Abstract
Renal fibrosis is the culmination of processes driven by signaling pathways involving transforming growth factor (TGF)-β family of cytokines, connective-tissue growth factor, nuclear factor κB, Wnt/β-catenin, Notch, and other growth factors. Many studies in experimental animal models have directly targeted these pathways and demonstrated efficacy in mitigating renal fibrosis. However, only a small fraction of these approaches have been attempted in human and even fewer have been successfully translated to clinical use for patient with kidney diseases. Drugs with proven efficacy for treatment of kidney diseases and tissue fibrosis exert some of their effects by interfering with components of these pathways. This review considers key molecular mediators of renal fibrosis and their potential as targets for treatment of renal fibrosis.
Keywords: kidney, fibrosis, HIPK2, signaling pathways, treatment
Introduction
Renal fibrosis is considered the final convergent pathway for progressive kidney diseases due to a wide range of pathophysiologically distinct processes [1]. The extent of renal fibrosis is not only a marker of injury, but also predicts the loss of function and progression of damage in the kidney [2–4]. Renal fibrogenesis involves cells from all compartments of the kidney (tubulointerstitial, glomerular, and vascular) as well as bone marrow-derived cells recruited to the kidney (i.e. mononuclear cells and fibrocytes). Although much has been learned of the molecular mechanisms underlying renal fibrogenesis, there is still a paucity of success in translating this knowledge to clinical application. Reviews of therapeutic targets in renal fibrosis have been undertaken in the past [5, 6]. The scope of this review is to summarize key molecular pathways driving renal fibrogenesis and to highlight novel molecular mediators of renal fibrosis. For detail description of cellular and molecular mechanisms of renal fibrosis readers are referred to recent reviews [7, 8].
Molecular mediators of renal fibrogenesis
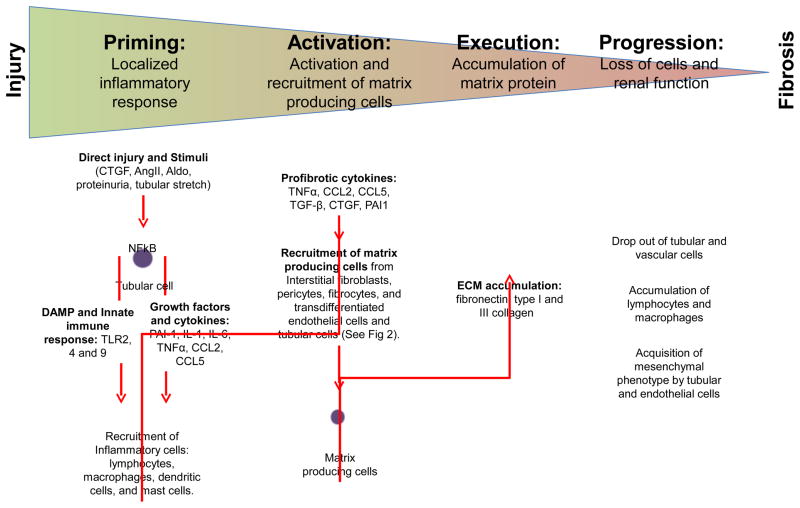
Fibrosis is considered an abnormal wound healing response to tissue injury where the balance between self-limited repair and exuberant accumulation of extracellular matrix (ECM) has been tipped in favor of the latter. The pathogenesis of renal fibrosis has been depicted as a continuum of four overlapping phases: priming, activation, execution and progression [8] (Figure 1). In the priming phase, tissue injury triggers an inflammatory response at the site of injury to recruit lymphocytes, monocytes/macrophages, dendritic cells, and mast cells. Nuclear factor (NF) κB is a key driver of this inflammatory response. NFκB signaling in tubular epithelial cells is triggered by CTGF [9], angiotensin II [10], aldosterone [11], or proteins from tubular fluid [12]. Activation of NFκB signaling drives the production of pro-inflammatory molecules such as plasminogen activator inhibitor (PAI)-1 [13], interleukin (IL)-1 [14], IL-6 [15], chemokine (C-C motif) ligand 2 (CCL2; also known as monocyte chemotactic protein 1) [16, 17], CCL5 [17], and tumor necrosis factor (TNF) α [17] by the injured tubular epithelial cells. Injured tubular cells also release Danger Associated Molecular Pattern molecules, which exert their effects on neighboring tubular epithelial cells and inflammatory cells through toll-like receptors to promulgate innate immune response by increasing the production of pro-inflammatory mediators and recruitment of leukocytes [18]. A profibrotic role has been ascribed to infiltrating CD4+ lymphocytes [19], CD3+ lymphocytes [20], M1-type macrophages [21, 22], and fibrocytes [23]. However, not all infiltrating cells are profibrotic: regulatory T cells [24], M2-type macrophages [22], and mast cells [25] have been shown to mitigate renal fibrosis.
Figure 1.
Four overlapping phases of renal fibrosis: priming, activation, execution, and progression. Direct tubular epithelial cell injury or cellular stimuli triggers a pro-inflammatory response involving activation of the innate immune response and production of growth factors and cytokines, which result in the recruitment of inflammatory cells. Localized accumulation of profibrotic cytokines promotes activation and recruitment of matrix-producing cells from different sources. Accumulation of extracellular matrix proteins is observed in renal fibrosis in conjunction with loss of tubular and vascular cells, accumulation of lymphocytes and macrophages, and acquisition of mesenchymal cellular phenotype by tubular and endothelial cells, which are associated with loss of kidney function. CTGF: connective tissue growth factor, AngII: angiotensin II, Aldo: aldosterone, AGEs: advanced glycation endproducts, NFκB: nuclear factor kappa B, TLR: toll-like receptors, DAMP: danger associated molecular pattern molecules, ROS: reactive oxygen species, IL: interleukin, TGF: transforming growth factor, TNF: tumor necrosis factor, CCL: chemokine C-C motif ligand, PAI: plasminogen activator inhibitor, and ECM: extracellular matrix.
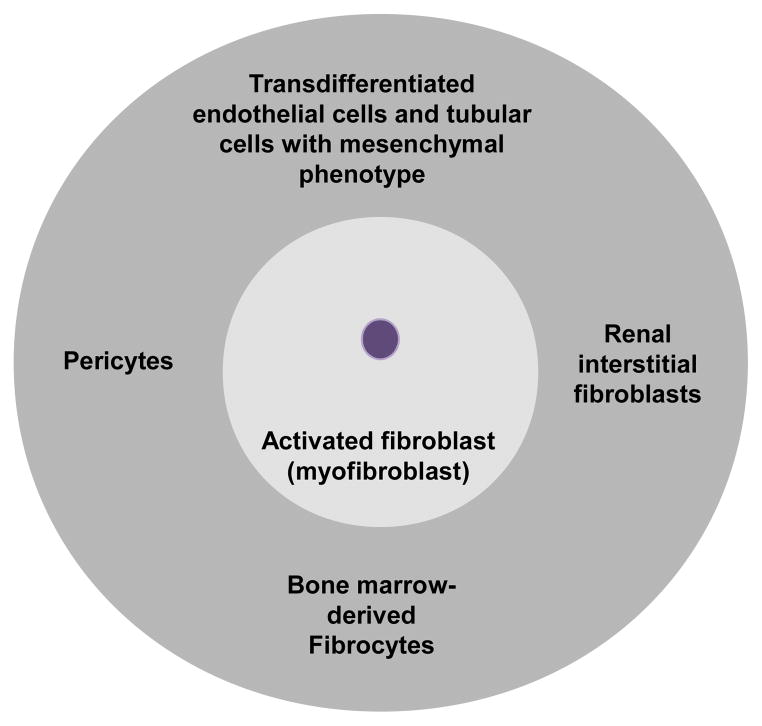
In the activation phase, profibrotic cytokines generated by injured tubular cells and inflammatory cells contribute to the activation of matrix-producing cells. Although multiple cell types are capable of producing extracellular matrix (ECM), renal interstitial fibroblast is considered the principal source of matrix production. A subpopulation of activated fibroblasts, called myofibroblasts, display increased proliferative activity and acquire the expression of α-smooth muscle actin (α-SMA) [26]. In renal fibrosis, cells from different origins contribute to the pool of myofibroblasts: renal interstitial fibroblasts [27]; bone-marrow-derived fibrocytes [28]; vascular pericytes [29]; and endothelial [30] and tubular [31] cells that had undergone transdifferentiation and acquired a mesenchymal phenotype (Figure 2). Tubular epithelial cells have the capacity to acquire a mesenchymal cell phenotype (i.e. epithelial-to-mesenchymal transdifferentiation, EMT) in the injured kidney [32], but whether tubular cells with mesenchymal marker expression can fully differentiate into interstitial myofibroblasts in vivo and how the process contributes to the pathogenesis of renal fibrosis has been debated [7, 33].
Figure 2.
