Abstract
Trypanosoma cruzi the agent of Chagas disease is a monophyletic but heterogeneous group conformed by several Discrete Typing Units (DTUs) named TcI to TcVI characterized by genetic markers. The trans-sialidase (TS) is a virulence factor involved in cell invasion and pathogenesis that is differentially expressed in aggressive and less virulent parasite stocks. Genes encoding TS-related proteins are included in a large family divided in several groups but only one of them contains TS genes. Two closely related genes differing in a T/C transition encode the enzymatically active TS (aTS) and a lectin-like TS (iTS). We quantified the aTS/iTS genes from TcII and TcVI aggressive and TcI low virulent strains and found variable aTS number (1–32) per haploid genome. In spite of being low TS enzyme-expressers, TcI strains carry 28–32 aTS gene copies. The intriguing absence of iTS genes in TcI strains together with the presence of aTS/iTS in TcII and TcVI strains (virulent) were observed. Moreover, after sequencing aTS/iTS from 38 isolates collected along the Americas encompassing all DTUs, the persistent absence of the iTS gene in TcI, TcIII and TcIV was found. In addition, the sequence clustering together with T/C transition analysis correlated to DTUs of T. cruzi. The consistence of TS results with both evolutionary genome models proposed for T. cruzi, namely the “Two Hybridization” and the “Three Ancestor” was discussed and reviewed to fit present findings. Parasite stocks to attempt genetic KO or to assay the involvement of iTS in parasite biology and virulence are finally available.
Introduction
Chagas disease is a chronic disabling disease caused by the protozoon Trypanosoma cruzi. With an estimated 8–10 million people already infected, and about 40,000 new cases/year, Chagas disease represents a major public health, social and economic problem in Latin America where about 100 million people are at risk [1]. Besides the vectorial spread, blood transfusion, organ transplants and congenital transmission increase the worldwide dissemination risk due to migratory processes as in the USA where it has been estimated that 300,000 people are infected [2].
T. cruzi constitutes a monophyletic but genetically heterogeneous group. Based on various genetic markers and evolutive and population genetics interpretations of data, T. cruzi populations have been classified into six Discrete Typing Units (DTU) namely TcI to TcVI. Recently a seventh group sampled in bats and named TcBat has been added [3], [4]. Because of the predominantly clonal evolution of the parasite, these DTUs are rather stable in space and time, constituting a useful framework for epidemiological and evolutionary analysis [5]. This genetic diversity seems to be correlated with a geographical distribution [3], [6],[7] and with biological characteristics of the parasite such as culture growth, pathogenicity in mice, evolution in the insect vector, susceptibility to antichagasic drugs and tissular tropism in animal and human infections. Human infection displays different clinical evolutions ranging from asymptomatic to cardiomyopathy, megaviscera or even death. Different outcome incidences are also determined by host genetics, the presence of mixed infections, cultural factors, etc. [8]. Within the endemic area, heterogeneous geographical distribution of DTUs has been extensively described suggesting that the genetic composition of the parasite could be partly responsible for the different manifestations of Chagas disease. Broadly, TcI is found from the south of the USA in the sylvatic environment to northern South America where it seems to be responsible for chagasic cardiomyopathy [7], [9]–[11]. In Southern Cone countries, TcI is usually associated to the sylvatic cycle whereas TcII, TcV, and TcVI are relatively more abundant than TcI in the domestic cycle [3], [12], [13]. In this region, human infections present higher rates of severe heart affectation [7], [14]–[18] and digestive abnormalities, which are exceptional in northern South America and Central America [19], [20]. TcIII which is usually isolated from vectors and sylvatic reservoirs has a low prevalence in human infections [11], [21], [22] whereas TcIV shows a similar geographical distribution but higher incidence in human infection [15], [23]–[25].
Although sialic acid is crucial for the life cycle of T. cruzi, being involved in host cell adhesion/invasion processes and escape from the complement, the parasite is unable to synthesize this sugar de novo. To circumvent this gap, the parasite expresses the trans-sialidase (TS), that transfers α(2,3)-linked sialyl residues among glycoproteins or glycolipids. Circulating TS activity alters the sialylation pattern of the cellular glycoconjugates leading to hematological and immunological abnormalities associated to the disease [26]–[28].
Genes encoding TS are included in a large family composed of at least 1439 members [29], a figure certainly underestimated due to the expected collapse when assembling closely similar sequences. Although several different groups of genes can be discerned, only one of them includes those that code for the TS proteins [30], [31]. It has been estimated that as many as 150 genes of this group are included in the genome [32] where two TS isoforms, the active enzyme (aTS) and an enzymatically inactive TS (iTS) are encoded. Comparison of the aTS vs. iTS deduced amino acid sequences shows variations in 20 residues, although the inactivation is entirely due to the single crucial Tyr342His replacement as a consequence of a T/C transition [33]. The replacement by histidine renders the protein enzymatically inactive but allows retaining the substrate binding ability conferring therefore a lectin-like activity [32], [34]. This strongly suggests a physiologic role for iTS in parasite attachment to substrates or cell surface receptors that might explain its conservation. Crystallographic analyses and enzyme kinetic assays [35] have recently shown that iTS retains residual hydrolytic activity. By using the recombinant iTS, a co-stimulating host T-cells effect have been adscribed [36].
Previous efforts to associate parasite genetic classification and biological features have allowed us to determine the expression/shed of aTS as a marker of pathogenicity that segregates strains belonging to different lineages [37]. In this study our aim was to analyze the distribution of genes encoding the virulence factor TS among DTU-representative isolates collected along the Americas in the context of their evolution. We found aTS in all analyzed stocks and the striking absence of iTS genes in TcI, TcIII and TcIV DTUs. The consistence of the TS results with current T. cruzi evolutionary genome models was reviewed to fit findings. Parasite stocks to attempt genetic KO or to assay the involvement of iTS in parasite biology and virulence are now available.
Materials and Methods
Trypanosoma cruzi isolates
The study was carried out in a panel of 38 parasite isolates encompassing all DTUs (nine TcI stocks, seven TcII, two TcIII, five TcIV, six TcV, and nine TcVI) obtained from various ecological origins (vectors, animal reservoirs and human infections) spanning all the endemic area from Argentina to the USA.
Trypanosoma cruzi genomic DNA purification
DNA from Ac, Hc, K-98, SN, Br, CMA, ChVal, HE, HT, RA, Q501/3, Tulahuen, ML, Alf, FAL and Cvd parasite strains was obtained from peripheral blood trypomastigotes. DNA from Silvio X10, Tu18, M5631, Can III, CL Brener, CID, H1, QUE, CBBcl2, ESMcl3Z2, IVVcl4, MAS1cl1, MVBcl8, X109/2, 3.1, 92122102R, STC10R, STC16Rcl1, MNcl2, SC43cl1, CA15, P63cl1 strains was obtained from epimastigotes. The Blood and Cell Culture DNA Purification Kit (Qiagen) or conventional phenol-chloroform DNA extraction methods were used.
