Abstract
Glutamatergic and dopaminergic neurotransmission is modulated by adenosine, whose ambient level in the brain is in turn regulated by the metabolic enzyme, adenosine kinase (ADK). Brain adenosinergic tone can therefore be effectively reduced and increased by up- and down-regulation of ADK expression, respectively. Although changes in brain ADK levels can yield multiple behavioral effects, the precise functional significance of telencephalon (neocortical and limbic structures) adenosine remains ill-defined. Amongst the phenotypes identified in transgenic mice with brain-wide ADK overexpression (ADKTG mice) and reduced adenosinergic tone, working memory deficiency and potentiated response to systemic N-methyl-D-aspartate receptor blockade were exacerbated by the introduction of local ADK disruption (elevated adenosinergic tone) restricted to the telencephalon (ADKTG:ADKTel-def mice). These two phenotypes, which are central to schizophrenia cognitive/negative symptoms, appear to be regulated by adenosinergic activities within and outside the telencephalon in a complementary manner. Here, we extended this unique comparison between ADKTG mice ADKTG:ADKTel-def mice to another prominent phenotype previously documented in ADKTG mice – namely, impaired Pavlovian freezing. We found that ADKTG:ADKTel-def mice again were associated with a more severe phenotype while sharing a similar phenotype profile. Furthermore, we qualified that this Pavlovian phenotype did not translate into a general deficiency in associative learning, since no such deficit was evident in three other (aversive and appetitive) Pavlovian learning paradigms. The present study has thus identified a hitherto unknown function of brain adenosine: the execution of conditioned freezing behavior, which is dependent on the balance of adenosinergic changes between the telencephalon and the rest of the brain.
Keywords: Adenosine, adenosine kinase, associative learning, conditioned freezing, memory, mutant mouse
1. Introduction
As an integrator of glutamatergic and dopaminergic signaling in the brain, the neuromodulator adenosine can exert extensive influence over behavioral outputs including cognitive performance (e.g., Boison et al., 2012; Fredholm et al., 2005; Sebastião and Ribeiro, 2009). Adenosine binds to G-protein coupled adenosine receptors, A1R and A2AR, each with a distinct expression pattern in the brain, and both separately linked to specific interactions with dopamine and glutamate receptors (for reviews, see Jacobson and Gao, 2006; Ribeiro et al., 2003). The extracellular neuro-active pools of adenosine in the brain are mainly controlled by adenosine kinase (ADK), an astrocytic enzyme that catalyzes the phosphorylation of adenosine thereby driving the influx of adenosine into astrocytes through passive transporters (Boison, 2006; Etherington et al., 2009). Up-regulation of ADK facilitates adenosine clearance and therefore reduces extracellular adenosine levels as demonstrated in the ADKTG mice, in which the endogenous ADK gene was replaced by a ubiquitin-driven ADK transgene (Fedele et al., 2005). The resulting brain-wide decrease in adenosine impaired working memory function and sensitized the motor response to systemic N-methyl-D-aspartate receptor (NMDAR) blockade (Singer et al., 2012; Yee et al., 2007) – phenotypes that are relevant to the negative and cognitive symptoms of schizophrenia (Boison et al., 2012). In an attempt to delineate the contribution of adenosine overexpression in the telencephalon (neocortical and limbic structures) to these phenotypes, a new mouse line was created by introducing a selective disruption of the ADK transgene into the telencephalon within the ADKTG background. These new mutant mice (ADKTG:ADKTel-def) had elevated extracellular adenosine throughout the cortical mantle, hippocampus and amygdala, while the rest of the brain remained as adenosine-deficient as in the original ADKTG line (Shen et al., 2011; Singer et al., 2012). Comparison between the two mutants thus provides a unique contrast between up- and down-regulation of telencephalon ADK against a background of adenosine deficiency in the rest of the brain – out of which the striatum is the structure with the highest expression of adenosine receptors, especially A2ARs (e.g. Ribeiro et al., 2003; Svenningsson et al., 1999b).
The rationale was as follows: If the overexpression of ADK (deficiency of adenosine) within corticolimbic structures critically underlies the working memory and NMDAR blockade-induced phenotypes in ADKTG mice, then reversing the changes in ADK/adenosine (i.e., disruption of ADK and elevation of adenosine) within these brain structures should yield phenotypes in the opposite direction or at least severely attenuating their phenotypic expression. However, both phenotypes were found to be exacerbated in the ADKTG:ADKTel-def mice compared with ADKTG mice (Singer et al., 2012). This unexpected finding has led to the conclusion that, against the backdrop of adenosine deficiency outside the telencephalon (primarily in the striatum), efficient working memory performance and the integrity of NMDAR function is sensitive to both up- and down-regulation of corticolimbic adenosinergic tone, perhaps via A1R- and A2AR-dependent mechanisms, respectively. The more severe effects of corticolimbic adenosinergic tone up-regulation might stem from a stronger striatal-cortical imbalance of adenosinergic tone in ADKTG:ADKTel-def mice. These insights gleaned from the unique comparison between ADKTG and ADKTG:ADKTel-def mice were further tested here by examining another prominent phenotype previously identified in ADKTG mice – namely, impaired Pavlovian conditioned freezing (Yee et al., 2007). To gauge the importance of this specific phenotype to Pavlovian learning in general, we extended the test to other associative learning paradigms, including two-way conditioned active avoidance, conditioned taste aversion and appetitive conditioned approach response. Possible confounds in locomotor activity, unconditioned fear/anxiety and motor coordination were assessed using the open field, elevated plus maze and accelerating rotarod, respectively. The present study adds to the argument that understanding adenosinergic regulation of behavior ought to take into account the intricate adenosinergic balance between telencephalon and structures beyond – in particular the striatum.
2. Materials and methods
2.1 Animals
ADKTG mice were created by breeding a loxP-flanked ADK transgene under the control of a human ubiquitin promoter into ADK knockout mice (Fedele et al., 2005, Yee et al., 2007). ADKTG:ADKTel-def mice were generated by breeding Emx1-Cre-TG3 mice, which express Cre-recombinase in neurons and astrocytes of the telencephalon (Iwasato et al., 2004), with ADKTG mice (Li et al., 2008). ADKTG and ADKTG:ADKTel-def mice were produced as littermates and maintained on a C57BL/6 genetic background (Singer et al., 2012). Strain- and age-matched WT C57BL6/129P3 mice served as controls. Littermates of the same sex were kept in groups of four to six in Type-III cages (Techniplast, Milan, Italy) housed in a temperature- and humidity-controlled (at 22°C and 55% R.H.) animal vivarium under a reversed light-dark cycle (lights off from 0800–2000 hr).
2.2 Behavioral testing
Behavioral testing began when the animals were approximately 12 weeks old, with all tests conducted in the dark phase of the light cycle. Detailed information on animal cohorts, group sizes, and sequence of behavioral experiments is provided in Table 1. All experiments were performed in accordance to protocols approved by the Institutional Animal Care and Use Committee adhering to NIH regulations and guidelines on the humane use of animals in research.
Table 1.
