Abstract
More than four decades ago, it was shown that RNA stably associates with chromatin. These studies indicated that chromatin-associated RNAs (caRNA) might be involved in the organization of chromatin structure. However, it is only recently that pools of chromatin-associated RNAs were characterized and functional studies were initiated. In Drosophila cells, an RNP complex consisting of snoRNAs and Decondensation factor 31 (Df31) is stably tethered to chromatin, mediated by the RNA- and histone-binding activities of Df31. Biochemical and functional characterizations suggest a structural role of this complex in chromatin organization. The binding of the Df31-snoRNA complex to chromatin results in the opening and the maintenance of accessible higher order structures of chromatin. We suggest that different classes of chromatin-associated RNPs are required for the targeted opening of higher order structures of chromatin, enabling the activation of DNA-dependent processes such as transcription.
Keywords: Chromatin-associated RNAs, snoRNAs, Decondensation factor 31, higher order structures of chromatin, chromatin organization, chromatin structure, euchromatin
Introduction
Chromatin represents the packaged and highly compacted form of our genome in the cell nucleus. The fundamental unit of chromatin is the nucleosome, composed of 147 bp of DNA wrapped around an octamer of H2A, H2B, H3 and H4 core histone proteins. Millions of nucleosomes are arranged like “pearls on a string,” separated by a short linker sequence. This nucleosomal chain is folded into several higher order levels of chromatin structure, further compacting the DNA.1-3 The structural organization of the different levels of higher order structures remains enigmatic; however, these structures obviously present a significant barrier for sequence-specific recognition, impeding the access of regulatory proteins to DNA. However, chromatin is the natural substrate for DNA-dependent processes, such as control of gene expression, DNA replication, recombination and repair.2,4-6 Therefore, active mechanisms to alter the higher order structures of chromatin to regulate DNA accessibility, such as the ATP-dependent chromatin remodeling of nucleosomes, must exist.
The co-fractionation of RNA molecules with chromatin was shown more than four decades ago. Chromatin isolated from different organisms, such as pea, calf, chicken and fruit fly, exhibited 2–10% of the total nucleic acids found in chromatin being stably associated RNA.7-11 Initially, it was suggested that these chromatin-associated RNAs (caRNAs) were nascent transcripts still being tethered to chromatin via RNA polymerase or contaminants from the isolation procedure.12,13 However, studies from the late 1970s described a possible role of these caRNAs in chromatin organization. It was hypothesized that caRNAs might play an activating role in the regulation of transcription.14 In this hypothesis, caRNA functions as an “activator” for the transcription of an “acceptor gene” by sequence-specific interactions between RNA and DNA, suggesting that “chromosomal RNAs may function as a sequence detector for chromosomal proteins”15. Recent studies shed light onto the function of caRNAs in chromatin organization. Chromatin-associated RNAs were isolated from different human cell lines, such as HeLa, human skin fibroblast and K562 cells. High throughput analyses revealed that a heterogeneous pool of RNAs was associated with chromatin. Furthermore, initial functional characterizations indicated roles for caRNAs in chromatin organization and in the regulation of genomic activity.16-18
We recently addressed the potential function of chromatin-associated RNA in Drosophila SL2 cells.19 The packaging of chromatin into the different levels of higher order structures can be partially assessed by its sensitivity to endonucleases. Compact chromatin is less accessible to hydrolysis by the endonuclease micrococcal nuclease (MNase), an enzyme that preferentially hydrolyses DNA with a preference for accessible open chromatin structures.20 We initiated our study by asking how depletion of nuclear RNA would influence MNase-dependent chromatin accessibility. To our surprise, chromatin formed a heavily compacted structure in the absence of RNA, suggesting an important role of RNA in maintaining open chromatin structures. We were able to reconstitute a close to native chromatin system in vitro, including chromatin-associated RNA, using a Drosophila embryo extract. Using this in vitro system, we could show the reversible effect of RNA withdrawal and addition on the opening and closing of the higher order structures of chromatin. Decreased MNase accessibility of chromatin directly correlated with RNA depletion and was shown to depend on a change in the chromatin conformation. The in vitro system was used to identify the players that modulate changes in higher order structures of chromatin. The chromatin-associated RNAs were identified by high-throughput sequencing. RNA analysis revealed that a fraction of low-abundant snoRNAs is bound to chromatin (46 out of 186 in the transcriptome). Individual snoRNAs of this pool were shown to be capable of opening higher order structures of chromatin in the in vitro assay. In addition, two of these snoRNAs, MeS28-U2134b and MeS28-G980, were verified by RNA FISH experiments and could be identified in the active, euchromatic interbands of Drosophila polytene chromosomes.