Multiple origins of myofibroblasts in renal fibrosis. Renal tubular interstitial fibroblasts, bone-marrow-derived fibrocytes, vascular pericytes, and transdifferentiated endothelial cells and tubular cells with mesenchymal phenotype have been shown to contribute to the population of myofibroblasts in the fibrotic kidney.
In the execution phase, myofibroblasts produce ECM. Although the accumulation of matrix proteins, such as fibronectin, and type I and III collagen, is a prominent feature of fibrosis, it is probably not the sole factor contributing to the progressive loss of renal function associated with renal fibrosis. Tubular cell apoptosis and atrophy, lymphocytes and macrophages infiltration, tubular epithelial cells and endothelial cells transdifferentiation, and peritubular vasculature rarefaction are also present in the fibrotic kidney and could also contribute to the progressive loss of renal function [7, 34].
In the progression phase, there is a shift from normal wound healing to over-exuberant inflammatory response resulting in the undesirable consequence of fibrosis and functional loss. Although the molecular tipping point that dictates this shift is still unclear, the duration and severity of injury are likely major determinants whether a successful repair is possible and dysregulated tissue fibrosis is averted.
Molecular targets for treatment of renal fibrosis
Several key mediators of kidney fibrosis were identified based on their contribution to fibrosis in other tissue types and/or involvement in EMT during development. These mediators have been targeted for treatment of renal fibrosis in human (Table 1). Here, we summarize key molecular pathways of renal fibrosis and highlight new molecular mediators of renal fibrosis (Figure 3).
Table 1.
Drugs with efficacy in human with renal diseases and fibrosis.
| Drug class/Drug | Human renal diseases | Outcomes studied in human trials | Experimental Models of Renal fibrosis |
|---|---|---|---|
| Tranilast | DKD [65, 66] | UACR, GFR decline, Urinary Type IV Collagen excretion | Sub-total nephrectomy [69] |
| Pyridone: Pirfenidone and fluorofenidone | FSGS [67], DKD [73] | UACR, GFR decline, Urinary TGF-β excretion | UUO [71], Subtotal Nephrectomy [72] |
| Renin-Angiotensin-Aldosterone system blockade | Diabetic, Non-diabetic, and Glomerular kidney diseases [74–78] | UACR, GFR decline | Subtotal Nephrectomy [135, 136] |
| Anti-CTGF monoclonal antibody (FG-3019) | DKD [98] | UACR | UUO [96, 97], DKD[95] |
DKD: Diabetic kidney disease, FSGS: focal segmental glomerular sclerosis, CKD: chronic kidney disease, IgAN: IgA nephropathy, GFR: glomerular filtration rate, UUO: unilateral ureteral obstruction, UACR: urinary albumin to creatinine ratio.
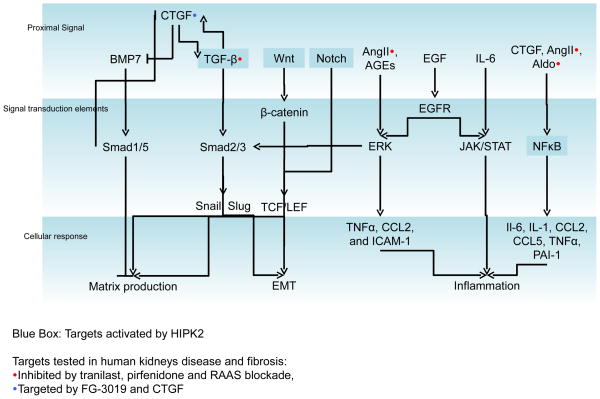
Figure 3.
Schematic of key mediators of kidney fibrosis. Proximal signals from growth factors, cytokines and signaling molecules activate cellular signal transduction elements in tubular cells as well as other cell types to promote profibrotic cellular responses that include extracellular matrix production, epithelial-mesenchymal transdifferentiation, and inflammation. Signaling transduction elements are activated by different proximal signals.
Homeo-domain interacting protein kinase (HIPK) 2 interfaces with nuclear factor (NF)-κB, Wnt/β-catenin, and Notch signaling components to promote renal fibrosis (highlighted in blue boxes). Approaches to antagonize CTGF, TGF-β, and renin-angiotensin-aldosterone signaling in renal disease and fibrosis have demonstrated clinical efficacy.
Transforming Growth Factor (TGF)-β and bone morphogenetic protein (BMP) Signaling
The TGF-β family of structurally related cytokines includes TGF-βs, activins and BMPs [35]. Several lines of evidence suggest that TGF-β-induced activation of downstream Smad signaling contributes to kidney fibrosis [36, 37]: the expression of TGF-β and TGF-β receptor is increased in human and experimental models of chronic kidney disease [38, 39]; overexpression of active TGF-β in mice promotes the development of glomerulosclerosis and tubulointerstitial fibrosis [40]; and inhibition of TGF-β activity attenuates renal disease in experimental models of renal fibrosis [41–45]. TGF-β released from injured tubular cells or inflammatory cells promotes tissue fibrosis by increasing ECM gene transcription, tubular cell apoptosis, ECM turnover, myofibroblast differentiation, and EMT depending on the cellular context of TGF-β action [39].
TGF-β exerts its action by promoting dimerization of type I and type II TGF-β receptors and subsequent phosphorylation of Smad2 and Smad3 [46]. Phosphorylated Smad2 and Smad3 complex with Smad4 in the cytosol then translocate into the nucleus to regulate gene expression. Alternatively, phosphorylated Smad2 and Smad3 can also complex with a subgroup of inhibitory Smads (Smad6 and Smad7) to recruit Smad ubiquitination regulatory factor ubiquitin ligases to the ligand-receptor complexes and initiate receptor degradation [47]. The role of TGF-β/Smad signaling in renal fibrosis, however, is likely more complex than the canonical TGF-β/Smad pathway described above. For instance, Smad2 and 3 can be directly activated by advanced glycation endproducts and angiotensin II through ERK/p38 mitogen-activated protein kinases in a TGF-β-independent manner [48–51]. Furthermore, TGF-β can control the action of several microRNAs (-192 [52–55], -29 [56], -216a [57], -21 [58], -377 [59]) that are involved in the regulation of ECM gene expression and/or EMT [60, 61].
Multiple approaches to block TGF-β-mediated response by blocking TGF-β directly or intracellular mediators of TGF-β-signaling have been investigated in animal models and small clinical trials. Approaches to abrogate TGF-β-mediated responses include direct targeting of TGF-β using antibodies, soluble receptors, or natural TGF-β-binding proteins (e.g. decorin); inhibition of TGF-β receptor using small molecular inhibitors; activation of inhibitory Smads (e.g. Smad7); enhancement of BMP signaling; and targeting of microRNAs that are regulated by TGF-β [5, 61]. In addition to its profibrotic action, TGF-β also possesses anti-inflammatory properties [62–64]. Therefore, direct and complete blockade of TGF-β could have serious effects on the immune system making it undesirable as a therapeutic approach. However, it has been suggested that the complexity of TGF-β regulation could afford specific antifibrotic targets without affecting immune-regulatory functions of TGF-β [6].
Several drugs with unintended anti-TGF-β activity have been studied in human kidney diseases. Two antifibrotic drugs with anti-TGF-β activity, tranilast [65, 66] and pirfenidone [67, 68], have demonstrated modest benefit in animal models of renal fibrosis and small pilot studies of diabetic kidney diseases. Tranilast treatment of rats with subtotal nephrectomy, a model of renal mass reduction leading to progressive renal fibrosis, resulted in an improvement of proteinuria, renal function, and histologic features of renal fibrosis [69]. A study of nine patients with advanced diabetic nephropathy, who demonstrated a progressive decline in renal function despite receiving renin angiotensin aldosterone system (RAAS) blockade, when treated with tranilast had a slower decline in renal function than before treatment [65]. Another study of ten patients with early diabetic nephropathy treated with tranilast for a year found that urinary albumin and type-IV collagen excretion after tranilast treatment were significantly reduced compared to pre-treatment values [66]. Interestingly, the PRESTO trial, a large randomized trial on effects of tranilast in preventing major cardiovascular events and restenosis after percutaneous coronary intervention, reported elevation in serum creatinine as an adverse event [70]. Although results from small studies are encouraging, studies with a focus on renal outcomes are needed to ascertain tranilast’s efficacy in renal fibrosis.
Pirfenidone belongs to a class of drugs, called the pyridones, which exhibit anti-TGF-β activity and have established safety and efficacy for treatment of pulmonary fibrosis and cirrhosis. Pyridones is effective in preventing the development of fibrosis in unilateral ureteral obstruction [71] and subtotal nephrectomy [72] animal models. In a single open-label study of 18 patients with refractory focal segmental glomerulosclerosis who received pirfenidone for 12-months, the rate of renal function decline before enrollment was significantly more than when on pirfenidone [67]. More recently, in a randomized, double-blinded, placebo-controlled trial of 77 patients with diabetic nephropathy, pirfenidone improved renal function at 1 year when compared to placebo [73]. However, no significant reduction in proteinuria was observed in the pirfenidone group. Studies with longer follow up and clinically relevant endpoints are needed to confirm the benefit of pirfenidone in fibrotic renal diseases.