Overview on experimental design, sequence of behavioral paradigms, number of cohorts, and group sizes in each test.
| Experiment | Duration (days) | Rest days before next test | Cohort | WT | ADKTG | ADKTG:ADKTel-def | |||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Female | Male | Female | Male | Female | Male | ||||
| Elevated plus maze | 1 | 2 | 1 | 8 | 8 | 71 | 7 | 4 | 7 |
|
| |||||||||
| Open field activity | 1 | 2 | 1 | 8 | 8 | 8 | 7 | 4 | 7 |
|
| |||||||||
| Rotarod | 1 | - | 1 | 8 | 8 | 8 | 7 | 4 | 7 |
|
| |||||||||
| Conditioned freezing | |||||||||
| CS freezing | 3 | - | 1+22 | 7 | 7 | 7 | 6 | 5 | 7 |
| Context freezing | 5 | - | 1+2 | 7 | 7 | 4 | 6 | 7 | 7 |
|
| |||||||||
| Appetitive conditioning | 24 | - | 3 | 33 | 3 | 5 | 3 | 5 | 3 |
|
| |||||||||
| Taste aversion | 8 | - | 4 | - | 9 | - | 10 | - | 10 |
|
| |||||||||
| Active avoidance | 5 | - | 5 | 3 | 3 | 4 | 4 | 4 | 4 |
one female ADKTG mouse fell from the plus maze and was therefore excluded from statistical analysis.
Animals from cohorts 1 and 2 were equally distributed between the two freezing experiments.
One female WT subject failed to learn the nose-poke response and was stopped from testing after pre-training.
2.2.1 Open field locomotor activity
Four identical squared arenas (40 × 40 cm) as fully described by Hauser et al. (2005) were used. Locomotor activity was indexed by the distance travelled recorded in successive bins of 10 min over a test period of 1 h. Derivation of raw data was performed by the Ethovision tracking software (Noldus Technology, Wageningen, The Netherlands).
2.2.2 Elevated plus maze test of anxiety
The apparatus consisted of two exposed and two enclosed arms extending from a central square platform as described in detail elsewhere (Yee et al., 2004). The test lasted for 5 min during which the animal was allowed to freely explore the maze. Two anxiety-related measures were calculated: (i) percentage of time spent in the open arms = [time in open arms/time in open and enclosed arms × 100%], and (ii) percentage of entries made into the open arms = [number of entries into open arms/number of entries into open and enclosed arms × 100%]. In addition, the total distance travelled in the entire maze was recorded to index locomotor activity. All data were collected by the Ethovision tracking software (Noldus Technology, Wageningen, The Netherlands).
2.2.3 Accelerating rotarod
The Ugo-Basile Model 7650 (Comerio, VA, Italy) accelerating rotarod for mice was used to assess motor coordination and motor learning. Five subjects were tested concurrently and placed on the rotating drum at a baseline speed of 5 rounds per minute (rpm). During the 5-min testing period, the speed was linearly increased to 40 rpm. The mice were given three trials with an inter-trial interval (ITI) of 24 h. The latency to fall from the rotating rod was recorded, with a maximum time of 300 s.
2.2.4 Cued and contextual conditioning in the conditioned freezing paradigm
The apparatus consisted of two sets of four conditioning chambers. The two sets were distinct from each other, and installed in separate testing rooms, providing two distinct contexts (as fully described before: Pietropaolo et al., 2007; Yee et al., 2006). The first set of chambers (context ‘A’) comprised four Coulbourn Instruments (Allentown, PA, USA) operant chambers (Model E10-10) each equipped with a grid floor made of stainless steel rods through which scrambled electric shocks (the unconditioned stimulus, US) could be delivered (model E13–14; Coulbourn Instruments). A transparent Plexiglas enclosure confined the animals to a rectangular region (17.5 × 13 cm). The inside of the chambers was illuminated by a house light (2.8 W) positioned on the panel wall, 21 cm above the grid floor. The second set of chambers (context ‘B’) comprised four cylindrical (19 cm in diameter) enclosures made of clear Plexiglas resting on a white plastic floor. Illumination inside the chamber was provided by an infrared light source instead of a visible light. Here, we avoided the use of an auditory stimulus as conditioned stimulus (CS) because of a severe hearing impairment previously detected in the ADKTG and ADKTG: ADKTel-def mice (data not shown). Instead, we employed a tactile CS similar to the one used in our previous study (Yee et al., 2007). Each of the eight chambers was equipped with a miniature digital camera mounted 30 cm directly above the area of interest. Successive images captured at 1 s intervals were compared to allow the indexation of freezing according to the algorithm described by Richmond et al. (1998). In brief, when the number of pixels difference between adjacent frames was less than 0.05% of the total number of pixels in a frame, then the animal was considered to be freezing in that 1-s interval. Conditioning to the tactile CS (cued conditioning) or the contextual cues in the absence of any discrete CS (context foreground conditioning) was evaluated in two separate experiments (see Table 1).
Cued conditioning
The experiment consisted of three phases (see Yee et al., 2006): conditioning, context-test, and CS-test. On day 1, conditioning was conducted in context A, comprising three discrete trials of CS-US pairing. Each trial began with a 30 s tone CS followed immediately by the delivery of a 1-s foot-shock set at 0.3 mA. Each trial was preceded and followed by an ITI of 180 s. 24 h later, the animals were returned to context A for a period of 8 min in the absence of any discrete stimulus except the house light to assess freezing to the background contextual cues. Another 24 h later, expression of CS-freezing was assessed in the neutral (non-shocked) context B. Following a 120-s acclimatization period, the CS was presented for 8 min. The pre-CS and CS periods were evaluated separately. To measure extinction of CS freezing the CS-test was repeated on the next two days.
Context conditioning
The procedure has been fully described by Pietropaolo et al. (2007). On day 1, the animals experienced three un-signaled foot shocks (1 s, 0.3 mA) in context A. Each foot shock was preceded and followed by an inter-shock interval (ISI) of 180 s. Following this, context freezing was evaluated by placing the animals in contexts A and B on alternate days (in the order of A-B-A-B across days 2–5), thus allowing the assessment of the context-specificity of the freezing response. Each context test lasted for 4 min.
2.2.5 Appetitive conditioning
Four mouse operant chambers (Model E10-10, Habitest System, Coulbourn Instruments, Allentown, PA, USA) were used, each placed inside a ventilated and sound-attenuated box. The chambers measured 33 cm in height and a Plexiglas wall was positioned 10 cm away from the panel wall creating a floor space of 16 × 25 cm. Dim Illumination (5 lux) inside each operant chamber was provided indirectly by an external 2.8 W light source mounted on the sound-attenuated box. A food magazine tray (Model H14-22M-20) containing a 1.4 W stimulus light inside was positioned in the middle of the panel wall, 2 cm above the grid floor. To reduce the brightness of the stimulus light the bulb was painting black by a waterproof pen. Nose-poke into the magazines was detected by an infrared photocell beam placed at the magazine’s entrance. The magazine was equipped with a liquid dipper dispenser (Model H14-05R) delivering 0.01 ml of liquid reward (20% condensed milk solution, Milch Lait, Schweizer Milchproduzenten, Switzerland). All raw data were calculated by the Graphic State (Version 1.013) software.
