Abstract
Objective
To define the optimal treatment for women with stage III or locally advanced breast cancer (LABC).
Evidence
Systematic review of English-language literature retrieved from MEDLINE (1984 to June 2002) and CANCERLIT (1983 to June 2002). A nonsystematic review of the literature was continued through December 2003.
Recommendations
· The management of LABC requires a combined modality treatment approach involving surgery, radiotherapy and systemic therapy.
Systemic therapy: chemotherapy
Operable tumours
· Patients with operable stage IIIA disease should be offered chemotherapy. They should receive adjuvant chemotherapy following surgery, or primary chemotherapy followed by locoregional management.
· Chemotherapy should contain an anthracycline. Acceptable regimens are 6 cycles of FAC, CAF, CEF or FEC. Taxanes are under intense investigation.
Inoperable tumours
· Patients with stage IIIB or IIIC disease, including those with inflammatory breast cancer and those with isolated ipsilateral internal mammary or supraclavicular lymph-node involvement, should be treated with primary anthracycline-based chemotherapy.
· Acceptable chemotherapy regimens are FAC, CAF, CEF or FEC. Taxanes are under intense investigation.
· Patients with stage IIIB or IIIC disease who respond to primary chemotherapy should be treated until the response plateaus or to a maximum of 6 cycles (minimum 4 cycles). Patients with stage IIIB disease should then undergo definitive surgery and irradiation. The locoregional management of patients with stage IIIC disease who respond to chemotherapy should be individualized. In patients with stage IIIB or IIIC disease who achieve maximum response with fewer than 6 cycles, further adjuvant chemotherapy can be given following surgery and irradiation. Patients whose tumours do not respond to primary chemotherapy can be treated with taxane chemotherapy or can proceed directly to irradiation followed by modified radical mastectomy, if feasible.
Systemic therapy: hormonal therapy
Operable and inoperable tumours
· Tamoxifen for 5 years should be recommended to pre- and postmenopausal women whose tumours are hormone responsive.
Locoregional management
Operable tumours
· Patients with stage IIIA disease should receive both modified radical mastectomy (MRM) and locoregional radiotherapy if feasible. They may be managed with MRM followed by chemotherapy and locoregional radiotherapy, or chemotherapy first followed by MRM and locoregional radiotherapy. Breast-conserving surgery is currently not a standard approach.
· Locoregional radiotherapy should be delivered to the chest wall and to the supraclavicular and axillary nodes. The role of internal mammary irradiation is unclear.
Inoperable tumours
· Patients with stage IIIB disease who respond to chemotherapy should receive surgery plus locoregional radiotherapy.
· The locoregional management of patients with stage IIIC disease who respond to chemotherapy is unclear and should be individualized.
· Patients whose disease remains inoperable following chemotherapy should receive locoregional radiotherapy with subsequent surgery, if feasible.
Validation
The authors' original text was revised by members of the Steering Committee on Clinical Practice Guidelines for the Care and Treatment of Breast Cancer. Subsequently, feedback was provided by 9 oncologists from across Canada. The final document was approved by the steering committee.
Sponsor
The Steering Committee on Clinical Practice Guidelines for the Care and Treatment of Breast Cancer was convened by Health Canada.
Completion date
December 2003.
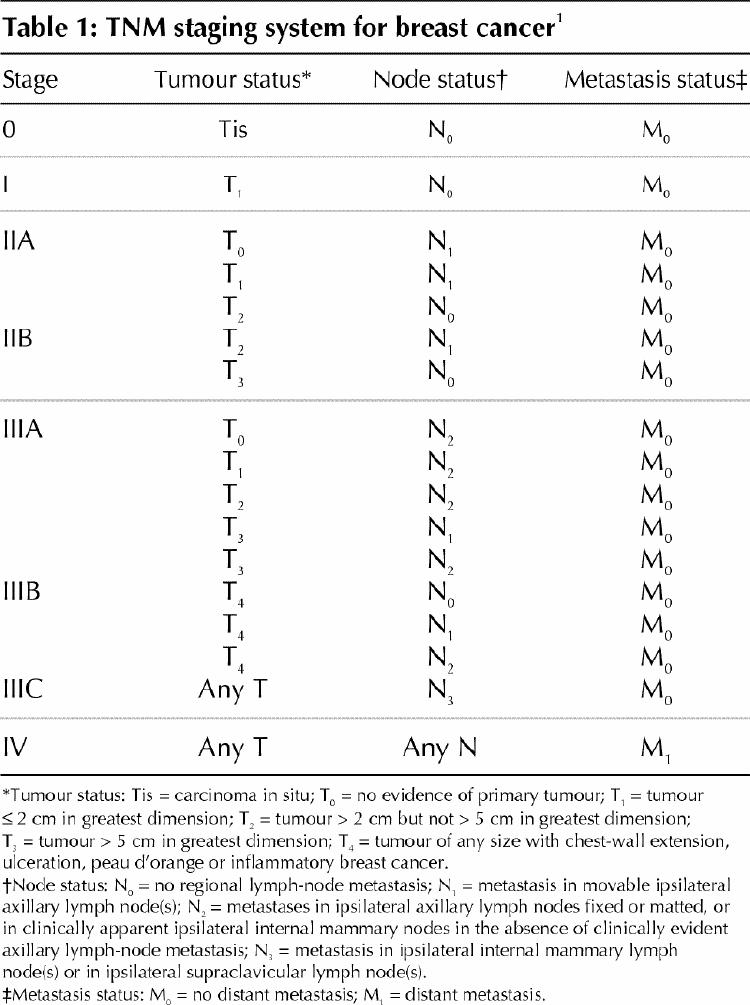
Locally advanced breast cancer (LABC) occurs relatively infrequently, but it poses a significant clinical challenge. LABC refers to large breast tumours (> 5 cm in diameter) associated with either skin or chest-wall involvement or with fixed (matted) axillary lymph nodes or with disease spread to the ipsilateral internal mammary or supraclavicular nodes.1 Inflammatory breast cancer, which manifests as a red swollen breast, is considered a type of LABC. The Tumour–Node–Metastasis (TNM) system is used to classify breast cancer into stages (Table 1).1 According to this system, LABC is stage III.
Table 1
During the last 60 years, the management of LABC has evolved considerably. Initially, patients with LABC were treated with radical mastectomy.2 Based on the disappointing results of surgery and radiotherapy2,3,4 in patients with LABC, and the early promising results of adjuvant systemic therapy in women with axillary node-positive breast cancer,5,6 systemic therapy was subsequently incorporated along with surgery and radiotherapy into the management of patients with LABC, termed “combined modality therapy.” Even with such combined modality therapy, the long-term survival rate is approximately 50% among patients with LABC.7 The focus of this guideline is to determine the optimal therapeutic approach for patients who present with LABC.
Methods
We conducted a systematic review of the English-language literature retrieved from MEDLINE (1984 to June 2002) and CANCERLIT (1983 to June 2002). Search terms used were “breast neoplasms,” “locally advanced breast cancer,” “stage III breast cancer,” “drug therapy,” “neo-adjuvant,” “primary systemic therapy,” “radiotherapy or irradiation,” “surgery,” “randomized trials” and “high-dose therapy.” A nonsystematic review of the literature was continued through December 2003. Additional data were identified by reviewing references in retrieved reports and by monitoring major conferences on breast cancer. The quality of the evidence on which conclusions are based is categorized into 5 levels.8 The main outcomes considered are locoregional control (defined as freedom from recurrence in the breast, chest wall or regional lymph nodes), disease-free survival (DFS; defined as survival free of breast cancer recurrence) and overall survival (OS).