The quantitative mass spectrometry approach identified 59 proteins with the potential to interact in an RNA-dependent manner. One of the most interesting candidates was Decondensation factor 31 (Df31), which was identified in a Dam-ID screen to be exclusively present at euchromatic regions.21 The knock down of Df31 in Sl2 cells resulted in chromatin compaction. In vitro assays identified the capability of this protein to bind single stranded RNA, with a preference for the snoRNAs, and we showed that Df31 preferentially binds to H3 in vitro. The interaction of Df31 to histone octamers is stabilized by RNA, as indicated by pull-down experiments. Thus, Df31 forms an RNA-dependent complex tethered to chromatin in vivo and in vitro, suggesting that this snoRNA-Df31 complex establishes and maintains accessible euchromatic domains. Further studies will address the molecular mechanisms and ask whether the RNP complexes initiate chromatin opening and/or if they are required to maintain an accessible structure.
Novel snoRNP Complexes and Their Chromatin Specific Functions
We hypothesize that the targeting of snoRNP complexes to chromatin distorts the higher order structures of chromatin, resulting in an open structure that allows the binding of regulatory factors (Fig. 1). In our study, we identified one complex consisting of Df31 and snoRNA, however, we suggest that many different complexes consisting of Df31-like proteins and specific RNA molecules exist that target specific genomic regions to establish an accessible chromatin configuration. The snoRNAs would be stably tethered to chromatin by specific proteins simultaneously binding to RNA and chromatin, such as Df31 (Fig. 1). Evidence supporting this assumption is the fact that the snoRNAs tested in FISH experiments target specific loci on Drosophila polytene chromosomes. The targeting mechanism of the snoRNP complex remains unclear. Df31 was shown to bind to the genomic regions characterized as red and yellow chromatin, two distinct forms of euchromatin.21 Df31 interacts with histone H3 and could be recruited by specific posttranslational modifications. The mechanism of chromatin opening is potentially initiated by the cooperative binding of the snoRNP complex to chromatin, forming an RNP network that interferes with the ordered folding of higher order structures, an effect observed in our in vitro assays (Fig. 1). The question whether these RNP complexes are involved in chromatin opening and/or maintain chromatin accessible has to be addressed in further studies.
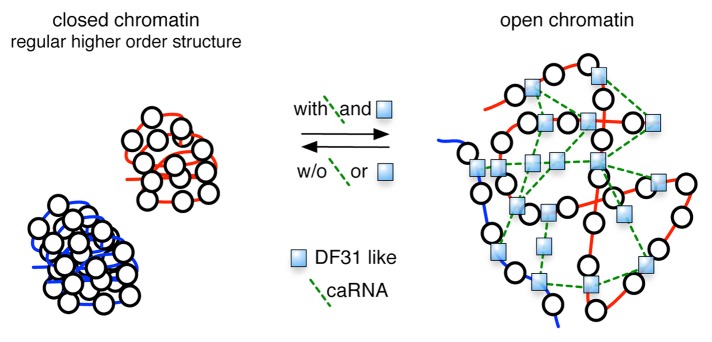
Figure 1. Model depicting the function of the chromatin associated RNP complex on the higher order structure of chromatin. Different complexes consisting of Df31-like proteins and specific RNA molecules exist that target specific genomic regions to establish an accessible chromatin configuration. The RNA and chromatin binding proteins like Df31 could tether the snoRNAs stably to chromatin. The mechanism of chromatin opening is potentially initiated by the cooperative binding of the snoRNP complex to chromatin, forming an RNP network that interferes with the ordered folding of higher order structures.