Food deprivation
Five days prior to operant testing, the animals were gradually introduced to a food restriction regime by progressively reducing the available feeding time (12 hr, 8 hr, 4 hr, 3 hr, 3 hr). At the same time, the animals were familiarized with the liquid reward in the home cage to minimize food neophobia. Afterwards, daily food rations (food pellets, Kliba 3430, Klibamühlen, Kaiseraugst, Switzerland) were calculated as a function of each animal’s weight loss/gain from the previous day in order to maintain a stable weight of not less than 85% of the ad lib weight. Drinking water was available throughout.
Pre-training
On the first day, the animals were habituated to the chambers and consuming the liquid reward from the dipper. To this end, the dipper was programmed to be raised and illuminated by the magazine light until a nose poke into the magazine was detected, after which time the dipper remained raised for another 3 s allowing the animal to consume the liquid reward. This procedure was repeated, with a variable ITI of 15 ± 5 s. The session ended either when the animal collected 20 rewards or 15 min had elapsed. All animals learned to consume the liquid food.
Appetitive conditioning
Conditioning began from the next day onward and lasted for 18 days. Each daily session comprised 10 conditioning trials separated by a variable ITI with a mean of 90 s (ranged 30–120 s). The CS was a 5-s illumination of a 2.8 V stimulus light flashing at 1 Hz (0.5 s on and 0.5 s off) which was mounted 10 cm directly above the magazine tray. The US consisted of 0.01 ml liquid reward made available for a period 5 s. During the CS and US periods, the magazine stimulus light was turned on. The US period immediately followed the CS period. Appetitive conditioning was indexed by the ratio of nose pokes made during the 5 s CS presentation and the 5 s immediately preceding the onset of CS. This was expressed in percent using the following formula: CS nose pokes/(CS nose pokes + pre-CS nose pokes) × 100%. A score of 50% denotes chance level performance in which the probability of responding was indifferent between the pre-CS and CS periods.
2.2.6 Conditioned taste aversion
This is a one-trial conditioning paradigm, in which a single pairing of a taste CS and gastric malaise leads to a lasting aversion to the taste. The procedure was adopted from Meyer et al. (2004). Throughout the experiment, the animals were housed individually in Makrolon cages (1291H, Eurostandard type III, H:425 × 266 × 185 mm; Tecniplast S.p.a., Milan, Italy). After habituation to individual housing for 2 days, access to water was gradually restricted over a period of 5 days as described by Meyer et al. (2004). On the 5th day, the water restriction was reduced to 1 h. Thereafter and until the end of experiment, the animals were allowed two 30 min drinking periods per day, separated by 4 h. During the drinking periods, two 15 ml drinking tubes were inserted into the cage, and the animals could freely consume liquid from either tube. The drinking holes (2.5 mm diameter) of the tubes were 40 mm apart and 50 mm above the cage floor. During the second drinking period, both tubes always contained water. Before conditioning, the animals were given three days of baseline drinking to stabilize daily water intake. A 10% (w/v) D-sucrose solution served as the taste CS. Gastric malaise, induced by systemic injection of lithium chloride (LiCl) solution, served as the US. LiCl (Sigma-Aldrich, Buchs, Switzerland) was dissolved in sterile saline to a final concentration of 0.25 M on the day of conditioning. The volume of LiCl injection was 2% v/w of the body weight and was administered via the intraperitoneal route. On the conditioning day, the animals were provided with sucrose solution in both tubes during the first drinking period. All animals received a LiCl injection 5 min afterwards. 24 h later, Conditioned taste aversion was measured by the amount of sucrose consumption in a two-choice test in which one tube contained sucrose solution and the other water.
2.2.7 Two-way active avoidance learning
This task captures both elements of classical and instrumental conditioning as the animal has to learn to perform a specific operant act in response to a visual stimulus to avoid the delivery of an aversive foot shock. The apparatus consisted of four identical two-way shuttle boxes (model H10–11M-SC; Coulbourn Instruments) as fully described by Yee et al. (2006). The internal dimensions of each box were 35.5 × 18 × 32.5 cm. The box was separated into two identical compartments with an interconnecting opening allowing the animal to move freely from one compartment to the other (i.e., a shuttle response). Through the stainless steel grid floor of the box, electric shocks (0.3 mA) could be delivered by a current shock generator (model H10–1M-XX-SF; Coulbourn Instruments). The visual CS consisted of two 2.8 W lights flashing a frequency of 1 Hz (0.5 s on and 0.5 s off), mounted on the left and right panel walls, 21 cm above the grid floor. Shuttle responses were detected by a series of photocells (H20–95X; Coulbourn Instruments) attached on the side of both shuttle compartments. Across 5 days, the animals underwent 40 conditioned avoidance trials per day administered at variable ITIs (mean of 40 s, ± 15 s). A trial began with the presentation of the flashing light CS. If the animal shuttled within 5 s from CS onset, the CS was terminated and the animal avoided the electric shock on that trial. Avoidance failure led immediately to an electric foot shock presented in coincidence to the CS. This could last for a maximum of 2 s but could be terminated by a shuttle response during this period (i.e., an escape response). Two-way avoidance learning was indexed by the number of avoided trials per day. In addition, the number of spontaneous ITI shuttles was calculated as a measure of locomotor activity.
2.3. Statistical analysis
Parametric analysis of variance (ANOVA) was used to determine statistically significant differences. To further delineate the nature of significant outcomes, we conducted additional restricted analyses to subsets of the data included in the overall ANOVA, or pair-wise comparisons (Fisher’s LSD) based on the associated error terms taken from the overall ANOVA. The results are presented as mean ± standard error (SE). All statistical analyses were carried out using PASW Statistics (version 18, SPSS Inc. Chicago IL, USA).
3. Results
3. Conditioned freezing to discrete and contextual CSs
Conditioned freezing behavior was assessed to either a discrete tactile CS or the conditioning context alone in the absence of any discrete CS. The two freezing experiments were carried out in two separate cohorts of animals.
3.1.1 Conditioned freezing to discrete CS
First, the development of the conditioned freezing response to the tactile CS was evaluated by the amount of freezing exhibited in the presence of the CS over three successive CS-US pairings. As illustrated in Figure 1A, WT mice showed a rapid increase in freezing time across CS presentations which was attenuated in ADKTG mice and completely absent in ADKTG:ADKTel-def mice. This impression was supported by a significant main effect of genotype [F(2,33) = 16.91, p < 0.001] and trials [F(2,66) = 9.96, p < 0.001] in a 3 × 2 × 3 (genotype × sex × trials) ANOVA of CS freezing. Subsequent post hoc comparisons confirmed the significant reduction in freezing time in either of the two mutant groups relative to WT mice (p’s < 0.005), and in ADKTG:ADKTel-def mice compared with ADKTG mice (p < 0.05). In addition, freezing behavior across the four ITI periods was examined. Again, freezing levels increased as a function of ITI periods, and this increase was weaker in ADKTG mice relative to WT controls, and absent in ADKTG:ADKTel-def mice. This gave rise to a significant main effect of genotype [F(2,33) = 33.87, p < 0.001] and ITI-periods [F(2,66) = 43.87, p < 0.001] as well as a genotype × ITI-periods interaction [F(2,66) = 14.77, p < 0.001] in a 3 × 2 × 4 (genotype × sex × ITI-periods) ANOVA of ITI freezing. Additional post hoc pair-wise comparisons verified the significant differences between ADKTG and WT mice, between ADKTG:ADKTel-def and WT mice, and between the two mutant groups (all p’s < 0.05).
