Abstract
Chemical cross-linking of proteins followed by proteolysis and mass spectrometric analysis of the resulting cross-linked peptides provides powerful insight into the quaternary structure of protein complexes. Mixed-isotope cross-linking (a method for distinguishing intermolecular cross-links) was coupled with liquid chromatography and ion mobility separations and mass spectrometry (LC-IMS-MS) to provide an additional separation dimension to the traditional cross-linking approach. This method produced multiplet m/z peaks that are aligned in the IMS drift time dimension and serve as signatures of intermolecular cross-linked peptides. We developed an informatics tool to use the amino acid sequence information inherent in the multiplet spacing for accurate identification of the cross-linked peptides. Because of the separation of peptides and cross-linked peptides in drift time, our LC-IMS-MS approach was able to confidently detect more intermolecular cross-linked peptides than LC-MS alone.
Introduction
Cross-linking mass spectrometry has long been used as a tool to probe protein structure.[1-5] Recent advances in cross-linking mass spectrometry (XL-MS) suggest that this technique will become an increasingly important tool for structural proteomics [6-7]. In XL-MS, protein complexes are treated with a bifunctional cross-linking reagent that creates a covalent link between adjacent portions of the peptide chain. Proteolytic digests are then analyzed by mass spectrometry (MS) to identify cross-linked peptides, which in turn provide structural information about the protein complex. Although the challenges of XL-MS (high complexity of reaction mixtures, low abundance of cross-linked peptides, high charge and mass of cross-linked peptides, and complex fragmentation spectra) are well known and remain formidable, promising advances are being described [6-8].
One challenge in XL-MS, particularly relevant to structural elucidation, is the problem of distinguishing intermolecular from intramolecular cross-links. Many protein complexes contain multiple copies of the same subunit, and unless the two linked peptides share overlapping, non-repeated sequences, intermolecular and intramolecular cross-links are indistinguishable, leading to ambiguous structural restraints. To date, the only reported method able to distinguish these types of cross-linked peptides is mixed-isotope cross-linking (MIX) [9]. In this technique, isotopically labeled (heavy) proteins are mixed with unlabeled (light) proteins under conditions that allow subunit exchange. The complex is then cross-linked, proteolytically digested, and analyzed using MS. In the mass spectra, intermolecular cross-linked peptides appear as quadruplet peaks, whereas intramolecular peptides appear as doublets. The original MIX application used 15N enrichment; we have used uniform 13C,15N labeling.
We introduce two improvements to the MIX method to facilitate identification of intermolecular cross-links. First, we have combined the MIX method with ion mobility spectrometry (IMS). IMS-MS has previously been used to separate positional isomers of “dead-end” cross-linker modified peptides [10], and to study complexes of a single amyloidogenic peptide [11], but not yet to study a digest of a cross-linked protein complex. The combination of IMS-MS with MIX is particularly powerful. Since the MIX heavy-isotope labels do not change IMS drift time (td), the multiplet signals are aligned in td, providing a spectral signature for cross-linked peptides that is readily detected both visually and algorithmically. Second, we developed an informatics tool to take advantage of the information contained in the spacing of the MIX multiplets. This spacing (determined by the number of heavy-isotope atoms) encodes information about the amino acid composition, which constrains peptide identification. Unlike previous MIX studies, which used protein labeled only with 15N [9, 12], we use both 15N and 13C to maximize the information content. The combination of mass shift and accurate mass often uniquely identifies the cross-linked peptide.
Here we demonstrate the MIX-LC-IMS-MS approach with two different homodimeric proteins (SrfN from Salmonella enterica subsp. enterica serovar Typhimurium [9.2 kDa per monomer] and SO_2176 from Shewanella oneidensis [8.9 kDa per monomer]) and compare the results with those obtained with no IMS separation. The results illustrate that the additional IMS separation provides improved specificity and detection of intermolecular cross-links over MIX-LC-MS alone.