RNAs Regulating Chromatin Organization and Genomic Activity
Despite the snoRNAs, characterized in our studies, which influence chromatin higher order structures, several other RNA species were shown to regulate chromatin organization and to enhance gene activity (Table 1). Exemplarily, a fraction of long ncRNAs in human fibroblasts, called CARs, was shown to enhance the activity of nearby genes in cis.16 Furthermore, non-coding transcripts originating from transcribed enhancers (eRNAs) were shown being markers for the activity of adjacent genes in mouse neurons.22-25 Another fraction of human long ncRNAs, called ncRNA-a, was identified to possess enhancer like functions, thereby increasing target gene activity in cis and trans.26 The lincRNA HOTTIP was shown to recruit a transcriptional activator complex to human target genes.27 Similarly, the roX-RNAs are part of a RNP-complex enabling hypertranscription of the single male x-chromosome in dosage compensation in Drosophila.28,29 Furthermore, the HSR1 ncRNA was shown to change the localization and activity of the HSF1 transcription factor upon heat shock in mammals.30 The examples show that non-coding RNAs employ different mechanisms to regulate gene activity and global chromatin structure. However, also coding RNAs seem to regulate gene activity as ciRNAs were detected to be part of transcription factories in human and mouse.18,31 Our recent study amplified the set of RNAs influencing gene activity by the snoRNAs and add an additional mechanism in that RNP-complexes directly alter the compaction state of higher order structures of chromatin.19
Table 1. RNAs influencing chromatin organization thereby enhancing gene activity.
| RNA name | Length | Properties | Organisms | Influence on chromatin organization | Publications |
|---|---|---|---|---|---|
| CARs |
Long |
ncRNA |
Human |
The presence of the CAR Intergenic10 positively correlates with the dimethylation of H3K4 at gene promoters and gene transcription. |
16
|
| Enhancer RNAs |
50–2000 nt |
ncRNA |
Mouse |
The eRNA dependent looping between enhancer and promotor positively influences transcription of adjacent genes. |
22
-
25
|
| ncRNA-a |
Long |
ncRNAs, capped, poly A |
Human |
The expression of target genes is potentiated in a ncRNA-a dependent manner. |
26
|
| HOTTIP (lincRNA) |
3764 nt |
ncRNA, capped, poly A |
Human |
Chromosomal looping brings HOTTIP into close proximity to its target genes. HOTTIP RNA binds the adaptor protein WDR5 driving histone H3 lysine 4 trimethylation and gene transcription. |
27
|
| roX RNAs |
600–3700 nt |
ncRNA |
Drosophila |
RNA-dependent recruitment of the activator MSL-complex leads to changes in histone marks resulting in enhanced x-chromosomal transcription. |
28
,
29
|
| HSR1 | Long | ncRNA | Mammals | HSR1 changes the localization and activity of the activating TF (HSF1) thereby enhancing gene activity. | 30 |
snoRNAs Acting on Chromatin
So far, the highly abundant and well-structured snoRNA molecules were shown to play a role in RNA editing and ribosome biogenesis.32 In eukaryotes, two specific modifications, 2'-O-methylation and pseudouridinilation, are directed by the box C/D and H/ACA snoRNAs.33
Potential new functions of the snoRNAs may arise from several high throughput RNA sequencing studies that revealed the tight association of snoRNAs and other RNA species with chromatin.16,18 In addition, several protein components of C/D snoRNPs were linked with chromatin remodeling and with transcription. The yeast homologs of the human snoRNP proteins p55 and p50, called Rvb1 and Rvb2, have been shown to form a dimer with ATPase activity in vitro and to possess chromatin-remodeling activity in vivo. These proteins affect the transcription of 5% of the yeast genome.34-36 In addition, Rvb1p and Rvb2p are components of the chromatin-remodeling complex INO80 that is involved in transcription regulation and genomic stability.37,38 Human p50 and p55 were also found to interact with the histone acetylase TIP60, acetylating nucleosomes in vitro and possessing ATPase and DNA helicase activity.39 Furthermore, the well-described snoRNA binding proteins Nop56p/Nop58p interact with matrix-attachment regions (MARs) in plants, which are thought to organize chromatin domains.40 Additionally, p55 was identified in the MARs, suggesting a function of the snoRNA-binding proteins in the structural organization of chromatin.41 A link to transcription activation is provided by the association of rat p55 with TBP and RNA polymerase II.42,43
