Abstract
Women experience dramatic changes in hormones, mood and cognition through different periods of their reproductive lives, particularly during pregnancy and giving birth. While limited human studies of early pregnancy and motherhood showed alteration of cognitive function in later life, research conducted on rodents showed a persistent improvement of learning and memory performance in females with history of giving birth (primiparous or multiparous) compared to virgin controls (nulliparous). In this mini review, we will focus on the effect of early motherhood on cognitive function later in life, which would provide insight on how reproductive experiences influence women’s health during ageing.
Keywords: pregnancy, motherhood, steroids, neurogenesis, gene regulation, cognition
Introduction
Reproductive experiences in females, pregnancy and giving birth, constitute as special conditions in women’s lives that affect various physiology and endocrinology systems. The reproductive hormones produced during pregnancy and postpartum period have profound and distinct effects on brain. Those changes include increasing of the size of the brain cell body, extending the length of dendritic branches, and altering of neurogenesis in several brain regions [1–3]. The effects of pregnancy and motherhood on learning and memory are associated, but different. These differences are attributed to an adaptive function in preparation for parturition, changes of associated hormones and specific brain functions. The effects are also different in human studies compared to rodent experiments. However, some of the reproductive experience induced behavioral changes last longer than the postpartum or mother care periods and may have important impacts later in life. In this mini review, we will discuss reproductive experiment associated changes in neurogenesis in different brain regions, learning and memory performances, as well as regulation of cognitive associated genes during pregnancy and postpartum period.
Parenting and the brain
It is known that the changes in cognitive function induced by pregnancy and motherhood are partially due to dramatic fluctuations in sex hormones as well as neuropeptides, which may alter the morphology and structure of various brain regions which are directly or indirectly involved in learning and memory functions. For instance, olfaction plays a central role in numerous basic roles in many animal species as a function of social recognition. Increasing evidence in animal studies suggest that neural plasticity in the olfactory bulb is important for maternal recognition and for the production of normal maternal behaviors [4,5]. While dendritic spines have been considered as one of the cellular substrates underlying experience-related plasticity and their morphology has been related with synaptic stability and strength, a recent study using in vivo time-lapse imaging technology in rats reported a significant increase in the number of stable dendritic spines of adult-born neurons in the olfactory bulb in lactating mothers compared with naive virgins [6]. However, reproductive experiences can cause more changes in brain regions beyond the olfactory bulb. The reproductive experience-induced morphology changes are also found in cognition-associated brain regions, such as the hippocampus, medial prefrontal cortex, and medial preoptic area [7–10]. In parallel with neurogenesis observed in the olfactory blub, studies also showed an increase in the dendritic spine density in the CA1 stratum lacunosum-moleculare in late-pregnant and lactating experimental rats [11]. Compared with virgin females, postpartum rats showed more dendritic spines in the anterodorsal and fewer in the posterodorsal but no difference was found in the posteroventral area of amygdale [12], suggesting brain-region specific changes of synaptic plasticity are induced by maternal care. As shown in figure 1, we found more BrdU positive cells in the hippocampus dentate gyrus region in older female mice with history of giving birth (6–9 months after parturition with more than 1 litter) compared to the age-matched virgin mice at 12 months old. While estradiol, corticosterone and prolactin play the main roles in the maternal associated regulation of neurogenesis in various brain regions [13], our data is in line of supporting the hypothesis that although the hormone surges are transient during pregnancy and postpartum, the reproductive experience-induced changes on brain function and behavior have lifelong effects [14]. The long lasting effects of the maternal experiment is not only supported by morphological studies, but also evidenced by changes in brain function later in life. For example, the motherhood experience during early life increased sensitivity in response to estrogen treatment-induced increase in cell proliferation in the dentate gyrus of hippocampus at an older age in rats [15], a higher tolerance to stress-induced learning impairment [16], and a permanent change in the responsiveness of the nervous system [17], suggesting a long lasting neurogenesis effect of the maternal experience on brain function. These structural or morphological changes in the brain, particularly in the hippocampus are believed to lead to behavioral implications, such as improvement in learning and memory activities.
Figure 1.
