Abstract
The organ of Corti in the mammalian inner ear is comprised of mechanosensory hair cells (HCs) and nonsensory supporting cells (SCs), both of which are believed to be terminally postmitotic beyond late embryonic ages. Consequently, regeneration of HCs and SCs does not occur naturally in the adult mammalian cochlea, though recent evidence suggests that these cells may not be completely or irreversibly quiescent in at earlier postnatal ages. Furthermore, regenerative processes can be induced by genetic and pharmacological manipulations, but, more and more reports suggest that regenerative potential declines as the organ of Corti continues to age. In numerous mammalian systems, such effects of aging on regenerative potential are well established. However, in the cochlea, the problem of regeneration has not been traditionally viewed as one of aging. This is an important consideration as current models are unable to elicit widespread regeneration or full recovery of function at adult ages yet regenerative therapies will need to be developed specifically for adult populations. Still, the advent of gene targeting and other genetic manipulations has established mice as critically important models for the study of cochlear development and HC regeneration and suggests that auditory HC regeneration in adult mammals may indeed be possible. Thus, this review will focus on the pursuit of regeneration in the postnatal and adult mouse cochlea and highlight processes that occur during postnatal development, maturation, and aging that could contribute to an age-related decline in regenerative potential. Second, we will draw upon the wealth of knowledge pertaining to age related senescence in tissues outside of the ear to synthesize new insights and potentially guide future research aimed at promoting HC regeneration in the adult cochlea.
Keywords: hair cell, stem cell, proliferation, regeneration, senescence, quiescence
1. Introduction
Hearing in mammals is dependent upon transduction of sound in the organ of Corti, a highly specialized structure within the cochlea of the inner ear. The organ of Corti is primarily comprised of two main cell types: sensory hair cells (HCs) and nonsensory supporting cells (SCs), both of which are believed to arise from the same pool of precursor cells during development, and therefore represent potential targets for HC regeneration (Dabdoub et al., 2008; Fekete et al., 1998). While the vast majority of HCs and SCs are formed during embryonic development in the mouse cochlea (Lee et al., 2006; Ruben, 1967), the organ of Corti continues to undergo processes of development, maturation, and aging well after birth. Although questions of cochlear aging have typically focused on age-related hearing loss (Bovo et al., 2011; Fetoni et al., 2011) and the declining success of cochlear implants with recipient age (Lee et al., 2012; McRackan et al., 2012; Nakashima et al., 2012; Noble et al., 2009), recent advances in our understanding of postnatal cochlear development suggest that increasing attention should be paid to postnatal aging and its effects on preventing regeneration. In numerous mammalian systems, such effects of aging to gradually diminish regenerative potential are well established (Chien et al., 2005; Cnop et al., 2011; Luo et al., 2006; Timchenko, 2009), though in the auditory epithelium of the cochlea, aging has not traditionally been considered in this context. This is most likely due to the fact that terminal mitosis of sensory precursors is believed to be a nearly singular event that takes place during embryonic development (Lee et al., 2006; Ruben, 1967). However, several recent studies suggest that HCs and SCs in the postnatal rodent cochlea undergo endogenous and ectopically induced processes of proliferation and differentiation, though many of these studies also suggest that the ability of cells within the organ of Corti to re-enter the cell cycle or to give rise to new HCs declines with postnatal age. This is an important consideration as the more well known effects of aging and hearing loss suggest that most regenerative therapies will need to be developed specifically for adult populations, and, even though new genetic tools and mutant models have led to many advances in HC regeneration, the ectopic activation of proliferation or differentiation in quiescent cochlear cells has yet to yield sufficient numbers of new HCs as to constitute functional recovery in an adult cochlea. Still, the advent of gene targeting has established mice as critically important models for the study of cochlear development and HC regeneration, and several of these models suggest that HC regeneration and recovery of hearing function may indeed be possible, but are currently limited by aging. This review aims to identify factors that could contribute to an age-related decline in regenerative potential by describing known effects of postnatal aging in the rodent cochlea, but also by examining tissues outside of the ear to synthesize new insights and potentially guide future research aimed at promoting HC regeneration in the adult organ of Corti.
2. Permanent cell cycle exit during the development of inner ear sensory epithelia: a gradually shifting paradigm
Since 1967, it has become widely accepted that HC and SC precursors in the mouse cochlea permanently exit the cell cycle just prior to their differentiation during embryonic development (Ruben, 1967). From this, it has been supposed that such early quiescence directly translates into an inability of HCs and SCs to regenerate beyond such point in time. However, studies from non-mammalian models show that SCs in juvenile and adult animals can proliferate and differentiate to give rise to new HCs suggesting that SC quiescence does not necessarily preclude HC regeneration (Brignull et al., 2009). Indeed, studies of the balance organs of mice and other mammals have shifted our understanding of HC regeneration, demonstrating that, even in mammals, cell proliferation can persist postnatally, new HCs may continue to be added, and some regenerative ability may be maintained into adulthood (Burns et al., 2012a; Burns et al., 2012b; Forge et al., 1993; Kawamoto et al., 2009; Li et al., 1997; Warchol et al., 1993). While mammalian cochlear cells are still largely thought to be excluded from any such proliferative or regenerative processes, recent evidence, primarily from mouse models, suggests another paradigm shift, where cochlear HCs and SCs exhibit signs of proliferation and differentiation, and yield new HCs at various postnatal ages.
Some of the first evidence for regenerative potential at late embryonic and neonatal ages came from explant cultures of rat (Lefebvre et al., 1993) and mouse cochleae (Kelley et al., 1995) where it was shown that HCs could be regenerated by both mitotic and non-mitotic processes. However, an inability to recapitulate definitive HC regeneration in vivo (Lenoir et al., 1997; Parietti et al., 1998) cast doubt on the true regenerative potential of the neonatal rodent cochlea, and suggested that either culture conditions do not accurately recapitulate the native cochlear environment or that a method to damage HCs more acutely in vivo was required. Indeed, recent data from our lab and others suggest that, while constitutive proliferation of HCs and SCs has eluded detection beyond embryonic day (E)14.5 in the intact mouse cochlea, the expression of proliferating cell nuclear antigen (PCNA) persists postnatally (see Figure 1), new HCs continue to be added to the mouse cochlea when examined at postnatal day (P) 0 and P6 (Jan et al., Unpublished), and rapidly acute HC loss during the first postnatal week, in vivo, results in proliferation, differentiation, and a robust, if transient, regeneration of lost HCs (Cox et al., 2012a). Despite such promising findings, this first postnatal week appears to represent a critical window during which such endogenous regeneration and proliferation can occur (Cox et al., 2012a). Expression of PCNA in C57BL/6 mice is no longer detectable in HCs or SCs by P5 (Figure 1), examination of mouse cochleae after P6 do not show any further addition of HCs (Jan et al., Unpublished), and regenerative processes in response to HC-loss appear to cease beyond P6-P7 (Cox et al., 2012a).
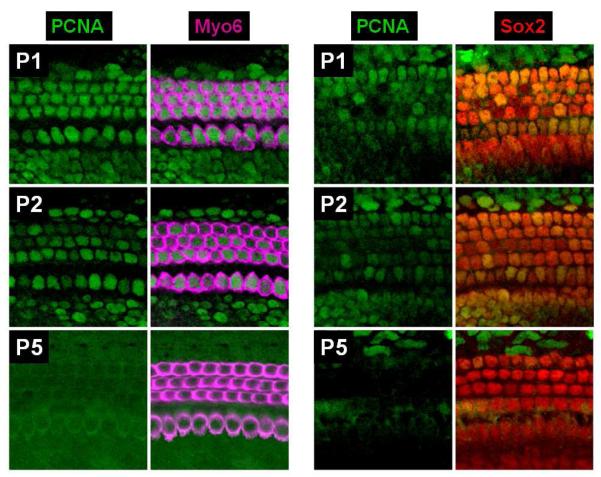
Figure1. Expression of Proliferating Cell Nuclear Antigen (PCNA) persists in the neonatal mouse cochlea.
PCNA is a cyclin protein that is a co-factor for DNA polymerase and thus critical for cell proliferation. Whole mount preparations from C57Bl/6 mouse cochleae at postnatal days 1 and 2 (P1 & P2 respectively) exhibit PCNA positive nuclei (green) in hair cells (purple) and supporting cells (red), labeled by hair cell specific myosin VI (Myo6) and the supporting cell marker SRY-box 2 (Sox2), respectively. By P5, no PCNA positive hair cells or supporting cells were detected. The persistence of PCNA suggests that postnatal cochlear hair cells and supporting cells may retain some of the factors necessary for cell cycle entry.
This loss of innate regeneration and HC addition is intriguing as, in many other studies where SC or HC proliferation and differentiation have been induced using pharmacological or genetic approaches, the level of proliferation or differentiation achieved also appears to diminish with postnatal age. More specifically, when inducible Cre lines are used to alter gene expression and induce proliferation or transdifferentiation within the organ of Corti, there is a consistent critical window during the first 2-4 postnatal weeks after which the genetic manipulations have little to no effect (Huang et al., 2011; Kelly et al., 2012; Liu et al., 2012b; Liu et al., 2012c; Oesterle et al., 2011). Additionally, it has been shown that a stem cell pool resides within the mouse cochlea (Chai et al., 2012; Savary et al., 2007; Shi et al., 2012; Sinkkonen et al., 2011; Wang et al., 2006; White et al., 2006; Yerukhimovich et al., 2007), and that a similar window, i.e. the first 2-3 postnatal weeks, constrains the ability to isolate and propagate these cells (Martinez-Monedero et al., 2007; Oshima et al., 2007). These findings suggest that multiple processes, which may be similar to those symptomatic of more gradual aging in other tissues, are occurring within the murine organ of Corti between E14 and P30, and are converging to inhibit processes of proliferation, differentiation, and regeneration. Thus, viewing cochlear development and regeneration in such a context of aging may generate new insights, new targets, and new intersections for known factors aimed at the regeneration of mammalian auditory HCs in the adult cochlea.
3. Endogenous regenerative capacity during early postnatal development
As mentioned previously, early lines of evidence pointed to ~E14.5 as the timepoint for terminal mitosis of mouse cochlear HC and SC progenitors (Ruben, 1967). However, several recent findings suggest that this initial exit from the cell cycle may only be the beginning of a more drawn out process of quiescence, where HCs and SCs retain some regenerative capacity beyond E14.5, potentially into the second postnatal week. For example, HCs and SCs continue to express the cell cycle protein PCNA well after E14.5, with expression still detectable in HCs and SCs as late as P2 (Figure 1) while Sox2 positive cells in the greater epithelial ridge (GER) and lesser epithelial ridge (LER) are readily labeled by the S-phase marker bromo-deoxyuridine (BrdU) into the 2nd postnatal week (Kamiya et al., 2001). While positive PCNA staining at these later ages may be indicative of slow degradation of the protein rather than active proliferation of the cells, it is interesting to note that PCNA is also expressed in SCs of the post-hatch chicken basilar papilla (BP) (Bhave et al., 1995) and in the sensory maculae of posthatch zebrafish (Bang et al., 2001), both of which add new SCs and in some cases HCs after hatching (Higgs et al., 2002; Oesterle, 1993). Furthermore, PCNA is a well characterized marker of proliferating cells with a well defined role in cell cycle progression (Moldovan et al., 2007; Strzalka et al., 2011). Thus, while the persistent expression of PCNA by itself does not suggest mouse cochlear cells are still actively proliferating at P2, it does demonstrate that not all of the machinery for cell proliferation has been completely eliminated by E14.5, let alone by the end of gestation.
In further support of the innate plasticity of neonatal HCs and SCs, Jan et al. (Unpublished) have recently characterized the addition of new HCs to the mouse cochlea between P0 and P6, which is similar to previous findings that suggest HCs continue to be added to the rat cochlea and the hamster cochlea up until P3 and P4, respectively (Kaltenbach et al., 1994; Mu et al., 1997). Also, several recent reports have demonstrated that stem and progenitor cells can be isolated from the postnatal mouse cochlea and placed into non-adherent culture conditions where they proliferate and can ultimately give rise to new HCs and SCs (Chai et al., 2012; Martinez-Monedero et al., 2007; Oshima et al., 2007; Savary et al., 2007; Shi et al., 2012; Wang et al., 2006; White et al., 2006; Yerukhimovich et al., 2007). As is typically seen in other systems (e.g. neurospheres), the ability to obtain spheres and/or new HCs and SCs declines with the age of the donor mice, exhibiting a dramatic decrease during the first 2-3 weeks postnatally (Martinez-Monedero et al., 2007; Oshima et al., 2007; White et al., 2006). Since many tissues that are capable of regeneration do so by means of a stem cell niche or progenitor cell population, this persistence of such a pool of otic precursors, and then its disappearance, further highlights the importance of postnatal development and maturation and its potential implications for HC regeneration.
While the expression of PCNA, the constitutive addition of HCs, and the presence of a potential pool of stem or progenitor cells all indirectly suggest the persistence of regenerative processes in the postnatal murine cochlea, there has been a dearth of direct evidence for such innate regeneration in vivo. Indeed, it has been particularly difficult to obtain any evidence for HC or SC regeneration that passes the litmus test of thymidine analog incorporation. That is, until very recently, when it was demonstrated that the neonatal mouse cochlea can and does give rise to new HCs via both proliferation and transdifferentiation following HC loss using a model of temporally controlled, cell autonomous expression of diphtheria toxin fragment A (DTA) (Cox et al., 2012a). Upon P0-P1 induction of DTA expression, cells that are positive for several HC markers (e.g myosinVIIa, calbindin, parvalbumin, prestin) incorporate ethynyl deoxyuridine (EdU) when injected between P3 and P5, and can also be co-labeled for PCNA. Furthermore, these cells express the stereocilia protein espin, take up lipophilic FM dyes, and their generation leads to near complete restoration of HC numbers in the apical turn of the cochlea between P3 and P6. Additionally, fate mapping suggests that, at least a portion of newly generated HCs are derived from SCs (Cox et al., 2012a). These data not only corroborate the in vitro work done previously (Kelley et al., 1995), but conclusively show that HCs and SCs in the mouse cochlea are capable of undergoing proliferation and/or differentiation well after E14.5. However, attempts to replicate such regenerative processes by induction of DTA at P6-P7 have met with failure, suggesting that this native regenerative response persists for most of the first postnatal week, but surpasses a critical threshold after this point. Still it is intriguing that such regeneration happens at all, and suggests that quiescence in HCs and SCs is a lengthy process that is merely initiated at ~E14, and that whatever checks on cell cycle are established at this point can be overcome for many days to follow. As cells lose their regenerative response to HC ablation, it is likely that factors suppressing regeneration are increasingly expressed and/or that factors that promote regeneration are decreased. Indeed, the evidence presented so far suggests that the downregulation of PCNA between P2 and P5 may be one such factor that further promotes quiescence.
4. Genetic mouse models of SC proliferation and differentiation and pharmacological experiments reveal a prohibitive effect of aging
In addition to the persistence of endogenous regenerative potential in postnatal cochlear HCs and SCs, several recent studies have used genetic manipulations to induce these cells to proliferate or transdifferentiate at later and later ages. Strikingly, many of these studies reveal prominent effects of aging that are reflective of phenotypes seen in numerous other tissues that exhibit declining regenerative ability with age. For example, the cyclin dependent kinase inhibitor p27Kip1 has been implicated as a critical factor in the quiescence of HCs and SCs, where germline deletion of p27Kip1 results in the continued proliferation of both HCs and SCs postnatally (Chen et al., 1999; Lowenheim et al., 1999). However, a dramatic decrease in the number of proliferative cells was observed when BrdU was administered to p27Kip1 knockout mice at 4 months of age as compared to P7-P10 (Lowenheim et al., 1999). Using inducible Cre models to ablate p27Kip1 at various ages, both Oesterle et al (2011) and Liu et al. (2012c) similarly noted that cells within the organ of Corti exhibit robust proliferation in response to neonatal p27Kip1 ablation, but display a dramatic decrease in the number of proliferative cells when p27Kip1 is ablated at P30 or P42. These findings suggest that factors other than p27Kip1 may be upregulated in the organ of Corti between P10 and P30, and that such factors compensate for p27Kip1 disruption by further dampening the proliferative ability of these cells. Another example for this can be found in the conditional ablation of Sox2 from cochlear SCs, which can induce multiple rounds of proliferation in inner PCs when deleted at P0-P1, but results in a failure of these cells to complete cycle when induced at P6-P7, and a dramatically reduced level of cell cycle entry when Sox2 is deleted at P30 (Liu et al., 2012c). In another recent paper, beta catenin was permanently overexpressed in Lgr5 positive SCs beginning at P0-P1, which resulted in robust SC proliferation by P8-P15, but a dramatic decline in proliferation, and increase in cell death, resulted in a complete absence of proliferation or of new cells by P21 (Chai et al., 2012). Additionally, when beta catenin overexpression is induced at adult ages, in the context of ototoxic HC damage, SC proliferation is not immediately apparent (Kuo, unpublished data). As another example, the neonatal ablation of the retinoblastoma protein (pRb) from HCs (Weber et al., 2008) or SCs (Yu et al., 2010) results in the proliferation of these cells between P3 and P6, yet ablation of pRb at 3 months of age does not appear to elicit any signs of proliferation (Huang et al., 2011). Similarly, germline deletion of the related retinoblastoma-like 2 protein (p130) results in supernumerary HCs and SCs that incorporate EdU when the mice are injected with the thymidine analog at P21 but not when they are injected at P30 (Rocha-Sanchez et al., 2011). Taken together, these reports strongly suggest an age-related decline in proliferative potential of cells in the organ of Corti as well as the combined role of several different factors to promote quiescence in the postnatal mouse cochlea.
Furthermore, proliferation is not the only regenerative process to be affected by age in the murine cochlea. As several recent studies suggest, the potential for SCs to differentiate or transdifferentiate into HCs is also severely hampered during the first few postnatal weeks. For example, inhibition of Notch signaling via treatment with a gamma secretase inhibitor or acute ablation of the canonical mediator RBPj results in supernumerary HCs in explants from neonatal rodent cochleae (Yamamoto et al., 2006; Zhao et al., 2011). However, in vivo Notch inhibition in an adult rodent cochlea failed to yield a significant increase in the numbers of cochlear HCs (Hori et al., 2007), suggesting that other factors come on that either act in conjunction with, or replace Notch signaling to maintain SC fate. This latter hypothesis, that Notch may no longer be required for SC maintenance with age, is suggested by the postnatal downregulation of markers of Notch signaling/activity in mouse cochlear SCs (Hartman et al., 2007; Hartman et al., 2009; Murata et al., 2006). In addition to Notch signaling, much attention has focused on overexpression of the basic helix loop helix (bHLH) transcription factor Atoh1 as a means to induce transdifferentiation of SCs to HCs. However, two recent reports of Atoh1 overexpression in mouse models both noted a decreased efficiency for SC to HC conversion with age (Kelly et al., 2012; Liu et al., 2012b). Specifically, Kelly et al. (2012) were able to induce Sox2 positive nonsensory cells to express HC markers by inducing a Tet-On-Atoh1 construct via doxycycline treatment between P0-P3. However, when inducing the Atoh1 overexpression at P8 and at P14, the numbers of supernumerary HCs declined with the age of induction. Similarly, Liu et al. (2012b), using a Cre-ER mediated method for Atoh1 overexpression, saw SC to HC conversion when they induced Atoh1 at P0-P1 and at P12-P13, but were unable to detect any converted cells when inductions were carried out at P30, despite the maintenance of high levels of Cre activity at later ages.