Drugs that inhibit the renin angiotensin and aldosterone system (RAAS) also exhibit anti-TGF-β activity. These drugs have demonstrated efficacy for diabetic and non-diabetic kidney diseases when studied in well-conducted, long-term, randomized trials. RAAS blockade mitigates the progression of chronic kidney disease and proteinuria [74–78]. Inhibitors of RAAS abrogate pro-fibrotic effects of angiotensin-II [79], which include induction of TGF-β and TGF-β receptor levels in the kidney [80–82], enhancement of CTGF expression [83–85], and augmentation of pro-inflammatory mediators through NFkB signaling in renal tissue [86]. The salutary effects of RAAS blockers on renal fibrosis, however, are not solely attributable to and likely extend beyond their anti-TGF-β action [87].
BMP belongs to the TGF-β superfamily of cytokines. BMP7 counteracts TGF-β-induced EMT and kidney fibrosis [88]. BMP7 exerts its action by binding to activin-like kinase (ALK) 3 and ALK 6 type I serine-threonine kinase receptors and activating intracellular Smad-dependent signaling pathway [85]. Activation of ALK3 receptor using a small peptide agonist of BMP signaling suppressed inflammation, apoptosis and EMT program and reversed renal fibrosis in models of acute and chronic renal injury [89]. Targeting of BMP7-ALK3 signaling in human with renal fibrosis has not been reported.
Connective-tissue growth factor (CTGF) signaling
CTGF is a heparin-binding glycoprotein of the CCN (CYR61/CTGF/NOV) family of intercellular signaling proteins. CTGF antagonizes BMP signaling and enhances TGF-β signaling by preventing BMP binding to BMP receptors and facilitating TGF-β binding to TGF-β receptors, respectively [90]. Since TGF-β is a potent inducer of CTGF and correlative studies in human and animals with tissue fibrosis support a link between CTGF and TGF-β, most models postulate that CTGF acts either downstream or cooperatively with TGF-β [91, 92]. CTGF expression is upregulated in kidneys of human renal disease and not expressed in normal kidneys. Furthermore, the level of expression correlates with the severity of renal fibrosis [93, 94]. Reduction of CTGF in murine models of kidney disease prevents renal fibrosis [95–97]. FG-3019, a human monoclonal antibody against CTGF, was studied in a small phase-I clinical trial on patients with diabetic nephropathy and found to significantly reduce albuminuria [98]. A phase 2 study of FG-3019 in subjects with type 2 diabetes and kidney disease was recently terminated by the sponsor due to suboptimal study design (ClinicalTrials.gov identifier NCT00913393).
Pentoxifylline is a nonselective phosphodiesterase inhibitor known to inhibit cell proliferation, inflammation, and ECM accumulation [99]. Pentoxyfiylline treatment of rat models of remnant kidney and obstructive nephropathy attenuated renal tubulointerstitial fibrosis, myofibroblast accumulation, and the upregulation of CTGF [100, 101]. Small trials of human subjects with chronic kidney disease treated with pentoxifylline have also shown to reduce proteinuria [102, 103] and significantly decrease the rate of renal function decline [104]. A recent systematic review including 991 patients from 17 trials guardedly concluded that pentoxifylline use may reduce proteinuria in diabetic patients with chronic kidney disease [105].
NFκB Signaling
Excessive, prolonged inflammation is a major driver of fibrosis [106, 107]. NFκB regulates the expression of multiple genes that play a key role in the inflammatory response during kidney injury. NFκB inhibition tend to attenuate inflammation and tubulointerstitial injury in some, but not all disease models [108]. Although some of the beneficial effects of RAAS blockade in kidney disease have been attributed to the inhibition of angiotensin II-mediated and/or aldosterone-mediated NFκB activation [11] [87], no data exists to support direct NFκB inhibition for treatment of renal fibrosis. Since knockout mice with deficiency of the p65 subunit of the NFκB family member develop liver degeneration and reduction in lymphocyte proliferation [109], complete and direct inhibition of NFκB signaling will likely have serious side effects.
Wnt/β-catenin Signaling
Wnt proteins are secreted lipid-modified glycoproteins that bind to cell surface receptors to initiate outside-in signaling. Wnt signaling regulates mesenchymal-to-epithelial transdifferentiation that occurs during normal kidney development, which mirrors EMT of tubular cells in the process of renal fibrogenesis [110]. Wnt/β-catenin signaling is active in the developing kidney, silenced in normal adult kidney, and reactivated in diseased adult kidney [111]. In canonical Wnt/β-catenin signaling, binding of Wnt to its transmembrane receptors Frizzled and low density lipoprotein receptor-related protein (LRP) results in activation and phosphorylation of LRP. Activated LRP6 recruits Dishvelled and Axin and inhibits glycogen synthase kinase-3β-mediated phosphorylation and proteosomal degradation of β-catenin. In the presence of Wnt, β-catenin accumulates and regulates gene expression through transcription factor T cell factor (TCF) and/or lymphoid enhancer factor (LEF).
In the obstructive nephropathy model of renal fibrosis, several members of the Wnt family are upregulated in the fibrotic kidney and β-catenin accumulates in the cytoplasm and nuclei of renal tubular epithelial cells [112]. Targeted inhibition of β-catenin-mediated gene transcription using a peptidomimetic small molecule ICG-001 in obstructive nephropathy attenuated fibrotic lesions and abolished TGF-β1-induced expression of profibrotic markers without disrupting Smad Signaling [113]. Targeted inhibition of the Wnt/β-catenin pathway has not been studied in human kidney fibrosis. Although strategies for indirect inhibition of Wnt signaling in renal fibrosis by targeting β-catenin and TCF/LEF interaction or at the receptor level have been proposed [114].
Notch Signaling
Notch signaling is required for normal kidney development. In a wide variety of kidney diseases, the expression of certain components of Notch signaling pathway correlated with the degree of proteinuria, glomerulosclerosis, tubulointerstitial fibrosis, and estimated glomerular filtration rate [115]. Inhibition of Notch signaling by either pharmacologic or genetic approach reduced the development of fibrosis in experimental animal models of renal fibrosis [116]. Tubule-specific over-expression of the Notch intracellular domain (NICD) in mice triggered the development of tubulointerstitial fibrosis without affecting expression of key drivers of EMT [116]. Efforts to abrogate Notch signaling in tubular epithelial cells by either genetic deletion or pharmacologic inhibition of Notch activation ameliorated tubulointerstitial fibrosis in murine models [116]. In addition, Notch signaling also plays a role in acute kidney injury and glomerular diseases [117]. Even though suppression of Notch signaling using inhibitors of γ-secretase, which is required for the activation and processing of NICD, have been studied for treatment of solid tumors, their role in human kidney diseases has yet to be explored.
Other growth factors
Fibroblast growth factor (FGF) -1 and 2 have cell proliferative effects on epithelial cells and fibroblasts. FGF-1 is expressed in infiltrating lymphocytes and macrophages; and the expression of FGF receptor-1 is upregulated in tubules of kidneys with inflammatory disease [118]. FGF-2 is able to induce EMT of tubular epithelial cells [119], which could be the causal link for the correlation between FGF-2 expression and the degree of renal fibrosis observed in human kidneys [120]. However, direct causal relation between FGF2 and renal fibrosis in vivo has yet to be demonstrated.
Platelet-derived growth factor (PDGF) isoforms stimulate the replication, survival and migration of myofibroblasts in fibrotic processes including renal fibrosis [121]. Antagonism of the PDGF-D isoform prevented renal fibrosis in an experimental model of glomerulonephritis [122, 123] and anti-PDGF-C treatment reduced fibrosis in obstructive nephropathy [124]. No clinical data exists currently to support using anti-PDGF treatment in renal fibrosis.
Epidermal growth factor receptor (EGFR) signaling mediates fundamental cellular functions such as proliferation, growth, and differentiation. Inhibition of EGFR protected against the development of renal fibrosis in models of hypertensive renal injury and renal mass reduction [125]. EGFR signaling crosstalks with angiotensin II-mediated pathway [126] and is crucial for sustained TGFβ expression [127]. Genetic or pharmacologic blockade of EGFR inhibited the development and progression of renal fibrotic in the rodent model of obstructive nephropathy [128]. Although anti-EGFR pharmacologic agents have demonstrated efficacy in the treatment of certain malignancies, they have not been studied in patients with kidney disease.
Hepatocyte growth factor (HGF) exerts antifibrotic properties in several murine models of chronic kidney disease by binding and inactivating Smad2 [129]. However, other studies suggest that HGF overexpression or its presence in the glomerular ultrafiltration is pro-fibrotic and could contribute to tubular injury [130, 131]. Since HGF also promotes metastasis and tumor growth [132], it is not likely that HGF signaling will be targeted in long-term clinical trials of renal fibrosis.