Figure 1.
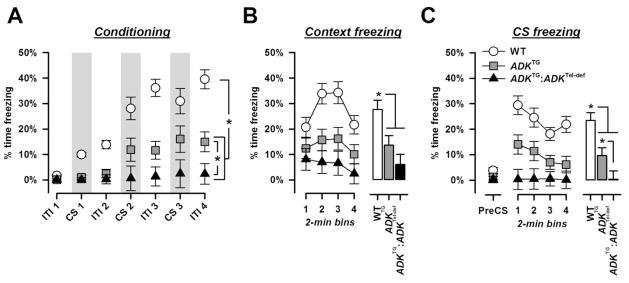
Cued conditioning using a vibrotactile CS. A, CS- and ITI-freezing strongly increased across the three pairings in WT mice which was reduced in the ADKTG mice and almost completely absent in the ADKTG:ADKTel-def mice. B, context background freezing is expressed in 2-min bins on the left and averaged across the four bins in the histogram on the right. The freezing response elicited by the background contextual cues was decreased in both mutant groups. C, freezing to the vibrotactile CS. The histogram on the right illustrates the mean freezing level per 2-min bin of the 8-min test period. Pre-CS freezing levels were equally low in all groups. Conditioned CS freezing was reduced in ADKTG mice and completely absent in ADKTG:ADKTel-def mice. * denotes a significant group differences (p’s < 0.05) based on post-hoc pair-wise comparisons following the emergence of a significant genotype effect in the respective overall ANOVAs.
24 h later, freezing to the background contextual cues was evaluated across a period of 8 min. WT mice exhibited a strong freezing response which showed a U-shaped profile (Figure 2A). A similar freezing response was seen in ADKTG mice albeit with a lower magnitude than in WT mice. Context freezing was further decreased in ADKTG:ADKTel-def mice showing a weak freezing response in the beginning which then gradually decreased to almost zero freezing by the end of the test period. Consistent with this observation, a 3 × 2 × 4 (genotype × sex × 2-min bins) ANOVA of percent time freezing yielded a significant main effect of genotype [F(2,33) = 8.80, p = 0.001] and bins [F(3,99) = 9.61, p < 0.001] and a genotype × bins interaction [F(6,99) = 3.00, p < 0.05]. Subsequent post hoc comparisons confirmed the significant decrease in freezing time in ADKTG and ADKTG:ADKTel-def mice relative to WT mice (all p’s < 0.05). However, the difference between ADKTG and ADKTG:ADKTel-def mice was not statistically significant (p = 0.11).
Figure 2.
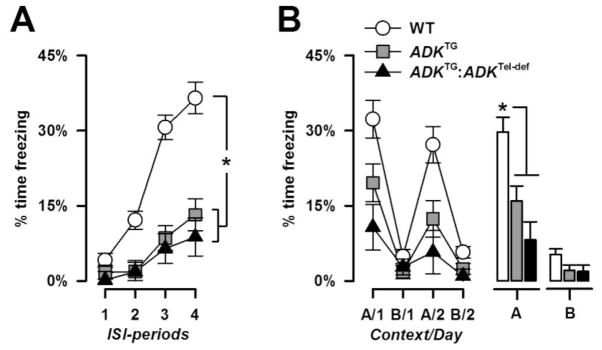
Context foreground conditioning. A, ISI-freezing during conditioning. The development of contextual fear was indicated by a gradual increase in percent freezing across ISI-periods, which was weaker in the mutant groups. B, freezing behavior in the shocked (‘A’) and neutral (‘B’) context is shown across the four test days. The histograms on the right illustrate the mean freezing levels in each context averaged across days. Increased freezing in the shocked context relative to the neutral context indicates the presence of a context-specific conditioned fear response. Contextual fear was substantially weaker in the two mutant groups compared with WT mice. * denotes a significant group difference (p’s < 0.05) based on post hoc comparison following a significant genotype effect in the overall ANOVA.
The conditioned freezing response to the vibrotactile CS was evaluated another 24 h later (Figure 1C). Pre-CS freezing levels were generally low and comparable between groups. Presentation of the CS evoked a pronounced freezing response in the WT mice which gradually decreased over time reflecting the presence of within-session extinction. A similar response but with a much lower magnitude was detected in ADKTG mice. By contrast, there was no sign of any conditioned freezing response to the CS in the ADKTG:ADKTel-def mice showing zero-freezing across the entire test period. This pattern of results led to a significant main effect of genotype [F(2,33) = 14.24, p < 0.001] and bin [F(3,99) = 7.66, p < 0.001] and their interaction [F(6,99) = 2.27, p < 0.05] in a 3 × 2 × 4 (genotype × sex × 2-min bins) ANOVA of CS freezing. Additional post hoc comparisons revealed that the level of CS freezing was significantly lower in either of the two mutant groups relative to WT mice, and in ADKTG:ADKTel-def mice compared to ADKTG mice (all p’s < 0.05).
3.1.2. Contextual conditioning
On day 1, percent freezing across the four ISI periods was subjected to a 3 × 2 × 4 (genotype × sex × ISI-periods) ANOVA. WT mice showed a strong increase in freezing time across ISI periods reflecting the development of contextual fear (Figure 2A). Freezing levels similarly increased in the two mutant groups but at a much lower rate. In agreement with our interpretation a significant main effect genotype [F(2,32) =28.52, p < 0.001] and ISI-periods [F(3,96) = 49.30, p < 0.001] as well as a genotype × ISI- periods interaction [F(6,96) = 9.87, p < 0.001] emerged. Post hoc pair-wise comparisons against WT controls confirmed the significant decrease in ISI freezing in both mutant groups (all p’s < 0.001).
Expression of contextual fear to the shocked context A in comparison to the neutral (non-shocked) context B was examined across the next four days (Figure 2B). WT mice exhibited a context-specific freezing response (days 1 and 3) to the shocked context A without generalizing to the neutral context B (days 2 and 4). The two mutant groups also showed stronger freezing in the shocked relative to the non-shocked context but the magnitude of the conditioned freezing response was substantially weaker than in WT mice. In support of this, a 3 × 2 × 4 (genotype × sex × days) ANOVA of percent freezing revealed a significant main effect of genotype [F(2,32) = 11.05, p < 0.001] and days [F(3,96) = 35.57, p < 0.001], and a genotype × days interaction [F(6,96) = 4.08, p < 0.005]. Additional restricted 3 × 2 (genotype × days) ANOVAs to each context revealed a significant genotype effect only in context A [F(2,32) = 11.65, p < 0.001]. Subsequent post hoc comparisons confirmed that both mutant lines exhibited lower levels of freezing relative to WT mice (all p’s < 0.005).
3.2 Appetitive conditioning
To evaluate whether impaired conditioned freezing in ADKTG and ADKTG:ADKTel-def mice might reflect a general Pavlovian learning deficit, we next conducted another conditioning experiment using different CS and US. Here, a light CS was paired with a food US, which was expected to lead to the development of a conditioned approach response (nose-poke) to the light CS in hungry mice. This was evident in all groups without suggestion any significant difference between genotypes.