Protein components of the snoRNA-containing RNP complexes were identified in chromatin-specific processes, raising the possibility that they function in conjunction with the snoRNA. However, direct evidence for the presence of the snoRNAs is missing, and it is possible that the proteins function in absence of the RNA molecules. With respect to the chromatin-specific function of snoRNAs, it is possible that the chromatin-associated snoRNAs form novel RNP complexes containing chromatin-specific proteins, replacing the classical snoRNA-binding proteins.
Potential Mechanisms of caRNA-Mediated Chromatin Opening
Further studies are needed to address the mechanism of chromatin opening by the snoRNA-containing complexes. We envision five potential ways by which this snoRNA-Df31 complex may function. (1) The 30 nm fiber, representing the first level of higher order structure of chromatin, is stabilized by internucleosomal interactions. The interaction of the histone H4 N-terminal domain (NTD) with a “charge patch” on the surface of H2A represents one type of such direct internucleosomal contact.44,45 The binding of Df31 to the H3 tail of the nucleosome that is near the H4 NTD may disturb these internucleosomal interactions by steric hindrance and indirectly impair the folding of chromatin.46,47 It was previously shown that histone NTDs do specifically bind to the sugar-phosphate backbone of nucleic acids.48 Therefore, the presence of the snoRNA in the complex could present a binding site for the histone NTDs, resulting in the stabilization of snoRNA-Df31 binding with chromatin and the distortion of internucleosomal interactions. (2) The histone H3 NTD also contributes to the formation of the higher order structures of chromatin by stabilizing the 30 nm fiber through interarray interactions.49-51 The known interaction of Df31 with this histone tail would destabilize the higher order structures of chromatin. (3) The higher order structures of chromatin correlate with the presence of specific histone modifications and proteins, such as HP1 and H1, associated with chromatin. Df31 was shown to interact with Su(var)3–9, which acts with H3K9 methyltransferase in heterochromatin formation.52 On one hand, Df31 could bind Su(var)3–9 and prevent histone H3K9 methylation, which is necessary for the HP1-mediated chromatin compaction.53 On the other hand, the highly abundant Df31 may compete with Su(var)3–9 for histone binding because both proteins target the H3 tail.
Df31 was also shown to interact with the chromatin-remodeling enzyme Iswi, an enzyme that is required for the compaction and H1 incorporation of chromosomes in Drosophila.52,54,55 The highly abundant Df31 protein could compete with H1 for Iswi binding or modulate the activity of the enzyme, thereby impairing the local assembly of heterochromatin. (4) Two additional features of Df31 could contribute to its chromatin-opening activity. First, it was shown that the protein contains histone-chaperoning activity, and second, Df31 is capable of mediating interstrand bridging.46,56 Both activities could introduce irregularities into the chromatin fiber and destabilize the higher order structures of chromatin. Such irregularities are present in euchromatic regions, exhibiting nucleosome free regions and irregularly spaced nucleosomes.57 (5) Cooperative binding of Df31 and RNA to chromatin in vitro, together with the interstrand bridging activity of Df31, suggest the spreading of the snoRNA-RNP within a targeted locus. The formation of a RNP network could stably maintain the accessibility of higher order structures of a larger chromatin domain, enabling DNA-dependent processes, such as transcription and replication (Fig. 1).