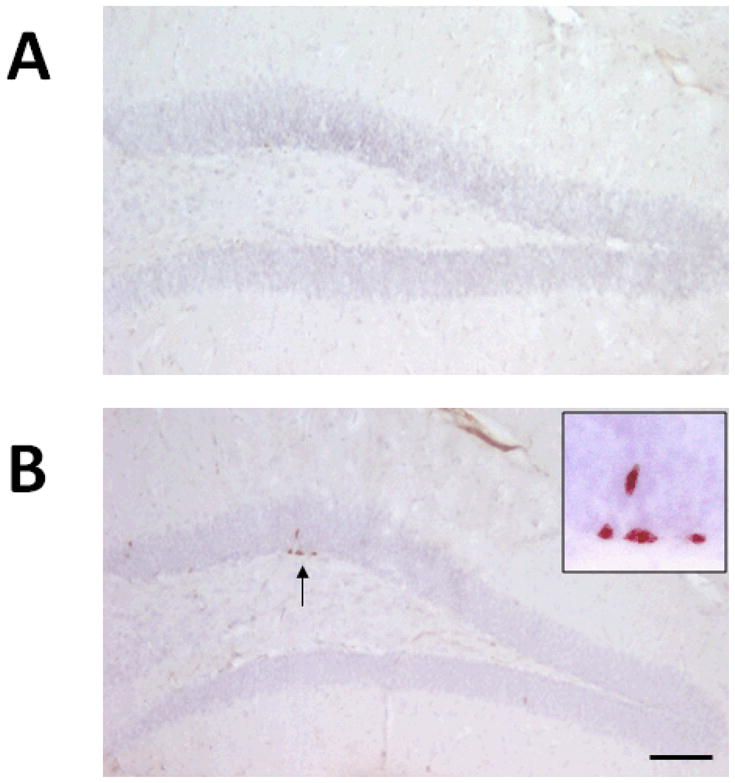
Representative images show that the number of BrdU-staining positive cells located in the subgranular zone of the dentate gyrus in hippocampus from multiparous mice (B) and age-matched nulliparous littermates (A) at 12 months old. BrdU was injected intraperitoneally with 50 mg/kg 4 hrs before sacrifice. Following 2N HCl treatment in 37°C for 30 min, the primary antibody against BrdU was applied (1:500, Abcam). After DAB step the sections were counter-stained with hemotaxyin. Bar: 100 um.
Parenting and cognitive function
As a consequence of the reproductive experience-induced brain structure changes, studies showed better learning and memory in mothers compared to virgins both in human and animal studies. The maternal experience associated behavioral changes include an improved ability for mothers to navigate in their surrounding environment [14,16,18], as well as an enhanced spatial and working cognitive function [19–21]. Part of the behavioral changes are an adaptive function for mothers who are forced to find and remember the location of food and water nearby their home (nest) in order to ensure the survival of their offspring. Behavioral changes are also demonstrated by an improved ability in preventing stress-induced learning deficits which often occur in virgins [22].
Pregnancy raises estrogen and progesterone to higher levels for much longer period of time compared to the estrous cycle. The increased hormone levels may contribute to a prolonged improvement of learning and memory. However, whether the improved learning and memory found in animal studies is related to elevated levels of steroid hormones such as estrogen and progesterone remains unknown. In addition, there is a large discrepancy between human studies and animal research. In human studies, pregnant women showed impairments in verbal memory [23], word fluency, word list learning [24,25], and performance on priming tasks and incidental learning tasks [26–28] as compared with non-pregnant controls. A recent longitudinal study of 302 women (254 pregnant and 48 non-pregnant) showed the transition of memory function from gestations during pregnancy into the postpartum period [29]. This study showed that recall memory was adversely affected during pregnancy and postpartum period while working memory was unaffected, and prenatal estradiol and cortisol levels predicted memory performance during pregnancy and into the postpartum period, suggesting that the exposure to high levels estrogen during pregnancy does not help learning and memory but causes specific memory impairment. Other human studies with a smaller sample size showed no differences in memory performance between pregnant subjects and non-pregnant controls [30,31]. However, findings from animal studies showed a different picture. The cognitive performance not only improved in pregnant rats, but the improvements also persist through postpartum period and even longer in rodents [14, 18–21]. Furthermore, rats with completed motherhood showed fewer errors and performed better than virgin rats while “pregnancy only” female rats (pups removed after parturition) failed to complete spatial learning and memory tests [14,32]. These discrepancies in findings of cognitive function between human and animal studies during pregnancy and motherhood are more likely due to different time points of examination. For human studies, examinations often occurred shortly after pregnancy or parturition while lacking further examinations of the long-term effects months or years after parturition and motherhood were completed. Another possible reason that could account for the inconsistency in the reproductive experience impact on cognitive function between human and rodents is whether birth control pills were used by the non-pregnant control subjects in human studies, as females taking oral contraceptives showed improvement of cognitive performance [33]. These discrepancies in findings of cognitive function between human and animal studies may also be related to the difference in level and change patterns of sex hormones during pregnancy, as well as very different social and living environments between human and animal [34, 35]. In contrast, the reproductive experiences are well controlled in animal studies and there are no concerns of extra hormonal treatments.
