Abstract
We assessed the acceptability of three of over-the-counter products representative of potential rectal microbicide (RM) delivery systems. From 2009 to 2010, 117 HIV-uninfected males (79 %) and females (21 %) who engage in receptive anal intercourse participated in a 6-week randomized crossover acceptability trial. Participants received each of three products (enema, lubricant-filled applicator, suppository) every 2 weeks in a randomized sequence. CASI and T-ACASI scales assessed product acceptability via Likert responses. Factor analysis was used to identify underlying factors measured by each scale. Random effects models were fit to examine age and gender effects on product acceptability. Three underlying factors were identified: Satisfaction with Product Use, Sexual Pleasure, and Ease of Product Use. For acceptability, the applicator ranked highest; however, differences between product acceptability scores were greatest among females and younger participants. These findings indicate that RM delivery systems impact their acceptability and should be considered early in RM development to enhance potential use.
Keywords: Anorectal products, Receptive anal intercourse, HIV prevention
Introduction
Rectal microbicides (RM) can be formulated as gels, creams, or enemas for application inside the rectum to prevent HIV infection during anal intercourse (AI). Among 24,787 men who have sex with men (MSM) who completed an online survey in the United States in 2010, 36 and 34 % reported having receptive anal intercourse (RAI) and insertive anal intercourse (IAI) at last sex, respectively [1]. Further, of those who reported having AI at last sex, 55 % reported not using condoms. Although AI is more frequently practiced among MSM, many heterosexual males and females also engage in AI. In a nationally representative survey conducted among adults and adolescents in 2009, 12.7 % of females reported having AI in the past year while 3.6 and 15.9 % of males reported having RAI and IAI, respectively, in the past year [2]. However, among males 25–49 years of age and females 20–39 years of age, >20 % reported having AI in the past year [3]. Further, among those who reported engaging in RAI at last sex, only 44 % of males and 11 % of females reported using condoms [2]. Compared to unprotected vaginal and IAI, unprotected RAI confers a greater risk of HIV infection per sex act [4, 5]. Given the low rate of condom use during AI and the risk associated with unprotected RAI, methods to prevent HIV infection during AI, such as RMs, are greatly needed.
The potential impact of RMs on HIV infection rates will depend on whether high-risk individuals are willing to use them [6]. While early RM acceptability studies evaluated the acceptability of a single placebo product among MSM [7, 8], more recent studies have measured the acceptability of active microbicide products among both males and females who practice RAI [9–11]. One study evaluated the safety and acceptability of UC781 gel relative to a placebo gel and observed high acceptability of the active gel [9]. In the recent RMP-02/MTN-006 trial of rectally applied but vaginally formulated 1 % tenofovir gel, fewer participants found the active gel acceptable compared to HEC placebo (25 vs. 50 %, respectively) [10]. However, rectal application of a reduced glycerin formulation of 1 % tenofovir gel in MTN-007 was well tolerated and highly acceptable relative to HEC placebo gel, 2 % nonoxynol-9, and no treatment [11].
A range of delivery systems, (i.e., formulations and devices), can be used to administer RMs, and in many cases the device used is dependent on the substance or consistency of the formulation to be applied. Yet, despite this range, few studies have compared the acceptability of a variety of potential RM delivery systems [12]. Carballo-Dieguez et al. were the first to measure the acceptability of more than one candidate RM delivery system in a randomized crossover trial. They reported that 75 % of participants preferred using a lubricant (35 mL) inserted using an enema bottle compared to only 25 % who preferred using a suppository (8 g) (p <0.001) [12]. However, this was a small study restricted to MSM (N = 77). No studies have compared the acceptability of multiple RM delivery systems among females [9, 13].
The goal of this study was to measure the acceptability of three candidate RM products representing distinct rectal delivery systems among males and females who practice RAI and examine the effects of various characteristics on product acceptability within this population.
Methods
Study Design and Population
Between February 2009 and September 2010, 117 HIV-uninfected males and females were enrolled in the Anorectal Microbicide Product (AMP) Study, a randomized crossover clinical trial, at the UCLA Center for Clinical AIDS Research and Education. The AMP Study was part of UCLAs ongoing U19 Microbicide Development Program (MDP), a DAIDS-supported Integrated Pre-Clinical/Clinical Program (IPCP) focused on the development of RMs (#AI0606414). All participants signed written informed consents approved by the UCLA Human Subjects Review Committee.
Participants were recruited from the Network for AIDS Research in Los Angeles’ research registry, ongoing MDP studies conducted at UCLA, from the UCLA CFAR Mucosal Immunology Core’s registry, and through advertisements posted on the UCLA campus, at local community-based organizations, on social networking sites (e.g., GRINDR) and classifieds (e.g., LA Weekly and craigs-list.org) websites. Potential participants were consented and screened at Visit 1. Eligible participants were ≥ 18 -years of age, HIV and STI (syphilis, rectal Neisseria gonorrhea, and rectal Chlamydia trachomatis) negative, had recently engaged in RAI (past month for males; past year for females), had a personal telephone for completing telephone questionnaires, understood local HIV/STI reporting requirements, and were willing and able to give informed consent, try all three anorectal products at least once, meet study visits, and complete study questionnaires. Final eligibility was determined 2 weeks later at the enrollment visit (Visit 2). Potential participants were excluded if they were homeless, pregnant or breastfeeding (females only), had an active rectal infection, reported a symptomatic anorectal herpes outbreak in the past month, reported anorectal pain or bleeding at Visit 1 or 2 (Grade 2 or higher, based on DAIDS rectal adverse events grading tables for microbicides studies, addendum 3 at http://rsc.tech-res.com/safetyandpharmacovigilance), had anorectal signs detected by visual and/or high resolution anoscopy examination at Visit 1 or 2 (Grade 2 or higher), had known allergies to any of the study products or components of the study products, were currently participating in another clinical trial of an investigational anorectal product, and/or had any other clinical conditions or prior therapy that would make them unsuitable for the study or unable to comply with study requirements.
Study Products
The following non-prescription, over-the-counter (OTC) anorectal products were compared in this study: (i) a disposable enema bottle, similar to Fleets®, pre-filled with 125 mL of a clear isotonic liquid (Normosol-R), (ii) a disposable vaginal applicator pre-filled with 4 mL of a clear, water-based, water-soluble, condom compatible isotonic gel (Pre-Seed™), and (iii) a 1.4 g anorectal suppository (Tucks™). Astroglide™ (0.4 mL packets), a water-based, water-soluble, condom compatible, OTC lubricant was also distributed with the enema and applicator to facilitate insertion. Although neither the enema nor the applicator under study are widely used in the community, they represent potentially acceptable RM products as many MSM use enemas or rectal douches in preparation for RAI [14] and lubricants similar to that contained in Pre-Seed™ are widely used by both males and females during RAI [15–19]. Moreover, 73 % of males and 39 % of females who practice RAI participating in the Rectal Health and Behaviors Study (RHBS), completed as the first phase of our project under UCLAs U19 MDP in Los Angeles and Baltimore, reported rectal douching in the past 6 months [20]. Tucks™ suppositories are commonly used to treat hemorrhoids, and 38 % of males and 40 % of females enrolled in the RHBS reported ever using a suppository in their rectum [20]. Further, these products were chosen because they are generally regarded as safe by the US Food and Drug Administration and represent three distinct anorectal delivery systems.