DTU characterization
All T. cruzi DNA samples were genotyped using polymerase chain reaction (PCR) strategies following Burgos et al [17] algorithm of classification. Some T. cruzi stocks (CID, H1, QUE, CBBcl2, ESMcl3Z2, IVVcl4, MAS1cl1, MVBcl8, X109/2, 3.1, 92122102R, STC10R, STC16Rcl1, MNcl2, SC43cl1, CA15, P63cl1) were also characterized by MLEE [38]–[40] for DTU assignment.
For PCR characterization, five reactions targeted to the intergenic region of spliced leader genes (SL-IR), 24sα rDNA and the A10 fragment were carried out on each DNA sample to determine the parasite DTU. The PCR amplicon size for each DTU was: Tc I: SL-IRac (150 bp), SL-IR-I (475 bp), and 24sα HnPCR (140 bp); TcII: SL-IRac (157 bp), SL-IR-II (425 bp), 24sα HnPCR (140 bp) and A10-PCR (690 bp); TcIII: SL-IRac (200 bp), 24sα HnPCR (125 bp), and A10-PCR (630 bp); TcIV: SL-IRac (200 bp) and 24sα HnPCR (140/145 bp); TcV: SL-IRac (157 bp), SL-IR-II (425 bp), 24sα HnPCR (125 or 125+140 bp), and A10-PCR (630 bp); TcVI: SL-IRac (157 bp), SL-IR-II (425 bp), 24sα HnPCR (140 bp), and A10-PCR (630 bp).
Quantification of aTS and iTS genes
To assess the number of genes per haploid genome coding for aTS and iTS in each parasite isolate, a real-time PCR-based strategy was applied. These assays were performed at the facilities of Eurofins Medigenomix GmbH (Germany) on an ABI 7900 HT Sequence Detection System (Applied Biosystems) using the universal mix of ABI TaqMan reagents (Applied Biosystems). Briefly, the region containing the mutation was amplified with the following flanking oligonucleotides: 5'-TGGGCAAGTATCCATTGGTGATG-3' and 5'-TGATCTCATGCAAACAGTACAGCTT-3'. In the same reaction, a pair of fluorescent-labeled probes specific for the two possible sequences at the mutation position (5′-AATTCCGCCTACAGCT-3′ coupled to FAM to detect aTS and 5′-AAAATTCCGCCCACAGCT-3′ coupled to VIC which binds to iTS) allowed the quantification of both genes. The analysis was normalized by quantification of the T. cruzi pyruvate dehydrogenase (PVDH)-encoding gene present as a single copy per haploid genome [41]. This reaction included a set of primers (5'-CGGCGTACCAGCCTGAGAT-3' and 5'-ACCTGAAGGCCCGGAATG-3') and a labeled probe (5'-TACCGTCGTGGCGACT-3') that hybridizes the pvdh gene.
Plasmids containing the aTS or iTS genes were used as control and during test standardization. In the data analysis, the intensity of each signal was a definite value (Ct, cycle of threshold), which is inversely related to the amount of complementary DNA. The proportion of both genes was calculated for each T. cruzi isolate as the average of two independent determinations.
Amplification and sequencing of TS genes
Two upstream and two downstream primers to the region containing the T/C transition of TS-encoding genes were designed (5′ primers: TS-51, 5′-GGAGGCTGTCGGCACGCTCTC-3′ and TS-5, 5′-GCTTCACTGCCGTGACCATCG-3′; 3′ primers: TS-31, 5′-TCACGCAGCGGTACGCATCCT-3′ and TS-3, 5′-CAGCGGGACCACAACCACGCT-3′), so that all the TS sequences annotated in the GenBank were targeted in at least one of the four PCR reactions to be performed. Amplification was carried out with 0.4 µM of each primer, 5 U of Pfu polymerase enzyme (Promega), 2.5 mM of dNTPs, and 100 ng of genomic T. cruzi DNA as template in 50 µl final reaction volume. The PCR cycle consisted of 30 rounds of 94°C for 45 s, 63°C for 45 s, and 72°C for 45 s, with a first step of 2 min at 94°C and one last step of 5 min at 72°C. PCR products were analyzed by electrophoresis in 2% agarose gel. Amplicons were purified and both strands sequenced with the primers used for amplification. Chromatograms were visually examined to determine the presence of C and/or T in the first position of the codon 342. Firstly TS-51/TS-31 primer set was used and those genomes rendering only the Tyr342 codon were then subjected to the other three PCR reactions. The IUPAC nomenclature for the genetic code was used to define single nucleotide polymorphism (SNP) positions with mixed base identification set to 15% of the highest peak. Sequences were deposited in the GenBank with the accession numbers KC286514, KC286515, KC286516, KC286517, KC286518, KC286519, KC286520, KC286521, KC286522, KC286523, KC286524, KC286525, KC286526, KC286527, KC286528, KC286529, KC286530, KC286531, KC286532, KC286533, KC286534, KC286535, KC286536, KC286537, KC286538, KC286539, KC286540, KC286541, KC286542, KC286543, KC286544, KC286545, KC286546, KC286547, KC286548, KC286549, KC294586 and KC294587.
Sequence comparison of the region around the codon 342
A 455-bp consensus sequence of each T. cruzi stock was obtained by comparison of forward and reverse sequences of the TS-51/TS-31 PCR reactions. Sequences from the different stocks were aligned and compared by using ClustalW2 program [42]. A clustering tree was built by using SplitsTree4 [43] with the following options: i) the “UncorrectedP” method which computes the proportion of positions at which two sequences differ was used; ii) the ambiguous state codes (such as W, M, V …) were handled with the option ‘Average’ meaning that the contribution at a site is averaged over all possible resolutions of the ambiguous codes, with the exception that sites having the same ambiguous code contribute zero; iii) the distance-based method used was UPGMA because we considered that evolution rate must be the same upon all branches; the bootstrap was conducted with 1,000 iterations.