Together, the studies reviewed here all appear to converge on the fact that postnatal aging progressively restricts the regenerative potential of the mammalian organ of Corti. Furthermore, since aging affects both proliferation and differentiation of cells, it suggests that there is either a large degree of interrelatedness between both processes, or that multiple senescence-related changes are taking place within cochlear cells as they age. Indeed, some evidence exists for both of these hypotheses. For example, simultaneous knockdown of two cyclin dependent kinase inhibitors (CDKNs) (p21Cip1 and p19INK4d) promotes greater proliferation in the mouse organ of Corti than deletion of either CDKN individually (Laine et al., 2007). This suggests that multiple factors are acting synergistically to restrict proliferation. Conversely, within the cochlea, Atoh1 is thought to influence differentiation only, but ectopic Atoh1 expression in neonatal mice also causes some cochlear cells to re-enter cycle (Kelly et al., 2012). Similarly, the sphere forming potential of otic cells is related to Notch signaling, which is also traditionally viewed as a differentiation cue in the inner ear (Savary et al., 2008). These findings suggest that processes of differentiation and proliferation are linked by pathways common to both. Therefore, it is likely that both an interrelatedness of proliferation and differentiation and a culmination of multiple repressive factors are at work in the aging cochlea, representing as yet unidentified challenges in the pursuit of auditory HC regeneration and functional recovery in the adult mammalian cochlea.
5. Postnatal cochlear development, maturation, and aging
5.1 Structural and anatomical changes
In light of the findings presented, it becomes important to appraise many of the changes known to occur during postnatal maturation and aging in an attempt to identify factors that could be repressing regenerative processes (Figure 2). One primary set of changes that is immediately apparent is the structural and anatomical remodeling that takes place during the postnatal maturation of the cochlea. Perhaps most striking are the opening of the tunnel of Corti and the formation of the spaces of Nuel between P6 and P10 (Kikuchi et al., 1965) which require much refinement of SC cytoarchitecture. Around a similar time, the tectorial membrane detaches from the apical surfaces of SCs and GER cells (Lim, 1987), and the basilar membrane (BM) develops, adding laminin and other basement membrane components (Cosgrove et al., 1997; Rodgers et al., 2001). This maturation of the BM may be particularly important in postnatal senescence as it physically separates the organ of Corti from a potential pool of stem cells or from mitogenic signals originating in the underlying mesenchyme. Some evidence for this is provided by the fact that dissections for otosphere culture typically do not eliminate the mesenchymal cells that reside beneath the BM, suggesting their potential contribution to sphere forming cells (Oshima et al., 2009; Parker et al., 2010). Indeed, when CD146 positive cells that reside below the BM are isolated specifically, they do generate spheres, though they do not proliferate as well or give rise to as many HC-like cells as Sox2 positive cells from above the BM (Sinkkonen et al., 2011). However, as CD146 does not label all of the cell types beneath the BM, the contribution of CD146 negative cells from beneath the BM to sphere forming cultures is as yet unknown. Additionally, the BM could also restrict signaling molecules from cells under the organ of Corti that might promote the proliferation, differentiation, or migration of SCs. Although it has yet to be demonstrated in the cochlea, such signaling through basement membranes is critical to regenerative processes in the kidney (Mollura et al., 2003), and the intestine (Kedinger et al., 1998). Furthermore, processes of proliferation, differentiation, and migration could be hindered by constraints placed upon SCs via their attachments to the BM itself. Indeed, in the mouse utricle, it has been shown that interactions of integrins and laminin at the BM are at least partly responsible for the reduction of proliferative SCs in explant cultures between E18 and P6 (Davies et al., 2007). Along these lines, another factor that could similarly act to inhibit regenerative processes during postnatal development is cadherin expression and cell-cell junctions. While E-cadherin is expressed at SC junctions in the postnatal and adult mouse organ of Corti (Whitlon, 1993; Whitlon et al., 1999), any quantitative changes in E-cadherin expression in the cochlea have not yet been well described. However, the mouse utricle demonstrates increased expression of E-cadherin with age (Collado et al., 2011) and a thickening of F-actin belts at apical junctions between SCs (Burns et al., 2008), both of which have been correlated to a decreased regenerative capacity with age in the mammalian utricle. Notably, several other studies have implicated cadherins in the prevention of proliferation via their actions upon beta catenin, p27Kip1, and pRb (Day et al., 1999; Orsulic et al., 1999; St Croix et al., 1998; Stockinger et al., 2001), all of which affect the quiescence of cells in the organ of Corti (Chai et al., 2012; Chen et al., 1999; Liu et al., 2012c; Lowenheim et al., 1999; Oesterle et al., 2011; Shi et al., 2012; Weber et al., 2008; Yu et al., 2010). Another attribute of the postnatal development of cell-cell junctions that could be important for regeneration is the changing expression of connexin (Cx) proteins with age and the establishment and maturation of gap junctions between SCs and among epithelial cells along the cochlear lateral wall. For example, the expression of Cx26 in the cochlea increases with age (Frenz et al., 2000; Xia et al., 1999; Xia et al., 2001), and Cx26 has been shown in multiple other tissues to antagonize proliferation (Braeuning et al., 2010; Momiyama et al., 2003; Muramatsu et al., 2002; Ott et al., 2006; Torres et al., 2005; Winterhager et al., 2000). Conversely, Cx43 and Cx45 appear to be downregulated with age in the cochlea (Cohen-Salmon et al., 2004), and Cx43 has been shown to stimulate proliferation and maintenance of progenitor cells in several tissue types (Ehmann et al., 2001; Lambe et al., 2006; Oviedo-Orta et al., 2010; Taniguchi Ishikawa et al., 2012; Zhang et al., 2006b). Another particularly notable change that occurs in the rodent cochlea during postnatal development (and one that is directly related to the changes in Cx expression) is the maturation of the endocochlear potential (EP), which takes place in the mouse up until approximately P14 (Anniko, 1985). The generation of a mature EP is dependent upon a high K+ concentration and low Na+ and Ca2+ concentrations in the endolymph, with a high HCO −3 concentration to buffer the pH. These are all important factors to consider in the pursuit of auditory hair cell regeneration. For example, recent evidence suggests that calcium signaling is critical for the switch from a highly proliferative precursor state to differentiated HCs in the zebrafish lateral line (Go et al., 2010). And while electrophysiological studies suggest that SCs may not respond to Na+ or K+, even when they are giving rise to new HCs (Masetto et al., 1997), levels of Na+ or K+ critically affect regenerative processes in some systems (Sundelacruz et al., 2008; Trinh et al., 2008; Tseng et al., 2010; Zhang et al., 2012a). While some of the hypotheses presented here are more speculative than others, it seems clear that the increasingly stringent constraints placed upon cells by the complex structure and unique extracellular environment of the organ of Corti that matures during this postnatal period could be critical to the regenerative potential of HCs and SCs.
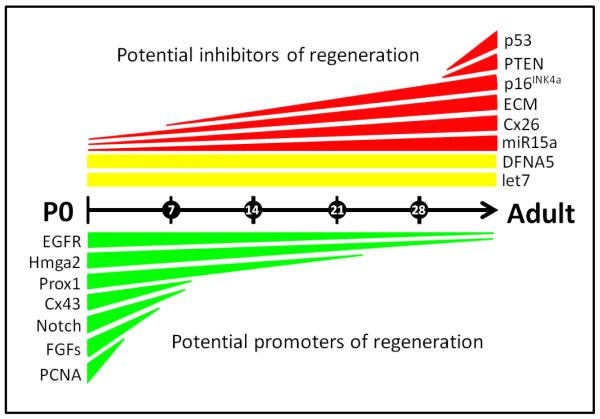
Figure 2. Several factors that may promote or inhibit auditory hair cell regeneration during postnatal development and aging of the rodent cochlea.
Factors are grouped by predicted roles of either preventing regenerative processes (red and yellow, above the age line) or promoting regenerative processes (green, below the age line). Factors with expression that increases with age are shown in red, while those with decreasing expression are shown in green. Factors whose expression persists with postnatal age are shown in yellow. The age line runs from birth, i.e postnatal day zero (P0), to adulthood, with each hatch mark representing 7 days. In most cases, the lengths of the colored bars cover the distance between timepoints at which expression levels were measured. However, for ease of visual representation, both p53 and PTEN have been added in their current positions despite the fact that their levels of expression were not measured at 1 month of age but rather between ~3 months of age and 18 months of age for PTEN, and ~3 months of age and 30 months of age for p53. Abbreviations: p53 = tumor protein 53, PTEN = phosphatase and tensin homolog, p16INK4a = cyclin dependent kinase inhibitor 2a, ECM = components of the extracellular matrix such as laminins and integrins, Cx26 = connexin 26, miR15a = microRNA 15a, DFNA5 = deafness autosomal dominant gene 5, let 7 = the let 7 family of microRNAs, EGFR = epidermal growth factor receptor, Hmga2 = high mobility group AT-hook 2, Prox1 = prospero related homeobox 1, Cx43 = connexin 43, Notch = activated Notch (and its canonical mediator Hes5), FGFs = acidic fibroblast growth factor and fibroblast growth factor receptor 1, PCNA = proliferating cell nuclear antigen.
5.2 Signaling molecules
In parallel with the maturation of cochlear anatomy and structure and the establishment of a mature EP, there are many changes in cell signaling that occur within the organ of Corti during postnatal development. One such change appears to be the rather dramatic decline of activated Notch signaling, suggested by the loss of activated Notch1 expression by P7 (Murata et al., 2006), and the loss of Hes5 reporter expression along a similar timeframe (Hartman et al., 2009). Since the presence of Notch signaling is important for the differentiation of prosensory cells into HCs (Hartman et al., 2010; Kiernan et al., 2005; Liu et al., 2012a; Pan et al., 2010; Yamamoto et al., 2011), the transdifferentiation of SCs into HCs (Yamamoto et al., 2006; Zhao et al., 2011), and possibly even the proliferation of otic stem cells and differentiating HCs (Kelly et al., 2012; Savary et al., 2008), the decline in Notch signaling is likely to have profound effects on the ability of cochlear cells to be induced to give rise to new auditory HCs.
Another particularly important signaling pathway that exhibits dynamic changes during late embryonic and postnatal cochlear development is the fibroblast growth factor (FGF) signaling pathway. While expression of FGF receptor 3 (FGFR3) appears to persist in pillar cells (PCs) and Deiters cells (DCs) at least to P30 (Liu et al., 2012b; Liu et al., 2012c), FGFR1 expression, is present in the sensory epithelium of the cochlea between E13 and E16, but gone from the organ of Corti by P3 (Hayashi et al., 2010). Similarly, in the developing rat cochlea, acidic FGF expression in HCs was readily detectable at perinatal ages, but was undetectable by P6 (Luo et al., 1993). This could be important for HC regeneration since FGF signaling has been shown to be critical for the establishment of the prosensory domain and the differentiation of HCs and SCs during development (Hayashi et al., 2007; Hayashi et al., 2008; Mueller et al., 2002; Puligilla et al., 2007). Additionally, while not all of the data concerning FGF signaling and regeneration has been positive (David et al., 2002; Oesterle et al., 2000), expression of genes relating to FGF signaling in the chick BP after ototoxic damage implicates them in HC regeneration (Pickles et al., 1997; Umemoto et al., 1995). As does the fact that addition of basic FGF to dissociated cultures from neonatal mouse cochleae increases sphere forming ability (Wang et al., 2006) and the addition of FGF2 to mouse utricular explants significantly increases proliferation, suggesting a role for FGF signaling in stem cell maintenance and/or proliferation ability (Zheng et al., 1997). Additionally, FGF may also be important for promoting SC to HC transdifferentiation as studies from the zebrafish inner ear suggest that FGFs can regulate Atoh1 expression and enhance the responsiveness of nonsensory cells to differentiate into HCs when Atoh1 is ectopically expressed (Millimaki et al., 2007; Sweet et al., 2011). This could be particularly important in the context of ectopic Atoh1-induced HC regeneration where overexpression of FGFR1, and/or additional FGF ligands, could increase the competency of nonsensory cells to respond to Atoh1 overexpression and turn on HC specific genes.
Another signaling pathway that may be particularly important for postnatal and adult cochlear HC regeneration is epidermal growth factor receptor (EGFR) mediated signaling. First, there is some data to suggest that EGFR is not expressed in the organ of Corti at 3-4 months of age despite its expression in HCs and SCs at earlier postnatal ages (Daudet et al., 2002; Zine et al., 2000). This pattern of expression suggests the loss of EGFR signaling as an important factor in the age-related decline in regenerative potential. Indeed, many studies in neonatal rodent cochleae have demonstrated a pronounced effect of EGF signaling to promote proliferation and the production of supernumerary HCs (Chardin et al., 1997; Doetzlhofer et al., 2004; Lefebvre et al., 2000; Malgrange et al., 2002; White et al., 2012), and EGF signaling has been shown to promote SC proliferation in the regenerating chick BP (White et al., 2012). Thus, the data presented suggest a role for EGFR in HC regeneration, and hint that existing models of induced SC proliferation may be improved at later ages via the reinstatement or enhancement of EGFR signaling.
Additionally, a case could be made for the importance of Retinoic Acid (RA) signaling, for, even though RA signaling is upregulated in the auditory sensory epithelium between E14 and P1, both the responsiveness to and the synthesis of RA appear to be largely diminished at later postnatal and adult ages (Kelley et al., 1993; Raz et al., 1999). Additionally, the RA binding protein, CRABP1 has been shown to be markedly downregulated between neonatal and adult cochleae (Kelley et al., 1993; Smeti et al., 2012). Although the picture is not completely clear, as some RA synthesizing and metabolizing enzymes are still expressed at P20 (Romand et al., 2006), and some RA responsiveness persists in adult mouse cochleae since RA is protective against noise exposure (Ahn et al., 2005; Shim et al., 2009). However, such persistence in the adult cochlea only demonstrates that HCs can respond to RA, while SCs may be unable to respond due to receptor downregulation. Although more information is needed to determine whether or not RA signaling is lost in adult SCs, a decline in RA signaling in the organ of Corti could be a major contributing factor to age-related senescence in the cochlea. In fact, a role for RA signaling in HC regeneration was demonstrated in one of the first papers to reveal the persistence of regenerative processes in the neonatal rodent cochlea, where mitotic addition of new HCs was enhanced by RA after ototoxic damage (Lefebvre et al., 1993). Furthermore, RA is important in the differentiation of HCs during embryonic development (Raz et al., 1999), promotes the formation of supernumerary HCs in neonatal explants (Kelley et al., 1993), and has been shown in other systems, notably the olfactory epithelium, to promote regenerative processes at adult ages (Rawson et al., 2007). Also, RA signaling is maintained at posthatch and adult ages in the chicken BP, where HC regeneration is known to occur (Kelley et al., 1993; Lee et al., 1996), and though the addition of supraphysiological RA to regenerating chick sensory epithelia does not appear to have any added beneficial effect (Warchol, 2002), the reinstatement of RA signaling in a mammalian cochlea otherwise deprived may still be able to promote proliferation or regeneration, particularly in the context of genetic ablation of cell cycle inhibitors.
Finally, it is important to keep in mind that many other changes occur in the cochlea during postnatal development, and these may also promote or inhibit regeneration, though the lack of direct evidence for such roles in the ear means that much further investigation will be needed. For example, expression of the nerve growth factor receptor TrkA increases with postnatal age in HCs and SCs (Dai et al., 2004). Additionally, there is a clear maturation of synaptic connections occurring in the organ of Corti during postnatal development (Sobkowicz et al., 1986), with changes occurring in the expression levels of neurotransmitters and their synthetic or catabolic enzymes, such as gamma-Aminobutyric acid (GABA) and acetylcholinesterase (AChE) (Emmerling et al., 1988; Emmerling et al., 1990; Sobkowicz et al., 1989; Whitlon et al., 1989). Though the potential roles of such neurotransmitters in regenerative processes are not readily apparent, there is a fair amount of literature demonstrating the effects, both positive and negative, of neurotransmitters on regeneration in numerous tissues (Cooke et al., 2008; Faroni et al., 2012; Faroni et al., 2011; Lou et al., 1999; Maouche et al., 2009; Papadimas et al., 2012; Soltani et al., 2011). Thus, these changes may be important in the consideration of the loss of regenerative potential with postnatal age in the mouse cochlea.
5.3 The loss of pluripotent otic stem cells
Until recently, it was unknown whether or not a true stem cell pool existed within the postnatal mammalian cochlea, however, numerous reports within the past decade have demonstrated the robust ability of postnatal cochlear epithelia to generate sphere cultures comprised of pluripotent cells that can give rise to HCs, SCs, and several other cell types (Chai et al., 2012; Martinez-Monedero et al., 2007; Oshima et al., 2007; Savary et al., 2007; Shi et al., 2012; Wang et al., 2006; Yerukhimovich et al., 2007). However, similar to what is seen in many other tissues, the cochlea appears to suffer from a decline in stem cell numbers with age. Perhaps the most direct evidence of this is the decline in sphere forming ability of cells isolated from rodent cochleae at 2-3 weeks postnatal age (Martinez-Monedero et al., 2007; Oshima et al., 2007; Zhong et al., 2010). This decline in sphere forming ability is correlated with a number of studies that record decreasing numbers of cells positive for traditional stem cell markers within the cochlear duct, such as Nestin (Lopez et al., 2004; Smeti et al., 2011; Watanabe et al., 2012), Lgr5 (Chai et al., 2011; Chai et al., 2012; Shi et al., 2012), GFAP (Rio et al., 2002; Smeti et al., 2011), Sox2 (Smeti et al., 2011; Waldhaus et al., 2012), and Abcg2 (Smeti et al., 2011). It is encouraging to note, however, that most, if not all, of these markers are still expressed at adult ages, if only in a restricted population of cells, suggesting that there may still be viable stem cells in the adult cochlea that can be purposed toward regeneration. Though not all of these markers have been verified as true stem cell markers in the inner ear, it would be particularly interesting to see if the SCs that do respond to p27Kip1 ablation at adult ages could be fate mapped from cells expressing any or all of these markers. Additionally, while the persistence of a restricted population of cells expressing stem cell markers is encouraging, it will still be important to expand the progenitor cell pool to a point where widespread HC regeneration and full recovery of function can be achieved.