Methods
Recombinant, His6-tagged, uniformly 13C,15N-labeled and unlabeled proteins were expressed inEscherichia coli and purified using standard methods [13-14]. A purified, 1:1 mixture of unlabeled and uniformly 13C,15N-labeled SO_2176 protein also was supplied by NESG (target SoR77). The plasmid construct for SrfN was provided by the Northeast Structural Genomics Consortium (NESG); SrfN is NESG target StR109. A mixture of 1:1 unlabeled: uniformly 13C,15N-labeled SrfN was prepared and allowed to equilibrate at 4 °C in phosphate-buffered saline. The samples were treated with the amine-reactive cross-linker bis(sulfosuccinimidyl)suberate (BS3) at 35-fold molar excess for 30 min at room temperature, and the reactions quenched by the addition of 1 M glycine to a final concentration of 20 mM. The sample was concentrated to <400 μL, denatured by adding solid urea for a final concentration of ~6-7 M, and then incubated at 60 °C for 30 min. The denatured protein was diluted 10-fold with 100 mM ammonium bicarbonate buffer (pH 8) and digested overnight with sequencing grade modified trypsin (Promega). The sample as acidified with trifluoroacetic acid (0.05% final concentraton) and desalted by solid-phase extraction on C18 hydrophobic resin (Supelco, St. Louis, MO).
Samples were subjected to liquid chromatography using a custom system described previously [15]. The LC column was packed in-house with Jupiter C18 stationary phase (3 μm particle size, Phenomenex, Torrance, CA), and consists of a fused silica capillary (60 cm × 75 μm i.d., Polymicro Technologies, Phoenix, AZ). Analysis of the MIX labeled samples was performed using an in-house built IMS-TOF MS instrument that couples a 1-m IMS drift tube with an Agilent 6224 TOF MS upgraded to a 1.5 meter flight tube providing resolution of ~25,000 in enhanced dynamic range mode.[16-17] To perform the IMS-MS measurements, the LC stream was ionized utilizing electrospray ionization and the ions were passed through a heated inlet capillary and focused by a high pressure ion funnel. Ions exiting the high pressure funnel where then accumulated in a trapping ion funnel before being released into the IMS drift tube filled with 4 Torr nitrogen. After separating according to mobility, ions in the drift tube were focused by a final ion funnel and transferred by way of a short quadrupole followed by a segmented quadrupole into the TOF MS for m/z analysis. Drift times were not calibrated, but the deviations of the observed drift times of nine standard peptides from known values, measured immediately before and after each experiment, were negligible. Drift times were also standardized to 298 K and 4 Torr. Raw LC-IMS-MS data were processed with Decon2LS [18], and LCIMS-MS Feature Finder [19] was used to group isotopic profiles across LC elution time and drift time into features (peaks in the elution time/drift time/mass space representing distinct chemical species).
We created a C# .NET 4.0 application called CrossLinkingIMS to search for cross-linked peptides in the LC-IMS-MS Feature Finder results. This software uses the known protein sequence to calculate both the mass of the predicted unlabeled tryptic cross-linked peptides, and the predicted mass shifts due to heavy isotope labeling. The protein N-terminus, and lysine, serine, threonine, and tyrosine residues were considered as potential cross-linking sites.[20-21] This list of theoretical cross-linked peptides was matched against the LC-IMS-MS features, using a mass tolerance of 10 ppm. When a match was found, the raw data were checked for matches to the theoretical masses of the corresponding labeled isotopic peaks in the relevant (m/z, tdrift, elution time) space. A cross-link was considered identified if all the expected peaks were present and the mass measurement error of the light peak was <10 ppm. Matches were visually examined to confirm intensity relationships (expected to be 1:1:1:1 for quadruplets and 1:2:1 for triplets; features passed the inspection if the observed ratio was within a factor of three of the expected and if peaks were clearly visible above the noise). This software and the data files used in this study are available at http://omics.pnl.gov/software/CrossLinkingIMS.php.
Distances between potential cross-linked residues in SO_2176 were calculated using both the NMR structure (PDB ID 2JUW) and the crystal structure (PDB ID 2QTI). Distances between cross-linked residues in SrfN were evaluated using the solution structure that was re-refined to account for in vivo proteolytic cleavage of the 21-residue N-terminal signal peptide (unpublished). In addition to calculating the linear (Euclidean) distance between cross-linked residues from these structures, we calculated the solvent-accessible surface distances (SASD) using the program xWalk [22]. SASD was calculated between Cβ atoms with the atomic radius expanded to 2.0 Å, and a maximum SASD of 34 Å as described. Cross-linked residue pairs were judged to be within range if the SASD was less than the sum of the length of the two side chains, the linker length (11.4 Å), and a 3 Å tolerance (e.g., 24.4 Å for a lysine-to-lysine cross-link) [23].
