Abstract
Benefit from cytotoxic therapy in myeloma may be limited by the persistence of residual tumour cells within protective niches. We have previously shown that monocytes/macrophages acquire a proinflammatory transcriptional profile in the myeloma microenvironment. Here we report constitutive activation of MAP3K8 kinase-dependent pathways that regulate the magnitude and extent of inflammatory activity of monocytes/macrophages within myeloma niches. In myeloma tumour cells, MAP3K8 acts as mitogen-induced MAP3K in mitosis and is required for TNFα-mediated ERK activation. Pharmacological MAP3K8 inhibition results in dose-dependent, tumour cell-autonomous apoptosis despite contact with primary stroma. MAP3K8 blockade may disrupt crucial macrophage-tumour cell interactions within myeloma niches.
Keywords: myeloma, bone marrow pathology, macrophages
INTRODUCTION
Whereas the role of tumour-associated macrophages in local propagation and metastasis of solid tumours is well established (Qian and Pollard 2010, Sica and Mantovani 2012), the contribution of macrophages to the natural history of haematopoietic cancers is inadequately explored. Myeloma provides an excellent model because of the dependence of malignant plasma cells on their bone marrow microenvironment. Myeloma tumours are rich in resident macrophages that provide growth-promoting, angiogenic and anti-apoptotic signals to malignant plasma cells (Kim, J., et al 2012, Ribatti and Vacca 2009, Zheng, et al 2009). We recently demonstrated that myeloma-associated monocytes/macrophages display a proinflammatory (M1) transcriptional profile associated with production of pro-myeloma inflammatory cytokines tumour necrosis factor-α (TNFα), interleukin (IL) 6, IL1β and IL8 (Kim, J., et al 2012). We also found concurrent transcription of genes characteristic of “alternative macrophage activation” (M2 phenotype) such as IL10 and the locus encoding IL1-receptor antagonist (IL1RN), suggesting significant plasticity between the M1-M2 phenotypic extremes.
To obtain insights into the underlying mechanisms, we examined the role of MAP3K8 (mitogen-activated protein kinase kinase kinase 8, Cot, TPL2), a serine/threonine kinase with central and non-redundant roles in regulating innate immune responses (Gantke, et al 2011). Map3k8-null mice are resistant to endotoxic shock induced by lipopolysaccharide, owing to a defect in TNFα processing by macrophages (Dumitru, et al 2000). In addition to its role in inflammatory signal transduction, MAP3K8 can efficiently transmit growth signals that activate extracellular signal-regulated kinase (ERK) (Johannessen, et al 2010). We hypothesized that MAP3K8 may regulate crucial macrophage-myeloma tumour cell interactions within the bone marrow microenvironment.
MATERIALS AND METHODS
Cell culture and MAP3K8 inhibition
Cell culture conditions are detailed in Supplementary Methods. MAP3K8 inhibitor (616373) was obtained from Calbiochem (Billerica, MA, USA).
Patient samples
Clinical characteristics of the patients are given in supplementary Table I. Bone marrow aspirates were collected following informed consent under an Institutional Review Board-approved protocol (HO07403) and processed as detailed in Supplementary Methods.
Expression of MAP3K8 transcripts in myeloma and other malignancies
Publicly available gene expression data (https://array.nci.nih.gov/caarray/project/woost-00041) of cell lines from diverse haematological malignancies and solid tumours were downloaded and analysed through Oncomine 4.4 (www.oncomine.com). The log2-transformed median-centred expression of MAP3K8 transcript was compared between different types of tumours using GraphPad Prism 5, using the Kruskal-Wallis test for one-way analysis of variance and the Dunn's multiple comparison test for post-hoc analysis.