5.4 Changes in gene/protein expression
In addition to structural changes and environmental changes that the HCs and SCs experience during the postnatal maturation of the organ of Corti, many cells also exhibit changes in gene and protein expression. In the mouse cochlea, expression of the Prospero related homeobox 1 (Prox1) protein decreases with age. During late embryonic and postnatal development, Prox1 exhibits a pattern of expression that becomes progressively restricted to SCs and then to subsets of SCs before finally becoming undetectable between P14 and P21 (Bermingham-McDonogh et al., 2006). Since Prox1 has been implicated in asymmetric cell division and differentiation (Elsir et al., 2012), it could play a vital role in HC regeneration, and may be necessary to promote asymmetric division and thus transdifferentiation of SCs to HCs. This may be particularly important in models where SCs are induced to proliferate, however, in one model of induced SC proliferation, where Sox2 was ablated in PCs and DCs, Prox1 immunoreactivity was detected in proliferating cells, despite no immediate transdifferentiation being observed (Liu et al., 2012c). While this suggests that Prox1 may not be sufficient to drive asymmetric division of SCs, it may still be necessary; or, it may be that a longer timeframe is needed for Prox1 mediated transdifferentiation to occur. Interestingly, Prox1 is also downregulated with age in the chick BP (Bermingham-McDonogh et al., 2006), however, it is still detectable in a population of SCs in 1 month old birds, and is upregulated in proliferating SCs and newly generated HCs after ototoxic damage (Stone et al., 2004). These findings suggest that Prox1 is indeed involved in HC regeneration, however whether or not, or to what extent Prox1 expression is necessary for such regeneration remains to be tested.
The phosphatase and tensin homolog (PTEN) protein is expressed in HCs and SCs and increases with age in the CBA/J mouse cochlea (Sha et al., 2010). Since PTEN is a tumor suppressor that acts via PI3K/pAkt signaling to prevent cell proliferation, such increases in PTEN expression could contribute to the senescence of cells in the organ of Corti with age. Indeed, it has been shown that PTEN regulates the proliferation of cells in the auditory epithelium since PTEN haploinsufficiency results in prosensory cells exiting the cycle later in development and in the production of supernumerary HCs (Dong et al., 2010b). Though some studies suggest that PTEN levels may be more variable at earlier postnatal ages (Dong et al., 2010a; Dong et al., 2010b), its increased expression at later ages and its demonstrated effects during development suggest that it may be a valuable target for inducing or enhancing regenerative processes in the cochlea at adult ages.
Finally, it may also be important to consider genes whose expression does not change with age, but merely persists. After all, if a known tumor suppressor is expressed in the organ of Corti from late embryonic through adult ages, it may not singlehandedly prevent proliferation, but may contribute to such effects in the context of increasing levels of CDKNs or decreasing levels of cyclins or other pro-mitogenic factors. As an example, the deafness, autosomal dominant 5 gene (DFNA5) is expressed in the murine cochlea from E15 through adulthood (Maeda et al., 2001), whereas epigenetic silencing of DFNA5 in multiple tissues and cell types leads to cell proliferation and tumor formation (Akino et al., 2007; de Beeck et al., 2012; Kim et al., 2008). Indeed, DFNA5 null mouse strains have been developed, and interestingly show no defects in hearing, though, on the C57Bl/6J background, they demonstrate supernumerary HCs (Van Laer et al., 2005), suggesting that DFNA5 does play a role in limiting HC morphogenesis, and could be similarly limiting regenerative potential in the postnatal and adult cochlea.
5.4 MicroRNAs and epigenetics
In recent years, more and more investigators have begun to look at the expression levels of microRNAs (miRs), which are small non-coding RNA molecules that can each target multiple messenger RNA molecules for degradation and/or disruption of protein expression (Heinrich et al., 2012; Ke et al., 2003). In the cochlea it seems that the expression levels for several miRs vary in a dynamic fashion with age (Friedman et al., 2009; Weston et al., 2006). Perhaps of particular interest is the pattern of expression of miR-15a, which exhibits increased expression between P0 and P30, and, based on seed sequence complementarity, miR15a is predicted to degrade several proliferation promoting transcripts, including several cyclins, cyclin dependent kinases, and E2F and Ras family members (Friedman et al., 2009). Indeed this is corroborated by reports in other systems that show an anti-mitotic effect of miR-15a via its degradation of E2F family members (Ofir et al., 2011), cyclinD1 (Bonci et al., 2008), Wnt3a (Bonci et al., 2008), and cMyb (Zhao et al., 2009). Moreover, since most of the models of induced SC proliferation have promoted cell cycle re-entry via ablation of either cyclin dependent kinase inhibitors (p27Kip1, p21Cip1, p19INK4d, etc.) or pRb (which acts to inhibit E2F family members in its hypophsophorylated state), the current data strongly suggest miR-15a, and its increasing expression in the cochlea with age, as a potential cause for age-related decline of cell cycle re-entry in these models.
Another area of research that is garnering more and more interest in cochlear development and regeneration is epigenetics, particularly, the methylation of genomic DNA. For example, the DNA methyltransferases Dnmt3a and Dnmt3b, which repress transcription by catalyzing the addition of methyl groups to DNA, exhibit increased expression in outer HCs and DCs between P1 and P14 in the rat cochlea (Mutai et al., 2009). Additionally, there is a strong correlation between increased methylation of Sox2 enhancer regions and the declining proliferative and otosphere-forming abilities of cells in the organ of Corti as they age (Waldhaus et al., 2012). Indeed, the Sox2 enhancer sites NOP1 and NOP2 show increasing methylation status that inversely correlates with proliferation in vitro and with postnatal age in vivo (Waldhaus et al., 2012). Furthermore, methylation of the promoter regions of the stem cell genes Nanog and Oct4 is also increased in the mouse cochlea between E13.5 and P21, suggesting further repression of pluripotency promoting factors with age (Waldhaus et al., 2012). Since epigenetic regulation of gene expression by methylation has been shown to be important for age-related senescence (Casillas et al., 2003; Li et al., 2012; Lopatina et al., 2002; So et al., 2011), these findings may be critical to our understanding of declining regenerative potential within the organ of Corti. However, despite such strong correlations, a direct causal relationship between gene methylation and the lack of HC regeneration still needs to be demonstrated.
It is clear from the review of the literature presented so far that there are many important changes that occur during the postnatal development, maturation, and aging of the cochlea (Figure 2). As such, it is likely that many of the changes are interrelated and may be acting synergistically. Such orchestration of factors and developmental events will also be an important consideration for attempts at regeneration, where manipulations to only one aspect or one factor may have a pronounced effect during development, or even at neonatal ages, but such effects may be hidden by compensative action of other factors or changes that occur simultaneously. Thus, in order to achieve HC regeneration in the adult mammalian cochlea, it is likely that several processes of aging or maturation that culminate to synergistically oppose regenerative processes will have to be overcome.
6. The more global aspects of aging suggest other potential targets for HC regeneration
Numerous mammalian tissues exhibit declining proliferation and/or regeneration with age, including the brain, the olfactory epithelium, the pancreas, the liver, cardiac muscle, skeletal muscle, and bone (Chien et al., 2005; Galvan et al., 2007; Porrello et al., 2011; Salpeter et al., 2010; Suzukawa et al., 2011; Timchenko, 2009). Interestingly, there are many common and overlapping factors of regeneration in these systems as well as in their age-related senescence. Here we review some of the more common aspects of regeneration and the factors that act to hinder it with age.
In mammals, tissue regeneration is almost always accomplished via a population of stem or progenitor cells that must proliferate and then differentiate into the appropriate cell type. In the cochlea, a progenitor cell population may indeed exist, though it appears to lay dormant until the appropriate stimuli activate the cells to proliferate or transdifferentiate (e.g. culture conditions or manipulation of p27Kip1 or Atoh1 expression). Despite this, one aspect that is already apparent is that the aging of cochlear SCs is a contributing factor to the lack of regeneration seen in the late postnatal and adult cochlea. This age-related decline in regenerative potential is exactly what is seen in numerous other tissues, where regenerative ability declines with age due to one of 3 main causes: (1) the loss of the stem or progenitor cell pool due to cell death, (2) loss of multipotency or differentiation ability, and, perhaps the most common, or most well-known: (3) loss of proliferative ability and/or self-renewal. Indeed, research in other systems suggests that some or all of these events occur throughout the lifetime of the tissue to diminish regenerative ability.
6.1 Depletion of the stem cell and/or progenitor cell pool via cell death
In many tissues, most notably skeletal muscle, liver, and kidneys, the age-related decline of regenerative potential is correlated with the loss of stem or progenitor cells via necrotic or apoptotic cell death (Collins et al., 2007; Giampietri et al., 2010; Menthena et al., 2011; Suzuki et al., 2012; Yang et al., 2010). In the case of the cochlea, such cell death may also be playing a role. For example, cells in the GER may represent a significant portion of stem or progenitor cells as they can be isolated mechanically and cultured to give rise to spheres (Sinkkonen et al., 2011), appear to continue proliferating in vivo during the first postnatal week (Kamiya et al., 2001) and are easily induced to differentiate into HCs at late embryonic (Malgrange et al., 2003) and postnatal ages (Huang et al., 2009; Kelly et al., 2012; Zheng et al., 2000). Yet these cells are known to undergo a wave of apoptotic cell death after the first postnatal week in the mouse cochlea (Kamiya et al., 2001; Takahashi et al., 2001), which correlates with the decline in sphere forming efficiency of cells isolated from the cochlea during the 2nd and 3rd postnatal week (Martinez-Monedero et al., 2007; Oshima et al., 2007; Zhong et al., 2010). Furthermore, it has been suggested that with continued aging, SCs such as PCs and DCs are also lost via apoptosis (Sha et al., 2009; Usami et al., 1997), suggesting even further decline of the potential pool of progenitor cells. These correlations suggest several important and unanswered questions. First, though GER cells and SCs clearly contribute to the potential pool of progenitor cells, the full extent of their respective contributions is unknown, as is whether or not their death contributes to the age-related decline in induced regenerative ability or ability to isolate otospheres from the cochlea. Also, if the loss of the GER contributes to the loss of regenerative potential with age, then experimental approaches for regeneration in adult cochleae would likely require re-instating a population of GER-like cells. While it may be possible to accomplish this by various means, e.g. with surgical delivery of embryonic stem (ES) cells or induced pluripotent stem (IPS) cells, or via dedifferentiation of existing cells (Choi et al., 2012; Gunewardene et al., 2012; Nishimura et al., 2012; Nishimura et al., 2009; Oshima et al., 2010; Takahashi et al., 2006), the re-instatement of an entire stem cell niche may represent a significant challenge for efforts aimed at HC regeneration.
6.2 Loss of multipotency with age
Another primary mechanism for the loss of regenerative potential with age appears to be the loss of differentiation capacity in stem or progenitor cell populations. However, this mechanism may not be truly global, since the majority of characterized examples of such losses in potency come from muscle and connective tissues, such as hematopoietic stem cells, muscle progenitors, mesenchymal stromal cells, fibroblasts, and melanocyte stem cells (de Girolamo et al., 2009; Inomata et al., 2009; Lees et al., 2006; Lescale et al., 2010; Norddahl et al., 2011; Siddappa et al., 2007; Simpson et al., 2010; Wang et al., 2012a). Other cell types, like neural stem cells for example, generally only exhibit small declines in differentiation ability with age (Gritti et al., 2009). Additionally, while differentiation ability of mammalian utricular SCs has not been directly correlated with age, some ability to transdifferentiate into HCs persists at adult ages, where Notch inhibition, ectopic Atoh1 expression, and HC damage can all induce the production of new HCs from underlying SCs (Li et al., 1997; Lin et al., 2011; Schlecker et al., 2011; Staecker et al., 2007). However, it is clear from the lack of any significant change to SC fate in the damaged mouse cochlea (Oesterle et al., 2009) that SCs in the late postnatal/adult mouse cochlea are not naturally capable of transdifferentiating into HCs or other morphologically distinct cell types. Indeed, the finding that ectopic Atoh1 expression in SCs becomes less efficient at inducing differentiation with age (Kelly et al., 2012; Liu et al., 2012b) does suggest that SCs do lose their ability to be differentiated into HCs. This suggests that the route to transdifferentiation of cochlear SCs in adults may lie in uncovering key differences between cochlear and utricular sensory epithelia or key similarities between cochlear cells and cells in connective tissues that similarly lose their ability to differentiate with age. In many of the tissues where multipotency is lost, the most commonly cited cause is DNA damage, both nuclear and mitochondrial (Alves et al., 2010; Inomata et al., 2009; Norddahl et al., 2011; Wang et al., 2012a). Although it has been suggested that some types of platinum based chemotherapeutics can cause DNA damage in cochlear SCs (Thomas et al., 2006), the extent of DNA damage that occurs naturally in SCs with age, or in response to noise, is not well characterized, suggesting that a progressive loss of differentiation ability could possibly be the result of accumulated DNA damage, though more research is needed to establish whether or not this is true.
6.3 Loss of proliferative potential and/or self-renewal
Perhaps the most well described cause for declining regenerative potential with age is the loss of proliferative ability and self-renewal in the stem cell or progenitor cell pool. Clearly, from data already presented, SCs are not only quiescent following development, but even upon reinstatement of proliferation by genetic manipulation, SCs eventually become quiescent again, suggesting that they do lose proliferative ability with time and that successful regeneration will depend upon being able to induce SCs to re-enter cycle at later and later ages. Indeed, while attempts to induce SCs to re-enter the cell cycle via ablation of the CDKN family members p21Cip1 (aka CDKN1a), p19INK4d (aka CDKN2d), or p27Kip1 (aka CDKN2b) (Chen et al., 1999; Chen et al., 2003; Laine et al., 2007; Liu et al., 2012c; Lowenheim et al., 1999; Oesterle et al., 2011) have all achieved some success, the increased expression of all 3 of these genes between E17.5 and P7 and the synergistic effect on proliferation seen when p19INK4d and p21Cip1 are knocked out together (Laine et al., 2007) strongly suggest that both redundancy and age-related increases in the expression of CDKN family members act to synergistically inhibit proliferation with postnatal age.
In the case of many tissues that retain postnatal regenerative ability, age-induced senescence is related to the upregulation of yet another CDKN, the tumor supressor p16INK4a (a.k.a. CDKN2a) (Dhawan et al., 2009; Janzen et al., 2006; Krishnamurthy et al., 2006; Molofsky et al., 2006; Rodriguez-Menocal et al., 2009; Wang et al., 2012b). Indeed, p16INK4a is expressed in the mouse cochlea, demonstrates increased expression with age, and its germline deletion results in proliferative cells in the adult organ of Corti after damage (Cox et al., 2012b; Waldhaus et al., 2012). These findings suggest that p16INK4a could be playing a role in the aging of the “stem” cell population in the cochlea. However, more work is needed to determine the extent to which p16INK4a plays a role in SC quiescence, and whether or not p16INK4a ablation could have a synergistic effect on proliferation when combined with the disruption of other CDKNs such as p21Cip1 or p27Kip1.
Similarly, other known tumor suppressors have been implicated in the decline of regenerative potential with age, though perhaps the most prominent example (after p16INK4a) appears to be tumor protein 53 (p53), which has been shown to play a role in decreased proliferation and regeneration with age in the skin, kidney, liver, brain, and spleen (Gannon et al., 2011; Hinkal et al., 2009; Medrano et al., 2009). Indeed, some studies suggest that overactivation of p53 can induce upregulation of p16INK4a and p21Cip1 (Figure 3), suggesting that it may be acting upstream of p16INK4a as well as other CDKN family members and therefore could represent a valuable single target that could affect multiple other genes known to contribute to age-induced senescence (Hinkal et al., 2009; Muthna et al., 2010). It is interesting to note that previous studies have shown that p53 expression in the cochlea increases in response to aging (Tadros et al., 2008) and to cisplatin ototoxicity (Devarajan et al., 2002; Zhang et al., 2003), though whether or not p53 is acting only in its role as a mediator of apoptosis or if it is preventing proliferation and regeneration has yet to be determined. Thus, whether or not p53 disruption could enhance proliferation in existing models at older ages remains an open and tantalizing question.
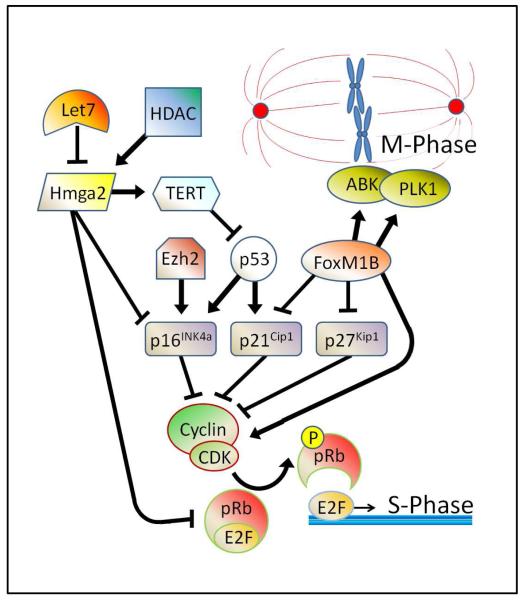
Figure 3. Putative effectors of senescence and their potential interactions in promoting or preventing cell proliferation.
At the bottom of the figure, entry of cells into the synthesis phase (S-phase) of the cell cycle is inhibited by the interaction of the retinoblastoma protein (pRb) and members of the E2F family of transcription factors (E2F). When pRb is hyperphosphorylated (P) by cyclin/cyclin dependent kinase (CDK) complexes, it dissociates from E2F transcription factors, resulting in progression into the cell cycle. Several cyclin dependent kinase inhibitors (e.g. p16INK4a, p21Cip1, p27Kip1) are known to be upregulated with age in many cell types, and act to repress cell proliferation by preventing cyclin-CDK dependent phosphorylation of pRb. The histone-lysine N-methyltransferase Ezh2 may prevent proliferation by promoting increased expression of the cyclin dependent kinase inhibitor (CDKN) p16INK4a. Similarly, the tumor (suppressor) protein 53 also prevents cell cycle entry by upgregulating the CDKNs p16INK4a and p21Cip1. However, p53 itself may be inhibited by high expression or activity of the telomerase reverse transcriptase (TERT) enzyme, which thus promotes cell cycle entry. TERT itself can be upregulated by the high mobility group AT-hook 2 protein (Hmga2) which has also been shown to inhibit p16INK4a and the interaction between pRb proteins and E2F family members, thus promoting cell proliferation. Let7 microRNA family members can target and degrade Hmga2 thus preventing proliferation, while histone deacetylases (HDAC) have been shown to increase Hmga2 expression, thus promoting cell cycle entry. Seemingly separate from these factors, the forkhead box M1 protein isoform B, FoxM1B, promotes cell proliferation by multiple mechanisms. First, FoxM1B upregulates cyclins and CDKs while simultaneously preventing p21Cip1 and p27kip1 from inhibiting the cyclin/CDK complexes. Second, FoxM1B upregulates Aurora B kinase (ABK) and polo-like kinase 1 (PLK1) which are both necessary for progression into the mitosis phase (M-phase) of the cell cycle. Arrows denote upregulation or activation of one factor by another, while flat bars denote the downregulation or inhibition of one factor by another.
