Abstract
Background
Stimulation of a variety of brain sites electrically or by opiates activates descending inhibitory pathways to attenuate noxious input to the spinal cord dorsal horn and produce analgesia. Analgesia induced by electrical stimulation of the periaqueductal gray of the midbrain (PAG) or medial rostral ventral medulla (RVM) matures late, towards the end or past the preweaning period. Descending facilitation takes precedence over inhibition. Yet opiates injected ICV or directly into the PAG induce analgesia relatively early in development. Our goal was to reexamine the role of opiates specific to individual receptor types in analgesia at several supraspinal sites.
Methods
Antinociception was tested following microinjection of DAMGO (μ opiate agonist), DPDPE (∂ opiate agonist) or U50,488 (κ opiate agonist) into the PAG, RVM, or dorsal lateral pons (DLP) in 3-, 10- and 14-day-old rats.
Results
DAMGO produced analgesia at 3 days of age at each brain area; the RVM was the most effective and the dorsal PAG the least effective site. DPDPE produced modest analgesia at 14 days of age at the ventral PAG, RVM or DLP but not the dorsal PAG. U50,488H was ineffective at all sites and all ages.
Conclusions
Antinociception could be elicited at all three sites by DAMGO as early as 3 days of age and DPDPE at 10 and 14 days of age. The degree of analgesia increased gradually during the first two weeks of life and likely reflects the maturation of connections within the brain and of descending inhibitory paths from these sites.
Introduction
Opiates produce analgesia at brain sites that activate descending inhibitory pathways [for review (Stamford, 1995; Vanegas, 2004; Willis, 1988)]. Electrical and opiate stimulation of the dorsal or ventral periaqueductal gray of the midbrain (dPAG/vPAG), rostral ventral medulla (RVM), or dorsal lateral pons (DLP) produce analgesia in the adult [for reviews (D’Mello and Dickenson, 2008 ; Rosenfeld, 1994)]. The dPAG and vPAG are distinct structures with different anatomy, projections and behavioral functions (Bandler et al., 1985; Cameron et al., 1995b; Morgan and Liebeskind, 1987). The PAG projects indirectly to the spinal cord through medullary structures to inhibit nociception (Beitz, 1982; Cameron et al., 1995b; Chieng and Christie, 1994b; Gallagher and Pert, 1978; Jones and Gebhart, 1988; Morgan and Liebeskind, 1987; Morgan et al., 2008). In the infant rat, electrical stimulation of the PAG (van Praag and Frenk, 1991) or the RVM (Hathway et al., 2009) is not antinociceptive until the third postnatal week. This was attributed to the dominance of descending facilitation over descending inhibition (Hathway et al., 2009). In contrast, morphine injected into the ventricular system or the ventral PAG produces analgesia at considerably earlier ages (Barr et al., 1992; Kehoe and Blass, 1986; Tive and Barr, 1992; Tseng et al., 1995). Therefore, one goal was to investigate opiate stimulation of the PAG, RVM and DLP to determine the developmental course of pain inhibition from these supraspinal sites.
Opioid receptor types develop differentially and opiates selective for different receptors have developmentally unique behavioral functions (Carden et al., 1991; Jackson and Kitchen, 1989b; McLaughlin and Dewey, 1994). μ- and k-Opioid receptors are present in brain at birth and mature slowly over the first two postnatal weeks. ∂-Opioid receptors appear in substantial numbers only towards the end of the second postnatal week (Jackson and Kitchen, 1989a; Leslie et al., 1982). There are no data on the analgesic effects of specific opioid ligands in any brain region in the developing animal. A second goal was to define the development of analgesia mediated by specific opioid receptors.
Analgesia occurs earlier when noxious stimuli are applied to the forepaw than to the hindpaw or tail. One explanation is the slow development of functional descending inhibition of dorsal horn neurons (Barr et al., 1992; Fitzgerald, 1986). However, maturational differences in the projections of the PAG to rostral medullary sites could also account for this difference. If the early developing rostral (e.g. forepaw) analgesia were due solely to the maturation of descending caudally projecting pathways, then the same pattern of analgesia should be seen following stimulation of the DLP and RVM as the PAG. If analgesia from PAG stimulation were due to differential maturation of projections from the PAG to the RVM or DLP, then the pattern of analgesia following stimulation of the these sites would differ. Thus, a third goal was to compare the development of analgesia from the PAG and downstream targets, the RVM and DLP, which send serotonergic and noradrenergic projections, respectively, to the spinal cord dorsal horn.
Methods
Subjects
Animals were male and female offspring of Long Evans hooded rats bred in our animal colony. We analyzed all baseline (e.g. vehicle) and drug response (DAMGO and DPDPE) at each site and age. We did not observe any sex differences, which is consistent with our prior work. Therefore we combined the data from both males and females together. The sex difference latency data and analyses are presented Supplemental Figure S1. Animals had free access to food and water. Pups remained with their dam in a temperature and humidity controlled breeding colony until the day of surgery. Four hundred eight pups from 120 litters were used, with five to 7 litters tested at each site and age for each drug, except as noted below. All experiments followed the ethical guidelines of the Society for Neuroscience and Society for Developmental Psychobiology and were approved by IACUC.
Surgery
The surgical methods used here are similar to those used to implant cannulas into brain sites in adult animals and modifications for infants have been described in detail previously (Barr, 1991; Capuano et al., 1992a, b; Chan et al., 2011). All surgery, testing and perfusions occurred on the same day. Briefly, four pups were removed from a single litter in the early morning and kept together in an incubator. Each pup in the single litter was implanted at a single anatomical target. Individual pups were deeply anesthetized by inhalation and the skull exposed. Pups were mounted in a modified stereotaxic device (Heller et al., 1979). A hole was drilled through the skull and the dura punctured. A 26-gauge guide cannula was lowered (PlasticsOne) and cemented to the skull using a thin layer of Caulk grip cement and a thicker layer of dental acrylic cement. No sutures were required. After the cement dried a stylet was placed in the guide cannula to keep it patent. Guide cannulas were implanted 0.75 to 1.0 mm above the target site; Supplemental Table S1 lists the co-ordinates. Pups were kept together in the litter in a warm chamber to reduce stress with access to milk. At the time of testing, approximately 8 hours after surgery, all pups were normally active and awake.
Drugs and Injections
Different litters were used at different ages. Each pup in each litter received a single dose of one drug and all doses including vehicle were given within a single litter. DAMGO (μ-opiate agonist) was tested at 3, 10 and 14 days of age at all sites; these ages were chosen on the basis of prior data with morphine injected into brain and because of evidence that descending inhibition begins to mature during the latter part of this developmental period (Fitzgerald, 1986; Hathway et al., 2009; Tive and Barr, 1992; Tseng et al., 1995; van Praag and Frenk, 1991). DPDPE (∂-opiate agonist) was tested only at 10 and 14 days of age for the ventral PAG, RVM and DLP because of the paucity of receptors in brain at younger ages (Kornblum et al., 1987; McDowell and Kitchen, 1986, 1987; Spain et al., 1985). DPDPE was tested with four litters at 10 days of age at the dorsal PAG site. It was without effect and not tested further at that age. U50,488 (k-opiate agonist) was tested at 3 and 14 days of age. It was non-analgesic at 14 days, and only two litters were tested at the 3-day-old age. It was also without effect in those two litters and testing ended for that drug.
Individual pups were removed from the incubator, a 30 gauge injector cannula (PlasticsOne) filled with drug or vehicle was lowered into the guide cannula. The drug or vehicle was slowly infused using a 1.0 μl syringe. The injector extended 0.75-1.0 mm beyond the guide cannula. The injector was slowly removed after 2 minutes and the stylet replaced. Drugs were the saline vehicle, DAMGO (0.05, 0.15 or 0.50 μg/ 200 nl), DPDPE 1.0, 3.0 or 10.0 μg/ 400 nl), or U50,488 (0.3, 1.0 or 3.0 μg/ 200 nl), which are selective for μ-, ∂- and k-opioid receptors specifically. The 200 nl volume was based on prior work that showed limited spread as measured by radiolabeled injected ligands and anatomical specificity in behavioral studies (Capuano et al., 1992b; Capuano et al., 1992c; Chen et al., 2006). The different injection volume for DPDPE was due to the limited solubility of the ∂- opioid peptide. The experimenter knew which compound was tested that day but was blind to the dose, including the vehicle.