RESULTS AND DISCUSSION
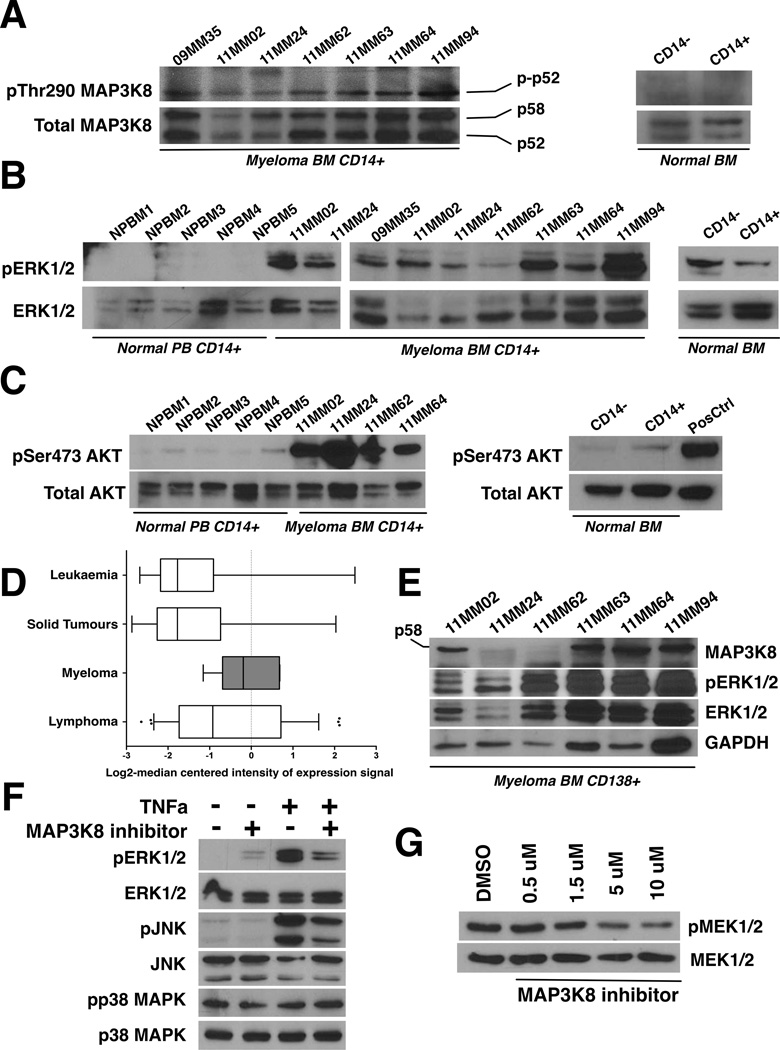
To interrogate the mechanisms underlying macrophage activation/polarization within myeloma niches, we investigated the role of MAP3K8 kinase, a master regulator of cytokine processing by activated macrophages (Lopez-Pelaez, et al 2012). We probed lysates of CD14+ cells isolated from myeloma marrow mononuclear fractions with an antibody directed against activated (phospho-Thr290) MAP3K8 (see Supplementary file for details). MAP3K8 was universally activated by phosphorylation in purified CD14+ cells, regardless of clinical stage of disease or prior treatments (Figure 1A). CD14+ cells expressed both MAP3K8 isoforms in comparable abundance. However, the phosphorylated p52 isoform was mostly detectable, possibly due to the inherent instability of phospho-p58 (Gantke, et al 2011).
Figure 1.
A. MAP3K8 kinase is constitutively active in myeloma-associated monocytes/macrophages. Bone marrow (BM) aspirates were collected with informed consent from 7 patients (“MM” lanes). Fresh BM from normal donors was obtained commercially. CD14+ mononuclear cells were purified by immunomagnetic separation and lysates were immunoblotted using antisera against phosphorylated (phospho-Thr290) and total MAP3K8. For the normal bone marrow control, CD14+ denotes the CD14+ mononuclear cell fraction (positive isolation) and CD14- denotes the CD14-depleted mononuclear fraction. Two different normal marrow donors gave identical results. Both p58 and p52 MAP3K8 isoforms are expressed in CD14+ cells at comparable rates. The phosphorylated p58 isoform is prone to rapid degradation (Gantke, et al 2011).