Glossary
Abbreviations:
- caRNA
chromatin-associated RNA
- snoRNA
small nucleolar RNA
- MNase
micrococcal nuclease
- MARs
matrix attached regions
- NTD
N-terminal domain
- caRNA
chromatin-associated RNA
- ciRNA
chromatin-interacting RNA
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/rnabiology/article/23175
References
- 1.Li G, Reinberg D. Chromatin higher-order structures and gene regulation. Curr Opin Genet Dev. 2011;21:175–86. doi: 10.1016/j.gde.2011.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Woodcock CL, Ghosh RP. Chromatin higher-order structure and dynamics. Cold Spring Harb Perspect Biol. 2010;2:a000596. doi: 10.1101/cshperspect.a000596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McGhee JD, Rau DC, Charney E, Felsenfeld G. Orientation of the nucleosome within the higher order structure of chromatin. Cell. 1980;22:87–96. doi: 10.1016/0092-8674(80)90157-9. [DOI] [PubMed] [Google Scholar]
- 4.van-Holde KE. Chromatin. Springer Verlag 1989:497. [Google Scholar]
- 5.Felsenfeld G, Groudine M. Controlling the double helix. Nature. 2003;421:448–53. doi: 10.1038/nature01411. [DOI] [PubMed] [Google Scholar]
- 6.Khorasanizadeh S. The nucleosome: from genomic organization to genomic regulation. Cell. 2004;116:259–72. doi: 10.1016/S0092-8674(04)00044-3. [DOI] [PubMed] [Google Scholar]
- 7.Bonner J, Widholm J. Molecular complementarity between nuclear DNA and organ-specific chromosomal RNA. Proc Natl Acad Sci U S A. 1967;57:1379–85. doi: 10.1073/pnas.57.5.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holoubek V, Deacon NJ, Buckle DW, Naora H. A small chromatin-associated RNA homologous to repetitive DNA sequences. Eur J Biochem. 1983;137:249–56. doi: 10.1111/j.1432-1033.1983.tb07822.x. [DOI] [PubMed] [Google Scholar]
- 9.Huang RC, Bonner J. Histone-bound RNA, a component of native nucleohistone. Proc Natl Acad Sci U S A. 1965;54:960–7. doi: 10.1073/pnas.54.3.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang RC, Huang PC. Effect of protein-bound RNA associated with chick embryo chromatin on template specificity of the chromatin. J Mol Biol. 1969;39:365–78. doi: 10.1016/0022-2836(69)90323-4. [DOI] [PubMed] [Google Scholar]
- 11.Bynum JW, Volkin E. Chromatin-associated RNA: differential extraction and characterization. Biochim Biophys Acta. 1980;607:304–18. doi: 10.1016/0005-2787(80)90083-0. [DOI] [PubMed] [Google Scholar]
- 12.Artman M, Roth JS. Chromosomal RNA: an artifact of preparation? J Mol Biol. 1971;60:291–301. doi: 10.1016/0022-2836(71)90295-6. [DOI] [PubMed] [Google Scholar]
- 13.Bonner J. Problematic chromosomal RNA. Nature. 1971;231:543–4. doi: 10.1038/231543c0. [DOI] [PubMed] [Google Scholar]
- 14.Britten RJ, Davidson EH. Gene regulation for higher cells: a theory. Science. 1969;165:349–57. doi: 10.1126/science.165.3891.349. [DOI] [PubMed] [Google Scholar]
- 15.Bonner J, Dahmus ME, Fambrough D, Huang RC, Marushige K, Tuan DY. The Biology of Isolated Chromatin: Chromosomes, biologically active in the test tube, provide a powerful tool for the study of gene action. Science. 1968;159:47–56. doi: 10.1126/science.159.3810.47. [DOI] [PubMed] [Google Scholar]
