Abstract
Obesity and associated dyslipidemia is the fastest growing health problem throughout the world. The combination of exercise and low-level laser therapy (LLLT) could be a new approach to the treatment of obesity and associated disease. In this work, the effects of LLLT associated with exercises on the lipid metabolism in regular and high-fat diet rats were verified. We used 64 rats divided in eight groups with eight rats each, designed: SC, sedentary chow diet; SCL, sedentary chow diet laser, TC, trained chow diet; TCL, trained chow diet laser; SH, sedentary high-fat diet; SHL, sedentary high-fat diet laser; TH, trained high-fat diet; and THL, trained high-fat diet laser. The exercise used was swimming during 8 weeks/90 min daily and LLLT (GA-Al-As, 830 nm) dose of 4.7 J/point and total energy 9.4 J per animal, applied to both gastrocnemius muscles after exercise. We analyzed biochemical parameters, percentage of fat, hepatic and muscular glycogen and relative mass of tissue, and weight percentage gain. The statistical test used was ANOVA, with post hoc Tukey–Kramer for multiple analysis between groups, and the significant level was p<0.001, p<0.01, and p<0.05. LLLT decreased the total cholesterol (p<0.05), triglycerides (p<0.01), low-density lipoprotein cholesterol (p<0.05), and relative mass of fat tissue (p<0.05), suggesting increased metabolic activity and altered lipid pathways. The combination of exercise and LLLT increased the benefits of exercise alone. However, LLLT without exercise tended to increase body weight and fat content. LLLT may be a valuable addition to a regimen of diet and exercise for weight reduction and dyslipidemic control.
Keywords: Exercise, Metabolism, LLLT, Photobiomodulation, Obesity, Dyslipidemia
Introduction
The pathogenesis of obesity is complex and not well understood. It is fundamentally a problem of energy balance, which can develop only when energy intake is in excess of energy expenditure. This fact has led to a major focus on the mechanisms controlling food intake and the components and regulatory mechanisms of energy expenditure [1]. Eating high-fat or high-calorie food associated with a sedentary lifestyle facilitates the development of a positive energy balance [1]. Obesity is strongly associated with many chronic diseases, such as hypertension, diabetes, coronary heart disease, cancer, nonalcoholic fatty liver disease, and dyslipidemias [2, 3]
Physical activity is already established as an important nonpharmacological strategy for control of obesity or high body-fat percentage and for the treatment of associated diseases [4]. Several clinical and experimental studies have demonstrated that a moderate exercise regimen combined with a normocaloric diet resulted in the reduction of adiposity and improved lipid profile [5, 6]. However, due to the increased incidence of obesity in the world, it becomes necessary to seek new noninvasive and nonpharmacological strategies to increase the physiological effects of exercises.
Several studies have investigated low-level laser therapy (LLLT) or light-emitting diode (LED) therapy and have made advances in the understanding of the underlying mechanisms LLLT in biological systems [7–9]. The main characteristics of photobiomodulation or photobiostimulation are the induction and stimulation of many aspects of cellular processes. According to Karu [10], the cellular redox state is an important determinant of the final response, and there are three signaling pathways that operate relating to cell attachment, mitochondrial respiratory chain, and Na, K-ATPase. Moreover, results suggest that specific wavelengths, such as red and near-infrared radiation, can create, regulate, or activate enzymatic processes in cells to improve metabolism [8].
There is still much to elucidate about the mechanisms underlying LLLT and how it acts on cells and tissues, but there is evidence that the response usually exhibit a biphasic dose–response profile [11]. In the adipose tissues, some authors have attempted to modify their metabolism using LLLT, and some clinical studies [12, 13] tried to explain how there could be the reduction of body contours promoted by the LLLT, including the transitory induction of pores in the membranes of the adipocytes and consequently liberation of intracellular constituents (fat) and its removal and metabolism; however, the mechanisms still remain unclear.
For this reason, parameters such as quantity hepatic and muscle glycogen, the percentage of lipids in different tissues, as well as triglycerides, total cholesterol, high-density lipoprotein (HDL) cholesterol and low-density lipoprotein (LDL) cholesterol may provide the state of health of individuals, as well as can be used to monitor the systemic of conditions, which are distributed the products of the metabolism of fat and sugar [5, 14, 15].
Nevertheless, only a few studies have described LLLT combined with exercise training, for example, in young males [8], postmenopausal women [9], or overweight individuals [16]. Thus, our goal was to perform a randomized controlled trial to investigate the effects of the LLLT associated with exercise training on lipid profile in rats fed with different diets. Our hypothesis was that combined exercise with LLLT could control the serum lipids and modify the lipid metabolism in animals with normocaloric and hypercaloric diet. If successful, this combination could play an important role in control of diseases associated with obesity.
Methods and procedures
Animals
All animal procedures were performed according to the principles in the Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Ethics Committee (number 067/2010). Sixty-four male Wistar rats (90 days old and weight of 317.00±19.16 g) were included in this study. Before beginning the experimental protocol, all of the groups [except for the normocaloric groups (N)] were fed ad libitum with the hyperlipidic diet (H) for 3 weeks [14] for the development of obesity and dyslipidemia. The animals were randomized according to diet into eight groups with eight rats each (n=8): normocaloric diet (N) groups—sedentary normal diet (SN), trained normal diet (TN), sedentary normal diet plus laser (SNL), and trained normal diet plus laser (TNL); hypercaloric high-fat diet (H) groups—sedentary high-fat diet (SH), trained high-fat diet (TH), sedentary high-fat diet plus laser (SHL), and trained high-fat diet plus laser (THL). Rats were kept one per cage with food and water ad libitum (8 weeks), on a 12:12-h light–dark cycle at 23±1 °C.
Diet
The experimental groups received the following diet: the normocaloric diet (N)—MP-77 standard rat chow diet provided in pellet form (Primor®, São Paulo, Brazil) containing 23 g of protein, 49 g of carbohydrate, 4 g of total fat, 5 g of fiber, 7 g of ash, and 6 g of vitamins per 100 g diet. The hypercaloric diet consisted of the same commercial rat chow plus peanuts, milk chocolate, and sweet biscuit in a proportion of 3:2:2:1. It contained 5.12 kcal/g (35 % of calories as fat) for the hypercaloric high-fat diet and 4.07 kcal/g for the normocaloric diet [14].