B. Constitutive ERK activation in myeloma-associated monocytes/macrophages. The activation status of ERK was probed using antibodies against phosphorylated and total ERK. CD14+ peripheral blood monocytes from 5 different normal donors are shown to the far left (NPBM1-5) next to CD14+ bone marrow mononuclear cells from myeloma patients (“MM” lanes). For the normal bone marrow control, CD14+ denotes the CD14+ mononuclear cell fraction (positive isolation) and CD14- denotes the CD14-depleted mononuclear fraction. Two different normal marrow donors gave identical results.
C. Constitutive AKT activation at Ser473 in myeloma-associated monocytes/macrophages. The activation status of AKT was probed using antibodies against phosphorylated (phospho-Ser473 AKT) and total AKT. CD14+ peripheral blood monocytes from 5 different normal donors are shown to the far left (NPBM1-5) next to CD14+ bone marrow mononuclear cells from myeloma patients (“MM” lanes). For the normal bone marrow control, CD14+ denotes the CD14+ mononuclear cell fraction (positive isolation) and CD14- denotes the CD14-depleted mononuclear fraction. Two different normal marrow donors gave identical results.
D. MAP3K8 expression levels are elevated in myeloma cells compared to non-lymphoid and non-haematopoietic tumour cells. MAP3K8 transcript expression data in multiple myeloma, leukaemia, lymphoma and solid tumour cell lines. Results are depicted in boxplots, which highlight the median and interquartile range of expression of MAP3K8 in each group. The whiskers of each boxplot represent the minimum and maximum expression of MAP3K8 for the respective group. The values for the log2-transformed median-centered expression of MAP3K8 transcript among myeloma cell lines was higher compared to solid tumours (p<0.0001, Kruskal-Wallis test for one-way analysis of variance, and p<0.05, post-hoc Dunn's multiple comparison test).
E. MAP3K8 protein expression and ERK activation in primary myeloma tumour cells. Lysates from primary CD138+ myeloma tumour cells were immunoblotted using antisera against total MAP3K8, phospho-ERK, total ERK and GAPDH as a loading control. In myeloma cells, the p58 MAP3K8 isoform was preferentially expressed.
F. MAP3K8 promotes ERK and JNK activation in response to TNF receptor stimulation in myeloma cells. MM1.S cells were serum-starved overnight in the presence or absence of MAP3K8 inhibitor (5 µM). A 5-min pulse of recombinant human TNFα (50 ng/ml) was given at the end of the incubation and cells were immediately lysed in protein buffer. MAP3K8 inhibition resulted in a reduction of phospho-ERK and phospho-JNK induction following stimulation with TNFα whereas p38MAPK activation status remained unaffected.
G. Treatment of myeloma cells with a small-molecule MAP3K8 kinase inhibitor results in dose-dependent reduction in phosphorylation of the MAP3K8 direct substrate, MEK. U266 myeloma cells were incubated with escalating concentrations of MAP3K8 inhibitor for 24 h in the presence of continuous mitogenic stimulation (10% fetal calf serum). Whole cell lysates were subsequently immunoblotted and probed with antisera against phospho-MEK1/2 and total MEK1/2. DMSO, dimethyl sulfoxide.
MAP3K8-dependent ERK activity promotes the synthesis of important pro-myeloma inflammatory mediators, including TNFα and IL-6 (Lopez-Pelaez, et al 2012). Accordingly, we found constitutive ERK phosphorylation in myeloma-associated, but not normal, monocytes/macrophages (Figure 1B). A critical target of ERK in activated macrophages is ADAM17 (TACE), the protease responsible for cleavage and processing of pre-TNFα (Gantke, et al 2011). We found constitutive TACE phosphorylation at Thr735, a site regulated by MAP3K8 signalling, in myeloma marrow-derived CD14+ mononuclear cells (Supplementary Figure 1).