Test for antinociception
Tests were conducted 15 minutes after drug injection using three water baths maintained at 44°, 47° and 52° C. The different temperatures were used because the intensity of the stimuli has been shown to be a factor in whether or not certain opiates are analgesic (Saeki and Yaksh, 1993; Wheeler-Aceto and Cowan, 1991). The forepaw, hindpaw and tail were each tested at the three temperatures for each pup. The entire test procedure for each animal took less than 5 minutes. The baseline latencies in the 44°C test were long and variable, ranging between 5-15 seconds, and likely were not reflective of withdrawal from a noxious stimulus. Thus only the 47°C and the 52°C tests are reported here. The latencies for these temperatures are very comparable to those of naïve, non-implanted animals under very similar testing conditions (Hughes and Barr, 1988) suggesting that the surgery and cannula implant had little effect on baseline responding.
The pup was picked up and gently held in position for testing. The index finger was placed on one side of the neck and the thumb and two other fingers wrapped around the torso. The pinky finger was placed between the tail and hindpaw. In this position, the paws and tail were extruded from the experimenter’s hand and could easily be manipulated for the test. The distal 2/3 of each appendage was then immersed sequentially in each water bath. The latency to initiate withdrawal was electronically measured using a foot operated digital timer. Latencies were rounded to the nearest 10th second and were used in the statistical analyses. Cutoff was 20 seconds. The order of testing for both the water temperature and the limb was randomly determined for each animal. No test temperature produced tissue damage.
Histology
After the litter was tested, each pup was overdosed with an anesthetic and perfused with saline followed by 10% formalin. Thirty μm frozen sections were cut, stained with cresylviolet, and examined under a light microscope. The location of each injection site was plotted on existing atlases (Paxinos et al., 1991; Sherwood and Timiras, 1971). The histological analysis was conducted without knowledge of the behavioral results. For brevity, we present the placement data for the 3-day-old pups only at the 47° temperature. Data from the 10- and 14-day-old animals showed virtually identical patterns and those data did not alter our interpretations and conclusions.
Data analysis of withdrawal latencies
The latency to withdraw the appendage was the dependent measure. An overall analysis of variance was conducted for each drug. For DPDPE this was for 14 days of age only. Subsequent analyses were performed for each site. The factors were the age of the pup, dose of the drug, test bath temperature and limb. Age was a between subjects variable; dose was a within litter variable. Temperature and appendage were within subject variables. For the initial analysis, the injection site was also a between subject factor. Both within litter and within subject variables were treated as repeated measures variables. Posthoc tests were by Newman-Keul statistics. The alpha level was 0.05.
Data analysis of cannula placements
To ascertain whether or not there were topographic patterns within a brain site, the histological results were analyzed. For this purpose, an analgesic response was defined as a latency of greater than 10 seconds. The selection of this response latency to define analgesia for the purposes of localizing effective sites was somewhat arbitrary but small changes in the definition made little difference in the results. For example, changing the definition from >10 seconds to >5 seconds would have added fewer than 6% analgesic sites for all temperatures. The latency data were analyzed for all animals regardless of whether the site was accurately placed. Because most sites were on-target, those few data points did not greatly influence the results and because of the within litter design, elimination of a single animal would necessitate discarding data from all animals in the litter or would require imputing missing data [see (Festing, 2006) for a discussion ]. The inclusion of the few misses is in general a conservative approach and the specific placements and their effects are evident on the histological plots.
Results
Overall Analyses
The omnibus ANOVA for DAMGO showed a significant four-way interaction among the appendage tested, dose of DAMGO, region stimulated, and age (F(36,372)=1.76; p<.01) There were significant main effects for the region stimulated, age of the animal, and dose of DAMGO (F(3,62)= 19.66; F(2,62)=12.25; F(3,186)=247.37; all p<.0001). Posthoc analyses of the regions showed that the stimulation of the NRM was most effective and the dorsal PAG the least effective. The DLP and ventral PAG did not differ from each other.
The overall analysis of variance for DPDPE for the 14-day-old pups showed a significant difference among regions (F(3,36)=4.50; p<.01). Posthoc analysis showed that stimulation of the dorsal PAG was less antinociceptive than stimulation of other areas, which did not differ from each other. The analgesic effects of DPDPE in the ventral PAG, RVM and DLP, though statistically significant, were modest.
The κ agonist U50,488 was ineffective in all regions (Supplemental Table S2) and is not discussed further.
We present the following results by implantation site and then by drug.
Periaqueductal gray
DAMGO
Behavioral data-Ventral PAG
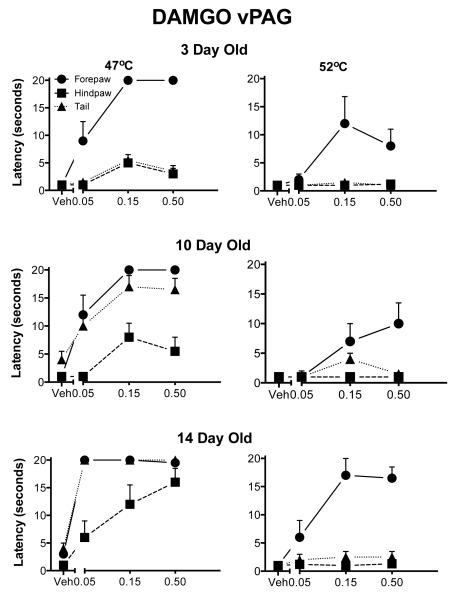
The main interaction of interest was the four-way interaction of dose, appendage, test temperature and age (F(12,102)=2.95; p<.005; Fig. 1). The main effects of dose, age and appendage were all significant. There was a general rostral to caudal maturation. DAMGO was antinociceptive in the forepaw test at 3 days of age across temperatures. Analgesia in the hindpaw and tail was not seen at this age. As the animal matured, the withdrawal response latency lengthened in the tail and the hindpaw tests. Stimulation of the ventral PAG was more effective for the tail than for the hindpaw at the older ages, as reported previously for morphine (Barr et al., 1992).
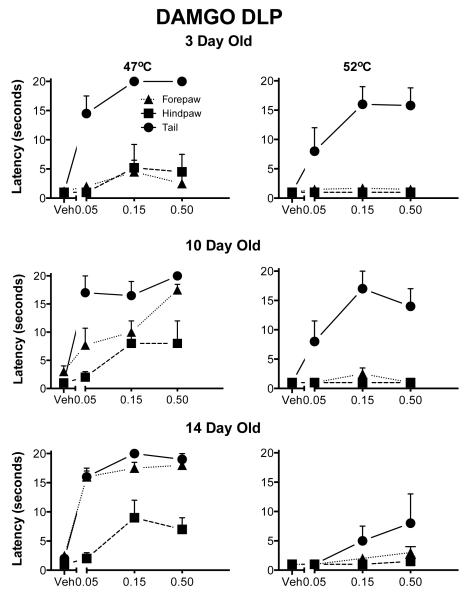
Fig. 1. Withdrawal latencies following injection of DAMGO into the ventral aspects of the PAG.
The three rows correspond to the three ages. The two columns show the two different test temperatures. The Y-axis is the mean withdrawal latency (± 1 SEM) and the dose of the drug is on the X-axis, including vehicle (Veh). The forepaw is denoted by circles, the hindpaw by squares and the tail by triangles. There was a general increase in antinociception in all three appendages with age. Withdrawal of the forepaw was increased consistently by DAMGO even at the highest temperature.