Exercise and LLLT protocols
The exercise program consisted of swimming in individual tanks (Fig. 1) filled with water, maintained at 28–32 °C. The animals of the trained groups swam for 30, 60, and 90 min on the first, second, and third days to adapt. The swimming period was then increased to 90 min/day, during 5 days/week. All rats swam with a load of 3–5 % body mass attached to the trunk by a jacket. The exercise protocols were performed for 5 days/week during 8 weeks. This program is considered to be of moderate intensity [5].
Fig. 1.

The training of swimming in individual tanks at controlled temperature (T=30 °C)±2. It is possible to see the jacket attached to the trunk during the training session
The LLLT parameters are shown in Table 1. It was irradiated transcutaneously on the muscles of the rat's paw (one point on quadriceps and other point on gastrocnemius). The energy density of laser irradiation and the anatomical points were chosen based on previous studies [8]. We decided not to use sham group because we have a positive control (hypercaloric diet and treated with LLLT), a negative control (hypercaloric diet and not treated with LLLT), as well as positive (hypercaloric) and negative (normocaloric) controls for the factor diet, with or without laser treatment.
Table 1.
Characteristics of the laser used in the experimental procedures
| Type | Ga-Al-As | Treatment time | 47 s |
|---|---|---|---|
| Wavelength | 830 nm (infrared) | Number of points | 2 points |
| Frequency | Continuous wave (CW) | Total energy delivered | 9.4 J |
| Optical output | 100 mW | Application mode: probe held stationary in skin contact with a 90° angle and slight pressure. Used always after the training session. | |
| Spot diameter | 0.6 mm | ||
| Power density | 35.36 W/cm2 | ||
| Energy per point | 4.7 J/point | ||
| Energy density | 1,662 J/cm2 |
These are the characteristics of equipment and wavelength used during the study. All applications were realized to the same person (Theralase, DMC, Equipment, São Carlos, SP, Brazil)
The laser was applied after the exercises because the advantage of using the stress induced to get the maximum of absorption and effects on metabolism. It was used the same protocol before in different papers from our laboratory [17].
Experimental procedure
At the end of 8 weeks of training and after a 24-h rest period, analysis was performed. All animals were euthanized by decapitation. The collected blood was immediately centrifuged and frozen at −80 °C. The heart (H), liver (L), gastrocnemius muscle (GAST), soleus muscle (SOL), the white adipose tissues (epididymal (EPI) retroperitoneal (RET), visceral (VIS)), and the interscapular brown adipose tissue (BAT) were immediately removed, weighted, and frozen at −20 °C.
Hepatic and muscular glycogen and percentage of lipids of tissues
The muscle and liver glycogen was determined by colorimetric method, which assesses the concentration of glycosyl-glucose using a standard of 100 nmol of glucose and determined using a ultraviolet/visible spectrophotometer Biospectro® SP220 [18]. The percentage of lipid content in the tissue was determined by the gravimetric method [6].
Serum analysis
Total cholesterol (CHOL-total), triacylglycerol (TG), and high-density lipoprotein cholesterol (HDL-c) in the serum were determined enzymatically (Laborlab® kits) using a spectrophotometer [6]. The low-density lipoprotein (LDL-c) was calculated by Friedewald equation [19].
Statistical analysis
All data were expressed as a mean and standard deviation. The Kolmogorov–Smirnov test was used to analyze the normality. For statistical evaluation of the metabolic parameters, a one-way ANOVA test with post hoc analysis (Tukey–Kramer multiple comparisons) was used between groups. Instat 3.0 for Windows 7 (Graph Pad, San Diego, CA, USA, 1998) was used for the statistical analysis and the significance level was given as p<0.001, p<0.01, and p<0.05.
Results
Body mass and relative mass of tissues
Effects of type diet and exercise
The body mass gain differences are shown in Fig. 2 as the percentage of gain depending on diet used and treatments. High-fat diet promoted in the sedentary group an increase in EPI, RET, and BAT relative weight compared with as observed in the sedentary rats fed with chow diet group (SN), but the relative weight of liver, VIS, GAST, SOL, and heart was not significantly affected by this diet (Table 2). In the TH group, there was a significant reduction in relative weight of RET when compared with SH group. In the same comparison, the EPI declined 18 % in their relative weight. In contrast, no difference was observed in relative weight of tissues when comparing SN and TN groups (Table 2).
Fig. 2.
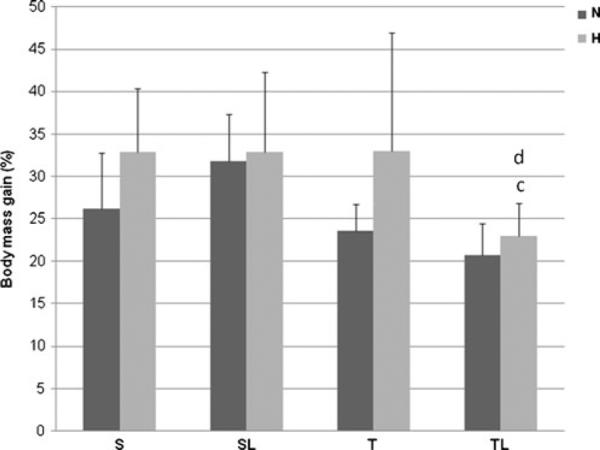
Values of weight gain in percentage of two series of animals: normocaloric diet (N) and hypercaloric diet (H). Different superscripts (a S versus SL; b S versus T; c SL versus TL; d T versus TL; e effects of high-fat diet in the different protocols) are significantly different (Tukey-Kramer multiple comparisons for p<0.05 except the specific comparisons: SHL×THL p<0.05, TH×THL p<0.05). The groups are designed: S sedentary; SL sedentary laser; T trained; TL trained laser
Table 2.