MAP3K8 also controls AKT phosphorylation at Ser473 following Toll receptor stimulation (Lopez-Pelaez, et al 2011). This event is thought to be a crucial part of a signalling cascade that limits the magnitude and duration of innate immune responses, in part through AKT/mTOR/STAT3-mediated synthesis of the anti-inflammatory cytokine IL10 (Serebrennikova, et al 2012, Weichhart, et al 2008). Myeloma-associated monocytes/macrophages displayed strong, constitutive AKT phosphorylation at Ser473 (Figure 1C). These results suggest that MAP3K8 has a role in fine-tuning macrophage activation within myeloma niches.
Furthermore, we sought to explore a potential cell-autonomous role of MAP3K8 activity in myeloma growth regulation. Expression levels of MAP3K8 are higher in multiple myeloma cells compared to non-lymphoid haematopoietic tumour cells or solid tumour cells, as determined by integration of publicly available expression data (https://array.nci.nih.gov/caarray/project/woost-00041) (Figure 1D). At the protein level, primary CD138+ cells derived from myeloma bone marrows predominantly expressed the p58 MAP3K8 isoform (Figure 1E). In 4 out of 6 available patients, MAP3K8 protein was readily detected by immunoblot analysis, in concert with activated ERK (Figure 1E). Lack of MAP3K8 expression with simultaneous ERK activation may reflect epistatic RAS or RAF mutations (Johannessen, et al 2010). Because MAP3K8 constitutes an essential component of a mechanism that leads to MAPK pathway activation downstream of the TNF receptor in naïve murine splenocytes (Eliopoulos, et al 2003), we sought to determine whether MAP3K8 can transmit inflammatory signals in human myeloma tumour cells, using human MM1.S myeloma cells as a model. Serum-deprived MM1.S cells respond to exogenous TNFα stimulation by phosphorylating ERK (Hideshima, et al 2001). MM1.S cells were starved of serum overnight in the presence or absence of a MAP3K8 kinase inhibitor at low micromolar concentrations and subsequently delivered a pulse of recombinant TNFα. Serum-starved MM1.S cells displayed low levels of basal ERK activation. Upon stimulation with TNFα, ERK was strongly phosphorylated. However, ERK phosphorylation was, at least partially, inhibited in the presence of MAP3K8 inhibitor (Figure 1F). These results demonstrate that in myeloma cells, MAP3K8 is required for MAPK pathway activation in response to TNFα stimulation. Moreover, we determined that MAP3K8 transmits signals that activate JNK upon TNFα stimulation (Figure 1F).
We next investigated whether MAP3K8 kinase blockade affected phosphorylation of the MAP3K8 direct substrate, MEK kinase, under conditions of continuous mitogenic stimulation. We incubated U266 cells (a myeloma cell line that lacks RAS mutations) in the presence of escalating doses of MAP3K8 inhibitor under standard culture conditions. We observed a dose-dependent reduction in phosphorylation of MEK1/2 (Figure 1G). In concert with reduced MEK phosphorylation, we observed a dose-dependent reduction in the growth of U266 cells as well as two other myeloma cell lines (MM1.S, RPMI8226) (Figure 2A). The attenuated effect of MAP3K8 kinase inhibition on RPMI8226 cells may be explained by constitutive MAPK pathway activity in this cell line, secondary to gain-of-function KRAS mutation. We conclude that MAP3K8 kinase blockade results in cell-autonomous growth inhibition of myeloma cells associated with reduction in MEK phosphorylation.