Results and Discussion
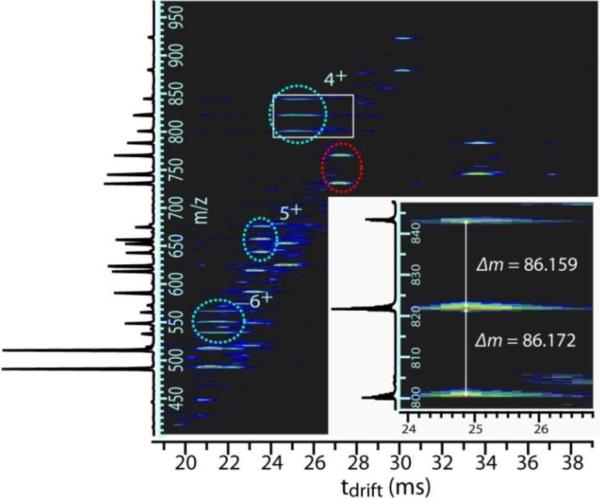
For simplicity, we designate the light and heavy versions of the longer peptide of a cross-linked pair as a and A, respectively, and the light and heavy versions of the shorter peptide as b and B, respectively. Figure 1 shows the nested IMS-MS drift time spectrum assigned to the MIX-labeled homodimeric cross-linked peptide AEQVSKQEISHFK/AEQVSKQEISHFK from SrfN (underlined residue indicates the presumed site of cross-linking). The homodimeric peptide appears as distinctive triplets (cyan circles), contrasting with the numerous doublets present (e.g., red circle). The aa, aA/Aa and AA forms (in this symmetrical case, both peptides are a or A) all have identical IMS drift time distributions (Figure 1, inset), supporting the assignment of all three peaks to the same cross-linked peptide. The spacing between the peaks corresponds to mass shifts of 86.159 and 86.172 Da, consistent with the expected increase in mass of 86.168 Da resulting from the 67 13C and 19 15N atoms in the peptide AEQVSKQEISHFK. The ratio of the aa:aA/Aa:AA peak heights (1:1.8:0.7) is ~ 1:2:1, as expected for a homodimeric cross-link. In addition, four charge states (3+ to 6+) are detected (3+ charge state not shown), each with correct m/z, peak spacing, and intensity ratios. Thus, the identification of AEQVSKQEISHFK/AEQVSKQEISHFK is deemed highly confident.
Figure 1.
MIX-IMS-MS identification of a cross-linked peptide. The IMS-mass spectrum depicts doublets (e.g., red circle) arising from light/heavy peptide pairs, and triplets (cyan circles) arising from the cross-linked peptide AEQVSKQEISHFK/AEQVSKQEISHFK in the 4+, 5+, and 6+ charge states. Inset: close-up of the 4+ charge state triplet (area in white box) that illustrates peak spacing and drift time profiles.
As a second example, Figure 2 illustrates the spectrum of the heterodimeric cross-link AEQVSKQEISHFK/AEQVSK4+ from SrfN. The m/z spacing between the pairs matches very well to the predicted values. The ab-Ab and aB-AB peak pairs have shifts of 86.15 and 86.17 Da, respectively (predicted, 86.168 Da); and the ab-aB and Ab-AB mass shifts are 35.06 and 35.08 Da (predicted, 35.067 Da). The IMS drift times of the four peaks are again identical, and the peak intensities are approximately equal, as expected for a heterodimeric cross-link. Taken together, these data provide a highly confident cross-link identification.
Figure 2.
Identification of a non-symmetrical cross-linked peptide by MIX-IMS-MS. Both the accurate mass (0.2 ppm, Table 1) and the spacing of the quadruplet support the assignment of this feature as the cross-linked peptide AEQVSKQEISHFK/AEQVSK4+.
Our search software identified nine intermolecular cross-linked peptides for SrfN and six cross-links for SO_2176 (Table 1). Out of the fifteen cross-links observed, only six could be identified as inter- and not intramolecular cross-links by sequence alone (i.e., because the two peptides share all or part of their sequence, indicating that they arise from different copies of the same polypeptide). MIX labeling clearly reveals the intermolecular nature of the other nine peptides.