Results
Quantification of aTS and iTS genes in the genome of T. cruzi
To analyze the distribution of TS-encoding genes in the genome of parasites analyzed by Risso et al [37], we performed a quantitative analysis of aTS and iTS by real-time PCR on DNA samples. The single copy Pvdh gene [41] was included as internal reference to standardize the number of haploid genome copies in the test. Primers that amplify the region containing the single nucleotide polymorphism (SNP) that determines the loss of enzymatic activity were used together with two probes that differ in only one base (T/C transition) and comprise the Tyr codon (to hybridize aTS genes) or His codon (complementary to the sequence of iTS genes), respectively. No cross-recognition between the aTS and iTS probes under test conditions was found, as assayed with plasmids harboring the corresponding gene. No Ct could be determined with the iTS-complementary probe for low-virulence TcI strains, indicating no detection of iTS genes carrying the T/C transition. As shown in Table 1, the genome of these T. cruzi isolates harbors 28 to 32 copies of aTS-coding genes. On the other hand, data obtained from the aggressive strains RA, Cvd, CL Brener and the clone Q501/3 (all belonging to TcVI) and Br (TcII) indicated that both the aTS and iTS genes were present with high variability in the gene copy number (Table 1). In these genomes, the aTS/iTS rate was high for TcII (3/1) and quite balanced in TcVI (2/1 and 1/1).
Table 1. Quantification of aTS and iTS in parasites representing high and low TS activity producers.
| T. cruzi | pvdh | aTS | iTS | |||
| DTU | Isolatea | Ct | Ct | Gene copies | Ct | Gene copies |
| TcI | Ac | 39.004±0.606 | 23.907±0.146 | 30 (100%) | ND | 0 |
| Hc | 34.172±0.098 | 18.473±0.001 | 32 (100%) | ND | 0 | |
| K-98 | 36.529±0.186 | 22.679±0.102 | 28 (100%) | ND | 0 | |
| TcII | Br | 18.269±0.022 | 16.912±0.032 | 3 (75%) | 17.611±0.029 | 1 (25%) |
| TcVI | RA | 28.925±0.018 | 14.767±0.065 | 28 (64%) | 20.900±0.877 | 16 (36%) |
| Q501/3 | 20.465±0.155 | 17.717±0.066 | 4 (67%) | 19.043±0.048 | 2 (33%) | |
| Cvd | 17.791±0.094 | 17.074±0.378 | 1 (50%) | 17.867±0.159 | 1 (50%) | |
| CL Brener | 29.132±0.161 | 14.719±0.032 | 29 (60%) | 19.494±0.141 | 19 (40%) | |
a) Parasites correspond to low (TcI) and high (TcII and TcVI) TS activity producer stocks as described by Risso et al [37].
Parasite DNA was subjected to quantitative real time PCR and aTS/iTS presence was determined by using probes labeled with reporter dyes. Gene number per haploid genome was determined by Ct comparison with that obtained for the pvdh single copy gene. Ct: cycle of threshold; ND: not detectable.
Presence of the T/C SNP in codon 342 of TS genes
The findings presented above strongly suggest the intriguing absence of iTS genes in TcI parasite genome. To further test the differential distribution of aTS and iTS among T. cruzi stocks belonging to different DTUs, the T/C SNP was directly searched by sequencing PCR-amplified gene fragments comprising the surrounding region. To assess the representation of the Tyr342His mutation among these different parasite stocks, two primers were designed upstream and two other downstream the target codon. All TS genes deposited at GenBank were covered given that all these known sequences were targeted at least once in the PCR strategy designed. Table 2 summarizes the biological sources and geographical origins of the parasite isolates tested and the findings observed. Notably, whereas all stocks from the DTUs TcII, TcV and TcVI contained both aTS and iTS genes (with T and C in the first position encoding codon 342 observed as a mixed peak in the chromatograms, see Figure 1), TcI, TcIII and TcIV parasites tested depicted only T (corresponding to aTS genes) indicating the absence of genes coding for iTS in agreement with results shown in Table 1.
Table 2. Origin and DTU classification of parasite isolates with TS isoforms predicted presence.
| T. cruzi isolate | Source | Countrya/Area | DTU | TSb |
| Ac | Human | Ar/Chaco | I | aTS |
| Hc | Human | Ar/unknown | I | aTS |
| SN | Human | Ar/Misiones | I | aTS |
| K-98 | Human | Ar/San Luis | I | aTS |
| FAL | Human | Ar/Chaco | I | aTS |
| Silvio X10 | Human | Br/Para | I | aTS |
| CID | Human | Me/Oaxaca | I | aTS |
| H1 | Human | Me/Yucatan | I | aTS |
| QUE | Vectorc | Me/Queretaro | I | aTS |
| Br | Human | Ar/unknown | II | aTS/iTS |
| Tu18 | Vectord | Bo/Tupiza | II | aTS/iTS |
| CBBcl2 | Human | Ch/Region IV | II | aTS/iTS |
| IVVcl4 | Human | Ch/Region IV | II | aTS/iTS |
| MVBcl8 | Human | Ch/Region IV | II | aTS/iTS |
| ESMcl3 | Human | Br/Bahia | II | aTS/iTS |
| MAS1cl1 | Human | Br/Fed. District | II | aTS/iTS |
| M5631 | Armadilloe | Br/I. Marajo | III | aTS |
| X109/2 | Dog | Pa/Pte. Hayes | III | aTS |
| Can III | Human | Br/Belem | IV | aTS |
| 3.1 | Vectord | Pe/Arequipa | IV | aTS |
| 92122102R | Raccoon | USA/Georgia | IV | aTS |
| STC10R | Raccoon | USA/Georgia | IV | aTS |
| STC16Rcl1 | Raccoon | USA/Georgia | IV | aTS |
| CMA | Human | Ar/Chaco | V | aTS/iTS |
| ChVAL | Human | Ar/unknown | V | aTS/iTS |
| HE | Human | Ar/Chaco | V | aTS/iTS |
| HT | Human | Ar/Chaco | V | aTS/iTS |
| MNcl2 | Human | Ch/Region IV | V | aTS/iTS |
| SC43cl1 | Vectord | Bo/Chuquisaca | V | aTS/iTS |
| RA | Human | Ar/Chaco | VI | aTS/iTS |
| Q501/3 | Human | Ch/Region IV | VI | aTS/iTS |
| Cvd | Human | Ar/Mendoza | VI | aTS/iTS |
| ALF | Human | Ar/Chaco | VI | aTS/iTS |
| ML | Human | Ar/unknown | VI | aTS/iTS |
| Tulahuen | Human | Ch/Region IV | VI | aTS/iTS |
| CL Brener | Vectord | Br/RG do Sul | VI | aTS/iTS |
| CA15 | Vectord | Bo/Santa Cruz | VI | aTS/iTS |
| P63cl1 | Vectord | Pa/Pte. Hayes | VI | aTS/iTS |
a) Ar: Argentina; Br: Brasil; Me: México; Bo: Bolivia; Ch: Chile; Pe: Perú; Pa: Paraguay. b) TS isoforms predicted presence. aTS: active trans-sialidase; iTS inactive trans-sialidase. c) unidentified blood-sucking vector; d) Triatoma infestans (vector bug). e) Dasypus novemcinctus.