Hormones, peptides and genes
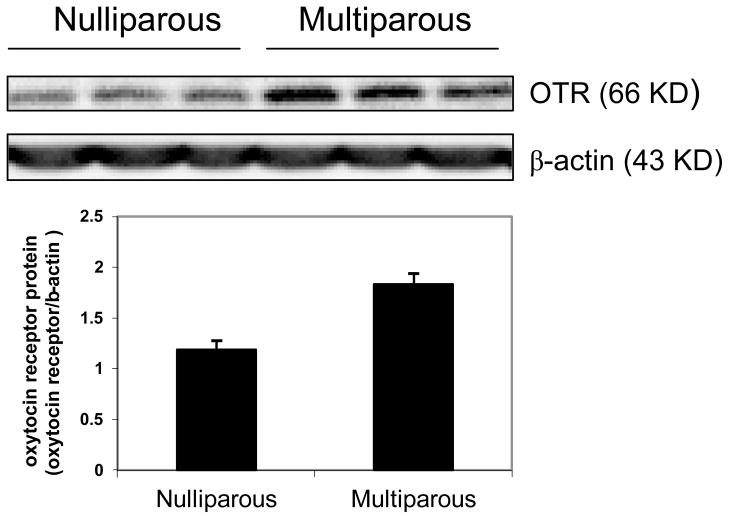
Pregnancy and postpartum periods are characterized by robust hormonal changes. By the end of the third trimester, levels of estrogen and progesterone in circulation increase dramatically and reach levels about 50-fold and 10-fold higher than the maximal menstrual cycle levels, respectively. The levels of both hormones drop sharply after parturition and in the early postpartum period. Such a drastic change of hormone levels between period of pregnancy and postpartum caused significant changes in brain functions as well as immediate behavioral changes. While estradiol is well recognized as a neurotrophic factor and promotes cell proliferation and survival in general, some studies reported that estrogen down-regulates cell proliferation in the hippocampus and subventricular zone during pregnancy in rodents [36,37]. Similarly, progesterone and its metabolites also suppress newborn cell numbers in the hippocampus [38,39]. The effects of estrogen and progesterone on brain cell proliferation might be dependent on reproductive experience, as studies in rats found no or little change in cell proliferation in the hippocampus during pregnancy while a significant decrease in hippocampal cell proliferation is reported after the parturition or early postpartum periods [13]. It is possible that the estrogen-induced changes of cell proliferation in the hippocampus may be compromised by other hormones and peptides which promote cell proliferation. In addition, studies showed that elevated levels of estradiol during pregnancy initially increase neurogenesis in the hippocampus of the female, but repeated exposure to estradiol during later pregnancy reduces cell death [37,40]. There are other hormones and peptides that also play roles in promoting cell proliferation, such as prolactin [1], oxytocin [3] and vasopressin [41]. Indeed, in addition to reproductive hormones such as estrogen, studies also found that neuropeptides such as oxytocin and vasopressin are also responsible for social recognition. Oxytocin working through the oxytocin receptor (OTR) located in medial preoptic area (MPOA) clearly increases maternal behavior with dependency upon estrogen priming [42–44]. The interaction between oxytocin and estrogen makes maternal and social behavior especially interesting. Our preliminary data also shows an elevation of OTR protein expression in multiparous mice compared to nulliparous mice (Figure 2).
Figure 2.
Oxytocin receptor (OTR) expression in brain of nulliparious and multiparous mice at 12 months old. All multiparous mice completed their last maternal experience at least 6 months ago and had more than 1 litter. Oxytocin receptor expression was detected by polyclonal anti-oxytocin receptor antibody (1:500, Santa Cruz) and the bar graph showed density analysis of western blot image of OTR.