As learning progressed, nose-poke responses during the CS increased relative to the baseline rate indexed in the pre-CS periods. The percent nose-poke measure showed a steady increase from 50% chance levels to >70% by the end of training (Figure 3). This led to a significant effect of blocks [F(5,80) = 5.67, p = 0.001] in a 2 × 6 (genotype × blocks) ANOVA, and the rate of learning did not significantly differ between genotypes. Supplementary ANOVAs of the absolute numbers of nose pokes in the CS, pre-CS and US periods all failed to reveal a significant genotype effect (data not shown).
Figure 3.
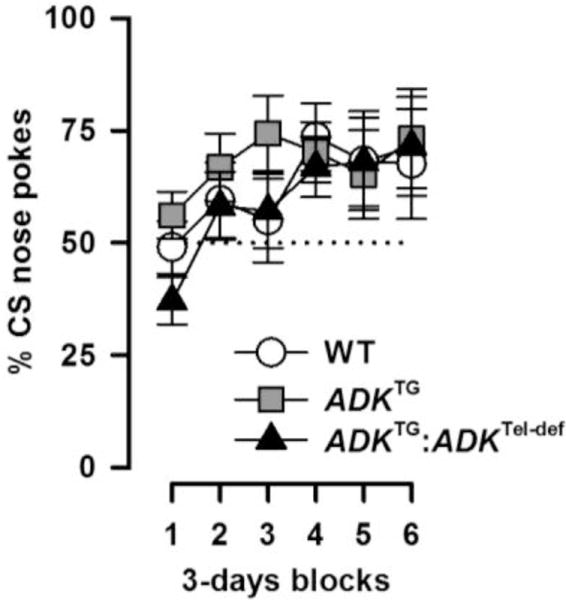
Appetitive conditioning is indexed by percent CS nose pokes [CS/(CS + pre-CS) × 100%]. The dotted line denotes chance performance (50%). The development of the conditioned approach response indexed in 3-days blocks was comparable between groups.
3.3 Conditioned taste aversion
The pattern of impaired conditioned freezing but intact appetitive conditioning in the ADKTG and ADKTG:ADKTel-def mice may point to a specific impairment in aversive conditioning involving negative reinforcers. To test this possibility we employed a conditioned taste aversion paradigm, which is also aversive but has different motor requirements.
The conditioning session was uneventful. One-way ANOVA of sucrose consumption yielded no significant effect (Table 2). Likewise, the amount of sucrose consumption did not differ between groups on the CS test session, in absolute amount as well as in terms of percentage of total liquid intake (Table 2). However, the total amount of liquid consumption (sucrose solution and water) differed between the three groups [F(2,26)=5.25, p=0.012] with ADKTG:ADKTel-def mice consuming more liquid than WT and ADKTG mice, respectively. This was confirmed by post hoc comparison revealing significantly lower liquid intake in ADKTG:ADKTel-def mice compared with the other two groups (all p’s <0.05). Thus, the null-effect on conditioned taste aversion does not support a general deficit in aversive conditioning in the ADKTG and ADKTG:ADKTel-def mice.
Table 2.
Drinking behavior in the conditioning and test phases of the taste aversion experiment. Sucrose and total liquid consumption (sucrose and water) is indexed in gram. On the test day, sucrose intake is additionally indexed as percentage of the total amount of liquid consumed. No differences between genotypes were detected.
| Experimental phase | Liquid | WT (n=9) | ADKTG (n=10) | ADKTG:ADKTel-def (n=10) |
|---|---|---|---|---|
| Conditioning | Sucrose | 1.98 ± 0.19 | 2.42 ± 0.18 | 2.41 ± 0.18 |
|
| ||||
| Test | Sucrose | 0.12 ± 0.06 | 0.11 ± 0.06 | 0.13 ± 0.06 |
| % sucrose | 7.28 ± 4.04% | 10.96 ± 3.84% | 6.35 ± 0.84% | |
| Total liquid | 1.28 ± 0.18 | 1.56 ± 0.17 | 2.08 ± 0.17 | |
3.4 Two-way conditioned active avoidance learning
Next, fear conditioning was assessed using the two-way active avoidance paradigm. Animals were trained to shuttle in response to the shock-predicting CS and thereby terminating the CS and avoiding the shock US. Unlike the conditioned freezing paradigm in which the critical performance measure (i.e., freezing) is negatively biased by confounding hyperactivity, active avoidance performance if anything would be facilitated due to the same confound. Avoidance learning was evident by the gradual increase in successful avoidances across the five conditioning sessions (Figure 4), and this was comparable observed between groups. A 3 × 2 × 5 (genotype × sex × days) ANOVA of the number of successful avoidances per day yielded only a significant effect of days [F(4,64) = 46.15, p < 0.001]. The number of spontaneous shuttles recorded during the ITIs also did not differ significantly between groups. The average number of ITI shuttles per day (±SE) was: WT = 8.63 ± 1.67, ADKTG = 7.45 ± 1.44, ADKTG:ADKTel-def = 7.85 ± 1.44. Intact active avoidance learning in the mutants provides further evidence that their freezing phenotypes do not reflect a general deficit in Pavlovian learning.
Figure 4.

Conditioned Active avoidance learning is indexed by the number of avoidance responses per day. Avoidance learning generally increased across the five daily training sessions.
3.5. Locomotor activity
Spontaneous locomotor activity in the open field was similarly enhanced in the two mutant groups compared with WT mice yielding a significant main effect of genotype [F(2,36) = 5.75, p < 0.01] in a 3 × 2 × 6 (genotype × sex × bins) ANOVA of the distance travelled per 10-min bins (Figure 5A). Subsequent post hoc comparison verified the significant differences between WT mice and each of the two mutant groups (all p’s < 0.01). Habituation of locomotor activity over time was present in all groups as indicated by a general decrease in activity across bins [F(5,180) = 60.85, p < 0.001]. The rate of locomotor habituation appeared higher in the two mutant lines compared with WT controls giving rise to a significant genotype × bins interaction [F(10,189) = 2.50, p < 0.05].
Figure 5.
A, locomotor activity is indexed by the distance travelled across successive 10-min bins (left) or averaged across bins in the bar blot on the right. ADKTG and ADKTG:ADKTel-def mice were equally hyperactive compared with WT mice (*, p < 0.05 based on post hoc comparison). B, motor performance on the rotarod as measured by the latency to fall was comparable between genotypes.
3.6. Motor functions
Motor coordination on the accelerating rotarod did not differ between genotypes. The latency to fall generally increased over days reflecting motor skill learning (Figure 5B). A 3 × 2 × 3 (genotype × days) ANOVA of the latency to fall revealed only a significant main effect of days [F(2,78)=27.30, p<0.001].
