Abstract
The spliceosomal component U1snRNP commits pre-mRNAs to the splicing pathway. Recently, a nuclear RNA surveillance function has been ascribed to U1, namely the suppression of intronic polyadenylation sites. This surveillance holds regulatory potential as it alters the 3′ ends of certain receptor tyrosine kinase mRNAs. However, suppression of 3′ end processing by U1 snRNP is also the cause of a severe genetic disorder. We described a 3′UTR point mutation creating a 5′SS leading to U1-mediated suppression of 3′ end formation. Thus, the inhibitory function of U1 is both beneficial and deleterious where misled. The exact mechanism of how U1 interferes with 3′ end processing remains unclear. According to our data, U1 snRNP already interferes with cleavage or poly(A) site selection instead of directly inhibiting poly(A) polymerase as previously assumed. Here, we present alternative models for U1-mediated poly(A) site suppression and discuss the implications for RNA quality control and disease-related mutations.
Keywords: 3′ UTR, RNA processing, U1 site, genetic disease, polyadenylation
Results and Discussion
Splicing is executed by a macromolecular machinery termed spliceosome. This apparatus is not recruited to the mRNA en bloc but rather undergoes stepwise assembly to form a catalytically active complex.1 Although the active spliceosome represents a 1:1 stoichiometry regarding its core components, their overall concentrations vary substantially in the nucleus.2 Commitment to the splicing pathway starts with binding of the uridine-rich small nuclear ribonucleoprotein 1 (U1 snRNP) to the 5′ splice sites (SS) via RNA-RNA-interaction.1 Interestingly, U1 snRNP is present in large excess over the other snRNPs.2 While this has been known for many years, conclusive explanations were missing. In addition, 13 y after its initial identification3 a new role for U1 snRNP emerged, i.e. interference with 3′ end processing first demonstrated in bovine papilloma virus (BPV; ref. 4). The virus uses this strategy to inhibit the expression of the structural (late) genes in the early phase of infection.5
3′ end formation is initiated by 3′ end processing factors binding to conserved sequence elements. Subsequent cleavage of the transcript is followed by poly(A) tail addition.6 The key element is the AAUAAA hexamer representing the polyadenylation signal (PAS), which is recognized by the cleavage and polyadenylation specificity factor (CPSF). Upon interaction with the cleavage-stimulating factor (CstF), which binds to the downstream sequence element (DSE), cleavage of the transcript is executed by a subunit of CPSF preferentially at a CA dinucleotide ~20–25 nts downstream of the PAS.7 After cleavage, a poly(A) tail is added to the nascent transcript in an untemplated reaction by the poly(A) polymerase (PAP; ref. 8).
In addition to the elements mentioned above, other auxiliary motifs are described to influence 3′ end processing. The most common one is the upstream sequence element (USE), which enhances 3′ end processing by recruiting splicing factors such as the U2 snRNP associated factor (U2AF) or hnRNP I (PTB; ref. 9).