We were faced with a number of challenges when trying to synthesize the results of the studies from the review of the literature. These included:
· Many studies were case series (levels IV and V evidence).
· The studies included different populations of patients with differing prognoses; for example, some studies included patients with inflammatory breast cancer whereas other studies did not.
· In studies evaluating systemic therapies, local therapy (surgery/radiotherapy) was often not standardized.
· The TNM tumour-staging system changed, in that tumours associated with ipsilateral supraclavicular nodal involvement that were initially considered LABC were considered metastatic breast cancer between 1987 and 2002 and are now considered LABC again.1
· The randomized trials that were available were old, had small patient numbers and used systemic therapy combinations that are often not used today.
We developed this guideline using a framework based on the operability of the tumour. The current TNM staging system, which is based on clinical characteristics of the primary tumour and regional lymph nodes, is used to help determine operability (Table 1).1 Large operable tumours include stage IIB and IIIA disease. Nonoperable tumours include stage IIIB or stage IIIC disease. Patients with ipsilateral supraclavicular lymph-node involvement as their sole site of metastases have in the past been classified as having stage IV breast cancer, but they have a better prognosis than patients with other sites of metastases and are included in the category of inoperable LABC (stage IIIC disease) within this guideline.1,9,10
Recommendations (including evidence and rationale)
· The management of LABC requires a combined modality treatment approach involving surgery, radiotherapy and systemic therapy.
The clinical management of LABC is complex and should be tailored to the individual patient. Frequently, surgery, radiotherapy and systemic therapy (chemotherapy, hormone therapy) are used. A multidisciplinary approach to LABC is recommended in which treatment is based on the combined opinions of a surgeon, radiation oncologist and medical oncologist. The initial management of LABC requires histological confirmation (e.g., core biopsy, incisional biopsy or skin biopsy) for diagnosis and for determination of hormone receptor and HER-2 neu oncogene status. Cytological evaluation by fine-needle aspiration is insufficient.
Systemic therapy: chemotherapy
Operable tumours
· Patients with operable stage IIIA disease should be offered chemotherapy. They should receive adjuvant chemotherapy following surgery, or primary chemotherapy followed by locoregional management.
Patients with stage IIIA breast cancer have potentially operable tumours. There are 2 approaches for treating these patients. The first is modified radical mastectomy (MRM) followed by adjuvant systemic therapy and radiotherapy, and the second is preoperative chemotherapy followed by surgery and radiotherapy.
Surgery followed by adjuvant chemotherapy
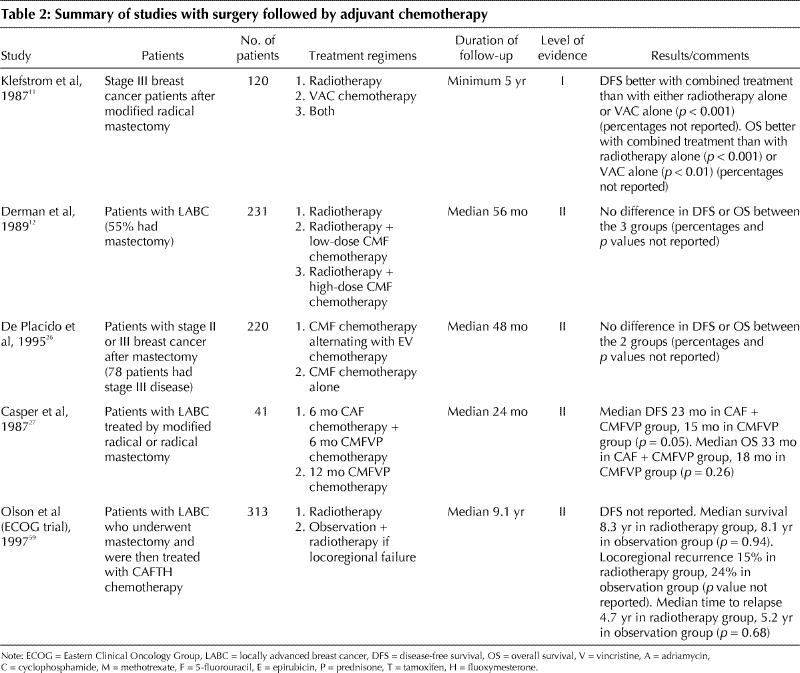
There have been only 2 randomized clinical trials of adjuvant chemotherapy exclusively in patients with stage III disease (Table 2).11,12 One of these studies11 reported a benefit with chemotherapy (level I evidence). The meta-analysis of the Early Breast Cancer Trialists' Collaborative Group (EBCTCG)5,6 included a subset of patients with large operable tumours. Overall, all patients benefited from adjuvant systemic chemotherapy regardless of tumour size (level I evidence). Therefore, it is reasonable to extrapolate the overall conclusions of the meta-analysis to patients with operable stage IIIA disease.