Study Procedures
Eligible participants were assigned to one of six potential product sequences at enrollment (Visit 2) using a crossover design randomized by gender in blocks of six. The first randomized study product was dispensed at Visit 2 (Fig. 1). Participants returned to the clinic every 2 weeks for 6 weeks to receive each of the next products in their sequence. At each study visit, participants were given five enemas, applicators, or suppositories and shown how to use them. Participants were instructed to use each product anorectally three to five times during the subsequent 2 weeks as part of their normal sexual routine or their normal routine in preparation for RAI, but were not required to use the products in the context of RAI. At each study visit, participants were also given condoms and reminded that the products do not protect against STIs. Because latex condoms are not compatible with the oil-based suppository (Tucks™), latex condoms were only distributed with the enema and the applicator while poly-urethane condoms were distributed with the suppository.
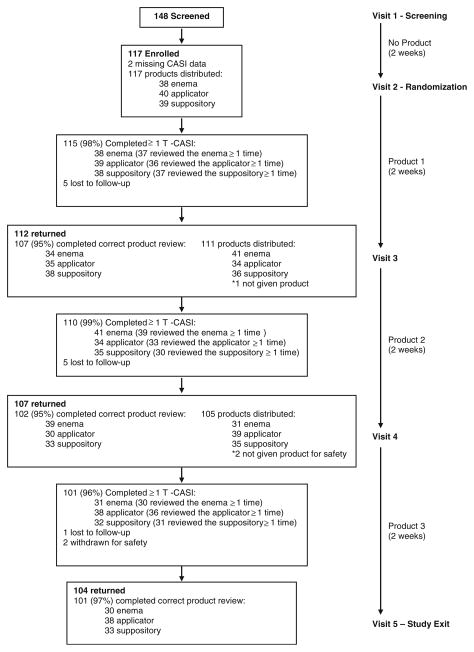
Fig. 1.
AMP Study profile
At each study visit, participants completed questionnaires using computer-assisted self-interviews (CASI), which collected information on demographics (screening visit only), sexual behavior, anorectal symptoms and their severity (mild, moderate, strong or severe), and product use and acceptability in the past 2 weeks. Brief face-to-face (FTF) interviews were also conducted at each study visit to obtain information on abnormal anorectal symptoms, adverse events (AEs), and/or new medications used in the past 2 weeks. Study staff graded the severity of reported AEs based on DAIDS AE grading tables for microbicides studies: Grade 1 (mild), Grade 2 (moderate), Grade 3 (severe), and Grade 4 (potentially life threatening). During each 2 week product use period, participants also completed two brief telephone questionnaires (each 5 days apart) using telephone audio computer-assisted self-interviews (T-ACASI), which collected information on sexual behavior and experiences using the products between study visits.
Acceptability Assessment
Multi-item scales designed to measure various aspects of product acceptability were included in CASI (11 items) and T-ACASI (17 items) questionnaires. Participants were asked to evaluate study products by responding to each of the items via a Likert scale (strongly agree, agree, neither agree nor disagree, disagree, or strongly disagree). The items included in CASI and T-ACASI questionnaires were not identical. CASI questionnaires asked participants to consider all the times the product was used in the past 2 weeks when responding to each item. T-ACASI questionnaires asked participants to consider only the last time the product was used and were programmed to skip product evaluations if participants did not report using the product since their last telephone interview.
Likert scale responses were reverse coded where necessary and used to create item scores on a scale of 1 (negative review) to 5 (positive review). Individual item scores were then averaged to create overall mean product acceptability scores for each data collection mode. Four items related to product use in the context of intercourse (anal and/or vaginal) were included in both CASI and T-ACASI questionnaires; however, not all participants reported having intercourse (anal and/or vaginal) in the past 2 weeks (CASI) or since their last telephone interview (T-ACASI). Scores for these four items were treated as missing when calculating overall mean CASI and/or T-ACASI product acceptability scores for individuals who did not report having intercourse (anal and/or vaginal) in either or both questionnaires.
Because acceptability scales and the larger study questionnaires were constructed to ascertain information on product use in the context of intercourse, and not just RAI, the term intercourse will be used to refer to anal and/or vaginal intercourse throughout the rest of the article, unless explicitly stated otherwise.
Statistical Analysis
To compare product use, anorectal symptoms, and AEs related to product use across products, logistic random effects models were used to account for the correlation between repeated measurements on the same individual. Cronbach α’s were computed by the study products to determine the internal consistency of CASI and T-ACASI acceptability scales. To identify underlying factors measured by CASI and T-ACASI acceptability scales, factor analyses stratified by the study products were conducted. Items loading on a particular factor (i.e., factor loading ≥0.40) were used to create subscales. Overall CASI and T-ACASI mean acceptability scores, mean acceptability scores for each of the subscales, and mean item scores were then compared across products using normal random effects models.
Efforts were made to determine whether observed differences between overall CASI and T-ACASI mean product acceptability scores were due to differential reporting by data collection mode or the slightly different structures of the acceptability scales included in each questionnaire. Correlations between mean product acceptability scores obtained via each data collection mode were examined by modeling partial CASI and T-ACASI acceptability scores (based on responses to similar items included in each questionnaire) as a function of product and data collection mode using a repeated measures bivariate regression model assuming an unstructured covariance matrix [21].
To estimate overall mean product acceptability scores, complete CASI and T-ACASI acceptability scores (based on responses to all items included in each questionnaire) were modeled as a function of product and data collection mode using a repeated measures bivariate random intercept model. This approach enabled the use of information for all participants with at least one score from either data collection mode and accounted for (i) the correlation between product acceptability scores obtained via each mode and (ii) the correlation across product acceptability scores for the same individual.
To investigate the effect of various covariates of interest on product acceptability, the following covariates were added one at a time to the repeated measures bivariate random intercept model for complete CASI and T-ACASI acceptability scores: age, gender, race/ethnicity, education, prior product use, intercourse in the past 2 weeks/since the last telephone interview, RAI in the past 2 weeks/since last telephone interview, last product use in the context of intercourse (only considered the effect on CASI acceptability scores), reported anorectal symptoms in the past 2 weeks, AEs related to product use in the past 2 weeks, and order (to determine whether acceptability of the first product differed from later products). To determine (i) whether the effect of these covariates on overall acceptability differed by data collection mode and (ii) whether patterns in product acceptability scores differed by any of these covariates, interaction terms between each covariate listed above and data collection mode and product, respectively, were added to the model one at a time.
Significant covariates and interaction terms at the 0.05 alpha level were considered for inclusion in the final model. If covariates were highly correlated, only one was included in the final model. Interactions that were no longer significant were also excluded from the final model. Residuals were inspected to assess adequacy of the final model. Differences between estimated complete product acceptability scores were derived from our final model and used as a proxy measure of product preferences. All analyses assume that data are missing at random and were conducted using Proc Mixed or Proc Glimmix in SAS 9.2 (SAS Institute, Cary NC).
Results
Of the 148 individuals screened for eligibility, 117 were enrolled and randomized to a product sequence (Fig. 1). Over the course of follow-up, 11 participants were lost to follow-up and two participants were withdrawn for safety. One of these experienced multiple Grade 1 gastrointestinal AEs (i.e., fullness, bloating, constipation and discolored stool) possibly related to product use, as well as a shingles outbreak unrelated to product use. The other participant also experienced multiple Grade 1 gastrointestinal AEs (i.e., fullness, urgency, bloating diarrhea) related to his diet, and unrelated to product use. Nine participants were accidentally asked to complete incorrect product reviews at ≥1 study visit. For example, in one instance a participant was given the enema at his last study visit, but was mistakenly asked to review the applicator during his current visit. These reviews were excluded from all analyses as it was unclear whether participants were evaluating the product listed in the survey or the product they were given at their last visit. Consequently, reported acceptability analyses are based on 109 participants with ≥1 correct CASI product review (103, 103, and 104 participant reviews for the enema, applicator, and suppository, respectively) and 116 participants with ≥1 T-ACASI review (110, 111, and 105 participant reviews for the enema, applicator, and suppository, respectively).