Table 1.
Cross-linked Peptides Identified by MIX-IM Method from SrfN and SO_2176
| Sitea | Peptide Ab | Peptide Bb | z | Δm (ppm) | Elution Time (min) | Drift Time (ms) | Relative Abundance |
|---|---|---|---|---|---|---|---|
| SrfN | |||||||
| A22c-K27 | AEQVSK | AEQVSKQEISHFK | 3 | 2. 6 | 24 .2 | 25.0 | 0.10 |
| 4 | 0.2 | 24.2 | 21.9 | 0.44 | |||
| 5 | 7.1 | 24.2 | 20.4 | 0.33 | |||
| A22-K59 | AEQVSK | EKLSELADAK | 3 | 6.0 | 25.4 | 22.6 | 0.06 |
| K27-K27 | AEQVSKQEISHFK | AEQVSKQEISHFK | 3 | 5.1 | 28.1 | 31.0 | 0.02 |
| 4 | 3.8 | 28.1 | 24.7 | 0.55 | |||
| 5 | 5.8 | 28.1 | 23.2 | 1.00 | |||
| 6 | 5.8 | 28.0 | 21.2 | 0.47 | |||
| K27-K70 | AEQVSKQEISHFK | GGKYYHIIAAR | 5 | 6.1 | 28.7 | 24.1 | 0.24 |
| K27-S61 | AEQVSKQEISHFK | LSELADAK | 4 | 5.7 | 28.8 | 23.8 | 0.13 |
| K27-K67 | AEQVSKQEISHFK | LSELADAKGGK | 3 | 0.5 | 27.8 | 28.4 | 0.04 |
| 3 | 0.1 | 27.8 | 27.5 | 0.04 | |||
| 5 | 4.5 | 27.8 | 22.1 | 0.19 | |||
| K27-S? | AEQVSKQEISHFK | VGTINVSQSGGQISSPSDLR | 5 | 5.4 | 31.1 | 25.0 | 0.38 |
| K27-Y71/Y72 | AEQVSKQEISHFK | YYHIIAAR | 4 | 1.5 | 31.9 | 24.6 | 0.06 |
| 5 | 5.5 | 31.9 | 22.6 | 0.10 | |||
| K67-K67 | LSELADAKGGK | LSELADAKGGK | 3 | 0.5 | 26.9 | 25.6 | 0.14 |
| 4 | 0.2 | 27.0 | 22.8 | 0.12 | |||
| SO_2176 | |||||||
| K48-S68 | KVPSESR | ALAQSVK | 3 | 4 | 21.3 | 21.3 | 0.04 |
| K48-K70 | KVPSESR | ALAQSVKSNLEHHHHHH | 4 | 3 | 33.0 | 23.3 | 0.38 |
| 5 | 7 | 33.0 | 22.9 | 1.00 | |||
| K48-S71 | KVPSESR | SNLEHHHHHH | 4 | 9 | 29.1 | 21.3 | 0.56 |
| 4 | 9 | 29.1 | 23.1 | 0.35 | |||
| K63-S68 | QAVAEQFAKALAQSVK | ALAQSVK | 4 | 8 | 40.4 | 22.9 | 0.03 |
| K63-K70 | QAVAEQFAKALAQSVK | ALAQSVKSNLEHHHHHH | 4 | 5 | 45.6 | 28.7 | 0.18 |
| 5 | 9 | 45.3 | 25.1 | 0.93 | |||
| K63-K63 | QAVAEQFAKALAQSVK | QAVAEQFAKALAQSVK | 4 | 2 | 48.3 | 27.4 | 0.10 |
| 4 | 4 | 48.4 | 28.6 | 0.21 | |||
Lysine (K) to lysine cross-linking sites are presumed where present, since NHS esters are primarily amine-reactive. Alternative cross-linking sites involving S, Y, or T residues are also possible.[20-21] C-terminal lysine residues are assumed not to participate in cross-links, since modified lysine is not a trypsin cleavage site.
Presumed cross-link sites are indicated in bold.
As the N-terminus of the mature, processed protein, A22 has an amino group available for cross-linking.