Homeo-domain interacting protein kinase (HIPK) 2 signaling
We recently found that HIPK2, a previously unrecognized kinase in the context of kidney disease, is upregulated in murine models of kidney injury and fibrosis as well as human with human immunodeficiency virus associated nephropathy (HIVAN), focal segmental glomerulosclerosis, diabetic nephropathy and IgA nephropathy [133]. HIPK2 is known to interact with transcription factors and functions as both a corepressor and a coactivator depending on the transcription factor and its subcellular localization [134]. HIPK2 interfaces with multiple profibrotic pathways that are known to mediate renal injury and fibrosis (i.e. Notch, Wnt/β-catenin, TGF-β, NFκB and p53) (Figure 3). Kidneys of HIPK2−/− mice subjected to experimental injury developed less up-regulation of mesenchymal cell markers and activation of Notch, TGF-β, p53, and NFκB signaling. Furthermore, HIPK2−/− mice were protected against the development of renal fibrosis in models of obstructive nephropathy, folic acid nephropathy, and HIVAN, suggesting that HIPK2 regulates the expression of genes engaged in kidney injury and fibrosis. We believe that HIPK2 is a potential therapeutic target for renal fibrosis since HIPK2 signaling impinges on multiple pro-fibrotic pathways and protein kinases as a group are important drug targets. Since HIPK2−/− mice did not exhibit significant phenotype at baseline, we do not anticipate pharmacologic inhibition of HIPK2 to cause significant side effects. However, long-term studies to monitor effects of HIPK2 suppression on immunity and carcinogenesis will need to be performed.
Conclusion
An overwhelming number of studies targeting key fibrogenic pathways have demonstrated efficacy in mitigating renal fibrosis in experimental models. Only a small fraction of these approaches, however, have been studied in human and even fewer have been successfully translated to clinical use. Medications with proven anti-fibrotic effects impact on these molecular mediators of kidney fibrosis. Complete and direct targeting of these profibrotic mediators, which also regulate other fundamental cellular processes, could have undesirable consequences. However, the complexity of regulation and crosstalk between pathways could afford a therapeutic window. Future efforts to better understand and identify novel molecular regulators of these profibrotic pathways could yield targets with improved therapeutic profile and efficacy.
Acknowledgments
Due to space limitations, the authors could not individually acknowledge work of many investigators in the field of renal fibrosis. We have cited articles that review multiple publications on a topic and we encourage the readers to use them as sources for further study.
JCH is supported by NIH 1R01DK078897 and 1R01DK088541-01A1 and a Veterans Affairs Merit Review Award 1I01BX000345; PYC is supported by NIH 5K08DK082760.
References
- 1.Harris RC, Neilson EG. Toward a unified theory of renal progression. Annual review of medicine. 2006;57:365–380. doi: 10.1146/annurev.med.57.121304.131342. [DOI] [PubMed] [Google Scholar]
- 2.Striker GE, Schainuck LI, Cutler RE, Benditt EP. Structural-functional correlations in renal disease. I. A method for assaying and classifying histopathologic changes in renal disease. Human pathology. 1970;1:615–630. doi: 10.1016/s0046-8177(70)80060-0. [DOI] [PubMed] [Google Scholar]
- 3.Nath KA. Tubulointerstitial changes as a major determinant in the progression of renal damage. American journal of kidney diseases : the official journal of the National Kidney Foundation. 1992;20:1–17. doi: 10.1016/s0272-6386(12)80312-x. [DOI] [PubMed] [Google Scholar]
- 4.Mackensen-Haen S, Bader R, Grund KE, Bohle A. Correlations between renal cortical interstitial fibrosis, atrophy of the proximal tubules and impairment of the glomerular filtration rate. Clinical nephrology. 1981;15:167–171. [PubMed] [Google Scholar]
- 5.Boor P, Sebekova K, Ostendorf T, Floege J. Treatment targets in renal fibrosis. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2007;22:3391–3407. doi: 10.1093/ndt/gfm393. [DOI] [PubMed] [Google Scholar]
- 6.Boor P, Ostendorf T, Floege J. Renal fibrosis: novel insights into mechanisms and therapeutic targets. Nature reviews Nephrology. 2010;6:643–656. doi: 10.1038/nrneph.2010.120. [DOI] [PubMed] [Google Scholar]
- 7.Zeisberg M, Neilson EG. Mechanisms of tubulointerstitial fibrosis. Journal of the American Society of Nephrology : JASN. 2010;21:1819–1834. doi: 10.1681/ASN.2010080793. [DOI] [PubMed] [Google Scholar]
- 8.Liu Y. Cellular and molecular mechanisms of renal fibrosis. Nature reviews Nephrology. 2011;7:684–696. doi: 10.1038/nrneph.2011.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanchez-Lopez E, Rayego S, Rodrigues-Diez R, Rodriguez JS, Rodrigues-Diez R, Rodriguez-Vita J, Carvajal G, Aroeira LS, Selgas R, Mezzano SA, et al. CTGF promotes inflammatory cell infiltration of the renal interstitium by activating NF-kappaB. Journal of the American Society of Nephrology : JASN. 2009;20:1513–1526. doi: 10.1681/ASN.2008090999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Esteban V, Lorenzo O, Ruperez M, Suzuki Y, Mezzano S, Blanco J, Kretzler M, Sugaya T, Egido J, Ruiz-Ortega M. Angiotensin II, via AT1 and AT2 receptors and NF-kappaB pathway, regulates the inflammatory response in unilateral ureteral obstruction. Journal of the American Society of Nephrology : JASN. 2004;15:1514–1529. doi: 10.1097/01.asn.0000130564.75008.f5. [DOI] [PubMed] [Google Scholar]
- 11.Leroy V, De Seigneux S, Agassiz V, Hasler U, Rafestin-Oblin ME, Vinciguerra M, Martin PY, Feraille E. Aldosterone activates NF-kappaB in the collecting duct. Journal of the American Society of Nephrology : JASN. 2009;20:131–144. doi: 10.1681/ASN.2008020232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abbate M, Zoja C, Remuzzi G. How does proteinuria cause progressive renal damage? Journal of the American Society of Nephrology : JASN. 2006;17:2974–2984. doi: 10.1681/ASN.2006040377. [DOI] [PubMed] [Google Scholar]
- 13.Zoja C, Garcia PB, Remuzzi G. The role of chemokines in progressive renal disease. Frontiers in bioscience : a journal and virtual library. 2009;14:1815–1822. doi: 10.2741/3343. [DOI] [PubMed] [Google Scholar]
- 14.Jones LK, O’Sullivan KM, Semple T, Kuligowski MP, Fukami K, Ma FY, Nikolic-Paterson DJ, Holdsworth SR, Kitching AR. IL-1RI deficiency ameliorates early experimental renal interstitial fibrosis. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2009;24:3024–3032. doi: 10.1093/ndt/gfp214. [DOI] [PubMed] [Google Scholar]
- 15.Semedo P, Correa-Costa M, Antonio Cenedeze M, Maria Avancini Costa Malheiros D, Antonia dos Reis M, Shimizu MH, Seguro AC, Pacheco-Silva A, Saraiva Camara NO. Mesenchymal stem cells attenuate renal fibrosis through immune modulation and remodeling properties in a rat remnant kidney model. Stem Cells. 2009;27:3063–3073. doi: 10.1002/stem.214. [DOI] [PubMed] [Google Scholar]
- 16.Shimizu H, Maruyama S, Yuzawa Y, Kato T, Miki Y, Suzuki S, Sato W, Morita Y, Maruyama H, Egashira K, Matsuo S. Anti-monocyte chemoattractant protein-1 gene therapy attenuates renal injury induced by protein-overload proteinuria. Journal of the American Society of Nephrology : JASN. 2003;14:1496–1505. doi: 10.1097/01.asn.0000069223.98703.8e. [DOI] [PubMed] [Google Scholar]
- 17.Gong R, Rifai A, Tolbert EM, Biswas P, Centracchio JN, Dworkin LD. Hepatocyte growth factor ameliorates renal interstitial inflammation in rat remnant kidney by modulating tubular expression of macrophage chemoattractant protein-1 and RANTES. Journal of the American Society of Nephrology : JASN. 2004;15:2868–2881. doi: 10.1097/01.ASN.0000141962.44300.3A. [DOI] [PubMed] [Google Scholar]
- 18.Anders HJ, Schlondorff D. Toll-like receptors: emerging concepts in kidney disease. Current opinion in nephrology and hypertension. 2007;16:177–183. doi: 10.1097/MNH.0b013e32803fb767. [DOI] [PubMed] [Google Scholar]
