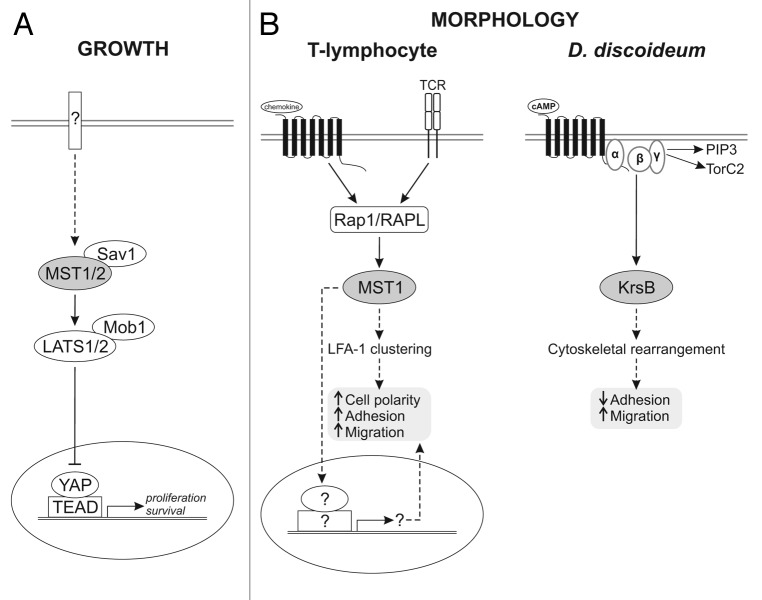
Hippo and its mammalian homologs MST1 and MST2 are important tumor suppressors best known for their effects on cell proliferation and survival; however, accumulating evidence suggests that these kinases are involved in a wide range of cellular processes, including regulation of cell morphology, adhesion and migration. In the canonical pathway activated Hippo/MST1/2 kinase phosphorylates and activates Warts/LATS kinase, which phosphorylates a transcriptional co-activator Yorkie (Yki) /YAP, retaining it in the cytoplasm. In the unphosphorylated state, Yki/YAP translocates to the nucleus and drives transcription of various genes involved in cell cycle progression (Fig. 1). The function of Hippo/MST1/2 in processes other than growth control is less clear and appears to be cell type-specific. For example, in HeLa and NIH-3T3 cell lines, activation of MST1 induces cell rounding and detachment, while in T-cells inactivation of MST1 leads to similar phenotypes.1-3 Furthermore, it is unclear whether these growth-independent actions of Hippo/MST1/2 rely on gene transcription.
Figure 1. A schematic of MST1/2 and KrsB signaling in mammalian and D. discoideum cells. (A) Canonical pathway regulating growth. Activated MST1/2 kinase interacts with Sav1 to phosphorylate Mob1 and LATS1/2 kinase, which work together to phosphorylate a transcriptional coactivator YAP, leading to its retention in the cytoplasm. Unphosphorylated YAP translocates to the nucleus, where, together with TEAD, it drives transcription of various genes important for cell proliferation and survival. For simplicity the schematic does not include upstream regulators of MST1/2. (B) Emerging pathways regulating cell morphology in T-lymphocytes and D. discoideum. In T-lymphocytes T cell receptor (TCR) ligation or stimulation with chemokines leads to MST1 activation downstream of Rap1 and its binding partner RAPL. Active MST1 promotes cell polarization, substrate adhesion and migration in part by inducing LFA-1 clustering. Whether MST1 functions independently of transcription in T cells is unclear. In Dictyostelium stimulation with a chemoattractant cAMP triggers transient activation of KrsB in a G-protein-dependent and PI3K- and TORC2-independent manner. In contrast to T cells, active KrsB negatively regulates cell substrate adhesion in part due to changes in the cytoskeleton. Solid lines with arrows and blunted ends refer to positive and inhibitory actions, respectively. Dotted arrows indicate less well-characterized pathways.