The observed cross-links are mapped onto the known protein structures in Figure 3. In addition, we calculated the theoretical solvent-accessible surface distances (SASD) for the observed cross-links with the xWalk program[22] (Tables S1 and S2). The SASD between two cross-linked residues describes the path of the cross-linker and the two linked side chains over the surface of the protein. This measure is more meaningful than simple Euclidean distance, which may take a physically unrealistic path through the center of the protein.
Figure 3.
Structural analysis of observed intermolecular cross-links. Cross-linking sites were assigned from MIX-LC-IMS-MS-identified cross-linked peptides by presuming that the link was between amino groups (lysine side chains and the N-terminus) where present, and serine and tyrosine side chains if no amino groups were present in the cross-linked peptide. Observed cross-links are indicated by solid lines. Red lines indicate that the cross-link distance is too great for the span of the BS3 linker, suggesting that cross-linking induced structural perturbation or aggregation, or possibly indicating regions of flexibility. See text and Supporting Material for further discussion. Tan lines also indicate cross-links for which the amino groups in the cross-linked peptide are out of range, but for which some combination of serine, tyrosine, lysine/amino terminus is in range. Blue lines indicate distances shorter than the linker length. Top, SrfN NMR structure. Bottom, SO_2176 crystal structure (PDB code 2QTI).
By either Euclidean distance or SASD, some of the observed cross-linked pairs in Table 1 are too distant for the length of the linker. Of the six cross-linked peptides identified for SO_2176, three contain residue pairs within the expected SASD (Tables S1 and S2). Nine intermolecular cross-linked peptides for SrfN were identified; only seven of these cross-linked peptides contain potential pairs of reactive residues that are within the expected maximum SASD. The identification of these peptides is well supported by the IMS-MS data; however, without LC-MS/MS experiments to pinpoint the cross-linked residues, any pair inter-peptide pair of amino or hydroxyl residues could be the linked site. Separate MS/MS experiments with cross-linked SrfN peptides (data not shown) suggest that the cross-linked residues are usually lysine. Four of the identified SrfN peptides have a pair of lysine residues; only one of these (AEQSVKQEISHFK/ AEQSVKQEISHFK) has the two lysine residues within range.
Many cross-linking studies have reported that some confidently observed cross-links are “out-of-range” [7-8, 24]. Possible reasons for this discrepancy include structural perturbation by excessive cross-linking[25], oligomerization/aggregation, rapid subunit exchange, and structural flexibility of the protein[26]. These possibilities are discussed in greater detail in the Supporting Information. We briefly note here that cross-linking induced oligomerization was observed in some SrfN preparations, although not the one used for MIX-LC-IMS-MS (Figure S1), and that the out-of-range cross-links in SO_2176 occur in a flexible region of the protein (Figure S2). Furthermore, we did not fully optimize the cross-linking conditions used in this study, which may have led to artifacts. Given these considerations, the observation of cross-links that appear inconsistent with the structure does not detract from the utility of the MIX-LC-IMS-MS method.
In IMS-MS, similar chemical compounds group in defined (m/z, td) regions or “trend lines,” based on their charge state and chemical identity [27-28]. The observed m/z and td values of the 15 cross-linked peptides in this study fall on the same charge-state dependent trend lines [27-28] as non-cross-linked peptides (Figure S3). Seeing this trend further increases confidence in the cross-linked peptide identifications.
To evaluate the contributions of the IMS separation to the specificity and sensitivity of the cross-link search, we compared MIX-LC-IMS-MS data to simulated MIX-LC-MS data (Supplementary Information). We estimated the false discovery rate (FDR) of our MIX-LC-IMS-MS and MIX-LC-MS search results using a decoy method in which 100 randomized variants of the target sequence were used as decoys. The results in Table 2 show that the inclusion of the IMS separation dramatically increased the number of cross-linked peptides detected for both proteins; and that for SO_2176, the IMS separation also considerably decreased the FDR. To understand why IMS-MS outperformed MS alone, we evaluated the spectra of cross-linked peptides found in the IMS-MS search but not in the MS-only search. We found that interfering, unrelated peaks overlapped with peaks belonging to the cross-linked peptides in the m/z dimension (Supplementary Information Figure S4), a situation problematic for automated spectrum analysis. In contrast, separation in the IMS td dimension removed the interfering signals, allowing unambiguous identification. These observations are in line with previous work showing that LC-IMS-MS enhances sensitivity and dynamic range compared to LC-MS because overlapping LC-MS features are separated in the td dimension [29].