While most of the splicing factors, which are also involved in 3′ end processing, have beneficial effects,7 U1 snRNP binding to the 3′ untranslated region (UTR) suppresses 3′ end formation and thereby decreases mRNA quantity in bovine and human papilloma viruses.4,10 This effect of U1 snRNP was shown to be independent of splicing, but requires base pairing of U1 snRNA with a 5′SS located in proximity of the PAS.4 Also, human immunodeficiency virus (HIV) depends on U1 snRNP-mediated suppression of 3′ end processing. Retroviruses harbor duplicated terminal repeats, including a duplication of the PAS. The promoter proximal HIV 5′SS is used to suppress the upstream PAS to ensure full-length transcription of the HIV genome.11 Furthermore, U1 snRNP participates in the autoregulation of U1A expression by binding to the U1A 3′UTR.12
U1 snRNP-mediated suppression was even utilized in gene silencing experiments by recruitment of U1 snRNP to specific target sites located in the 3′UTR of any gene of interest via synthetic U1 adaptors.13
Novel data on U1 snRNP interference with the cleavage/polyadenylation machinery was published 2 y ago by the Dreyfuss laboratory demonstrating that U1 snRNP is able to suppress the usage of intronic PAS to prevent premature cleavage and polyadenylation (PCPA) and the formation of truncated transcripts.14 In the case of tyrosine kinase receptors, PCPA may serve a regulatory function by producing shortened mRNAs. In some of these mRNAs one or more exons encoding the C terminus of the receptors are missing, leading to constitutively active receptors, which are related to particular types of cancer.15
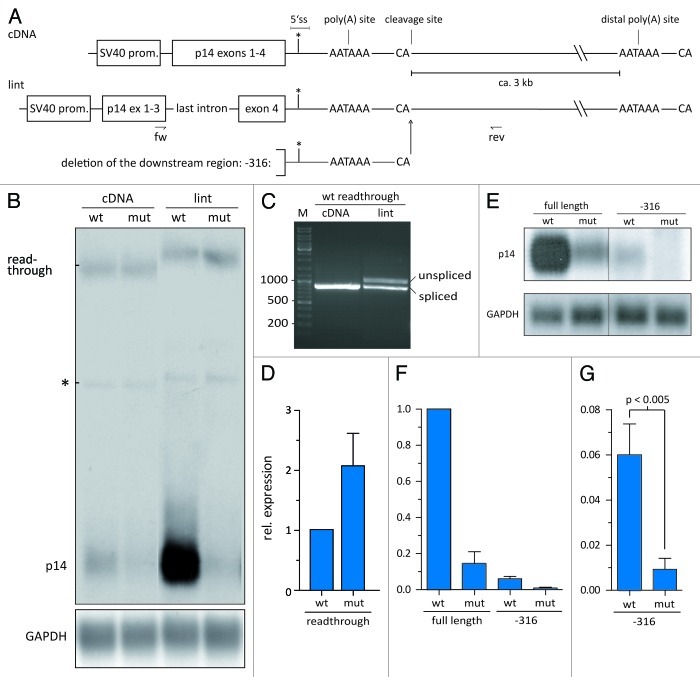
The other side of this type of quality control is represented by a point mutation creating an U1 snRNP binding site in the 3′UTR of the p14/robld3 gene, which leads to a severe immunodeficiency syndrome.16 U1 snRNP-mediated PAS suppression, also in the case of p14, is independent of splicing, but rather interferes with 3′ end processing.16 In our experimental system, we took advantage of minigenes consisting of p14 cDNA, the 3′UTR plus part of the downstream genomic region (Fig. 1A). This system can recapitulate the effect of the mutation (Fig. 1B). However, we did not observe the same extend of suppression as in patient’s cells.17 Taken into account the coupling of terminal intron splicing and 3′ end processing,18 we included the last intron of p14 (Fig. 1A) and observed a strong upregulation of wild type p14 mRNA expression (Fig. 1B). However, the mutated p14 mRNA levels harboring the 3′UTR with the U1 site are as low as in the cDNA context. The intron-containing minigenes now recapitulate the same downregulation as observed in patient-derived cells (Fig. 1B; ref. 17). In fact, p14 expression is as dependent on terminal intron splicing as β-globin mRNA.19,20 U1 snRNP binding does not inhibit splicing of the terminal intron (Fig. 1B), but we recognized a higher migrating band for the read-through RNA (Fig. 1A and B). A closer examination of this RNA species revealed a mixture of spliced and unspliced read-through transcripts (Fig. 1C). The mechanistic details of this finding are currently under investigation. A quantification of the read-through RNA shows a 2-fold increase in case of the mutation (Fig. 1D). Also, the amount of unprocessed, unspliced RNA at the proximal p14-specific PAS increased by 1.5-fold.16 Thus, skipping of the natural p14 PAS cannot fully account for the observed reduction in RNA levels (Fig. 1B and D) and a substantial amount of p14 RNA must be degraded after failure of 3′ end processing. In summary, the strong positive effect of terminal intron splicing on 3′ end processing is dismissed in case of U1 binding.