3.7. Anxiety-like Behavior
Unconditioned fear and anxiety-like Behavior were assessed in the elevated plus maze. WT, ADKTG, and ADKTG:ADKTel-def mice were comparable in both anxiety measures, percent open arm time (WT = 35.2 ± 6.5%, ADKTG = 47.4 ± 6.9%, ADKTG:ADKTel-def = 37.5 ± 8.1%), and percent open arm entries (WT = 48.3 ± 5.3%, ADKTG = 51.0 ± 5.7%, ADKTG:ADKTel-def = 42.7 ± 6.6%). Likewise, locomotor activity as indexed by the total distance travelled did not differ between groups (WT = 7.43 ± 0.56 m, ADKTG = 6.91 ± 0.60 m ADKTG:ADKTel-def = 7.82 ± 0.75 m). Separate one-way ANOVAs of all three measures failed to yield a significant genotype effect (all F’s <1).
4. DISCUSSION
The present study not only replicated the severe performance deficit by ADKTG mice in the acquisition and expression of the conditioned freezing response (Yee et al., 2007), but also revealed three novel findings. First, a more severe conditioned freezing deficit was seen in ADKTG:ADKTel-def mice when adenosine concentration in the telencephalon was raised above physiological levels by local ADK deletion. Second, we qualify here that the freezing phenotype should not be interpreted as a general Pavlovian learning deficit, because similar deficits were not observed in three alternative associative learning paradigms. Third, the two mutant lines were similarly hyperactive in the open field, suggesting that this phenotype is indifferent to the adenosinergic tone in the telencephalon, and most likely stems from the common up-regulation of ADK and adenosine deficiency in the striatum (Chen et al., 2010; Huang et al., 2005; Shen et al., 2008b). These findings reinforce the impression that ADKTG and ADKTG:ADKTel-def mice shared a similar phenotypic profile (Singer et al., 2012) – a similarity in form and direction that is punctuated with more intense phenotypic expression in the ADKTG:ADKTel-def mouse line due to the opposite changes in ADK expression in the telencephalon between the two lines. The present study extended the list of phenotypes that fall into this category from working memory deficit, and potentiated motor response to NMDAR blockade (Singer et al., 2012), to impaired conditioned freezing. Although the present study cannot offer a mechanistic account of the observations that seemingly opposite changes in telencephalon ADK expression can be linked to similar phenotypes (against the background of striatal ADK over-expression), our data further emphasize the importance and need to develop more refined models to fully address this intriguing possibility.
Control of locomotor activity by striatal ADK
The similar levels of hyperactivity in ADKTG and ADKTG:ADKTel-def mice likely stemmed from the shared hypofunction of subcortical adenosinergic signaling. A candidate brain region is the striatum which plays a key role in the control of locomotor behavior (Alexander et al., 1986, 1990; DeLong and Wichmann, 2007; DeLong et al., 1986). A1Rs and A2ARs are highly expressed in medium spiny neurons in the dorsal striatum where they jointly regulate locomotor activity via multiple mechanisms (Ferré et al., 1997; Fuxe et al., 1998). It is widely accepted that blockade of postsynaptic A2ARs located in striatopallidal neurons led to locomotor hyperactivity (e.g., Ferre et al., 2008; Svenningsson et al., 1999a). One may therefore speculate that striatal A2ARs hypofunction is likely a parsimonious explanation for the common hyperlocomotor phenotype.
At the same time, A2ARs also regulate the release of dopamine in the nucleus accumbens (Gomes et al., 2006, 2009) – a critical component of the mesolimbic dopamine system implicated in normal motor activity as well as the motor-stimulant effects of low doses of amphetamine (Creese and Iversen, 1974, 1975). The motor stimulant effect of the mixed A1R/A2AR antagonist, caffeine, has been linked to an increase in accumbal dopamine release (Lazarus et al., 2011; Solinas et al., 2002). Thus, accumbal A2AR underactivity might contribute to the hyperlocomotor activity phenotypes present in ADKTG and ADKTG:ADKTel-def mice. Lastly, a contribution of A1R-dependent mechanisms cannot be dismissed because locomotor activity is also regulated by A1Rs through synergistic interaction with A2AR and other independent pathways (Jacobson et al., 1993; Kurzim et al., 2006; Popoli et al., 1996).
In the adult brain, ADK is almost exclusively expressed in astrocytes (Boison, 2008b; Studer et al., 2006) suggesting that the extra-cellular adenosine concentration regulating the activity of afferent excitatory fibers into the dorsal striatum is most likely controlled by reuptake into astrocytes within the striatal network. Thus, striatal astrocytes can modify motor activity through their homeostatic control of extra-cellular adenosine via ADK. This regulation appears robust against divergent alterations in ADK expression in the telencephalon. Targeting striatal ADK (in astrocytes) may provide a novel approach to suppress motor disturbance in conditions such as Parkinson’s and Huntington’s diseases.
Is the conditioned freezing phenotype indicative of a general associative learning deficit?
Answer to this question would be critical to the subsequent discussion on the possible neural basis of this phenotype. A similar concern has been raised in the first demonstration of impaired conditioned freezing in ADKTG mice (Yee et al., 2007), when it was noted that ADKTG mice were also spontaneously hyperactive. The authors addressed this concern by normalizing the indexation of CS-freezing with respect to the first 2-minute period of Pre-CS freezing in the CS-test session, and showed that the ADKTG mice’s corrected levels of CS-freezing remained significantly below that of controls (see Figure 6D of Yee et al., 2007). Here, no such difference in Pre-CS freezing was observed, yet a similar confounding increase in activity was present in ADKTG as well as ADKTG:ADKTel-def mice in the open field. Importantly, the hyperactivity phenotype was similar in magnitude in the two mutant lines, and therefore cannot by itself explain the differential expression of the conditioned freezing phenotype, even though the confounding hyperactivity might equally contribute to the freezing impairment observed in the two mutant lines.
Yee et al. (2007) initially found that the ADKTG mice showed a specific deficit in the conditioned freezing response to a discrete (tactile) CS. Here, the freezing deficit was unequivocally extended to contextual conditioning, which was not observed before. This extension is a critical addition to Yee et al.’s (2007) study, where the assessment of context freezing was confounded by the fact that the animals had been pre-exposed to the context prior to conditioning. Thus, the present results indicate that the freezing phenotype did not discriminate between discrete CS and contextual CSs. To test the speculation by Yee et al. (2007) that this phenotype might be indicative of a general associative learning deficit, we first extended our evaluation to an appetitive conditioned approach paradigm and revealed that both mutant lines learned as well as controls did. We initially suspected that the Pavlovian phenotype might be specific for aversive conditioning, and thus not readily translatable to an appetitive paradigm with food reward as the US. However, the mutant lines again performed similarly to controls in the conditioned taste aversion and the conditioned avoidance tests, in which associative learning was assessed in terms of retention and acquisition, respectively. Even though the normal avoidance performance might conceivably be explained by the compensatory non-specific increase in activity levels, such an argument is untenable for the normal conditioned response seen in the conditioned taste aversion test. Taken together, the three additional tests of associative learning convincingly showed that the development of conditioned response was not impaired in either mutant line irrespective of whether the conditioned response was learned rapidly on a single trial (conditioned taste aversion) or gradually across many trials (active avoidance learning and appetitive conditioning). These new data critically revise the interpretation initially adopted by Yee et al. (2007) when the freezing phenotype in the ADKTG mice was first identified. It represents neither a general associative learning deficit nor a specific deficiency in the acquisition of conditioned fear.