- 16.Mondal T, Rasmussen M, Pandey GK, Isaksson A, Kanduri C. Characterization of the RNA content of chromatin. Genome Res. 2010;20:899–907. doi: 10.1101/gr.103473.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tilgner H, Knowles DG, Johnson R, Davis CA, Chakrabortty S, Djebali S, et al. Deep sequencing of subcellular RNA fractions shows splicing to be predominantly co-transcriptional in the human genome but inefficient for lncRNAs. Genome Res. 2012;22:1616–25. doi: 10.1101/gr.134445.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caudron-Herger M, Müller-Ott K, Mallm JP, Marth C, Schmidt U, Fejes-Tóth K, et al. Coding RNAs with a non-coding function: maintenance of open chromatin structure. Nucleus. 2011;2:410–24. doi: 10.4161/nucl.2.5.17736. [DOI] [PubMed] [Google Scholar]
- 19.Schubert T, Pusch MC, Diermeier S, Benes V, Kremmer E, Imhof A, et al. Df31 Protein and snoRNAs Maintain Accessible Higher-Order Structures of Chromatin. Mol Cell. 2012;48:434–44. doi: 10.1016/j.molcel.2012.08.021. [DOI] [PubMed] [Google Scholar]
- 20.Henikoff JG, Belsky JA, Krassovsky K, MacAlpine DM, Henikoff S. Epigenome characterization at single base-pair resolution. Proc Natl Acad Sci U S A. 2011;108:18318–23. doi: 10.1073/pnas.1110731108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Filion GJ, van Bemmel JG, Braunschweig U, Talhout W, Kind J, Ward LD, et al. Systematic protein location mapping reveals five principal chromatin types in Drosophila cells. Cell. 2010;143:212–24. doi: 10.1016/j.cell.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim TK, Hemberg M, Gray JM, Costa AM, Bear DM, Wu J, et al. Widespread transcription at neuronal activity-regulated enhancers. Nature. 2010;465:182–7. doi: 10.1038/nature09033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Djebali S, Davis CA, Merkel A, Dobin A, Lassmann T, Mortazavi A, et al. Landscape of transcription in human cells. Nature. 2012;489:101–8. doi: 10.1038/nature11233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang D, Garcia-Bassets I, Benner C, Li W, Su X, Zhou Y, et al. Reprogramming transcription by distinct classes of enhancers functionally defined by eRNA. Nature. 2011;474:390–4. doi: 10.1038/nature10006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanyal A, Lajoie BR, Jain G, Dekker J. The long-range interaction landscape of gene promoters. Nature. 2012;489:109–13. doi: 10.1038/nature11279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ørom UA, Derrien T, Beringer M, Gumireddy K, Gardini A, Bussotti G, et al. Long noncoding RNAs with enhancer-like function in human cells. Cell. 2010;143:46–58. doi: 10.1016/j.cell.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang KC, Yang YW, Liu B, Sanyal A, Corces-Zimmerman R, Chen Y, et al. A long noncoding RNA maintains active chromatin to coordinate homeotic gene expression. Nature. 2011;472:120–4. doi: 10.1038/nature09819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Amrein H, Axel R. Genes expressed in neurons of adult male Drosophila. Cell. 1997;88:459–69. doi: 10.1016/S0092-8674(00)81886-3. [DOI] [PubMed] [Google Scholar]
- 29.Franke A, Baker BS. The rox1 and rox2 RNAs are essential components of the compensasome, which mediates dosage compensation in Drosophila. Mol Cell. 1999;4:117–22. doi: 10.1016/S1097-2765(00)80193-8. [DOI] [PubMed] [Google Scholar]