Sample Characteristics
Study participants were racially and ethnically diverse (49 white, 39 % African-American, and 26 % Hispanic), mostly male at birth (79 %), single (69 %), and highly educated (73 % had at least some college education) (Table 1). The mean age of study participants was 39.6 years (SD = 12.1); 27 % were under 30 years of age. At baseline, 78 % of participants reported having AI in the past 2 weeks, of whom 67 % reported always using a lubricant during AI and 79 % reported having RAI a mean of 2.8 times (SD = 2.9). While 31 % of participants reported no prior experience using any of the study’s anorectal products at baseline, 58 % of males and 56 % of females had previously used an enema.
Table 1.
Baseline characteristics of AMP Study participants
| Characteristic | Males (N = 92) | Females (N = 25) | Total (N = 117) |
|---|---|---|---|
| n (%) | n (%) | n (%) | |
| Mean age in years (SD) | 39.7 (12.2) | 39.6 (11.9) | 39.6 (12.1) |
| Race | |||
| White | 35 (44.3) | 14 (63.6) | 49 (48.5) |
| African-American | 32 (40.5) | 7 (31.8) | 39 (38.6) |
| Other | 12 (15.2) | 1 (4.6) | 13 (12.9) |
| Hispanic | 21 (23.3) | 9 (36.0) | 30 (26.1) |
| Single | 71 (78.0) | 9 (36.0) | 80 (69.0) |
| Education | |||
| <High school graduate | 3 (3.3) | 1 (4.0) | 4 (3.5) |
| High school graduate or GED | 21 (23.3) | 6 (24.0) | 27 (23.5) |
| Some college | 23 (25.6) | 10 (40.0) | 33 (28.7) |
| College graduate | 31 (34.4) | 8 (32.0) | 39 (33.9) |
| Professional or graduate degree | 12 (13.3) | 0 (0.0) | 12 (10.4) |
| Intercoursea (past 2 weeks) | 70 (77.8) | 22 (88.0) | 92 (80.0) |
| Anal intercourse (past 2 weeks) | 69 (77.5) | 20 (80.0) | 89 (78.1) |
| Receptive anal intercourse | 50 (72.5) | 20 (100.0) | 70 (78.7) |
| Mean # of times (SD) | 2.8 (3.0) | 2.6 (2.8) | 2.8 (2.9) |
| Insertive anal intercourse | 47 (68.1) | – | 47 (68.1) |
| Mean # of times (SD) | 3.4 (3.5) | – | 3.4 (3.5) |
| Lubricant use during anal intercourse (past 2 weeks) | |||
| Never | 10 (14.9) | 2 (10.0) | 12 (13.8) |
| Some of the time | 6 (9.0) | 4 (20.0) | 10 (11.5) |
| Most of the time | 4 (6.0) | 3 (15.0) | 7 (8.1) |
| Every time | 47 (70.2) | 11 (55.0) | 58 (66.7) |
| Prior anorectal product use | |||
| Enema | 50 (58.1) | 14 (56.0) | 64 (57.7) |
| Applicator | 33 (38.4) | 9 (36.0) | 42 (37.8) |
| Suppository | 18 (20.9) | 4 (16.0) | 22 (19.8) |
| No prior anorectal product use | 26 (30.2) | 8 (32.0) | 34 (30.6) |
Numbers may not sum to total due to missing data; percents may not sum to 100 % due to rounding
Intercourse = anal and/or vaginal intercourse
Product Use
During follow-up, 75, 76, and 66 % of participants reported using the enema, applicator, and suppository, respectively, ≥3 times in the past 2 weeks (Table 2). No significant differences across products were detected in the frequency of product use, intercourse, or condom or lubricant use at last intercourse during or after product use in the past 2 weeks. Among those who reported having intercourse in the past 2 weeks, the applicator was used during intercourse more than any other product (applicator = 43 %, enema = 12 %, and suppository = 24 %; t test value for applicator compared to enema = 4.33, p value <0.0001; t test value for applicator compared to suppository = 2.7, p value = 0.01). Regardless of the product used, most participants reported using products during intercourse with main or regular partners. Of those reporting intercourse in the past 2 weeks, 81 % reported last using the applicator in the context of intercourse (i.e., before, during or after intercourse), while only 70 and 55 % of participants reported last using the enema and suppository, respectively, in the context of intercourse (t test value for enema compared to applicator = 1.8, p value = 0.08; t test value for enema compared to suppository = 1.8, p value = 0.07; t test value for applicator compared to suppository = 3.5, p value = 0.001).
Table 2.
Product use and intercourse by product used in the past 2 weeks among AMP Study participants
| No producta (N = 117) | Product
|
Test of fixed effectb F value (p) |
|||
|---|---|---|---|---|---|
| Enema (N = 103) | Applicator (N = 103) | Suppository (N = 104) | |||
| n (%) | n (%) | n (%) | n (%) | ||
| Used product ≥ 3 times (past 2 weeks) | – | 76 (75.3) | 77 (75.5) | 69 (66.4) | 1.3 (0.28) |
| Intercourse (past 2 weeks) | 95 (82.6) | 86 (83.5) | 86 (83.5) | 89 (85.6) | 0.2 (0.93) |
| Vaginal intercourse (past 2 weeks) | 60 (65.2) | 46 (54.1) | 47 (54.7) | 52 (59.1) | 0.9 (0.45) |
| Receptive anal intercourse (past 2 weeks) | 76 (80.0) | 72 (83.7) | 76 (88.4) | 73 (82.0) | 0.8 (0.52) |
| Insertive anal intercourse (past 2 weeks) | 56 (60.2) | 49 (57.0) | 50 (58.1) | 53 (60.2) | 0.1 (0.99) |
| Used product during intercourse (past 2 weeks)c,d,e | – | 10 (11.9) | 36 (43.4) | 21 (23.6) | 10.1 (<0.0001) |
| Partner type at last use during intercourse | |||||
| Main or regular | – | 6 (60.0) | 26 (72.2) | 16 (76.2) | – |
| Friend or acquaintance | – | 3 (30.0) | 7 (19.4) | 4 (19.0) | – |
| One-time partner | – | 1 (10.0) | 3 (8.3) | 1 (4.8) | – |
| At last intercourse during or after product use | |||||
| Condom used | – | 51 (60.0) | 59 (68.6) | 61 (70.1) | 1.4 (0.26) |
| Lubricant used | – | 65 (78.3) | 59 (70.2) | 65 (76.5) | 0.8 (0.45) |
| Last product use in the context of intercoursee | – | 60 (69.8) | 70 (81.4) | 49 (55.1) | 6.2 (0.003) |
Intercourse = anal and/or vaginal intercourse; context = before, during, or after intercourse; p = p value
Initial 2 week screening period between Visits 1 and 2 during which participants were not asked to use any products
Test of equality across products based on results from logistic random effects models
Significant difference (p <0.05) between the enema and applicator
Significant difference (p <0.05) between the enema and suppository
Significant difference (p <0.05) between the applicator and suppository
Anorectal Symptoms and AEs
During the initial 2 week screening period (no product), 18 % of participants reported experiencing ≥1 anorectal symptom via CASI (Table 3). Anorectal symptoms (≥1 symptom in the past 2 weeks) were also reported via CASI by participants using the enema (15 %), applicator (13 %), and suppository (22 %). However, none of these proportions were significantly different from one another, nor were there significant differences when compared with anorectal symptoms reported during the screening period. Most symptoms were of mild or moderate severity and no severe symptoms were reported for any of the products. During FTF interviews, AEs related to product use (≥1 AE in the past 2 weeks) were reported by participants using the enema (12 %), applicator (9 %), and suppository (16 %); although these differences were not significant. All AEs related to product use were Grade 1 (mild: enema = 21 AEs in 12 participants; applicator = 14 AEs in 8 participants; suppository = 32 AEs in 17 participants) or Grade 2 (moderate: enema = 1 AE in 1 participant; applicator = 1 AE in 1 participant), and mostly affected the gastrointestinal system.