Relative mass of tissues in rat fed with normocaloric or hypercaloric diet (g/100 g of body weight)
| Mass of | SN | SNL | TN | TNL | SH | SHL | TH | THL |
|---|---|---|---|---|---|---|---|---|
| Heart | 0.28±0.10 | 0.35±0.02 | 0.36±0.01 | 0.38±0.04 | 0.33±0.03 | 0.33±0.02 | 0.35±0.04 | 0.36±0.02 |
| Liver | 2.66±0.29 | 2.52±0.34 | 2.28±0.32 | 2.54±0.20 | 2.28±0.32 | 2.54±0.20 | 2.28±0.18 | 2.48±0.12 |
| GAST | 0.45±0.10 | 0.49±0.03 | 0.55±0.03 | 0.52±0.04 | 0.49±0.03 | 0.47±0.01 | 0.52±0.02 | 0.51±0.02 |
| SOL | 0.04±0.00 | 0.04±0.00 | 0.04±0.01 | 0.04±0.00 | 0.04±0.00 | 0.04±0.00 | 0.04±0.00 | 0.04±0.00 |
| BAT | 0.07±0.02 | 0.06±0.01 | 0.09±0.01 | 0.09±0.02 | 0.11e±0.04 | 0.08±0.01 | 0.10±0.01 | 0.10±0.01 |
| EPI | 0.78±0.11 | 1.13a±0.26 | 0.79±0.12 | 0.67c±0.09 | 1.38e±0.23 | 1.47±0.07 | 1.13±0.14 | 1.29e±0.21 |
| RET | 0.74±0.16 | 1.37a±0.26 | 1.04±0.29 | 0.77c±0.17 | 2.09e±0.50 | 2.19e±0.37 | 1.47b±0.19 | 1.53ce±0.12 |
| VIS | 0.69±0.16 | 0.81±0.11 | 0.70±0.25 | 0.83±0.29 | 0.99±0.16 | 1.14±0.58 | 1.00±0.18 | 1.05±0.35 |
Values are expressed as mean±standard deviation (n=8/group). Different superscripts
S versus SL;
S versus T;
SL versus TL;
T versus TL;
effects of hypercaloric diet in the different protocols are significantly different (Tukey–Kramer multiple comparisons for p <0.001 except the specific comparisons: Brown adipose tissue, SC × SH p<0.05; epididymal, SN × SNL p<0.05; retroperitoneal, SH × TH and TH × THL p<0.05.
The groups are designed: SN sedentary normocaloric diet; SNL sedentary normocaloric diet laser; TN trained normocaloric diet; TNL trained normocaloric diet laser; SH sedentary hypercaloric diet; SHL sedentary hypercaloric diet laser; TH trained hypercaloric diet; THL trained hypercaloric diet laser. The variables are the mass for: heart; liver; GAST gastrocnemius muscle; SOL soleus muscle; BAT brown Adipose Tissue; EPI: Epididymal adipose tissue; RET: Retroperitoneal adipose tissue; VIS Visceral adipose tissue
Effects of LLLT combined with diet and exercise
In the LLLT sedentary groups (SNL and SHL), the relative weight of EPI was greater than respective controls (SN and SH). In the SCL group, the relative weight of RET also showed an increase compared with the SN group (Table 2). Exercise associated with LLLT promoted a significant reduction in the relative weight of adipose tissues (EPI, RET) in rats fed with chow diet when compared with the sedentary (SNL). In the THL group, EPI declined 12 % in relative weight, and the relative weight of RET was lower than SHL group (Table 2). In the other tissues, no difference was observed in the relative weight in SHL, SNL, THL, and TNL groups (Table 2).
Glycogen content and percentage of lipid in the tissues
Effects of type diet and exercise
The glycogen content in liver, GAST, and SOL did not differ significantly across sedentary and exercise groups during the experimental period when compared with respective control groups (Table 3). Nevertheless, exercise increased the glycogen content in the liver of the groups TN and TH by 32 and 28 %, respectively. In the TH group, the SOL glycogen content was 40 % smaller when compared with SH group (Table 3). High-fat diet promoted a significant increase in fatty liver of the sedentary rats. On the other hand, exercise promoted a significant reduction in the fat content in liver in TH group. No significant differences occurred in lipid content in GAST in the SN, SH, TN, and TH groups.
Table 3.
Glycogen hepatic/muscle (μml/g) and percentage of fat in the tissues of rats fed with normocaloric or hypercaloric diet
| SN | SNL | TN | TNL | SH | SHL | TH | THL | |
|---|---|---|---|---|---|---|---|---|
| Glycogen content | ||||||||
| Liver | 0.80±0.41 | 0.87±0.23 | 1.06±0.42 | 1.04±0.23 | 0.78±0.13 | 0.87±0.13 | 1.00±0.08 | 1.68cde±0.34 |
| GAST | 0.29±0.03 | 0.31 ±0.02 | 0.31 ±0.02 | 0.28±0.03 | 0.30±0.02 | 0.39ea±0.06 | 0.29±0.03 | 0.30c±0.02 |
| SOL | 0.38±0.09 | 0.35±0.16 | 0.36±0.05 | 0.35 ±0.08 | 0.37±0.07 | 0.38±0.14 | 0.22±0.04 | 0.57d±0.17 |
| Percentage of fat | ||||||||
| Liver | 1.43±0.26 | 1.56±0.23 | 1.67±0.17 | 1.63 ±0.32 | 2.74e±1.39 | 2.74e±0.43 | 1.47b±0.25 | 1.87±0.32 |
| Gast | 0.36±0.11 | 0.37±0.11 | 0.34±0.07 | 0.37±0.06 | 0.43±0.10 | 0.43±0.15 | 0.39±0.10 | 0.37±0.09 |
Values are expressed as mean± standard deviation (n=8/group). The differences are highlighted in italics. Different superscripts
S versus SL;
S versus T;
SL versus TL;
T versus TL;
effects of hypercaloric diet in the different protocols are significantly different (Tukey–Kramer multiple comparisons for p<0.001 except the specific comparisons for glycogen: liver, TH × THL p<0.01 and TNL × THL p<0.05; gastrocnemius muscle, SNL × SHL p<0.01 and SHL × THL p<0.01; for percentage of fat: liver, SN SH p<0.01, SNL × SHL p<0.05 and SH × TH p<0.01).
