Abstract
Tumor microenvironment plays a central role in the development and dissemination of cancer cells. In addition to study each specific cellular component of the microenvironment, it has become clear that it is the type and amount of information that cells exchange that ultimately affects cancer phenotype. Recently, it has been discovered that intercellular communication occurs through the release of microvesicles and exosomes, whose cargo represents the information released by one cell to a recipient cell. A key component of this cargo is represented by microRNAs (miRNAs), small non-coding RNAs with gene regulatory functions. We discovered that miRNAs released by cancer cells within microvesicles can reach and bind to Toll-like receptors (TLRs) in surrounding immune cells, and activate them in a paracrine loop. As a result, immune cells produce cytokines that increase cell proliferation and metastatic potential. This discovery provides the rationale for the development of new drugs that might be used in the treatment of cancer as well as other inflammation-related diseases.
Keywords: IL-6, TNF-α, Toll-like receptors, exosomes, metastasis, microRNAs, tumor microenvironment
MicroRNAs as Cancer Biomarkers and Cell-Cell Cross-Talk Mediators
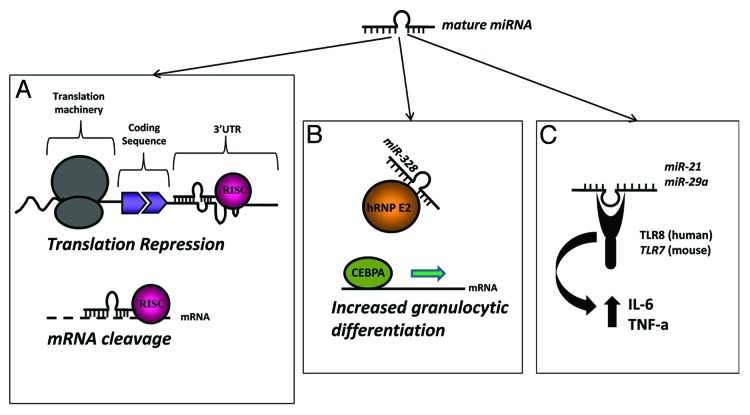
MicroRNAs (miRNAs) are a large family of non-coding, single stranded RNAs of 19–24 nucleotides in length which regulate gene expression both at the transcriptional and translational level.1,2 Such regulation occurs by binding to complementary sequences in the coding, 5′- or 3′-untranslated region (UTR) of target mRNAs (mRNAs)3-5 (Fig. 1A). MiRNAs regulate nearly 30% of the human genome1 and play a pivotal role in most critical biological processes, including differentiation, development, proliferation, cell cycle, metabolism and host-viral interactions.4,6-8 They are also involved in the onset of several human diseases (such as cancer, diabetes and neurodegenerative disorders).9-13 Over the past few years it has been demonstrated that several miRNAs contribute to cancer development through gain and loss of function mechanisms.14 MiRNAs are in fact frequently located in cancer-associated genomic regions, such as minimal regions of amplification, loss of heterozygosity, fragile sites and common breakpoint regions in or in proximity of oncogenes or tumor suppressor genes. During the last decade miRNA profiling studies have been performed on different kind of human tumors pointing out which miRNAs are dys-regulated with respect to normal tissues, and shedding light on their involvement in cancer formation and progression.It is possible to identify “miRNA signatures” for different types of cancer with diagnostic, prognostic and in some cases predictive-of-response-to-treatment implications. As cancer biomarkers, miRNAs can be detected not only in primary tumors (vs. the normal tissue counterpart) but also in circulating body fluids, where they have been also found differentially expressed in cancer patients with respect to healthy donors.15
Figure 1. Different mechanisms of action of mature microRNAs. (A) The “classical” mechanism of action of mature miRNAs consists in their binding to a partially (upper panel) or completely (lower panel) complementary sequence in a target mRNA leading to translational repression or mRNA cleavage, respectively. (B) MiRNAs can also bind to proteins and affect their function. The case of miR-328 is here described. By directly binding to hRNP E2, miR-328 functions as a decoy and subtracts hRNP E2 from binding to and inhibiting CEBPA. As a result of miR-328-hRNP E2 interaction, CEBPA is able to bind to target mRNAs exerting its transcription factor function leading to increased granulocytic differentiation. (C) MiRNAs can also bind to proteic receptors and activate them. Here we describe the mechanism of action of miR-21 and -29a, released by cancer cells within MVs, and able to bind to TLR8 (in humans) or TLR7 (in mice) in surrounding immune cells. As a result of this interaction, immune cells release IL-6 and TNF-α which promote cancer growth.