Table 2
Primary chemotherapy followed by surgery
A number of case series have reported on preoperative chemotherapy in patients with operable stage IIIA breast cancer (level IV evidence).13,14,15,16 Table 3 summarizes one retrospective study and the randomized trials that compared preoperative and postoperative chemotherapy.15,16,17,18,19,20,21,22,23,24,25 The primary purpose of these studies was to determine whether preoperative chemotherapy, compared with postoperative chemotherapy, improved DFS and OS. These studies involved patients mainly with stage I or stage II disease and included a small proportion of women with tumours greater than 5 cm in diameter. No difference in DFS and OS was detected between the preoperative and postoperative chemotherapy groups (level II evidence). Preoperative chemotherapy often caused shrinkage of the tumour and permitted the performance of breast-conserving surgery (BCS) when a mastectomy was originally planned.17,18,19,20,21,22,23,24,25 However, results from 2 trials suggested that patients whose tumours were down-staged so that BCS could be performed when it was not initially planned were at higher risk of local recurrence19 and had worse survival.20
Table 3
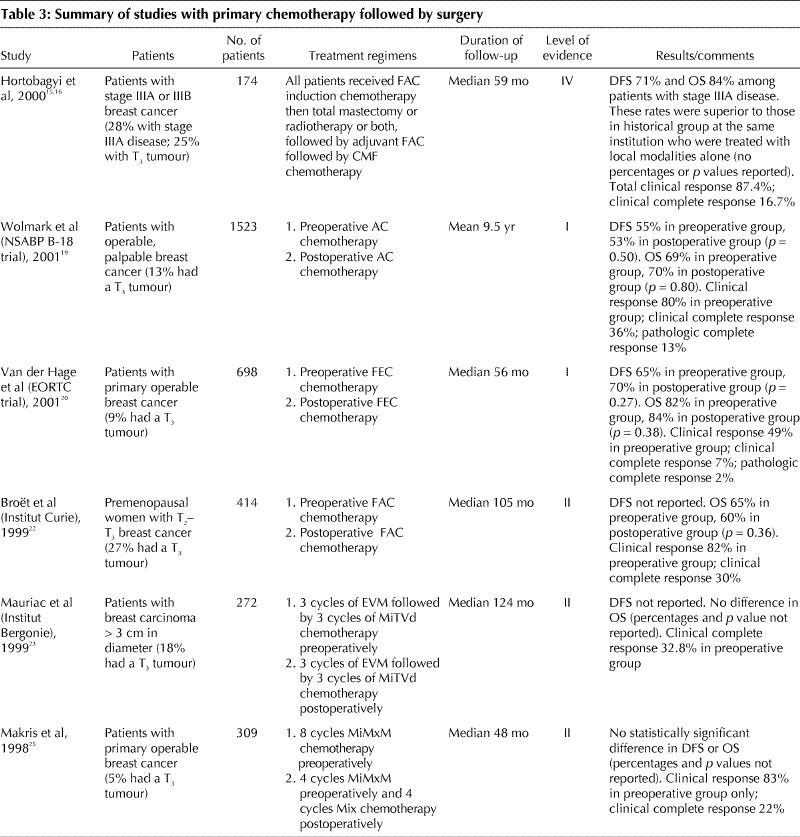
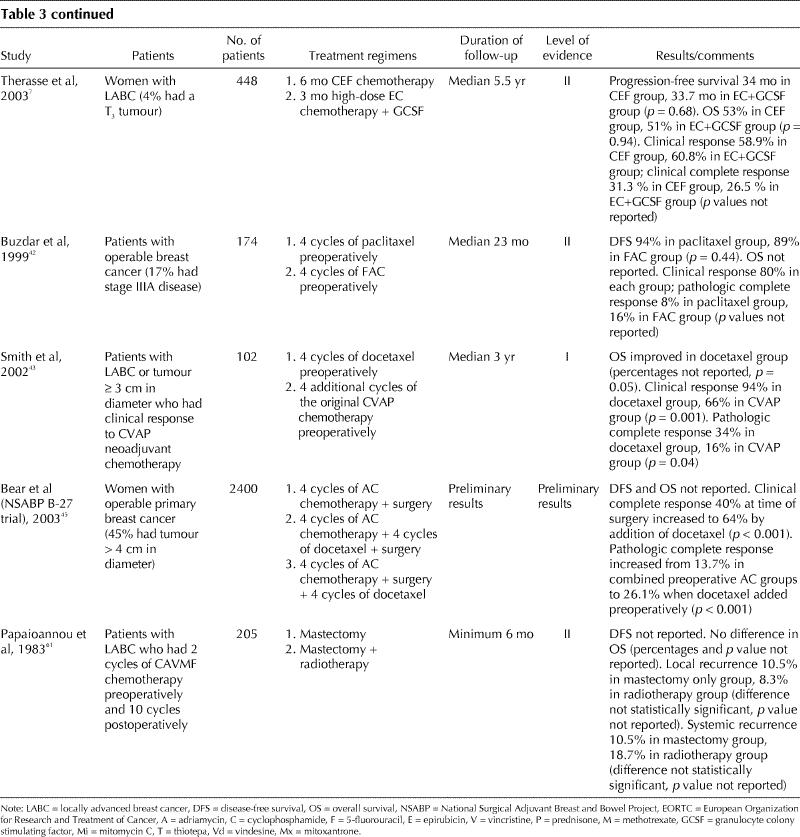
Table 3 continued
Choice of chemotherapy
· Chemotherapy should contain an anthracycline. Acceptable regimens are 6 cycles of FAC, CAF, CEF or FEC (see Box 1). Taxanes are under intense investigation.
Box 1.
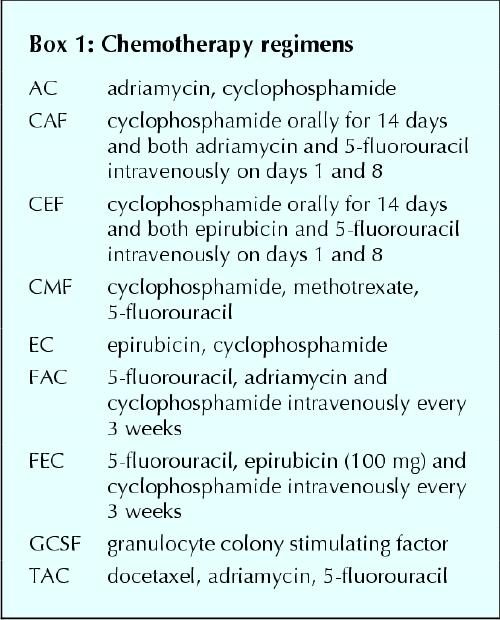
There have been relatively few trials specifically involving women with operable stage IIIA breast cancer that have addressed the issue of the type of chemotherapy to be used.11,26,27 The number of patients in these trials was small. Two trials showed improved DFS with anthracycline-based chemotherapy over no chemotherapy (level I evidence)11 and over a CMF–vincristine–prednisone regimen (level II evidence).27 A third trial showed no difference in DFS or OS between CMF and a CMF–epirubicin–vincristine regimen (level II evidence) (Table 2).26 Hence, recommendations concerning the type of chemotherapy regimens to be used in stage IIIA disease are based on extrapolation from trials in women with metastatic breast cancer and node-positive breast cancer.
Results of randomized trials involving women with metastatic breast cancer have shown a superior response rate and prolonged DFS with anthracycline-containing chemotherapy regimens than with CMF.28,29 Randomized trials have confirmed the superiority of anthracycline-containing regimens such as CEF and CAF over conventional CMF in women with node-negative and node-positive breast cancer.30,31,32 In contrast, in the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-15 trial, 4 cycles of AC chemotherapy was equivalent to 6 months of CMF.33 In the most recent EBCTCG overview,5 there was a statistically significant improvement in survival with anthracycline-based chemotherapy compared with CMF in early stage breast cancer. Although there are limitations to cross-study comparisons, it is reasonable to consider that 4 cycles of AC, although equivalent to 6 months of CMF,33 is probably inferior to 6 cycles of anthracycline-containing drug regimens such as FAC,16 CAF,28,30 CEF32 and FEC.34 In women who cannot receive anthracyclines because of underlying cardiac disease, CMF chemotherapy can be considered.
Six cycles of chemotherapy should be administered. This is based on the trials of adjuvant chemotherapy that showed that 6 cycles of CAF or CEF was superior to 6 cycles of CMF30,32 and that 6 cycles of FEC was superior to 3 cycles of FEC.35
In a multicentre trial, 448 women with LABC were randomly allocated to receive either CEF, or EC plus GCSF (Table 3).7 At a median follow-up of 5.5 years, no difference in survival was detected between the 2 groups (53% and 51% respectively; level II evidence).