The groups are designed: SN sedentary normocaloric; SNL sedentary normocaloric laser; TN trained normocaloric; TNL trained normocaloric laser; SH sedentary hypercaloric; SHL sedentary hypercaloric laser; TH trained hypercaloric; THL trained hypercaloric laser. The variables are: GAST gastrocnemius muscle; SOL soleus muscle
Effects of LLLT combined with diet and exercise
In rats fed with chow diet, LLLT did not promote changes in the glycogen and lipid content in the tissues. The same effect was observed in the fat content of the gastrocnemius muscle in SHL and THL groups (Table 3). SHL showed higher GAST glycogen content when compared with SNL and SH groups. Exercise and LLLT in rats fed with high-fat diet showed higher glycogen content in the liver when compared with SHL, TH and TNL groups. Exercise and LLLT, in rats fed with high-fat diet, also promoted an increase in glycogen content in SOL when compared with the TH group. On the other hand, in the GAST, exercise and LLLT promoted a significant decrease in glycogen content when compared with its respective control group (SHL) (Table 3). Fatty liver in THL was 32 % smaller when compared with the SHL group.
Lipid profile
Effects of type diet and exercise
The consumption of a high-fat diet, compared to a chow diet, in sedentary rats promoted an increase in the total amount of plasma cholesterol (CHOL-total), TG and low density lipoprotein cholesterol (LDL-c). HDL-c concentrations did not show a statistically significant difference. On the other hand, exercise promoted a significant reduction in the CHOL-total, TG, HDL-c, and LDL-c concentrations in both diets (Fig. 3a–d).
Fig. 3.
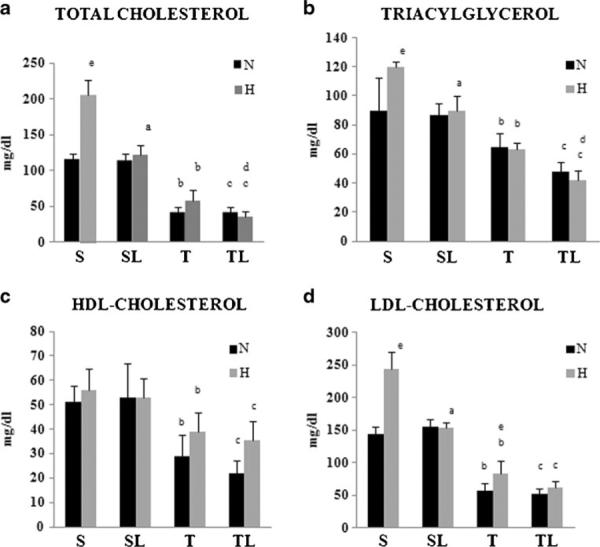
Blood lipid analysis. C denotes normocaloric diet and H denotes hypercaloric diet. The groups are designated: S sedentary; SL sedentary laser; T trained; TL trained laser. Values are expressed as mean±standard deviation (n=8/group). Different superscripts (a S versus SL; b S versus T; c SL versus TL; d T versus TL; e effects of hypercaloric diet in the different protocols) are significantly different (Tukey–Kramer multiple comparisons for p<0.001 except the specific comparisons for cholesterol total: TH×THL p<0.05; for triglycerides: SN×TN p<0.01; and TH×THL p<0.01; for HDL-cholesterol: SN×TN p< 0.01, SHL×THL p<0.05 and SH×TH p<0.05; LDL-cholesterol: TN×TH p<0.05). a Total cholesterol. b Triacylglycerol. c HDL-cholesterol. d LDL-cholesterol
Effects of LLLT combined with diet and exercise
No difference was observed in this parameter in the group SLC. However, in the sedentary rats fed with high-fat diet (SHL), LLLT promoted a significant decrease in CHOL-total, TG, and LDL-c concentrations when compared with SH group. HDL-c concentrations do not change in the same comparison. Exercise and LLLT in both diets promoted a significant reduction in CHOL-total, TG, LDL-c, and HDL-c concentrations when compared with the respective control groups. THL in rats fed with high-fat diet also showed a significant decrease in serum cholesterol and TG concentrations compared with the TH group (Fig. 3a–d).
Discussion
This is the first randomized controlled experimental study evaluating effects of combined LLLT and training exercise on lipid pathways. We found a decreased lipid profile, and these results suggest that this LLLT with exercise training as a new alternative for dyslipidemic control.
Recent research has discussed the effects of high-fat diet consumption on fatty liver, intramuscular fat, lipid profile, glycogen concentration in the muscle or liver, and their relation to the development of chronic diseases and obesity [20, 21]. Moreover, there is recent interest in the role of physical exercise combined with LLLT as an adjunct approach to reduce the adverse effects of high-fat diet and sedentary life-style. However, the intensity, frequency, and duration of the exercise and the kind of diet promote different metabolic adaptations [15, 22, 23]. It is questionable whether all models of physical exercise have the same beneficial effects on adiposity, fatty liver, lipid profile, and glycogen concentration. Several studies have reported that moderate swimming exercise in animals (90 min) promotes reduction in adiposity, fatty liver, and increase in glycogen concentration and improvement in lipid profile [2, 5, 24–26].
In relation to the high-fat diet (SH group), experimental studies demonstrated that the intake of this diet was related to the dyslipidemic profile and was observed in our results by an increase in total cholesterol, TG, and LDL-c concentrations (see Fig. 3a–d)[5, 6, 14, 25]. Besides, due to the higher energy content of the high-fat diet compared with the chow diet, this diet induced increases in adiposity and fatty liver [2, 5, 6]. These alterations were observed in the present study (see Table 2 and 3).