- 19.Tapmeier TT, Fearn A, Brown K, Chowdhury P, Sacks SH, Sheerin NS, Wong W. Pivotal role of CD4+ T cells in renal fibrosis following ureteric obstruction. Kidney international. 2010;78:351–362. doi: 10.1038/ki.2010.177. [DOI] [PubMed] [Google Scholar]
- 20.Kelly CJ, Zurier RB, Krakauer KA, Blanchard N, Neilson EG. Prostaglandin E1 inhibits effector T cell induction and tissue damage in experimental murine interstitial nephritis. The Journal of clinical investigation. 1987;79:782–789. doi: 10.1172/JCI112885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ko GJ, Boo CS, Jo SK, Cho WY, Kim HK. Macrophages contribute to the development of renal fibrosis following ischaemia/reperfusion-induced acute kidney injury. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2008;23:842–852. doi: 10.1093/ndt/gfm694. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y, Wang YP, Zheng G, Lee VW, Ouyang L, Chang DH, Mahajan D, Coombs J, Wang YM, Alexander SI, Harris DC. Ex vivo programmed macrophages ameliorate experimental chronic inflammatory renal disease. Kidney international. 2007;72:290–299. doi: 10.1038/sj.ki.5002275. [DOI] [PubMed] [Google Scholar]
- 23.Wada T, Sakai N, Matsushima K, Kaneko S. Fibrocytes: a new insight into kidney fibrosis. Kidney international. 2007;72:269–273. doi: 10.1038/sj.ki.5002325. [DOI] [PubMed] [Google Scholar]
- 24.Gandolfo MT, Jang HR, Bagnasco SM, Ko GJ, Agreda P, Satpute SR, Crow MT, King LS, Rabb H. Foxp3+ regulatory T cells participate in repair of ischemic acute kidney injury. Kidney international. 2009;76:717–729. doi: 10.1038/ki.2009.259. [DOI] [PubMed] [Google Scholar]
- 25.Miyazawa S, Hotta O, Doi N, Natori Y, Nishikawa K, Natori Y. Role of mast cells in the development of renal fibrosis: use of mast cell-deficient rats. Kidney international. 2004;65:2228–2237. doi: 10.1111/j.1523-1755.2004.00629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grande MT, Lopez-Novoa JM. Fibroblast activation and myofibroblast generation in obstructive nephropathy. Nature reviews Nephrology. 2009;5:319–328. doi: 10.1038/nrneph.2009.74. [DOI] [PubMed] [Google Scholar]
- 27.Picard N, Baum O, Vogetseder A, Kaissling B, Le Hir M. Origin of renal myofibroblasts in the model of unilateral ureter obstruction in the rat. Histochemistry and cell biology. 2008;130:141–155. doi: 10.1007/s00418-008-0433-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iwano M, Fischer A, Okada H, Plieth D, Xue C, Danoff TM, Neilson EG. Conditional abatement of tissue fibrosis using nucleoside analogs to selectively corrupt DNA replication in transgenic fibroblasts. Molecular therapy : the journal of the American Society of Gene Therapy. 2001;3:149–159. doi: 10.1006/mthe.2000.0251. [DOI] [PubMed] [Google Scholar]
- 29.Lin SL, Kisseleva T, Brenner DA, Duffield JS. Pericytes and perivascular fibroblasts are the primary source of collagen-producing cells in obstructive fibrosis of the kidney. The American journal of pathology. 2008;173:1617–1627. doi: 10.2353/ajpath.2008.080433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zeisberg EM, Potenta SE, Sugimoto H, Zeisberg M, Kalluri R. Fibroblasts in kidney fibrosis emerge via endothelial-to-mesenchymal transition. Journal of the American Society of Nephrology : JASN. 2008;19:2282–2287. doi: 10.1681/ASN.2008050513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zeisberg M, Kalluri R. Fibroblasts emerge via epithelial-mesenchymal transition in chronic kidney fibrosis. Frontiers in bioscience : a journal and virtual library. 2008;13:6991–6998. doi: 10.2741/3204. [DOI] [PubMed] [Google Scholar]
- 32.Zeisberg M, Duffield JS. Resolved: EMT produces fibroblasts in the kidney. Journal of the American Society of Nephrology : JASN. 2010;21:1247–1253. doi: 10.1681/ASN.2010060616. [DOI] [PubMed] [Google Scholar]
- 33.Kriz W, Kaissling B, Le Hir M. Epithelial-mesenchymal transition (EMT) in kidney fibrosis: fact or fantasy? The Journal of clinical investigation. 2011;121:468–474. doi: 10.1172/JCI44595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schlondorff DO. Overview of factors contributing to the pathophysiology of progressive renal disease. Kidney international. 2008;74:860–866. doi: 10.1038/ki.2008.351. [DOI] [PubMed] [Google Scholar]
- 35.Massague J. TGF-beta signal transduction. Annual review of biochemistry. 1998;67:753–791. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- 36.Bottinger EP. TGF-beta in renal injury and disease. Seminars in nephrology. 2007;27:309–320. doi: 10.1016/j.semnephrol.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 37.Lan HY. Diverse roles of TGF-beta/Smads in renal fibrosis and inflammation. International journal of biological sciences. 2011;7:1056–1067. doi: 10.7150/ijbs.7.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Border WA, Noble NA. Transforming growth factor beta in tissue fibrosis. The New England journal of medicine. 1994;331:1286–1292. doi: 10.1056/NEJM199411103311907. [DOI] [PubMed] [Google Scholar]
- 39.Bitzer M, Sterzel RB, Bottinger EP. Transforming growth factor-beta in renal disease. Kidney & blood pressure research. 1998;21:1–12. doi: 10.1159/000025837. [DOI] [PubMed] [Google Scholar]
- 40.Bottinger EP, Kopp JB. Lessons from TGF-beta transgenic mice. Mineral and electrolyte metabolism. 1998;24:154–160. doi: 10.1159/000057364. [DOI] [PubMed] [Google Scholar]
- 41.Border WA, Okuda S, Languino LR, Sporn MB, Ruoslahti E. Suppression of experimental glomerulonephritis by antiserum against transforming growth factor beta 1. Nature. 1990;346:371–374. doi: 10.1038/346371a0. [DOI] [PubMed] [Google Scholar]
- 42.Border WA, Noble NA, Yamamoto T, Harper JR, Yamaguchi Y, Pierschbacher MD, Ruoslahti E. Natural inhibitor of transforming growth factor-beta protects against scarring in experimental kidney disease. Nature. 1992;360:361–364. doi: 10.1038/360361a0. [DOI] [PubMed] [Google Scholar]
- 43.Sharma K, Jin Y, Guo J, Ziyadeh FN. Neutralization of TGF-beta by anti-TGF-beta antibody attenuates kidney hypertrophy and the enhanced extracellular matrix gene expression in STZ-induced diabetic mice. Diabetes. 1996;45:522–530. doi: 10.2337/diab.45.4.522. [DOI] [PubMed] [Google Scholar]
- 44.Isaka Y, Akagi Y, Ando Y, Tsujie M, Sudo T, Ohno N, Border WA, Noble NA, Kaneda Y, Hori M, Imai E. Gene therapy by transforming growth factor-beta receptor-IgG Fc chimera suppressed extracellular matrix accumulation in experimental glomerulonephritis. Kidney international. 1999;55:465–475. doi: 10.1046/j.1523-1755.1999.00275.x. [DOI] [PubMed] [Google Scholar]
- 45.Ziyadeh FN, Hoffman BB, Han DC, Iglesias-De La Cruz MC, Hong SW, Isono M, Chen S, McGowan TA, Sharma K. Long-term prevention of renal insufficiency, excess matrix gene expression, and glomerular mesangial matrix expansion by treatment with monoclonal antitransforming growth factor-beta antibody in db/db diabetic mice. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:8015–8020. doi: 10.1073/pnas.120055097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wrana JL, Attisano L, Wieser R, Ventura F, Massague J. Mechanism of activation of the TGF-beta receptor. Nature. 1994;370:341–347. doi: 10.1038/370341a0. [DOI] [PubMed] [Google Scholar]
- 47.Kavsak P, Rasmussen RK, Causing CG, Bonni S, Zhu H, Thomsen GH, Wrana JL. Smad7 binds to Smurf2 to form an E3 ubiquitin ligase that targets the TGF beta receptor for degradation. Molecular cell. 2000;6:1365–1375. doi: 10.1016/s1097-2765(00)00134-9. [DOI] [PubMed] [Google Scholar]
- 48.Li JH, Huang XR, Zhu HJ, Oldfield M, Cooper M, Truong LD, Johnson RJ, Lan HY. Advanced glycation end products activate Smad signaling via TGF-beta-dependent and independent mechanisms: implications for diabetic renal and vascular disease. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2004;18:176–178. doi: 10.1096/fj.02-1117fje. [DOI] [PubMed] [Google Scholar]
