicoccus jeotgali B3T, an extremely halophilic archaeon. J Bacteriol 192: 4528–4529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Anderson I, Tindall BJ, Rohde M, Lucas S, Han J, et al. (2012) Complete genome sequence of Halopiger xanaduensis type strain (SH-6T). Stand Genomic Sci 6: 31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Feng DF, Doolittle RF (1987) Progressive sequence alignment as a prerequisite to correct phylogenetic trees. J Mol Evol 25: 351–360. [DOI] [PubMed] [Google Scholar]
- 62. Henikoff S, Henikoff JG (1992) Amino acid substitution matrices from protein blocks. Proc Natl Acad Sci U S A 89: 10915–10919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Monera OD, Sereda TJ, Zhou NE, Kay CM, Hodges RS (1995) Relationship of sidechain hydrophobicity and alpha-helical propensity on the stability of the single-stranded amphipathic alpha-helix. J Pept Sci 1: 319–329. [DOI] [PubMed] [Google Scholar]
- 64. Hidaka M, Fushinobu S, Ohtsu N, Motoshima H, Matsuzawa H, et al. (2002) Trimeric crystal structure of the glycoside hydrolase family 42 β-galactosidase from Thermus thermophilus A4 and the structure of its complex with galactose. J Mol Biol 322: 79–91. [DOI] [PubMed] [Google Scholar]
- 65. Guex N, Peitsch MC (1997) SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modeling. Electrophoresis 18: 2714–2723. [DOI] [PubMed] [Google Scholar]
- 66. Serada TJ, Mant CT, Sönnichsen FD, Hodges RS (1994) Reversed-phase chromatography of synthetic amphipathic α-helical peptides as a model for ligand/receptor interactions. Effect of changing hydrophobic environment on the relative hydrophilicity/hydrophobicity of amino acid side-chains. J Chromatogr A 676: 139–153. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table of 604 orthologous proteins from 12 mesophilic Haloarchaea and H. lacusprofundi .
(DOC)
Genome-wide tally and percent of amino acid substitutions between invariant mesophilic haloarchaeal proteins (vertical) and H. lacusprofundi (horizontal) for 604 selected cHOGs.
(DOC)