Figure 1. Chromatograms from the region flanking the T/C SNP.
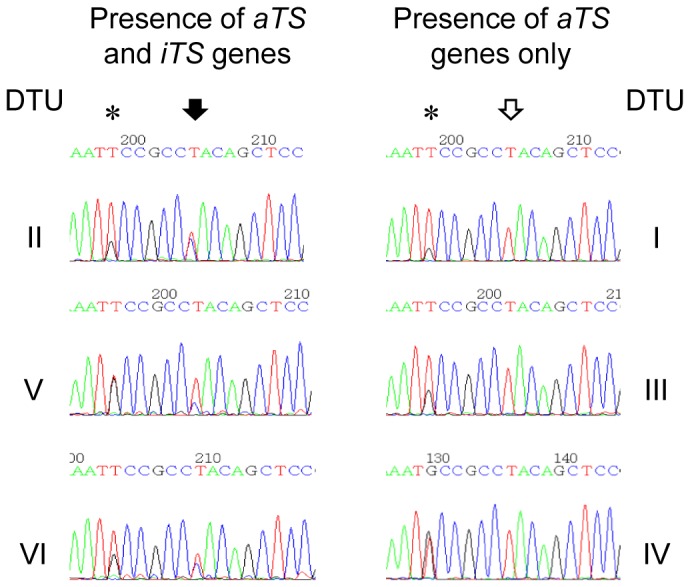
Sequencing examples from parasites belonging to the six DTUs are shown. Black arrow points T and C nucleotides in TcII, TcV and TcVI PCR products. Empty arrow points the same position in TcI, TcIII and TcIV amplicons, where only T was observed. Star indicates a T/G SNP (K in IUPAC code) present in all tested parasites.
Sequence comparison of TSs amplicons
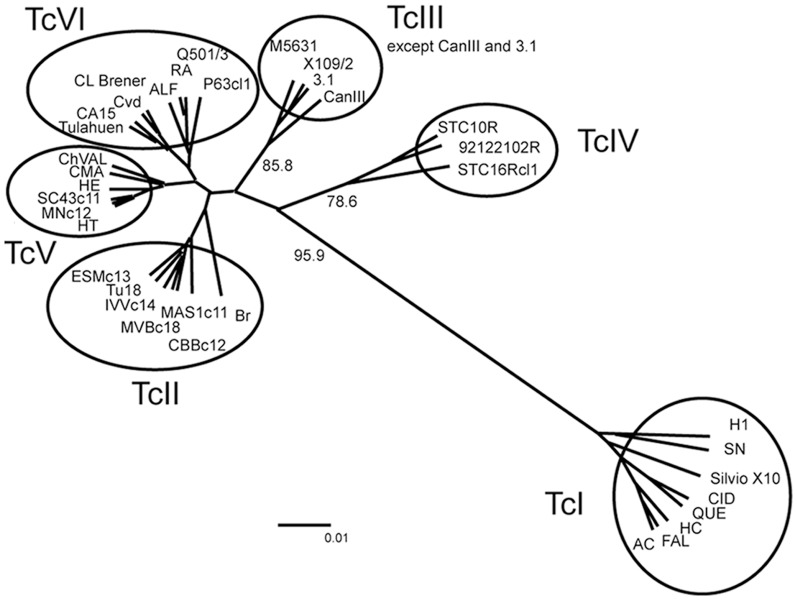
To further analyze the region in search for other SNPs that might be useful to classify parasites, we compared the 455 bp TS gene region sequenced from the 38 T. cruzi stocks belonging to the six DTUs. Besides the nucleotide position corresponding to the T/C transition (Figure 1), sequences also include the TGG codon for Trp312, one of the two aromatic residues that participate in substrate binding [35]. Interestingly, all stocks from every DTU encoded the Trp312 codon sequence in that position (see Figure S1). On the other hand, sequences revealed several differences among the six DTUs as other SNPs beyond the T/C transition. Nine SNPs were synapomorphs of DTUs or groups of DTUs. Based on these SNPs and other less conserved polymorphisms (asterisks in Figure S1), the sequence clustering analysis as an UPGMA tree (Figure 2) showed that parasite strains were differentiated into six groups that coincide with the previous DTU assignment, TcI, TcII, TcIII, TcIV, TcV and TcVI with only two exceptions (CANIII and 3.1 strains which are classified as TcIV but here clustered with TcIII). However, bootstrap values were remarkably significant for TcI, TcIII and TcIV and lower (<50) for the other DTUs.
Figure 2. UPGMA tree based on TS genes sequence alignment (with ambiguous states).
Each circle grouped all 38 T. cruzi strains in their respective previous assigned DTU, except CAN III and 3.1 that were previously assigned to TcIV. Significant bootstrap values for TcI, TcIII and TcIV are reported, bootstrap values for other DTUs were <50.