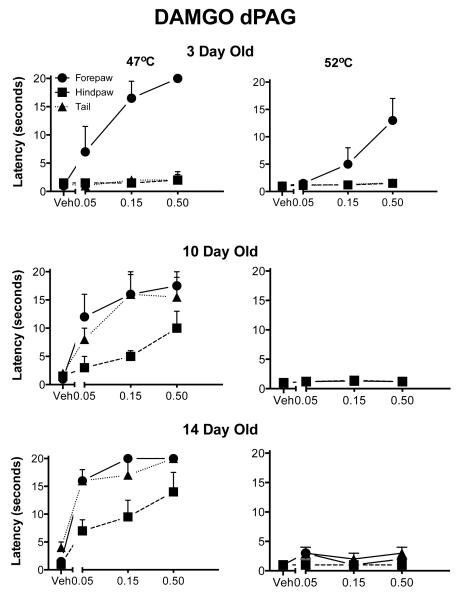
Behavioral data-Dorsal PAG
There was a significant three-way interaction among dose, appendage and age (F(12,84)=5.39; p<.000l; Fig. 2). The development of analgesia for the 47°C temperature was comparable to that of the ventral PAG. At the higher temperature, DAMGO stimulation of the dorsal PAG was not analgesic with the single exception of the highest dose in the 3-day-old pups. The differences between the dorsal PAG and the ventral PAG were due to this ineffectiveness of DAMGO at the higher temperature.
Fig. 2. Withdrawal latencies following injection of DAMGO into the dorsal aspects of the PAG.
The details are as in Fig. 1. There was significant analgesia in the forepaw at three days of age and increased antinociception in the hindpaw and tail with increasing age. At the highest temperature, only the 3 day old pups showed any analgesia and then only in the forepaw test.
Histology
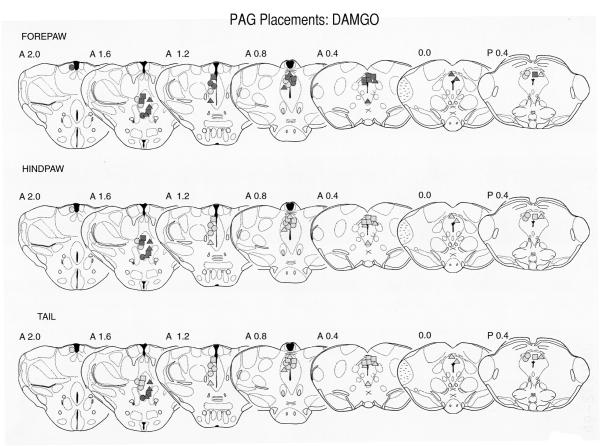
Data from the 3-day-old pups at the 47° temperature only are presented for clarity (Fig. 3). Placements and data for the 10- and 14-day-old pups were analyzed and the results were quite similar to those of the 3-day-old.
Fig. 3.
Localization of DAMGO injection sites within the PAG of three day old pups. Data are from the 47° C. test only. Sections are adapted from the Sherwood and Timiras atlas (Sherwood and Timiras, 1971) and the numbers to the upper left of each section are the AP planes from that atlas. The data are plotted by both dose and by effectiveness in the forepaw, hindpaw and tail withdrawal tests. The different appendages are depicted in the three rows of sections. The circles represent the lowest dose (0.05 μg), squares the middle dose (0.15 μg), and the triangles the highest dose (0.50 μg). Lightly shaded symbols represent ineffective sites in which the withdrawal latencies were less than 10 seconds. The more darkly shaded symbols represent sites with withdrawal latencies greater than 10 seconds. In general, higher doses were more effective and the forepaw more sensitive to the effects of DAMGO. This agrees with the latency data (Fig. 2). Both dorsal and ventral sites were effective, although there is suggestion that the more caudal sites were less analgesic (e.g. p0.4 in the forepaw withdrawal test and caudal to A1.2 in the hindpaw and tail tests.).
Most sites were located in the rostral half of the PAG, although there was a tendency for the ventral sites to be more rostral than the dorsal sites. It is not likely that this is the reason that the ventral sites were more effective since ventral sites were more effective even when they were in the same rostral/caudal plane (e.g. Fig. 3, section 1.6). There was a significant dose dependent effect for the forepaw.
DPDPE
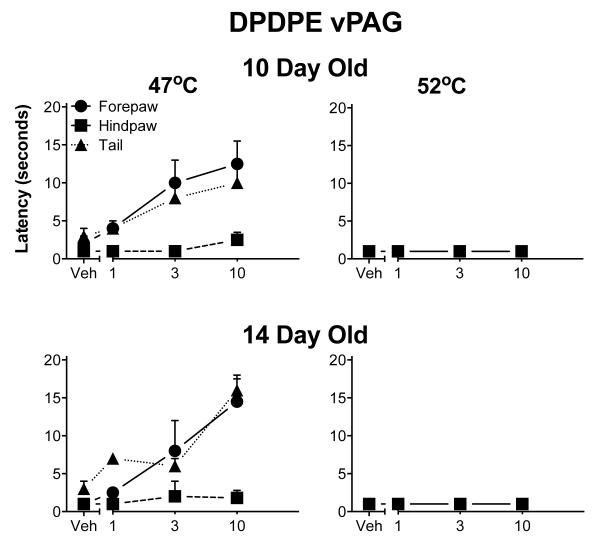
Behavioral data-Ventral PAG
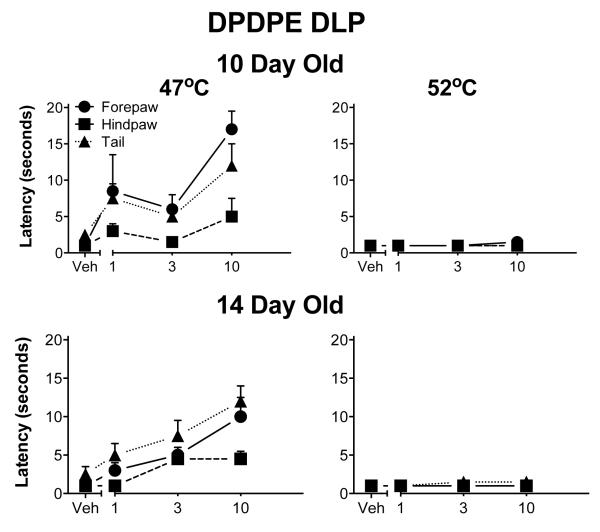
DPDPE produced a limited degree of analgesia, mostly noted at the lower 47°C test temperature. The 10 and 14-day-old pups did not differ from each other. The antinociceptive effect was modest and limited to the forepaw and tail. DPDPE was totally without effect at the highest test temperature (Fig. 4). No analgesia was noted at any temperature following DPDPE injection into the dorsal PAG in 14-day-old pups (data not shown). No other age was tested.
Fig. 4.
Withdrawal latencies following injection of DPDPE into the ventral aspects of the PAG. The details are as in Fig. 1, except that 3-day-old pups were not tested. DPDPE was less effective than DAMGO. DPDPE was never fully effective at 47° C and not analgesic at all at the highest temperature. Furthermore, DPDPE was minimally effective in the hindpaw except at the lowest temperature.
Histology
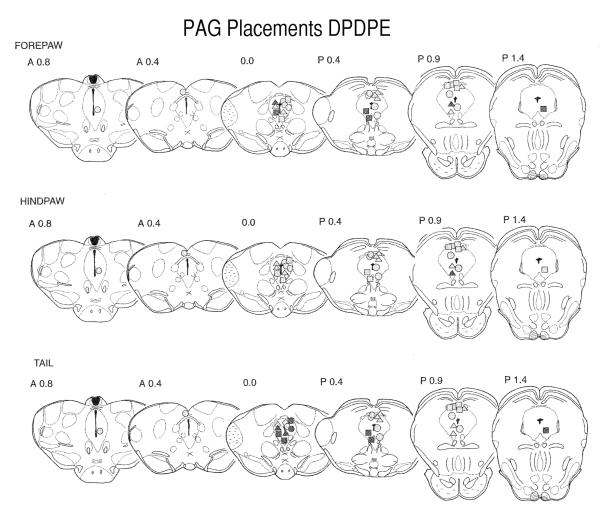
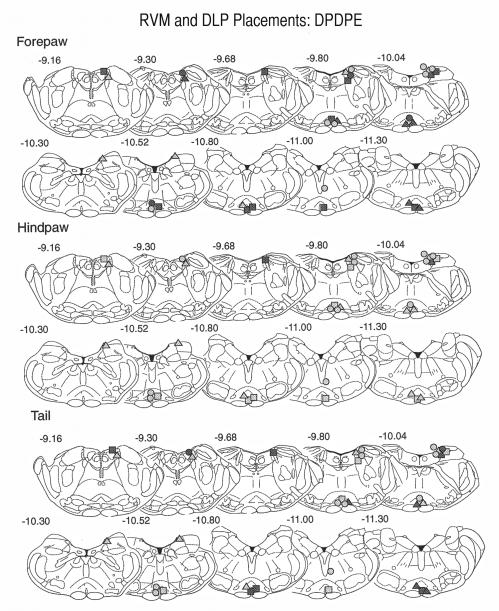
The histology was plotted for 14-day-old pups to include both the ventral and dorsal PAG sites (Fig. 5). Injections were, on average, slightly caudal to the DAMGO sites, although most sites were still in the rostral half of the PAG. Positive (analgesic) sites were located almost exclusively in the ventral PAG.