It has been common belief that miRNAs regulate gene expression within the cell. However, recent findings show that these small molecules can also be transferred from cell to cell through mechanisms involving shedding extracellular vesicles (EVs). Although a true consensus has not been reached among scientists yet, EVs include exosomes, microvesicles, membrane microparticles and apoptotic vesicles. The distinctions among these different types of EVs are based on their size and gradient density. While exosomes are 30–100 nm in diameter, microvesicles are 100–1,000 nm, membrane microparticles are 50–80 nm and apoptotic bodies 50–500 nm.16 EVs are secreted17 by different kind of cells: epithelial cells,18 dendritic cells,19 B- and T- cells,20,21 mast cells22 and tumor cells.23 Secreted EVs may remain in proximity of the cells which have generated them or may reach more distant sites through biological fluids. This may explain the presence of EVs in plasma, urine, milk, semen and cerebrospinal fluid.24 Although the mechanism of EV formation and secretion is far from being elucidated, it has been demonstrated that ceramide is involved in this process.26
Although EVs were originally considered a “disposal system” for unnecessary membrane proteins,27-29 they have recently captured much attention as a vehicle involved in cell-to-cell communication.30-33 Exosomes can transfer viral RNA (from HCV infected cells) to nonpermissive plasmacytoid dendritic cells (pDCs) to evade pathogen recognition.26 In 2007 Valadi et al.34 isolated exosomes from a mouse and human mast cell line, and from primary bone marrow-derived mouse mast cells. They then performed a microarray analysis finding that the isolated vesicles contained not only proteins but also the mRNAs of approximately 1,300 genes, and the in vitro translation assay showed that these mRNAs were functional. Moreover, the authors found small RNAs corresponding to approximately 121 miRNAs (including miR-1, miR-18, miR-181 and miR-375), which are thought to be involved in processes like angiogenesis, tumorigenesis and exocytosis.34-36 Also, some of these miRNAs were expressed at higher levels in exosomes than in the cell of origin, suggesting that some miRNAs may be uniquely packed into exosomes.
Kosaka et al.25 showed that miR-146a secreted by COS-7 cell line targets ROCK1-encoding mRNA when delivered to recipient PC-3M prostate cells, validating the fact that also secreted miRNAs take part on this novel mechanism of intercellular communication. In 2012, Montecalvo and coworkers37 showed that dendritic cells (DCs) secrete exosomes that are loaded with distinct sets of miRNAs, dependent on the status of DC activation and that such exosomes can fuse with target cells, thereby delivering their membranous and cytosolic contents. Finally, they demonstrated that transferred miRNAs can repress target mRNAs in the recipient cells.
Although new findings are unveiling the secrets of this novel mechanism of cell-cell communication, there are still a lot of points to be elucidated. For example, it is still not clear how miRNAs are sorted into EVs and which proteins are involved in this process.
Cancer-secreted microRNAs Activate a TLR-mediated Pro-Tumoral Inflammatory Response
The hypothesis
The identification of miRNAs within EVs and exosomes and the fact that they are secreted by cells and can exert a function on other cells in a paracrine fashion, prompted us to investigate whether miRNAs can work by triggering a receptor-mediated response. The ability of miRNAs to bind to proteins has already been previously shown by Eiring et al., who demonstrated that miR-328 directly binds to hRNP E2 and subtracts this protein from its inhibitory effect on the transcription of CEBPA, a factor that promotes granulocytic differentiation38 (Fig. 1B). Among the proteic receptors that might be able to bind to miRNAs, we decided to focus on a group of receptors that are known to bind single-stranded RNAs (ssRNAs) of a size (19–24 nucleotides) similar to a mature miRNA: the Toll-like receptors (TLRs).
Toll-like receptors and their relevance in cancer
The TLR family consists of 10 members (13 in mice), which enable innate immune cells and other specialized cell subsets such as epithelial cells to respond to a variety of pathogen-associated molecular patterns (PAMPs).39 TLR3, TLR7, TLR8 and TLR9 form a subgroup of TLRs for structural and functional similarities. All of them, in fact, recognize viral nucleic acid and are located in the endosomal membrane. While TLR3 and -9 bind double-stranded RNA (dsRNA) and DNA-containing CpG motives, respectively, TLR7 and TLR8 are able to recognize ssRNAs.40,41
TLR7 and TLR8 were initially identified as receptors for antiviral small molecules such as imidazoquinoline derivates,42,43 but subsequently were found to be responsible for the detection of ssRNA derived from the human immunodeficiency virus (HIV) and the influenza virus.40,41 Different studies in humans showed that both TLR7 and TLR8 transfer responsiveness to ssRNA, but in mice this is true only for TLR7,40,41 since TLR8 is not functional.
TLR7 and TLR8 activation induces the recruitment of the adaptor MyD88 followed by the formation of a complex with IRAK1, IRAK4 and TRAF6, which results in NF-κB (nuclear factor-kappa B) activation. NF-κB plays a critical role in the development of tumors and in the context of chronic inflammation.44,45
Although TLR expression was first observed in immune cells, several reports have described the expression of TLRs in non-malignant and malignant epithelial cells. The role of TLRs in cancer is still controversial, since it has not been convincingly determined whether they favor or inhibit cancer development.