- 49.Chung AC, Zhang H, Kong YZ, Tan JJ, Huang XR, Kopp JB, Lan HY. Advanced glycation end-products induce tubular CTGF via TGF-beta-independent Smad3 signaling. Journal of the American Society of Nephrology : JASN. 2010;21:249–260. doi: 10.1681/ASN.2009010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang W, Huang XR, Canlas E, Oka K, Truong LD, Deng C, Bhowmick NA, Ju W, Bottinger EP, Lan HY. Essential role of Smad3 in angiotensin II-induced vascular fibrosis. Circulation research. 2006;98:1032–1039. doi: 10.1161/01.RES.0000218782.52610.dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang F, Chung AC, Huang XR, Lan HY. Angiotensin II induces connective tissue growth factor and collagen I expression via transforming growth factor-beta-dependent and -independent Smad pathways: the role of Smad3. Hypertension. 2009;54:877–884. doi: 10.1161/HYPERTENSIONAHA.109.136531. [DOI] [PubMed] [Google Scholar]
- 52.Kato M, Zhang J, Wang M, Lanting L, Yuan H, Rossi JJ, Natarajan R. MicroRNA-192 in diabetic kidney glomeruli and its function in TGF-beta-induced collagen expression via inhibition of E-box repressors. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:3432–3437. doi: 10.1073/pnas.0611192104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang B, Herman-Edelstein M, Koh P, Burns W, Jandeleit-Dahm K, Watson A, Saleem M, Goodall GJ, Twigg SM, Cooper ME, Kantharidis P. E-cadherin expression is regulated by miR-192/215 by a mechanism that is independent of the profibrotic effects of transforming growth factor-beta. Diabetes. 2010;59:1794–1802. doi: 10.2337/db09-1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chung AC, Huang XR, Meng X, Lan HY. miR-192 mediates TGF-beta/Smad3-driven renal fibrosis. Journal of the American Society of Nephrology : JASN. 2010;21:1317–1325. doi: 10.1681/ASN.2010020134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Krupa A, Jenkins R, Luo DD, Lewis A, Phillips A, Fraser D. Loss of MicroRNA-192 promotes fibrogenesis in diabetic nephropathy. Journal of the American Society of Nephrology : JASN. 2010;21:438–447. doi: 10.1681/ASN.2009050530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu Y, Taylor NE, Lu L, Usa K, Cowley AW, Jr, Ferreri NR, Yeo NC, Liang M. Renal medullary microRNAs in Dahl salt-sensitive rats: miR-29b regulates several collagens and related genes. Hypertension. 2010;55:974–982. doi: 10.1161/HYPERTENSIONAHA.109.144428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kato M, Wang L, Putta S, Wang M, Yuan H, Sun G, Lanting L, Todorov I, Rossi JJ, Natarajan R. Post-transcriptional up-regulation of Tsc-22 by Ybx1, a target of miR-216a, mediates TGF-{beta}-induced collagen expression in kidney cells. The Journal of biological chemistry. 2010;285:34004–34015. doi: 10.1074/jbc.M110.165027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang Z, Peng H, Chen J, Chen X, Han F, Xu X, He X, Yan N. MicroRNA-21 protects from mesangial cell proliferation induced by diabetic nephropathy in db/db mice. FEBS letters. 2009;583:2009–2014. doi: 10.1016/j.febslet.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 59.Wang Q, Wang Y, Minto AW, Wang J, Shi Q, Li X, Quigg RJ. MicroRNA-377 is up-regulated and can lead to increased fibronectin production in diabetic nephropathy. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2008;22:4126–4135. doi: 10.1096/fj.08-112326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kasinath BS, Feliers D. The complex world of kidney microRNAs. Kidney international. 2011;80:334–337. doi: 10.1038/ki.2011.165. [DOI] [PubMed] [Google Scholar]
- 61.Kantharidis P, Wang B, Carew RM, Lan HY. Diabetes complications: the microRNA perspective. Diabetes. 2011;60:1832–1837. doi: 10.2337/db11-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Letterio JJ, Roberts AB. Regulation of immune responses by TGF-beta. Annual review of immunology. 1998;16:137–161. doi: 10.1146/annurev.immunol.16.1.137. [DOI] [PubMed] [Google Scholar]
- 63.Yaswen L, Kulkarni AB, Fredrickson T, Mittleman B, Schiffman R, Payne S, Longenecker G, Mozes E, Karlsson S. Autoimmune manifestations in the transforming growth factor-beta 1 knockout mouse. Blood. 1996;87:1439–1445. [PubMed] [Google Scholar]
- 64.Wan YY, Flavell RA. TGF-beta and regulatory T cell in immunity and autoimmunity. Journal of clinical immunology. 2008;28:647–659. doi: 10.1007/s10875-008-9251-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Soma J, Sugawara T, Huang YD, Nakajima J, Kawamura M. Tranilast slows the progression of advanced diabetic nephropathy. Nephron. 2002;92:693–698. doi: 10.1159/000064071. [DOI] [PubMed] [Google Scholar]
- 66.Soma J, Sato K, Saito H, Tsuchiya Y. Effect of tranilast in early-stage diabetic nephropathy. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2006;21:2795–2799. doi: 10.1093/ndt/gfl325. [DOI] [PubMed] [Google Scholar]
- 67.Cho ME, Smith DC, Branton MH, Penzak SR, Kopp JB. Pirfenidone slows renal function decline in patients with focal segmental glomerulosclerosis. Clinical journal of the American Society of Nephrology : CJASN. 2007;2:906–913. doi: 10.2215/CJN.01050207. [DOI] [PubMed] [Google Scholar]
- 68.Sharma K, Ix JH, Mathew AV, Cho M, Pflueger A, Dunn SR, Francos B, Sharma S, Falkner B, McGowan TA, et al. Pirfenidone for diabetic nephropathy. Journal of the American Society of Nephrology : JASN. 2011;22:1144–1151. doi: 10.1681/ASN.2010101049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kelly DJ, Zhang Y, Gow R, Gilbert RE. Tranilast attenuates structural and functional aspects of renal injury in the remnant kidney model. Journal of the American Society of Nephrology : JASN. 2004;15:2619–2629. doi: 10.1097/01.ASN.0000139066.77892.04. [DOI] [PubMed] [Google Scholar]
- 70.Holmes DR, Jr, Savage M, LaBlanche JM, Grip L, Serruys PW, Fitzgerald P, Fischman D, Goldberg S, Brinker JA, Zeiher AM, et al. Results of Prevention of REStenosis with Tranilast and its Outcomes (PRESTO) trial. Circulation. 2002;106:1243–1250. doi: 10.1161/01.cir.0000028335.31300.da. [DOI] [PubMed] [Google Scholar]
- 71.Li BX, Tang YT, Wang W, Xie YY, Wang NS, Yuan QJ, Zhang FF, Peng ZZ, Hu GY, Tao LJ. Fluorofenidone attenuates renal interstitial fibrosis in the rat model of obstructive nephropathy. Molecular and cellular biochemistry. 2011;354:263–273. doi: 10.1007/s11010-011-0826-1. [DOI] [PubMed] [Google Scholar]
- 72.Takakuta K, Fujimori A, Chikanishi T, Tanokura A, Iwatsuki Y, Yamamoto M, Nakajima H, Okada M, Itoh H. Renoprotective properties of pirfenidone in subtotally nephrectomized rats. European journal of pharmacology. 2010;629:118–124. doi: 10.1016/j.ejphar.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 73.RamachandraRao SP, Zhu Y, Ravasi T, McGowan TA, Toh I, Dunn SR, Okada S, Shaw MA, Sharma K. Pirfenidone is renoprotective in diabetic kidney disease. Journal of the American Society of Nephrology : JASN. 2009;20:1765–1775. doi: 10.1681/ASN.2008090931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vilayur E, Harris DC. Emerging therapies for chronic kidney disease: what is their role? Nature reviews Nephrology. 2009;5:375–383. doi: 10.1038/nrneph.2009.76. [DOI] [PubMed] [Google Scholar]
- 75.Randomised placebo-controlled trial of effect of ramipril on decline in glomerular filtration rate and risk of terminal renal failure in proteinuric, non-diabetic nephropathy. The GISEN Group (Gruppo Italiano di Studi Epidemiologici in Nefrologia) Lancet. 1997;349:1857–1863. [PubMed] [Google Scholar]
- 76.Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. The New England journal of medicine. 1993;329:1456–1462. doi: 10.1056/NEJM199311113292004. [DOI] [PubMed] [Google Scholar]
- 77.Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB, Ritz E, Atkins RC, Rohde R, Raz I. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. The New England journal of medicine. 2001;345:851–860. doi: 10.1056/NEJMoa011303. [DOI] [PubMed] [Google Scholar]