Table 3.
Anorectal symptoms and AEs related to product use in the past 2 weeks among AMP Study participants
| No producta (N = 117) | Product
|
Test of fixed effectb F value (p) |
|||
|---|---|---|---|---|---|
| Enema (N = 103) | Applicator (N = 103) | Suppository (N = 104) | |||
| n (%) | n (%) | n (%) | n (%) | ||
| Anorectal symptoms—past 2 weeks | |||||
| Reported ≥1 anorectal symptom | 20 (17.5) | 15 (14.9) | 13 (12.6) | 23 (22.1) | 1.3 (0.3) |
| # Anorectal symptoms reported | 34 | 27 | 20 | 39 | |
| Swelling | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Itching | 5 (14.7) | 5 (18.5) | 3 (15.0) | 7 (17.9) | |
| Pain | 2 (5.9) | 0 (0.0) | 2 (10.0) | 1 (2.6) | |
| Needing to have a bowel movement | 5 (14.7) | 5 (18.5) | 2 (10.0) | 2 (5.1) | |
| Constipation | 5 (14.7) | 1 (3.7) | 2 (10.0) | 2 (5.1) | |
| Diarrhea | 6 (17.6) | 4 (14.8) | 5 (25.0) | 8 (20.5) | |
| Bloody bowel movement | 1 (2.9) | 3 (11.1) | 1 (5.0) | 2 (5.1) | |
| Increased stool frequency | 1 (2.9) | 1 (3.7) | 0 (0.0) | 0 (0.0) | |
| Increased farting | 3 (8.8) | 3 (11.1) | 2 (10.0) | 5 (12.8) | |
| Wetness/discharge | 0 (0.0) | 0 (0.0) | 1 (5.0) | 4 (10.3) | |
| Burning inside butt | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (5.1) | |
| Burning outside or on edge of butt | 1 (2.9) | 0 (0.0) | 0 (0.0) | 1 (2.6) | |
| Irritation | 2 (5.9) | 4 (14.8) | 0 (0.0) | 5 (12.8) | |
| Hemorrhoids | 1 (2.9) | 1 (3.7) | 1 (5.0) | 0 (0.0) | |
| Bumps/lumps inside butt | 1 (2.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Bumps/lumps outside butt | 1 (2.9) | 0 (0.0) | 1 (5.0) | 0 (0.0) | |
| Severity of symptoms | |||||
| Mild | 26 (76.5) | 19 (70.4) | 11 (55.5) | 22 (56.4) | |
| Moderate | 7 (20.6) | 6 (22.2) | 6 (30.0) | 16 (41.0) | |
| Strong | 1 (2.9) | 2 (7.4) | 3 (15.0) | 1 (2.6) | |
| Severe | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Adverse events (AEs)—past 2 weeks | |||||
| Reported ≥ 1 AE related to product use | – | 12 (11.7) | 9 (8.7) | 17 (16.4) | 1.4 (0.3) |
| # AEs related to product use reported | – | 22 | 15 | 32 | |
| Bloating/fullness | 5 (22.7) | 2 (13.3) | 5 (15.6) | ||
| Bloody bowel movement | 1 (4.6) | 1 (6.7) | 1 (3.1) | ||
| Constipation | 0 (0.0) | 1 (6.7) | 1 (3.1) | ||
| Diarrhea | 4 (18.2) | 3 (20.0) | 6 (18.8) | ||
| Irritation | 3 (13.6) | 0 (0.0) | 4 (12.5) | ||
| Itching | 2 (9.1) | 1 (6.7) | 5 (15.6) | ||
| Burning | 1 (4.6) | 0 (0.0) | 2 (6.3) | ||
| Soiling | 1 (4.6) | 0 (0.0) | 0 (0.0) | ||
| Abdominal pain | 1 (4.6) | 0 (0.0) | 0 (0.0) | ||
| Urgency | 2 (9.1) | 1 (6.7) | 2 (6.3) | ||
| Wetness/discharge | 1 (4.6) | 2 (13.3) | 4 (12.5) | ||
| Flatulence | 0 (0.0) | 1 (6.7) | 0 (0.0) | ||
| Incontinence | 0 (0.0) | 1 (6.7) | 0 (0.0) | ||
| Pain | 1 (4.6) | 1 (6.7) | 1 (3.1) | ||
| Discolored stool | 0 (0.0) | 0 (0.0) | 1 (3.1) | ||
| Headache | 0 (0.0) | 1 (6.7) | 0 (0.0) | ||
| Severity of AEs related to product use | |||||
| Grade 1—mild | – | 21 (95.5) | 14 (93.3) | 32 (100.0) | |
| Grade 2—moderate | – | 1 (4.6) | 1 (6.7) | 0 (0.0) | |
| Grade 3—severe | – | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Grade 4—potentially life threatening | – | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
p = p value
Initial 2 week screening period between Visits 1 and 2 during which participants were not asked to use any products
Test of equality across products based on results from logistic random effects models
Components of Acceptability
Both acceptability scales had high internal consistency. Cronbach α’s ranged from 0.82 to 0.84 across products for the CASI scale and 0.79–0.86 for the T-ACASI scale. Factor analysis identified two underlying factors measured by the CASI acceptability scale across all products: Satisfaction with Product Use loading on seven items and Sexual Pleasure loading on three items (Table 4). Based on CASI acceptability scores, the applicator was the most acceptable product overall. The applicator also received the highest CASI score for Satisfaction with Product Use, while CASI scores for Sexual Pleasure showed the applicator and enema were preferred to the suppository. Using the T-ACASI acceptability scale data, factor analysis identified three underlying factors measured across all products: Satisfaction with Product Use loading on ten items, Ease of Product Use loading on five items, and Sexual Pleasure loading on two items. Based on overall T-ACASI acceptability scores, both the applicator and suppository were preferred to the enema and no preference was identified between the applicator and the suppository. The applicator and suppository also received the highest T-ACASI scores for Ease of Product Use, while the applicator alone received the highest T-ACASI score for Sexual Pleasure. There were no significant differences between the three product T-ACASI scores for Satisfaction with Product Use.
Table 4.