Figure 1. U1-mediated PAS suppression in the p14 minigene context. (A) The p14 minigene construct is depicted displaying the authentic p14 PAS and a distal PAS ca. 3 kb downstream. The location of the U1 snRNP binding site, i.e., a 5′ splice sites (5′SS) is indicated by a vertical line. The mutation is marked by an asterisk. From top to bottom, the p14 cDNA, last intron (lint) and the deletion construct (-316) are shown. (B) Northern blot using total RNA from HeLa cells transiently transfected with the indicated constructs. The p14 RNA is indicated on the left. The read-through band, which is processed at the distal PAS is named. The asterisk indicates a DNA contamination. GAPDH serves as a loading control. (C) RT-PCR performed on poly(A)+-mRNA isolated from HeLa cells transfected with the indicated constructs. The positions of the primers are given by arrows in (A; middle panel). (D) Quantification of the read-through band by phosphoimager analysis. The wild-type was set to 1. Mean and standard deviation represents the results of three independent experiments. (E) Northern blot performed as in (B) comparing the -316 deletion mutant to the wild-type and the mutated constructs. (F and G) Quantification of the northern blot in (E) by phosphoimager analyses. The wild-type was set to 1. (G) Visualizes the effect of the mutation in the -316 deletion in a higher resolution. Means and standard deviations represent the results of three independent experiments.
Another question is if regulatory sequences downstream of the cleavage site (CS) are involved in U1-mediated suppression. HPV contains four partially overlapping weak U1 snRNP binding sites in the 3′ UTR that inhibit expression of the late genes. Auxiliary CUGBP1 binding sites are present downstream of the U1 cluster. They enhance U1 snRNP binding and, thus, enforce inhibition of late gene expression.21 We asked whether sequences downstream of the p14 CS are important for U1-mediated suppression as our original constructs harbor 316 nts of the genomic downstream region (Fig. 1A). Deletion of this region leads to a dramatic decrease in mRNA quantity for both wt and mut p14 RNA (Fig. 1E and F). This indicates the presence of a DSE and other auxiliary elements.16 Most importantly, the ratio between wt and mut mRNA is not affected (Fig. 1G). Thus, the deleterious effect of U1 on p14 expression is independent of the downstream genomic region, or in other words, suppression of PCPA relies on U1 snRNP binding and the core PAS, but not on sequences 3′ of the CS in the case of p14.
In order to get a deeper understanding of the mechanism of U1-dependent PAS suppression, we replaced the 3′ end processing signals in the p14 gene with histone elements. Interestingly, U1 snRNP-mediated suppression of p14 RNA cleavage/polyadenylation does not affect histone 2A 3′ end processing.16 However, factors such as CPSF and CstF participate in both processes and the cleavage of polyadenylated and histone mRNA is executed by the same endonuclease.22 Even the beneficial effect of splicing factors such as U2 snRNP can be transferred to histone 3′ end processing.23 Both pathways mainly differ in the mode of recognition of the processing signals and in the manner of recruitment. While CPSF is recruited by the conserved PAS in polyadenylated transcripts, in histone genes, binding occurs via protein-protein interaction of histone-specific 3′ end processing factors.24 So U1 may disturb the recruitment of CPSF and other factors unique to poly(A) mRNA processing (Fig. 2B and C).
