Body temperature in mammals is homeostatically regulated around a set point, which renders it independent of large variations in environmental temperature. Hypothalamic regions, including the preoptic area, act as thermoregulatory centers that receive temperature inputs from the core body through hypothalamic receptors and from the environment through skin receptors. Thermogenic and heat-dissipative processes in tissues, such as muscles, brown adipose tissue, skin and vascular tissue, are regulated to counterbalance temperature deviations in order to maintain a relatively constant body temperature.1 While the thermoregulatory system aims to maintain the body temperature at a fixed target value, this set point itself fluctuates throughout the course of a day, resulting in circadian variations of body temperature. In humans, the maximal body temperature is reached in the late afternoon and drops to the nadir value at the end of the sleep phase. The circadian component in body temperature is under the control of the suprachiasmatic nuclei (SCN), which project to the preoptic area.2 The SCN is continuously entrained by light signals from the retinohypothalamic tract and, in turn, synchronizes circadian body temperature cycles to environmental light-dark cycles. In addition to body temperature cycles, the SCN governs a plethora of neuronal and humoral signals with daily oscillations, and these rhythmic SCN outputs control diverse physiological functions, either directly or indirectly through the synchronization of circadian oscillators in peripheral tissues. Peripheral clocks, which are operative in most body cells, and central oscillators in SCN neurons have a similar if not identical molecular makeup. Moreover, both central and peripheral oscillators are self-sustained and cell-autonomous. However, while SCN neurons are coupled through paracrine and electrical signals and therefore never loose phase coherence, peripheral cells do not appear to exchange phase information and thus must be continuously synchronized by the SCN pacemaker.3 Body temperature cycles have recently been demonstrated to function as systemic cues that efficiently phase entrain individual oscillators in cultured cells and tissue explants.4-6 Moreover, the survey of circadian mRNA expression in livers of mice with genetically arrested hepatocyte oscillators, but still functional SCN clocks, revealed a subgroup of transcripts whose accumulation cycles are likely driven by body temperature rhythms.7
We reconstituted the systemic circadian regulation of one of these genes, cold-inducible RNA-binding protein (Cirp), in cultured mouse fibroblasts entrained by simulated body temperature cycles. Cirp mRNA and protein abundance oscillated throughout the day in cells synchronized by body temperature rhythms (38°C–34°C) but not in cells kept at 36.5°C after synchronization of the core clock machinery by serum stimulation.8 In animals Cirp must therefore be regulated by body temperature cycles under the control of the master clock in the SCN rather than by peripheral oscillators. Conversely, if the temperature-regulated gene Cirp was involved in the regulation of peripheral clock factors, this would provide means by which circadian clocks can be influenced by temperature cues. Indeed, the reduction of CIRP protein levels in fibroblasts by RNA interference showed that CIRP is required for high-amplitude circadian gene expression. Moreover, this protein appears to confer robustness to circadian clocks toward phase-shifting cues.8 Thus, CIRP-deficient cells adjust their phase much more rapidly to altered temperature cycles than CIRP-proficient cells.
To further characterize the molecular interactions between CIRP and circadian clock components, we identified RNAs specifically interacting with CIRP and profiled the transcriptomes from CIRP-proficient and -deficient cells by deep RNA sequencing. CIRP exhibited preferential binding to 3′ untranslated regions of RNAs close to stop codons and 3′ ends. As expected, these CIRP target RNAs were more often misregulated in CIRP-depleted cells than transcripts not interacting with CIRP.8 Among CIRP-interacting and -regulated transcripts we identified Clock mRNA and a few other transcripts encoding core clock components. Single RNA molecule counting revealed that CIRP-regulated cytoplasmic Clock transcript abundance, which largely accounted for the defects in circadian gene expression cycles observed in CIRP-depleted cells.8
In addition to modulating phase and amplitude of circadian oscillators CIRP may also influence other cellular processes. For example, gene ontology analyses of CIRP-interacting and -regulated transcripts unveiled an enrichment in functions related to cell cycle progression and cell adhesion.8 Liver regeneration, which relays on such processes, follows a diurnal rhythm and depends on a functional clockwork circuitry.9 Taken together, cell proliferation and cell-cell contact remodelling might well be regulated by systemic cues emanating from the SCN, such as body temperature cycles, in addition to or instead of mechanisms depending on local liver clocks.
