Our life starts with the fusion of oocytes with spermatozoa, eventually leading to the development of an embryo. Though the cellular events involved in fertilization are well-described, their molecular underpinnings remain poorly understood.1 Substances controlling the elementary fusion processes are of utmost socioeconomic and medical relevance. Spermidine is a naturally occurring polyamine vital for life,2 regulating multiple cellular processes, including gene expression, autophagy and aging.2,3 In this issue, Bauer et al.4 establishes a fundamental role for spermidine in this fusion process in budding yeast S. cerevisiae.4 Subsequently, the authors also extended the role of the polyamine spermidine for oocyte fertilization in the nematode Caenorhabditis elegans as well, implying that the fundamental mechanisms underlying fertilization are highly conserved across phlyla.
Fertilization efficiency has been reported to decline gradually with advanced paternal age,5 in line with the age-related decrease in polyamines level.6 However, it remained unclear whether the very fusion process of oocytes and spermatozoa is in fact directly modulated by polyamines. To test this, Bauer et al.4 studied sexual reproduction in the baker’s yeast S. cerevisiae, during which two haploid cells of opposed mating type (“MATa” and “MATα”) combine to generate one diploid cell. To specifically determine the role of the spermidine SPE2, an enzyme essential for spermidine production was deleted in one of the mating strains (“MATa”Δspe2). This disruption resulted in drastic reduction of overall mating efficiency. Interestingly, supplementation of exogenous spermidine in cultures of “MATα” wild type and “MATa”Δspe2 cells restored the mating efficiency, indicating that spermidine is necessary for efficient mating in S. cerevisiae.
In response to mating pheromone, sexually reproducing yeast cells of opposite mating type differentiate into a specialized pear-shaped functional form, known as “shmoo.” Consistent with the idea, while the phermone-treated viable “MATa”Δspe2 cells hardly developed shmoos, the administration of spermidine rescued the deficient shmoo formation in SPE2 disruptants to wild-type control levels. Thus, polyamines appear largely indispensable for shmoo formation, explaining the reduced mating efficacy in SPE2 disruptants.
The authors then extended their finding to a metazoan animal, C. elegans. Here, impairing spermidine biosynthesis by deleting of spermidine synthase (Spds-1), resulted in a significant reduction of the total number of fertilized eggs laid, indicating spermidine to be important for effective fertilization in worms.
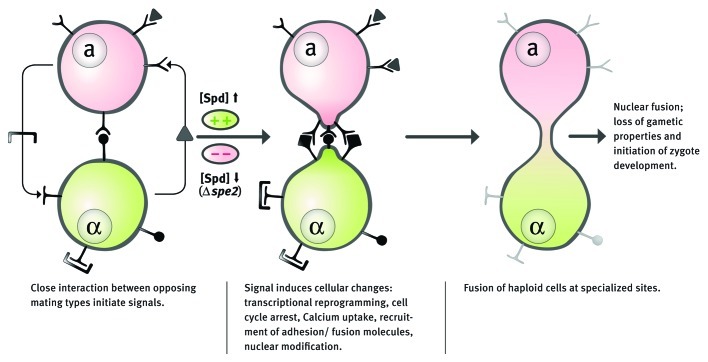
How about the mechanistic underpinnings of the spermidine effects on fertilization? Spermidine was recently shown to induce autophagy in a range of model organisms, including mice, worms, flies and yeast.3 However, deletion of ATG7, which is indispensable for autophagy-mediated clearance in S cerevisiae, did not influence its mating efficiency. Similarly, RNAi-driven knockdown of beclin-1, an autophagic regulator in C. elegans, did not influence egg fertilization rates. Thus, in both cases, spermidine-mediated effects on fertilization seem autophagy-independent. Alternative pathways involving different molecular target(s) should then be responsible for these spermidine-mediated effects. In yeast, when opposite mating type are mixed together, reciprocal pheromone stimulation via a GTP-binding protein-mediated pathway triggers a plethora of responses: increased expression of agglutinins, cell division arrest, increased Ca2+ uptake, competence for nuclear fusion, transcriptional reprogramming and formation of a projection that becomes the site of cell fusion.7,8 A key question for the future is how and which response(s) of fertilization processes are regulated by spermidine?
Recently, structural similarities between proteins involved in sperm-egg recognition in eukaryotes and such involved in fusion of haploid yeasts was proposed.8 This study here demonstrated the power of the “simple” yeast model in the genetic dissection of fundamental eukaryotic fertilization mechanisms.4 An important preset for male fertility in higher eukaryotes is the physiological priming of spermatozoa, collectively referred to as capacitation; during capacitation, several signaling cascades are initiated, thereby rendering spermatozoa competent for acrosome reaction, a process driving the sperm to penetrate the oocyte.1 Ca2+ is reported to play a critical role for both capacitation as well as acrosome reaction.1 As Ca2+ channels are known to be regulated by spermidine, an interesting (though speculative) possibility is that Ca2+ influx could be modulated by spermidine. In addition, cross-talk between cAMP and phosphatidylinositol pathways is highly critical for the fertilization process1 and, again, spermidine is reported to modulate these pathways.
With fertility rates steadily decreasing in western societies, a better understanding of the mechanistic details that underlie the pro-fertilization effects is needed. It might not be too far-fetched to see a role of polyamines for fertilization medicine one day. (Fig. 1)
Figure 1. Model representing the major events of fertilization in yeast. Several responses are induced in response to reciprocal pheromone stimulation in oppositely mating strains, which might be regulated by spermidine (Spd).
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/23676
References
- 1.Abou-haila A, Tulsiani DR. Signal transduction pathways that regulate sperm capacitation and the acrosome reaction. Arch Biochem Biophys. 2009;485:72–81. doi: 10.1016/j.abb.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Igarashi K, Kashiwagi K. Modulation of cellular function by polyamines. Int J Biochem Cell Biol. 2010;42:39–51. doi: 10.1016/j.biocel.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 3.Eisenberg T, Knauer H, Schauer A, Büttner S, Ruckenstuhl C, Carmona-Gutierrez D, et al. Induction of autophagy by spermidine promotes longevity. Nat Cell Biol. 2009;11:1305–14. doi: 10.1038/ncb1975. [DOI] [PubMed] [Google Scholar]
- 4.Bauer MA, Carmona-Gutiérrez D, Ruckenstuhl C, Reisenbichler A, Megalou EV, Eisenberg T, et al. Spermidine promotes mating and fertilization efficiency in model organisms. Cell Cycle. 2013;12:346–52. doi: 10.4161/cc.23199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stewart AF, Kim ED. Fertility concerns for the aging male. Urology. 2011;78:496–9. doi: 10.1016/j.urology.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 6.Scalabrino G, Ferioli ME. Polyamines in mammalian ageing: an oncological problem, too? A review. Mech Ageing Dev. 1984;26:149–64. doi: 10.1016/0047-6374(84)90090-3. [DOI] [PubMed] [Google Scholar]
- 7.Cross F, Hartwell LH, Jackson C, Konopka JB. Conjugation in Saccharomyces cerevisiae. Annu Rev Cell Biol. 1988;4:429–57. doi: 10.1146/annurev.cb.04.110188.002241. [DOI] [PubMed] [Google Scholar]
- 8.Swanson WJ, Aagaard JE, Vacquier VD, Monné M, Sadat Al Hosseini H, Jovine L. The molecular basis of sex: linking yeast to human. Mol Biol Evol. 2011;28:1963–6. doi: 10.1093/molbev/msr026. [DOI] [PMC free article] [PubMed] [Google Scholar]