Mean scores for CASI and T-ACASI acceptability subscales and individual scale items from the AMP Acceptability Study
| CASI | Mean Scorea (95 % CI)
|
||
|---|---|---|---|
| Enema (N = 103) | Applicator (N = 103) | Suppository (N = 104) | |
| Overall acceptabilityb,d | 3.5 (3.4, 3.6) | 3.7 (3.6, 3.9) | 3.4 (3.3, 3.6) |
| Cronbach α | 0.82 | 0.84 | 0.84 |
| Subscales | |||
| Satisfaction with product useb,d | 3.6 (3.5, 3.8) | 3.9 (3.8, 4.1) | 3.7 (3.5, 3.8) |
| I liked using the product (reverse)c,d | 3.6 (3.4, 3.8) | 3.8 (3.6, 4.0) | 3.2 (3.0, 3.4) |
| The product was difficult to use | 3.6 (3.4, 3.8) | 3.9 (3.7, 4.1) | 3.8 (3.6, 4.0) |
| The product was inconvenient to useb,d | 3.3 (3.1, 3.5) | 3.7 (3.5, 3.9) | 3.4 (3.1, 3.6) |
| The product was painful to useb | 4.0 (3.8, 4.1) | 4.2 (4.0, 4.4) | 4.1 (3.9, 4.3) |
| Using the product irritated my buttb,d | 3.9 (3.8, 4.1) | 4.2 (4.1, 4.4) | 4.0 (3.8, 4.2) |
| The product was too runnyb,c | 3.4 (3.2, 3.6) | 3.8 (3.6, 4.0) | 3.7 (3.5, 3.9) |
| The product interrupted sexe | 3.7 (3.5, 3.9) | 3.9 (3.7, 4.1) | 3.7 (3.5, 3.9) |
| Sexual pleasuree,c,d | 3.0 (2.8, 3.2) | 3.1 (2.9, 3.4) | 2.7 (2.5, 2.9) |
| The product increased my sexual pleasure (reverse)e,c,d | 3.0 (2.7, 3.2) | 3.0 (2.8, 3.3) | 2.5 (2.3, 2.8) |
| The product increased my partner’s sexual pleasure (reverse)e,c,d | 3.0 (2.7, 3.2) | 3.1 (2.8, 3.3) | 2.7 (2.5, 2.9) |
| My partner liked it when I used the product (reverse)e,c,d | 3.2 (2.9, 3.4) | 3.3 (3.1, 3.6) | 2.9 (2.7, 3.1) |
| I had no problems using the product (reverse)f | 3.4 (3.2, 3.7) | 3.6 (3.4, 3.9) | 3.4 (3.2, 3.7) |
| T-ACASI | Mean Scorea (95 % CI)
|
||
|---|---|---|---|
| Enema (n = 110) | Applicator (n = 111) | Suppository (n = 105) | |
| Overall acceptabilityb,c | 3.8 (3.7, 3.9) | 4.0 (3.9, 4.1) | 3.9 (3.8, 4.0) |
| Cronbach α | 0.79 | 0.85 | 0.86 |
| Subscales | |||
| Satisfaction with product use | 3.9 (3.8, 4.0) | 3.9 (3.8, 4.1) | 3.9 (3.7, 4.0) |
| I felt pain when I used the product | 4.0 (3.8, 4.2) | 4.2 (4.0, 4.4) | 4.1 (3.9, 4.3) |
| I felt irritation when I used the productb | 4.0 (3.8, 4.2) | 4.2 (4.0, 4.4) | 4.1 (3.9, 4.3) |
| I felt irritation after I used the product | 4.1 (3.9, 4.3) | 4.2 (4.0, 4.4) | 4.0 (3.9, 4.2) |
| The product was too runnyc | 3.4 (3.2, 3.6) | 3.6 (3.4, 3.8) | 3.7 (3.5, 3.9) |
| The product leaked outb,c | 3.3 (3.1, 3.5) | 3.7 (3.5, 3.9) | 3.7 (3.5, 3.9) |
| The product made me feel very stickyb,c | 4.1 (3.9, 4.2) | 3.6 (3.4, 3.8) | 3.7 (3.5, 3.9) |
| The product interrupted sexe | 3.9 (3.7, 4.1) | 4.0 (3.8, 4.2) | 3.9 (3.6, 4.1) |
| The smell bothered mec | 4.1 (3.9, 4.3) | 4.0 (3.8, 4.2) | 3.9 (3.7, 4.1) |
| The taste bothered mec | 4.1 (3.9, 4.3) | 4.0 (3.8, 4.1) | 3.9 (3.7, 4.1) |
| My partner did not like the producte | 3.8 (3.6, 4.0) | 3.8 (3.6, 4.0) | 3.6 (3.4, 3.8) |
| Ease of product useb,c | 3.8 (3.7, 3.9) | 4.4 (4.2, 4.5) | 4.3 (4.1, 4.4) |
| The product was easy to use (reverse)b,d | 4.3 (4.2, 4.4) | 4.5 (4.3, 4.6) | 4.3 (4.2, 4.4) |
| The product was easy to carry around (reverse)b,c | 3.2 (3.0, 3.4) | 4.2 (4.0, 4.4) | 4.3 (4.1, 4.5) |
| The product was easy to store (reverse)b,c | 3.8 (3.6, 4.0) | 4.4 (4.2, 4.5) | 4.4 (4.2, 4.6) |
| The product was easy to put or throw away (reverse)b,c | 4.0 (3.8, 4.1) | 4.5 (4.4, 4.7) | 4.4 (4.2, 4.5) |
| The product was convenient to use (reverse)b,c,d | 3.7 (3.5, 3.9) | 4.2 (4.0, 4.4) | 4.0 (3.8, 4.1) |
| Sexual pleasuree,b,d | 2.8 (2.6, 3.0) | 3.2 (3.0, 3.5) | 2.9 (2.7, 3.2) |
| The product increased my sexual pleasure (reverse)e,b,d | 2.8 (2.5, 3.0) | 3.2 (2.9, 3.5) | 2.8 (2.6, 3.1) |
| The product increased my partner’s sexual pleasure (reverse)e,b | 2.8 (2.6, 3.1) | 3.3 (3.0, 3.5) | 3.0 (2.7, 3.3) |
Likert scale response values were reverse coded where necessary such that lower scores correspond to a negative review and higher scores correspond to a positive review
Significant difference (p <0.05) in means for the enema and applicator
Significant difference (p <0.05) in means for the enema and suppository
Significant difference (p <0.05) in means for the applicator and suppository
Among those who had intercourse (anal and/or vaginal) in the past 2 weeks/since their last telephone interview
Not correlated with either of the identified factors
Product Acceptability: Partial Scores
To determine whether differences in overall mean product acceptability scores obtained from the two data collection modes were due to differential reporting by mode or the slightly different acceptability scales implemented in each mode, we examined partial CASI and T-ACASI product acceptability scores (based on responses to similar items included in each questionnaire). Estimated mean product acceptability scores from our repeated measures bivariate model for partial CASI and T-ACASI scores were: CASI enema = 3.5 (95 % CI: 3.4, 3.6), CASI applicator = 3.7 (95 % CI: 3.6, 3.8), CASI suppository = 3.5 (95 % CI: 3.4, 3.6), T-ACASI enema = 3.7 (95 % CI: 3.6, 3.8), T-ACASI applicator = 4.0 (95 % CI: 3.9, 4.1), and T-ACASI suppository = 3.8 (95 % CI: 3.7, 3.9) (data not shown). Although partial T-ACASI product acceptability scores were slightly higher than partial CASI product acceptability scores, the correlations between partial CASI and T-ACASI product scores were >0.5 regardless of whether scores were examined based on alphabetic order (Normosol-R [enema], Pre-Seed [applicator], Tucks [suppository]) or the temporal order in which products were received (data not shown). Further, there was no evidence to suggest that patterns in partial product acceptability scores differed by data collection mode (F test value for product by data collection mode interaction = 2.5, p value = 0.09).
Product Acceptability: Complete Scores
Estimated mean product acceptability scores from our repeated measures bivariate random intercept model for complete CASI and T-ACASI scores were: CASI enema = 3.4 (95 % CI: 3.3, 3.5), CASI applicator = 3.7 (95 % CI: 3.6, 3.8), CASI suppository = 3.5 (95 % CI: 3.4, 3.7), T-ACASI enema = 3.8 (95 % CI: 3.7, 3.9), T-ACASI applicator = 4.0 (95 % CI: 3.9, 4.1), and T-ACASI suppository = 3.9 (95 % CI: 3.8, 4.0) (data not shown). When the covariates of interest were added one at a time to this model, estimated complete product acceptability scores were higher among those who did not experience any anorectal symptoms in the past 2 weeks (regression coefficient = 0.23, F test value = 8.0, p value = 0.005), those who did not experience any AEs related to product use in the past 2 weeks (regression coefficient = 0.26, F test value = 8.2, p value = 0.004), those who did not have intercourse in the past 2 weeks/ since their last telephone interview (regression coefficient = 0.23, F test value = 21.7, p value <0.0001), and those who did not have RAI in the past 2 weeks/since their last telephone interview (regression coefficient = 0.17, F test value = 13.3, p value = 0.0003) (data not shown). However, among those who did have intercourse in the past 2 weeks, complete CASI acceptability scores were higher among those who last used products in the context of intercourse (regression coefficient = 0.30, F test value = 10.1, p value = 0.002).