Fig. 5.
Placement of DPDPE injection sites within the PAG of 14-day-old pups. The sections are adapted from the Sherwood and Timiras atlas (Sherwood and Timiras, 1971). Other details are as for the legend in Fig. 3. Fewer sites were positive, even at this age, than for DAMGO. With two exceptions, there were no sites above the aqueduct that produced latencies greater than 10 seconds and only one site was positive in the hindpaw test.
Rostral ventral medulla
DAMGO
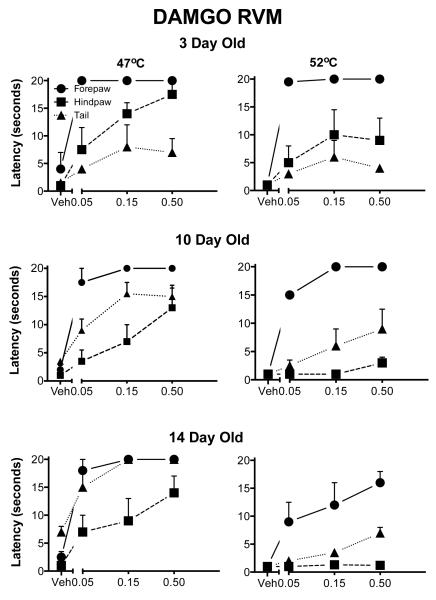
Behavioral Data
The main effects of appendage, temperature and dose were significant (all p<.000l; Fig. 6). However, there was no significant age effect; nor was there a significant interaction of age with the dose of DAMGO. There were significant three-way interactions of appendage, dose and age (p=0.02), suggesting that the development of analgesia depended, in part, on the appendage tested. At all ages the forepaw latencies were close to the cutoff, although analgesia was seen also in the hindpaw and tail.
Fig. 6.
Withdrawal latencies following injection of DAMGO into the RVM. The details are as in Fig. 1. At three days of age DAMG0 induced analgesia in all appendages although it was more effective in the forepaw. Unlike other sites, even at the highest test temperatures, DAMGO was analgesic at all ages.
Histology
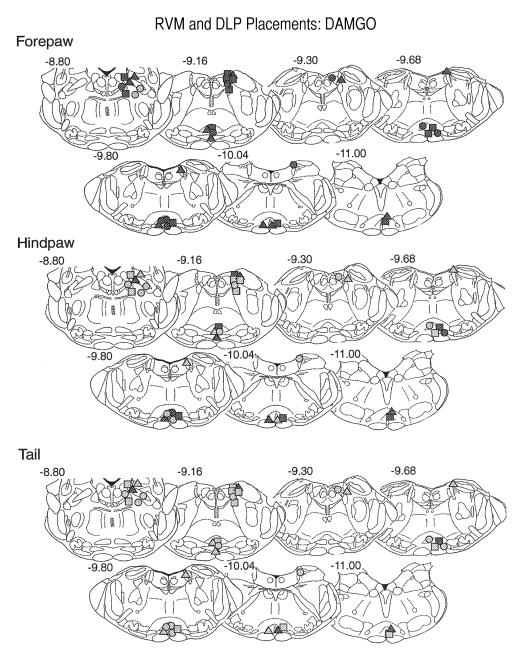
Virtually all sites were within the medial RVM, and in particular in the nucleus raphe magnus. There was no obvious site specificity, in part because of the close proximity of cannula placements in the dorsal/ventral and lateral planes (e.g. Fig. 7). Analgesia was noted at all placements in the forepaw, regardless of dose. Few sites were positive in the tail withdrawal test and no low dose placements produced analgesia. The histological results for the hindpaw were in between (as were the latency results), with most of the medium and high doses producing analgesia but with only one active low dose site.
Fig. 7.
Placement of DAMG0 injection sites within the RVM and the DLP of three-day-old pups. The sections are adapted from the Paxinos et al. atlas (Paxinos et al., 1991) and the numbers in the upper right of the section are the AP plane from that atlas. Other details are as for the legend in Fig. 3. In general, higher doses were more effective and the forepaw test more sensitive to the effects of DAMG0. This agrees with the latency data. More rostral sites were more effective than caudal sites, especially for the hindpaw and tail. Few low-dose sites were positive in the RVM in the hindpaw and tail test.
DPDPE
Behavioral data
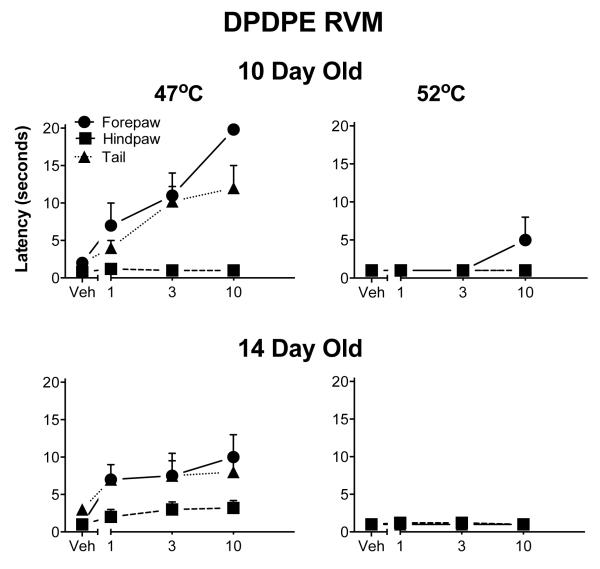
As in the ventral PAG, DPDPE produced but a modest analgesia in the RVM, again mostly noted at the 47° C test temperature. Even that effect was small and limited to the forepaw and tail. DPDPE was again without effect at the highest test temperature (Fig. 8).
Fig. 8.
Withdrawal latencies following injection of DPDPE into the RVM. The details are as in Fig. 1, except that 3-day-old pups were not tested. DPDPE was also less effective than DAMGO at this site. DPDPE was never fully effective at 47° C and not analgesic at all at the highest temperature. Furthermore, it was minimally effective in the hindpaw.
Histology
Most placements were analgesic in the forepaw withdrawal test (Fig. 9). The only exceptions were the low dose sites, one of which was dorsal to the intended placement. Only the medium and high doses of DPDPE were effective in the tail flick test and no sites were analgesic in the hindpaw withdrawal test.
Fig. 9.
Placement of DPDPE injection sites within the DLP and RVM of 10-day-old pups. The sections are as described in Fig. 7 and are from the Paxinos et al atlas (Paxinos et al., 1991). The numbers to the upper right of each section or the AP plane from the atlas. Other details are as for the legend for Fig. 4. For the DLP, the majority of positive sites were at or rostral to section −10.04. Few sites were positive in the hindpaw test and unlike DAMGO, there was little evidence of effects for either the forepaw or tail tests. Most RVM sites were positive in forepaw, except at the lowest dose, but no site was analgesic in the hindpaw test.