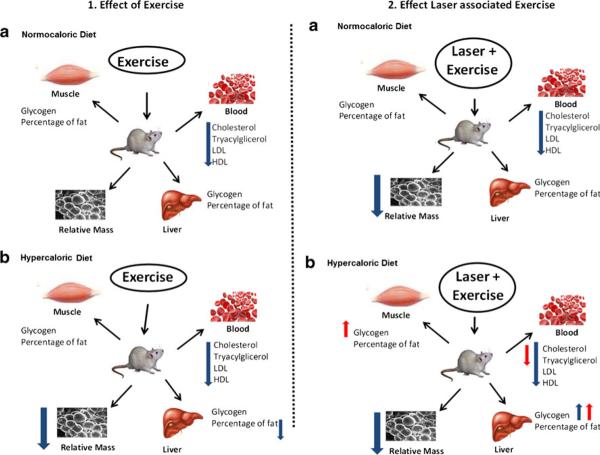
A high-fat diet has been associated with a decrease in the rate of glycolysis and glycogen synthesis, leading to a lower content of glycogen in tissues [15, 22, 23]. However, this fact was not observed in our results when the glycogen content in the muscle and liver did not differ significantly from the control group (see Table 3). Several studies have reported that moderate exercise promotes reduction in body adiposity, dyslipidemia and fatty liver in rats [5, 24–26]. It has been reported that high-fat diets result in an increase in lipid oxidation during exercise [27]. It is known that both increased lipolysis and the consequent increase in plasma fatty acids during exercise facilitate this change [28]. However, these same two factors act in an opposite direction with respect to the metabolism of carbohydrates. Physical training increases the glycogen content stored, while the high-fat diet has been associated with a decreased rate of glycolysis and glycogen synthesis [29]. Thus, our finding agrees with that those found in the literature demonstrated by improvement in lipid profile in trained groups (see Fig. 3a, b), reduction in relative weight of EPI, RET (see Table 2), and fatty liver and glycogen content (see Table 3). In Fig. 4 we give a summary of our key findings related to kind of diet and training.
Fig. 4.
The summary of key findings related to kind of diet and training. The blue arrows showed the comparisons control groups×trained and the red arrows the comparisons with trained groups×trained and laser groups. The comparison with laser effects associated to exercise for hypercaloric groups showed a red arrow and a blue arrow for the variables of blood. The red arrow includes the variables cholesterol, and tryacylglicerol as the blue arrow includes all variables
It is known that exercise improves the lipid profile and lipid metabolism. However, the effect of LLLT on the metabolic activity is not yet established. Jackson et al. [12] performed a noncontrolled and nonrandomized pilot clinical study that investigated the effects of the LLLT (635 nm) on lipid parameters. The individuals were able to maintain a regular diet and exercise regimen during the study. The LLLT was applied around the patient abdomen (five independent diode lasers with power output of 17 mW each was applied for 20 min leading to 6.6 J/cm2 fluence) during 2 weeks (three sessions per week with duration of 20 min each) with the subject at rest. Their results showed a significant reduction in cholesterol and triglyceride levels.
In a similar, but this time-controlled and randomized study, Rushdi [30] showed that LLLT (four laser pads with 38 diodes laser at each pad, 650–660 nm and 1.3 W) of total energy applied on the abdomen, for 55 min, two times per week for 2 weeks, could reduce cholesterol and triglyceride levels as well as reduce LDL levels while preserving HDL levels.
The hypothesis proposed by authors [30, 31] was that the LLLT could alter the mitochondrial membrane potential and the intracellular redox state with a resultant increase in ADP-ATP exchange rate. These mitochondrial changes may suppress cholesterologenesis by altering the transcription factors responsible for the expression of essential genes involved in the biosynthetic process.
We agree partly with this hypothesis. Our results showed that the LLLT did not suppress cholesterol synthesis, but caused a redirection of serum lipids to fat reserves (in sedentary conditions) and an improved supply of substrate for energy expenditure (in trained conditions). In addition, we believe that the exercise training combined with LLLT could have increased mitochondrial metabolism, as well as increased mitochondrial number and/or caused the fusion of smaller mitochondria to form giant mitochondria [32]. These effects could increase physical performance [7, 33–35].
Cytochrome c oxidase is unit IV of the electron transport chain of mitochondria and is also a chromophore for LLLT. It had been speculated that LLLT increases the rate at which cytochrome c oxidase transfers electrons and could cause the reduction of the catalytic center of cytochrome c oxidase, thus making more electrons available for the reduction of dioxygen [36, 37]. This mechanism of action of LLLT causes an increase in the electron and proton transfer, an increased quantity of ATP, and an initially increased production of reactive oxygen species (ROS). Elevated ROS concentration increases the lipid peroxidation, and this event occurs where ROS reacts with lipids found within cell membranes, temporarily damaging them [38]. Transitory pores created on adipocytes' membrane have been shown on several studies through scanning electron microscopy and transmission electron microscopy [39]. In addition to this, when irradiated adipocytes were cultured, they were shown to be able to recover to their original cell membrane structure and remain alive or viable.
A controlled and randomized trial showed that LLLT combined with aerobic or strength training in humans had long-lasting effects with improvement of muscle performance over three months [7]. Leal Junior et al. [33] and De Marchi et al. [7] showed that LLLT applied before exercise had acute effects with reduction of blood lactate, creatine kinase, and C-reactive protein levels with accelerated postexercise recovery in athletes, and showed that inflammation was reduced.
There is an extensive literature showing a high correlation between obesity and inflammatory activity [40]. Many of these papers correlate various adipokines as responsible for this pathophysiologic state [41]. Adiponectin is one of the adipokines that is responsible for response to exercise, leading to upregulation of its receptors, apparently related to increased mitochondrial metabolism [42]. It is a hypothesis that may explain how LLLT interacts with mitochondria, especially when combined with exercise [43]. LLLT is known to have a modulatory effect on inflammation, which could in turn affect the action of adiponectin on fat metabolism.