Discussion
The T. cruzi current classification into six principal DTUs, mainly based on genetic characteristics, proved to be a valid framework to study the biological variability of T. cruzi that is widely recognized [3]. Indeed genetic diversity of the parasite is undoubtedly an important factor influencing many biological parameters (reviewed in [3]), and it has been suggested that it could be partly responsible for the different clinical outcomes of Chagas disease [8]. No strong correlation has yet been observed between the development of pathology and parasite's DTUs. However, most clinical and epidemiological studies in human infections associate TcI with infections in patients living from Colombia northwards, whereas TcII, TcV and TcVI have been detected as the most prevalent etiological agents at the south of South America, where Chagas disease presents high rates of severe heart affectation [15]–[18]. In particular, digestive syndromes, also observed in southern countries, appear to be associated only to TcII, TcV and TcVI parasites [19], [20]. Although T. cruzi populations display differential virulence and pathogenic characteristics, genetic markers linked with the evolution of the infection and their outcomes have not been identified to date. Different efforts have been made to find virulence factors that correlate with the current parasite classification [37], [44]–[46]. A recent study [47] has shown differential expression of 29 out of 261 proteins that are overexpressed in T. cruzi stocks belonging to a given DTU. We have previously shown [26], [37] that virulent parasites currently studied that belong to TcII or TcVI, express/shed more TS activity than the less aggressive TcI stocks. In addition, increased circulating TS activity correlates with several abnormalities observed early during the infection [26], [28]. The involvement of shed aTS in the alterations of the histoarchitecture of the spleen, thymus and ganglia, as well as in the induction of thrombocytopenia has been evaluated by both the administration of recombinant aTS to naive mice and the neutralization of the enzymatic activity during the acute infection [26], [28], [48]. Here the fine quantitative SNP mapping allowed us to identify aTS/iTS differences that remain hidden in genomic analyses [49] because iTS genes are not included in databases taken as reference. The present quantification of TS genes in parasite stocks exposed the absence of iTS genes together with the presence of similar copy number of aTS in all TcI parasite stocks tested (28 to 32 copies/haploid genome). On the other hand, variable aTS and iTS copy number (from 1 to 29 and 1 to 19 copies/haploid genome, respectively) were found in TcII and TcVI. These DTUs showed an aTS/iTS ratio that ranged from 1 to 3 (Table 1). These current observations allow us to conclude that the actual protein expression is independent of the number of aTS genes because genomes from high aTS producer parasites contain similar or even lower aTS gene copy numbers than those from TcI parasites with little production of aTS [37]. Moreover, the absence of iTS genes in this group raises the possibility of a correlation between this gap and the lower virulence previously observed for the TcI parasites assayed [37]. Considering that the aggressive strains [37] contain genes encoding iTS isoform, a role for this protein in the virulent behavior could be inferred. The analysis of iTS/aTS genes was then extended to representative parasite stocks encompassing the six DTUs, isolated from several sources (insect vectors, animal reservoirs and human infections) in different geographical areas (from the USA to Argentina). We found that aTS genes were present in all 38 parasite populations, emphasizing the central role of this enzyme in parasite biology. It is worth noting that iTS was observed exclusively in stocks from DTUs TcII, TcV and TcVI but intriguingly absent in all TcI, TcIII and TcIV stocks analyzed. The absence of cumulated mutations or stop codons in iTS sequences, together with the fact that we have always found the same T/C transition that encodes the Tyr342His amino acid replacement as the enzyme inactivation mechanism, indicate that the same iTS genes, conserved among all the TcII, TcV and TcVI parasite populations, are probably expressed. The Trp312 and Tyr 119 codons that are crucial in creating the two-aromatic residue-stacking site for the galactosyl portion of the substrate [50] are also conserved in aTS and iTS proteins from all DTUs. In support, a residual enzyme activity has been recently found for iTS protein [35] emphasizing that it has similar properties to aTS in sequence and folding. Furthermore, in vitro assays have demonstrated the co-stimulatory properties of iTS proteins on the immune system [36]. The strong sequence conservation in all iTS genes supports that iTS plays an evolutionary selectable role, instead of representing just a collection of pseudogenes. Therefore, an involvement in parasite attachment/invasion to host cells can be postulated because iTS acts as a lectin, able to bind not only small oligosaccharides but also sialylated glycoproteins [32], [34], a relevant feature in the physiological scenario of parasite infection.
Interestingly, our findings also reveal the existence of parasites with highly reduced TS genes content that provide models to develop genomic KO, a largely expected tool to extend the study of the biological relevance of TS whose generation has been hampered by the high gene copy numbers always reported for TS. Moreover, the ongoing transfection assays with the iTS gene might provide with a nice opportunity to test the actual relevance of iTS in parasite biology and pathogenesis.
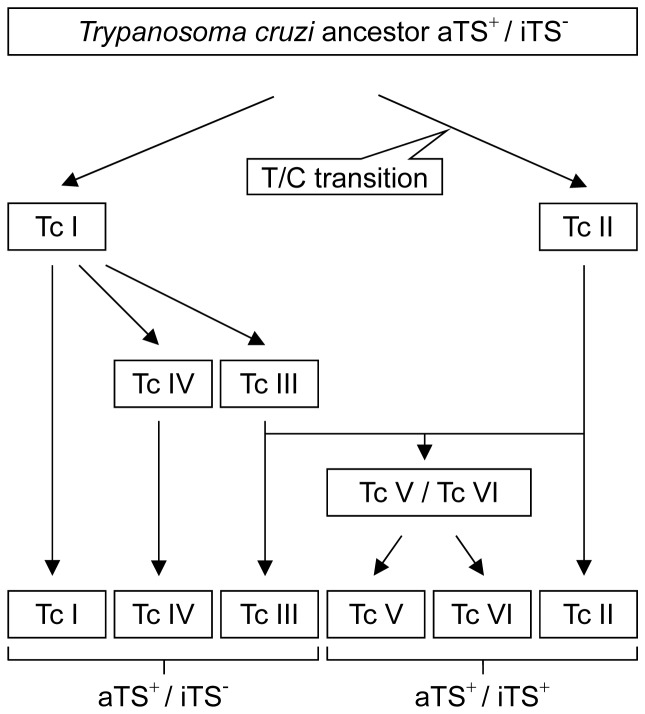
In 2009, an expert committee revised the information available about T. cruzi evolution and clustering. They remember that the partition of T. cruzi in six principal DTUs could be explained by two alternative models for their origin: the ‘Two Hybridization’ model giving rise to TcIII and then to TcV and TcVI through hybridization of two ancestors (TcI and TcII) [51] and the ‘Three Ancestor’ where the ancestors TcI, TcII and TcIII gave rise to the hybrids TcV and TcVI [52]. The current distribution of aTS/iTS suggests a closer relationship of TcI with TcIII-TcIV than with the other DTUs as well as a related evolution of TcII, TcV and TcVI. Indeed, the sequence analysis that reflect the variability of a set of genes coding for the same virulence factor (TS) fits with the six DTUs clustering, although TcII, TcV and TcVI DTU were not supported by significant bootstrap values because the hybrid nature of TcV and TcVI, reduces the bootstrap values, and if these strains are removed from the analysis (see Figure S2), TcI and TcII DTUs are everyone very well supported by high bootstrap value (93.8 and 98.3 respectively), and TcIII and TcIV are grouped together with a lower bootstrap value (60.9). However, this group is further divided into two clusters, one including CanIII, M5631, X109/2 and 3.1 strains (bootstrap value of 95.8) and the other comprising STC16Rcl1, STC10R and 92122102R strains (bootstrap value of 71.4). Although several scenarios can explain the current variability of the TS genes within DTUs, considering that TcI and TcII are ancestors [51], [52] and that iTS may have originated from aTS genes through a single mutation event, the common ancestor of TcI and TcII should not have had iTS. After iTS consolidation in TcII, its delivery during subsequent hybridization events could explain its presence in TcV and TcVI parasites (newest hybrid groups [51], [52]). Further, considering the “Two Hybridization” model, the absence of iTS in TcIII and TcIV could be explained by an inequitable ancient recombination, gene conversion or by loss of iTS corresponding genes. In the “Three Ancestor” model [52] TcIII-TcIV could have early diverged from TcI and propagated without iTS genes. The close relationship between TcIII and IV with TcI is also supported by findings with cruzipain and TSSA antigens [45], [53]. As shown in Figure 3, an alternative picture of T. cruzi evolution might be drawn that fits the previously obtained data plus that reported here. Ancestor parasites lack iTS, then TcII acquired iTS and both TcI and TcII became ancestors of all the other DTUs. A single hybridization event is postulated between TcII and TcIII that rendered TcV and VI, TcIII and IV seem to have evolved from TcI instead from hybridization of TcI with TcII because this hypothesis requires two events, the hybridization itself followed by the lost of the iTS genes contributed by TcII genome.