Conversely, oncogenes may also play a role in age-related decline of regenerative potential. For example, loss of the forkhead family transcription factor Foxm1b (a.k.a. HFH-11B, Trident, Win, or MPP2) is correlated with age-related decline in regenerative potential, and both loss and gain of function experiments demonstrate its necessity for proliferation and regenerative processes in a number of different tissues (Ackermann Misfeldt et al., 2008; Kalinichenko et al., 2003; Ly et al., 2000; Schmucker et al., 2011; Wang et al., 2001; Zhang et al., 2006a; Zhao et al., 2006). Indeed multiple lines of evidence suggest that Foxm1b is upstream of several cyclin dependent processes (Figure 3), where it promotes cell proliferation and regeneration via decreased nuclear expression of p21Cip1 and p27Kip1 (Kalinichenko et al., 2003; Wang et al., 2001; Wang et al., 2002; Zhang et al., 2006a; Zhao et al., 2006), increased expression of Cdc25a and Cdc25b (which activate the cyclin dependent kinase Cdc2) and increased expression of several cyclins, most notably cyclin D1, cyclin A2, cyclin F, cyclin B1, and cyclin B2 (Kalinichenko et al., 2003; Wang et al., 2001; Wang et al., 2002; Zhao et al., 2006). In addition, Foxm1b not only promotes G1/S phase transition (or cell cycle entry), but also promotes progression through G2/M phase by regulating factors such as Polo-like kinase 1 (PLK1) and Aurora B kinase (ABK) (Gusarova et al., 2007; Krupczak-Hollis et al., 2004; Laoukili et al., 2005; Nakamura et al., 2010). As such, Foxm1b represents a potentially more potent target than p27Kip1 or p21Cip1 alone for the induction of regenerative processes in the cochlea. However, little is known about Foxm1b’s expression and function in the aging cochlea. What is clear is that the Foxm1 gene is expressed in the embryonic mouse cochlea, and that knockdown of insulin-like growth factor 1 (IGF1) increases Foxm1 expression which correlates with decreased nuclear localization of p27Kip1 (Sanchez-Calderon et al., 2010). This suggests that Foxm1b could play a similar role in the cochlea as it does in other tissues, though whether or not Foxm1b expression changes with age, or whether it plays a role in maintaining SC quiescence, however remain important unanswered questions.
Another potential target for age-related decline of regenerative potential is the high mobility group family member Hmga2. While Hmga2 has not been correlated with age-related decline in as many tissue types as some of the other candidate presented, it has been demonstrated that Hmga2 is highly expressed in embryonic neural stem cells and that its expression declines significantly with age (Nishino et al., 2008). Furthermore, knockdown of Hmga2 dramatically reduces neural stem cell self renewal in young and middle aged mice, but not in old mice, where levels in wild-type mice are already low (Nishino et al., 2008). Indeed, Hmga2 has been shown to promote maintenance of stem cell populations and proliferation by multiple means, including maintaining the expression of pluripotency genes like Sox2 and UTF (Li et al., 2007), enhancing E2F1 activity (Fedele et al., 2006), and downregulating the expression of p16INK4a and p14ARF (Nishino et al., 2008). Interestingly, a very recent paper suggests that a similar pattern of declining expression with age occurs in the mouse cochlea, where Hmga2 expression is almost 27 times higher at P3 than at adult ages (Smeti et al., 2012). This last piece of data, as well as the demonstrated effects of Hmga2 on numerous factors known to affect cell proliferation and stem cell self renewal, suggest Hmga2 as an attractive target for manipulation in the mouse cochlea to ascertain whether or not its downregulation with age could be inhibiting stem cell maintenance or SC proliferation. Indeed, mouse models exist that allow for both loss and gain of function studies of Hmga2 function (Ashar et al., 2010), and thus such questions are readily testable with currently available tools.
If we move even further upstream from p16 and Hmga2, we find that both miRNAs and histone acetylation have a demonstrated role in Hmga2 (and thus p16) expression levels (Figure 3). For example, histone deacetylase enzymes (HDACs) have been shown to play a role in the regulation of Hmga2, where inhibition of HDACs with the HDAC inhibitor Trichostatin A results in decreased Hmga2 expression in several different cell lines (Ferguson et al., 2003). This may be important as the application of HDAC inhibitors to regenerating chicken utricles significantly reduced the numbers of proliferating SCs (Slattery et al., 2009). Additionally, several reports demonstrate that members of the let7 family of miRNAs target Hmga2 resulting in its posttranscriptional downregulation (Lee et al., 2007; Mayr et al., 2007; Nishino et al., 2008; Park et al., 2007; Yu et al., 2007). Thus let7 miRs are appealing potential targets for preventing or reducing the effects of aging on regeneration as they prevent proliferation not only via loss of Hmga2 and all of its downstream effects, but also via degradation of the known oncogene hRas (Yu et al., 2007). Additionally, the expression of several let7 miRs are significantly decreased during HC regeneration in the adult newt (Tsonis et al., 2007), suggesting that let7 miRs may indeed be important for the maintenance of quiescence in mechanosensory epithelia. Furthermore, it has been shown that several let7 family members are expressed in the postnatal mouse cochlea, and that their expression levels persist to at least P100 (Weston et al., 2006), suggesting that let7 miRs could be playing an accessory role in age-related senescence of cochlear SCs.
In addition to the potential effects of miRs and histone deacetylation, several studies suggest that histone methylation status is also important for aging and regeneration. Primarily, the polycomb group 2 protein complex and its histone methyltransferase component Ezh2 (enhancer of zeste homolog 2) have been identified in multiple systems as declining with age and as being important regulators of cell proliferation and stem/progenitor cell maintenance (Agherbi et al., 2009; Cakouros et al., 2012; Chen et al., 2009). One potential mechanism for Ezh2’s effects on senescence and regeneration is the methylation of histone 3 (H3) at the locus of p16INK4a and p14ARF, preventing their expression. Conversely, when Ezh2 is downregulated, the histone demethylase Jmjd3 is upregulated, leading to demethylation of H3 and increased expression of p16INK4a and p14ARF (Agger et al., 2009; Agherbi et al., 2009; Barradas et al., 2009; Cakouros et al., 2012; Chen et al., 2009).
Finally, perhaps the one cellular change that is most characteristic of aging is the length of chromosomal telomeres, and their maintenance by telomerase. In many systems and cell types, even those that do not continue to proliferate, aging is correlated with reduced expression and/or activity of the catalytic component of the telomerase complex: telomerase reverse transcriptase (TERT) (Eitan et al., 2012; Ju et al., 2011; Kajstura et al., 2010; Wang et al., 2012a; Wootton et al., 2003; Zhu et al., 2007). Furthermore, decreased expression or function of TERT and the shortening of telomeres has been shown to greatly diminish regenerative potential in numerous tissues via stem or progenitor cell senescence, or in some cases, apoptotic cell death (Ju et al., 2011; Kajstura et al., 2010; Nitta et al., 2011; von Figura et al., 2011; Wang et al., 2012a; Watabe-Rudolph et al., 2011). While some studies have suggested that the effects of TERT downregulation or telomere shortening to prevent regeneration may be mediated by p53 and p21Cip1 (Wang et al., 2012a; Watabe-Rudolph et al., 2011; Westhoff et al., 2010) others have suggested that such effects can be independent of these tumor suppressors (Nitta et al., 2011; von Figura et al., 2011). One additional mechanism may be mediated by p16INK4a expression as loss of p16INK4a can reinstate proliferation in mouse embryonic fibroblasts despite shortened telomeres and dysfunctional telomerase (Nitta et al., 2011; Zhang et al., 2012b). Interestingly, some evidence suggests that Hmga2 may be upstream of TERT, where increased expression of Hmga2 results in increased expression of TERT and longer telomeres, allowing cells to avoid senescence and continue to proliferate (Li et al., 2011). Despite such an established role for TERT and telomere length in regeneration in other systems, very little is known about changes in TERT or telomere length with age in the cochlea. However, TERT expression in the lateral wall of the cochlea does appear to be downregulated in response to cisplatin damage (Guthrie, 2009) suggesting that telomere length could be an important consideration for HC regeneration.
On a final note, there are some rare examples of tissues that do not experience age related declines in regenerative potential (Brann et al., 2010; Stern et al., 2007; Zouboulis et al., 2008), though the reasons for this are not yet well understood. Perhaps, with further study, some of the factors identified here will be revealed to be absent in such systems, or perhaps new and different mechanisms for promoting regeneration will be discovered. What is clear, however, is that the organ of Corti now appears to fall squarely in the group of tissues that exhibit an age-related decline in regenerative ability, and because of this, hopefully the field of auditory HC regeneration will benefit from the findings in these other tissues that face similar obstacles to their recovery.
6. Conclusion
In summary, cells within the postnatal murine organ of Corti are capable of some degree of proliferation and differentiation, but exhibit declining regenerative potential with age, suggesting that multiple processes of aging are likely accumulating to hinder this plasticity over time. The decline in regenerative potential and its correlation with many changes relevant to proliferation and differentiation suggest that the targeting of a single gene, or single mechanism, will likely fail to elicit widespread regeneration or recovery of function in an adult cochlea. Rather, approaches that target multiple aspects of this rapid aging and senescence should be considered. Indeed the current review, which aims to identify commonalities among aging phenotypes in regeneration across multiple mammalian tissues, suggests several targets that have yet to be studied simultaneously or in great detail within the aging cochlea. Among these are: growth factors, epigenetic regulation, micro RNAs, redundancy of CDKN genes, and telomere length/telomerase activity. In discovering which aspects of aging are most detrimental to HC regeneration, we may identify new and significant challenges along the road to therapeutic regeneration (e.g., a requirement of reinstating the GER or of regressing the cochlea to a flat epithelial sheet), or we may find that widespread regeneration and recovery of function lay within easier reach (e.g. targeting one or two miRs or epigenetic mechanisms). Given the previously demonstrated successes of many investigators to induce proliferation and differentiation in the postnatal cochlea, and the successes outlined here pertaining to other cell types whose resistance to regeneration can be overcome, the authors remain wholly optimistic that extensive HC regeneration in an adult mammalian cochlea and complete (or near complete) recovery of hearing function are readily attainable goals.
Highlights for “Postnatal development, maturation and aging of the mouse cochlea and their effects on hair cell regeneration”.
Brad Walters and Jian Zuo
Recent models show cells in the postnatal cochlea can give rise to new hair cells.
Aging decreases the ability to induce cochlear cell proliferation/differentiation.
Many postnatal changes that might dampen cochlear regeneration are described.
Many effectors of age-related senescence in other systems are also described.
Acknowledgements
The authors would like to thank Brandon Cox, Bryan Kuo, Taha Jan, and Alan Cheng for sharing and allowing us to present findings that are not yet published, as well as an additional thanks to Brandon Cox for helpful comments in the preparation of this manuscript. This work was supported by grants from the National Institutes of Health: DC06471 (J.Z.) and CA21765; Office of Naval Research: N000140911014, N000141210191, and N000141210775 (J.Z.); the American Lebanese Syrian Associated Charities (ALSAC) of St. Jude Children’s Research Hospital; The Hartwell Individual Biomedical Research Award (J.Z.); The National Organization For Hearing Research (B.J.W.) and The Hearing Health Foundation 2012 Emerging Research Grant (B.J.W.)
Abbreviations
- HCs
hair cells
- SCs
supporting cells
- E
embryonic day
- P
postnatal day
- PCNA
proliferating cell nuclear antigen
- GER
greater epithelial ridge
- LER
lesser epithelial ridge
- BP
basilar papilla
- DTA
diphtheria toxin fragment A
- EdU
ethynyl-deoxyuridine
- BrdU
bromo-deoxyuridine
- pRb
retinoblastoma protein
- p130
retinoblastoma-like 2 protein
- bHLH
basic helix-loop-helix transcription factor
- CDKN
cyclin dependent kinase inhibitor
- BM
basilar membrane
- Cx
connexin
- EP
endocochlear potential
- FGF
fibroblast growth factor
- FGFR
fibroblast growth factor receptor
- PC
pillar cell
- DC
Deiters cell
- EGFR
epidermal growth factor receptor
- RA
retinoic acid
- GABA
gamma-aminobutyric acid
- AChE
acetylcholinesterase
- Prox1
prospero-related homeobox 1
- PTEN
phosphatase and tensin homolog
- miR
micro-RNA
- p53
tumor protein 53
- PLK1
polo-like kinase 1
- ABK
aurora B kinase
- IGF1
insulin-like growth factor 1
- HDAC
histone deacetylase
- H3
histone 3
- TERT
telomerase reverse transcriptase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ackermann Misfeldt A, Costa RH, Gannon M. Beta-cell proliferation, but not neogenesis, following 60% partial pancreatectomy is impaired in the absence of FoxM1. Diabetes. 2008;57:3069–77. doi: 10.2337/db08-0878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agger K, Cloos PA, Rudkjaer L, Williams K, Andersen G, Christensen J, Helin K. The H3K27me3 demethylase JMJD3 contributes to the activation of the INK4A-ARF locus in response to oncogene- and stress-induced senescence. Genes & development. 2009;23:1171–6. doi: 10.1101/gad.510809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agherbi H, Gaussmann-Wenger A, Verthuy C, Chasson L, Serrano M, Djabali M. Polycomb mediated epigenetic silencing and replication timing at the INK4a/ARF locus during senescence. PloS one. 2009;4:e5622. doi: 10.1371/journal.pone.0005622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn JH, Kang HH, Kim YJ, Chung JW. Anti-apoptotic role of retinoic acid in the inner ear of noise-exposed mice. Biochemical and biophysical research communications. 2005;335:485–90. doi: 10.1016/j.bbrc.2005.07.114. [DOI] [PubMed] [Google Scholar]
- Akino K, Toyota M, Suzuki H, Imai T, Maruyama R, Kusano M, Nishikawa N, Watanabe Y, Sasaki Y, Abe T, Yamamoto E, Tarasawa I, Sonoda T, Mori M, Imai K, Shinomura Y, Tokino T. Identification of DFNA5 as a target of epigenetic inactivation in gastric cancer. Cancer science. 2007;98:88–95. doi: 10.1111/j.1349-7006.2006.00351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves H, Munoz-Najar U, De Wit J, Renard AJ, Hoeijmakers JH, Sedivy JM, Van Blitterswijk C, De Boer J. A link between the accumulation of DNA damage and loss of multi-potency of human mesenchymal stromal cells. Journal of cellular and molecular medicine. 2010;14:2729–38. doi: 10.1111/j.1582-4934.2009.00931.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anniko M. Histochemical, microchemical (microprobe) and organ culture approaches to the study of auditory development. Acta Otolaryngol Suppl. 1985;421:10–8. doi: 10.3109/00016488509121752. [DOI] [PubMed] [Google Scholar]
- Ashar HR, Chouinard RA, Jr., Dokur M, Chada K. In vivo modulation of HMGA2 expression. Biochimica et biophysica acta. 2010;1799:55–61. doi: 10.1016/j.bbagrm.2009.11.013. [DOI] [PubMed] [Google Scholar]
- Bang PI, Sewell WF, Malicki JJ. Morphology and cell type heterogeneities of the inner ear epithelia in adult and juvenile zebrafish (Danio rerio) The Journal of comparative neurology. 2001;438:173–90. doi: 10.1002/cne.1308. [DOI] [PubMed] [Google Scholar]
- Barradas M, Anderton E, Acosta JC, Li S, Banito A, Rodriguez-Niedenfuhr M, Maertens G, Banck M, Zhou MM, Walsh MJ, Peters G, Gil J. Histone demethylase JMJD3 contributes to epigenetic control of INK4a/ARF by oncogenic RAS. Genes & development. 2009;23:1177–82. doi: 10.1101/gad.511109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermingham-McDonogh O, Oesterle EC, Stone JS, Hume CR, Huynh HM, Hayashi T. Expression of Prox1 during mouse cochlear development. The Journal of comparative neurology. 2006;496:172–86. doi: 10.1002/cne.20944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhave SA, Stone JS, Rubel EW, Coltrera MD. Cell cycle progression in gentamicin-damaged avian cochleas. J Neurosci. 1995;15:4618–28. doi: 10.1523/JNEUROSCI.15-06-04618.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonci D, Coppola V, Musumeci M, Addario A, Giuffrida R, Memeo L, D’Urso L, Pagliuca A, Biffoni M, Labbaye C, Bartucci M, Muto G, Peschle C, De Maria R. The miR-15a-miR-16-1 cluster controls prostate cancer by targeting multiple oncogenic activities. Nature medicine. 2008;14:1271–7. doi: 10.1038/nm.1880. [DOI] [PubMed] [Google Scholar]