On one hand, TLR stimulation has a pro-tumoral effect, favoring tumor initiation,46,47 development,48 invasion,49,50 resistance to chemotherapy,51-53 and escape from the immune system.52,54 On the other hand, TLR stimulation can lead to tumor regression either by direct induction of tumor cell apoptosis55-57 or by activation of anti-tumoral immune responses. Conforti et al. showed that uncoupling the immunostimolatory and immunosuppressive effects of TLR3 activation by manipulating the released cytokines upon the receptor activation is a feasible and attractive new anticancer approach.58
Several studies strongly suggest that chronic inflammation (i.e., chronic bronchitis, chronic obstructive diseases, emphysema, asbestos or tobacco smoke) increases the risk of carcinogenesis.59,60 Lungs are frequently exposed to RNA viruses (such as respiratory syncytial and influenza viruses) and pathogens that are recognized by TLR7 and TLR8,40,42 suggesting that these TLRs are present on lung epithelial cells. A link between TLR7 and TLR8 signaling and inflammation, tumor growth, and chemoresistance has been observed. Moreover, it is known that the expression of TLR7 and TLR8 in lung cancer cells and their stimulation results in activation of NF-κB and upregulation of Bcl-2 expression. This was associated with increased tumor cell survival and resistance to apoptosis induced by chemotherapy in vitro.51 These data emphasize that TLR signaling can directly interfere with the tumor cell either by increasing cell survival or by inducing resistance to cell death.
Increased scientific and clinical interest in TLR7 and TLR8 for cancer biology has originated from the discovery of antitumoral activity of some small-molecule compounds61 which have been shown to act as agonists for one or both receptors.43,62 Most of the findings concerning the antitumoral mode of action of TLR7/8 agonists have been obtained with the nucleoside analog imiquimod.63 Imiquimod activates preferentially TLR7; its agonistic activity on TLR8 appears to be much weaker.64 Another molecule named Resiquimod is a selective ligand for TLR7 in mice and for TLR7 and TLR8 in humans. Resiquimod induces more pronounced cytokine secretion, macrophage activation and enhancement of cellular immunity as compared with imiquimod.65,66 Gardiquimod is another imidazoquinoline derivative that, similar to imiquimod, induces activation of NF-κB in cells expressing human or murine TLR7. At high concentration (that is ≥ 3 mg ml−1), gardiquimod also activates TLR8.
Imiquimod induces expression of proinflammatory cytokines including IFN-α, TNF-α, IL-2, -6, -8, -12, G-CSF and GM-CSF, as well as chemokines such as CCL3 (MIP-1α), CCL4 (MIP-1β) and CCL2 (MCP-1).67-69 Rettig et al. have shown that nanometric protamine-RNA particles induce production of IFN-α, whereas micrometric particles mainly induce the production of TNF-α in human immune cells. This difference is explained by the fact that nanoparticles (but not microparticles) are selectively phagocytosed by pDCs, which produce IFN-α, whereas monocytes (that mainly produce TNF-α) have a higher activation threshold than pDCs.70 More recently, Schiller et al. have shown that apoptotic cells release DNA in membrane microparticles, able to bind to TLR9 in pDCs and inducing IFN-α secretion.71 These findings are of great value, and provide important insights on the role of pro-inflammatory cytokines as also able to trigger an anti-tumoral activity, and on how to develop new delivery systems able to trigger a specific cocktail of cytokines with anti-tumoral properties. In addition to the NF-κB-mediated transcription of proinflammatory mediators, it appears that TLR7- (and TLR8)-agonistic activities of imiquimod induce some proinflammatory cytokines, such as IFNγ, in a NF-κB-independent fashion. The known functions of these mediators explain, at least in part, many cellular responses to imiquimod including activation and chemotactic properties on dendritic cells and their precursors as well as on cytotoxic T-lymphocytes and other immune cells.
MiRNAs can bind and activate TLR8 in human (TLR7 in mice)
Our first approach to assess whether cancer-released miRNAs can bind to TLRs in recipient surrounding immune cells was to determine which miRNAs are secreted by cancer released exosomes. We used non-small cell lung cancer (NSCLC) cell lines as a model and were able to demonstrate that among the secreted miRNAs, miR-16, -29a, -21 and -27b were highly represented.33 By performing confocal microscopy experiments we were able to show that these miRNAs can reach the content of endosomes in the recipient cells and co-localize with ssRNA binding TLRs within them. Subsequently, we performed co-immunoprecipitation experiments and observed that while miR-21 and miR-29a bind to TLR8, miR-16 does not.33 We partially tried to address this differential behavior of miR-16 by performing a mutational analysis. To this aim, we changed several bases in the 3′ region of miR-21 and miR-29a (which contains a GU motif known to activate TLRs) with the corresponding ones in miR-16 sequence. Our data indicate that while miR-21 U20G mutant, miR-29a U20G mutant and miR-29a U21C mutant significantly reduce TLR8-mediated NF-κB activation, the miR-21 G18U mutant exerted an opposite effect,33 suggesting that more than the GU content, it is the tridimensional structure of the miRNAs (as dictated by its nucleotide sequence) to affect miRNA ability to functionally activate TLRs. However, further experiments are warranted to address this specific issue. In humans we observed that miR-21 and miR-29a activate TLR8, but not TLR7, suggesting a selectivity of ssRNA-binding TLR activation by miRNAs, whose significance still needs to be better understood. Interestingly, another group has shown that in TLR7 knockout mice, let-7 (whose expression is upregulated in the cephalorachidian liquid of patients affected by Alzheimer disease) does not produce the neurodegenerative effects observed in presence of TLR7.72 These data support an unconventional mechanism of action for let-7, at least in part mediated by its interaction with TLR7. Overall, these findings also indicate that miRNA-TLR binding interactions have implications for human pathology that go even beyond cancer.