The role of taxanes in LABC and as preoperative chemotherapy in women with early breast cancer is currently under investigation. Studies involving women with metastatic breast cancer indicate that taxanes can cause significant tumour regression and improvement in symptoms in patients with anthracycline-resistant disease.36,37 Taxane-containing regimens have been evaluated in randomized trials involving patients with axillary node-positive breast cancer. Compared with AC, AC followed by paclitaxel was associated with improved DFS in 2 trials38,39 and with improved OS in 1 trial.38 A recent trial suggested that AC administered every 2 weeks followed by paclitaxel every 2 weeks (dose dense chemotherapy) was associated with improved DFS compared with the usual AC followed by paclitaxel, both administered every 3 weeks.40 Another recently reported trial involving women with node-positive breast cancer showed that, compared with FAC, the chemotherapy regimen TAC improved DFS and OS.41
In a trial of FAC versus paclitaxel, both administered preoperatively, no difference was detected in clinical or pathologic response rate and DFS (Table 3).42 Results from 2 studies suggested that the addition of a taxane sequentially to an anthracycline-based regimen can improve clinical and pathologic response (Table 3).43,44,45
Currently, there are insufficient data to make definitive recommendations concerning the use of taxane-containing regimens in LABC. However, this is an evolving area of investigation. Management of LABC with systemic therapy remains an area for further research, and randomized studies are needed to identify optimal strategies. Participation in clinical trials is encouraged.
Inoperable tumours
· Patients with stage IIIB or IIIC disease, including those with inflammatory breast cancer and those with isolated ipsilateral internal mammary or supraclavicular lymph-node involvement, should be treated with primary anthracycline-based chemotherapy.
· Acceptable chemotherapy regimens are FAC, CAF, CEF or FEC. Taxanes are under intense investigation.
[See the discussion of chemotherapy in the section “operable tumours.”] The results of trials of chemotherapy in women with metastatic breast cancer and axillary node-positive breast cancer have demonstrated the superiority of anthracycline-containing combination chemotherapy over CMF.28,29,30,31,32 On the basis of this evidence, when chemotherapy is used for treating stage IIIB or IIIC breast cancer, an anthracycline-containing regimen should be used if there are no contraindications to its use. A regimen of FAC, CAF, CEF or FEC is preferred.16,30,32,34 An adequate dose intensity and total dose of anthracycline should be used.34,46 CMF chemotherapy can be used in women who cannot receive anthracycline-containing chemotherapy because of underlying heart disease. The role of taxanes is currently under investigation, and no recommendations can be made at present for the incorporation of taxanes in primary chemotherapy for inoperable LABC.
There is currently no evidence to support the use of high-dose chemotherapy with stem cell support for this group of patients, as the randomized trials involving women with metastatic breast cancer and breast cancer with extensive nodal involvement have demonstrated no difference in survival between high-dose chemotherapy and standard-dose chemotherapy (level II evidence).47,48
· Patients with stage IIIB or IIIC disease who respond to primary chemotherapy should be treated until the response plateaus or to a maximum of 6 cycles (minimum 4 cycles). Patients with stage IIIB disease should then undergo definitive surgery and irradiation. The locoregional management of patients with stage IIIC disease who respond to chemotherapy should be individualized. In patients with stage IIIB or IIIC disease who achieve maximum response with fewer than 6 cycles, further adjuvant chemotherapy can be given following surgery and irradiation. Patients whose tumours do not respond to primary chemotherapy can be treated with taxane chemotherapy or can proceed directly to irradiation followed by modified radical mastectomy, if feasible.
Patients with stage III disease who are treated with primary chemotherapy need to be followed carefully for evidence of response. Multivariate analyses in several studies17,18,19,49,50 have shown that the primary tumour response is correlated with patient outcome and that patients who have pathological evidence of a complete response following primary therapy have a superior DFS and OS compared with those who do not have such a response (level III evidence). Clinical response is seen in about 80% of patients who receive primary chemotherapy. To ascertain response, at least 2–3 cycles of chemotherapy should be administered.
The treatment of patients with LABC whose tumours do not respond to anthracycline-containing chemotherapy is unclear. It is reasonable to try taxane chemotherapy42,43,44,45 or to proceed directly to locoregional therapy including irradiation and MRM, if possible.
Patients with stage IIIB or IIIC disease who do have a clinical response to primary chemotherapy should receive ongoing treatment until the manifested response clearly plateaus or to a maximum of 6 cycles, whichever comes first. The threshold for anthracycline-associated cardiac toxicity should not be exceeded. The optimal duration of primary chemotherapy is unclear. However, the results of studies involving women with node-positive breast cancer who received adjuvant chemotherapy showed that a regimen of 6 cycles of CMF was superior to that of 3 cycles of CMF51 and that a regimen of 6 cycles of FEC was superior to that of 3 cycles of FEC.35
It is unclear whether additional chemotherapy should be administered following primary chemotherapy and definitive locoregional therapy. This has been examined in several case series (level V evidence).15,16,49
Systemic therapy: hormonal therapy
Operable and inoperable tumours
· Tamoxifen for 5 years should be recommended to pre-and postmenopausal women whose tumours are hormone responsive.
Following completion of chemotherapy, pre- or postmenopausal patients with LABC and hormone-responsive tumours should receive adjuvant tamoxifen therapy, 20 mg/d, for 5 years (see guideline 8).5,52 Tamoxifen should be started after completion of chemotherapy.53 The aromatase inhibitor, anastrozole, has been compared with tamoxifen in postmenopausal women with early breast cancer following surgery.54 The early results of that study showed that, compared with tamoxifen, anastrozole was associated with improved DFS and had fewer side effects. The role of aromatase inhibitors as adjuvant therapy in breast cancer is evolving.55 The role of luteinizing hormone-releasing hormone agonists in premenopausal patients is evolving as new data emerge (see guideline 8).
Patients who are not candidates for any chemotherapy can be managed with hormonal treatment and then receive locoregional management as described below.56,57,58
Locoregional management
Operable tumours
· Patients with stage IIIA disease should receive both modified radical mastectomy (MRM) and locoregional radiotherapy if feasible. They may be managed with MRM followed by chemotherapy and locoregional radiotherapy, or chemotherapy first followed by MRM and locoregional radiotherapy. Breast-conserving surgery is currently not a standard approach.
MRM (mastectomy plus a level 1 and level 2 axillary dissection) remains the standard surgical treatment for operable locally advanced disease. The role of BCS is unclear and the subject of research. Previous studies demonstrating equivalence of BCS to mastectomy were performed in patients with stage I and II disease (see guideline 3). In the trials that compared preoperative chemotherapy with chemotherapy administered postoperatively, the proportion of women with tumours greater than 5 cm in diameter ranged from 5% to 27%. Patients with operable stage III disease who desire to preserve their breast should be made aware that BCS is currently not a standard approach and is generally not recommended. There is a lack of evidence concerning breast reconstruction surgery in women with LABC. Reconstruction is generally performed after completion of chemotherapy and radiotherapy, because of the concern that postoperative complications could delay chemotherapy and radiotherapy.
In the largest study evaluating locoregional therapy,59,60 eligible patients with operable LABC had a mastectomy, 6 cycles of anthracycline-based chemotherapy and, if disease-free, were randomly assigned to receive radiotherapy or observation with radiotherapy at isolated sites of locoregional failure (Table 2). Locoregional recurrences were reduced from 24% to 15% with immediate radiotherapy (level I evidence).