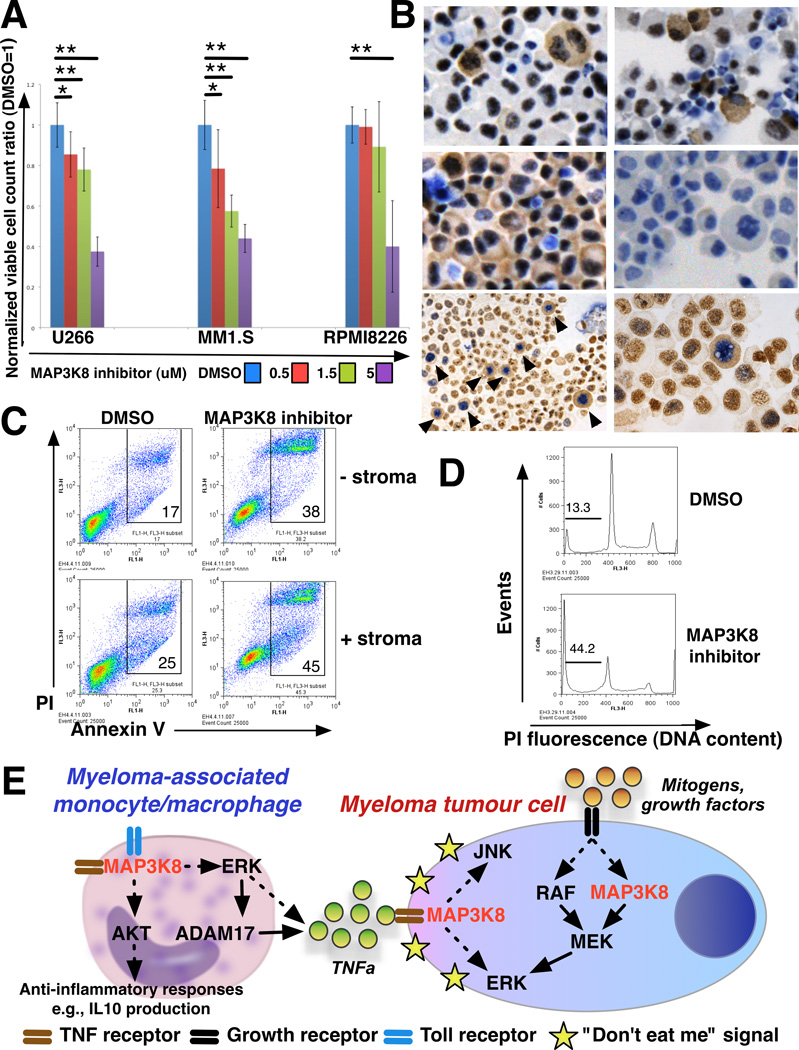
Figure 2.
A. MAP3K8 kinase inhibition results in dose-dependent growth inhibition of human myeloma cells. Human myeloma cell lines U266, MM1.S and RPMI8226 were plated at a starting concentration of 100,000 cells/ ml and treated with escalating concentrations of MAP3K8 kinase inhibitor as shown. Cultures were terminated at 96 h and viable cell counts performed. Each value represents the average of 6 counts obtained from 3 independent experiments, normalized to the untreated (DMSO-only) control. Error bars denote one standard deviation above and below the mean. Statistical significance is indicated with single asterisks at threshold p<0.05 and double asterisks at threshold p<0.01.
B. MAP3K8 phosphorylation peaks in myeloma cells in mitosis. Cytospin preparations from U266 cells were immunostained using antisera against phospho-Thr290 MAP3K8 and total MAP3K8. Top left, actively growing U266 cells were stained with an antibody against phospho-Thr290 MAP3K8. Note that cytoplasmic staining correlates with mitotic nuclear morphology. Top right, U266 cells were pretreated with nocodazole overnight and immunostained with an anti-phospho-Thr290 antibody. Nocodazole inhibits spindle formation and results in G2/M arrest. Treatment with nocodazole results in cytoplasmic staining of numerous G2/M arrested cells. Middle left, actively growing U266 cells stained with an antibody against total MAP3K8. Almost all cells exhibit cytoplasmic staining, regardless of cell cycle status. Middle right, secondary antibody-only control. Bottom panels, cells were stained with antisera against phospho-Thr290 MAP3K8 (two different antibodies giving identical results, see Supplementary file for details) (cytoplasmic brown staining, DAB chromogen) followed by an antibody against phospho-Histone H3 (Ser10) (nuclear blue/violet staining, BCIP/NBT chromogen). Low and high power views are shown. Cells that exhibit cytoplasmic phospho-MAP3K8 staining also stain positive for nuclear phospho-Histone H3. Specific cytoplasmic staining for phospho-MAP3K8 is distinct from variable diffuse nuclear brown staining (background).