- 30.Shamovsky I, Ivannikov M, Kandel ES, Gershon D, Nudler E. RNA-mediated response to heat shock in mammalian cells. Nature. 2006;440:556–60. doi: 10.1038/nature04518. [DOI] [PubMed] [Google Scholar]
- 31.Cook PR. A model for all genomes: the role of transcription factories. J Mol Biol. 2010;395:1–10. doi: 10.1016/j.jmb.2009.10.031. [DOI] [PubMed] [Google Scholar]
- 32.Bachellerie JP, Cavaillé J, Hüttenhofer A. The expanding snoRNA world. Biochimie. 2002;84:775–90. doi: 10.1016/S0300-9084(02)01402-5. [DOI] [PubMed] [Google Scholar]
- 33.Watkins NJ, Bohnsack MT. The box C/D and H/ACA snoRNPs: key players in the modification, processing and the dynamic folding of ribosomal RNA. Wiley Interdiscip Rev RNA. 2012;3:397–414. doi: 10.1002/wrna.117. [DOI] [PubMed] [Google Scholar]
- 34.Jónsson ZO, Dhar SK, Narlikar GJ, Auty R, Wagle N, Pellman D, et al. Rvb1p and Rvb2p are essential components of a chromatin remodeling complex that regulates transcription of over 5% of yeast genes. J Biol Chem. 2001;276:16279–88. doi: 10.1074/jbc.M011523200. [DOI] [PubMed] [Google Scholar]
- 35.King TH, Decatur WA, Bertrand E, Maxwell ES, Fournier MJ. A well-connected and conserved nucleoplasmic helicase is required for production of box C/D and H/ACA snoRNAs and localization of snoRNP proteins. Mol Cell Biol. 2001;21:7731–46. doi: 10.1128/MCB.21.22.7731-7746.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Avner P, Heard E. X-chromosome inactivation: counting, choice and initiation. Nat Rev Genet. 2001;2:59–67. doi: 10.1038/35047580. [DOI] [PubMed] [Google Scholar]
- 37.Papamichos-Chronakis M, Watanabe S, Rando OJ, Peterson CL. Global regulation of H2A.Z localization by the INO80 chromatin-remodeling enzyme is essential for genome integrity. Cell. 2011;144:200–13. doi: 10.1016/j.cell.2010.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shen X, Mizuguchi G, Hamiche A, Wu C. A chromatin remodelling complex involved in transcription and DNA processing. Nature. 2000;406:541–4. doi: 10.1038/35020123. [DOI] [PubMed] [Google Scholar]
- 39.Ikura T, Ogryzko VV, Grigoriev M, Groisman R, Wang J, Horikoshi M, et al. Involvement of the TIP60 histone acetylase complex in DNA repair and apoptosis. Cell. 2000;102:463–73. doi: 10.1016/S0092-8674(00)00051-9. [DOI] [PubMed] [Google Scholar]
- 40.Hatton D, Gray JC. Two MAR DNA-binding proteins of the pea nuclear matrix identify a new class of DNA-binding proteins. Plant J. 1999;18:417–29. doi: 10.1046/j.1365-313X.1999.00468.x. [DOI] [PubMed] [Google Scholar]
- 41.Holzmann K, Gerner C, Korosec T, Pöltl A, Grimm R, Sauermann G. Identification and characterization of the ubiquitously occurring nuclear matrix protein NMP 238. Biochem Biophys Res Commun. 1998;252:39–45. doi: 10.1006/bbrc.1998.9604. [DOI] [PubMed] [Google Scholar]
- 42.Kanemaki M, Makino Y, Yoshida T, Kishimoto T, Koga A, Yamamoto K, et al. Molecular cloning of a rat 49-kDa TBP-interacting protein (TIP49) that is highly homologous to the bacterial RuvB. Biochem Biophys Res Commun. 1997;235:64–8. doi: 10.1006/bbrc.1997.6729. [DOI] [PubMed] [Google Scholar]
- 43.Qiu XB, Lin YL, Thome KC, Pian P, Schlegel BP, Weremowicz S, et al. An eukaryotic RuvB-like protein (RUVBL1) essential for growth. J Biol Chem. 1998;273:27786–93. doi: 10.1074/jbc.273.43.27786. [DOI] [PubMed] [Google Scholar]