The effect of product (F test value for product by data collection mode interaction = 6.1, p value = 0.003) and prior product use (F test value for prior product use by data collection mode interaction = 6.8, p value = 0.01) on acceptability differed by data collection mode. Further, product preferences depended on age (F test value for age by product interaction = 5.1, p value = 0.01), prior product use (F test value for prior product use by product interaction = 3.3, p value = 0.04), and RAI in the past 2 weeks/since the last telephone interview (F test value for RAI by product interaction = 4.1, p value = 0.02). Further, there was evidence of a three-way interaction between product, gender, and data collection mode (F test value for three-way interaction = 3.1, p value = 0.04). That is, complete product acceptability scores differed by gender and gender patterns also differed by data collection mode.
After considering all significant covariates and interaction terms, our final model included product, data collection mode, age, gender, prior product use, intercourse in the past 2 weeks/since the last telephone interview, anorectal symptoms, and the following interaction terms: age by product, gender by product, gender by data collection mode, product by data collection mode, prior product use by data collection mode, and product by gender by data collection mode (Table 5). Intercourse and RAI in the past 2 weeks/since the last telephone interview were highly correlated (~0.9) across products. Because CASI and T-ACASI questionnaires asked about product use “in the context of sex” without differentiating between vaginal intercourse, IAI, or RAI, only intercourse (vaginal and/or anal) in the past 2 weeks/since the last telephone interview was included in the final model. Anorectal symptoms and AEs were also highly correlated (~0.8) across products. Because there were more anorectal symptoms reported than related AEs at each visit, only anorectal symptoms were included in the final model. An examination of the residuals revealed that this model adequately fit the data.
Table 5.
Regression coefficients from final bivariate random intercept model for CASI and T-ACASI product acceptability scores from the AMP Study (N = 104)
| Effect | CASI—specific
|
Common
|
T-ACASI—specific
|
Test of Fixed Effecta F value (p) |
|||
|---|---|---|---|---|---|---|---|
| Estimate | 95 % CI | Estimate | 95 % CI | Estimate | 95 % CI | ||
| Intercept | 3.40 | 3.17, 3.64 | 3.73 | 3.51, 3.95 | |||
| Product | 4.7 (0.01) | ||||||
| Enema | 0.07 | −0.12, 0.26 | −0.21 | −0.37, −0.06 | |||
| Applicator | 0.18 | 0.01, 0.36 | −0.11 | −0.26, 0.03 | |||
| Suppository | Ref | – | Ref | – | |||
| Female | −0.17 | −0.48, 0.14 | 0.21 | −0.05, 0.46 | 3.7 (0.06) | ||
| Age (years) | 0.010 | 0.001, 0.020 | 2.4 (0.12) | ||||
| No prior product use | −0.18 | −0.35, −0.02 | 0.12 | −0.01, 0.25 | 3.0 (0.08) | ||
| No symptoms (past 2 weeks) | 0.23 | 0.07, 0.39 | 7.7 (0.01) | ||||
| No intercourseb | 0.23 | 0.12, 0.33 | 19.2 (<0.0001) | ||||
| Age by product interaction | 3.3 (0.04) | ||||||
| Age × Enema | −0.002 | −0.012, 0.008 | |||||
| Age × Applicator | −0.012 | −0.022, −0.002 | |||||
| Gender by product interaction | 3.5 (0.03) | ||||||
| Female × Enema | −0.33 | −0.70, 0.04 | 0.14 | −0.17, 0.44 | |||
| Female × Applicator | 0.25 | −0.12, 0.62 | −0.26 | −0.56, 0.05 | |||
p = p value
Test of fixed effects based on results from bivariate random intercept model
No intercourse (anal and/or vaginal) in the past 2 weeks/since last telephone interview
Overall participants found the applicator most acceptable. However, the magnitude of differences between complete product acceptability scores differed by age, gender, and data collection mode (Fig. 2). Significant differences in both CASI and T-ACASI complete product acceptability scores disappeared with age among males. Among females, significant differences in both CASI and T-ACASI complete product acceptability scores also diminished with age; however, patterns in complete product acceptability scores were not consistent across data collection modes.
Fig. 2.
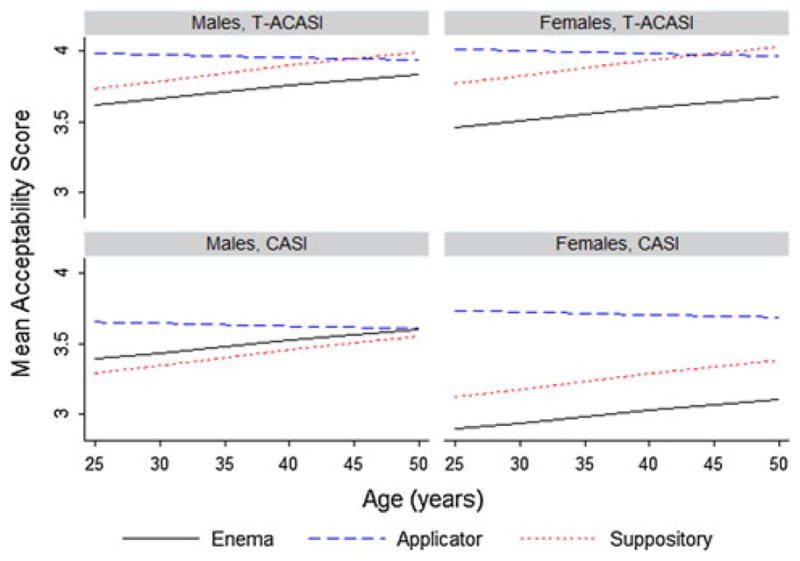
Estimated mean product acceptability scores from final model by age, gender, and data collection mode among AMP Study participants with no prior product use experience, who did not report having intercourse (anal and/or vaginal) in the past 2 weeks/since their last telephone interview, and who did not report any anorectal symptoms in the past 2 weeks (N = 104)
Discussion
This was the first trial to compare the acceptability of three distinct anorectal delivery systems (enema, lubricant-filled applicator, suppository) for the application of future, approved RMs. Information was acquired for at-risk HIV-uninfected males and females who practice RAI. Compared to the enema and the suppository, the applicator filled with lubricant received the highest overall acceptability score. This is consistent with the results from the only other randomized crossover acceptability trial comparing two candidate RM products (lubricant vs. suppository) [12], despite differences in the study population, the size of delivery devices, and carrier volumes between the two trials.
Age and gender significantly impacted patterns in product acceptability scores or the strength of product preferences. Across both data collection modes (CASI and T-CASI), females’ preferences were stronger than males’. Among females, the applicator was the preferred product across all ages based on CASI acceptability scores, whereas both the applicator and the suppository were preferred to the enema among older females based on T-ACASI acceptability scores. Acceptability scores from both modes suggest that younger males preferred the applicator to the enema and suppository, while older males did not appear to prefer any product over another. These findings suggest that the marketing of RM products and the counseling of adherence to their use for HIV prevention may need to be age and gender specific.