Dorsal lateral pons
DAMGO
Behavioral Data
There were significant main effects of appendage and dose but not of age. The interaction of appendage, dose and age was significant (p<.05). The forepaw showed almost full analgesia at all ages and there was a rostral to caudal maturation of analgesia in the hindpaw and tail tests (Fig. 10). At the highest temperature, there was no analgesia noted in the hindpaw or tail test at any age.
Fig. 10.
Withdrawal latencies following injection DAMGO into the DLP. The details are as in Fig. 1. These data are similar to those seen for the PAG. At 3 days of age, DAMGO induced analgesia mostly in the forepaw test except at the lowest temperature. At the highest temperature DAMGO was analgesic only in the forepaw withdrawal test. There was an increase in the effectiveness of DAMGO for hindpaw and tail.
Histology
Compared to the other sites there was more rostral to caudal variability in the DLP placements (Fig. 9), although most placements accurately targeted the LC. In the forepaw test, almost all sites were positive. The two exceptions were low dose placements that were lateral to the LC. Others have also found specificity within the DLP with LC and dorsal noradrenergic bundle targeted sites more effective than sites outside the LC, such as the parabrachial nucleus (Rossi et al., 1993). Low dose sites within the LC were positive, as were all medium and high dose placements. When the hindpaw and tail were tested, the highest dose produced analgesia, but only at more rostral placements.
DPDPE
Behavioral Data
DPDPE produced a modest analgesia at the 47° C temperature in the forepaw, hindpaw and tail tests but only at the highest dose. Ten and 14 day old pups did not differ from each other. DPDPE was totally without effect at the highest temperature (Fig. 11).
Fig. 11.
Withdrawal latencies following injection of DPDPE into the DLP. The details are as in Fig. 1, except that 3-day-old pups were not tested. DPDPE was less effective than DAMGO at this site. DPDPE was never fully effective at 47° C, and virtually without effect at the higher temperatures. Furthermore, it was minimally effective in the hindpaw at any temperature.
Histology
These results were similar to those of others described above: the higher doses were more effective and the forepaw was the most sensitive appendage (Fig. 9). For all three tests, sites at the caudal extent of the LC and parabrachial region were ineffective, even at the highest doses. This agrees with the results from the DAMGO experiments in which the rostral sites were more effective. Sites dorsal to the DLP or dorsal within the LC area were less effective in the forepaw withdrawal tests.
Discussion and conclusions
DAMGO and to a significantly lesser degree, DPDPE, were analgesic when microinjected into the PAG, RVM or DLP, sites that are important in the analgesic actions of opiates in the adult. DAMGO was most effective, active at both temperatures and all three appendages. The RVM was the most effective site and the ventral PAG and DLP were similar in their effects.
Methodological considerations
Localization of the injection
The unique properties of the neural microenvironment of the infant (e.g. fewer lipids, different cellular density) make it difficult to define the exact spread of drug. In prior studies we used radiolabeled drugs or stimulation of adjacent sites to show anatomical specificity. Radiolabeled muscimol injected into the prefrontal cortex in the volume used here (200 nl) spread to a maximum radius of 0.5 mm from the cannula tip (Chen et al., 2006). Tritiated dopamine (2 μl) injected into the amygdala remained localized within that structure (Barr et al., 2009). To assess specificity functionally, injection of epinephrine (200 nl) into the perifornical lateral hypothalamus, but not the adjacent anterior hypothalamus or ventricle, enhanced feeding in two-day-old rat pups (Capuano et al., 1992b; Capuano et al., 1992c).
Receptor specificity
Although drugs may be specific in adults, their receptor specificity has been tested less often in infants. We have no guarantees that the drugs used here are equally specific in infants. For a large number of ligands, the affinity of the ligands remains constant whereas the density of binding sites changes (Desprat and Zajac, 1994; Koch et al., 1980; Spain et al., 1983; Tempel and Zukin, 1987; Zhang and Pasternak, 1981). Therefore we have some confidence that the drugs are specific in the infant as well as the adult.
Similarities and differences with prior literature
Facilitation vs. Inhibition
Injection opiates into the PAG or the aqueduct produces analgesia in animals as young as three days of age (Barr et al., 1992; Blass et al., 1993; Kehoe and Blass, 1986; Tive and Barr, 1992; Tseng et al., 1995). In an ex vivo isolated brain stem-spinal cord preparation from a neonatal rat, spinal cord transection or inactivation of the brain stem increased the area of the slow ventral root potential (sVRP), arguing for tonic descending inhibition (Tarasiuk et al., 1996). In contrast, electrical stimulation of the PAG or RVM produced a late maturation of analgesia in the hindlimb or tail (Hathway et al., 2009; van Praag and Frenk, 1991). Moreover, electrical stimulation of the RVM produced only descending facilitation of the hindlimb response throughout the entire preweaning period. Bipolar electrical stimulation may preferentially activate facilitatory paths through local fibers of passage as well as cell bodies or fail to target specific types of neurons as chemical stimulation might. Because opiates act within the PAG and RVM to inhibit GABA release in the adult (Fields et al., 1983; Heinricher et al., 2004), electrical stimulation in the infant could override that inhibition. However, this interpretation is complicated because: 1) electrical stimulation of the PAG or RVM in adults produces both facilitation and inhibition [reviewed in (Fields, 2000)]; 2) excitotoxic lesions of the RVM prolong hindpaw withdrawal latencies at 3 and 21 days of age and reduce those latencies at 40 days of age (Hathway et al., 2009); and 3) GABA neurons in the PAG are late to appear (Barbaresi, 2010). Given that individual neurons are both facilitated and inhibited by RVM stimulation in the infant and the adult (Hathway et al., 2009), the descending influences on spinal cord activity and behavior are clearly changing during development. There may be preferential activation of facilitatory paths in the infant but inhibitory circuits can be active and activated.
Receptor specific effects
DAMGO was the most effective and potent of the three drugs tested. DPDPE was without effect in the dorsal PAG, and only moderately analgesic when injected into the ventral PAG, RVM or DLP. U50,488H was without effect. These differences are consistent with the data in the adult animal that suggest that μ opioids are more efficacious than are ∂ opioids when injected into supraspinal sites but that k-opioid receptors are unimportant under conditions tested here (Smith et al., 1988; Watkins et al., 1992).
The conditions under which ∂-opioid receptor activation induces analgesia are not well defined. Most studies find supraspinal administration of DPDPE or DSLET to be antinociceptive [ (Chieng and Christie, 1994a; Heyman et al., 1987; Jensen and Yaksh, 1986; Lunzer and Portoghese, 2007; Moreau and Fields, 1986; Porreca et al., 1987 ; ; Reichling and Basbaum, 1990; Takagi et al., 1978; Vaught et al., 1988) for review (Heyman et al., 1988)]. DPDPE was analgesic in the present studies under limited conditions – without effect at higher temperatures or when injected into the dorsal PAG. Even when analgesic, it was not fully effective at any site. Differences in types of analgesic tests and/or differences in the intensity of the stimulus could account for these differences. Bodnar (Bodnar et al., 1988) used a tail flick test with baseline latencies consistently around 2 -3 seconds and did not find ∂-opiate mediated analgesia. The tail withdrawal latency for the 47° C temperature, at which analgesia was noted here, was within that range, 2.8 seconds. But comparisons of stimulus intensities across ages are difficult. Furthermore, baseline latencies here did not necessary correlate to whether or not the opiate induced analgesia. Alternatively, ∂-opioid receptors may subserve antinociception in the young but not the more mature animal. We know of no evidence for or against that hypothesis. Note also that DPDPE is a selective ∂1-opioid agonist. We have no data on ∂2-opioid agonists, such as deltorphin, which may be more effective analgesics (Rossi et al., 1994).