Table 2.
Comparison of MIX-LC-IMS-MS and MIX-LC-IMS decoy search and false discovery rate analysis.
| SrfN | SO_2176 | |||
|---|---|---|---|---|
| IMS | No IMS | IMS | No IMS | |
| Forward Hits | 18 | 8 | 12 | 2 |
| Peptides | 9 | 4 | 8 | 1 |
| Decoy Hits/100a | 0 | 0 | 0.44 | 0.42 |
| Peptides/100a | 0 | 0 | 0.37 | 0.36 |
| % FDR (Features)b | 0 | 0 | 3.7 | 21 |
| % FDR (Peptides) | 0 | 0 | 4.6 | 36 |
The decoy set consisted of 100 randomized variants of the target sequence, and was therefore 100 times larger than the target search space. Division by a factor of 100 normalizes this difference.
Calculated as (Decoy Hits/100)/Forward Hits*100.
Conclusions
Our results show that MIX specifically distinguishes intermolecular cross-links from intramolecular cross-links, a useful feature for the analysis of homo-multimeric complexes. By utilizing the MIX-LC-IMS-MS approach, we were able to increase both the number and specificity of cross-linked peptide identifications relative to MIX-LC-MS alone. This approach could easily be extended to larger, heteromeric complexes by applying the MIX labeling to a subset of the subunits, and the advantages of the additional IMS separation also apply to cross-linking experiments in which the cross-linking reagent, rather than the protein, is isotopically labeled.
Supplementary Material
Acknowledgments
We thank Dr. Guy Montelione and the NESG for providing the plasmid construct for expressing SrfN/STM0082 and the mixture of unlabeled and 13C,15N- SO_2176. We thank Drs. Sam Payne and Gordon Slysz for helpful discussions, and Abdullah Kahraman for assistance with the xWalk program. This research was supported by the National Institute of General Medical Sciences (NIGMS grant GM094623). SrfN protein was produced in a project funded by the National Institute of Allergy and Infectious Diseases (IAA Y1-AI-8401-01). The work used instrumentation and capabilities developed with support from the NIGMS grant 8 P41 GM103493-10, and the U.S. Department of Energy/Office of Biological and Environmental Research (DOE/BER). This work was performed in EMSL, a DOE/BER national scientific user facility located at PNNL in Richland, Washington.
References
- 1.Rappsilber J. The beginning of a beautiful friendship: Cross-linking/mass spectrometry and modelling of proteins and multi-protein complexes. J. Struct. Biol. 2011;173(3):530–540. doi: 10.1016/j.jsb.2010.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sinz A. Chemical cross-linking and mass spectrometry to map three-dimensional protein structures and protein-protein interactions. Mass Spectrom. Rev. 2006;25(4):663–682. doi: 10.1002/mas.20082. [DOI] [PubMed] [Google Scholar]
- 3.Leitner A, Walzthoeni T, Kahraman A, Herzog F, Rinner O, Beck M, Aebersold R. Probing Native Protein Structures by Chemical Cross-linking, Mass Spectrometry, and Bioinformatics. Molecular & Cellular Proteomics. 2010;9(8):1634–1649. doi: 10.1074/mcp.R000001-MCP201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schilling B, Row RH, Gibson BW, Guo X, Young MM. MS2Assign, automated assignment and nomenclature of tandem mass spectra of chemically crosslinked peptides. J. Am. Soc. Mass Spectrom. 2003;14(8):834–850. doi: 10.1016/S1044-0305(03)00327-1. [DOI] [PubMed] [Google Scholar]
- 5.Young MM, Tang N, Hempel JC, Oshiro CM, Taylor EW, Kuntz ID, Gibson BW, Dollinger G. High throughput protein fold identification by using experimental constraints derived from intramolecular cross-links and mass spectrometry. Proc. Natl. Acad. Sci. U. S. A. 2000;97(11):5802–5806. doi: 10.1073/pnas.090099097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walzthoeni T, Claassen M, Leitner A, Herzog F, Bohn S, Forster F, Beck M, Aebersold R. False discovery rate estimation for cross-linked peptides identified by mass spectrometry. Nat. Methods. 2012;9(9):901–903. doi: 10.1038/nmeth.2103. [DOI] [PubMed] [Google Scholar]