Figure 3. Parasite DTU evolution model proposed.
Considering the previously proposed evolution models [3] together with data reported here, an evolution model is drawn where the acquisition of the iTS gene by a single mutation event by TcII places TcI and TcII as the only ancestors for all the other DTUs. A single hybridization event of TcIII and TcII derivates in TcV and VI as previously proposed.
Finding an association between clinical manifestations and parasite genotype is a difficult task. The multiclonal nature of most natural infections and the histotropic behavior of different parasites lead to partial characterizations when bloodstream and/or other infected tissue samples are analyzed [54], [55]. The regional diversity of Chagas disease outcomes has been attributed to a set of complex interactions where the parasite genetic makeup, as well as the environmental and the host immunogenetic background are some of the factors involved (reviewed by [56]). In the challenge to identify links between the infecting DTUs and the pathogenesis induced by T. cruzi we presented for the first time the differential distribution among parasite populations of iTS/aTS, a virulence factor-related gene that is well correlated with the evolutionary history of the parasite. The expression of this complex (aTSa/iTS) of virulent genes may be a key to better understand the mechanism of virulence and its relationship with T. cruzi evolution.
Supporting Information
Consensus sequence of TS gene internal region. Sequence alignment of T. cruzi stocks encompassing the 6 DTUs (TcI to TcVI). (.): conserved sites; (▾): SNPs that identify a group of parasites (inter-DTU polymorphism). In those positions, depicted nucleotide for each DTU was present in all sequences obtained from all parasites of each DTU (named as IUPAC code); (*): other polymorphic positions not shared by all stocks within a DTU (intra-DTU polymorphisms); TGG: Trp312 codon conserved in all stocks from all DTUs; Box: Tyr342His codon where Thymidine (encoding Tyr) and Cytosine (encoding His) are present in all stocks belonging to TcII, TcV and TcVI whereas only Thymidine was found in TcI, TcIII and TcIV genomes. No other mutations were found in this codon.
(TIF)
UPGMA tree based on TS genes sequence alignment (with ambiguous states) not including hybrid DTUs. To avoid deviations induced by the hybrid nature of T. cruzi TcV and TcVI DTUs, UPGMA tree was built excluding these DTUs.
(TIF)
Acknowledgments
Agustina Scaraffia and Carla Pascuale help in parasite typing assays and the critical reading of the manuscript by Dr. ACC Frasch are highly appreciated.
Funding Statement
This work was supported by grants from Agencia Nacional de Promoción Científica y Tecnológica and Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) from Argentina, and National Institutes of Health (NIH) [Grant R01AI075589 to OC]. The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. JMB, MGR, OC and MSL are Researchers from CONICET, Argentina.
References
- 1. Rassi A Jr, Rassi A, Marin-Neto JA (2010) Chagas disease. Lancet 375: 1388–1402. [DOI] [PubMed] [Google Scholar]
- 2. Bern C, Montgomery SP (2009) An estimate of the burden of Chagas disease in the United States. Clin Infect Dis 49: e52–54. [DOI] [PubMed] [Google Scholar]
- 3. Zingales B, Miles MA, Campbell DA, Tibayrenc M, Macedo AM, et al. (2012) The revised Trypanosoma cruzi subspecific nomenclature: rationale, epidemiological relevance and research applications. Infect Genet Evol 12: 240–253. [DOI] [PubMed] [Google Scholar]
- 4. Cosentino RO, Agüero F (2012) A simple strain typing assay for Trypanosoma cruzi: discrimination of major evolutionary lineages from a single amplification product. PLoS Negl Trop Dis 6: e1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tibayrenc M (1998) Genetic epidemiology of parasitic protozoa and other infectious agents: the need for an integrated approach. Int J Parasitol 28: 85–104. [DOI] [PubMed] [Google Scholar]
- 6. Miles MA, Llewellyn MS, Lewis MD, Yeo M, Baleela R, et al. (2009) The molecular epidemiology and phylogeography of Trypanosoma cruzi and parallel research on Leishmania: looking back and to the future. Parasitology 136: 1509–1528. [DOI] [PubMed] [Google Scholar]
- 7. Risso MG, Sartor PA, Burgos JM, Briceño L, Rodriguez EM, et al. (2011) Immunological identification of Trypanosoma cruzi lineages in human infection along the endemic area. Am J Trop Med Hyg 84: 78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Macedo AM, Machado CR, Oliveira RP, Pena SD (2004) Trypanosoma cruzi: genetic structure of populations and relevance of genetic variability to the pathogenesis of chagas disease. Mem Inst Oswaldo Cruz 99: 1–12. [DOI] [PubMed] [Google Scholar]
- 9. Bosseno MF, Barnabe C, Magallon Gastelum E, Lozano Kasten F, Ramsey J, et al. (2002) Predominance of Trypanosoma cruzi lineage I in Mexico. J Clin Microbiol 40: 627–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Añez N, Crisante G, da Silva FM, Rojas A, Carrasco H, et al. (2004) Predominance of lineage I among Trypanosoma cruzi isolates from Venezuelan patients with different clinical profiles of acute Chagas' disease. Trop Med Int Health 9: 1319–1326. [DOI] [PubMed] [Google Scholar]
- 11. Ramirez JD, Guhl F, Rendon LM, Rosas F, Marin-Neto JA, et al. (2010) Chagas cardiomyopathy manifestations and Trypanosoma cruzi genotypes circulating in chronic Chagasic patients. PLoS Negl Trop Dis 4: e899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yeo M, Acosta N, Llewellyn M, Sanchez H, Adamson S, et al. (2005) Origins of Chagas disease: Didelphis species are natural hosts of Trypanosoma cruzi I and armadillos hosts of Trypanosoma cruzi II, including hybrids. Int J Parasitol 35: 225–233. [DOI] [PubMed] [Google Scholar]
- 13. Gaunt M, Miles M (2000) The ecotopes and evolution of triatomine bugs (triatominae) and their associated trypanosomes. Mem Inst Oswaldo Cruz 95: 557–565. [DOI] [PubMed] [Google Scholar]