Functionally, we observed that miR-21 and -29a binding to human TLR8 (or murine TLR7) induces NF-κB activation and increased secretion of pro-inflammatory and pro-metastatic citokynes IL-6 and TNF-α, which increase the metastatic potential of LLC (Lewis Lung Carcinoma) cells. These effects are significantly reduced in TLR7 knockout mice or when mice were treated with compounds such as Bafilomycin A and GW4869, which affect exosome-endosome fusion and exosome secretion, respectively.33 Interestingly, we also observed that while lung cancer cells are the main producers of TLR-activating miRNAs, a co-localization of secreted miRNAs and TLRs occurs mainly within the macrophages located at the tumor interface. Since this co-localization is not observed in the normal tissue distant from the tumor, nor in the central part of the tumor, it seems reasonable to conclude the miRNA-TLR interaction (at least for miR-21 and miR-29a) affects more the biology of the periphery of the tumor. It still remains to be determined what is the relevance of the miRNA-TLR interaction for other miRNAs and in a physiological setting.
Conclusions and Future Perspectives
Innate immune system activation is tightly related to the inflammation-cancer correlation. Interestingly, in several types of cancer, immune cells represent up to 50% of tumor mass.73 A specific population of macrophages named tumor associated macrophages (TAMs) plays a pivotal role in the tumor growth promoting cancer cell survival, neoangiogenesis and dissemination.73,74 TAM infiltration has been described as an early phenomenon in the metastatic process.75 In particular, these cells seem to prepare a niche to sustain cancer cell growth producing matrix proteases and angiogenic chemokines that promote endothelial cell recruitment and proliferation.76,77 Besides their role in tumor support for preexisting metastatic cells, macrophages have been implicated in the establishment of a constitutive inflammatory process that induces oncogenic mutation in the surrounding epithelial cells particularly through the secretion of highly reactive compounds like reactive oxygen and nitrogen species.78 TAM recruitment in the tumor site is suspected to be a part of an innate immune response but the factors involved in the initiation of this process have not been fully elucidated.
With the exception of the well known microorganism-driven cancers,79 in the majority of inflammation-related cancers the source of the chronic inflammatory status is unknown and this particular situation is known as sterile inflammation.80 There is now considerable evidence that self-recognition through receptors of innate immunity, such as TLRs, can occur and significantly contribute to sterile inflammation and autoimmunity. We described a completely novel mechanism of action through which cancer cells promote their growth and dissemination. A summary of “traditional” and newly discovered mechanisms of action for miRNAs is summarized in Figure 1. By secreting exosomes containing miRNAs able to reach ssRNA-binding TLRs in the endosomes of surrounding immune cells, cancer cells can promote an increased immune-cell mediated secretion of IL-6 and TNF-α within the tumor microenvironment (Fig. 1C). As a result, cancer cells increase proliferation and dissemination. Interestingly, this new discovery harbors several translational implications. It is indeed conceivable to interrupt this aberrant cross-talk between cancer cells and surrounding immune cells within the tumor microenvironment, for instance by using molecules able to interfere with the capacity of cancer cells to release exosomes. Another approach might be creating genetically engineered TLRs that conserve the ability to bind to the exosomic miRNAs but do not trigger the intracellular signal transduction that leads to increased cytokine production by the immune cells. Although in its infancy, these strategies will certainly provide new molecular targets to develop new anticancer drugs. Finally, and probably even more interestingly, being able to interfere with this exosomic miRNA-mediated intercellular communication will provide new therapeutic avenues also in neurodegenerative diseases and other inflammation-related diseases. While the activation of ssRNA-binding TLRs by miRNAs released by lung cancer cells has important implications for cancer growth and dissemination, it is still unknown whether the same paracrine cross-talk between cancer cells and surrounding cells occurs also in other types of cancers and within other cellular components of the tumor microenvironment. More intriguingly, it can be hypothesized that the activation of receptors by cell-released miRNAs might also involve other receptors and could also occur in physiological conditions. These perspectives are exciting and will lead to a better understanding of cancer’s and other diseases’ pathology, leading to the identification of new therapeutic targets.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/rnabiology/article/23144