- 7.Yang B, Wu Y-J, Zhu M, Fan S-B, Lin J, Zhang K, Li S, Chi H, Li Y-X, Chen H-F, Luo S-K, Ding Y-H, Wang L-H, Hao Z, Xiu L-Y, Chen S, Ye K, He S-M, Dong M-Q. Identification of cross-linked peptides from complex samples. Nat Meth. 2012;9(9):904–906. doi: 10.1038/nmeth.2099. [DOI] [PubMed] [Google Scholar]
- 8.Herzog F, Kahraman A, Boehringer D, Mak R, Bracher A, Walzthoeni T, Leitner A, Beck M, Hartl F-U, Ban N, Malmström L, Aebersold R. Structural Probing of a Protein Phosphatase 2A Network by Chemical Cross-Linking and Mass Spectrometry. Science. 2012;337(6100):1348–1352. doi: 10.1126/science.1221483. [DOI] [PubMed] [Google Scholar]
- 9.Taverner T, Hall NE, O'Hair RAJ, Simpson RJ. Characterization of an Antagonist Interleukin-6 Dimer by Stable Isotope Labeling, Cross-linking, and Mass Spectrometry. J. Biol. Chem. 2002;277(48):46487–46492. doi: 10.1074/jbc.M207370200. [DOI] [PubMed] [Google Scholar]
- 10.Santos LFA, Iglesias AH, Pilau EJ, Gomes AF, Gozzo FC. Traveling-Wave Ion Mobility Mass Spectrometry Analysis of Isomeric Modified Peptides Arising from Chemical Cross-Linking. J. Am. Soc. Mass Spectrom. 2010;21(12):2062–2069. doi: 10.1016/j.jasms.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 11.Smith DP, Anderson J, Plante J, Ashcroft AE, Radford SE, Wilson AJ, Parker MJ. Trifluoromethyldiazirine: an effective photo-induced cross-linking probe for exploring amyloid formation. Chem. Commun. 2008;(44):5728–5730. doi: 10.1039/b813504e. [DOI] [PubMed] [Google Scholar]
- 12.Soderberg CAG, Lambert W, Kjellstrom S, Wiegandt A, Wulff RP, Mansson C, Rutsdottir G, Emanuelsson C. Detection of Crosslinks within and between Proteins by LC-MALDI-TOFTOF and the Software FINDX to Reduce the MSMS-Data to Acquire for Validation. PLoS One. 2012;7(6) doi: 10.1371/journal.pone.0038927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Acton TB, Xiao R, Anderson S, Aramini J, Buchwald WA, Ciccosanti C, Conover K, Everett J, Hamilton K, Huang YJ, Janjua H, Kornhaber G, Lau J, Lee DY, Liu GH, Maglaqui M, Ma LC, Mao L, Patel D, Rossi P, Sahdev S, Shastry R, Swapna GVT, Tang YF, Tong SC, Wang DY, Wang H, Zhao L, Montelione GT. Kuo LC, editor. Preparation of Protein Samples For NMR Structure, Function, and Small-Molecule Screening Studies. Fragment-Based Drug Design: Tools, Practical Approaches, and Examples. 2011;493:21–60. doi: 10.1016/B978-0-12-381274-2.00002-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cort JR, Selan U, Schulte A, Grimm F, Kennedy MA, Dahl C. Allochromatium vinosum DsrC: Solution-state NMR structure, redox properties, and interaction with DsrEFH, a protein essential for purple sulfur bacterial sulfur oxidation. J. Mol. Biol. 2008;382(3):692–707. doi: 10.1016/j.jmb.2008.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Livesay EA, Tang K, Taylor BK, Buschbach MA, Hopkins DF, LaMarche BL, Zhao R, Shen Y, Orton DJ, Moore RJ, Kelly RT, Udseth HR, Smith RD. Fully Automated Four-Column Capillary LC–MS System for Maximizing Throughput in Proteomic Analyses. Anal. Chem. 2007;80(1):294–302. doi: 10.1021/ac701727r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ibrahim Y, Belov ME, Tolmachev AV, Prior DC, Smith RD. Ion Funnel Trap Interface for Orthogonal Time-of-Flight Mass Spectrometry. Anal. Chem. 2007;79(20):7845–7852. doi: 10.1021/ac071091m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ibrahim YM, Prior DC, Baker ES, Smith RD, Belov ME. Characterization of an ion mobility-multiplexed collision-induced dissociation-tandem time-of-flight mass spectrometry approach. Int. J. Mass spectrom. 