- Bovo R, Ciorba A, Martini A. Environmental and genetic factors in age-related hearing impairment. Aging clinical and experimental research. 2011;23:3–10. doi: 10.1007/BF03324947. [DOI] [PubMed] [Google Scholar]
- Braeuning A, Singh Y, Rignall B, Buchmann A, Hammad S, Othman A, von Recklinghausen I, Godoy P, Hoehme S, Drasdo D, Hengstler JG, Schwarz M. Phenotype and growth behavior of residual beta-catenin-positive hepatocytes in livers of beta-catenin-deficient mice. Histochemistry and cell biology. 2010;134:469–81. doi: 10.1007/s00418-010-0747-1. [DOI] [PubMed] [Google Scholar]
- Brann JH, Firestein S. Regeneration of new neurons is preserved in aged vomeronasal epithelia. J Neurosci. 2010;30:15686–94. doi: 10.1523/JNEUROSCI.4316-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brignull HR, Raible DW, Stone JS. Feathers and fins: non-mammalian models for hair cell regeneration. Brain research. 2009;1277:12–23. doi: 10.1016/j.brainres.2009.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns JC, Cox BC, Thiede BR, Zuo J, Corwin JT. In vivo proliferative regeneration of balance hair cells in newborn mice. J Neurosci. 2012a;32:6570–7. doi: 10.1523/JNEUROSCI.6274-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns JC, On D, Baker W, Collado MS, Corwin JT. Over Half the Hair Cells in the Mouse Utricle First Appear After Birth, with Significant Numbers Originating from Early Postnatal Mitotic Production in Peripheral and Striolar Growth Zones. J Assoc Res Otolaryngol. 2012b doi: 10.1007/s10162-012-0337-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns JC, Christophel JJ, Collado MS, Magnus C, Carfrae M, Corwin JT. Reinforcement of cell junctions correlates with the absence of hair cell regeneration in mammals and its occurrence in birds. The Journal of comparative neurology. 2008;511:396–414. doi: 10.1002/cne.21849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cakouros D, Isenmann S, Cooper L, Zannettino A, Anderson P, Glackin C, Gronthos S. Twist-1 induces Ezh2 recruitment regulating histone methylation along the Ink4A/Arf locus in mesenchymal stem cells. Molecular and cellular biology. 2012;32:1433–41. doi: 10.1128/MCB.06315-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casillas MA, Jr., Lopatina N, Andrews LG, Tollefsbol TO. Transcriptional control of the DNA methyltransferases is altered in aging and neoplastically-transformed human fibroblasts. Molecular and cellular biochemistry. 2003;252:33–43. doi: 10.1023/a:1025548623524. [DOI] [PubMed] [Google Scholar]
- Chai R, Xia A, Wang T, Jan TA, Hayashi T, Bermingham-McDonogh O, Cheng AG. Dynamic expression of Lgr5, a Wnt target gene, in the developing and mature mouse cochlea. J Assoc Res Otolaryngol. 2011;12:455–69. doi: 10.1007/s10162-011-0267-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai R, Kuo B, Wang T, Liaw EJ, Xia A, Jan TA, Liu Z, Taketo MM, Oghalai JS, Nusse R, Zuo J, Cheng AG. Wnt signaling induces proliferation of sensory precursors in the postnatal mouse cochlea. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:8167–72. doi: 10.1073/pnas.1202774109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chardin S, Romand R. Factors modulating supernumerary hair cell production in the postnatal rat cochlea in vitro. Int J Dev Neurosci. 1997;15:497–507. doi: 10.1016/s0736-5748(96)00106-2. [DOI] [PubMed] [Google Scholar]
- Chen H, Gu X, Su IH, Bottino R, Contreras JL, Tarakhovsky A, Kim SK. Polycomb protein Ezh2 regulates pancreatic beta-cell Ink4a/Arf expression and regeneration in diabetes mellitus. Genes & development. 2009;23:975–85. doi: 10.1101/gad.1742509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Segil N. p27(Kip1) links cell proliferation to morphogenesis in the developing organ of Corti. Development (Cambridge, England) 1999;126:1581–90. doi: 10.1242/dev.126.8.1581. [DOI] [PubMed] [Google Scholar]
- Chen P, Zindy F, Abdala C, Liu F, Li X, Roussel MF, Segil N. Progressive hearing loss in mice lacking the cyclin-dependent kinase inhibitor Ink4d. Nature cell biology. 2003;5:422–6. doi: 10.1038/ncb976. [DOI] [PubMed] [Google Scholar]
- Chien KR, Karsenty G. Longevity and lineages: toward the integrative biology of degenerative diseases in heart, muscle, and bone. Cell. 2005;120:533–44. doi: 10.1016/j.cell.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Choi BY, Song JJ, Chang SO, Kim SU, Oh SH. Intravenous administration of human mesenchymal stem cells after noise- or drug-induced hearing loss in rats. Acta oto-laryngologica. 2012;132(Suppl 1):S94–102. doi: 10.3109/00016489.2012.660731. [DOI] [PubMed] [Google Scholar]
- Cnop M, Igoillo-Esteve M, Hughes SJ, Walker JN, Cnop I, Clark A. Longevity of human islet alpha- and beta-cells. Diabetes, obesity & metabolism. 2011;13(Suppl 1):39–46. doi: 10.1111/j.1463-1326.2011.01443.x. [DOI] [PubMed] [Google Scholar]
- Cohen-Salmon M, Maxeiner S, Kruger O, Theis M, Willecke K, Petit C. Expression of the connexin43- and connexin45-encoding genes in the developing and mature mouse inner ear. Cell and tissue research. 2004;316:15–22. doi: 10.1007/s00441-004-0861-2. [DOI] [PubMed] [Google Scholar]
- Collado MS, Thiede BR, Baker W, Askew C, Igbani LM, Corwin JT. The postnatal accumulation of junctional E-cadherin is inversely correlated with the capacity for supporting cells to convert directly into sensory hair cells in mammalian balance organs. J Neurosci. 2011;31:11855–66. doi: 10.1523/JNEUROSCI.2525-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins CA, Zammit PS, Ruiz AP, Morgan JE, Partridge TA. A population of myogenic stem cells that survives skeletal muscle aging. Stem cells (Dayton, Ohio) 2007;25:885–94. doi: 10.1634/stemcells.2006-0372. [DOI] [PubMed] [Google Scholar]
- Cooke JP, Ghebremariam YT. Endothelial nicotinic acetylcholine receptors and angiogenesis. Trends in cardiovascular medicine. 2008;18:247–53. doi: 10.1016/j.tcm.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove D, Rodgers KD. Expression of the major basement membrane-associated proteins during postnatal development in the murine cochlea. Hearing research. 1997;105:159–70. doi: 10.1016/s0378-5955(96)00203-1. [DOI] [PubMed] [Google Scholar]
- Cox BC, Lenoir A, Zhang L, Steigelman K, Fang J, Zuo J. In Vivo Hair Cell Regeneration in the Neonatal Mouse Cochlea. Abstracts of the 35th Annual Midwinter Research Meeting of the Association for Research in Otolaryngology.2012a. p. 374. [Google Scholar]
- Cox BC, Dearman JA, Papal S, Steigelman K, Valentine MB, Zuo J. The Role of P16Ink4a in Mammalian Hair Cell Regeneration. Abstracts of the 35th Annual Midwinter Research Meeting of the Association for Research in Otolaryngology.2012b. p. 373. [Google Scholar]
- Dabdoub A, Puligilla C, Jones JM, Fritzsch B, Cheah KS, Pevny LH, Kelley MW. Sox2 signaling in prosensory domain specification and subsequent hair cell differentiation in the developing cochlea. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:18396–401. doi: 10.1073/pnas.0808175105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai CF, Steyger PS, Wang ZM, Vass Z, Nuttall AL. Expression of Trk A receptors in the mammalian inner ear. Hearing research. 2004;187:1–11. doi: 10.1016/s0378-5955(03)00277-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daudet N, Ripoll C, Lenoir M. Transforming growth factor-alpha-induced cellular changes in organotypic cultures of juvenile, amikacin-treated rat organ of corti. The Journal of comparative neurology. 2002;442:6–22. doi: 10.1002/cne.1418. [DOI] [PubMed] [Google Scholar]
- David EA, Jackson-Boeters L, Daley T, MacRae DL. Cochlear delivery of fibroblast growth factor 1 and its effects on apoptosis and cell cycling in noise-exposed guinea pig ears. The Journal of otolaryngology. 2002;31:304–12. doi: 10.2310/7070.2002.34330. [DOI] [PubMed] [Google Scholar]
- Davies D, Magnus C, Corwin JT. Developmental changes in cell-extracellular matrix interactions limit proliferation in the mammalian inner ear. The European journal of neuroscience. 2007;25:985–98. doi: 10.1111/j.1460-9568.2007.05355.x. [DOI] [PubMed] [Google Scholar]
- Day ML, Zhao X, Vallorosi CJ, Putzi M, Powell CT, Lin C, Day KC. E-cadherin mediates aggregation-dependent survival of prostate and mammary epithelial cells through the retinoblastoma cell cycle control pathway. The Journal of biological chemistry. 1999;274:9656–64. doi: 10.1074/jbc.274.14.9656. [DOI] [PubMed] [Google Scholar]
- de Beeck KO, Van Laer L, Van Camp G. DFNA5, a gene involved in hearing loss and cancer: a review. The Annals of otology, rhinology, and laryngology. 2012;121:197–207. doi: 10.1177/000348941212100310. [DOI] [PubMed] [Google Scholar]
- de Girolamo L, Lopa S, Arrigoni E, Sartori MF, Baruffaldi Preis FW, Brini AT. Human adipose-derived stem cells isolated from young and elderly women: their differentiation potential and scaffold interaction during in vitro osteoblastic differentiation. Cytotherapy. 2009;11:793–803. doi: 10.3109/14653240903079393. [DOI] [PubMed] [Google Scholar]
- Devarajan P, Savoca M, Castaneda MP, Park MS, Esteban-Cruciani N, Kalinec G, Kalinec F. Cisplatin-induced apoptosis in auditory cells: role of death receptor and mitochondrial pathways. Hearing research. 2002;174:45–54. doi: 10.1016/s0378-5955(02)00634-2. [DOI] [PubMed] [Google Scholar]
- Dhawan S, Tschen SI, Bhushan A. Bmi-1 regulates the Ink4a/Arf locus to control pancreatic beta-cell proliferation. Genes & development. 2009;23:906–11. doi: 10.1101/gad.1742609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetzlhofer A, White PM, Johnson JE, Segil N, Groves AK. In vitro growth and differentiation of mammalian sensory hair cell progenitors: a requirement for EGF and periotic mesenchyme. Developmental biology. 2004;272:432–47. doi: 10.1016/j.ydbio.2004.05.013. [DOI] [PubMed] [Google Scholar]
- Dong Y, Sui L, Yamaguchi F, Kamitori K, Hirata Y, Suzuki A, Holley M, Tokuda M. Role of phosphatase and tensin homolog in the development of the mammalian auditory system. Neuroreport. 2010a;21:731–5. doi: 10.1097/WNR.0b013e32833bfb5e. [DOI] [PubMed] [Google Scholar]
- Dong Y, Sui L, Yamaguchi F, Kamitori K, Hirata Y, Hossain MA, Suzuki A, Holley MC, Tokuda M. Phosphatase and tensin homolog deleted on chromosome 10 regulates sensory cell proliferation and differentiation of hair bundles in the mammalian cochlea. Neuroscience. 2010b;170:1304–13. doi: 10.1016/j.neuroscience.2010.08.024. [DOI] [PubMed] [Google Scholar]
- Ehmann UK, Calderwood SK, Stevenson MA. Gap-junctional communication between feeder cells and recipient normal epithelial cells correlates with growth stimulation. In vitro cellular & developmental biology. 2001;37:100–10. doi: 10.1290/1071-2690(2001)037<0100:GJCBFC>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Eitan E, Tichon A, Daniel G, Priel E. Telomerase expression in adult and old mouse Purkinje neurons. Rejuvenation research. 2012;15:206–9. doi: 10.1089/rej.2011.1285. [DOI] [PubMed] [Google Scholar]
- Elsir T, Smits A, Lindstrom MS, Nister M. Transcription factor PROX1: its role in development and cancer. Cancer metastasis reviews. 2012 doi: 10.1007/s10555-012-9390-8. [DOI] [PubMed] [Google Scholar]
- Emmerling MR, Sobkowicz HM. Differentiation and distribution of acetylcholinesterase molecular forms in the mouse cochlea. Hearing research. 1988;32:137–45. doi: 10.1016/0378-5955(88)90086-x. [DOI] [PubMed] [Google Scholar]
- Emmerling MR, Sobkowicz HM, Levenick CV, Scott GL, Slapnick SM, Rose JE. Biochemical and morphological differentiation of acetylcholinesterase-positive efferent fibers in the mouse cochlea. Journal of electron microscopy technique. 1990;15:123–43. doi: 10.1002/jemt.1060150205. [DOI] [PubMed] [Google Scholar]
- Faroni A, Terenghi G, Magnaghi V. Expression of functional gamma-aminobutyric acid type a receptors in Schwann-like adult stem cells. J Mol Neurosci. 2012;47:619–30. doi: 10.1007/s12031-011-9698-9. [DOI] [PubMed] [Google Scholar]
- Faroni A, Mantovani C, Shawcross SG, Motta M, Terenghi G, Magnaghi V. Schwann-like adult stem cells derived from bone marrow and adipose tissue express gamma-aminobutyric acid type B receptors. Journal of neuroscience research. 2011;89:1351–62. doi: 10.1002/jnr.22652. [DOI] [PubMed] [Google Scholar]
- Fedele M, Visone R, De Martino I, Troncone G, Palmieri D, Battista S, Ciarmiello A, Pallante P, Arra C, Melillo RM, Helin K, Croce CM, Fusco A. HMGA2 induces pituitary tumorigenesis by enhancing E2F1 activity. Cancer cell. 2006;9:459–71. doi: 10.1016/j.ccr.2006.04.024. [DOI] [PubMed] [Google Scholar]
- Fekete DM, Muthukumar S, Karagogeos D. Hair cells and supporting cells share a common progenitor in the avian inner ear. J Neurosci. 1998;18:7811–21. doi: 10.1523/JNEUROSCI.18-19-07811.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson M, Henry PA, Currie RA. Histone deacetylase inhibition is associated with transcriptional repression of the Hmga2 gene. Nucleic acids research. 2003;31:3123–33. doi: 10.1093/nar/gkg403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetoni AR, Picciotti PM, Paludetti G, Troiani D. Pathogenesis of presbycusis in animal models: a review. Experimental gerontology. 2011;46:413–25. doi: 10.1016/j.exger.2010.12.003. [DOI] [PubMed] [Google Scholar]
- Forge A, Li L, Corwin JT, Nevill G. Ultrastructural evidence for hair cell regeneration in the mammalian inner ear. Science (New York, N.Y. 1993;259:1616–9. doi: 10.1126/science.8456284. [DOI] [PubMed] [Google Scholar]
- Frenz CM, Van De Water TR. Immunolocalization of connexin 26 in the developing mouse cochlea. Brain Res Brain Res Rev. 2000;32:172–80. doi: 10.1016/s0165-0173(99)00078-8. [DOI] [PubMed] [Google Scholar]
- Friedman LM, Dror AA, Mor E, Tenne T, Toren G, Satoh T, Biesemeier DJ, Shomron N, Fekete DM, Hornstein E, Avraham KB. MicroRNAs are essential for development and function of inner ear hair cells in vertebrates. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:7915–20. doi: 10.1073/pnas.0812446106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan V, Jin K. Neurogenesis in the aging brain. Clinical interventions in aging. 2007;2:605–10. doi: 10.2147/cia.s1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon HS, Donehower LA, Lyle S, Jones SN. Mdm2-p53 signaling regulates epidermal stem cell senescence and premature aging phenotypes in mouse skin. Developmental biology. 2011;353:1–9. doi: 10.1016/j.ydbio.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giampietri C, Petrungaro S, Coluccia P, Antonangeli F, Giannakakis K, Faraggiana T, Filippini A, Cossu G, Ziparo E. c-Flip overexpression affects satellite cell proliferation and promotes skeletal muscle aging. Cell death & disease. 2010;1:e38. doi: 10.1038/cddis.2010.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go W, Bessarab D, Korzh V. atp2b1a regulates Ca(2+) export during differentiation and regeneration of mechanosensory hair cells in zebrafish. Cell calcium. 2010;48:302–13. doi: 10.1016/j.ceca.2010.09.012. [DOI] [PubMed] [Google Scholar]
- Gritti A, Dal Molin M, Foroni C, Bonfanti L. Effects of developmental age, brain region, and time in culture on long-term proliferation and multipotency of neural stem cell populations. The Journal of comparative neurology. 2009;517:333–49. doi: 10.1002/cne.22153. [DOI] [PubMed] [Google Scholar]
- Gunewardene N, Dottori M, Nayagam BA. The convergence of cochlear implantation with induced pluripotent stem cell therapy. Stem cell reviews. 2012;8:741–54. doi: 10.1007/s12015-011-9320-0. [DOI] [PubMed] [Google Scholar]
- Gusarova GA, Wang IC, Major ML, Kalinichenko VV, Ackerson T, Petrovic V, Costa RH. A cell-penetrating ARF peptide inhibitor of FoxM1 in mouse hepatocellular carcinoma treatment. The Journal of clinical investigation. 2007;117:99–111. doi: 10.1172/JCI27527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie OW. DNA repair proteins and telomerase reverse transcriptase in the cochlear lateral wall of cisplatin-treated rats. Journal of chemotherapy (Florence, Italy) 2009;21:74–9. doi: 10.1179/joc.2009.21.1.74. [DOI] [PubMed] [Google Scholar]
- Hartman BH, Reh TA, Bermingham-McDonogh O. Notch signaling specifies prosensory domains via lateral induction in the developing mammalian inner ear. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:15792–7. doi: 10.1073/pnas.1002827107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman BH, Hayashi T, Nelson BR, Bermingham-McDonogh O, Reh TA. Dll3 is expressed in developing hair cells in the mammalian cochlea. Dev Dyn. 2007;236:2875–83. doi: 10.1002/dvdy.21307. [DOI] [PubMed] [Google Scholar]
- Hartman BH, Basak O, Nelson BR, Taylor V, Bermingham-McDonogh O, Reh TA. Hes5 expression in the postnatal and adult mouse inner ear and the drug-damaged cochlea. J Assoc Res Otolaryngol. 2009;10:321–40. doi: 10.1007/s10162-009-0162-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Cunningham D, Bermingham-McDonogh O. Loss of Fgfr3 leads to excess hair cell development in the mouse organ of Corti. Dev Dyn. 2007;236:525–33. doi: 10.1002/dvdy.21026. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Ray CA, Bermingham-McDonogh O. Fgf20 is required for sensory epithelial specification in the developing cochlea. J Neurosci. 2008;28:5991–9. doi: 10.1523/JNEUROSCI.1690-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Ray CA, Younkins C, Bermingham-McDonogh O. Expression patterns of FGF receptors in the developing mammalian cochlea. Dev Dyn. 2010;239:1019–26. doi: 10.1002/dvdy.22236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich EM, Dimmeler S. MicroRNAs and stem cells: control of pluripotency, reprogramming, and lineage commitment. Circulation research. 2012;110:1014–22. doi: 10.1161/CIRCRESAHA.111.243394. [DOI] [PubMed] [Google Scholar]