References
- 1.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–97. doi: 10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 2.Denli AM, Tops BB, Plasterk RH, Ketting RF, Hannon GJ. Processing of primary microRNAs by the Microprocessor complex. Nature. 2004;432:231–5. doi: 10.1038/nature03049. [DOI] [PubMed] [Google Scholar]
- 3.Lagos-Quintana M, Rauhut R, Lendeckel W, Tuschl T. Identification of novel genes coding for small expressed RNAs. Science. 2001;294:853–8. doi: 10.1126/science.1064921. [DOI] [PubMed] [Google Scholar]
- 4.Lau NC, Lim LP, Weinstein EG, Bartel DP. An abundant class of tiny RNAs with probable regulatory roles in Caenorhabditis elegans. Science. 2001;294:858–62. doi: 10.1126/science.1065062. [DOI] [PubMed] [Google Scholar]
- 5.Lee RC, Ambros V. An extensive class of small RNAs in Caenorhabditis elegans. Science. 2001;294:862–4. doi: 10.1126/science.1065329. [DOI] [PubMed] [Google Scholar]
- 6.Carleton M, Cleary MA, Linsley PS. MicroRNAs and cell cycle regulation. Cell Cycle. 2007;6:2127–32. doi: 10.4161/cc.6.17.4641. [DOI] [PubMed] [Google Scholar]
- 7.Harfe BD. MicroRNAs in vertebrate development. Curr Opin Genet Dev. 2005;15:410–5. doi: 10.1016/j.gde.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 8.Boehm M, Slack FJ. MicroRNA control of lifespan and metabolism. Cell Cycle. 2006;5:837–40. doi: 10.4161/cc.5.8.2688. [DOI] [PubMed] [Google Scholar]
- 9.Poy MN, Eliasson L, Krutzfeldt J, Kuwajima S, Ma X, Macdonald PE, et al. A pancreatic islet-specific microRNA regulates insulin secretion. Nature. 2004;432:226–30. doi: 10.1038/nature03076. [DOI] [PubMed] [Google Scholar]
- 10.Landthaler M, Yalcin A, Tuschl T. The human DiGeorge syndrome critical region gene 8 and Its D. melanogaster homolog are required for miRNA biogenesis. Curr Biol. 2004;14:2162–7. doi: 10.1016/j.cub.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 11.Jin P, Alisch RS, Warren ST. RNA and microRNAs in fragile X mental retardation. Nat Cell Biol. 2004;6:1048–53. doi: 10.1038/ncb1104-1048. [DOI] [PubMed] [Google Scholar]
- 12.Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, et al. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–8. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 13.Volinia S, Calin GA, Liu CG, Ambs S, Cimmino A, Petrocca F, et al. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci U S A. 2006;103:2257–61. doi: 10.1073/pnas.0510565103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Croce CM. Causes and consequences of microRNA dysregulation in cancer. Nat Rev Genet. 2009;10:704–14. doi: 10.1038/nrg2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci U S A. 2008;105:10513–8. doi: 10.1073/pnas.0804549105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cocucci E, Racchetti G, Meldolesi J. Shedding microvesicles: artefacts no more. Trends Cell Biol. 2009;19:43–51. doi: 10.1016/j.tcb.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 17.Henderson MC, Azorsa DO. The genomic and proteomic content of cancer cell-derived exosomes. Front Oncol. 2012;2:38. doi: 10.3389/fonc.2012.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Niel G, Raposo G, Candalh C, Boussac M, Hershberg R, Cerf-Bensussan N, et al. Intestinal epithelial cells secrete exosome-like vesicles. Gastroenterology. 2001;121:337–49. doi: 10.1053/gast.2001.26263. [DOI] [PubMed] [Google Scholar]
- 19.Théry C, Regnault A, Garin J, Wolfers J, Zitvogel L, Ricciardi-Castagnoli P, et al. Molecular characterization of dendritic cell-derived exosomes. Selective accumulation of the heat shock protein hsc73. J Cell Biol. 1999;147:599–610. doi: 10.1083/jcb.147.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, et al. B lymphocytes secrete antigen-presenting vesicles. J Exp Med. 1996;183:1161–72. doi: 10.1084/jem.183.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blanchard N, Lankar D, Faure F, Regnault A, Dumont C, Raposo G, et al. TCR activation of human T cells induces the production of exosomes bearing the TCR/CD3/zeta complex. J Immunol. 2002;168:3235–41. doi: 10.4049/jimmunol.168.7.3235. [DOI] [PubMed] [Google Scholar]