2010;293(1-3):34–44. doi: 10.1016/j.ijms.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jaitly N, Mayampurath A, Littlefield K, Adkins JN, Anderson GA, Smith RD. Decon2LS: An open-source software package for automated processing and visualization of high resolution mass spectrometry data. BMC Bioinformatics. 2009;10 doi: 10.1186/1471-2105-10-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crowell KL, Shah AR, Slysz G, Lamarche BL, Meng D, Baker ES, Monroe ME, Anderson GA, Smith RD. LC-IMS-MS Feature Finder: Detecting Multidimensional Features in IMS-TOF MS Data.. The American Society for Mass Spectrometry Conference; Denver, CO. 2011. [Google Scholar]
- 20.Mädler S, Bich C, Touboul D, Zenobi R. Chemical cross-linking with NHS esters: a systematic study on amino acid reactivities. J. Mass Spectrom. 2009;44(5):694–706. doi: 10.1002/jms.1544. [DOI] [PubMed] [Google Scholar]
- 21.Kalkhof S, Sinz A. Chances and Pitfalls of chemical cross-linking with amine-reactive N-hydroxysuccinimide esters. Analytical Bioanalytical Chemistry. 2008;392:305–312. doi: 10.1007/s00216-008-2231-5. [DOI] [PubMed] [Google Scholar]
- 22.Kahraman A, Malmström L, Aebersold R. Xwalk: computing and visualizing distances in cross-linking experiments. Bioinformatics. 2011;27(15):2163–2164. doi: 10.1093/bioinformatics/btr348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leitner A, Reischl R, Walzthoeni T, Herzog F, Bohn S, Förster F, Aebersold R. Expanding the Chemical Cross-Linking Toolbox by the Use of Multiple Proteases and Enrichment by Size Exclusion Chromatography. Molecular & Cellular Proteomics. 2012;11(3) doi: 10.1074/mcp.M111.014126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen ZA, Jawhari A, Fischer L, Buchen C, Tahir S, Kamenski T, Rasmussen M, Lariviere L, Bukowski-Wills J-C, Nilges M, Cramer P, Rappsilber J. Architecture of the RNA polymerase II-TFIIF complex revealed by cross-linking and mass spectrometry. EMBO J. 2010;29(4):717–726. doi: 10.1038/emboj.2009.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fabris D, Yu ET. Elucidating the higher-order structure of biopolymers by structural probing and mass spectrometry: MS3D. J. Mass Spectrom. 2010;45(8):841–860. doi: 10.1002/jms.1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li H, Wells SA, Jimenez-Roldan JE, Römer RA, Zhao Y, Sadler PJ, O'Connor PB. Protein flexibility is key to cisplatin crosslinking in calmodulin. Protein Sci. 2012;21(9):1269–1279. doi: 10.1002/pro.2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Valentine SJ, Counterman AE, Hoaglund CS, Reilly JP, Clemmer DE. Gas-phase separations of protease digests. J. Am. Soc. Mass Spectrom. 1998;9(11):1213–1216. doi: 10.1016/S1044-0305(98)00101-9. [DOI] [PubMed] [Google Scholar]
- 28.Woods A, Koomen J, Ruotolo B, Gillig K, Russel D, Fuhrer K, Gonin M, Egan T, Schultz J. A study of peptide-peptide interactions using MALDI ion mobility o-TOF and ESI mass spectrometry. J. Am. Soc. Mass Spectrom. 2002;13(2):166–169. doi: 10.1016/S1044-0305(01)00348-8. [DOI] [PubMed] [Google Scholar]
- 29.Baker ES, Livesay EA, Orton DJ, Moore RJ, Danielson WF, Prior DC, Ibrahim YM, LaMarche BL, Mayampurath AM, Schepmoes AA, Hopkins DF, Tang K, Smith RD, Belov ME. An LC-IMS-MS Platform Providing Increased Dynamic Range for High-Throughput Proteomic Studies. Journal of Proteome Research. 2009;9(2):997–1006. doi: 10.1021/pr900888b. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.