Several studies have demonstrated that LLLT alters cyclic adenosine monophosphate (cAMP) levels [44]. One mechanism to explain reduction in fat levels through the action of LLLT is that the adipocyte membrane is activated by raised cAMP concentrations that stimulate, in turn, cytoplasmic lipase that triggers the conversion of triglycerides into fatty acids and glycerol, both elements that can easily pass through the cell membrane. On the other hand, epinephrine is known to exert antilopolytic effects through its action on adrenergic receptors via increasing cAMP levels [45]. In addition to this, variations in types of adrenergic receptors and adrenergic receptor sensitivity on adipocytes of the abdominal and femoral regions in both males and females have been previously reported [39]. Based on these findings, it can be speculated that LLLT through increasing levels of cAMP might have enhancing effects on lipolysis and different amounts of fat reduction in different regions in the body might be explained by this hypothesis, which further confirms our results that showed variations in fat reduction among different regions.
Results of lipid profile can be changed by alteration in dietary habits and when patients perform exercise training. However, the studies discussed [30, 31] did not measure the aerobic fitness and dietary variables. In this context, our study is important because there was control of both diet and training.
Thus LLLT can improve pathways of energetic metabolism, mainly lipid metabolism, potentiating the effects of LLLT and, when combined with exercise of moderated intensity, could be used as a new approach to control dyslipidemia and consequently have a role in treatment of diseases related to dyslipidemia and obesity [2, 15]. The summary of our key findings of this study is shown in Fig. 4.
Acknowledgments
We are grateful to Dr. Pinar Avci for the interesting discussion about the mechanisms involved in the lipid metabolism.
References
- 1.Tock L, Prado WL, Caranti DA, Cristofalo DM, Lederman H, Fisberg M, Siqueira KO, Stella SG, Antunes HK, Cintra IP, Tufik S, de Mello MT, Damaso AR. Nonalcoholic fatty liver disease decrease in obese adolescents after multidisciplinary therapy. Eur J Gastroenterol Hepatol. 2006;18(12):1241–1245. doi: 10.1097/01.meg.0000243872.86949.95. doi:10.1097/01.meg.0000243872.86949.95 00042737-200612000-00001 [pii] [DOI] [PubMed] [Google Scholar]
- 2.Gauthier MS, Couturier K, Latour JG, Lavoie JM. Concurrent exercise prevents high-fat-diet-induced macrovesicular hepatic steatosis. J Appl Physiol. 2003;94(6):2127–2134. doi: 10.1152/japplphysiol.01164.2002. doi:10.1152/japplphysiol.01164.2002.01164.2002 [pii] [DOI] [PubMed] [Google Scholar]
- 3.Linsel-Nitschke P, Tall AR. HDL as a target in the treatment of atherosclerotic cardiovascular disease. Nat Rev Drug Discov. 2005;4(3):193–205. doi: 10.1038/nrd1658. doi:10.1038/nrd1658. [DOI] [PubMed] [Google Scholar]
- 4.Pappachan JM, Chacko EC, Arunagirinathan G, Sriraman R. Management of hypertension and diabetes in obesity: non-pharmacological measures. Int J Hypertens. 2011;2011:398065. doi: 10.4061/2011/398065. doi:10.4061/2011/398065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sene-Fiorese M, Duarte FO, Scarmagnani FR, Cheik NC, Manzoni MS, Nonaka KO, Rossi EA, de Oliveira Duarte AC, Damaso AR. Efficiency of intermittent exercise on adiposity and fatty liver in rats fed with high-fat diet. Obesity (Silver Spring) 2008;16(10):2217–2222. doi: 10.1038/oby.2008.339. doi:oby2008339 [pii] 10.1038/oby.2008.339. [DOI] [PubMed] [Google Scholar]
- 6.Duarte FO, Sene-Fiorese M, Manzoni MS, de Freitas LF, Cheik NC, de Oliveira G, Duarte AC, Nonaka KO, Damaso A. Caloric restriction and refeeding promoted different metabolic effects in fat depots and impaired dyslipidemic profile in rats. Nutrition. 2008;24(2):177–186. doi: 10.1016/j.nut.2007.10.012. doi:S0899-9007(07)00318-8 [pii] 10.1016/j.nut.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 7.De Marchi T, Leal EC, Junior, Bortoli C, Tomazoni SS, Lopes-Martins RA, Salvador M. Low-level laser therapy (LLLT) in human progressive-intensity running: effects on exercise performance, skeletal muscle status, and oxidative stress. Lasers Med Sci. 2012;27(1):231–236. doi: 10.1007/s10103-011-0955-5. doi:10.1007/s10103-011-0955-5. [DOI] [PubMed] [Google Scholar]
- 8.Ferraresi C, de Brito OT, de Oliveira ZL, de Menezes Reiff RB, Baldissera V, de Andrade Perez SE, Matheucci E, Junior, Parizotto NA. Effects of low level laser therapy (808 nm) on physical strength training in humans. Lasers Med Sci. 2011;26(3):349–358. doi: 10.1007/s10103-010-0855-0. doi:10.1007/s10103-010-0855-0. [DOI] [PubMed] [Google Scholar]
- 9.Paolillo FR, Milan JC, Aniceto IV, Barreto SG, Rebelatto JR, Borghi-Silva A, Parizotto NA, Kurachi C, Bagnato VS. Effects of infrared-LED illumination applied during high-intensity treadmill training in postmenopausal women. Photomed Laser Surg. 2011;29(9):639–645. doi: 10.1089/pho.2010.2961. doi:10.1089/pho.2010.2961. [DOI] [PubMed] [Google Scholar]