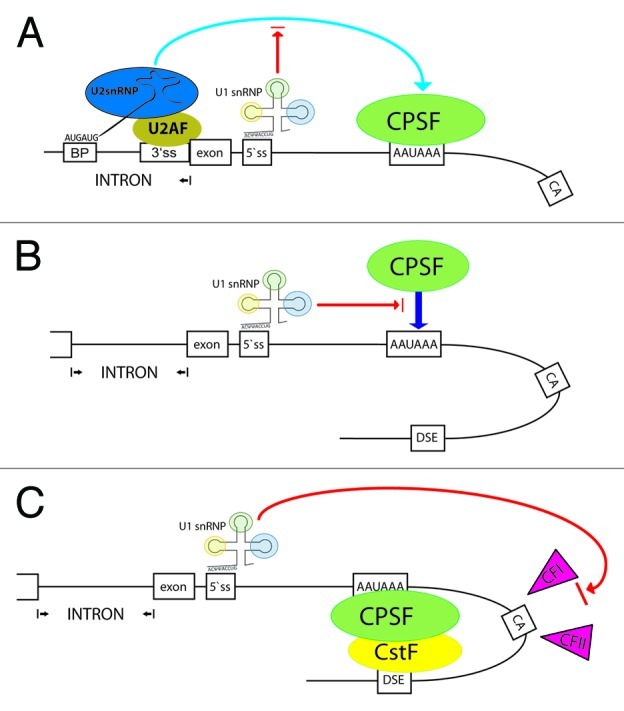
Figure 2. Models for U1 snRNP-mediated inhibition of 3′ end formation. The 3′ end of a gene is outlined including the terminal exon, the upstream intron and the core sequence elements for 3′ end processing (see Fig. 1). A 5′SS base-paired to U1 snRNP is drawn. CPSF binds to the hexamer. (A) U2AF is bound to the 3′SS and U2 snRNP to the branch point (BP). By interacting with CPSF, the terminal exon is defined. U1 snRNP may interfere indirectly with this process. (B) U1 snRNP binding to the 3′UTR leads to inhibition of the sequence dependent recruitment of CPSF. (C) Upon interaction of CPSF and CstF (bound to the downstream element; DSE), the PAS-specific 3′ end processing factors cleavage factor I (CFI) and II (CFII) are recruited. These factors could be potential targets of U1 snRNP. For details see text.
The inhibitory effect of U1 snRNP was previously adjudicated to a direct interaction of the U1 subunit U1 70K with the poly(A) polymerase.10 But our data revealed that the effect of U1 must occur prior to polyadenylation, e.g., inhibition of transcript cleavage or recruitment of particular factors to the PAS. An RNase protection assay (RPA) and RT-qPCR specific for unprocessed p14 mRNAs displayed more uncleaved p14 transcripts and increased read-through in the case of U1 binding.16 We have constructed a tandem PAS reporter harboring a duplication of the p14 3′UTR and, thus, two identical PAS. Preliminary data show that the U1 site located in the first 3′UTR is able to partially prevent 3′ end formation at the first and second PAS and on top leads to substantial read-through, which is incompatible with PAP inhibition alone (M.R., J.L. and J.B.; unpublished data). These observations lead us to hypothesize that the sequence-specific recruitment of CPSF or CstF is inhibited by U1 snRNP binding to the 3′UTR (Fig. 2B). Alternatively, U1 snRNP may interact with factors which are specifically involved in 3′ end processing of polyadenylated mRNAs. Candidates are the cleavage factors I (CFI) and II (CFII; Fig. 2C).
In contrast to BPV and HPV, HIV uses a 5′SS downstream of the PAS and CS to prevent PCPA of its pre-mRNA.11 Further in vitro analysis revealed that cleavage is inhibited by U1 snRNP.25,26 A similar case is also found in the most recent publication on PCPA. In one set of experiments, the authors placed an U1 site downstream of an intronic PAS and observed roughly 50% suppression. However, the same PAS is almost completely suppressed by the upstream 5′SS.27 The significance of this difference is unclear and it remains to be determined if and how the localization of U1 binding matters in terms of preventing PCPA.