Site differences
The mechanisms by which opiates induce antinociception following injection into the PAG, RVM and DLP are maturing in the first two weeks of life. The pattern of analgesia seen following PAG stimulation could be due to the maturation of descending pathways or the maturation of projections from the PAG to the RVM and DLP. It is likely both. DAMGO injected into the RVM was most effective since it induced analgesia in the hindpaw and tail at all ages and at the highest temperature. Thus, pathways from the PAG to the RVM are likely developing from 3 to 14 days of age. By 14 days of age there were minimum differences between the two sites. Because the ventral PAG and the DLP sites were equivalently effective, it is not likely that the increased effectiveness of opiate induced analgesia over the first two weeks can be attributed the PAG acting through the DLP or due to maturation of the PAG itself. These Although we interpret the delayed appearance of analgesia in the hindpaws and tail as evidence of delayed descending inhibition, other mechanisms are possible. We cannot rule out, for example, that stimulation of the PAG is recruiting other sites as the pup develops or that the developmental course of analgesia is modified by receptor development. For example, there are well-documented synergistic interactions of μ-opiates between the PAG and the DLP and RVM (Bodnar et al., 1988; Rossi et al., 1993, 1994). Determining synergistic effects among these three sites during early development might tease apart these interactions as they have in the adult (Bodnar et al., 1988; Rossi et al., 1993, 1994).
Localization within the PAG
There are a number of differences between the dorsal and ventral PAG in the adult. The projections of the dorsal lateral PAG are mainly to the locus ceruleus, subceruleus, the rostral paragigantocellular nucleus, and the region of the A5 noradrenergic cell group. In contrast, the major projections of the ventral lateral PAG are to the nucleus raphe magnus, the caudal aspects of the lateral paragigantocellular nucleus, and the rostral ventral lateral reticular nucleus (Cameron et al., 1995b). Stimulation of the ventral PAG by opiates, excitatory amino acids, or by electrical current induces analgesia mediated by opioid mechanisms. Electrical or glutamatergic stimulation of the dorsal PAG, in contrast, activates mechanisms that are neither blocked by naloxone nor cross-tolerant with morphine (Cannon et al., 1982; Carstens and Douglass, 1995; Nichols and Thorn, 1990). In the infant, morphine injected into the ventral PAG resulted in a pattern of early onset analgesia quite similar to that of DAMGO. However, morphine was ineffective until 14 days of age when injected into the dorsal PAG (Tive and Barr, 1992). Here, DAMGO was effective in the dorsal PAG in the forepaw test at three days of age. DAMGO is a more efficacious agonist than morphine and the difference could be due to that increased efficacy. In the present study, injection sites were more rostral than in the morphine study. There are differences in the function of the PAG along the rostral to caudal plane [see (Bandler and Shipley, 1994) for review], although few differences exist in the caudad projections from the rostral or caudal PAG (Cameron et al., 1995a; Cameron et al., 1995b). Resolution of these issues will require further work.
Supplementary Material
Stimulation of the midbrain and medulla is 1) facilitatory in infant rodents but inhibitory in older animals with electrical stimulation; or 2) inhibitory and analgesic with chemical stimulation using morphine.
We show here that using specific opiate agonists, there is a strong μ opiate mediated analgesia from the periaqueductal gray, rostral ventral medulla or dorsal lateral pons in the infant rat.
Thus the midbrain/medulla can be inhibitory in the neonate.
Acknowledgments
This work was conducted while the first author was on faculty in the Department of Psychology, Hunter College, City University of New York and the Department of Developmental Neuroscience, New York State Psychiatric Institute, Columbia University College of Physicians and Surgeons.
Roles: Barr conceived and designed the study. He performed the statistical analyses and wrote the manuscript. Wang helped develop the methods, performed the surgery and behavioral tests, did the histology and the histological data analysis.
Funding: NIH R01DA007341 and funds from the City University of New York to GA Barr.
Footnotes
Conflict of Interest: None
References
- Bandler R, Depaulis A, Vergnes M. Identification of midbrain neurones mediating defensive behaviour in the rat by microinjections of excitatory amino acids. Behav Brain Res. 1985;15:107–119. doi: 10.1016/0166-4328(85)90058-0. [DOI] [PubMed] [Google Scholar]
- Bandler R, Shipley MT. Columnar organization in the midbrain periaqueductal gray: modules for emotional expression? TINS. 1994;17:379–389. doi: 10.1016/0166-2236(94)90047-7. [DOI] [PubMed] [Google Scholar]
- Barbaresi P. Postnatal development of GABA-immunoreactive neurons and terminals in rat periaqueductal gray matter: a light and electron microscopic study. J Comp Neurol. 2010;518:2240–2260. doi: 10.1002/cne.22329. [DOI] [PubMed] [Google Scholar]
- Barr G, Moriceau S, Shionoya K, Muzny K, Gao P, Wang S, Sullivan R. Transitions in infant learning are modulated by dopamine in the amygdala. Nat Neurosci. 2009;12:1367–1369. doi: 10.1038/nn.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr GA. Neuropharmaco-ontogeny: concepts and methods of study. In: Shair HN, Barr GA, Hofer MA, editors. Developmental psychobiology: new methods and changing concepts. Oxford; New York: 1991. pp. 321–341. [Google Scholar]
- Barr GA, Miya DY, Paredes W. Analgesic effects of intraventricular and intrathecal injection of morphine and ketocyclazocine in the infant rat. Brain Res. 1992;584:83–91. doi: 10.1016/0006-8993(92)90881-9. [DOI] [PubMed] [Google Scholar]
- Beitz AJ. The nuclei of origin of brainstem serotonergic projections to the rodent spinal trigeminal nucleus. Neuroscience Letters. 1982;2:223–228. doi: 10.1016/0304-3940(82)90297-x. [DOI] [PubMed] [Google Scholar]
- Blass EM, Cramer CP, Fanselow MS. The development of morphine-induced antinociception in neonatal rats: a comparison of forepaw, hindpaw, and tail retraction from a thermal stimulus. Pharmacol Biochem Behav. 1993;44:643–649. doi: 10.1016/0091-3057(93)90180-2. [DOI] [PubMed] [Google Scholar]
- Bodnar RJ, Williams CL, Lee SJ, Pasternak GW. Role of mu 1-opiate receptors in supraspinal opiate analgesia: a microinjection study. Brain Res. 1988;447:25–34. doi: 10.1016/0006-8993(88)90962-6. [DOI] [PubMed] [Google Scholar]
- Cameron AA, Khan IA, Westlund KN, Cliffer KD, Willis WD. The efferent projections of the periaqueductal gray in the rat: a Phaseolus vulgaris-leucoagglutinin study. I. Ascending projections. J Comp Neurol. 1995a;351:568–584. doi: 10.1002/cne.903510407. [DOI] [PubMed] [Google Scholar]
- Cameron AA, Khan IA, Westlund KN, Willis WD. The efferent projections of the periaqueductal gray in the rat: a phaseolus vulgaris leucoagglutinin study .2. descending projections. Journal of Comparative Neurology. 1995b;351:585–601. doi: 10.1002/cne.903510408. [DOI] [PubMed] [Google Scholar]