- 22.Raposo G, Tenza D, Mecheri S, Peronet R, Bonnerot C, Desaymard C. Accumulation of major histocompatibility complex class II molecules in mast cell secretory granules and their release upon degranulation. Mol Biol Cell. 1997;8:2631–45. doi: 10.1091/mbc.8.12.2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mears R, Craven RA, Hanrahan S, Totty N, Upton C, Young SL, et al. Proteomic analysis of melanoma-derived exosomes by two-dimensional polyacrylamide gel electrophoresis and mass spectrometry. Proteomics. 2004;4:4019–31. doi: 10.1002/pmic.200400876. [DOI] [PubMed] [Google Scholar]
- 24.Camussi G, Deregibus MC, Bruno S, Cantaluppi V, Biancone L. Exosomes/microvesicles as a mechanism of cell-to-cell communication. Kidney Int. 2010;78:838–48. doi: 10.1038/ki.2010.278. [DOI] [PubMed] [Google Scholar]
- 25.Kosaka N, Iguchi H, Yoshioka Y, Takeshita F, Matsuki Y, Ochiya T. Secretory mechanisms and intercellular transfer of microRNAs in living cells. J Biol Chem. 2010;285:17442–52. doi: 10.1074/jbc.M110.107821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dreux M, Garaigorta U, Boyd B, Décembre E, Chung J, Whitten-Bauer C, et al. Short-range exosomal transfer of viral RNA from infected cells to plasmacytoid dendritic cells triggers innate immunity. Cell Host Microbe. 2012;12:558–70. doi: 10.1016/j.chom.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnstone RM. The Jeanne Manery-Fisher Memorial Lecture 1991. Maturation of reticulocytes: formation of exosomes as a mechanism for shedding membrane proteins. Biochem Cell Biol. 1992;70:179–90. doi: 10.1139/o92-028. [DOI] [PubMed] [Google Scholar]
- 28.Johnstone RM, Bianchini A, Teng K. Reticulocyte maturation and exosome release: transferrin receptor containing exosomes shows multiple plasma membrane functions. Blood. 1989;74:1844–51. [PubMed] [Google Scholar]
- 29.Johnstone RM, Mathew A, Mason AB, Teng K. Exosome formation during maturation of mammalian and avian reticulocytes: evidence that exosome release is a major route for externalization of obsolete membrane proteins. J Cell Physiol. 1991;147:27–36. doi: 10.1002/jcp.1041470105. [DOI] [PubMed] [Google Scholar]
- 30.Baj-Krzyworzeka M, Szatanek R, Weglarczyk K, Baran J, Zembala M. Tumour-derived microvesicles modulate biological activity of human monocytes. Immunol Lett. 2007;113:76–82. doi: 10.1016/j.imlet.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 31.Wysoczynski M, Ratajczak MZ. Lung cancer secreted microvesicles: underappreciated modulators of microenvironment in expanding tumors. Int J Cancer. 2009;125:1595–603. doi: 10.1002/ijc.24479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Castellana D, Zobairi F, Martinez MC, Panaro MA, Mitolo V, Freyssinet JM, et al. Membrane microvesicles as actors in the establishment of a favorable prostatic tumoral niche: a role for activated fibroblasts and CX3CL1-CX3CR1 axis. Cancer Res. 2009;69:785–93. doi: 10.1158/0008-5472.CAN-08-1946. [DOI] [PubMed] [Google Scholar]
- 33.Fabbri M, Paone A, Calore F, Galli R, Gaudio E, Santhanam R, et al. MicroRNAs bind to Toll-like receptors to induce prometastatic inflammatory response. Proc Natl Acad Sci U S A. 2012;109:E2110–6. doi: 10.1073/pnas.1209414109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–9. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 35.Wienholds E, Plasterk RH. MicroRNA function in animal development. FEBS Lett. 2005;579:5911–22. doi: 10.1016/j.febslet.2005.07.070. [DOI] [PubMed] [Google Scholar]
- 36.Alvarez-Garcia I, Miska EA. MicroRNA functions in animal development and human disease. Development. 2005;132:4653–62. doi: 10.1242/dev.02073. [DOI] [PubMed] [Google Scholar]
- 37.Montecalvo A, Larregina AT, Shufesky WJ, Stolz DB, Sullivan ML, Karlsson JM, et al. Mechanism of transfer of functional microRNAs between mouse dendritic cells via exosomes. Blood. 2012;119:756–66. doi: 10.1182/blood-2011-02-338004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eiring AM, Harb JG, Neviani P, Garton C, Oaks JJ, Spizzo R, et al. miR-328 functions as an RNA decoy to modulate hnRNP E2 regulation of mRNA translation in leukemic blasts. Cell. 2010;140:652–65. doi: 10.1016/j.cell.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 40.Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e Sousa C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 2004;303:1529–31. doi: 10.1126/science.1093616. [DOI] [PubMed] [Google Scholar]
- 41.Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S, et al. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004;303:1526–9. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- 42.Heil F, Ahmad-Nejad P, Hemmi H, Hochrein H, Ampenberger F, Gellert T, et al. The Toll-like receptor 7 (TLR7)-specific stimulus loxoribine uncovers a strong relationship within the TLR7, 8 and 9 subfamily. Eur J Immunol. 2003;33:2987–97. doi: 10.1002/eji.200324238. [DOI] [PubMed] [Google Scholar]