- Higgs DM, Souza MJ, Wilkins HR, Presson JC, Popper AN. Age- and size-related changes in the inner ear and hearing ability of the adult zebrafish (Danio rerio) J Assoc Res Otolaryngol. 2002;3:174–84. doi: 10.1007/s101620020035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkal GW, Gatza CE, Parikh N, Donehower LA. Altered senescence, apoptosis, and DNA damage response in a mutant p53 model of accelerated aging. Mechanisms of ageing and development. 2009;130:262–71. doi: 10.1016/j.mad.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori R, Nakagawa T, Sakamoto T, Matsuoka Y, Takebayashi S, Ito J. Pharmacological inhibition of Notch signaling in the mature guinea pig cochlea. Neuroreport. 2007;18:1911–4. doi: 10.1097/WNR.0b013e3282f213e0. [DOI] [PubMed] [Google Scholar]
- Huang M, Sage C, Tang Y, Lee SG, Petrillo M, Hinds PW, Chen ZY. Overlapping and distinct pRb pathways in the mammalian auditory and vestibular organs. Cell cycle (Georgetown, Tex. 2011;10:337–51. doi: 10.4161/cc.10.2.14640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Chi F, Han Z, Yang J, Gao W, Li Y. New ectopic vestibular hair cell-like cells induced by Math1 gene transfer in postnatal rats. Brain research. 2009;1276:31–8. doi: 10.1016/j.brainres.2009.04.036. [DOI] [PubMed] [Google Scholar]
- Inomata K, Aoto T, Binh NT, Okamoto N, Tanimura S, Wakayama T, Iseki S, Hara E, Masunaga T, Shimizu H, Nishimura EK. Genotoxic stress abrogates renewal of melanocyte stem cells by triggering their differentiation. Cell. 2009;137:1088–99. doi: 10.1016/j.cell.2009.03.037. [DOI] [PubMed] [Google Scholar]
- Jan TA, Chai R, Sayyid ZN, van Amerongen R, Xia A, Wang T, Ko P, Sinkkonen ST, Zeng YA, Levin JR, Heller S, Nusse R, Cheng AG. Tympanic border cells are Wnt-responsive and can act as progenitors for postnatal mouse cochlear cells. Unpublished. [DOI] [PMC free article] [PubMed]
- Janzen V, Forkert R, Fleming HE, Saito Y, Waring MT, Dombkowski DM, Cheng T, DePinho RA, Sharpless NE, Scadden DT. Stem-cell ageing modified by the cyclin-dependent kinase inhibitor p16INK4a. Nature. 2006;443:421–6. doi: 10.1038/nature05159. [DOI] [PubMed] [Google Scholar]
- Ju Z, Zhang J, Gao Y, Cheng T. Telomere dysfunction and cell cycle checkpoints in hematopoietic stem cell aging. International journal of hematology. 2011;94:33–43. doi: 10.1007/s12185-011-0882-z. [DOI] [PubMed] [Google Scholar]
- Kajstura J, Gurusamy N, Ogorek B, Goichberg P, Clavo-Rondon C, Hosoda T, D’Amario D, Bardelli S, Beltrami AP, Cesselli D, Bussani R, del Monte F, Quaini F, Rota M, Beltrami CA, Buchholz BA, Leri A, Anversa P. Myocyte turnover in the aging human heart. Circulation research. 2010;107:1374–86. doi: 10.1161/CIRCRESAHA.110.231498. [DOI] [PubMed] [Google Scholar]
- Kalinichenko VV, Gusarova GA, Tan Y, Wang IC, Major ML, Wang X, Yoder HM, Costa RH. Ubiquitous expression of the forkhead box M1B transgene accelerates proliferation of distinct pulmonary cell types following lung injury. The Journal of biological chemistry. 2003;278:37888–94. doi: 10.1074/jbc.M305555200. [DOI] [PubMed] [Google Scholar]
- Kaltenbach JA, Falzarano PR. Postnatal development of the hamster cochlea. I. Growth of hair cells and the organ of Corti. The Journal of comparative neurology. 1994;340:87–97. doi: 10.1002/cne.903400107. [DOI] [PubMed] [Google Scholar]
- Kamiya K, Takahashi K, Kitamura K, Momoi T, Yoshikawa Y. Mitosis and apoptosis in postnatal auditory system of the C3H/He strain. Brain research. 2001;901:296–302. doi: 10.1016/s0006-8993(01)02300-9. [DOI] [PubMed] [Google Scholar]
- Kawamoto K, Izumikawa M, Beyer LA, Atkin GM, Raphael Y. Spontaneous hair cell regeneration in the mouse utricle following gentamicin ototoxicity. Hearing research. 2009;247:17–26. doi: 10.1016/j.heares.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke XS, Liu CM, Liu DP, Liang CC. MicroRNAs: key participants in gene regulatory networks. Current opinion in chemical biology. 2003;7:516–23. doi: 10.1016/s1367-5931(03)00075-9. [DOI] [PubMed] [Google Scholar]
- Kedinger M, Duluc I, Fritsch C, Lorentz O, Plateroti M, Freund JN. Intestinal epithelial-mesenchymal cell interactions. Annals of the New York Academy of Sciences. 1998;859:1–17. doi: 10.1111/j.1749-6632.1998.tb11107.x. [DOI] [PubMed] [Google Scholar]
- Kelley MW, Talreja DR, Corwin JT. Replacement of hair cells after laser microbeam irradiation in cultured organs of corti from embryonic and neonatal mice. J Neurosci. 1995;15:3013–26. doi: 10.1523/JNEUROSCI.15-04-03013.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley MW, Xu XM, Wagner MA, Warchol ME, Corwin JT. The developing organ of Corti contains retinoic acid and forms supernumerary hair cells in response to exogenous retinoic acid in culture. Development (Cambridge, England) 1993;119:1041–53. doi: 10.1242/dev.119.4.1041. [DOI] [PubMed] [Google Scholar]
- Kelly MC, Chang Q, Pan A, Lin X, Chen P. Atoh1 directs the formation of sensory mosaics and induces cell proliferation in the postnatal mammalian cochlea in vivo. J Neurosci. 2012;32:6699–710. doi: 10.1523/JNEUROSCI.5420-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiernan AE, Cordes R, Kopan R, Gossler A, Gridley T. The Notch ligands DLL1 and JAG2 act synergistically to regulate hair cell development in the mammalian inner ear. Development (Cambridge, England) 2005;132:4353–62. doi: 10.1242/dev.02002. [DOI] [PubMed] [Google Scholar]
- Kikuchi K, Hilding D. The development of the organ of Corti in the mouse. Acta oto-laryngologica. 1965;60:207–22. doi: 10.3109/00016486509127003. [DOI] [PubMed] [Google Scholar]
- Kim MS, Chang X, Yamashita K, Nagpal JK, Baek JH, Wu G, Trink B, Ratovitski EA, Mori M, Sidransky D. Aberrant promoter methylation and tumor suppressive activity of the DFNA5 gene in colorectal carcinoma. Oncogene. 2008;27:3624–34. doi: 10.1038/sj.onc.1211021. [DOI] [PubMed] [Google Scholar]
- Krishnamurthy J, Ramsey MR, Ligon KL, Torrice C, Koh A, Bonner-Weir S, Sharpless NE. p16INK4a induces an age-dependent decline in islet regenerative potential. Nature. 2006;443:453–7. doi: 10.1038/nature05092. [DOI] [PubMed] [Google Scholar]
- Krupczak-Hollis K, Wang X, Kalinichenko VV, Gusarova GA, Wang IC, Dennewitz MB, Yoder HM, Kiyokawa H, Kaestner KH, Costa RH. The mouse Forkhead Box m1 transcription factor is essential for hepatoblast mitosis and development of intrahepatic bile ducts and vessels during liver morphogenesis. Developmental biology. 2004;276:74–88. doi: 10.1016/j.ydbio.2004.08.022. [DOI] [PubMed] [Google Scholar]
- Laine H, Doetzlhofer A, Mantela J, Ylikoski J, Laiho M, Roussel MF, Segil N, Pirvola U. p19(Ink4d) and p21(Cip1) collaborate to maintain the postmitotic state of auditory hair cells, their codeletion leading to DNA damage and p53-mediated apoptosis. J Neurosci. 2007;27:1434–44. doi: 10.1523/JNEUROSCI.4956-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambe T, Finlay D, Murphy M, Martin F. Differential expression of connexin 43 in mouse mammary cells. Cell biology international. 2006;30:472–9. doi: 10.1016/j.cellbi.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Laoukili J, Kooistra MR, Bras A, Kauw J, Kerkhoven RM, Morrison A, Clevers H, Medema RH. FoxM1 is required for execution of the mitotic programme and chromosome stability. Nature cell biology. 2005;7:126–36. doi: 10.1038/ncb1217. [DOI] [PubMed] [Google Scholar]
- Lee ER, Friedland DR, Runge CL. Recovery from forward masking in elderly cochlear implant users. Otol Neurotol. 2012;33:355–63. doi: 10.1097/MAO.0b013e318248ede5. [DOI] [PubMed] [Google Scholar]
- Lee KH, Cotanche DA. Potential role of bFGF and retinoic acid in the regeneration of chicken cochlear hair cells. Hearing research. 1996;94:1–13. doi: 10.1016/0378-5955(95)00220-0. [DOI] [PubMed] [Google Scholar]
- Lee YS, Dutta A. The tumor suppressor microRNA let-7 represses the HMGA2 oncogene. Genes & development. 2007;21:1025–30. doi: 10.1101/gad.1540407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YS, Liu F, Segil N. A morphogenetic wave of p27Kip1 transcription directs cell cycle exit during organ of Corti development. Development (Cambridge, England) 2006;133:2817–26. doi: 10.1242/dev.02453. [DOI] [PubMed] [Google Scholar]
- Lees SJ, Rathbone CR, Booth FW. Age-associated decrease in muscle precursor cell differentiation. Am J Physiol Cell Physiol. 2006;290:C609–15. doi: 10.1152/ajpcell.00408.2005. [DOI] [PubMed] [Google Scholar]
- Lefebvre PP, Malgrange B, Staecker H, Moonen G, Van de Water TR. Retinoic acid stimulates regeneration of mammalian auditory hair cells. Science (New York, N.Y. 1993;260:692–5. doi: 10.1126/science.8480180. [DOI] [PubMed] [Google Scholar]
- Lefebvre PP, Malgrange B, Thiry M, Van De Water TR, Moonen G. Epidermal growth factor upregulates production of supernumerary hair cells in neonatal rat organ of corti explants. Acta oto-laryngologica. 2000;120:142–5. doi: 10.1080/000164800750000784. [DOI] [PubMed] [Google Scholar]
- Lenoir M, Vago P. Does the organ of Corti attempt to differentiate new hair cells after antibiotic intoxication in rat pups? Int J Dev Neurosci. 1997;15:487–95. doi: 10.1016/s0736-5748(96)00105-0. [DOI] [PubMed] [Google Scholar]
- Lescale C, Dias S, Maes J, Cumano A, Szabo P, Charron D, Weksler ME, Dosquet C, Vieira P, Goodhardt M. Reduced EBF expression underlies loss of B-cell potential of hematopoietic progenitors with age. Aging cell. 2010;9:410–9. doi: 10.1111/j.1474-9726.2010.00566.x. [DOI] [PubMed] [Google Scholar]
- Li AY, Lin HH, Kuo CY, Shih HM, Wang CC, Yen Y, Ann DK. High-mobility group A2 protein modulates hTERT transcription to promote tumorigenesis. Molecular and cellular biology. 2011;31:2605–17. doi: 10.1128/MCB.05447-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Jiang TX, Hughes MW, Wu P, Widelitz RB, Fan G, Chuong CM. Progressive Alopecia Reveals Decreasing Stem Cell Activation Probability during Aging of Mice with Epidermal Deletion of DNA Methyltransferase 1. The Journal of investigative dermatology. 2012 doi: 10.1038/jid.2012.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Forge A. Morphological evidence for supporting cell to hair cell conversion in the mammalian utricular macula. Int J Dev Neurosci. 1997;15:433–46. doi: 10.1016/s0736-5748(96)00102-5. [DOI] [PubMed] [Google Scholar]
- Li O, Li J, Droge P. DNA architectural factor and proto-oncogene HMGA2 regulates key developmental genes in pluripotent human embryonic stem cells. FEBS letters. 2007;581:3533–7. doi: 10.1016/j.febslet.2007.06.072. [DOI] [PubMed] [Google Scholar]
- Lim DJ. Development of the tectorial membrane. Hearing research. 1987;28:9–21. doi: 10.1016/0378-5955(87)90149-3. [DOI] [PubMed] [Google Scholar]
- Lin V, Golub JS, Nguyen TB, Hume CR, Oesterle EC, Stone JS. Inhibition of Notch activity promotes nonmitotic regeneration of hair cells in the adult mouse utricles. J Neurosci. 2011;31:15329–39. doi: 10.1523/JNEUROSCI.2057-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Owen T, Fang J, Zuo J. Overactivation of Notch1 signaling induces ectopic hair cells in the mouse inner ear in an age-dependent manner. PloS one. 2012a;7:e34123. doi: 10.1371/journal.pone.0034123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Dearman JA, Cox BC, Walters BJ, Zhang L, Ayrault O, Zindy F, Gan L, Roussel MF, Zuo J. Age-dependent in vivo conversion of mouse cochlear pillar and Deiters’ cells to immature hair cells by Atoh1 ectopic expression. J Neurosci. 2012b;32:6600–10. doi: 10.1523/JNEUROSCI.0818-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Walters BJ, Owen T, Brimble MA, Steigelman KA, Zhang L, Mellado Lagarde MM, Valentine MB, Yu Y, Cox BC, Zuo J. Regulation of p27Kip1 by Sox2 Maintains Quiescence of Inner Pillar Cells in the Murine Auditory Sensory Epithelium. J Neurosci. 2012c;32:10530–10540. doi: 10.1523/JNEUROSCI.0686-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopatina N, Haskell JF, Andrews LG, Poole JC, Saldanha S, Tollefsbol T. Differential maintenance and de novo methylating activity by three DNA methyltransferases in aging and immortalized fibroblasts. Journal of cellular biochemistry. 2002;84:324–34. doi: 10.1002/jcb.10015. [DOI] [PubMed] [Google Scholar]
- Lopez IA, Zhao PM, Yamaguchi M, de Vellis J, Espinosa-Jeffrey A. Stem/progenitor cells in the postnatal inner ear of the GFP-nestin transgenic mouse. Int J Dev Neurosci. 2004;22:205–13. doi: 10.1016/j.ijdevneu.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Lou G, Zhang M, Minuk GY. Effects of acute ethanol exposure on polyamine and gamma-aminobutyric acid metabolism in the regenerating liver. Alcohol (Fayetteville, N.Y. 1999;19:219–27. doi: 10.1016/s0741-8329(99)00050-6. [DOI] [PubMed] [Google Scholar]
- Lowenheim H, Furness DN, Kil J, Zinn C, Gultig K, Fero ML, Frost D, Gummer AW, Roberts JM, Rubel EW, Hackney CM, Zenner HP. Gene disruption of p27(Kip1) allows cell proliferation in the postnatal and adult organ of corti. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:4084–8. doi: 10.1073/pnas.96.7.4084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Daniels SB, Lennington JB, Notti RQ, Conover JC. The aging neurogenic subventricular zone. Aging cell. 2006;5:139–52. doi: 10.1111/j.1474-9726.2006.00197.x. [DOI] [PubMed] [Google Scholar]
- Luo L, Koutnouyan H, Baird A, Ryan AF. Acidic and basic FGF mRNA expression in the adult and developing rat cochlea. Hearing research. 1993;69:182–93. doi: 10.1016/0378-5955(93)90106-b. [DOI] [PubMed] [Google Scholar]
- Ly DH, Lockhart DJ, Lerner RA, Schultz PG. Mitotic misregulation and human aging. Science (New York, N.Y. 2000;287:2486–92. doi: 10.1126/science.287.5462.2486. [DOI] [PubMed] [Google Scholar]
- Maeda Y, Fukushima K, Kasai N, Maeta M, Nishizaki K. Quantification of TECTA and DFNA5 expression in the developing mouse cochlea. Neuroreport. 2001;12:3223–6. doi: 10.1097/00001756-200110290-00016. [DOI] [PubMed] [Google Scholar]
- Malgrange B, Knockaert M, Belachew S, Nguyen L, Moonen G, Meijer L, Lefebvre PP. The inhibition of cyclin-dependent kinases induces differentiation of supernumerary hair cells and Deiters’ cells in the developing organ of Corti. Faseb J. 2003;17:2136–8. doi: 10.1096/fj.03-0035fje. [DOI] [PubMed] [Google Scholar]
- Malgrange B, Belachew S, Thiry M, Nguyen L, Rogister B, Alvarez ML, Rigo JM, Van De Water TR, Moonen G, Lefebvre PP. Proliferative generation of mammalian auditory hair cells in culture. Mechanisms of development. 2002;112:79–88. doi: 10.1016/s0925-4773(01)00642-6. [DOI] [PubMed] [Google Scholar]
- Maouche K, Polette M, Jolly T, Medjber K, Cloez-Tayarani I, Changeux JP, Burlet H, Terryn C, Coraux C, Zahm JM, Birembaut P, Tournier JM. {alpha}7 nicotinic acetylcholine receptor regulates airway epithelium differentiation by controlling basal cell proliferation. The American journal of pathology. 2009;175:1868–82. doi: 10.2353/ajpath.2009.090212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Monedero R, Oshima K, Heller S, Edge AS. The potential role of endogenous stem cells in regeneration of the inner ear. Hearing research. 2007;227:48–52. doi: 10.1016/j.heares.2006.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masetto S, Correia MJ. Electrophysiological properties of vestibular sensory and supporting cells in the labyrinth slice before and during regeneration. Journal of neurophysiology. 1997;78:1913–27. doi: 10.1152/jn.1997.78.4.1913. [DOI] [PubMed] [Google Scholar]
- Mayr C, Hemann MT, Bartel DP. Disrupting the pairing between let-7 and Hmga2 enhances oncogenic transformation. Science (New York, N.Y. 2007;315:1576–9. doi: 10.1126/science.1137999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRackan TR, Reda FA, Rivas A, Noble JH, Dietrich MS, Dawant BM, Labadie RF. Comparison of cochlear implant relevant anatomy in children versus adults. Otol Neurotol. 2012;33:328–34. doi: 10.1097/MAO.0b013e318245cc9f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medrano S, Burns-Cusato M, Atienza MB, Rahimi D, Scrable H. Regenerative capacity of neural precursors in the adult mammalian brain is under the control of p53. Neurobiology of aging. 2009;30:483–97. doi: 10.1016/j.neurobiolaging.2007.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menthena A, Koehler CI, Sandhu JS, Yovchev MI, Hurston E, Shafritz DA, Oertel M. Activin A, p15INK4b signaling, and cell competition promote stem/progenitor cell repopulation of livers in aging rats. Gastroenterology. 2011;140:1009–20. doi: 10.1053/j.gastro.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millimaki BB, Sweet EM, Dhason MS, Riley BB. Zebrafish atoh1 genes: classic proneural activity in the inner ear and regulation by Fgf and Notch. Development (Cambridge, England) 2007;134:295–305. doi: 10.1242/dev.02734. [DOI] [PubMed] [Google Scholar]
- Moldovan GL, Pfander B, Jentsch S. PCNA, the maestro of the replication fork. Cell. 2007;129:665–79. doi: 10.1016/j.cell.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Mollura DJ, Hare JM, Rabb H. Stem-cell therapy for renal diseases. Am J Kidney Dis. 2003;42:891–905. doi: 10.1016/j.ajkd.2003.07.018. [DOI] [PubMed] [Google Scholar]