- Cannon JT, Prieto GJ, Liebeskind JC. Evidence for opioid and non-opioid forms of stimulation produced analgesia in the rat. Brain Research. 1982;243:315–321. doi: 10.1016/0006-8993(82)90255-4. [DOI] [PubMed] [Google Scholar]
- Capuano CA, Leibowitz SF, Barr GA. The pharmaco-ontogeny of the paraventricular alpha 2-noradrenergic receptor system mediating norepinephrineinduced feeding in the rat. Brain Res Dev Brain Res. 1992a;68:67–74. doi: 10.1016/0165-3806(92)90248-u. [DOI] [PubMed] [Google Scholar]
- Capuano CA, Leibowitz SF, Barr GA. The pharmaco-ontogeny of the perifornical lateral hypothalamic beta 2-adrenergic and dopaminergic receptor systems mediating epinephrine- and dopamine-induced suppression of feeding in the rat. Brain Res Dev Brain Res. 1992b;70:1–7. doi: 10.1016/0165-3806(92)90098-h. [DOI] [PubMed] [Google Scholar]
- Capuano CA, Leibowitz SF, Barr GA. Pharmaco–ontogeny of hypothalamic induced feeding. Developmental Brain Research. 1992c;68:67–74. doi: 10.1016/0165-3806(92)90248-u. [DOI] [PubMed] [Google Scholar]
- Carden SE, Barr GA, Hofer MA. Differential effects of specific opioid receptor agonists on rat pup isolation calls. Brain Res Dev Brain Res. 1991;62:17–22. doi: 10.1016/0165-3806(91)90185-l. [DOI] [PubMed] [Google Scholar]
- Carstens E, Douglass DK. Midbrain suppression of limb withdrawal and tail flick reflexes in the rat: correlates with descending inhibition of sacral spinal neurons. Journal of Neurophysiology. 1995;73:2179–2194. doi: 10.1152/jn.1995.73.6.2179. [DOI] [PubMed] [Google Scholar]
- Chan T, Kyere K, Davis B, Shemyakin A, Kabitzke P, Shair H, Barr G, Wiedenmayer C. The role of the medial prefrontal cortex in innate fear regulation in infants, juveniles, and adolescents. J Neurosci. 2011;31:4991–4999. doi: 10.1523/JNEUROSCI.5216-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SW, Shemyakin A, Wiedenmayer CP. The role of the amygdala and olfaction in unconditioned fear in developing rats. J Neurosci. 2006;26:233–240. doi: 10.1523/JNEUROSCI.2890-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chieng B, Christie MJ. Hyperpolarization by opioids acting on mu-receptors of a sub-population of rat periaqueductal gray neurones in vitro. Br J Pharmacol. 1994a;113:121–128. doi: 10.1111/j.1476-5381.1994.tb16183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chieng B, Christie MJ. Inhibition by opioids acting on mu receptors of GABAergic and glutamatergic postsynaptic potentials in single rat periaqueductal gray neurones in vitro. Br J Pharmacol. 1994b;113:303–309. doi: 10.1111/j.1476-5381.1994.tb16209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Mello R, Dickenson AH. Spinal cord mechanisms of pain. Br J Anaesth. 2008;101:8–16. doi: 10.1093/bja/aen088. [DOI] [PubMed] [Google Scholar]
- Desprat C, Zajac JM. Ontogeny of neuropeptide FF pharmacology and receptors in mouse brain. Developmental Brain Research. 1994;82:118–126. doi: 10.1016/0165-3806(94)90154-6. [DOI] [PubMed] [Google Scholar]
- Festing MF. Design and statistical methods in studies using animal models of development. ILAR J. 2006;47:5–14. doi: 10.1093/ilar.47.1.5. [DOI] [PubMed] [Google Scholar]
- Fields HL. Pain modulation: expectation, opioid analgesia and virtual pain. Prog Brain Res. 2000;122:245–253. doi: 10.1016/s0079-6123(08)62143-3. [DOI] [PubMed] [Google Scholar]
- Fields HL, Vanegas H, Hentall ID, Zorman G. Evidence that disinhibition of brain stem neurones contributes to morphine analgesia. Nature. 1983;306:684–686. doi: 10.1038/306684a0. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M. Monoamines and descending control of nociception. Trends Neurosci. 1986:51–52. [Google Scholar]
- Gallagher DW, Pert A. Afferents to brain stem nuclei (brain stem raphae, nucleus reticularis pontis caudalis and nucleus gigantocellularis) in the rat as demonstrated by microiontophoretically applied horse radish peroxidase. Brain Research. 1978;144:257–275. doi: 10.1016/0006-8993(78)90153-1. [DOI] [PubMed] [Google Scholar]
- Hathway GJ, Koch S, Low L, Fitzgerald M. The changing balance of brainstem-spinal cord modulation of pain processing over the first weeks of rat postnatal life. J Physiol. 2009;587:2927–2935. doi: 10.1113/jphysiol.2008.168013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinricher MM, Martenson ME, Neubert MJ. Prostaglandin E2 in the midbrain periaqueductal gray produces hyperalgesia and activates pain-modulating circuitry in the rostral ventromedial medulla. Pain. 2004;110:419–426. doi: 10.1016/j.pain.2004.04.026. [DOI] [PubMed] [Google Scholar]
- Heller A, Hutchens JO, Kirby ML, Karapas F, Fernandez C. Stereotaxic electrode placement in the neonatal rat. J Neurosci Meth. 1979;1:41–76. doi: 10.1016/0165-0270(79)90006-2. [DOI] [PubMed] [Google Scholar]
- Heyman JS, Mulvaney SA, Mosberg HI, Porreca F. Opioid δ-receptor involvement in supraspinal and spinal antinociception in mice. Brain Research. 1987;420:100–108. doi: 10.1016/0006-8993(87)90244-7. [DOI] [PubMed] [Google Scholar]
- Heyman JS, Vaught JL, Raffa RB, Porreca F. Can supraspinal delta opioid receptor mediate antinociception? TIPS. 1988;9:134–138. doi: 10.1016/0165-6147(88)90195-2. [DOI] [PubMed] [Google Scholar]
- Hughes HE, Barr GA. Analgesic effects of intrathecally applied noradrenergic compounds in the developing rat: differences due to thermal and mechanical nociception. Developmental Brain Research. 1988;41:109–120. doi: 10.1016/0165-3806(88)90174-5. [DOI] [PubMed] [Google Scholar]
- Jackson HC, Kitchen I. Behavioural effects of selective μ, κ, and δ-opioid agonists in neonatal rats. Psychopharmacol. 1989a;97:404–409. doi: 10.1007/BF00439459. [DOI] [PubMed] [Google Scholar]
- Jackson HC, Kitchen I. Behavioural effects of selective mu-, kappa-, and delta-opioid agonists in neonatal rats. Psychopharmacology (Berl) 1989b;97:404–409. doi: 10.1007/BF00439459. [DOI] [PubMed] [Google Scholar]
- Jensen TS, Yaksh TL. Comparison of the antinociceptive action of mu and delta opioid receptor ligands in the periaqueductal gray matter, medial and paramedial ventral medulla in the rat as studied by the microinjection technique. Brain Res. 1986;372:301–312. doi: 10.1016/0006-8993(86)91138-8. [DOI] [PubMed] [Google Scholar]
- Jones SL, Gebhart GF. Inhibition of spinal nociceptive transmissin from the midbrain, pons and medulla in the rat: activation of descending inhibition my morphine, glutamate and electrical stimulation. Brain Research. 1988;460:281–296. doi: 10.1016/0006-8993(88)90373-3. [DOI] [PubMed] [Google Scholar]
- Kehoe P, Blass EM. Central nervous system mediation of positive and negative reinforcement in neonatal albino rats. Brain Res. 1986;392:69–75. doi: 10.1016/0165-3806(86)90233-6. [DOI] [PubMed] [Google Scholar]
- Koch B, Sakly M, Lutz-Bucher B. Ontogeny of opiate receptor sites in brain: apparent lack of low affinity sites during early neonatal life. Horm Metab Res. 1980;12:342–343. doi: 10.1055/s-2007-996287. [DOI] [PubMed] [Google Scholar]
- Kornblum HI, Hurlbut DE, Leslie FM. Postnatal development of multiple opioid receptors in rat brain. Brain Research. 1987;415:21–41. doi: 10.1016/0165-3806(87)90226-4. [DOI] [PubMed] [Google Scholar]
- Leslie FM, Tso S, Hurlbut DE. Differential appearnance of opiate receptor subtypes in neonatal rat brain. Life Sci. 1982;31:1393–1396. doi: 10.1016/0024-3205(82)90389-7. [DOI] [PubMed] [Google Scholar]
- Lunzer MM, Portoghese PS. Selectivity of delta- and kappa-opioid ligands depends on the route of central administration in mice. J Pharmacol Exp Ther. 2007;322:166–171. doi: 10.1124/jpet.107.120279. [DOI] [PubMed] [Google Scholar]