- 44.Dorigo B, Schalch T, Bystricky K, Richmond TJ. Chromatin fiber folding: requirement for the histone H4 N-terminal tail. J Mol Biol. 2003;327:85–96. doi: 10.1016/S0022-2836(03)00025-1. [DOI] [PubMed] [Google Scholar]
- 45.Dorigo B, Schalch T, Kulangara A, Duda S, Schroeder RR, Richmond TJ. Nucleosome arrays reveal the two-start organization of the chromatin fiber. Science. 2004;306:1571–3. doi: 10.1126/science.1103124. [DOI] [PubMed] [Google Scholar]
- 46.Guillebault D, Cotterill S. The Drosophila Df31 protein interacts with histone H3 tails and promotes chromatin bridging in vitro. J Mol Biol. 2007;373:903–12. doi: 10.1016/j.jmb.2007.07.049. [DOI] [PubMed] [Google Scholar]
- 47.Gordon F, Luger K, Hansen JC. The core histone N-terminal tail domains function independently and additively during salt-dependent oligomerization of nucleosomal arrays. J Biol Chem. 2005;280:33701–6. doi: 10.1074/jbc.M507048200. [DOI] [PubMed] [Google Scholar]
- 48.Arya G, Zhang Q, Schlick T. Flexible histone tails in a new mesoscopic oligonucleosome model. Biophys J. 2006;91:133–50. doi: 10.1529/biophysj.106.083006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kan PY, Lu X, Hansen JC, Hayes JJ. The H3 tail domain participates in multiple interactions during folding and self-association of nucleosome arrays. Mol Cell Biol. 2007;27:2084–91. doi: 10.1128/MCB.02181-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zheng C, Lu X, Hansen JC, Hayes JJ. Salt-dependent intra- and internucleosomal interactions of the H3 tail domain in a model oligonucleosomal array. J Biol Chem. 2005;280:33552–7. doi: 10.1074/jbc.M507241200. [DOI] [PubMed] [Google Scholar]
- 51.Yang Z, Zheng C, Thiriet C, Hayes JJ. The core histone N-terminal tail domains negatively regulate binding of transcription factor IIIA to a nucleosome containing a 5S RNA gene via a novel mechanism. Mol Cell Biol. 2005;25:241–9. doi: 10.1128/MCB.25.1.241-249.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guruharsha KG, Rual JF, Zhai B, Mintseris J, Vaidya P, Vaidya N, et al. A protein complex network of Drosophila melanogaster. Cell. 2011;147:690–703. doi: 10.1016/j.cell.2011.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schotta G, Ebert A, Reuter GSU. SU(VAR)3-9 is a conserved key function in heterochromatic gene silencing. Genetica. 2003;117:149–58. doi: 10.1023/A:1022923508198. [DOI] [PubMed] [Google Scholar]
- 54.Corona DF, Siriaco G, Armstrong JA, Snarskaya N, McClymont SA, Scott MP, et al. ISWI regulates higher-order chromatin structure and histone H1 assembly in vivo. PLoS Biol. 2007;5:e232. doi: 10.1371/journal.pbio.0050232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Siriaco G, Deuring R, Chioda M, Becker PB, Tamkun JW. Drosophila ISWI regulates the association of histone H1 with interphase chromosomes in vivo. Genetics. 2009;182:661–9. doi: 10.1534/genetics.109.102053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Worby CA, Simonson-Leff N, Dixon JE. RNA interference of gene expression (RNAi) in cultured Drosophila cells. Sci STKE. 2001;2001:pl1. doi: 10.1126/stke.2001.95.pl1. [DOI] [PubMed] [Google Scholar]
- 57.Mavrich TN, Jiang C, Ioshikhes IP, Li X, Venters BJ, Zanton SJ, et al. Nucleosome organization in the Drosophila genome. Nature. 2008;453:358–62. doi: 10.1038/nature06929. [DOI] [PMC free article] [PubMed] [Google Scholar]