- 78.Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar S. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. The New England journal of medicine. 2001;345:861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 79.Ruster C, Wolf G. Angiotensin II as a morphogenic cytokine stimulating renal fibrogenesis. Journal of the American Society of Nephrology : JASN. 2011;22:1189–1199. doi: 10.1681/ASN.2010040384. [DOI] [PubMed] [Google Scholar]
- 80.Liu Z, Huang XR, Lan HY. Smad3 mediates ANG II-induced hypertensive kidney disease in mice. American journal of physiology Renal physiology. 2012;302:F986–997. doi: 10.1152/ajprenal.00595.2011. [DOI] [PubMed] [Google Scholar]
- 81.Wolf G, Mueller E, Stahl RA, Ziyadeh FN. Angiotensin II-induced hypertrophy of cultured murine proximal tubular cells is mediated by endogenous transforming growth factor-beta. The Journal of clinical investigation. 1993;92:1366–1372. doi: 10.1172/JCI116710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wolf G, Ziyadeh FN, Stahl RA. Angiotensin II stimulates expression of transforming growth factor beta receptor type II in cultured mouse proximal tubular cells. J Mol Med (Berl) 1999;77:556–564. doi: 10.1007/s001099900028. [DOI] [PubMed] [Google Scholar]
- 83.Grotendorst GR. Connective tissue growth factor: a mediator of TGF-beta action on fibroblasts. Cytokine & growth factor reviews. 1997;8:171–179. doi: 10.1016/s1359-6101(97)00010-5. [DOI] [PubMed] [Google Scholar]
- 84.Liu BC, Sun J, Chen Q, Ma KL, Ruan XZ, Phillips AO. Role of connective tissue growth factor in mediating hypertrophy of human proximal tubular cells induced by angiotensin II. American journal of nephrology. 2003;23:429–437. doi: 10.1159/000074534. [DOI] [PubMed] [Google Scholar]
- 85.Massague J, Chen YG. Controlling TGF-beta signaling. Genes & development. 2000;14:627–644. [PubMed] [Google Scholar]
- 86.Phillips MI, Kagiyama S. Angiotensin II as a pro-inflammatory mediator. Curr Opin Investig Drugs. 2002;3:569–577. [PubMed] [Google Scholar]
- 87.Mezzano SA, Ruiz-Ortega M, Egido J. Angiotensin II and renal fibrosis. Hypertension. 2001;38:635–638. doi: 10.1161/hy09t1.094234. [DOI] [PubMed] [Google Scholar]
- 88.Zeisberg M, Hanai J, Sugimoto H, Mammoto T, Charytan D, Strutz F, Kalluri R. BMP-7 counteracts TGF-beta1-induced epithelial-to-mesenchymal transition and reverses chronic renal injury. Nature medicine. 2003;9:964–968. doi: 10.1038/nm888. [DOI] [PubMed] [Google Scholar]
- 89.Sugimoto H, LeBleu VS, Bosukonda D, Keck P, Taduri G, Bechtel W, Okada H, Carlson W, Jr, Bey P, Rusckowski M, et al. Activin-like kinase 3 is important for kidney regeneration and reversal of fibrosis. Nature medicine. 2012;18:396–404. doi: 10.1038/nm.2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Abreu JG, Ketpura NI, Reversade B, De Robertis EM. Connective-tissue growth factor (CTGF) modulates cell signalling by BMP and TGF-beta. Nature cell biology. 2002;4:599–604. doi: 10.1038/ncb826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Leask A, Abraham DJ. TGF-beta signaling and the fibrotic response. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2004;18:816–827. doi: 10.1096/fj.03-1273rev. [DOI] [PubMed] [Google Scholar]
- 92.Chen XM, Qi W, Pollock CA. CTGF and chronic kidney fibrosis. Front Biosci (Schol Ed) 2009;1:132–141. doi: 10.2741/S13. [DOI] [PubMed] [Google Scholar]
- 93.Ito Y, Aten J, Bende RJ, Oemar BS, Rabelink TJ, Weening JJ, Goldschmeding R. Expression of connective tissue growth factor in human renal fibrosis. Kidney international. 1998;53:853–861. doi: 10.1111/j.1523-1755.1998.00820.x. [DOI] [PubMed] [Google Scholar]
- 94.Yokoi H, Sugawara A, Mukoyama M, Mori K, Makino H, Suganami T, Nagae T, Yahata K, Fujinaga Y, Tanaka I, Nakao K. Role of connective tissue growth factor in profibrotic action of transforming growth factor-beta: a potential target for preventing renal fibrosis. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2001;38:S134–138. doi: 10.1053/ajkd.2001.27422. [DOI] [PubMed] [Google Scholar]
- 95.Guha M, Xu ZG, Tung D, Lanting L, Natarajan R. Specific down-regulation of connective tissue growth factor attenuates progression of nephropathy in mouse models of type 1 and type 2 diabetes. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2007;21:3355–3368. doi: 10.1096/fj.06-6713com. [DOI] [PubMed] [Google Scholar]
- 96.Yokoi H, Mukoyama M, Nagae T, Mori K, Suganami T, Sawai K, Yoshioka T, Koshikawa M, Nishida T, Takigawa M, et al. Reduction in connective tissue growth factor by antisense treatment ameliorates renal tubulointerstitial fibrosis. Journal of the American Society of Nephrology : JASN. 2004;15:1430–1440. doi: 10.1097/01.asn.0000130565.69170.85. [DOI] [PubMed] [Google Scholar]
- 97.Wang Q, Usinger W, Nichols B, Gray J, Xu L, Seeley TW, Brenner M, Guo G, Zhang W, Oliver N, Lin A, Yeowell D. Cooperative interaction of CTGF and TGF-beta in animal models of fibrotic disease. Fibrogenesis & tissue repair. 2011;4:4. doi: 10.1186/1755-1536-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Adler SG, Schwartz S, Williams ME, Arauz-Pacheco C, Bolton WK, Lee T, Li D, Neff TB, Urquilla PR, Sewell KL. Phase 1 study of anti-CTGF monoclonal antibody in patients with diabetes and microalbuminuria. Clinical journal of the American Society of Nephrology : CJASN. 2010;5:1420–1428. doi: 10.2215/CJN.09321209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tsai TJ, Lin RH, Chang CC, Chen YM, Chen CF, Ko FN, Teng CM. Vasodilator agents modulate rat glomerular mesangial cell growth and collagen synthesis. Nephron. 1995;70:91–99. doi: 10.1159/000188550. [DOI] [PubMed] [Google Scholar]
- 100.Lin SL, Chen RH, Chen YM, Chiang WC, Lai CF, Wu KD, Tsai TJ. Pentoxifylline attenuates tubulointerstitial fibrosis by blocking Smad3/4-activated transcription and profibrogenic effects of connective tissue growth factor. Journal of the American Society of Nephrology : JASN. 2005;16:2702–2713. doi: 10.1681/ASN.2005040435. [DOI] [PubMed] [Google Scholar]
- 101.Lin SL, Chen YM, Chien CT, Chiang WC, Tsai CC, Tsai TJ. Pentoxifylline attenuated the renal disease progression in rats with remnant kidney. Journal of the American Society of Nephrology : JASN. 2002;13:2916–2929. doi: 10.1097/01.asn.0000034909.10994.8a. [DOI] [PubMed] [Google Scholar]
- 102.Lin SL, Chen YM, Chiang WC, Wu KD, Tsai TJ. Effect of pentoxifylline in addition to losartan on proteinuria and GFR in CKD: a 12-month randomized trial. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2008;52:464–474. doi: 10.1053/j.ajkd.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 103.Navarro JF, Mora C, Rivero A, Gallego E, Chahin J, Macia M, Mendez ML, Garcia J. Urinary protein excretion and serum tumor necrosis factor in diabetic patients with advanced renal failure: effects of pentoxifylline administration. American journal of kidney diseases : the official journal of the National Kidney Foundation. 1999;33:458–463. doi: 10.1016/s0272-6386(99)70182-4. [DOI] [PubMed] [Google Scholar]
- 104.Perkins RM, Aboudara MC, Uy AL, Olson SW, Cushner HM, Yuan CM. Effect of pentoxifylline on GFR decline in CKD: a pilot, double-blind, randomized, placebo-controlled trial. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2009;53:606–616. doi: 10.1053/j.ajkd.2008.11.026. [DOI] [PubMed] [Google Scholar]
- 105.Shan D, Wu HM, Yuan QY, Li J, Zhou RL, Liu GJ. Pentoxifylline for diabetic kidney disease. Cochrane Database Syst Rev. 2012;2:CD006800. doi: 10.1002/14651858.CD006800.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 107.Nathan C, Ding A. Nonresolving inflammation. Cell. 2010;140:871–882. doi: 10.1016/j.cell.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 108.Sanz AB, Sanchez-Nino MD, Ramos AM, Moreno JA, Santamaria B, Ruiz-Ortega M, Egido J, Ortiz A. NF-kappaB in renal inflammation. Journal of the American Society of Nephrology : JASN. 2010;21:1254–1262. doi: 10.1681/ASN.2010020218. [DOI] [PubMed] [Google Scholar]