PCPA suppression in general was shown to require higher concentrations of U1 compared with basic splicing. A partial U1 inhibition caused mRNA isoform switching in neuronal differentiation, while splicing is not hampered.27 Therefore, the regulation of PCPA was termed “telescripting,” putting U1 into the role of a watchtower for mRNA length. While this is probably true for intronic PAS, attributing a regulatory function in alternative polyadenylation (APA) to U1 needs to be analyzed in more detail. APA mainly describes the switch between a proximal and a distal PAS in one 3′UTR.26,28 Very recently, the nuclear isoform of the polyA-binding protein (PABPN1) was shown to be a suppressor of proximal PAS usage.29 A genome-wide shortening of 3′UTRs was observed after knockdown of PABPN1. The levels of U1 snRNP were not assessed. We picked one gene (EIF2S3), which showed an identical 3′UTR shortening in both studies.27,29 In this particular case, both the proximal and the distal PAS are non-canonical. The proximal PAS is preceded by a strong USE and, more importantly, by a high-scoring 5′SS. The distal PAS is located more than 1 kb downstream and PCPA suppression by U1 might not reach that far. The question is if PABPN1 and U1 snRNP work together or if inhibition of one factor exerts secondary effects on the other. Yet another study implicated participation of cleavage factor I 68 in 3′UTR shortening.30
In general, we believe that regulation of APA solely by U1 might be hazardous, as a single U1 site is able to suppress at least two PAS in a given 3′UTR if the distance is short enough27 (M.R., J.L. and J. B; unpublished data.). More examples of APA have to be analyzed as exemplified above to address this question.
Neither the subunits of U1 snRNP nor the 3′ end processing factors involved in PCPA suppression could be clearly identified. Here, we present data that, in combination with published studies, allow containment and estimation of the processes likely to be targeted by U1 snRNP-mediated suppression of 3′ end processing (Fig. 2). According to the hypothesis of Martinson, intronic PAS are in competition with splicing.31 If splicing is fast due to strong splice sites, 3′ end processing is outcompeted. This is not the complete story as suppression of PCPA can function independent of splicing as shown by Dreyfuss and coworkers27 and by our p14 cDNA constructs (Fig. 1B; ref. 16). But for some intronic PAS flanked by strong splice sites, the competition model may hold true.
Terminal exon definition increases the efficiency of 3′ end processing (Fig. 1B). U1 snRNP binding to a 5′SS within the terminal exon would create a new “pseudo”-exon and render the PAS “intronic” (Fig. 2A). If splicing to a downstream 3′SS does not occur, the process of 3′ end formation might be poisoned at this point. However, we also observed PAS suppression in the p14 cDNA (Fig. 1B; ref. 16). Most compatible with PCPA might be the scenarios depicted in Figure 2B and C. Here, U1 snRNP prevents the initial recognition of the PAS by CPSF. Since RNA polymerase II already recruits parts of the cleavage/polyadenylation machinery at the promoter,32 a surveillance function is needed to prevent an early “drop-off” at intronic PAS. As a result from our studies with histone 3′ end reporters, we encircled the cleavage factors I and II as candidates since they are unique to PAS-dependent 3′ end formation. We speculate that U1 may inhibit their activity or recruitment (Fig. 2C). In the very same direction, cleavage factor I was recently identified as a regulator of 3′UTR length.30
Further experiments are required to get a better understanding of how PCPA suppression and, thus, an important surveillance mechanism functions in health and disease.
Acknowledgments
We would like to thank T. Schulz for ongoing support. This work was funded by Israeli-Lower Saxony cooperation grant VWZN2628 to J.B.