- 43.Jurk M, Heil F, Vollmer J, Schetter C, Krieg AM, Wagner H, et al. Human TLR7 or TLR8 independently confer responsiveness to the antiviral compound R-848. Nat Immunol. 2002;3:499. doi: 10.1038/ni0602-499. [DOI] [PubMed] [Google Scholar]
- 44.Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5:749–59. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- 45.Pikarsky E, Porat RM, Stein I, Abramovitch R, Amit S, Kasem S, et al. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature. 2004;431:461–6. doi: 10.1038/nature02924. [DOI] [PubMed] [Google Scholar]
- 46.Rakoff-Nahoum S, Medzhitov R. Regulation of spontaneous intestinal tumorigenesis through the adaptor protein MyD88. Science. 2007;317:124–7. doi: 10.1126/science.1140488. [DOI] [PubMed] [Google Scholar]
- 47.Xiao H, Gulen MF, Qin J, Yao J, Bulek K, Kish D, et al. The Toll-interleukin-1 receptor member SIGIRR regulates colonic epithelial homeostasis, inflammation, and tumorigenesis. Immunity. 2007;26:461–75. doi: 10.1016/j.immuni.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 48.Huang B, Zhao J, Unkeless JC, Feng ZH, Xiong H. TLR signaling by tumor and immune cells: a double-edged sword. Oncogene. 2008;27:218–24. doi: 10.1038/sj.onc.1210904. [DOI] [PubMed] [Google Scholar]
- 49.Ikebe M, Kitaura Y, Nakamura M, Tanaka H, Yamasaki A, Nagai S, et al. Lipopolysaccharide (LPS) increases the invasive ability of pancreatic cancer cells through the TLR4/MyD88 signaling pathway. J Surg Oncol. 2009;100:725–31. doi: 10.1002/jso.21392. [DOI] [PubMed] [Google Scholar]
- 50.Killeen SD, Wang JH, Andrews EJ, Redmond HP. Exploitation of the Toll-like receptor system in cancer: a doubled-edged sword? Br J Cancer. 2006;95:247–52. doi: 10.1038/sj.bjc.6603275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cherfils-Vicini J, Platonova S, Gillard M, Laurans L, Validire P, Caliandro R, et al. Triggering of TLR7 and TLR8 expressed by human lung cancer cells induces cell survival and chemoresistance. J Clin Invest. 2010;120:1285–97. doi: 10.1172/JCI36551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kelly MG, Alvero AB, Chen R, Silasi DA, Abrahams VM, Chan S, et al. TLR-4 signaling promotes tumor growth and paclitaxel chemoresistance in ovarian cancer. Cancer Res. 2006;66:3859–68. doi: 10.1158/0008-5472.CAN-05-3948. [DOI] [PubMed] [Google Scholar]
- 53.Szajnik M, Szczepanski MJ, Czystowska M, Elishaev E, Mandapathil M, Nowak-Markwitz E, et al. TLR4 signaling induced by lipopolysaccharide or paclitaxel regulates tumor survival and chemoresistance in ovarian cancer. Oncogene. 2009;28:4353–63. doi: 10.1038/onc.2009.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huang B, Zhao J, Li H, He KL, Chen Y, Chen SH, et al. Toll-like receptors on tumor cells facilitate evasion of immune surveillance. Cancer Res. 2005;65:5009–14. doi: 10.1158/0008-5472.CAN-05-0784. [DOI] [PubMed] [Google Scholar]
- 55.Salaun B, Coste I, Rissoan MC, Lebecque SJ, Renno T. TLR3 can directly trigger apoptosis in human cancer cells. J Immunol. 2006;176:4894–901. doi: 10.4049/jimmunol.176.8.4894. [DOI] [PubMed] [Google Scholar]
- 56.Paone A, Starace D, Galli R, Padula F, De Cesaris P, Filippini A, et al. Toll-like receptor 3 triggers apoptosis of human prostate cancer cells through a PKC-alpha-dependent mechanism. Carcinogenesis. 2008;29:1334–42. doi: 10.1093/carcin/bgn149. [DOI] [PubMed] [Google Scholar]
- 57.Galli R, Starace D, Busà R, Angelini DF, Paone A, De Cesaris P, et al. TLR stimulation of prostate tumor cells induces chemokine-mediated recruitment of specific immune cell types. J Immunol. 2010;184:6658–69. doi: 10.4049/jimmunol.0902401. [DOI] [PubMed] [Google Scholar]
- 58.Conforti R, Ma Y, Morel Y, Paturel C, Terme M, Viaud S, et al. Opposing effects of toll-like receptor (TLR3) signaling in tumors can be therapeutically uncoupled to optimize the anticancer efficacy of TLR3 ligands. Cancer Res. 2010;70:490–500. doi: 10.1158/0008-5472.CAN-09-1890. [DOI] [PubMed] [Google Scholar]
- 59.Littman AJ, Thornquist MD, White E, Jackson LA, Goodman GE, Vaughan TL. Prior lung disease and risk of lung cancer in a large prospective study. Cancer Causes Control. 2004;15:819–27. doi: 10.1023/B:CACO.0000043432.71626.45. [DOI] [PubMed] [Google Scholar]
- 60.Ardies CM. Inflammation as cause for scar cancers of the lung. Integr Cancer Ther. 2003;2:238–46. doi: 10.1177/1534735403256332. [DOI] [PubMed] [Google Scholar]