Several cognitive related genes were identified with association of the hormone changes in pregnancy and postpartum period by multiple studies. Ca2+/Calomoduline-dependent protein kinase II (CAMKII) is involved in synaptic plasticity and memory. A down-regulation of CAMKII is found in the brain of individuals with depression, Alzheimer’s disease as well as in early postpartum period [45–47]. Calcineurin is a Ca2+/calmodulin-dependent protein phosphatase and is also widely recognized for its abundance in the brain and its association with cognitive function. For example, overexpression of calcineurin in young adult animals leads to altered synaptic function, memory retention deficits and inhibition of calcineurin can rescue the memory decline in a mouse model of AD at a young age [48–50]. Furthermore, the level of Thr286 phosphorylation of CAMKII in the hippocampus is associated with faster hippocampal-dependent spatial memory formation reported from animal studies [51]. Recent animal studies showed that gene expression of calcineurin increases during pregnancy and parturition [52] and the levels of calcineurin protein vanished from MPOA brain region in the postpartum period [53]. The cAMP-response element-binding (CREB) protein is a crucial transcription factor regulating expression of genes involved in neuronal growth and plasticity and takes part in neuronal survival. Studies showed that estradiol induces spine plasticity in the hippocampus via rapid membrane effects and slower transcriptional regulation via the CREB pathway [54–56]. Furthermore, the number of cells with positive immunostaining for phospho-CREB in the medial preoptic area of the hypothalamus, a key region for the expression of maternal behavior, increased about three-fold in female maternal mice exposure to pups [57]. Brain-derived neurotrophic factor (BDNF) is a small protein for neuronal survival. BDNF is known to upregulate the induction of long-term potentiation, to enhance synaptic transmission, and also to increase neuronal plasticity in the central nervous system (CNS) [43,58]. The role of BDNF in learning and memory is also evidenced by genetic approaches, such as an age-dependent deficit in learning was found in BDNF(+/−) animals [59] and BDNF(−/−) mice showed impaired motor learning performance but not spatial learning and recognition memory compared with wildtype mice suggesting a specific neocortical dysfunction [60]. The level of BDNF expression increased during the pregnant period and decreased in the early postpartum period in a hormone-stimulated pseudopregancy and postpartum rat model [47]. In sheep, in the early postpartum period, the levels of BDNF mRNA were increased in the hippocampus CA1 region and temporal frontal cortex, but no changes were found in the olfactory bulbs, suggesting an independent change in visual recognition memory from classic maternal care behavior [61,62].
Conclusion
While limited human studies have been done in cognitive function changes long after giving birth to a child, findings from animal studies show that reproductive experiences improve learning and memory performance in mothers even long past the maternal care period. Although the molecular mechanisms of these persistent improvements in cognitive function found in primiparous or multiparous rodents remain unknown, dramatic changes of hormone levels, expression of steroid receptors, and regulation of cognitive associated genes have been identified and may contribute to the reproductive experience induced alteration of cognitive functions.
Acknowledgments
This work was supported by grants from the Alzheimer’s Association IIRG-07-59510 and IIRG-09-61521, American Health Assistance Foundation Grant G2006-118, and National Institute of Health (NIH R01AG032441-01, R01AG025888-01).