Using an unbiased screen for genes involved in chemotaxis of Dictyostelium discoideum, we identified Hippo/MST1/2 homolog KrsB as a novel regulator of multicellular pattern formation, cell substrate adhesion and directed migration (Fig. 1).4 Cells lacking KrsB kinase activity have increased contact with the substrate and are difficult to detach from the surface. This underlies defects in chemotaxis and random motility, which are corrected when cells are plated on a “slippery” surface. Changes in the actin cytoskeleton may account for the increased adhesion in cells lacking KrsB (unpublished observations). While our findings that KrsB negatively regulates adhesion are in contrast to those in T-cells, adhesion of T-cells in response to chemokines is mediated via integrins, which are absent in Dictyostelium.5 It would be interesting to test the role of MST1/2 in cells exhibiting amoeboid movement in an integrin-independent manner, e.g., neutrophils migrating in a 3D environment.6
The KrsB system in Dictyostelium has several unique features that contribute to our overall understanding of these pathways. First, KrsB regulates cell adhesion and migration but, unlike its mammalian counterparts, has no effect on cell growth, proliferation or cell survival, making this model organism uniquely suited for studies of growth-independent functions of the Hippo/MST1/2 family of kinases. Second, Dictyostelium does not have Warts/Lats, Yki/YAP or Sd/TEAD homologs.7 Although it is possible that there are functional orthologs of these proteins, the KrsB kinase may function independently of transcription in these cells. Consistently, the phenotypic changes in cell attachment disappear concurrently with the doxycycline-induced re-expression of KrsB in KrsB-deficient cells over a two hour period (unpublished observations). Thus, Dictyostelium might offer unique advantages for studying transcription-independent roles of Hippo. Third, KrsB lacks a SARAH domain, which mediates the dimerization thought to be required for MST1/2 activation by trans-autophosphorylation.8 However, KrsB does undergo a rapid, chemoattractant-induced trans-autophosphorylation on the conserved Thr residue in the activation loop (Thr 176). This suggests that either dimerization is not required for autophosphorylation or KrsB can form dimers without a SARAH domain. The autophosphorylation is functionally relevant since KrsB with a mutation (T176A) in the conserved phosphorylation site cannot rescue the phenotype of KrsB-deficient cells.
In summary, KrsB homologs Hippo and MST1/2 act as tumor suppressors in Drosophila and mammals and regulate cytoskeletal dynamics in several mammalian cell types but control only cytoskeletal activities in Dictyostelium. Interestingly, many other oncogenes and tumor suppressors have important roles in the maintenance of cytoskeletal dynamics and cell migration in addition to their roles in cell growth. For example, activation of the oncogenes Ras, PI3K and Akt, and inactivation of the tumor suppressor PTEN at the leading edge of a migrating cell is important for biasing pseudopod protrusion in the direction of a gradient. In Dictyostelium, as found for KrsB, these components do not alter cell growth. Identification of a Hippo homolog as a regulator of directed cell migration further highlights the overlap between the signaling networks regulating growth and the fast morphological changes observed in response to growth factor/chemokine stimulation.
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/23668
References
- 1.Glantschnig H, Rodan GA, Reszka AA. Mapping of MST1 kinase sites of phosphorylation. Activation and autophosphorylation. J Biol Chem. 2002;277:42987–96. doi: 10.1074/jbc.M208538200. [DOI] [PubMed] [Google Scholar]
- 2.Lee K-K, Ohyama T, Yajima N, Tsubuki S, Yonehara S. MST, a physiological caspase substrate, highly sensitizes apoptosis both upstream and downstream of caspase activation. J Biol Chem. 2001;276:19276–85. doi: 10.1074/jbc.M005109200. [DOI] [PubMed] [Google Scholar]
- 3.Katagiri K, Imamura M, Kinashi T. Spatiotemporal regulation of the kinase Mst1 by binding protein RAPL is critical for lymphocyte polarity and adhesion. Nat Immunol. 2006;7:919–28. doi: 10.1038/ni1374. [DOI] [PubMed] [Google Scholar]
- 4.Artemenko Y, Batsios P, Borleis J, Gagnon Z, Lee J, Rohlfs M, et al. Tumor suppressor Hippo/MST1 kinase mediates chemotaxis by regulating spreading and adhesion. Proc Natl Acad Sci USA. 2012;109:13632–7. doi: 10.1073/pnas.1211304109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sebé-Pedrós A, Roger AJ, Lang FB, King N, Ruiz-Trillo I. Ancient origin of the integrin-mediated adhesion and signaling machinery. Proc Natl Acad Sci U S A. 2010;107:10142–7. doi: 10.1073/pnas.1002257107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lämmermann T, Bader BL, Monkley SJ, Worbs T, Wedlich-Söldner R, Hirsch K, et al. Rapid leukocyte migration by integrin-independent flowing and squeezing. Nature. 2008;453:51–5. doi: 10.1038/nature06887. [DOI] [PubMed] [Google Scholar]
- 7.Sebé-Pedrós A, Zheng Y, Ruiz-Trillo I, Pan D. Premetazoan origin of the hippo signaling pathway. Cell Rep. 2012;1:13–20. doi: 10.1016/j.celrep.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Praskova M, Khoklatchev A, Ortiz-Vega S, Avruch J. Regulation of the MST1 kinase by autophosphorylation, by the growth inhibitory proteins, RASSF1 and NORE1, and by Ras. Biochem J. 2004;381:453–62. doi: 10.1042/BJ20040025. [DOI] [PMC free article] [PubMed] [Google Scholar]