Footnotes
Previously published online: www.landesbioscience.com/journals/rnabiology/article/23314
References
- 1.Wahl MC, Will CL, Lührmann R. The spliceosome: design principles of a dynamic RNP machine. Cell. 2009;136:701–18. doi: 10.1016/j.cell.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 2.Burge CB, Tuschl T, Sharp PA. Splicing of precursors to mRNAs by the spliceosomes. In: Cech TR, ed. The RNA world. Cold Spring Habor, NY, 1999:525-60. [Google Scholar]
- 3.Yang VW, Lerner MR, Steitz JA, Flint SJ. A small nuclear ribonucleoprotein is required for splicing of adenoviral early RNA sequences. Proc Natl Acad Sci USA. 1981;78:1371–5. doi: 10.1073/pnas.78.3.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Furth PA, Choe WT, Rex JH, Byrne JC, Baker CC. Sequences homologous to 5′ splice sites are required for the inhibitory activity of papillomavirus late 3′ untranslated regions. Mol Cell Biol. 1994;14:5278–89. doi: 10.1128/mcb.14.8.5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Furth PA, Baker CC. An element in the bovine papillomavirus late 3′ untranslated region reduces polyadenylated cytoplasmic RNA levels. J Virol. 1991;65:5806–12. doi: 10.1128/jvi.65.11.5806-5812.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Proudfoot NJ. Ending the message: poly(A) signals then and now. Genes Dev. 2011;25:1770–82. doi: 10.1101/gad.17268411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Danckwardt S, Hentze MW, Kulozik AE. 3′ end mRNA processing: molecular mechanisms and implications for health and disease. EMBO J. 2008;27:482–98. doi: 10.1038/sj.emboj.7601932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colgan DF, Manley JL. Mechanism and regulation of mRNA polyadenylation. Genes Dev. 1997;11:2755–66. doi: 10.1101/gad.11.21.2755. [DOI] [PubMed] [Google Scholar]
- 9.Danckwardt S, Kaufmann I, Gentzel M, Foerstner KU, Gantzert AS, Gehring NH, et al. Splicing factors stimulate polyadenylation via USEs at non-canonical 3′ end formation signals. EMBO J. 2007;26:2658–69. doi: 10.1038/sj.emboj.7601699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gunderson SI, Polycarpou-Schwarz M, Mattaj IW. U1 snRNP inhibits pre-mRNA polyadenylation through a direct interaction between U1 70K and poly(A) polymerase. Mol Cell. 1998;1:255–64. doi: 10.1016/S1097-2765(00)80026-X. [DOI] [PubMed] [Google Scholar]
- 11.Ashe MP, Pearson LH, Proudfoot NJ. The HIV-1 5′ LTR poly(A) site is inactivated by U1 snRNP interaction with the downstream major splice donor site. EMBO J. 1997;16:5752–63. doi: 10.1093/emboj/16.18.5752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guan F, Caratozzolo RM, Goraczniak R, Ho ES, Gunderson SI. A bipartite U1 site represses U1A expression by synergizing with PIE to inhibit nuclear polyadenylation. RNA. 2007;13:2129–40. doi: 10.1261/rna.756707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goraczniak R, Behlke MA, Gunderson SI. Gene silencing by synthetic U1 adaptors. Nat Biotechnol. 2009;27:257–63. doi: 10.1038/nbt.1525. [DOI] [PubMed] [Google Scholar]
- 14.Kaida D, Berg MG, Younis I, Kasim M, Singh LN, Wan L, et al. U1 snRNP protects pre-mRNAs from premature cleavage and polyadenylation. Nature. 2010;468:664–8. doi: 10.1038/nature09479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vorlová S, Rocco G, Lefave CV, Jodelka FM, Hess K, Hastings ML, et al. Induction of antagonistic soluble decoy receptor tyrosine kinases by intronic polyA activation. Mol Cell. 2011;43:927–39. doi: 10.1016/j.molcel.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Langemeier J, Schrom EM, Rabner A, Radtke M, Zychlinski D, Saborowski A, et al. A complex immunodeficiency is based on U1 snRNP-mediated poly(A) site suppression. EMBO J. 2012;31:4035–44. doi: 10.1038/emboj.2012.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bohn G, Allroth A, Brandes G, Thiel J, Glocker E, Schäffer AA, et al. A novel human primary immunodeficiency syndrome caused by deficiency of the endosomal adaptor protein p14. Nat Med. 2007;13:38–45. doi: 10.1038/nm1528. [DOI] [PubMed] [Google Scholar]