References
- 1.Shingo T, Gregg C, Enwere E, Fujikawa H, Hassam R, Geary C, Cross JC, Weiss S. Pregnancy-stimulated neurogenesis in the adult female forebrain mediated by prolactin. Science. 2003;299:117–20. doi: 10.1126/science.1076647. [DOI] [PubMed] [Google Scholar]
- 2.Prange-Kiel J, Rune GM. Direct and indirect effect of estrogen on rat hippocampus. Neuroscience. 2006;138:765–772. doi: 10.1016/j.neuroscience.2005.05.061. [DOI] [PubMed] [Google Scholar]
- 3.Tomizawa K, Iga N, Lu YF, Moriwaki A, Matsushita M, Li ST, Miyamoto O, Itano T, Matsui H. Oxytocin improves long-lasting spatial memory during motherhood through MAP kinase cascade. Nat Neurosci. 2003;6:384–90. doi: 10.1038/nn1023. [DOI] [PubMed] [Google Scholar]
- 4.Dickinson C, Keverne EB. Importance of noradrenergic mechanisms in the olfactory bulbs for the maternal behaviour of mice. Physiol Behav. 1988;43:13–316. doi: 10.1016/0031-9384(88)90193-x. [DOI] [PubMed] [Google Scholar]
- 5.Sakamoto M, Imayoshi I, Ohtsuka T, Yamaguchi M, Mori K, Kageyama R. Continuous neurogenesis in the adult forebrain is required for innate olfactory responses. Proc Natl Acad Sci USA. 2011;108:8479–8484. doi: 10.1073/pnas.1018782108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kopel H, Schechtman E, Groysman M, Mizrahi A. Enhanced synaptic integration of adult-born neurons in the olfactory bulb of lactating mothers. J Neurosci. 2012;32:7519–27. doi: 10.1523/JNEUROSCI.6354-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pereira M, Morrell JI. Functional mapping of the neural circuitry of rat maternal motivation: effects of site-specific transient neural inactivation. J Neuroendocrinol. 2011;23:1020–35. doi: 10.1111/j.1365-2826.2011.02200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Febo M, Segarra AC, Stolberg TL, Ferris CF. BOLD signal response to cocaine varies with sexual receptivity in female rats. Neuroreport. 2011;22:19–22. doi: 10.1097/WNR.0b013e3283416f81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kinsley CH, Lambert KG. Reproduction-induced neuroplasticity: natural behavioural and neuronal alterations associated with the production and care of offspring. J Neuroendocrinol. 2008;20:515–25. doi: 10.1111/j.1365-2826.2008.01667.x. Review. [DOI] [PubMed] [Google Scholar]
- 10.Pawluski JL, Galea LA. Hippocampal morphology is differentially affected by reproductive experience in the mother. J Neurobiol. 2006;66:71–81. doi: 10.1002/neu.20194. [DOI] [PubMed] [Google Scholar]
- 11.Kinsley CH, Trainer R, Stafisso-Sandoz G, Quadros P, Marcus LK, Hearon C, Meyer EA, Hester N, Morgan M, Kozub FJ, Lambert KG. Motherhood and the hormones of pregnancy modify concentrations of hippocampal neuronal dendritic spines. Horm Behav. 2006;49:131–142. doi: 10.1016/j.yhbeh.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 12.Rasia-Filho AA, Fabian C, Rigoti KM, Achaval M. Influence of sex, estrous cycle and motherhood on dendritic spine density in the rat medial amygdala revealed by the Golgi method. Neuroscience. 2004;126:839–847. doi: 10.1016/j.neuroscience.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 13.Lévy F, Gheusi G, Keller M. Plasticity of the parental brain: a case for neurogenesis. J Neuroendocrinol. 2011;23:984–93. doi: 10.1111/j.1365-2826.2011.02203.x. Review. [DOI] [PubMed] [Google Scholar]
- 14.Kinsley CH, Madonia L, Gifford GW, Tureski K, Griffin GR, Lowry C, Williams J, Collins J, McLearie H, Lambert KG. Motherhood improves learning and memory. Nature. 1999;402:137–8. doi: 10.1038/45957. [DOI] [PubMed] [Google Scholar]
- 15.Barha CK, Galea LA. Motherhood alters the cellular response to estrogens in the hippocampus later in life. Neurobiol Aging. 2011;32:2091–5. doi: 10.1016/j.neurobiolaging.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 16.Maeng LY, Shors TJ. Once a mother, always a mother: maternal experience protects females from the negative effects of stress on learning. Behav Neurosci. 2012;126:137–41. doi: 10.1037/a0026707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bellinger DL, Lubahn C, Lorton D. Maternal and early life stress effects on immune function: relevance to immunotoxicology. J Immunotoxicol. 2008;5:419–44. doi: 10.1080/15476910802483415. [DOI] [PubMed] [Google Scholar]