Commercial lubricants are commonly used during AI among both males and females [15–19]. This was also seen in this study with >85 % of participants reporting lubricant use during AI in the past 2 weeks at baseline. It is this widespread use of lubricants among individuals who practice AI that likely explains the high acceptability of the lubricant-filled applicator. Therefore, the observed differences in the strength of product preferences across age, gender, and data collection mode may be due to age and gender differences in familiarity with products similar to those under study.
Given that the questionnaires implemented in each data collection mode were not identical and that product acceptability scores based only on similar items included in each questionnaire (i.e., partial product acceptability scores) were positively correlated, it is unlikely that the differences in the strength of product preferences across modes were entirely due to differential reporting by mode. Some of the items unique to the T-ACASI questionnaire that were correlated with the Ease of Product Use factor referred to product portability (e.g., ease of carrying, storing, and disposing of products). Many females are accustomed to using and carrying feminine hygiene products similar in size and shape to the applicator and suppository under evaluation. On the other hand, many MSM practice rectal douching using enemas in preparation for AI, and often begin douching in their late 20s [14]. While less is known about rectal douching practices among females in preparation for AI, in the RHBS fewer females reported rectal douching in the past 6 months than males (39 vs. 73 %) [20]. Further, rectal douching may be less common among women due to the known association between vaginal douching and vaginal infections [22]. Given that enema use is common among MSM [14], it is plausible that a reasonably designed applicator or suppository would not be more burdensome for men to carry. Again, age and gender differences in familiarity with similar products may be contributing to (i) the observed differences in product preferences between males and females and (ii) the inconsistency between CASI and T-ACASI product preferences among older females.
Although the prevalence of reported anorectal symptoms during each product use period did not differ from that during the screening period, there was an association between experiencing anorectal symptoms or AEs related to product use and product acceptability. Thus, participants may have been ascribing symptoms not outside of their norm to product use. Participants enrolled in a recent clinical trial of active RMs reported a high willingness to use the products in the future if there was some indication of actual protection despite reporting a significant number of Grade 3 AEs [10]. This suggests that people may be more tolerant of side effects from active RM products than from placebo products. It also suggests that risk perception of HIV may affect toleration and attitudes towards AEs if those using active RM products practice higher risk behaviors or feel more vulnerable to HIV infection than those in our study. More gender specific qualitative research on the expectation and acceptability of symptoms surrounding active RM product use is needed.
Higher complete product acceptability scores were observed among individuals who did not have intercourse in the past 2 weeks/since their last telephone interview. However, analyses of complete CASI acceptability scores alone among those who did have intercourse in the past 2 weeks suggest that last product use in the context of intercourse was actually associated with higher CASI product acceptability scores. Because detailed data on product acceptability after each product use were not collected, it is unclear whether those who last used products in the context of intercourse found them more acceptable and were thus willing to use them in the context of intercourse or whether use in the context of intercourse enhanced product acceptability. However, a subset analysis (data not shown) revealed that product acceptability scores were lower among those who had intercourse in the past 2 weeks but did not last use the product in the context of intercourse than scores among those who did not have intercourse at all in the past 2 weeks. Thus, findings from our subset analysis suggest that those who found the products more acceptable may have been more willing to use them in the context of intercourse.
Moreover, most participants who reported using products during intercourse reported use with main/regular partners. Although data on all partner types in the past 2 weeks were not collected, this finding may be due to the fact that participants were more likely to have told their main/regular partners about their study participation, and thus used study products with those partners more frequently than with other partners. However, the fact that fewer participants tried the products with non-main/regular partners also suggests that use of RM products with less familiar partners may be more challenging. Further, acceptability of product use with less familiar partners may be different from that with regular partners, as shown with vaginal microbicides [23]. This could be a concern for the potential widespread use of RMs for HIV prevention if individuals are not willing to use any new products with unfamiliar partners, although the delivery system used may not be a major factor. Qualitative data on the motivations behind product use and non-use with specific partner types are needed.
Product acceptability was assessed in this study by use of two acceptability scales that measured Satisfaction with Product Use, Ease of Product Use, and Sexual Pleasure. While such an approach reflects the multi-dimensional nature of acceptability, it does not consider the relative weight of such items in the ultimate result of acceptability—product use. We only observed a weak association between product acceptability and frequency of product use (data not shown). Thus, it is unclear whether there is no association or there was just insufficient variation in the frequency of product use (i.e., all participants were instructed to use products three to five times in a 2 week period) to observe the true strength of the association. To evaluate this association, data on adherence to use of products either at every sexual encounter or following a regular schedule over a period of sustained use are needed. Until such analyses can be conducted, it is not known if these acceptability scores will predict eventual use patterns or product adherence. However, assessment of acceptability as a range of factors rather than as a single item, such as intention to use, provides more detailed indications of preferences and concerns regarding the product that may influence continued use.
This study has several limitations. First, because our goal was to evaluate potential RM delivery systems as a whole, the acceptability scales implemented in this study were not designed to measure the acceptability of product formulations and delivery devices separately. Thus, we cannot differentiate between formulation and delivery device acceptability in our analyses. Second, product acceptability was not specifically evaluated in the context of RAI. Although the ultimate goal is to understand acceptability in the context of RAI, it is important to measure acceptability in general early in product development. For example, as our findings suggest, side effects of product use likely impact acceptability. Therefore, it is important to identify side effects or other problems associated with product use outside of RAI because they will likely occur during or be exacerbated by RAI as well. Third, acceptability scales and larger study questionnaires only asked about product use in the context of intercourse, thus the level of detail surrounding use and acceptability in the context of RAI that we are able to present is restricted by this limitation. Fourth, participants were not directly asked about their preferences between the three study products. Instead, we identified product preferences based on differences in estimated product acceptability scores. Finally, we did not collect detailed data on condom or lubricant use during or after product use; however, participants were asked whether they used condoms and lubricant at last intercourse during or after product use. Our findings indicate that there were no significant differences in condom or lubricant use at last intercourse during or after product use. Thus, even if condom and lubricant use affected product acceptability they did not do so differentially across the study products.
This study evaluated the acceptability of three potential RM delivery systems among individuals who practice RAI and are thus at greatest risk of HIV infection. Further, by including younger males and females in this study, insight was gained about preferences between the different delivery systems across populations of risk. Thus, our findings should be considered in the development of RMs to enhance acceptability among those most likely to benefit from an effective RM.
Acknowledgments
This research was supported by UCLA MDP funding from the National Institutes of Health (NIAID IPCP U19 #AI0606414), Center for HIV Identification, Prevention and Treatment Services (CHIPTS) funding from the National Institutes of Health (P30 #MH58107), and UCLA Center for AIDS Research (CFAR) funding from the National Institutes of Health (NIAID #AI28697: Mucosal Immunology Laboratory Core (MICL)). The authors would like to recognize the contributions of Elena Khanukhova and Edward Robbie to this study. The authors would also like to thank all study participants, without whom this study would not have been possible.
Contributor Information
Heather A. Pines, Email: hpines@ucla.edu, Department of Epidemiology, Fielding School of Public Health, University of California-Los Angeles, 650 Charles E. Young Dr., South CHS 41-295A, P.O. Box 951772, Los Angeles, CA 90095-1772, USA
Pamina M. Gorbach, Department of Epidemiology, Fielding School of Public Health, University of California-Los Angeles, 650 Charles E. Young Dr., South CHS 41-295A, P.O. Box 951772, Los Angeles, CA 90095-1772, USA
Robert E. Weiss, Department of Biostatistics, Fielding School of Public Health, University of California-Los Angeles, Los Angeles, CA, USA
Kristen Hess, Department of Epidemiology, Fielding School of Public Health, University of California-Los Angeles, 650 Charles E. Young Dr., South CHS 41-295A, P.O. Box 951772, Los Angeles, CA 90095-1772, USA.