- 18.Rigo F, Martinson HG. Functional coupling of last-intron splicing and 3′-end processing to transcription in vitro: the poly(A) signal couples to splicing before committing to cleavage. Mol Cell Biol. 2008;28:849–62. doi: 10.1128/MCB.01410-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu S, Cullen BR. Analysis of the stimulatory effect of splicing on mRNA production and utilization in mammalian cells. RNA. 2003;9:618–30. doi: 10.1261/rna.5260303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nott A, Meislin SH, Moore MJ. A quantitative analysis of intron effects on mammalian gene expression. RNA. 2003;9:607–17. doi: 10.1261/rna.5250403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goraczniak R, Gunderson SI. The regulatory element in the 3′-untranslated region of human papillomavirus 16 inhibits expression by binding CUG-binding protein 1. J Biol Chem. 2008;283:2286–96. doi: 10.1074/jbc.M708789200. [DOI] [PubMed] [Google Scholar]
- 22.Kolev NG, Steitz JA. Symplekin and multiple other polyadenylation factors participate in 3′-end maturation of histone mRNAs. Genes Dev. 2005;19:2583–92. doi: 10.1101/gad.1371105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Friend K, Lovejoy AF, Steitz JA. U2 snRNP binds intronless histone pre-mRNAs to facilitate U7-snRNP-dependent 3′ end formation. Mol Cell. 2007;28:240–52. doi: 10.1016/j.molcel.2007.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dominski Z, Marzluff WF. Formation of the 3′ end of histone mRNA: getting closer to the end. Gene. 2007;396:373–90. doi: 10.1016/j.gene.2007.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vagner S, Rüegsegger U, Gunderson SI, Keller W, Mattaj IW. Position-dependent inhibition of the cleavage step of pre-mRNA 3′-end processing by U1 snRNP. RNA. 2000;6:178–88. doi: 10.1017/S1355838200991854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shi Y. Alternative polyadenylation: new insights from global analyses. RNA. 2012;18:2105–17. doi: 10.1261/rna.035899.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berg MG, Singh LN, Younis I, Liu Q, Pinto AM, Kaida D, et al. U1 snRNP determines mRNA length and regulates isoform expression. Cell. 2012;150:53–64. doi: 10.1016/j.cell.2012.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Di Giammartino DC, Nishida K, Manley JL. Mechanisms and consequences of alternative polyadenylation. Mol Cell. 2011;43:853–66. doi: 10.1016/j.molcel.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jenal M, Elkon R, Loayza-Puch F, van Haaften G, Kühn U, Menzies FM, et al. The poly(A)-binding protein nuclear 1 suppresses alternative cleavage and polyadenylation sites. Cell. 2012;149:538–53. doi: 10.1016/j.cell.2012.03.022. [DOI] [PubMed] [Google Scholar]
- 30.Martin G, Gruber AR, Keller W, Zavolan M. Genome-wide analysis of pre-mRNA 3′ end processing reveals a decisive role of human cleavage factor I in the regulation of 3′ UTR length. Cell Rep. 2012;1:753–63. doi: 10.1016/j.celrep.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 31.Martinson HG. An active role for splicing in 3′-end formation. Wiley Interdiscip Rev RNA. 2011;2:459–70. doi: 10.1002/wrna.68. [DOI] [PubMed] [Google Scholar]
- 32.Calvo O, Manley JL. Strange bedfellows: polyadenylation factors at the promoter. Genes Dev. 2003;17:1321–7. doi: 10.1101/gad.1093603. [DOI] [PubMed] [Google Scholar]