- 18.Lemaire V, Billard JM, Dutar P, George O, Piazza PV, Epelbaum J, Le Moal M, Mayo W. Motherhood-induced memory improvement persists across lifespan in rats but is abolished by a gestational stress. Eur J Neurosci. 2006;23:3368–74. doi: 10.1111/j.1460-9568.2006.04870.x. [DOI] [PubMed] [Google Scholar]
- 19.Paris JJ, Frye CA. Estrous cycle, pregnancy, and parity enhance performance of rats in object recognition or object placement tasks. Reproduction. 2008;136:105. doi: 10.1530/REP-07-0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Darnaudéry M, Perez-Martin M, Del Favero F, Gomez-Roldan C, Garcia-Segura LM, Maccari S. Early motherhood in rats is associated with a modification of hippocampal function. Psychoneuroendocrinology. 2007;32:803–12. doi: 10.1016/j.psyneuen.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 21.Gatewood JD, Morgan MD, Eaton M, McNamara IM, Stevens LF, Macbeth AH, Meyer EA, Lomas LM, Kozub FJ, Lambert KG, Kinsley CH. Motherhood mitigates aging-related decrements in learning and memory and positively affects brain aging in the rat. Brain Res Bull. 2005;66:91–8. doi: 10.1016/j.brainresbull.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 22.Maeng LY, Waddell J, Shors TJ. The prefrontal cortex communicates with the amygdala to impair learning after acute stress in females but not in males. J Neurosci. 2010;30:16188–96. doi: 10.1523/JNEUROSCI.2265-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Henry JD, Rendell PG. A review of the impact of pregnancy on memory function. J Clin Exp Neuropsychol. 2007;29:793–803. doi: 10.1080/13803390701612209. [DOI] [PubMed] [Google Scholar]
- 24.de Groot RH, Hornstra G, Roozendaal N, Jolles J, de Groot RHM, Hornstra G, Roozendaal N, Jolles J. Memory performance, but not information processing speed, may be reduced during early pregnancy. J Clin Exp Neuropsychol. 2003;25:482–8. doi: 10.1076/jcen.25.4.482.13871. [DOI] [PubMed] [Google Scholar]
- 25.Henry JF, Sherwin BB. Hormones and cognitive functioning during late pregnancy and postpartum: a longitudinal study. Behav Neurosci. 2012;126:73–85. doi: 10.1037/a0025540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sharp K, Brindle PM, Brown MW, Turner GM. Memory loss during pregnancy. Br J Obstet Gynaecol. 1993;100:209–15. doi: 10.1111/j.1471-0528.1993.tb15232.x. [DOI] [PubMed] [Google Scholar]
- 27.Rendell PG, Henry JD. Prospective-memory functioning is affected during pregnancy and postpartum. J Clin Exp Neuropsychol. 2008;30:913–9. doi: 10.1080/13803390701874379. [DOI] [PubMed] [Google Scholar]
- 28.Buckwalter JG, Buckwalter DK, Bluestein BW, Stanczyk FZ. Pregnancy and post partum: changes in cognition and mood. Prog Brain Res. 2001;133:303–19. doi: 10.1016/s0079-6123(01)33023-6. Review. [DOI] [PubMed] [Google Scholar]
- 29.Glynn LM. Giving birth to a new brain: hormone exposures of pregnancy influence human memory. Psychoneuroendocrinology. 2010;35:1148–55. doi: 10.1016/j.psyneuen.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 30.McDowall J, Moriarty R. Implicit and explicit memory in pregnant women: an analysis of data-driven and conceptually driven processes. Q J Exp Psychol. 2000;53:729–40. doi: 10.1080/713755904. [DOI] [PubMed] [Google Scholar]
- 31.Christensen H, Leach LS, Mackinnon A. Cognition in pregnancy and motherhood: prospective cohort study. Br J Psychiatry. 2010;196:126–32. doi: 10.1192/bjp.bp.109.068635. [DOI] [PubMed] [Google Scholar]