However, we cannot explain why the conditioning phenotype was uniquely demonstrated in the conditioned freezing paradigm. Any account in terms of functional disturbances in corticolimbic structures implicated in conditioned freezing, viz., hippocampus and amygdala, cannot accommodate the null effects demonstrated in the other conditioning paradigms, in which these structures are to varying degrees also implicated (Everitt et al., 2003; Maren, 2001; Parkinson et al., 2000; Reilly and Bornovalova, 2005; Werka, 1998; Yamamoto et al., 1994). We must therefore consider the possibility that the freezing phenotype reflected primarily a deficit in the execution of the freezing response that was independent of learning or interference by confounding hyperactivity. One candidate mechanism might involve an effect on the periaqueductal grey (PAG) which is critical for the freezing response (Johnson and Le Doux, 2004; Le Doux, 2000; Maren, 2001). The expression of freezing is correlated with PAG activity: Freezing can be evoked or suppressed by stimulation and inhibition of PAG neural activity, respectively (Bandler and Depaulis, 1988; Brandao et al., 2008; Depaulis et al., 1989; Le Doux et al., 1988). Decrease of ambient adenosine in the PAG might be expected to reduce neuronal excitation (and thus attenuate freezing), since increase of ambient adenosine by intra-PAG infusion of dipyridamole, an inhibitor of adenosine re-uptake, has been shown to reduce the release of the inhibitory neurotransmitter glycine (de Novellis et al., 2002). However, PAG adenosine should be similarly reduced in ADKTG and ADKTG:ADKTel-def mice, and as such it could not account for the more severe conditioned freezing impairment in ADKTG:ADKTel-def mice.
ADK within and outside the telencephalon
Since ADKTG mice and ADKTG:ADKTel-def mice differed solely in terms of telencephalon expression of ADK (i.e., adenosine concentration), it is logical to attribute their differential performance to the opposite changes in telencephalon adenosinergic tone (being reduced in ADKTG mice, but elevated in ADKTG:ADKTel-def mice). According to this dissection, up-regulation of telencephalon adenosine would be disruptive for conditioned freezing. Confirmation of this speculation, however, would certainly require the evaluation of new engineered mice with telencephalon specific ADK disruption. Moreover, the present data set alone cannot decide on the relative contributions of ADK overexpression within and outside the telencephalon to the conditioned freezing impairment in ADKTG mice. Any speculation in this regard must be directly tested with additional models (see later).
Our most recent data revealed that genetic deletion of A2AR in the striatum enhanced conditioned freezing (Wei et al., unpublished data). Activation of striatal A2ARs is expected to be similarly reduced in ADKTG and ADKTG:ADKTel-def mice, and therefore should, if at all, lead to enhanced conditioned freezing. However, both mutant lines showed a freezing deficit, which was more severe in the ADKTG:ADKTel-def mice. Notably, a similar pattern was revealed in the assessment of working memory, which was enhanced in striatal A2AR knockouts (Wei et al., 2011) but impaired in the ADKTG as well as the ADKTG:ADKTel-def mice with the latter again more severely impaired (Singer et al., 2012). These findings collectively suggest that disturbance of telencephalon adenosine homeostasis in either direction might disrupt normal behavior, with up-regulation being more disruptive than down-regulation against the common background of adenosine depletion elsewhere in the brain. Bearing in mind the intrinsic limitations of our models, which nonetheless have yielded the predicted region-specific changes in ADK expression and adenosine concentration (Li et al., 2008; Shen et al., 2011; Singer et al., 2012), we wish to speculate here that an imbalance between the adenosine concentration within and beyond the telencephalon might parsimoniously account for the apparent paradoxical effects between the two transgenic models. This speculative characterization is corroborated by: First, systemic administration of agonists as well as antagonists of adenosine receptors disrupt conditioned freezing (Corodimas and Tomita, 2001; Corodimas et al., 2000), thus showing that both increase and decrease in global adenosinergic activity can similarly impair the development of Pavlovian conditioned freezing. Second, impaired motor response to amphetamine and working memory deficiency in ADKTG mice were antagonized by focal adenosine delivery into the hippocampus and striatum, respectively, thus demonstrating that increased adenosinergic tone confined to the telencephalon or the striatum can be dissociated at the behavioral levels (Shen et al., 2012).
The need to overcome limitations of the present models in future studies
The present study is the second demonstration of the intriguing outcomes wherein qualitatively opposite changes in telencephalon ADK expression apprently yielded only quantitative differences in the expression of essentially the same phenotypes. Before concluding that the phenotypic comparison between ADKTG and ADKTG:ADKTel-def are yielding paradoxical interpretations of telencephalon adenosine function, it is imperative to consider the limitations of the specific genetic models studied here. First, although telencephalon ADK/adenosine was manipulated in opposite directions in the two mutant lines, the changes were expressed against ADK over-expression in the rest of the brain. More refined models that can allow a direct and sole comparison between up- and down-regulation of ADK in the telencephalon alone (without any compromise to the rest of the brain) are necessary to test any predictions derived from the present and previous data sets (also see Singer et al., 2012). The existing Cre-loxP recombination system under the regulation of the EMX-1 promoter can confine the excision of the ADK gene to the dorsal telencephalon (Iwasato et al., 2004). Similarly, any conclusions drawn here regarding up- or down-regulation of ADK/adenosine outside the telencephalon, namely, the striatum, also require further validation with more refined tools. We have previously employed the Dlx5/6-Cre conditional knockout system to generate striatal specific A2AR knockout mice (Wei et al., 2011). However, this approach is unsuitable for targeting ADK because of its primary expression in astrocyter rather than neurons. Alternative methods currently underway in our laboratory include the transplantation of adenosine-releasing cells (Shen et al., 2012), the local application of viral vectors engineered to express sense/antisense Adk-cDNA (Theofilas et al., 2011) in wild type mice, or intracerebral infusion of Cre-expressing viral vectors (e.g., Muhia et al., 2012) in ADKfl/fl mice. These more refined techniques, can further address the common concern over developmental compensation in traditional mutant mouse models. Although no compensatory changes in A1R and A2AR expression have been reported in the ADKTG mice (Shen et al., 2012), these have not been examined in the ADKTG:ADKTel-def line.
5. Conclusions
Our study has added a new level of complexity and lent further credence to the general thesis that adenosine homeostasis is essential for healthy brain function (Boison et al., 2012), and that its dysregulation might be implicated in specific psychopathology present in mental disorders, such as schizophrenia (Boison, 2008a; Lara et al., 2006). Thus, corrective adenosinergic interventions may yield therapeutic efficacy, but dosage and regional specificity must be taken into consideration. This might represent a significant challenge for the successful translation into clinic-ready pharmacotherapy, and the evaluation of in vivo animal models will remain indispensable for the further delineation and mechanistic dissection of adenosinergic regulation of behavior and cognition.
Highlights.
Brain adenosine modulation on Pavlovian learning is investigated in two mutant models
Up- and down-regulation of cortical adenosine severely impaired conditioned freezing
The conditioned freezing impairment does not generalized to other Pavlovian paradigms
Adenosine homeostasis is critically important for maintaining normal brain function
Regional specific effects are relevant for development of adenosine-based therapies
Acknowledgments
This work was partly supported by NIH grant R01MH083973 and the Swiss Federal Institute of Technology Zurich (ETH Zurich). ChuChu Zhang was supported by an exchange program between ETH Zurich and Hong Kong University of Science and Technology. We are also grateful to Peter Schmid for his technical supports, and Joram Feldon for providing access to the necessary apparatus and animals-keeping facilities for the reported experiments.