- 10.Karu TI, Pyatibrat LV, Afanasyeva NI. Cellular effects of low power laser therapy can be mediated by nitric oxide. Lasers Surg Med. 2005;36(4):307–314. doi: 10.1002/lsm.20148. doi:10.1002/lsm.20148. [DOI] [PubMed] [Google Scholar]
- 11.Huang YY, Sharma SK, Carroll J, Hamblin MR. Biphasic dose response in low level light therapy—an update. Dose-Response. 2011;9(4):602–618. doi: 10.2203/dose-response.11-009.Hamblin. doi:10.2203/dose-response.11-009.Hamblin drp-09-602 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jackson RF, Dedo DD, Roche GC, Turok DI, Maloney RJ. Low-level laser therapy as a non-invasive approach for body contouring: a randomized, controlled study. Lasers Surg Med. 2009;41(10):799–809. doi: 10.1002/lsm.20855. doi:10.1002/lsm.20855. [DOI] [PubMed] [Google Scholar]
- 13.Jackson RF, Stern FA, Neira R, Ortiz-Neira CL, Maloney J. Application of low-level laser therapy for noninvasive body contouring. Lasers Surg Med. 2012;44(3):211–217. doi: 10.1002/lsm.22007. doi:10.1002/lsm.22007. [DOI] [PubMed] [Google Scholar]
- 14.Duarte FO, Sene-Fiorese M, Cheik NC, Maria AS, de Aquino AE, Jr, Oishi JC, Rossi EA, de Oliveira Duarte AC G, Damaso AR. Food restriction and refeeding induces changes in lipid pathways and fat deposition in the adipose and hepatic tissues in rats with diet-induced obesity. Exp Physiol. 2012;97(7):882–894. doi: 10.1113/expphysiol.2011.064121. doi: doi:10.1113/expphysiol.2011.064121. [DOI] [PubMed] [Google Scholar]
- 15.Horowitz JF. Fatty acid mobilization from adipose tissue during exercise. Trends Endocrinol Metab. 2003;14(8):386–392. doi: 10.1016/s1043-2760(03)00143-7. doi:Doi 10.1016/S1043-2760(03)00143-7. [DOI] [PubMed] [Google Scholar]
- 16.Caruso-Davis MK, Guillot TS, Podichetty VK, Mashtalir N, Dhurandhar NV, Dubuisson O, Yu Y, Greenway FL. Efficacy of low-level laser therapy for body contouring and spot fat reduction. Obes Surg. 2011;21(6):722–729. doi: 10.1007/s11695-010-0126-y. doi:10.1007/s11695-010-0126-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vieira WH, Ferraresi C, Perez SE, Baldissera V, Parizotto NA. Effects of low-level laser therapy (808 nm) on isokinetic muscle performance of young women submitted to endurance training: a randomized controlled clinical trial. Lasers Med Sci. 2012;27(2):497–504. doi: 10.1007/s10103-011-0984-0. doi:10.1007/s10103-011-0984-0. [DOI] [PubMed] [Google Scholar]
- 18.Moraes G, Altran AE, Avilez IM, Barbosa CC, Bidinotto PM. Metabolic adjustments during semi-aestivation of the marble swamp eel (Synbranchus marmoratus, Bloch 1795)—a facultative air breathing fish. Braz J Biol. 2005;65(2):305–312. doi: 10.1590/s1519-69842005000200015. doi:S1519-69842005000200015 [pii] [DOI] [PubMed] [Google Scholar]
- 19.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 20.Marchesini G, Bugianesi E, Forlani G, Cerrelli F, Lenzi M, Manini R, Natale S, Vanni E, Villanova N, Melchionda N, Rizzetto M. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 2003;37(4):917–923. doi: 10.1053/jhep.2003.50161. doi:10.1053/jhep. 2003.50161 S0270913903001216 [pii] [DOI] [PubMed] [Google Scholar]
- 21.Liao CC, Su TC, Chien KL, Wang JK, Chiang CC, Lin CC, Lin RS, Lee YT, Sung FC. Elevated blood pressure, obesity, and hyperlipidemia. J Pediatr. 2009;155(1):79–83. 83, e71. doi: 10.1016/j.jpeds.2009.01.036. doi:doi:10.1016/j.jpeds.2009.01.036. [DOI] [PubMed] [Google Scholar]
- 22.Ruby BC, Robergs RA. Gender differences in substrate utilization during exercise. Sports Med. 1994;17(6):393–410. doi: 10.2165/00007256-199417060-00005. [DOI] [PubMed] [Google Scholar]
- 23.Even PC, Rieth N, Roseau S, Larue-Achagiotis C. Substrate oxidation during exercise in the rat cannot fully account for training-induced changes in macronutrients selection. Metabolism. 1998;47(7):777–782. doi: 10.1016/s0026-0495(98)90111-1. doi:S0026-0495(98)90111-1 [pii] [DOI] [PubMed] [Google Scholar]
- 24.Schrauwen P, Westerterp KR. The role of high-fat diets and physical activity in the regulation of body weight. Br J Nutr. 2000;84(4):417–427. doi: 10.1017/s0007114500001720. doi:S0007114500001720 [pii] [DOI] [PubMed] [Google Scholar]
- 25.Do Nascimento CMO, Estadella D, Oyama LM, Damaso AR, Ribeiro EB. Effect of palatable hyperlipidic diet on lipid metabolism of sedentary and exercised rats. Nutrition. 2004;20(2):218–224. doi: 10.1016/j.nut.2003.10.008. doi:DOI 10.1016/j.nut.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 26.Novelli ELB, Burneiko RCM, Diniz YS, Galhardi CM, Rodrigues HG, Ebaid GMX, Faine LA, Padovani CR, Cicogna AC. Interaction of hypercaloric diet and physical exercise on lipid profile, oxidative stress and antioxidant defenses. Food Chem Toxicol. 2006;44(7):1167–1172. doi: 10.1016/j.fct.2006.01.004. doi:DOI 10.1016/j.fct.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 27.Helge JW, Watt PW, Richter EA, Rennie MJ, Kiens B. Fat utilization during exercise: adaptation to a fat-rich diet increases utilization of plasma fatty acids and very low density lipoproteintriacylglycerol in humans. J Physiol-London. 2001;537(3):1009–1020. doi: 10.1111/j.1469-7793.2001.01009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Helge JW. Long-term fat diet adaptation effects on performance, training capacity, and fat utilization. Med Sci Sport Exer. 2002;34(9):1499–1504. doi: 10.1097/00005768-200209000-00016. doi:Doi 10.1249/01.Mss.0000027691.95763.B5. [DOI] [PubMed] [Google Scholar]