- 32.Pawluski JL, Vanderbyl BL, Ragan K, Galea LA. First reproductive experience persistently affects spatial reference and working memory in the mother and these effects are not due to pregnancy or ‘mothering’ alone. Behav Brain Res. 2006;175:157–65. doi: 10.1016/j.bbr.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 33.Cicinelli E, De Tommaso M, Cianci A, Colacurci N, Rella L, Loiudice L, Cicinelli MV, Livrea P. Oral contraceptive therapy modulates hemispheric asymmetry in spatial attention. Contraception. 2011;84:634–6. doi: 10.1016/j.contraception.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 34.Jones A, Osmond C, Godfrey KM, Phillips DI. Evidence for developmental programming of cerebral laterality in humans. PLoS One. 2011;6:e17071. doi: 10.1371/journal.pone.0017071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Danilovich N, Ram Sairam M. Recent female mouse models displaying advanced reproductive aging. Exp Gerontol. 2006;41:117–22. doi: 10.1016/j.exger.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 36.Pawluski JL, van den Hove DL, Rayen I, Prickaerts J, Steinbusch HW. Stress and the pregnant female: Impact on hippocampal cell proliferation, but not affective-like behaviors. Horm Behav. 2011;59:572–80. doi: 10.1016/j.yhbeh.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 37.Barker JM, Galea LA. Repeated estradiol administration alters different aspects of neurogenesis and cell death in the hippocampus of female, but not male, rats. Neuroscience. 2008;152:888–902. doi: 10.1016/j.neuroscience.2007.10.071. [DOI] [PubMed] [Google Scholar]
- 38.Giachino C, Galbiati M, Fasolo A, Peretto P, Melcangi RC. Effects of progesterone derivatives, dihydroprogesterone and tetrahydroprogesterone, on the subependymal layer of the adult rat. J Neurobiol. 2004;58:493–502. doi: 10.1002/neu.10290. [DOI] [PubMed] [Google Scholar]
- 39.Tanapat P, Hastings NB, Gould E. Ovarian steroids influence cell proliferation in the dentate gyrus of the adult female rat in a dose- and time-dependent manner. J Comp Neurol. 2005;481:252–65. doi: 10.1002/cne.20385. [DOI] [PubMed] [Google Scholar]
- 40.Ormerod BK, Lee TT, Galea LA. Estradiol initially enhances but subsequently suppresses (via adrenal steroids) granule cell proliferation in the dentate gyrus of adult female rats. J Neurobiol. 2003;55:247–260. doi: 10.1002/neu.10181. [DOI] [PubMed] [Google Scholar]
- 41.Taylor PV, Veenema AH, Paul MJ, Bredewold R, Isaacs S, de Vries GJ. Sexually dimorphic effects of a prenatal immune challenge on social play and vasopressin expression in juvenile rats. Biol Sex Differ. 2012;3:15. doi: 10.1186/2042-6410-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pedersen CA, Ascher JA, Monroe YL, Prange AJ., Jr Oxytocin induces maternal behavior in virgin female rats. Science. 1982;216:648–650. doi: 10.1126/science.7071605. [DOI] [PubMed] [Google Scholar]
- 43.Shen Y, Li R. The role of neuropeptides in learning and memory: possible mechanisms. Med Hypotheses. 1995;45:529–38. doi: 10.1016/0306-9877(95)90235-x. [DOI] [PubMed] [Google Scholar]
- 44.Olazábal DE, Young LJ. Oxytocin receptors in the nucleus accumbens facilitate “spontaneous” maternal behavior in adult female prairie voles. Neuroscience. 2006;141:559–568. doi: 10.1016/j.neuroscience.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 45.Amada N, Aihara K, Ravid R, Horie M. Reduction of NR1 and phosphorylated Ca2+/calmodulin-dependent protein kinase II levels in Alzheimer’s disease. Neuroreport. 2005;16:1809–13. doi: 10.1097/01.wnr.0000185015.44563.5d. [DOI] [PubMed] [Google Scholar]
- 46.Novak G, Seeman P, Tallerico T. Increased expression of calcium/calmodulin-dependent protein kinase IIbeta in frontal cortex in schizophrenia and depression. Synapse. 2006;59:61–8. doi: 10.1002/syn.20211. [DOI] [PubMed] [Google Scholar]