C. Treatment of myeloma cells with a MAP3K8 kinase inhibitor results in apoptosis that is not rescued by the contact with myeloma-derived mesenchymal stromal cells. U266 myeloma cells were grown in the presence or absence of a monolayer of mesenchymal stromal cells derived from myeloma bone marrow. At 96 h of culture in the presence or absence of MAP3K8 inhibitor, cells were harvested, fixed and stained with Annexin V-fluorescein isothiocyanate and propidium iodide (PI) and analysed on a flow cytometer. Gates include early (Annexin Vhigh, PIlow) and late (Annexin Vhigh, PIhigh) apoptotic cells.
D. Cell-cycle profile of myeloma cells treated with a MAP3K8 kinase inhibitor. U266 myeloma cells were harvested at 96 h of culture in the presence or absence of MAP3K8 kinase inhibitor. Cells were fixed, stained with propidium iodide (PI) and analysed for DNA content on a flow cytometer. Treatment with MAP3K8 inhibitor results in an increase in the percentage of apoptotic cells (denoted by bar) without a corresponding G2/M arrest.
E. Schematic diagram summarizing the complex actions of MAP3K8 kinase in the myeloma niche. In the proposed model, MAP3K8 participates in mitogenic signal transduction through the MAPK pathway and regulates TNF receptor-mediated inflammatory signal transduction in myeloma tumour cells. Additionally, MAP3K8 regulates production of TNFα (and other myeloma-promoting inflammatory cytokines) by myeloma-associated monocytes/macrophages through ERK-mediated mechanisms. MAP3K8 also controls AKT-mediated attenuation of macrophage inflammatory activity in order to avoid overt tissue injury by activated macrophages. Direct interactions are denoted by solid lines. Indirect interactions are denoted by broken lines.
Using antisera against phospho-Thr290 MAP3K8, we observed intense cytoplasmic staining of myeloma cells in mitosis (Figure 2B, top left). By contrast, total MAP3K8 protein was detected in almost all cells regardless of nuclear morphology (Figure 2B, middle left). When treated with nocodazole, cells arrest with a G2/M DNA content but cannot form mitotic spindles. Upon nocodazole treatment, phospho-Thr290 MAP3K8 could be detected in numerous G2/M-arrested cells (Figure 2B, top right). To confirm these findings, cells were sequentially stained for phospho-MAP3K8 and phospho-Histone H3 (Ser10), a marker of mitosis (Figure 2B, bottom left and right panels). Cells displaying cytoplasmic staining for phospho-MAP3K8 also displayed nuclear staining for phospho-Histone H3. Thus MAP3K8 activation correlates with cell cycle status and/or increased ploidy of myeloma tumour cells.
To investigate the mechanism underlying growth inhibition upon MAP3K8 kinase blockade, we treated U266 cells with the MAP3K8 kinase inhibitor in the presence or absence of primary myeloma bone marrow-derived mesenchymal stomal cells as a source of disease-appropriate stroma. MAP3K8 kinase blockade augmented apoptotic death of myeloma cells, regardless of stromal contact (Figure 2C). Despite expression of activated MAP3K8 in mitosis, treatment with MAP3K8 inhibitor led to apoptosis of the treated cells without evidence of G2/M arrest (Figure 2D). We conclude that MAP3K8 kinase blockade leads to cell-autonomous demise of myeloma cells that cannot be rescued by the interactions afforded by myeloma marrow-derived stromal cells.