Although we gained insights into the effects of cold and heat shocks in mammalian cells and organs during the last few years, the regulation of gene expression and physiology exerted by body temperature cycles remains to be addressed. Understanding the functional consequences of circadian thermoregulation may reveal a circadian regulatory network involving both signals controlled by the SCN and local peripheral clocks. (Fig. 1)
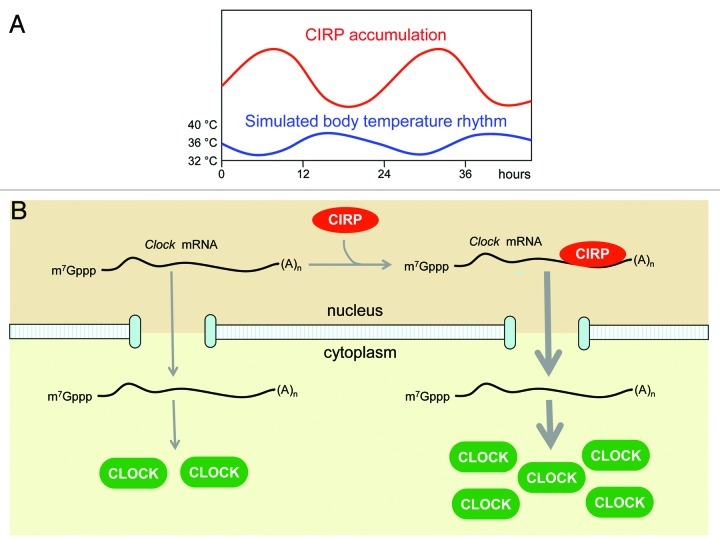
Figure 1. (A) Simulated mouse body temperature rhythms oscillating smoothly between 34°C and 38°C are sufficient to engender robust 24-h CIRP mRNA and protein accumulation cycles in cultured fibroblasts (schematic representation). (B) In the nucleus CIRP binds to 3′ untranslated regions (and, on occasion, intron sequences) of several hundred transcripts. One such transcript encodes the circadian core clock transcription factor CLOCK. If CIRP is depleted, less Clock mRNA reaches the cytoplasm and CLOCK protein concentrations drop to levels that are no longer capable of supporting high-amplitude circadian gene expression cycles. It still remains to be shown, however, whether the temperature-dependent CIRP accumulation rhythms shown schematically in panel (A) contribute to the temporal CLOCK protein expression pattern.
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/23670
References
- 1.Clapham JC. Central control of thermogenesis. Neuropharmacology. 2012;63:111–23. doi: 10.1016/j.neuropharm.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 2.Kräuchi K. The thermophysiological cascade leading to sleep initiation in relation to phase of entrainment. Sleep Med Rev. 2007;11:439–51. doi: 10.1016/j.smrv.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 3.Dibner C, Schibler U, Albrecht U. The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu Rev Physiol. 2010;72:517–49. doi: 10.1146/annurev-physiol-021909-135821. [DOI] [PubMed] [Google Scholar]
- 4.Brown SA, Zumbrunn G, Fleury-Olela F, Preitner N, Schibler U. Rhythms of mammalian body temperature can sustain peripheral circadian clocks. Curr Biol. 2002;12:1574–83. doi: 10.1016/S0960-9822(02)01145-4. [DOI] [PubMed] [Google Scholar]
- 5.Saini C, Morf J, Stratmann M, Gos P, Schibler U. Simulated body temperature rhythms reveal the phase-shifting behavior and plasticity of mammalian circadian oscillators. Genes Dev. 2012;26:567–80. doi: 10.1101/gad.183251.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buhr ED, Yoo SH, Takahashi JS. Temperature as a universal resetting cue for mammalian circadian oscillators. Science. 2010;330:379–85. doi: 10.1126/science.1195262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kornmann B, Schaad O, Bujard H, Takahashi JS, Schibler U. System-driven and oscillator-dependent circadian transcription in mice with a conditionally active liver clock. PLoS Biol. 2007;5:e34. doi: 10.1371/journal.pbio.0050034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morf J, Rey G, Schneider K, Stratmann M, Fujita J, Naef F, et al. Cold-inducible RNA-binding protein modulates circadian gene expression posttranscriptionally. Science. 2012;338:379–83. doi: 10.1126/science.1217726. [DOI] [PubMed] [Google Scholar]
- 9.Matsuo T, Yamaguchi S, Mitsui S, Emi A, Shimoda F, Okamura H. Control mechanism of the circadian clock for timing of cell division in vivo. Science. 2003;302:255–9. doi: 10.1126/science.1086271. [DOI] [PubMed] [Google Scholar]