- 47.Suda S, Segi-Nishida E, Newton SS, Duman RS. A postpartum model in rat: behavioral and gene expression changes induced by ovarian steroid deprivation. Biol Psychiatry. 2008;64:311–9. doi: 10.1016/j.biopsych.2008.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abdul HM, Sama MA, Furman JL, Mathis DM, Beckett TL, Weidner AM, Patel ES, Baig I, Murphy MP, LeVine H, 3rd, Kraner SD, Norris CM. Cognitive decline in Alzheimer’s disease is associated with selective changes in calcineurin/NFAT signaling. J Neurosci. 2009;29:12957–69. doi: 10.1523/JNEUROSCI.1064-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Taglialatela G, Hogan D, Zhang WR, Dineley KT. Intermediate- and long-term recognition memory deficits in Tg2576 mice are reversed with acute calcineurin inhibition. Behav Brain Res. 2009;200:95–9. doi: 10.1016/j.bbr.2008.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Foster TC, Sharrow KM, Masse JR, Norris CM, Kumar A. Calcineurin links Ca2+ dysregulation with brain aging. J Neurosci. 2001;21:4066–73. doi: 10.1523/JNEUROSCI.21-11-04066.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Takahashi E, Niimi K, Itakura C. Enhanced CaMKII activity and spatial cognitive function in SAMP6 mice. Behav Neurosci. 2009;123:527–32. doi: 10.1037/a0015119. [DOI] [PubMed] [Google Scholar]
- 52.Tabata C, Ogita K, Sato K, Nakamura H, Qing Z, Negoro H, Kumasawa K, Temma-Asano K, Tsutsui T, Nishimori K, Kimura T. Calcineurin/NFAT pathway: a novel regulator of parturition. Am J Reprod Immunol. 2009;62:44–50. doi: 10.1111/j.1600-0897.2009.00710.x. [DOI] [PubMed] [Google Scholar]
- 53.O’Day DH, Payne LA, Drmic I, Fleming AS. Loss of calcineurin from the medial preoptic area of primiparous rats. Biochem Biophys Res Commun. 2001;281:1037–40. doi: 10.1006/bbrc.2001.4471. [DOI] [PubMed] [Google Scholar]
- 54.Hardingham GE, Bading H. The Yin and Yang of NMDA receptor signalling. Trends Neurosci. 2003;26:81–9. doi: 10.1016/S0166-2236(02)00040-1. [DOI] [PubMed] [Google Scholar]
- 55.Luine V, Frankfurt M. Interactions between estradiol, BDNF and dendritic spines in promoting memory. Neuroscience. 2012 Oct; doi: 10.1016/j.neuroscience.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lonze BE, Ginty DD. Function and regulation of CREB family transcription factors in the nervous system. Neuron. 2002;35:605–23. doi: 10.1016/s0896-6273(02)00828-0. [DOI] [PubMed] [Google Scholar]
- 57.Jin SH, Blendy JA, Thomas SA. Cyclic AMP response element-binding protein is required for normal maternal nurturing behavior. Neuroscience. 2005;133:647–55. doi: 10.1016/j.neuroscience.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 58.Mizuno M, Yamada K, Olariu A, Nawa H, Nabeshima T. Involvement of brain-derived neurotrophic factor in spatial memory formation and maintenance in a radial arm maze test in rats. J Neurosci. 2000;20:7116–21. doi: 10.1523/JNEUROSCI.20-18-07116.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Endres T, Lessmann V. Age-dependent deficits in fear learning in heterozygous BDNF knock-out mice. Learn Mem. 2012;19:561–70. doi: 10.1101/lm.028068.112. [DOI] [PubMed] [Google Scholar]
- 60.Carretón O, Giralt A, Torres-Peraza JF, Brito V, Lucas JJ, Ginés S, Canals JM, Alberch J. Age-dependent decline of motor neocortex but not hippocampal performance in heterozygous BDNF mice correlates with a decrease of cortical PSD-95 but an increase of hippocampal TrkB levels. Exp Neurol. 2012;237:335–45. doi: 10.1016/j.expneurol.2012.06.033. [DOI] [PubMed] [Google Scholar]
- 61.Broad KD, Mimmack ML, Keverne EB, Kendrick KM. Increased BDNF and trk-B mRNA expression in cortical and limbic regions following formation of a social recognition memory. Eur J Neurosci. 2002;16:2166–74. doi: 10.1046/j.1460-9568.2002.02311.x. [DOI] [PubMed] [Google Scholar]
- 62.de Lima MN, Presti-Torres J, Vedana G, Alcalde LA, Stertz L, Fries GR, Roesler R, Andersen ML, Quevedo J, Kapczinski F, Schröder N. Early life stress decreases hippocampal BDNF content and exacerbates recognition memory deficits induced by repeated D-amphetamine exposure. Behav Brain Res. 2011;224:100–6. doi: 10.1016/j.bbr.2011.05.022. [DOI] [PubMed] [Google Scholar]