Two smaller randomized trials had conflicting results regarding the value of radiotherapy. These trials are summarized in Table 2 (level I evidence)11 and Table 3 (level II evidence).61
There is a large body of level I and level II evidence demonstrating that locoregional radiotherapy following mastectomy in patients with node-positive disease treated with systemic therapy is associated with not only a reduction in locoregional recurrence but also an increase in overall survival (see guideline on postmastectomy radiotherapy). In the 3 largest trials of postmastectomy radiotherapy, 12%–14% of patients had stage IIIA disease.62,63,64 A meta-analysis of all trials in which patients were treated with systemic therapy also confirmed the benefit of locoregional radiotherapy in improving disease-free and overall survival.65
In summary, the results of 2 randomized trials and data extrapolated from trials involving women with node-positive disease support the use of locoregional radiotherapy in patients with LABC who are treated with mastectomy.
· Locoregional radiotherapy should be delivered to the chest wall and to the supraclavicular and axillary nodes. The role of internal mammary irradiation is unclear.
When locoregional radiotherapy is delivered following MRM for locally advanced disease, radiation should be delivered to the chest wall and the supraclavicular and axillary nodes. Whether treatment to the internal mammary nodes is required is unclear.66 In many of the studies reviewed for this guideline, the internal mammary nodes were irradiated. However, there are no studies that examined the impact of such radiotherapy. It is not unreasonable to include radiotherapy to the internal mammary nodal region, provided that this can be done without treating an excessive amount of heart or lung tissue. Locoregional radiotherapy has been associated with a modest increase in late non-breast-cancer deaths of cardiac or vascular origin.66 The recommended dose of radiation is 50 Gy in 25 fractions or equivalent.
Inoperable tumours
· Patients with stage IIIB disease who respond to chemotherapy should receive surgery plus locoregional radiotherapy.
· The locoregional management of patients with stage IIIC disease who respond to chemotherapy is unclear and should be individualized.
· Patients whose disease remains inoperable following chemotherapy should receive locoregional radiotherapy with subsequent surgery, if feasible.
Three small trials compared MRM alone with locoregional radiotherapy alone following chemotherapy (Table 4).67,68,69 The results of these studies suggest that both treatments are equally effective after primary chemotherapy in inoperable disease (level II evidence).
Table 4
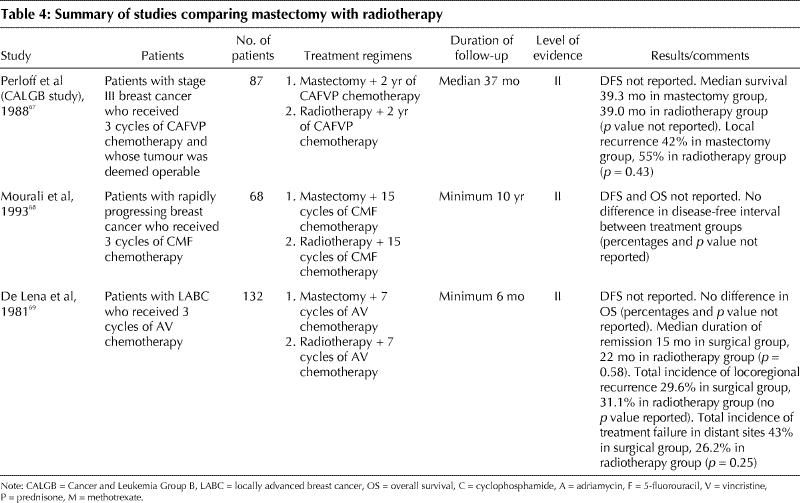
No randomized trials were found that compared mastectomy plus locoregional radiotherapy with mastectomy alone following chemotherapy, but 2 case series demonstrated that locoregional control was better if both mastectomy and radiotherapy were performed70,71 (level V evidence).
The results of these studies and data from randomized trials in operable disease support the use of both MRM and locoregional radiotherapy in achieving optimal local control when feasible. It is unknown whether the sequence of surgery and radiotherapy makes a difference. Technical and disease factors will usually influence the order of treatments. For example, patients who have a good response to primary chemotherapy may be best managed by MRM followed by radiotherapy. Patients who remain inoperable after chemotherapy could receive radiotherapy followed by surgery, if feasible. Again, similar to operable disease, the role of BCS in this situation is unclear and is the subject of research.
The locoregional management of patients with stage IIIC disease who respond to chemotherapy is unclear. In the absence of evidence on this subgroup of patients, it is reasonable that they receive locoregional radiotherapy (including nodal irradiation). The role of completion mastectomy should be individualized and based on such factors as response to chemotherapy and radiotherapy, absence of metastases on re-staging examinations and patient fitness.
Patients who are treated primarily with radiotherapy should be given tumouricidal doses to areas of bulk disease (60–66 Gy in 30 to 33 fractions or equivalent). Higher doses of radiation (70 Gy in 35 fractions by external beam or brachytherapy) to areas of bulk disease may be considered for patients if surgery is felt not to be an option and if tolerance of critical organs permits. Two case series have reported a dose-response relation with higher doses of radiation that resulted in decreased rates of local recurrence (level V evidence).72,73
Acknowledgments
We thank the following oncologists for their valuable advice: Dr. Vivien Bramwell, Tom Baker Cancer Centre, Calgary, Alta.; Dr. Margot Burnell, Atlantic Health Sciences Corporation, Saint John, NB; Dr. Karen Gelmon, BC Cancer Agency–Vancouver Cancer Centre, Vancouver, BC; Dr. Peter Lovrics, St. Joseph's Hospital, Hamilton, Ont.; Dr. John Mackey, Cross Cancer Institute, Edmonton, Alta.; Dr. Maureen Nolan, Nova Scotia Cancer Centre, Halifax, NS; Dr. Jean Robert, Hôpital du Saint-Sacrement, Québec, Que.; Dr. Katherine Vallis, Princess Margaret Hospital, Toronto, Ont.; and Dr. TheodoreVandenberg, London Regional Cancer Centre, London, Ont..
Appendix 1.

Footnotes
A patient version of these guidelines appears in Appendix 1.
A guide for patients on the treatment of locally advanced breast cancer appears on page 994.
This article has been peer reviewed.
Contributors: All of the authors were involved in reviewing the literature and categorizing the levels of evidence. They have all been involved in the writing of the many drafts of this article and all have given final approval of the version to be published.
Competing interests: None declared for Drs. Shenkier, Weir, Levine, Olivotto and Whelan. At the time of writing the guidelines, Dr. Reyno had no competing interests; he is now an employee of Aventis Pharmaceuticals Inc.