- 61.Sidky YA, Borden EC, Weeks CE, Reiter MJ, Hatcher JF, Bryan GT. Inhibition of murine tumor growth by an interferon-inducing imidazoquinolinamine. Cancer Res. 1992;52:3528–33. [PubMed] [Google Scholar]
- 62.Hemmi H, Kaisho T, Takeuchi O, Sato S, Sanjo H, Hoshino K, et al. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol. 2002;3:196–200. doi: 10.1038/ni758. [DOI] [PubMed] [Google Scholar]
- 63.Schön M, Schön MP. The antitumoral mode of action of imiquimod and other imidazoquinolines. Curr Med Chem. 2007;14:681–7. doi: 10.2174/092986707780059625. [DOI] [PubMed] [Google Scholar]
- 64.Lee J, Chuang TH, Redecke V, She L, Pitha PM, Carson DA, et al. Molecular basis for the immunostimulatory activity of guanine nucleoside analogs: activation of Toll-like receptor 7. Proc Natl Acad Sci U S A. 2003;100:6646–51. doi: 10.1073/pnas.0631696100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wagner TL, Ahonen CL, Couture AM, Gibson SJ, Miller RL, Smith RM, et al. Modulation of TH1 and TH2 cytokine production with the immune response modifiers, R-848 and imiquimod. Cell Immunol. 1999;191:10–9. doi: 10.1006/cimm.1998.1406. [DOI] [PubMed] [Google Scholar]
- 66.Imbertson LM, Beaurline JM, Couture AM, Gibson SJ, Smith RM, Miller RL, et al. Cytokine induction in hairless mouse and rat skin after topical application of the immune response modifiers imiquimod and S-28463. J Invest Dermatol. 1998;110:734–9. doi: 10.1046/j.1523-1747.1998.00174.x. [DOI] [PubMed] [Google Scholar]
- 67.Reiter MJ, Testerman TL, Miller RL, Weeks CE, Tomai MA. Cytokine induction in mice by the immunomodulator imiquimod. J Leukoc Biol. 1994;55:234–40. doi: 10.1002/jlb.55.2.234. [DOI] [PubMed] [Google Scholar]
- 68.Weeks CE, Gibson SJ. Induction of interferon and other cytokines by imiquimod and its hydroxylated metabolite R-842 in human blood cells in vitro. J Interferon Res. 1994;14:81–5. doi: 10.1089/jir.1994.14.81. [DOI] [PubMed] [Google Scholar]
- 69.Gibson SJ, Imbertson LM, Wagner TL, Testerman TL, Reiter MJ, Miller RL, et al. Cellular requirements for cytokine production in response to the immunomodulators imiquimod and S-27609. J Interferon Cytokine Res. 1995;15:537–45. doi: 10.1089/jir.1995.15.537. [DOI] [PubMed] [Google Scholar]
- 70.Rettig L, Haen SP, Bittermann AG, von Boehmer L, Curioni A, Krämer SD, et al. Particle size and activation threshold: a new dimension of danger signaling. Blood. 2010;115:4533–41. doi: 10.1182/blood-2009-11-247817. [DOI] [PubMed] [Google Scholar]
- 71.Schiller M, Parcina M, Heyder P, Foermer S, Ostrop J, Leo A, et al. Induction of type I IFN is a physiological immune reaction to apoptotic cell-derived membrane microparticles. J Immunol. 2012;189:1747–56. doi: 10.4049/jimmunol.1100631. [DOI] [PubMed] [Google Scholar]
- 72.Lehmann SM, Krüger C, Park B, Derkow K, Rosenberger K, Baumgart J, et al. An unconventional role for miRNA: let-7 activates Toll-like receptor 7 and causes neurodegeneration. Nat Neurosci. 2012;15:827–35. doi: 10.1038/nn.3113. [DOI] [PubMed] [Google Scholar]
- 73.Solinas G, Germano G, Mantovani A, Allavena P. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J Leukoc Biol. 2009;86:1065–73. doi: 10.1189/jlb.0609385. [DOI] [PubMed] [Google Scholar]
- 74.Leek RD, Harris AL, Lewis CE. Cytokine networks in solid human tumors: regulation of angiogenesis. J Leukoc Biol. 1994;56:423–35. doi: 10.1002/jlb.56.4.423. [DOI] [PubMed] [Google Scholar]
- 75.Clark CE, Hingorani SR, Mick R, Combs C, Tuveson DA, Vonderheide RH. Dynamics of the immune reaction to pancreatic cancer from inception to invasion. Cancer Res. 2007;67:9518–27. doi: 10.1158/0008-5472.CAN-07-0175. [DOI] [PubMed] [Google Scholar]
- 76.Balkwill F, Coussens LM. Cancer: an inflammatory link. Nature. 2004;431:405–6. doi: 10.1038/431405a. [DOI] [PubMed] [Google Scholar]
- 77.Allavena P, Sica A, Garlanda C, Mantovani A. The Yin-Yang of tumor-associated macrophages in neoplastic progression and immune surveillance. Immunol Rev. 2008;222:155–61. doi: 10.1111/j.1600-065X.2008.00607.x. [DOI] [PubMed] [Google Scholar]
- 78.De Marzo AM, Platz EA, Sutcliffe S, Xu J, Grönberg H, Drake CG, et al. Inflammation in prostate carcinogenesis. Nat Rev Cancer. 2007;7:256–69. doi: 10.1038/nrc2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Moore PS, Chang Y. Why do viruses cause cancer? Highlights of the first century of human tumour virology. Nat Rev Cancer. 2010;10:878–89. doi: 10.1038/nrc2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Trinchieri G. Cancer and inflammation: an old intuition with rapidly evolving new concepts. Annu Rev Immunol. 2012;30:677–706. doi: 10.1146/annurev-immunol-020711-075008. [DOI] [PubMed] [Google Scholar]