- 29.Lee KU, Kim CH, Youn JH, Park JY, Hong SK, Park KS, Park SW, Suh KI. Effects of high-fat diet and exercise training on intracellular glucose metabolism in rats. Am J Physiol-Endoc M. 2000;278(6):E977–E984. doi: 10.1152/ajpendo.2000.278.6.E977. [DOI] [PubMed] [Google Scholar]
- 30.Rushdi TA. Effect of low-level laser therapy on cholesterol and triglyceride serum levels in icu patients: a controlled, randomized study. EJCTA. 2010;4(2):5. [Google Scholar]
- 31.Jackson RF, Roche GC, Wisler K. Reduction in cholesterol and triglyceride serum levels following low-level laser irradiation: a noncontrolled, nonrandomized pilot study. Am J Cosmet Surg. 2010;27(4):177–184. [Google Scholar]
- 32.Bakeeva LE, Manteifel VM, Rodichev EB, Karu TI. [Formation of gigantic mitochondria in human blood lymphocytes under the effect of an He-Ne laser] Mol Biol (Mosk) 1993;27(3):608–617. [PubMed] [Google Scholar]
- 33.Leal EC, Junior, Lopes-Martins RA, Baroni BM, De Marchi T, Taufer D, Manfro DS, Rech M, Danna V, Grosselli D, Generosi RA, Marcos RL, Ramos L, Bjordal JM. Effect of 830 nm low-level laser therapy applied before high-intensity exercises on skeletal muscle recovery in athletes. Lasers Med Sci. 2009;24(6):857–863. doi: 10.1007/s10103-008-0633-4. doi:10.1007/s10103-008-0633-4. [DOI] [PubMed] [Google Scholar]
- 34.Sussai DA, Carvalho Pde T, Dourado DM, Belchior AC, dos Reis FA, Pereira DM. Low-level laser therapy attenuates creatine kinase levels and apoptosis during forced swimming in rats. Lasers Med Sci. 2010;25(1):115–120. doi: 10.1007/s10103-009-0697-9. doi:10.1007/s10103-009-0697-9. [DOI] [PubMed] [Google Scholar]
- 35.Paolillo FR, Corazza AV, Borghi-Silva A, Parizotto NA, Kurachi C, Bagnato VS. Infrared LED irradiation applied during high-intensity treadmill training improves maximal exercise tolerance in postmenopausal women: a 6-month longitudinal study. Lasers Med Sci. 2012 doi: 10.1007/s10103-012-1062-y. doi:10.1007/s10103-012-1062-y. [DOI] [PubMed] [Google Scholar]
- 36.Brunori M, Giuffre A, Sarti P. Cytochrome c oxidase, ligands and electrons. J Inorg Biochem. 2005;99(1):324–336. doi: 10.1016/j.jinorgbio.2004.10.011. doi: S0162-0134(04)00316-2 [pii] 10.1016/j.jinorgbio.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 37.Chen CH, Hung HS, Hsu SH. Low-energy laser irradiation increases endothelial cell proliferation, migration, and eNOS gene expression possibly via PI3K signal pathway. Lasers Surg Med. 2008;40(1):46–54. doi: 10.1002/lsm.20589. doi:10.1002/lsm.20589. [DOI] [PubMed] [Google Scholar]
- 38.Geiger PG, Korytowski W, Girotti AW. Photodynamically generated 3-beta-hydroxy-5 alpha-cholest-6-ene-5- hydroperoxide: toxic reactivity in membranes and susceptibility to enzymatic detoxification. Photochem Photobiol. 1995;62(3):580–587. doi: 10.1111/j.1751-1097.1995.tb02388.x. [DOI] [PubMed] [Google Scholar]
- 39.Neira R, Arroyave J, Ramirez H, Ortiz CL, Solarte E, Sequeda F, Gutierrez MI. Fat liquefaction: effect of low-level laser energy on adipose tissue. Plast Reconstr Surg. 2002;110(3):912–922. doi: 10.1097/00006534-200209010-00030. discussion 923-915. [DOI] [PubMed] [Google Scholar]
- 40.Athyros VG, Tziomalos K, Karagiannis A, Anagnostis P, Mikhailidis DP. Should adipokines be considered in the choice of the treatment of obesity-related health problems? Curr Drug Targets. 2010;11(1):122–135. doi: 10.2174/138945010790030992. [DOI] [PubMed] [Google Scholar]
- 41.Koh EH, Park JY, Park HS, Jeon MJ, Ryu JW, Kim M, Kim SY, Kim MS, Kim SW, Park IS, Youn JH, Lee KU. Essential role of mitochondrial function in adiponectin synthesis in adipocytes. Diabetes. 2007;56(12):2973–2981. doi: 10.2337/db07-0510. doi:db07-0510 [pii] 10.2337/db07-0510. [DOI] [PubMed] [Google Scholar]
- 42.Huang H, Iida KT, Sone H, Yokoo T, Yamada N, Ajisaka R. The effect of exercise training on adiponectin receptor expression in KKAy obese/diabetic mice. J Endocrinol. 2006;189(3):643–653. doi: 10.1677/joe.1.06630. doi:189/3/643 [pii] 10.1677/joe.1.06630. [DOI] [PubMed] [Google Scholar]
- 43.de Almeida P, Lopes-Martins RA, Tomazoni SS, Silva JA, Jr, de Carvalho PT, Bjordal JM, Leal EC., Junior Low-level laser therapy improves skeletal muscle performance, decreases skeletal muscle damage and modulates mRNA expression of COX-1 and COX-2 in a dose-dependent manner. Photochem Photobiol. 2011;87(5):1159–1163. doi: 10.1111/j.1751-1097.2011.00968.x. doi:10.1111/j.1751-1097.2011.00968.x. [DOI] [PubMed] [Google Scholar]
- 44.Franco W, Leite RS, Parizotto NA. Effects of low intensity infrared laser radiation on the water transport in the isolated toad urinary bladder. Lasers Surg Med. 2003;32(4):299–304. doi: 10.1002/lsm.10166. doi:10.1002/lsm.10166. [DOI] [PubMed] [Google Scholar]
- 45.Khan MH, Victor F, Rao B, Sadick NS. Treatment of cellulite: part II. advances and controversies. J Am Acad Dermatol. 2010;62(3):373–384. doi: 10.1016/j.jaad.2009.10.041. doi:doi:10.1016/j.jaad.2009.10.041, quiz 385-376. [DOI] [PubMed] [Google Scholar]