Correspondence to: Dr. Mark Levine, Rm. 104, Henderson Research Centre, Henderson Hospital, 711 Concession St., Hamilton ON L8V 1C3; fax 905 389-9288
References
- 1.Singletary SE, Allred C, Ashley P, Bassett LW, Berry D, Bland KI, et al. Revision of the American Joint Committee on Cancer Staging System for Breast Cancer. J Clin Oncol 2002;20(17);3628-36. [DOI] [PubMed]
- 2.Haagensen CD, Stout AP. Carcinoma of the breast. II: Criteria of operability. Ann Surg 1943;118:859-70, 1032-51. [DOI] [PMC free article] [PubMed]
- 3.Strom EA, McNeese MD, Fletcher GH, Romsdahl MA, Montague ED, Oswald MJ. Results of mastectomy and postoperative irradiation in the management of locoregionally advanced carcinoma of the breast. Int J Radiat Oncol Biol Phys 1991;21(2):319-23. [DOI] [PubMed]
- 4.Toonkel LM, Fix I, Jacobson LH, Bamberg N, Wallach CB. Locally advanced breast carcinoma: results with combined regional therapy. Int J Radiat Oncol Biol Phys 1986;12(9):1583-7. [DOI] [PubMed]
- 5.Early Breast Cancer Trialists' Collaborative Group. Polychemotherapy for early breast cancer: an overview of the randomised trials. Lancet 1998; 352:930-42. [PubMed]
- 6.Early Breast Cancer Trialists' Collaborative Group. Tamoxifen for early breast cancer: an overview of the randomised trials. Lancet 1998;351:1451-67. [PubMed]
- 7.Therasse P, Mauriac L, Welnicka-Jaskiewicz M, Bruning P, Cufer T, Bonnefoi H, et al. Final results of a randomized phase III trial comparing cyclophosphamide, epirubicin, and fluorouracil with a dose-intensified epirubicin and cyclophosphamide + filgrastim as neoadjuvant treatment in locally advanced breast cancer: an EORTC-NCIC-SAKK multicenter study. J Clin Oncol 2003;21(5):843-50. [DOI] [PubMed]
- 8.Sackett DL. Rules of evidence and clinical recommendations on the use of antithrombotic agents. Chest 1989;95(Suppl):2S-4S. [PubMed]
- 9.Brito RA, Valero V, Buzdar AU, Booser DJ, Ames F, Strom E, et al. Long-term results of combined-modality therapy for locally advanced breast cancer with ipsilateral supraclavicular metastases: the University of Texas M.D. Anderson Cancer Center experience. J Clin Oncol 2001;19(3):628-33. [DOI] [PubMed]
- 10.Olivotto IA, Chua B, Allan SJ, Speers CH, Chia S, Ragaz J. Long-term survival of patients with supraclavicular metastases at diagnosis of breast cancer. J Clin Oncol 2003;21(5):851-4. [DOI] [PubMed]
- 11.Klefstrom P, Grohn P, Heinonen E, Holsti L, Holsti P. Adjuvant postoperative radiotherapy, chemotherapy, and immunotherapy in stage III breast cancer. II. 5-year results and influence of levamisole. Cancer 1987;60(5):936-42. [DOI] [PubMed]
- 12.Derman DP, Browde S, Kessel IL, De Moor NG, Lange M, Dansey R, et al. Adjuvant chemotherapy (CMF) for stage III breast cancer: a randomized trial. Int J Radiat Oncol Biol Phys 1989;17:257-61. [DOI] [PubMed]
- 13.Harris JR, Lippman ME, Morrow M, Oxborne CK, editors. Diseases of the breast. 2nd ed. Philadelphia: Lippincott Williams and Wilkins; 2000.
- 14.Wolff AC, Davidson NE. Primary systemic therapy in operable breast cancer. J Clin Oncol 2000;18(7):1558-69. [DOI] [PubMed]
- 15.Hortobagyi GN, Singletary SE, Strom EA. Treatment of locally advanced and inflammatory breast cancer. In: Harris JR, Lippman ME, Morrow M, Oxborne CK, editors. Diseases of the breast. 2nd ed. Philadelphia: Lippincott Williams and Wilkins; 2000. p.645-60.
- 16.Hortobagyi GN, Ames FC, Buzdar AU, Kau SW, McNeese MD, Paulus D, et al. Management of stage III primary breast cancer with primary chemotherapy, surgery, and radiation therapy. Cancer 1988;62(12):2507-16. [DOI] [PubMed]
- 17.Fisher B, Brown A, Mamounas E, Wieand S, Robidoux A, Margolese RG, et al. Effect of preoperative chemotherapy of local-regional disease in women with operable breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-18. J Clin Oncol 1997;15:2483-93. [DOI] [PubMed]
- 18.Fisher B, Bryant J, Wolmark N, Mamounas E, Brown A, Fisher ER, et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol 1998;16(8):2672-85. [DOI] [PubMed]
- 19.Wolmark N, Wang J, Mamounas E, Bryant J, Fisher B. Preoperative chemotherapy in patients with operable breast cancer: nine-year results from National Surgical Adjuvant Breast and Bowel Project B-18. J Natl Cancer Inst Monogr 2001;30:96-102. [DOI] [PubMed]
- 20.Van der Hage JA, van de Velde CJH, Julien JP, Tubiana-Hulin M, Vandervelden C, Duchateau L, et al. Preoperative chemotherapy in primary operable breast cancer: results from the European Organization for Research and Treatment of Cancer trial 10902. J Clin Oncol 2001;19(22):4224-37. [DOI] [PubMed]
- 21.Scholl SM, Fourquet A, Asselain B, Pierga JY, Vilcoq JR, Durand JC, et al. Neoadjuvant versus adjuvant chemotherapy in premenopausal patients with tumours considered too large for breast conserving surgery: preliminary results of a randomised trial: S6. Eur J Cancer 1994;645-52. [DOI] [PubMed]
- 22.Broët P, Scholl SM, de la Rochefordière A, Fourquet A, Moreau T, De Rycke Y, et al. Short and long-term effects on survival in breast cancer patients treated by primary chemotherapy: an updated analysis of a randomized trial. Breast Cancer Res Treat 1999;58:151-6. [DOI] [PubMed]
- 23.Mauriac L, MacGrogan G, Avril A, Durand M, Floquet A, Debled M, et al. Neoadjuvant chemotherapy for operable breast carcinoma larger than 3 cm: a unicentre randomized trial with a 124-month median follow-up. Institut Bergonie Bordeaux Groupe Sein (IBBGS). Ann Oncol 1999;10(1):47-52. [DOI] [PubMed]
- 24.Powles TJ, Hickish TF, Makris A, Ashley SE, O'Brien ME, Tidy VA, et al. Randomized trial of chemoendocrine therapy started before or after surgery for treatment of primary breast cancer. J Clin Oncol 1995;13(3):547-52. [DOI] [PubMed]
- 25.Makris A, Powles TJ, Ashley SE, Chang J, Hickish T, Tidy VA, et al. A reduction in the requirements for mastectomy in a randomized trial of neoadjuvant chemoendocrine therapy in primary breast cancer. Ann Oncol 1998; 9(11):1179-84. [DOI] [PubMed]
- 26.De Placido S, Perrone F, Carlomagno C, Morabito A, Pagliarulo C, Lauria R, et al. CMF vs alternating CMF/EV in the adjuvant treatment of operable breast cancer. A single centre randomised clinical trial (Naples GUN-3 study). Br J Cancer 1995;71(6):1283-7. [DOI] [PMC free article] [PubMed]
- 27.Casper ES, Guidera CA, Bosl GJ, Hakes TB, Kaufman RJ, Shurgot B, et al. Combined modality treatment of locally advanced breast cancer: adjuvant combination chemotherapy with and without doxorubicin. Breast Cancer Res Treat 1987;9:39-44. [DOI] [PubMed]
- 28.Bull JM, Tormey DC, Li SH, Carbone PP, Falkson G, Blom J, et al. A randomized comparative trial of adriamycin versus methotrexate in combination drug therapy. Cancer 1978;41(5):1649-57. [DOI] [PubMed]
- 29.Falkson G, Tormey DC, Carey P, Witte R, Falkson HC. Long-term survival of patients treated with combination chemotherapy for metastatic breast cancer. Eur J Cancer 1991;27(8):973-7. [DOI] [PubMed]
- 30.Hutchins L, Green S, Ravdin P, Lew D, Martino S, Abeloff M, et al. CMF versus CAF with and without tamoxifen in high-risk node-negative breast cancer patients and a natural history follow-up study in low-risk node-negative patients: first results of Intergroup Trial INT 0102. Prog Proc Am Soc Clin Oncol 1998;1a.