- 14. Zingales B, Souto RP, Mangia RH, Lisboa CV, Campbell DA, et al. (1998) Molecular epidemiology of American trypanosomiasis in Brazil based on dimorphisms of rRNA and mini-exon gene sequences. Int J Parasitol 28: 105–112. [DOI] [PubMed] [Google Scholar]
- 15. Breniere SF, Bosseno MF, Noireau F, Yacsik N, Liegeard P, et al. (2002) Integrate study of a Bolivian population infected by Trypanosoma cruzi, the agent of Chagas disease. Mem Inst Oswaldo Cruz 97: 289–295. [DOI] [PubMed] [Google Scholar]
- 16. Diosque P, Barnabe C, Padilla AM, Marco JD, Cardozo RM, et al. (2003) Multilocus enzyme electrophoresis analysis of Trypanosoma cruzi isolates from a geographically restricted endemic area for Chagas' disease in Argentina. Int J Parasitol 33: 997–1003. [DOI] [PubMed] [Google Scholar]
- 17. Burgos JM, Altcheh J, Bisio M, Duffy T, Valadares HM, et al. (2007) Direct molecular profiling of minicircle signatures and lineages of Trypanosoma cruzi bloodstream populations causing congenital Chagas disease. Int J Parasitol 37: 1319–1327. [DOI] [PubMed] [Google Scholar]
- 18. del Puerto R, Nishizawa JE, Kikuchi M, Iihoshi N, Roca Y, et al. (2010) Lineage analysis of circulating Trypanosoma cruzi parasites and their association with clinical forms of Chagas disease in Bolivia. PLoS Negl Trop Dis 4: e687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Luquetti AO, Miles MA, Rassi A, de Rezende JM, de Souza AA, et al. (1986) Trypanosoma cruzi: zymodemes associated with acute and chronic Chagas' disease in central Brazil. Trans R Soc Trop Med Hyg 80: 462–470. [DOI] [PubMed] [Google Scholar]
- 20. Lages-Silva E, Ramirez LE, Pedrosa AL, Crema E, da Cunha Galvao LM, et al. (2006) Variability of kinetoplast DNA gene signatures of Trypanosoma cruzi II strains from patients with different clinical forms of Chagas' disease in Brazil. J Clin Microbiol 44: 2167–2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Llewellyn MS, Lewis MD, Acosta N, Yeo M, Carrasco HJ, et al. (2009) Trypanosoma cruzi IIc: phylogenetic and phylogeographic insights from sequence and microsatellite analysis and potential impact on emergent Chagas disease. PLoS Negl Trop Dis 3: e510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Marcili A, Lima L, Valente VC, Valente SA, Batista JS, et al. (2009) Comparative phylogeography of Trypanosoma cruzi TCIIc: new hosts, association with terrestrial ecotopes, and spatial clustering. Infect Genet Evol 9: 1265–1274. [DOI] [PubMed] [Google Scholar]
- 23. Miles MA, Cedillos RA, Povoa MM, de Souza AA, Prata A, et al. (1981) Do radically dissimilar Trypanosoma cruzi strains (zymodemes) cause Venezuelan and Brazilian forms of Chagas' disease? Lancet 1: 1338–1340. [DOI] [PubMed] [Google Scholar]
- 24. Marcili A, Valente VC, Valente SA, Junqueira AC, da Silva FM, et al. (2009) Trypanosoma cruzi in Brazilian Amazonia: Lineages TCI and TCIIa in wild primates, Rhodnius spp. and in humans with Chagas disease associated with oral transmission. Int J Parasitol 39: 615–623. [DOI] [PubMed] [Google Scholar]
- 25. Garzon EA, Barnabe C, Cordova X, Bowen C, Paredes W, et al. (2002) Trypanosoma cruzi isoenzyme variability in Ecuador: first observation of zymodeme III genotypes in chronic chagasic patients. Trans R Soc Trop Med Hyg 96: 378–382. [DOI] [PubMed] [Google Scholar]
- 26. Tribulatti MV, Mucci J, Van Rooijen N, Leguizamón MS, Campetella O (2005) The trans-sialidase from Trypanosoma cruzi induces thrombocytopenia during acute Chagas' disease by reducing the platelet sialic acid contents. Infect Immun 73: 201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mucci J, Risso MG, Leguizamón MS, Frasch AC, Campetella O (2006) The trans-sialidase from Trypanosoma cruzi triggers apoptosis by target cell sialylation. Cell Microbiol 8: 1086–1095. [DOI] [PubMed] [Google Scholar]
- 28. Risso MG, Pitcovsky TA, Caccuri RL, Campetella O, Leguizamón MS (2007) Immune system pathogenesis is prevented by the neutralization of the systemic trans-sialidase from Trypanosoma cruzi during severe infections. Parasitology 134: 503–510. [DOI] [PubMed] [Google Scholar]
- 29. El-Sayed NM, Myler PJ, Bartholomeu DC, Nilsson D, Aggarwal G, et al. (2005) The genome sequence of Trypanosoma cruzi, etiologic agent of Chagas disease. Science 309: 409–415. [DOI] [PubMed] [Google Scholar]
- 30. Campetella O, Sánchez DO, Cazzulo JJ, Frasch ACC (1992) A superfamily of Trypanosoma cruzi surface antigens. Parasitol Today 8: 378–381. [DOI] [PubMed] [Google Scholar]
- 31. Freitas LM, dos Santos SL, Rodrigues-Luiz GF, Mendes TA, Rodrigues TS, et al. (2011) Genomic analyses, gene expression and antigenic profile of the trans-sialidase superfamily of Trypanosoma cruzi reveal an undetected level of complexity. PLoS One 6: e25914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cremona ML, Campetella O, Sanchez DO, Frasch AC (1999) Enzymically inactive members of the trans-sialidase family from Trypanosoma cruzi display beta-galactose binding activity. Glycobiology 9: 581–587. [DOI] [PubMed] [Google Scholar]
- 33. Cremona ML, Sanchez DO, Frasch AC, Campetella O (1995) A single tyrosine differentiates active and inactive Trypanosoma cruzi trans-sialidases. Gene 160: 123–128. [DOI] [PubMed] [Google Scholar]
- 34. Todeschini AR, Girard MF, Wieruszeski JM, Nunes MP, DosReis GA, et al. (2002) trans-Sialidase from Trypanosoma cruzi binds host T-lymphocytes in a lectin manner. J Biol Chem 277: 45962–45968. [DOI] [PubMed] [Google Scholar]
- 35. Oppezzo P, Obal G, Baraibar MA, Pritsch O, Alzari PM, et al. (2011) Crystal structure of an enzymatically inactive trans-sialidase-like lectin from Trypanosoma cruzi: the carbohydrate binding mechanism involves residual sialidase activity. Biochim Biophys Acta 1814: 1154–1161. [DOI] [PubMed] [Google Scholar]
- 36. Todeschini AR, Nunes MP, Pires RS, Lopes MF, Previato JO, et al. (2002) Costimulation of host T lymphocytes by a trypanosomal trans-sialidase: involvement of CD43 signaling. J Immunol 168: 5192–5198. [DOI] [PubMed] [Google Scholar]