The data from our study support the hypothesis that MAP3K8 kinase activity contributes to myeloma growth and survival through both cell-autonomous and non-autonomous mechanisms (summarized in Figure 2E). MAP3K8 clearly transmits growth and inflammatory signals in myeloma cells, acting as a mitogen- and TNF receptor-activated MAP3K. Myeloma-associated monocytes/macrophages engage MAP3K8 to regulate production of pro-myeloma inflammatory cytokines through the ERK pathway and additionally, to modulate macrophage polarization through the AKT pathway in order to limit tissue injury by activated macrophages. Myeloma cells may be further shielded from cytopathic actions of inflammatory macrophages by expressing “don’t eat me” signals (Kim, D., et al 2012). Targeted MAP3K8 inhibition can thus interfere with critical macrophage-tumour cell interactions within myeloma niches. The clinical efficacy of targeted MAP3K8 inhibition may depend on RAS or RAF mutational status and may help prevent or circumvent acquired resistance to other MAPK pathway inhibitors (Johannessen, et al 2010). Because Map3k8 nullizygosity is compatible with normal haematopoietic development and function (Dumitru, et al 2000), MAP3K8 blockade is likely to be endowed with a wide therapeutic window. Constitutive activation of signalling pathways in tumour-accessory cells is extrinsic, in contrast to malignant cells where intrinsic activation usually results from mutations. Ultimate proof of the therapeutic potential of MAP3K8 loss/inhibition would thus require testing in appropriate in vivo systems in which microenvironmental cues are complex and physiological. This work is currently underway in the authors’ laboratory.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Emery Bresnick for critical review of the manuscript and the Bresnick laboratory members for valuable help with the experiments. FA is the recipient of a Leukemia Research Foundation New Investigator Award. CH is the recipient of a Kirschstein National Research Service Award (T32 HL007899-Hematology in Training-PI, John Sheehan). JJ is the recipient of a TL1 trainee award from the Clinical and Translational Science Award (CTSA) programme, previously through the National Center for Research Resources (NCRR) grant 1UL1RR025011, and now by the National Center for Advancing Translational Sciences (NCATS), grant 9U54TR000021 (PI, Marc Drezner). PH is recipient of NHLBI – NIH grant K08 HL081076. This work was supported in part by funds from the UWCCC Trillium Fund for Multiple Myeloma Research, the UW Horace Collins Research Fund, the Wisconsin Alumni Research Foundation through the UW Graduate School, the UW Carbone Cancer Center (Core Grant P30 CA014520) and the UW- Madison School of Medicine and Public Health.
Footnotes
CONFLICT OF INTEREST DISCLOSURE
The authors have no financial conflicts-of-interest to disclose.
AUTHOR CONTRIBUTIONS
EH, CH, JJ, CF and FA performed experiments. JK and NB performed patient sample preparations. NC provided patient samples and clinical data. IM, CM, SM and PH provided critical advice on study design. FA was overall responsible for study design and wrote the manuscript.