- 31.Mouridsen HT, Andersen J, Andersson M, Dombernowsky P, Ejlertsen B, Rose C, et al. Adjuvant anthracycline in breast cancer. Improved outcome in premenopausal patients following substitution of methotrexate in the CMF combination with epirubicin [abstract]. Prog Proc Am Soc Clin Oncol 1999;18:68a.
- 32.Levine MN, Bramwell VH, Pritchard KI, Norris BD, Shepherd LE, Abu-Zahra H, et al. Randomized trial of intensive cyclophosphamide, epirubicin, and fluorouracil chemotherapy compared with cyclophosphamide, methotrexate, and fluorouracil in premenopausal women with node-positive breast cancer. National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 1998;16(8):2651-8. [DOI] [PubMed]
- 33.Fisher B, Brown AM, Dimitrov NV, Poisson R, Redmond C, Margolese RG, et al. Two months of doxorubicin-cyclophosphamide with and without interval reinduction therapy compared with 6 months of cyclophosphamide, methotrexate, and fluorouracil in positive-node breast cancer patients with tamoxifen-nonresponsive tumors: results from the National Surgical Adjuvant Breast and Bowel Project B-15. J Clin Oncol 1990;8(9):1483-96. [DOI] [PubMed]
- 34.French Adjuvant Study Group. Benefit of a high-dose epirubicin regimen in adjuvant chemotherapy for node-positive breast cancer patients with poor prognostic factors: 5-year follow-up results of French Adjuvant Study Group 05 randomized trial. J Clin Oncol 2001;19(3):602-11. [DOI] [PubMed]
- 35.Fumoleau P, Kerbrat P, Romestaing P, Fargeot P, Brémond A, Namer M, et al. Randomized trial comparing six versus three cycles of epirubicin-based adjuvant chemotherapy in premenopausal, node-positive breast cancer patients: 10-year follow-up results of the French Adjuvant Study Group 01 trial. J Clin Oncol 2003;21:298-305. [DOI] [PubMed]
- 36.Seidman AD, Reichman BS, Crown JP, Yao TJ, Currie V, Hakes TB, et al. Paclitaxel as second and subsequent therapy for metastatic breast cancer: activity independent of prior anthracycline response. J Clin Oncol 1995;13 (5):1152-9. [DOI] [PubMed]
- 37.Valero V, Holmes FA, Walters RS, Theriault RL, Esparza L, Fraschini G, et al. Phase II trial of docetaxel: a new, highly effective antineoplastic agent in the management of patients with anthracycline-resistant metastatic breast cancer. J Clin Oncol 1995;13(12):2886-94. [DOI] [PubMed]
- 38.Henderson IC, Berry DA, Demetri GD, Cirrincione CT, Goldstein LJ, Martino S, et al. Improved outcomes from adding sequential paclitaxel but not from escalating doxorubicin dose in an adjuvant chemotherapy regimen for patients with node positive primary breast cancer. J Clin Oncol 2003;21:976-83. [DOI] [PubMed]
- 39.Mamounas E, Bryant J, Lembersky BC, Fisher B, Atkins JN, Fehrenbacher L, et al. Paclitaxel following doxorubicin/cyclophosphamide as adjuvant chemotherapy for node positive breast cancer: Results from NSABP B-28 [abstract]. Prog Proc Am Soc Clin Oncol 2003;22:4. [DOI] [PubMed]
- 40.Citron ML, Berry, DA, Cirrincione C, Hudis C, Winer EP, Gradishar WJ, et al. Randomized trial of dose-dense conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: first report of Intergroup Trial C9741/Cancer and Leukemia Group B Trial 9741. J Clin Oncol 2003; 21:1431-9. [DOI] [PubMed]
- 41.Nabholtz JM, Pienkowski T, Mackey J, Pawlicki M, Guastalla JP, Vogel C, et al. Phase III trial comparing TAC (docetaxel, doxorubicin, cyclophosphamide) with FAC (5-fluorouracil, doxorubicin, cyclophosphamide) in the adjuvant treatment of node positive breast cancer (BC) patients: interim analysis of the BCIRG 001 study [abstract]. Proc Am Soc Clin Oncol 2002; 21:36a.
- 42.Buzdar AU, Singletary SE, Theriault RL, Booser DJ, Valero V, Ibrahim N, et al. Prospective evaluation of paclitaxel versus combination chemotherapy with fluorouracil, doxorubicin, and cyclophosphamide as neoadjuvant therapy in patients with operable breast cancer. J Clin Oncol 1999;17(11):3412-17. [DOI] [PubMed]
- 43.Smith IC, Heys SD, Hutcheon AW, Miller ID, Payne S, Gilbert FJ, et al. Neoadjuvant chemotherapy in breast cancer: significantly enhanced response with docetaxel. J Clin Oncol 2002;20(6):1456-66. [DOI] [PubMed]
- 44.Heys SD, Hutcheon AW, Sarkar TK, Ogston KN, Miller ID, Payne S, et al. Neoadjuvant docetaxel in breast cancer: 3-year survival results from the Aberdeen Trial. Clinical Breast Cancer 2002;3 (Suppl 2):S69-74. [DOI] [PubMed]
- 45.Bear HD, Anderson S, Brown A, Smith R, Mamounas P, Fisher B, et al. The effect on tumor response of adding preoperative docetaxel to preoperative doxorubicin and cyclophosphamide: preliminary results from National Surgical Adjuvant Breast and Bowel Project protocol B-27. J Clin Oncol 2003; 21(22):4165-74. [DOI] [PubMed]
- 46.Wood WC, Budman DR, Korzun AH, Cooper MR, Younger J, Hart RD, et al. Dose and dose intensity of adjuvant chemotherapy for stage II, node-positive breast carcinoma [published erratum appears in N Engl J Med 1994; 331(2):139]. N Engl J Med 1994;330(18):1253-9. [DOI] [PubMed]
- 47.Stadtmauer EA, O'Neill A, Goldstein LJ, Crilley PA, Mangan KF, Ingle JN, et al. Conventional-dose chemotherapy compared with high-dose chemotherapy plus autologous hematopoietic stem-cell transplantation for metastatic breast cancer [comment in N Engl J Med 2000;342(15):1119-20]. N Engl J Med 2000;342(15):1069-76. [DOI] [PubMed]
- 48.Peters W, Rosner G, Vredenburgh J, Sphall E, Crump M, Richardson P, et al. A prospective randomized comparison of two doses of combination alkylating agents as consolidation after CAF in high-risk primary breast cancer involving ten or more axillary lymph nodes [abstract]. Proc Am Soc Clin Oncol 1999;18:2a.