- Molofsky AV, Slutsky SG, Joseph NM, He S, Pardal R, Krishnamurthy J, Sharpless NE, Morrison SJ. Increasing p16INK4a expression decreases forebrain progenitors and neurogenesis during ageing. Nature. 2006;443:448–52. doi: 10.1038/nature05091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momiyama M, Omori Y, Ishizaki Y, Nishikawa Y, Tokairin T, Ogawa J, Enomoto K. Connexin26-mediated gap junctional communication reverses the malignant phenotype of MCF-7 breast cancer cells. Cancer science. 2003;94:501–7. doi: 10.1111/j.1349-7006.2003.tb01473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu MY, Chardin S, Avan P, Romand R. Ontogenesis of rat cochlea. A quantitative study of the organ of Corti. Brain Res Dev Brain Res. 1997;99:29–37. doi: 10.1016/s0165-3806(96)00194-0. [DOI] [PubMed] [Google Scholar]
- Mueller KL, Jacques BE, Kelley MW. Fibroblast growth factor signaling regulates pillar cell development in the organ of corti. J Neurosci. 2002;22:9368–77. doi: 10.1523/JNEUROSCI.22-21-09368.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muramatsu A, Iwai M, Morikawa T, Tanaka S, Mori T, Harada Y, Okanoue T. Influence of transfection with connexin 26 gene on malignant potential of human hepatoma cells. Carcinogenesis. 2002;23:351–8. doi: 10.1093/carcin/23.2.351. [DOI] [PubMed] [Google Scholar]
- Murata J, Tokunaga A, Okano H, Kubo T. Mapping of notch activation during cochlear development in mice: implications for determination of prosensory domain and cell fate diversification. The Journal of comparative neurology. 2006;497:502–18. doi: 10.1002/cne.20997. [DOI] [PubMed] [Google Scholar]
- Mutai H, Nagashima R, Sugitani Y, Noda T, Fujii M, Matsunaga T. Expression of Pou3f3/Brn-1 and its genomic methylation in developing auditory epithelium. Developmental neurobiology. 2009;69:913–30. doi: 10.1002/dneu.20746. [DOI] [PubMed] [Google Scholar]
- Muthna D, Soukup T, Vavrova J, Mokry J, Cmielova J, Visek B, Jiroutova A, Havelek R, Suchanek J, Filip S, English D, Rezacova M. Irradiation of adult human dental pulp stem cells provokes activation of p53, cell cycle arrest, and senescence but not apoptosis. Stem cells and development. 2010;19:1855–62. doi: 10.1089/scd.2009.0449. [DOI] [PubMed] [Google Scholar]
- Nakamura S, Hirano I, Okinaka K, Takemura T, Yokota D, Ono T, Shigeno K, Shibata K, Fujisawa S, Ohnishi K. The FOXM1 transcriptional factor promotes the proliferation of leukemia cells through modulation of cell cycle progression in acute myeloid leukemia. Carcinogenesis. 2010;31:2012–21. doi: 10.1093/carcin/bgq185. [DOI] [PubMed] [Google Scholar]
- Nakashima T, Hattori T, Sone M, Asahi K, Matsuda N, Teranishi M, Yoshida T, Kato K, Sato E. Cochlear blood flow and speech perception ability in cochlear implant users. Otol Neurotol. 2012;33:165–8. doi: 10.1097/MAO.0b013e318241c0db. [DOI] [PubMed] [Google Scholar]
- Nishimura K, Nakagawa T, Sakamoto T, Ito J. Fates of murine pluripotent stem cell-derived neural progenitors following transplantation into mouse cochleae. Cell transplantation. 2012 doi: 10.3727/096368911X623907. [DOI] [PubMed] [Google Scholar]
- Nishimura K, Nakagawa T, Ono K, Ogita H, Sakamoto T, Yamamoto N, Okita K, Yamanaka S, Ito J. Transplantation of mouse induced pluripotent stem cells into the cochlea. Neuroreport. 2009;20:1250–4. doi: 10.1097/WNR.0b013e32832ff287. [DOI] [PubMed] [Google Scholar]
- Nishino J, Kim I, Chada K, Morrison SJ. Hmga2 promotes neural stem cell self-renewal in young but not old mice by reducing p16Ink4a and p19Arf Expression. Cell. 2008;135:227–39. doi: 10.1016/j.cell.2008.09.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitta E, Yamashita M, Hosokawa K, Xian M, Takubo K, Arai F, Nakada S, Suda T. Telomerase reverse transcriptase protects ATM-deficient hematopoietic stem cells from ROS-induced apoptosis through a telomere-independent mechanism. Blood. 2011;117:4169–80. doi: 10.1182/blood-2010-08-297390. [DOI] [PubMed] [Google Scholar]
- Noble W, Tyler RS, Dunn CC, Bhullar N. Younger- and older-age adults with unilateral and bilateral cochlear implants: speech and spatial hearing self-ratings and performance. Otol Neurotol. 2009;30:921–9. doi: 10.1097/MAO.0b013e3181b76b3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norddahl GL, Pronk CJ, Wahlestedt M, Sten G, Nygren JM, Ugale A, Sigvardsson M, Bryder D. Accumulating mitochondrial DNA mutations drive premature hematopoietic aging phenotypes distinct from physiological stem cell aging. Cell stem cell. 2011;8:499–510. doi: 10.1016/j.stem.2011.03.009. [DOI] [PubMed] [Google Scholar]
- Oesterle EC, Campbell S. Supporting cell characteristics in long-deafened aged mouse ears. J Assoc Res Otolaryngol. 2009;10:525–44. doi: 10.1007/s10162-009-0183-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesterle EC, Bhave SA, Coltrera MD. Basic fibroblast growth factor inhibits cell proliferation in cultured avian inner ear sensory epithelia. The Journal of comparative neurology. 2000;424:307–26. doi: 10.1002/1096-9861(20000821)424:2<307::aid-cne9>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Oesterle EC, Chien WM, Campbell S, Nellimarla P, Fero ML. p27 (Kip1) is required to maintain proliferative quiescence in the adult cochlea and pituitary. Cell cycle (Georgetown, Tex. 2011;10 doi: 10.4161/cc.10.8.15301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesterle EC, Rubel EW. Postnatal production of supporting cells in the chick cochlea. Hearing research. 1993;66:213–224. doi: 10.1016/0378-5955(93)90141-m. [DOI] [PubMed] [Google Scholar]
- Ofir M, Hacohen D, Ginsberg D. MiR-15 and miR-16 are direct transcriptional targets of E2F1 that limit E2F-induced proliferation by targeting cyclin E. Mol Cancer Res. 2011;9:440–7. doi: 10.1158/1541-7786.MCR-10-0344. [DOI] [PubMed] [Google Scholar]
- Orsulic S, Huber O, Aberle H, Arnold S, Kemler R. E-cadherin binding prevents beta-catenin nuclear localization and beta-catenin/LEF-1-mediated transactivation. Journal of cell science. 1999;112(Pt 8):1237–45. doi: 10.1242/jcs.112.8.1237. [DOI] [PubMed] [Google Scholar]
- Oshima K, Senn P, Heller S. Isolation of sphere-forming stem cells from the mouse inner ear. Methods in molecular biology (Clifton, N.J. 2009;493:141–62. doi: 10.1007/978-1-59745-523-7_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima K, Shin K, Diensthuber M, Peng AW, Ricci AJ, Heller S. Mechanosensitive hair cell-like cells from embryonic and induced pluripotent stem cells. Cell. 2010;141:704–16. doi: 10.1016/j.cell.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima K, Grimm CM, Corrales CE, Senn P, Martinez Monedero R, Geleoc GS, Edge A, Holt JR, Heller S. Differential distribution of stem cells in the auditory and vestibular organs of the inner ear. J Assoc Res Otolaryngol. 2007;8:18–31. doi: 10.1007/s10162-006-0058-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott T, Jokwitz M, Lenhard D, Romualdi A, Dombrowski F, Ittrich C, Schwarz M, Willecke K. Ablation of gap junctional communication in hepatocytes of transgenic mice does not lead to disrupted cellular homeostasis or increased spontaneous tumourigenesis. European journal of cell biology. 2006;85:717–28. doi: 10.1016/j.ejcb.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Oviedo-Orta E, Perreau M, Evans WH, Potolicchio I. Control of the proliferation of activated CD4+ T cells by connexins. Journal of leukocyte biology. 2010;88:79–86. doi: 10.1189/jlb.0909613. [DOI] [PubMed] [Google Scholar]
- Pan W, Jin Y, Stanger B, Kiernan AE. Notch signaling is required for the generation of hair cells and supporting cells in the mammalian inner ear. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:15798–803. doi: 10.1073/pnas.1003089107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadimas GK, Tzirogiannis KN, Mykoniatis MG, Grypioti AD, Manta GA, Panoutsopoulos GI. The emerging role of serotonin in liver regeneration. Swiss medical weekly. 2012;142:w13548. doi: 10.4414/smw.2012.13548. [DOI] [PubMed] [Google Scholar]
- Parietti C, Vago P, Humbert G, Lenoir M. Attempt at hair cell neodifferentiation in developing and adult amikacin intoxicated rat cochleae. Brain research. 1998;813:57–66. doi: 10.1016/s0006-8993(98)00965-2. [DOI] [PubMed] [Google Scholar]
- Park SM, Shell S, Radjabi AR, Schickel R, Feig C, Boyerinas B, Dinulescu DM, Lengyel E, Peter ME. Let-7 prevents early cancer progression by suppressing expression of the embryonic gene HMGA2. Cell cycle (Georgetown, Tex. 2007;6:2585–90. doi: 10.4161/cc.6.21.4845. [DOI] [PubMed] [Google Scholar]
- Parker M, Brugeaud A, Edge AS. Primary culture and plasmid electroporation of the murine organ of Corti. J Vis Exp. 2010 doi: 10.3791/1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickles JO, van Heumen WR. The expression of messenger RNAs coding for growth factors, their receptors, and eph-class receptor tyrosine kinases in normal and ototoxically damaged chick cochleae. Developmental neuroscience. 1997;19:476–87. doi: 10.1159/000111245. [DOI] [PubMed] [Google Scholar]
- Porrello ER, Mahmoud AI, Simpson E, Hill JA, Richardson JA, Olson EN, Sadek HA. Transient regenerative potential of the neonatal mouse heart. Science (New York, N.Y. 2011;331:1078–80. doi: 10.1126/science.1200708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puligilla C, Feng F, Ishikawa K, Bertuzzi S, Dabdoub A, Griffith AJ, Fritzsch B, Kelley MW. Disruption of fibroblast growth factor receptor 3 signaling results in defects in cellular differentiation, neuronal patterning, and hearing impairment. Dev Dyn. 2007;236:1905–17. doi: 10.1002/dvdy.21192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawson NE, LaMantia AS. A speculative essay on retinoic acid regulation of neural stem cells in the developing and aging olfactory system. Experimental gerontology. 2007;42:46–53. doi: 10.1016/j.exger.2006.05.021. [DOI] [PubMed] [Google Scholar]
- Raz Y, Kelley MW. Retinoic acid signaling is necessary for the development of the organ of Corti. Developmental biology. 1999;213:180–93. doi: 10.1006/dbio.1999.9364. [DOI] [PubMed] [Google Scholar]
- Rio C, Dikkes P, Liberman MC, Corfas G. Glial fibrillary acidic protein expression and promoter activity in the inner ear of developing and adult mice. The Journal of comparative neurology. 2002;442:156–62. doi: 10.1002/cne.10085. [DOI] [PubMed] [Google Scholar]
- Rocha-Sanchez SM, Scheetz LR, Contreras M, Weston MD, Korte M, McGee J, Walsh EJ. Mature mice lacking Rbl2/p130 gene have supernumerary inner ear hair cells and supporting cells. J Neurosci. 2011;31:8883–93. doi: 10.1523/JNEUROSCI.5821-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers KD, Barritt L, Miner JH, Cosgrove D. The laminins in the murine inner ear: developmental transitions and expression in cochlear basement membranes. Hearing research. 2001;158:39–50. doi: 10.1016/s0378-5955(01)00283-0. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Menocal L, Pham SM, Mateu D, St-Pierre M, Wei Y, Pestana I, Aitouche A, Vazquez-Padron RI. Aging increases p16 INK4a expression in vascular smooth-muscle cells. Bioscience reports. 2009;30:11–8. doi: 10.1042/BSR20080128. [DOI] [PubMed] [Google Scholar]
- Romand R, Kondo T, Fraulob V, Petkovich M, Dolle P, Hashino E. Dynamic expression of retinoic acid-synthesizing and -metabolizing enzymes in the developing mouse inner ear. The Journal of comparative neurology. 2006;496:643–54. doi: 10.1002/cne.20936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruben RJ. Development of the inner ear of the mouse: a radioautographic study of terminal mitoses. Acta oto-laryngologica. 1967;220(Suppl):1–44. [PubMed] [Google Scholar]
- Salpeter SJ, Klein AM, Huangfu D, Grimsby J, Dor Y. Glucose and aging control the quiescence period that follows pancreatic beta cell replication. Development (Cambridge, England) 2010;137:3205–13. doi: 10.1242/dev.054304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Calderon H, Rodriguez-de la Rosa L, Milo M, Pichel JG, Holley M, Varela-Nieto I. RNA microarray analysis in prenatal mouse cochlea reveals novel IGF-I target genes: implication of MEF2 and FOXM1 transcription factors. PloS one. 2010;5:e8699. doi: 10.1371/journal.pone.0008699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savary E, Hugnot JP, Chassigneux Y, Travo C, Duperray C, Van De Water T, Zine A. Distinct population of hair cell progenitors can be isolated from the postnatal mouse cochlea using side population analysis. Stem cells (Dayton, Ohio) 2007;25:332–9. doi: 10.1634/stemcells.2006-0303. [DOI] [PubMed] [Google Scholar]
- Savary E, Sabourin JC, Santo J, Hugnot JP, Chabbert C, Van De Water T, Uziel A, Zine A. Cochlear stem/progenitor cells from a postnatal cochlea respond to Jagged1 and demonstrate that notch signaling promotes sphere formation and sensory potential. Mechanisms of development. 2008;125:674–86. doi: 10.1016/j.mod.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Schlecker C, Praetorius M, Brough DE, Presler RG, Jr., Hsu C, Plinkert PK, Staecker H. Selective atonal gene delivery improves balance function in a mouse model of vestibular disease. Gene therapy. 2011;18:884–90. doi: 10.1038/gt.2011.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmucker DL, Sanchez H. Liver regeneration and aging: a current perspective. Current gerontology and geriatrics research. 2011;2011:526379. doi: 10.1155/2011/526379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha SH, Chen FQ, Schacht J. Activation of cell death pathways in the inner ear of the aging CBA/J mouse. Hearing research. 2009;254:92–9. doi: 10.1016/j.heares.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha SH, Chen FQ, Schacht J. PTEN attenuates PIP3/Akt signaling in the cochlea of the aging CBA/J mouse. Hearing research. 2010;264:86–92. doi: 10.1016/j.heares.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi F, Kempfle JS, Edge AS. Wnt-responsive lgr5-expressing stem cells are hair cell progenitors in the cochlea. J Neurosci. 2012;32:9639–48. doi: 10.1523/JNEUROSCI.1064-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim HJ, Kang HH, Ahn JH, Chung JW. Retinoic acid applied after noise exposure can recover the noise-induced hearing loss in mice. Acta oto-laryngologica. 2009;129:233–8. doi: 10.1080/00016480802226155. [DOI] [PubMed] [Google Scholar]
- Siddappa R, Licht R, van Blitterswijk C, de Boer J. Donor variation and loss of multipotency during in vitro expansion of human mesenchymal stem cells for bone tissue engineering. J Orthop Res. 2007;25:1029–41. doi: 10.1002/jor.20402. [DOI] [PubMed] [Google Scholar]
- Simpson RM, Wells A, Thomas D, Stephens P, Steadman R, Phillips A. Aging fibroblasts resist phenotypic maturation because of impaired hyaluronan-dependent CD44/epidermal growth factor receptor signaling. The American journal of pathology. 2010;176:1215–28. doi: 10.2353/ajpath.2010.090802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinkkonen ST, Chai R, Jan TA, Hartman BH, Laske RD, Gahlen F, Sinkkonen W, Cheng AG, Oshima K, Heller S. Intrinsic regenerative potential of murine cochlear supporting cells. Scientific reports. 2011;1:26. doi: 10.1038/srep00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slattery EL, Speck JD, Warchol ME. Epigenetic influences on sensory regeneration: histone deacetylases regulate supporting cell proliferation in the avian utricle. J Assoc Res Otolaryngol. 2009;10:341–53. doi: 10.1007/s10162-009-0166-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeti I, Assou S, Savary E, Masmoudi S, Zine A. Transcriptomic analysis of the developing and adult mouse cochlear sensory epithelia. PloS one. 2012;7:e42987. doi: 10.1371/journal.pone.0042987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeti I, Savary E, Capelle V, Hugnot JP, Uziel A, Zine A. Expression of candidate markers for stem/progenitor cells in the inner ears of developing and adult GFAP and nestin promoter-GFP transgenic mice. Gene Expr Patterns. 2011;11:22–32. doi: 10.1016/j.gep.2010.08.008. [DOI] [PubMed] [Google Scholar]
- So AY, Jung JW, Lee S, Kim HS, Kang KS. DNA methyltransferase controls stem cell aging by regulating BMI1 and EZH2 through microRNAs. PloS one. 2011;6:e19503. doi: 10.1371/journal.pone.0019503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobkowicz HM, Emmerling MR. Development of acetylcholinesterase-positive neuronal pathways in the cochlea of the mouse. Journal of neurocytology. 1989;18:209–24. doi: 10.1007/BF01206663. [DOI] [PubMed] [Google Scholar]
- Sobkowicz HM, Rose JE, Scott GL, Levenick CV. Distribution of synaptic ribbons in the developing organ of Corti. Journal of neurocytology. 1986;15:693–714. doi: 10.1007/BF01625188. [DOI] [PubMed] [Google Scholar]
- Soltani N, Qiu H, Aleksic M, Glinka Y, Zhao F, Liu R, Li Y, Zhang N, Chakrabarti R, Ng T, Jin T, Zhang H, Lu WY, Feng ZP, Prud’homme GJ, Wang Q. GABA exerts protective and regenerative effects on islet beta cells and reverses diabetes. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:11692–7. doi: 10.1073/pnas.1102715108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Croix B, Sheehan C, Rak JW, Florenes VA, Slingerland JM, Kerbel RS. E-Cadherin-dependent growth suppression is mediated by the cyclin-dependent kinase inhibitor p27(KIP1) The Journal of cell biology. 1998;142:557–71. doi: 10.1083/jcb.142.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staecker H, Praetorius M, Baker K, Brough DE. Vestibular hair cell regeneration and restoration of balance function induced by math1 gene transfer. Otol Neurotol. 2007;28:223–31. doi: 10.1097/MAO.0b013e31802b3225. [DOI] [PubMed] [Google Scholar]