- 37. Risso MG, Garbarino GB, Mocetti E, Campetella O, González Cappa SM, et al. (2004) Differential expression of a virulence factor, the trans-sialidase, by the main Trypanosoma cruzi phylogenetic lineages. J Infect Dis 189: 2250–2259. [DOI] [PubMed] [Google Scholar]
- 38. Breniere SF, Barnabe C, Bosseno MF, Tibayrenc M (2003) Impact of number of isoenzyme loci on the robustness of intraspecific phylogenies using multilocus enzyme electrophoresis: consequences for typing of Trypanosoma cruzi . Parasitology 127: 273–281. [DOI] [PubMed] [Google Scholar]
- 39. Barnabe C, Brisse S, Tibayrenc M (2000) Population structure and genetic typing of Trypanosoma cruzi, the agent of Chagas disease: a multilocus enzyme electrophoresis approach. Parasitology 120: 513–526. [DOI] [PubMed] [Google Scholar]
- 40. Breniere SF, Lopez J, Vargas F, Barnabe C (1997) Genetic variability and microdistribution of Triatoma infestans genotypes and Trypanosoma cruzi clones in Arequipa region (Peru). Mem Inst Oswaldo Cruz 92: 401–408. [DOI] [PubMed] [Google Scholar]
- 41. Buscaglia CA, Pollevick GD, Veloso C, Lorca M, Frasch AC, et al. (1996) A putative pyruvate dehydrogenase alpha subunit gene from Trypanosoma cruzi . Biochim Biophys Acta 1309: 53–57. [DOI] [PubMed] [Google Scholar]
- 42. Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, et al. (2007) Clustal W and Clustal X version 2.0. Bioinformatics 23: 2947–2948. [DOI] [PubMed] [Google Scholar]
- 43. Huson DH, Bryant D (2006) Application of phylogenetic networks in evolutionary studies. Mol Biol Evol 23: 254–267. [DOI] [PubMed] [Google Scholar]
- 44. Mathieu-Daude F, Bosseno MF, Garzon E, Lelievre J, Sereno D, et al. (2007) Sequence diversity and differential expression of Tc52 immuno-regulatory protein in Trypanosoma cruzi: potential implications in the biological variability of strains. Parasitol Res 101: 1355–1363. [DOI] [PubMed] [Google Scholar]
- 45. Lima L, Ortiz PA, da Silva FM, Alves JM, Serrano MG, et al. (2012) Repertoire, genealogy and genomic organization of cruzipain and homologous genes in Trypanosoma cruzi, T. cruzi-like and other trypanosome species. PLoS One 7: e38385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Soares RP, Torrecilhas AC, Assis RR, Rocha MN, Moura e Castro FA, et al. (2012) Intraspecies variation in Trypanosoma cruzi GPI-mucins: biological activities and differential expression of alpha-galactosyl residues. Am J Trop Med Hyg 87: 87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Telleria J, Biron DG, Brizard JP, Demettre E, Seveno M, et al. (2010) Phylogenetic character mapping of proteomic diversity shows high correlation with subspecific phylogenetic diversity in Trypanosoma cruzi . Proc Natl Acad Sci USA 107: 20411–20416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Leguizamón MS, Mocetti E, Garcia Rivello H, Argibay P, Campetella O (1999) trans-sialidase from Trypanosoma cruzi induces apoptosis in cells from the immune system in vivo. J Infect Dis 180: 1398–1402. [DOI] [PubMed] [Google Scholar]
- 49. Franzen O, Ochaya S, Sherwood E, Lewis MD, Llewellyn MS, et al. (2011) Shotgun sequencing analysis of Trypanosoma cruzi I Sylvio X10/1 and comparison with T. cruzi VI CL Brener. PLoS Negl Trop Dis 5: e984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Buschiazzo A, Muiá R, Larrieux N, Pitcovsky T, Mucci J, et al. (2012) Trypanosoma cruzi trans-sialidase in complex with a neutralizing antibody: structure/function studies towards the rational design of inhibitors. PLoS Pathog 8: e1002474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Westenberger SJ, Barnabe C, Campbell DA, Sturm NR (2005) Two hybridization events define the population structure of Trypanosoma cruzi . Genetics 171: 527–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. de Freitas JM, Augusto-Pinto L, Pimenta JR, Bastos-Rodrigues L, Goncalves VF, et al. (2006) Ancestral genomes, sex, and the population structure of Trypanosoma cruzi . PLoS Pathog 2: e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bhattacharyya T, Brooks J, Yeo M, Carrasco HJ, Lewis MD, et al. (2010) Analysis of molecular diversity of the Trypanosoma cruzi trypomastigote small surface antigen reveals novel epitopes, evidence of positive selection and potential implications for lineage-specific serology. Int J Parasitol 40: 921–928. [DOI] [PubMed] [Google Scholar]
- 54. Vago AR, Andrade LO, Leite AA, d'Avila Reis D, Macedo AM, et al. (2000) Genetic characterization of Trypanosoma cruzi directly from tissues of patients with chronic Chagas disease: differential distribution of genetic types into diverse organs. Am J Pathol 156: 1805–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Burgos JM, Diez M, Vigliano C, Bisio M, Risso M, et al. (2010) Molecular identification of Trypanosoma cruzi discrete typing units in end-stage chronic Chagas heart disease and reactivation after heart transplantation. Clin Infect Dis 51: 485–495. [DOI] [PubMed] [Google Scholar]
- 56. Campbell DA, Westenberger SJ, Sturm NR (2004) The determinants of Chagas disease: connecting parasite and host genetics. Curr Mol Med 4: 549–562. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Consensus sequence of TS gene internal region. Sequence alignment of T. cruzi stocks encompassing the 6 DTUs (TcI to TcVI). (.): conserved sites; (▾): SNPs that identify a group of parasites (inter-DTU polymorphism). In those positions, depicted nucleotide for each DTU was present in all sequences obtained from all parasites of each DTU (named as IUPAC code); (*): other polymorphic positions not shared by all stocks within a DTU (intra-DTU polymorphisms); TGG: Trp312 codon conserved in all stocks from all DTUs; Box: Tyr342His codon where Thymidine (encoding Tyr) and Cytosine (encoding His) are present in all stocks belonging to TcII, TcV and TcVI whereas only Thymidine was found in TcI, TcIII and TcIV genomes. No other mutations were found in this codon.
(TIF)
UPGMA tree based on TS genes sequence alignment (with ambiguous states) not including hybrid DTUs. To avoid deviations induced by the hybrid nature of T. cruzi TcV and TcVI DTUs, UPGMA tree was built excluding these DTUs.
(TIF)