- 49.Bonadonna G, Valagussa P, Brambilla C, Ferrari L, Moliterri A, Terenziani M, et al. Primary chemotherapy in operable breast cancer: eight-year experience at the Milan Cancer Institute. J Clin Oncol 1998;16(1):93-100. [DOI] [PubMed]
- 50.Kuerer HM, Newman LA, Smith TL, Ames FC, Hunt KK, Dhingra K, et al. Clinical course of breast cancer patients with complete pathologic primary tumor and axillary lymph node response to doxorubicin-based neoadjuvant chemotherapy. J Clin Oncol 1999;17(2):460-9. [DOI] [PubMed]
- 51.International Breast Cancer Study Group. Duration and reintroduction of adjuvant chemotherapy for node positive breast cancer patients: an update based on 10 years follow-up. J Clin Oncol 1996;14:1885-94. [DOI] [PubMed]
- 52.Bartelink H, Rubens RD, van der Schueren E, Sylvester R. Hormonal therapy prolongs survival in irradiated locally advanced breast cancer: a European Organization for Research and Treatment of Cancer randomized phase III trial. J Clin Oncol 1997;15(1):207-15. [DOI] [PubMed]
- 53.Albain KS, Green SJ, Ravdin PM, Cobau CD, Levine EG, Ingle JN, et al. Adjuvant chemohormonal therapy for primary breast cancer should be sequential instead of concurrent: initial results of Intergroup Trial 0100 (SWOG-8814) [abstract]. Prog Proc Am Soc Clin Oncol 2002;21:37.
- 54.ATAC (Arimidex, Tamoxifen Alone or in Combination) Trialists' Group. Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early breast cancer: first results of the ATAC randomised trial. Lancet 2002;359:2131-9. [DOI] [PubMed]
- 55.Winer EP, Hudis C, Burstein HJ, Chlebowski RT, Ingle JN, Edge SB, et al. American Society of Clinical Oncology technology assessment on the use of aromatase inhibitors as adjuvant therapy for women with hormone receptor-positive breast cancer: status report 2002. J Clin Oncol 2002;20:3317-27. [DOI] [PubMed]
- 56.Alagaratnam TT, Wong J. Tamoxifen versus chemotherapy as adjuvant treatment in stage III breast cancer. Aust N Z J Surg 1986;56(1):39-41. [DOI] [PubMed]
- 57.Willsher PC, Robertson JFR, Armitage NC, Morgan DAL, Nicholson RI, Blamey RW. Locally advanced breast cancer: early results of a randomized trial comparing primary treatment with tamoxifen or radiotherapy in post-menopausal women. Eur J SurgOncol 1996;22(1):34-7. [DOI] [PubMed]
- 58.Willsher PC, Robertson JFR, Chan SY, Jackson L, Blamey RW. Locally advanced breast cancer: early results of a randomised trial of multimodal therapy versus initial hormone therapy. Eur J Cancer 1997;33(1):45-9. [DOI] [PubMed]
- 59.Olson JE, Neuberg D, Pandya K, Richter MP, Solin LJ, Gilchrist KW, et al. The role of radiotherapy in the management of operable locally advanced breast carcinoma. Results of a randomized trial by the Eastern Cooperative Oncology Group. Cancer 1997;79(6):1138-49. [PubMed]
- 60.Fowble B. Postmastectomy radiation: a modest benefit prevails for high risk patients [editorial]. Cancer 1997;79(6):1061-6. [DOI] [PubMed]
- 61.Papaioannou A, Lissaios B, Vasilaros S, Miligos S, Papadimitriou G, Kondilis D, et al. Pre- and postoperative chemoendocrine treatment with or without postoperative radiotherapy for locally advanced breast cancer. Cancer 1983;51(7):1284-90. [DOI] [PubMed]
- 62.Ragaz J, Jackson SM, Le N, Plenderleith IH, Spinelli JJ, Basco VE, et al. Adjuvant radiotherapy and chemotherapy in node-positive premenopausal women with breast cancer. N Engl J Med 1997;337(14):956-62. [DOI] [PubMed]
- 63.Overgaard M, Hansen PS, Overgaard J, Rose C, Andersson M, Bach F, et al. Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. N Engl J Med 1997;337(14):949-55. [DOI] [PubMed]
- 64.Overgaard M, Jensen M-B, Overgaard J, Hansen PS, Rose C, Andersson M, et al. Postoperative radiotherapy in high-risk postmenopausal breast-cancer patients given adjuvant tamoxifen: Danish Breast Cancer Cooperative Group DBCG 82c randomised trial. Lancet 1999;353:1641-8. [DOI] [PubMed]
- 65.Whelan T, Julian J, Wright J, Jadad AR, Levine ML. Does locoregional radiation therapy improve survival in breast cancer? A meta-analysis. J Clin Oncol 2000;18(6):1220-9. [DOI] [PubMed]
- 66.Recht A, Edge SB, Solin LJ, Robinson DS, Estabrook A, Fine RE, et al. Postmastectomy radiotherapy: guidelines of the American Society of Clinical Oncology. J Clin Oncol 2001;19:1539-69. [DOI] [PubMed]
- 67.Perloff M, Lesnick GJ, Korzun A, Chu F, Holland JF, Thirlwell MP, et al. Combination chemotherapy with mastectomy or radiotherapy for stage III breast carcinoma: a Cancer and Leukemia Group B study. J Clin Oncol 1988;6 (2):261-9. [DOI] [PubMed]
- 68.Mourali N, Tabbane F, Muenz LR, Behi J, Moussa FB, Jaziri M, et al. Ten-year results utilizing chemotherapy as primary treatment in nonmetastatic, rapidly progressing breast cancer. Cancer Invest 1993;11(4):363-70. [DOI] [PubMed]
- 69.De Lena M, Varini M, Zucali R, Rovini D, Viganotti G, Valagussa P, et al. Multimodal treatment for locally advanced breast cancer. Results of chemotherapy-radiotherapy versus chemotherapy-surgery. Cancer Clin Trials 1981; 4(3):229-36. [PubMed]
- 70.Pierce LJ, Lippman M, Ben-Baruch N, Swain S, O'Shaughnessy J, Bader JL, et al. The effect of systemic therapy on local-regional control in locally advanced breast cancer. Int J Radiat Oncol Biol Phys 1992;23(5):949-60. [DOI] [PubMed]
- 71.Perez CA, Fields JN, Fracasso PM, Philpott G, Soares RL, Jr, Taylor ME, et al. Management of locally advanced carcinoma of the breast . II. Inflammatory carcinoma. Cancer 1994;74(1 Suppl):466-76. [DOI] [PubMed]
- 72.Arriagada R, Mouriesse H, Sarrazin D, Clark RM, Deboer G. Radiotherapy alone in breast cancer. I. Analysis of tumor parameters, tumor dose and local control. Int J Rad Oncol Biol Phys 1985;11:1751-7. [DOI] [PubMed]
- 73.Van Limbergen E, Van der Schueren E, Van den Bogaert W, Van Wing J. Local control of operable breast cancer after radiotherapy alone. Eur J Cancer 1990;26(6):674-9. [DOI] [PubMed]