- McDowell J, Kitchen I. Ontogenesis of d-opioid receptors in rat brain using [3H]-[D-Pen2, D-Pen5]enkephalin as a binding ligand. Europ J Pharmacol. 1986;128:287–289. doi: 10.1016/0014-2999(86)90780-6. [DOI] [PubMed] [Google Scholar]
- McDowell J, Kitchen I. Development of opioid systems: peptides, receptors and pharmacology. Brain Res Rev. 1987;12:397–421. doi: 10.1016/0165-0173(87)90006-3. [DOI] [PubMed] [Google Scholar]
- McLaughlin CR, Dewey WL. A comparison of the antinociceptive effects of opioid agonists in neonatal and adult rats in phasic and tonic nociceptive tests. Pharmacol Biochem Behav. 1994;49:1017–1023. doi: 10.1016/0091-3057(94)90258-5. [DOI] [PubMed] [Google Scholar]
- Moreau JL, Fields HL. Evidence for GABA involvement in midbrain control of medullary neurons that modulate nociceptive transmission. Brain Research. 1986;397:37–46. doi: 10.1016/0006-8993(86)91367-3. [DOI] [PubMed] [Google Scholar]
- Morgan MM, Liebeskind JC. Site specificity in the development of tolerance to stimulation-produced analgesia from the periaqueductal grey matter of the rat. Brain Research. 1987;425:356–359. doi: 10.1016/0006-8993(87)90519-1. [DOI] [PubMed] [Google Scholar]
- Morgan MM, Whittier KL, Hegarty DM, Aicher SA. Periaqueductal gray neurons project to spinally projecting GABAergic neurons in the rostral ventromedial medulla. Pain. 2008 doi: 10.1016/j.pain.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols DS, Thorn BE. Stimulation-produced analgesia and its cross-tolerance between dorsal and ventral PAG loci. Pain. 1990;41:347–352. doi: 10.1016/0304-3959(90)90011-2. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Tork I, Tecott L, Valentino K. Atlas of the developing rat brain. Academic Press; San Diego, CA: 1991. [Google Scholar]
- Porreca F, Heyman JS, Mosberg HI, Omnas JR, Vaught JL. Role of mu and delta receptors in the supraspinal and spinal analgesic effects of [D-Pen2, D-Pen5]enkephalin in the mouse. Journal Pharmacology & Experimental Therapeutics. 1987;241:393–400. [PubMed] [Google Scholar]
- Reichling DB, Basbaum AI. Contribution of brainstem GABAergic circuitry to descending antinociceptive controls: II. Electron microscopic immunocytochemical evidence of GABAergic control over the projection from the periaqueductal gray to the nucleus raphe magnus in the rat. Journal of Comparative Neurology. 1990;302:378–393. doi: 10.1002/cne.903020214. [DOI] [PubMed] [Google Scholar]
- Rosenfeld JP. Interacting brain stem components of opiate-activated, descending, pain-inhibitory systems. Neurosci Biobehav Rev. 1994;18:403–409. doi: 10.1016/0149-7634(94)90053-1. [DOI] [PubMed] [Google Scholar]
- Rossi GC, Pasternak GW, Bodnar RJ. Synergistic brainstem interactions for morphine analgesia. Brain Res. 1993;624:171–180. doi: 10.1016/0006-8993(93)90075-x. [DOI] [PubMed] [Google Scholar]
- Rossi GC, Pasternak GW, Bodnar RJ. Mu and delta opioid synergy between the periaqueductal gray and the rostro-ventral medulla. Brain Res. 1994;665:85–93. doi: 10.1016/0006-8993(94)91155-x. [DOI] [PubMed] [Google Scholar]
- Saeki S, Yaksh TL. Suppression of nociceptive responses by spinal mu opioid agonists - effects of stimulus intensity and agonist efficacy. Anesth Analg. 1993;77:265–274. doi: 10.1213/00000539-199308000-00010. [DOI] [PubMed] [Google Scholar]
- Sherwood N, Timiras PS. Stereotaxic atlas of the developing rat brain. University of California Press; Berkeley, Calif.: 1971. [Google Scholar]
- Smith DJ, Perrotti JM, Crisp T, Cabral ME, Long JT, Scalzitti JM. The mu opiate receptor is responsible for descending pain inhibition originating in the periaqueductal gray region of the rat brain. Eur J Pharmacol. 1988;156:47–54. doi: 10.1016/0014-2999(88)90145-8. [DOI] [PubMed] [Google Scholar]
- Spain JW, Bennett DB, Roth BL, Coscia CJ. Ontogeny of benzomorphan-selective (kappa) sites: a computerized analysis. Life Sci. 1983;33(Suppl 1):235–238. doi: 10.1016/0024-3205(83)90486-1. [DOI] [PubMed] [Google Scholar]
- Spain JW, Roth BL, Coscia CJ. Differential ontogeny of multiple opioid receptors (mu, delta, and kappa) J Neurosci. 1985;5:584–588. doi: 10.1523/JNEUROSCI.05-03-00584.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamford JA. Descending control of pain. British Journal of Anaesthesia. 1995;75:217–227. doi: 10.1093/bja/75.2.217. [DOI] [PubMed] [Google Scholar]
- Takagi H, Satoh M, Akaike A, Shibata T, Kuraishi Y. Analgesia by enkephalins injected into the nucleus reticularis gigantocellularis of rat medulla oblongata. EJP. 1978;49:113–116. doi: 10.1016/0014-2999(78)90229-7. [DOI] [PubMed] [Google Scholar]
- Tarasiuk A, Gibbs L, Kendig JJ. Descending inhibition in neonatal rat spinal cord: actions of pentobarbital and morphine. Brain Res Bull. 1996;41:39–45. doi: 10.1016/0361-9230(96)00168-2. [DOI] [PubMed] [Google Scholar]
- Tempel A, Zukin RS. Neuroanatomical patterns of the u, d, and k opioid receptors of rat brain as determinied by quantitative in vitro autoradiography. Proceedings of the National Academy of Sciences. 1987;84:4308–4312. doi: 10.1073/pnas.84.12.4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tive LA, Barr GA. Analgesia from the periaqueductal gray in the developing rat: focal injections of morphine or glutamate and effects of intrathecal injection of methysergide or phentolamine. Brain Res. 1992;584:92–109. doi: 10.1016/0006-8993(92)90882-a. [DOI] [PubMed] [Google Scholar]
- Tseng LF, Collins KA, Wang Q. Differential ontogenesis of thermal and mechanical antinociception induced by morphine and beta-endorphin. Europ J Pharmacol. 1995;277:71–76. doi: 10.1016/0014-2999(95)00064-r. [DOI] [PubMed] [Google Scholar]
- van Praag H, Frenk H. The development of stimulation-produced analgesia (SPA) in the rat. Brain Res Dev Brain Res. 1991;64:71–76. doi: 10.1016/0165-3806(91)90210-a. [DOI] [PubMed] [Google Scholar]
- Vanegas H. To the descending pain-control system in rats, inflammation-induced primary and secondary hyperalgesia are two different things. Neurosci Lett. 2004;361:225–228. doi: 10.1016/j.neulet.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Vaught JL, Mathiasen JR, Raffa RB. Examination of the involvement of supraspinal and spinal mu and delta opioid receptors in analgesia using the mu receptor deficient CXBK mouse. J Pharmacol Exp Ther. 1988;245:13–16. [PubMed] [Google Scholar]
- Watkins LR, Wiertelak EP, Maier SF. Kappa opiate receptors mediate tail-shock induced antinociception at spinal levels. Brain Res. 1992;582:1–9. doi: 10.1016/0006-8993(92)90310-6. [DOI] [PubMed] [Google Scholar]
- Wheeler-Aceto H, Cowan A. Standardization of the rat paw formalin test for the evaluation of analgesics. Psychopharmacol (Berlin) 1991;104:35–44. doi: 10.1007/BF02244551. [DOI] [PubMed] [Google Scholar]
- Willis W.D.j. Anatomy and physiology of descending control of nociceptive responses of dorsala horn neurons: comprehensive review. In: Fields HL, Besson J-M, editors. Progress in Brain Research. Elsevier Science Publishers; Amsterdam: 1988. pp. 1–29. [DOI] [PubMed] [Google Scholar]
- Zhang AZ, Pasternak GW. Ontogeny of opioid pharmacology and receptors: high and low affinity site differences. Eur J Pharmacol. 1981;73:29–40. doi: 10.1016/0014-2999(81)90142-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.