- 109.Doi TS, Takahashi T, Taguchi O, Azuma T, Obata Y. NF-kappa B RelA-deficient lymphocytes: normal development of T cells and B cells, impaired production of IgA and IgG1 and reduced proliferative responses. The Journal of experimental medicine. 1997;185:953–961. doi: 10.1084/jem.185.5.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Liu Y. New insights into epithelial-mesenchymal transition in kidney fibrosis. Journal of the American Society of Nephrology : JASN. 2010;21:212–222. doi: 10.1681/ASN.2008121226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pulkkinen K, Murugan S, Vainio S. Wnt signaling in kidney development and disease. Organogenesis. 2008;4:55–59. doi: 10.4161/org.4.2.5849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.He W, Dai C, Li Y, Zeng G, Monga SP, Liu Y. Wnt/beta-catenin signaling promotes renal interstitial fibrosis. Journal of the American Society of Nephrology : JASN. 2009;20:765–776. doi: 10.1681/ASN.2008060566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hao S, He W, Li Y, Ding H, Hou Y, Nie J, Hou FF, Kahn M, Liu Y. Targeted inhibition of beta-catenin/CBP signaling ameliorates renal interstitial fibrosis. Journal of the American Society of Nephrology : JASN. 2011;22:1642–1653. doi: 10.1681/ASN.2010101079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hwang I, Seo EY, Ha H. Wnt/beta-catenin signaling: a novel target for therapeutic intervention of fibrotic kidney disease. Archives of pharmacal research. 2009;32:1653–1662. doi: 10.1007/s12272-009-2200-3. [DOI] [PubMed] [Google Scholar]
- 115.Murea M, Park JK, Sharma S, Kato H, Gruenwald A, Niranjan T, Si H, Thomas DB, Pullman JM, Melamed ML, Susztak K. Expression of Notch pathway proteins correlates with albuminuria, glomerulosclerosis, and renal function. Kidney international. 2010;78:514–522. doi: 10.1038/ki.2010.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bielesz B, Sirin Y, Si H, Niranjan T, Gruenwald A, Ahn S, Kato H, Pullman J, Gessler M, Haase VH, Susztak K. Epithelial Notch signaling regulates interstitial fibrosis development in the kidneys of mice and humans. The Journal of clinical investigation. 2010;120:4040–4054. doi: 10.1172/JCI43025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sirin Y, Susztak K. Notch in the kidney: development and disease. The Journal of pathology. 2012;226:394–403. doi: 10.1002/path.2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rossini M, Cheunsuchon B, Donnert E, Ma LJ, Thomas JW, Neilson EG, Fogo AB. Immunolocalization of fibroblast growth factor-1 (FGF-1), its receptor (FGFR-1), and fibroblast-specific protein-1 (FSP-1) in inflammatory renal disease. Kidney international. 2005;68:2621–2628. doi: 10.1111/j.1523-1755.2005.00734.x. [DOI] [PubMed] [Google Scholar]
- 119.Strutz F, Zeisberg M, Ziyadeh FN, Yang CQ, Kalluri R, Muller GA, Neilson EG. Role of basic fibroblast growth factor-2 in epithelial-mesenchymal transformation. Kidney international. 2002;61:1714–1728. doi: 10.1046/j.1523-1755.2002.00333.x. [DOI] [PubMed] [Google Scholar]
- 120.Strutz F, Zeisberg M, Hemmerlein B, Sattler B, Hummel K, Becker V, Muller GA. Basic fibroblast growth factor expression is increased in human renal fibrogenesis and may mediate autocrine fibroblast proliferation. Kidney international. 2000;57:1521–1538. doi: 10.1046/j.1523-1755.2000.00997.x. [DOI] [PubMed] [Google Scholar]
- 121.Bonner JC. Regulation of PDGF and its receptors in fibrotic diseases. Cytokine & growth factor reviews. 2004;15:255–273. doi: 10.1016/j.cytogfr.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 122.Ostendorf T, Rong S, Boor P, Wiedemann S, Kunter U, Haubold U, van Roeyen CR, Eitner F, Kawachi H, Starling G, et al. Antagonism of PDGF-D by human antibody CR002 prevents renal scarring in experimental glomerulonephritis. Journal of the American Society of Nephrology : JASN. 2006;17:1054–1062. doi: 10.1681/ASN.2005070683. [DOI] [PubMed] [Google Scholar]
- 123.Boor P, Konieczny A, Villa L, Kunter U, van Roeyen CR, LaRochelle WJ, Smithson G, Arrol S, Ostendorf T, Floege J. PDGF-D inhibition by CR002 ameliorates tubulointerstitial fibrosis following experimental glomerulonephritis. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2007;22:1323–1331. doi: 10.1093/ndt/gfl691. [DOI] [PubMed] [Google Scholar]
- 124.Eitner F, Bucher E, van Roeyen C, Kunter U, Rong S, Seikrit C, Villa L, Boor P, Fredriksson L, Backstrom G, et al. PDGF-C is a proinflammatory cytokine that mediates renal interstitial fibrosis. Journal of the American Society of Nephrology : JASN. 2008;19:281–289. doi: 10.1681/ASN.2007030290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Melenhorst WB, Mulder GM, Xi Q, Hoenderop JG, Kimura K, Eguchi S, van Goor H. Epidermal growth factor receptor signaling in the kidney: key roles in physiology and disease. Hypertension. 2008;52:987–993. doi: 10.1161/HYPERTENSIONAHA.108.113860. [DOI] [PubMed] [Google Scholar]
- 126.Lautrette A, Li S, Alili R, Sunnarborg SW, Burtin M, Lee DC, Friedlander G, Terzi F. Angiotensin II and EGF receptor cross-talk in chronic kidney diseases: a new therapeutic approach. Nature medicine. 2005;11:867–874. doi: 10.1038/nm1275. [DOI] [PubMed] [Google Scholar]
- 127.Chen J, Chen JK, Nagai K, Plieth D, Tan M, Lee TC, Threadgill DW, Neilson EG, Harris RC. EGFR signaling promotes TGFbeta-dependent renal fibrosis. Journal of the American Society of Nephrology : JASN. 2012;23:215–224. doi: 10.1681/ASN.2011070645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Liu N, Guo JK, Pang M, Tolbert E, Ponnusamy M, Gong R, Bayliss G, Dworkin LD, Yan H, Zhuang S. Genetic or pharmacologic blockade of EGFR inhibits renal fibrosis. Journal of the American Society of Nephrology : JASN. 2012;23:854–867. doi: 10.1681/ASN.2011050493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Liu Y. Hepatocyte growth factor in kidney fibrosis: therapeutic potential and mechanisms of action. American journal of physiology Renal physiology. 2004;287:F7–16. doi: 10.1152/ajprenal.00451.2003. [DOI] [PubMed] [Google Scholar]
- 130.Takayama H, LaRochelle WJ, Sabnis SG, Otsuka T, Merlino G. Renal tubular hyperplasia, polycystic disease, and glomerulosclerosis in transgenic mice overexpressing hepatocyte growth factor/scatter factor. Laboratory investigation; a journal of technical methods and pathology. 1997;77:131–138. [PubMed] [Google Scholar]
- 131.Wang SN, LaPage J, Hirschberg R. Role of glomerular ultrafiltration of growth factors in progressive interstitial fibrosis in diabetic nephropathy. Kidney international. 2000;57:1002–1014. doi: 10.1046/j.1523-1755.2000.00928.x. [DOI] [PubMed] [Google Scholar]
- 132.Jiang WG, Martin TA, Parr C, Davies G, Matsumoto K, Nakamura T. Hepatocyte growth factor, its receptor, and their potential value in cancer therapies. Critical reviews in oncology/hematology. 2005;53:35–69. doi: 10.1016/j.critrevonc.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 133.Jin Y, Ratnam K, Chuang PY, Fan Y, Zhong Y, Dai Y, Mazloom AR, Chen EY, D’Agati V, Xiong H, et al. A systems approach identifies HIPK2 as a key regulator of kidney fibrosis. Nature medicine. 2012;18:580–588. doi: 10.1038/nm.2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Calzado MA, Renner F, Roscic A, Schmitz ML. HIPK2: a versatile switchboard regulating the transcription machinery and cell death. Cell Cycle. 2007;6:139–143. doi: 10.4161/cc.6.2.3788. [DOI] [PubMed] [Google Scholar]
- 135.Ots M, Mackenzie HS, Troy JL, Rennke HG, Brenner BM. Effects of combination therapy with enalapril and losartan on the rate of progression of renal injury in rats with 5/6 renal mass ablation. Journal of the American Society of Nephrology : JASN. 1998;9:224–230. doi: 10.1681/ASN.V92224. [DOI] [PubMed] [Google Scholar]
- 136.Adamczak M, Gross ML, Krtil J, Koch A, Tyralla K, Amann K, Ritz E. Reversal of glomerulosclerosis after high-dose enalapril treatment in subtotally nephrectomized rats. Journal of the American Society of Nephrology : JASN. 2003;14:2833–2842. doi: 10.1097/01.asn.0000095248.91994.d3. [DOI] [PubMed] [Google Scholar]