- Stern MM, Bickenbach JR. Epidermal stem cells are resistant to cellular aging. Aging cell. 2007;6:439–52. doi: 10.1111/j.1474-9726.2007.00318.x. [DOI] [PubMed] [Google Scholar]
- Stockinger A, Eger A, Wolf J, Beug H, Foisner R. E-cadherin regulates cell growth by modulating proliferation-dependent beta-catenin transcriptional activity. The Journal of cell biology. 2001;154:1185–96. doi: 10.1083/jcb.200104036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone JS, Shang JL, Tomarev S. cProx1 immunoreactivity distinguishes progenitor cells and predicts hair cell fate during avian hair cell regeneration. Dev Dyn. 2004;230:597–614. doi: 10.1002/dvdy.20087. [DOI] [PubMed] [Google Scholar]
- Strzalka W, Ziemienowicz A. Proliferating cell nuclear antigen (PCNA): a key factor in DNA replication and cell cycle regulation. Annals of botany. 2011;107:1127–40. doi: 10.1093/aob/mcq243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundelacruz S, Levin M, Kaplan DL. Membrane potential controls adipogenic and osteogenic differentiation of mesenchymal stem cells. PloS one. 2008;3:e3737. doi: 10.1371/journal.pone.0003737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzukawa K, Kondo K, Kanaya K, Sakamoto T, Watanabe K, Ushio M, Kaga K, Yamasoba T. Age-related changes of the regeneration mode in the mouse peripheral olfactory system following olfactotoxic drug methimazole-induced damage. The Journal of comparative neurology. 2011;519:2154–74. doi: 10.1002/cne.22611. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Sakaguchi T, Inaba K, Suzuki S, Konno H. Impact of cell cycle disruption on impaired hepatic regeneration in aged livers with ischemic insult. The Journal of surgical research. 2012;173:267–77. doi: 10.1016/j.jss.2010.10.012. [DOI] [PubMed] [Google Scholar]
- Sweet EM, Vemaraju S, Riley BB. Sox2 and Fgf interact with Atoh1 to promote sensory competence throughout the zebrafish inner ear. Developmental biology. 2011;358:113–21. doi: 10.1016/j.ydbio.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadros SF, D’Souza M, Zhu X, Frisina RD. Apoptosis-related genes change their expression with age and hearing loss in the mouse cochlea. Apoptosis. 2008;13:1303–21. doi: 10.1007/s10495-008-0266-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Kamiya K, Urase K, Suga M, Takizawa T, Mori H, Yoshikawa Y, Ichimura K, Kuida K, Momoi T. Caspase-3-deficiency induces hyperplasia of supporting cells and degeneration of sensory cells resulting in the hearing loss. Brain research. 2001;894:359–67. doi: 10.1016/s0006-8993(01)02123-0. [DOI] [PubMed] [Google Scholar]
- Taniguchi Ishikawa E, Gonzalez-Nieto D, Ghiaur G, Dunn SK, Ficker AM, Murali B, Madhu M, Gutstein DE, Fishman GI, Barrio LC, Cancelas JA. Connexin-43 prevents hematopoietic stem cell senescence through transfer of reactive oxygen species to bone marrow stromal cells. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:9071–6. doi: 10.1073/pnas.1120358109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JP, Lautermann J, Liedert B, Seiler F, Thomale J. High accumulation of platinum-DNA adducts in strial marginal cells of the cochlea is an early event in cisplatin but not carboplatin ototoxicity. Molecular pharmacology. 2006;70:23–9. doi: 10.1124/mol.106.022244. [DOI] [PubMed] [Google Scholar]
- Timchenko NA. Aging and liver regeneration. Trends in endocrinology and metabolism: TEM. 2009;20:171–6. doi: 10.1016/j.tem.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Torres LN, Matera JM, Vasconcellos CH, Avanzo JL, Hernandez-Blazquez FJ, Dagli ML. Expression of connexins 26 and 43 in canine hyperplastic and neoplastic mammary glands. Veterinary pathology. 2005;42:633–41. doi: 10.1354/vp.42-5-633. [DOI] [PubMed] [Google Scholar]
- Trinh NT, Prive A, Maille E, Noel J, Brochiero E. EGF and K+ channel activity control normal and cystic fibrosis bronchial epithelia repair. American journal of physiology. 2008;295:L866–80. doi: 10.1152/ajplung.90224.2008. [DOI] [PubMed] [Google Scholar]
- Tseng AS, Beane WS, Lemire JM, Masi A, Levin M. Induction of vertebrate regeneration by a transient sodium current. J Neurosci. 2010;30:13192–200. doi: 10.1523/JNEUROSCI.3315-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsonis PA, Call MK, Grogg MW, Sartor MA, Taylor RR, Forge A, Fyffe R, Goldenberg R, Cowper-Sal-lari R, Tomlinson CR. MicroRNAs and regeneration: Let-7 members as potential regulators of dedifferentiation in lens and inner ear hair cell regeneration of the adult newt. Biochemical and biophysical research communications. 2007;362:940–5. doi: 10.1016/j.bbrc.2007.08.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umemoto M, Sakagami M, Fukazawa K, Ashida K, Kubo T, Senda T, Yoneda Y. Hair cell regeneration in the chick inner ear following acoustic trauma: ultrastructural and immunohistochemical studies. Cell and tissue research. 1995;281:435–43. doi: 10.1007/BF00417861. [DOI] [PubMed] [Google Scholar]
- Usami S, Takumi Y, Fujita S, Shinkawa H, Hosokawa M. Cell death in the inner ear associated with aging is apoptosis? Brain research. 1997;747:147–50. doi: 10.1016/s0006-8993(96)01243-7. [DOI] [PubMed] [Google Scholar]
- Van Laer L, Pfister M, Thys S, Vrijens K, Mueller M, Umans L, Serneels L, Van Nassauw L, Kooy F, Smith RJ, Timmermans JP, Van Leuven F, Van Camp G. Mice lacking Dfna5 show a diverging number of cochlear fourth row outer hair cells. Neurobiology of disease. 2005;19:386–99. doi: 10.1016/j.nbd.2005.01.019. [DOI] [PubMed] [Google Scholar]
- von Figura G, Wagner M, Nalapareddy K, Hartmann D, Kleger A, Guachalla LM, Rolyan H, Adler G, Rudolph KL. Regeneration of the exocrine pancreas is delayed in telomere-dysfunctional mice. PloS one. 2011;6:e17122. doi: 10.1371/journal.pone.0017122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldhaus J, Cimerman J, Gohlke H, Ehrich M, Muller M, Lowenheim H. Stemness of the organ of Corti relates to the epigenetic status of Sox2 enhancers. PloS one. 2012;7:e36066. doi: 10.1371/journal.pone.0036066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Chen Q, Lee SH, Choi Y, Brad Johnson F, Pignolo RJ. Impairment of osteoblast differentiation due to proliferation-independent telomere dysfunction in mouse models of accelerated aging. Aging cell. 2012a;11:704–13. doi: 10.1111/j.1474-9726.2012.00838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Quail E, Hung NJ, Tan Y, Ye H, Costa RH. Increased levels of forkhead box M1B transcription factor in transgenic mouse hepatocytes prevent age-related proliferation defects in regenerating liver. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:11468–73. doi: 10.1073/pnas.201360898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Krupczak-Hollis K, Tan Y, Dennewitz MB, Adami GR, Costa RH. Increased hepatic Forkhead Box M1B (FoxM1B) levels in old-aged mice stimulated liver regeneration through diminished p27Kip1 protein levels and increased Cdc25B expression. The Journal of biological chemistry. 2002;277:44310–6. doi: 10.1074/jbc.M207510200. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zang X, Wang Y, Chen P. High expression of p16INK4a and low expression of Bmi1 are associated with endothelial cellular senescence in the human cornea. Molecular vision. 2012b;18:803–15. [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Jiang H, Yan Y, Wang Y, Shen Y, Li W, Li H. Characterization of proliferating cells from newborn mouse cochleae. Neuroreport. 2006;17:767–71. doi: 10.1097/01.wnr.0000215781.22345.8b. [DOI] [PubMed] [Google Scholar]
- Warchol ME. Cell density and N-cadherin interactions regulate cell proliferation in the sensory epithelia of the inner ear. J Neurosci. 2002;22:2607–16. doi: 10.1523/JNEUROSCI.22-07-02607.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warchol ME, Lambert PR, Goldstein BJ, Forge A, Corwin JT. Regenerative proliferation in inner ear sensory epithelia from adult guinea pigs and humans. Science (New York, N.Y. 1993;259:1619–22. doi: 10.1126/science.8456285. [DOI] [PubMed] [Google Scholar]
- Watabe-Rudolph M, Begus-Nahrmann Y, Lechel A, Rolyan H, Scheithauer MO, Rettinger G, Thal DR, Rudolph KL. Telomere shortening impairs regeneration of the olfactory epithelium in response to injury but not under homeostatic conditions. PloS one. 2011;6:e27801. doi: 10.1371/journal.pone.0027801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe R, Morell MH, Miller JM, Kanicki AC, O’Shea KS, Altschuler RA, Raphael Y. Nestin-expressing cells in the developing, mature and noise-exposed cochlear epithelium. Molecular and cellular neurosciences. 2012;49:104–9. doi: 10.1016/j.mcn.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber T, Corbett MK, Chow LM, Valentine MB, Baker SJ, Zuo J. Rapid cell-cycle reentry and cell death after acute inactivation of the retinoblastoma gene product in postnatal cochlear hair cells. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:781–5. doi: 10.1073/pnas.0708061105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westhoff JH, Schildhorn C, Jacobi C, Homme M, Hartner A, Braun H, Kryzer C, Wang C, von Zglinicki T, Kranzlin B, Gretz N, Melk A. Telomere shortening reduces regenerative capacity after acute kidney injury. J Am Soc Nephrol. 2010;21:327–36. doi: 10.1681/ASN.2009010072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston MD, Pierce ML, Rocha-Sanchez S, Beisel KW, Soukup GA. MicroRNA gene expression in the mouse inner ear. Brain research. 2006;1111:95–104. doi: 10.1016/j.brainres.2006.07.006. [DOI] [PubMed] [Google Scholar]
- White PM, Stone JS, Groves AK, Segil N. EGFR signaling is required for regenerative proliferation in the cochlea: conservation in birds and mammals. Developmental biology. 2012;363:191–200. doi: 10.1016/j.ydbio.2011.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White PM, Doetzlhofer A, Lee YS, Groves AK, Segil N. Mammalian cochlear supporting cells can divide and trans-differentiate into hair cells. Nature. 2006;441:984–7. doi: 10.1038/nature04849. [DOI] [PubMed] [Google Scholar]
- Whitlon DS. E-cadherin in the mature and developing organ of Corti of the mouse. Journal of neurocytology. 1993;22:1030–8. doi: 10.1007/BF01235747. [DOI] [PubMed] [Google Scholar]
- Whitlon DS, Sobkowicz HM. GABA-like immunoreactivity in the cochlea of the developing mouse. Journal of neurocytology. 1989;18:505–18. doi: 10.1007/BF01474546. [DOI] [PubMed] [Google Scholar]
- Whitlon DS, Zhang X, Pecelunas K, Greiner MA. A temporospatial map of adhesive molecules in the organ of Corti of the mouse cochlea. Journal of neurocytology. 1999;28:955–68. doi: 10.1023/a:1007038609456. [DOI] [PubMed] [Google Scholar]
- Winterhager E, Kaufmann P, Gruemmer R. Cell-cell-communication during placental development and possible implications for trophoblast proliferation and differentiation. Placenta. 2000;21(Suppl A):S61–8. doi: 10.1053/plac.1999.0516. [DOI] [PubMed] [Google Scholar]
- Wootton M, Steeghs K, Watt D, Munro J, Gordon K, Ireland H, Morrison V, Behan W, Parkinson EK. Telomerase alone extends the replicative life span of human skeletal muscle cells without compromising genomic stability. Human gene therapy. 2003;14:1473–87. doi: 10.1089/104303403769211682. [DOI] [PubMed] [Google Scholar]
- Xia A, Kikuchi T, Hozawa K, Katori Y, Takasaka T. Expression of connexin 26 and Na,K-ATPase in the developing mouse cochlear lateral wall: functional implications. Brain research. 1999;846:106–11. doi: 10.1016/s0006-8993(99)01996-4. [DOI] [PubMed] [Google Scholar]
- Xia A, Katori Y, Oshima T, Watanabe K, Kikuchi T, Ikeda K. Expression of connexin 30 in the developing mouse cochlea. Brain research. 2001;898:364–7. doi: 10.1016/s0006-8993(01)02216-8. [DOI] [PubMed] [Google Scholar]
- Yamamoto N, Chang W, Kelley MW. Rbpj regulates development of prosensory cells in the mammalian inner ear. Developmental biology. 2011;353:367–79. doi: 10.1016/j.ydbio.2011.03.016. [DOI] [PubMed] [Google Scholar]
- Yamamoto N, Tanigaki K, Tsuji M, Yabe D, Ito J, Honjo T. Inhibition of Notch/RBP-J signaling induces hair cell formation in neonate mouse cochleas. Journal of molecular medicine (Berlin, Germany) 2006;84:37–45. doi: 10.1007/s00109-005-0706-9. [DOI] [PubMed] [Google Scholar]
- Yang H, Fogo AB. Cell senescence in the aging kidney. J Am Soc Nephrol. 2010;21:1436–9. doi: 10.1681/ASN.2010020205. [DOI] [PubMed] [Google Scholar]
- Yerukhimovich MV, Bai L, Chen DH, Miller RH, Alagramam KN. Identification and characterization of mouse cochlear stem cells. Developmental neuroscience. 2007;29:251–60. doi: 10.1159/000096415. [DOI] [PubMed] [Google Scholar]
- Yu F, Yao H, Zhu P, Zhang X, Pan Q, Gong C, Huang Y, Hu X, Su F, Lieberman J, Song E. let-7 regulates self renewal and tumorigenicity of breast cancer cells. Cell. 2007;131:1109–23. doi: 10.1016/j.cell.2007.10.054. [DOI] [PubMed] [Google Scholar]
- Yu Y, Weber T, Yamashita T, Liu Z, Valentine MB, Cox BC, Zuo J. In vivo proliferation of postmitotic cochlear supporting cells by acute ablation of the retinoblastoma protein in neonatal mice. J Neurosci. 2010;30:5927–36. doi: 10.1523/JNEUROSCI.5989-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Ackermann AM, Gusarova GA, Lowe D, Feng X, Kopsombut UG, Costa RH, Gannon M. The FoxM1 transcription factor is required to maintain pancreatic beta-cell mass. Molecular endocrinology (Baltimore, Md. 2006a;20:1853–66. doi: 10.1210/me.2006-0056. [DOI] [PubMed] [Google Scholar]
- Zhang J, Chan YC, Ho JC, Siu CW, Lian Q, Tse HF. Regulation of cell proliferation of human induced pluripotent stem cell-derived mesenchymal stem cells via ether-a-go-go 1 (hEAG1) potassium channel. Am J Physiol Cell Physiol. 2012a;303:C115–25. doi: 10.1152/ajpcell.00326.2011. [DOI] [PubMed] [Google Scholar]
- Zhang M, Liu W, Ding D, Salvi R. Pifithrin-alpha suppresses p53 and protects cochlear and vestibular hair cells from cisplatin-induced apoptosis. Neuroscience. 2003;120:191–205. doi: 10.1016/s0306-4522(03)00286-0. [DOI] [PubMed] [Google Scholar]
- Zhang X, Wu X, Tang W, Luo Y. Loss of p16(Ink4a) Function Rescues Cellular Senescence Induced by Telomere Dysfunction. International journal of molecular sciences. 2012b;13:5866–77. doi: 10.3390/ijms13055866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Chen X, Wu D, Liu W, Wang J, Feng Z, Cai G, Fu B, Hong Q, Du J. Downregulation of connexin 43 expression by high glucose induces senescence in glomerular mesangial cells. J Am Soc Nephrol. 2006b;17:1532–42. doi: 10.1681/ASN.2005070776. [DOI] [PubMed] [Google Scholar]
- Zhao H, Kalota A, Jin S, Gewirtz AM. The c-myb proto-oncogene and microRNA-15a comprise an active autoregulatory feedback loop in human hematopoietic cells. Blood. 2009;113:505–16. doi: 10.1182/blood-2008-01-136218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao LD, Guo WW, Lin C, Li LX, Sun JH, Wu N, Ren LL, Li XX, Liu HZ, Young WY, Gao WQ, Yang SM. Effects of DAPT and Atoh1 overexpression on hair cell production and hair bundle orientation in cultured Organ of Corti from neonatal rats. PloS one. 2011;6:e23729. doi: 10.1371/journal.pone.0023729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao YY, Gao XP, Zhao YD, Mirza MK, Frey RS, Kalinichenko VV, Wang IC, Costa RH, Malik AB. Endothelial cell-restricted disruption of FoxM1 impairs endothelial repair following LPS-induced vascular injury. The Journal of clinical investigation. 2006;116:2333–43. doi: 10.1172/JCI27154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng JL, Gao WQ. Overexpression of Math1 induces robust production of extra hair cells in postnatal rat inner ears. Nature neuroscience. 2000;3:580–6. doi: 10.1038/75753. [DOI] [PubMed] [Google Scholar]
- Zheng JL, Helbig C, Gao WQ. Induction of cell proliferation by fibroblast and insulin-like growth factors in pure rat inner ear epithelial cell cultures. J Neurosci. 1997;17:216–26. doi: 10.1523/JNEUROSCI.17-01-00216.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong C, Han Y, Qiu J, Lu L, Chen Y, Chen J, Hei R, Mi W. A comparison of the proliferative capacity and ultrastructure of proliferative cells from the cochleae of newborn rats of different ages. International journal of pediatric otorhinolaryngology. 2010;74:192–7. doi: 10.1016/j.ijporl.2009.11.009. [DOI] [PubMed] [Google Scholar]
- Zhu CH, Mouly V, Cooper RN, Mamchaoui K, Bigot A, Shay JW, Di Santo JP, Butler-Browne GS, Wright WE. Cellular senescence in human myoblasts is overcome by human telomerase reverse transcriptase and cyclin-dependent kinase 4: consequences in aging muscle and therapeutic strategies for muscular dystrophies. Aging cell. 2007;6:515–23. doi: 10.1111/j.1474-9726.2007.00306.x. [DOI] [PubMed] [Google Scholar]
- Zine A, Nyffeler M, de Ribaupierre F. Spatial expression patterns of epidermal growth factor receptor gene transcripts in the postnatal mammalian cochlea. Hearing research. 2000;141:19–27. doi: 10.1016/s0378-5955(99)00203-8. [DOI] [PubMed] [Google Scholar]
- Zouboulis CC, Adjaye J, Akamatsu H, Moe-Behrens G, Niemann C. Human skin stem cells and the ageing process. Experimental gerontology. 2008;43:986–97. doi: 10.1016/j.exger.2008.09.001. [DOI] [PubMed] [Google Scholar]