Ryan Murphy, Department of Epidemiology, Fielding School of Public Health, University of California-Los Angeles, 650 Charles E. Young Dr., South CHS 41-295A, P.O. Box 951772, Los Angeles, CA 90095-1772, USA.
Terry Saunders, David Geffen School of Medicine, University of California-Los Angeles, Los Angeles, CA, USA.
Joelle Brown, Department of Epidemiology, Fielding School of Public Health, University of California-Los Angeles, 650 Charles E. Young Dr., South CHS 41-295A, P.O. Box 951772, Los Angeles, CA 90095-1772, USA.
Peter A. Anton, David Geffen School of Medicine, University of California-Los Angeles, Los Angeles, CA, USA
Ross D. Cranston, Department of Medicine, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA
References
- 1.Rosenberger JG, Reece M, Schick V, et al. Sexual behaviors and situational characteristics of most recent male-partnered sexual event among gay and bisexually identified men in the United States. J Sex Med. 2011;8(11):3040–50. doi: 10.1111/j.1743-6109.2011.02438.x. [DOI] [PubMed] [Google Scholar]
- 2.Reece M, Herbenick D, Schick V, Sanders SA, Dodge B, Fortenberry JD. Condom use rates in a national probability sample of males and females ages 14 to 94 in the United States. J Sex Med. 2010;7(Suppl 5):266–76. doi: 10.1111/j.1743-6109.2010.02017.x. [DOI] [PubMed] [Google Scholar]
- 3.Herbenick D, Reece M, Schick V, Sanders SA, Dodge B, Fortenberry JD. Sexual behavior in the United States: results from a national probability sample of men and women ages 14–94. J Sex Med. 2010;7(Suppl 5):255–65. doi: 10.1111/j.1743-6109.2010.02012.x. [DOI] [PubMed] [Google Scholar]
- 4.Vittinghoff E, Douglas J, Judson F, McKirnan D, MacQueen K, Buchbinder SP. Per-contact risk of human immunodeficiency virus transmission between male sexual partners. Am J Epidemiol. 1999;150(3):306–11. doi: 10.1093/oxfordjournals.aje.a010003. [DOI] [PubMed] [Google Scholar]
- 5.Varghese B, Maher JE, Peterman TA, Branson BM, Steketee RW. Reducing the risk of sexual HIV transmission: quantifying the per-act risk for HIV on the basis of choice of partner, sex act, and condom use. Sex Transm Dis. 2002;29(1):38–43. doi: 10.1097/00007435-200201000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Mantell JE, Myer L, Carballo-Dieguez A, et al. Microbicide acceptability research: current approaches and future directions. Soc Sci Med. 2005;60(2):319–30. doi: 10.1016/j.socscimed.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 7.Gross M, Celum CL, Tabet SR, Kelly CW, Coletti AS, Chesney MA. Acceptability of a bioadhesive nonoxynol-9 gel delivered by an applicator as a rectal microbicide. Sex Transm Dis. 1999;26(10):572–8. doi: 10.1097/00007435-199911000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Carballo-Dieguez A, Exner T, Dolezal C, Pickard R, Lin P, Mayer KH. Rectal microbicide acceptability: results of a volume escalation trial. Sex Transm Dis. 2007;34(4):224–9. doi: 10.1097/01.olq.0000233715.59239.83. [DOI] [PubMed] [Google Scholar]
- 9.Ventuneac A, Carballo-Dieguez A, McGowan I, et al. Acceptability of UC781 gel as a rectal microbicide among HIV-uninfected women and men. AIDS Behav. 2010;14(3):618–28. doi: 10.1007/s10461-009-9611-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anton PA, Cranston RD, Kashuba A, et al. RMP-02/MTN-006: a Phase 1 rectal safety, acceptability, pharmacokinetic, and pharmacodynamic study of tenofovir 1 % gel compared with oral tenofovir disoproxil fumarate. AIDS Res Hum Retroviruses. 2012 doi: 10.1089/aid.2012.0262. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McGowan I, Hoesley C, Andrew P, et al. MTN-007: a Phase 1 randomized, double-blind, placebo-controlled rectal safety and acceptability study of tenofovir 1 % Gel. 19th conference on retroviruses and opportunistic infections; Seattle. 2012. p. abstract 34LB. [Google Scholar]
- 12.Carballo-Dieguez A, Dolezal C, Bauermeister JA, O’Brien W, Ventuneac A, Mayer K. Preference for gel over suppository as delivery vehicle for a rectal microbicide: results of a randomised, crossover acceptability trial among men who have sex with men. Sex Transm Infect. 2008;84(6):483–7. doi: 10.1136/sti.2008.030478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coly A, Gorbach PM. Microbicide acceptability research: recent findings and evolution across phases of product development. Curr Opin HIV AIDS. 2008;3(5):581–6. doi: 10.1097/COH.0b013e32830aba00. [DOI] [PubMed] [Google Scholar]
- 14.Carballo-Dieguez A, Bauermeister J, Ventuneac A, Dolezal C, Mayer K. Why rectal douches may be acceptable rectal-microbicide delivery vehicles for men who have sex with men. Sex Transm Dis. 2009;37(4):228–33. doi: 10.1097/OLQ.0b013e3181bf9b2d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gross M, Buchbinder SP, Celum C, Heagerty P, Seage GR., 3rd Rectal microbicides for U.S. gay men. Are clinical trials needed? Are they feasible? HIVNET vaccine preparedness study protocol team. Sex Transm Dis. 1998;25(6):296–302. doi: 10.1097/00007435-199807000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Carballo-Dieguez A, Stein Z, Saez H, Dolezal C, Nieves-Rosa L, Diaz F. Frequent use of lubricants for anal sex among men who have sex with men: the HIV prevention potential of a microbicidal gel. Am J Public Health. 2000;90(7):1117–21. doi: 10.2105/ajph.90.7.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carballo-Dieguez A, O’Sullivan LF, Lin P, Dolezal C, Pollack L, Catania J. Awareness and attitudes regarding microbicides and Nonoxynol-9 use in a probability sample of gay men. AIDS Behav. 2007;11(2):271–6. doi: 10.1007/s10461-006-9128-0. [DOI] [PubMed] [Google Scholar]
- 18.Javanbakht M, Murphy R, Gorbach P, LeBlanc MA, Pickett J. Preference and practices relating to lubricant use during anal intercourse: implications for rectal microbicides. Sex Health. 2010;7(2):193–8. doi: 10.1071/SH09062. [DOI] [PubMed] [Google Scholar]
- 19.Gorbach PM, Weiss RE, Fuchs E, et al. The slippery slope: lubricant use and rectal sexually transmitted infections: a newly identified risk. Sex Transm Dis. 2012;39(1):59–64. doi: 10.1097/OLQ.0b013e318235502b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stahlman S, Gorbach PM. Microbicides. Vol. 2012. Sydney; 2012. Rectal behaviors: a global overview. [Google Scholar]
- 21.Weiss RE. Modeling longitudinal data. New York: Springer; 2005. [Google Scholar]
- 22.Martino JL, Vermund SH. Vaginal douching: evidence for risks or benefits to women’s health. Epidemiol Rev. 2002;24(2):109–24. doi: 10.1093/epirev/mxf004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gorbach PM, Borgerding JA, Coumi N, et al. Microbicides. Vol. 2010. Pittsburgh: 2010. Effects of Partnership Change on Gel Adherence in HPTN 035. [DOI] [PMC free article] [PubMed] [Google Scholar]