REFERENCES
- 1.Dumitru CD, Ceci JD, Tsatsanis C, Kontoyiannis D, Stamatakis K, Lin JH, Patriotis C, Jenkins NA, Copeland NG, Kollias G, Tsichlis PN. TNF-alpha induction by LPS is regulated posttranscriptionally via a Tpl2/ERK-dependent pathway. Cell. 2000;103:1071–1083. doi: 10.1016/s0092-8674(00)00210-5. [DOI] [PubMed] [Google Scholar]
- 2.Eliopoulos AG, Wang CC, Dumitru CD, Tsichlis PN. Tpl2 transduces CD40 and TNF signals that activate ERK and regulates IgE induction by CD40. EMBO J. 2003;22:3855–3864. doi: 10.1093/emboj/cdg386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gantke T, Sriskantharajah S, Ley SC. Regulation and function of TPL-2, an IkappaB kinase-regulated MAP kinase kinase kinase. Cell Res. 2011;21:131–145. doi: 10.1038/cr.2010.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hideshima T, Chauhan D, Schlossman R, Richardson P, Anderson KC. The role of tumor necrosis factor alpha in the pathophysiology of human multiple myeloma: therapeutic applications. Oncogene. 2001;20:4519–4527. doi: 10.1038/sj.onc.1204623. [DOI] [PubMed] [Google Scholar]
- 5.Johannessen CM, Boehm JS, Kim SY, Thomas SR, Wardwell L, Johnson LA, Emery CM, Stransky N, Cogdill AP, Barretina J, Caponigro G, Hieronymus H, Murray RR, Salehi-Ashtiani K, Hill DE, Vidal M, Zhao JJ, Yang X, Alkan O, Kim S, Harris JL, Wilson CJ, Myer VE, Finan PM, Root DE, Roberts TM, Golub T, Flaherty KT, Dummer R, Weber BL, Sellers WR, Schlegel R, Wargo JA, Hahn WC, Garraway LA. COT drives resistance to RAF inhibition through MAP kinase pathway reactivation. Nature. 2010;468:968–972. doi: 10.1038/nature09627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim D, Wang J, Willingham SB, Martin R, Wernig G, Weissman IL. Anti-CD47 antibodies promote phagocytosis and inhibit the growth of human myeloma cells. Leukemia. 2012 doi: 10.1038/leu.2012.141. Epub ahead of print, [DOI] [PubMed] [Google Scholar]
- 7.Kim J, Denu RA, Dollar BA, Escalante LE, Kuether JP, Callander NS, Asimakopoulos F, Hematti P. Macrophages and mesenchymal stromal cells support survival and proliferation of multiple myeloma cells. Br J Haematol. 2012;158:336–346. doi: 10.1111/j.1365-2141.2012.09154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lopez-Pelaez M, Soria-Castro I, Bosca L, Fernandez M, Alemany S. Cot/tpl2 activity is required for TLR-induced activation of the Akt p70 S6k pathway in macrophages: Implications for NO synthase 2 expression. Eur J Immunol. 2011;41:1733–1741. doi: 10.1002/eji.201041101. [DOI] [PubMed] [Google Scholar]
- 9.Lopez-Pelaez M, Fumagalli S, Sanz C, Herrero C, Guerra S, Fernandez M, Alemany S. Cot/tpl2-MKK1/2-Erk1/2 controls mTORC1-mediated mRNA translation in Toll-like receptor-activated macrophages. Mol Biol Cell. 2012;23:2982–2992. doi: 10.1091/mbc.E12-02-0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ribatti D, Vacca A. The role of monocytes-macrophages in vasculogenesis in multiple myeloma. Leukemia. 2009;23:1535–1536. doi: 10.1038/leu.2009.55. [DOI] [PubMed] [Google Scholar]
- 12.Serebrennikova OB, Tsatsanis C, Mao C, Gounaris E, Ren W, Siracusa LD, Eliopoulos AG, Khazaie K, Tsichlis PN. Tpl2 ablation promotes intestinal inflammation and tumorigenesis in Apcmin mice by inhibiting IL-10 secretion and regulatory T-cell generation. Proc Natl Acad Sci U S A. 2012;109:E1082–E1091. doi: 10.1073/pnas.1115098109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122:787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weichhart T, Costantino G, Poglitsch M, Rosner M, Zeyda M, Stuhlmeier KM, Kolbe T, Stulnig TM, Horl WH, Hengstschlager M, Muller M, Saemann MD. The TSC-mTOR signaling pathway regulates the innate inflammatory response. Immunity. 2008;29:565–577. doi: 10.1016/j.immuni.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 15.Zheng Y, Cai Z, Wang S, Zhang X, Qian J, Hong S, Li H, Wang M, Yang J, Yi Q. Macrophages are an abundant component of myeloma microenvironment and protect myeloma cells from chemotherapy drug-induced apoptosis. Blood. 2009;114:3625–3628. doi: 10.1182/blood-2009-05-220285. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.