Footnotes
Contributions: The study was conceived by BKY, and experiments performed by PS and CCZ together. BKY and PS performed all data analysis and interpretation. Manuscript was prepared by BKY and PS. The mutant lines reported in this study originated from DB’s laboratory.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander GE, Crutcher MD, DeLong MR. Basal ganglia-thalamocortical circuits: parallel substrates for motor, oculomotor, “prefrontal” and “limbic” functions. Prog Brain Res. 1990;85:119–46. [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–81. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Bandler R, Depaulis A. Elicitation of intraspecific defence reactions in the rat from midbrain periaqueductal grey by microinjection of kainic acid, without neurotoxic effects. Neurosci Lett. 1988;88:291–6. doi: 10.1016/0304-3940(88)90226-1. [DOI] [PubMed] [Google Scholar]
- Boison D, Singer P, Shen HY, Feldon J, Yee BK. Adenosine hypothesis of schizophrenia - Opportunities for pharmacotherapy. Neuropharmacology. 2012;62:1527–43. doi: 10.1016/j.neuropharm.2011.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boison D. Adenosine as a neuromodulator in neurological diseases. Cur Opin Pharmacol. 2008a;8:2–7. doi: 10.1016/j.coph.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boison D. The adenosine kinase hypothesis of epileptogenesis. Prog Neurobiol. 2008b;84:249–62. doi: 10.1016/j.pneurobio.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boison D. Adenosine kinase, epilepsy and stroke: mechanisms and therapies. Trends Pharmacol Sci. 2006;12:652–58. doi: 10.1016/j.tips.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Brandao ML, Zanoveli JM, Ruiz-Martinez RC, Oliveira LC, Landeira-Fernandez J. Different patterns of freezing Behavior organized in the periaqueductal gray of rats: Association with different types of anxiety. Behav Brain Res. 2008;188:1–13. doi: 10.1016/j.bbr.2007.10.018. [DOI] [PubMed] [Google Scholar]
- Chen JF, Yu L, Shen HY, He JC, Wang X, Zheng R. What knock-out animals tell us about the effects of caffeine. J Alzheimers Dis. 2010;20 (Suppl 1):17–24. doi: 10.3233/JAD-2010-1403. [DOI] [PubMed] [Google Scholar]
- Corodimas KP, Pruitt JC, Stieg JM. Acute exposure to caffeine selectively disrupts context conditioning in rats. Psychopharmacology (Berl) 2000;152:376–82. doi: 10.1007/s002130000557. [DOI] [PubMed] [Google Scholar]
- Corodimas KP, Tomita H. Adenosine A1 receptor activation selectively impairs the acquisition of contextual fear conditioning in rats. Behav Neurosci. 2001;115:1283–90. doi: 10.1037//0735-7044.115.6.1283. [DOI] [PubMed] [Google Scholar]
- Creese I, Iversen SD. The pharmacological and anatomical substrates of the amphetamine response in the rat. Brain Res. 1975;83:419–36. doi: 10.1016/0006-8993(75)90834-3. [DOI] [PubMed] [Google Scholar]
- Creese I, Iversen SD. The role of forebrain dopamine systems in amphetamine induced stereotyped behavior in the rat. Psychopharmacologia. 1974;39:345–57. doi: 10.1007/BF00422974. [DOI] [PubMed] [Google Scholar]
- de Novellis V, Marabese I, Uliano R, Palazzo E, Scafuro A, sca Rossi F, Maione S. Type I and II metabotropic glutamate receptors modulate periaqueductal grey glycine release: interaction between mGlu2/3 and A1 adenosine receptors. Neuropharmacology. 2002;43:1061–9. doi: 10.1016/s0028-3908(02)00227-7. [DOI] [PubMed] [Google Scholar]
- DeLong MR, Alexander GE, Mitchell SJ, Richardson RT. The contribution of basal ganglia to limb control. Prog Brain Res. 1986;64:161–74. doi: 10.1016/S0079-6123(08)63411-1. [DOI] [PubMed] [Google Scholar]
- DeLong MR, Wichmann T. Circuits and circuit disorders of the basal ganglia. Arch Neurol. 2007;64:20–4. doi: 10.1001/archneur.64.1.20. [DOI] [PubMed] [Google Scholar]
- Depaulis A, Bandler R, Vergnes M. Characterization of pretentorial periaqueductal gray matter neurons mediating intraspecific defensive Behaviors in the rat by microinjections of kainic acid. Brain Res. 1989;486:121–32. doi: 10.1016/0006-8993(89)91284-5. [DOI] [PubMed] [Google Scholar]
- Etherington LA, Patterson GE, Meechan L, Boison D, Irving AJ, Dale N, Frenguelli B. Astrocytic adenosine kinase regulates basal synaptic adenosine levels and seizure activity but not activity-dependent adenosine release in the hippocampus. Neuropharmacology. 2009;56:429–37. doi: 10.1016/j.neuropharm.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Cardinal RN, Parkinson JA, Robbins TW. Appetitive behavior: Impact of amygdala-dependent mechanisms of emotional learning. Ann NY Acad Sci. 2003;985:233–50. [PubMed] [Google Scholar]
- Fedele DE, Gouder N, Güttinger M, Gabernet L, Scheurer L, Rülicke T, Crestani F, Boison D. Astrogliosis in epilepsy leads to overexpression of adenosine kinase resulting in seizure aggravation. Brain. 2005;128:2383–95. doi: 10.1093/brain/awh555. [DOI] [PubMed] [Google Scholar]
- Ferré S, Fredholm BB, Morelli M, Popoli P, Fuxe K. Adenosine–dopamine receptor–receptor interactions as an integrative mechanism in the basal ganglia. Trends Neurosci. 1997;20:482–7. doi: 10.1016/s0166-2236(97)01096-5. [DOI] [PubMed] [Google Scholar]
- Ferré S, Quiroz C, Woods AS, Cunha R, Popoli P, Ciruela F, Lluis C, Franco R, Azdad K, Schiffmann SN. An update on adenosine A2A-dopamine D2 receptor interactions: Implications for the function of G protein-coupled receptors. Curr Pharm Des. 2008;14:1468–74. doi: 10.2174/138161208784480108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredholm BB, Chen JF, Cunha RA, Svenningsson P, Vaugeois JM. Adenosine and brain function. Int Rev Neurobiol. 2005;63:191–270. doi: 10.1016/S0074-7742(05)63007-3. [DOI] [PubMed] [Google Scholar]
- Fuxe K, Ferré S, Zoli M, Agnati LF. Integrated events in central dopamine transmission as analyzed at multiple levels. Evidence for intramembrane adenosine A2A/dopamine D2 and adenosine A1/dopamine D1 receptor interactions in the basal ganglia. Brain Res Brain Res Rev. 1998;26:258–73. doi: 10.1016/s0165-0173(97)00049-0. [DOI] [PubMed] [Google Scholar]
- Gomes CA, Simões PF, Canas PM, Quiroz C, Sebastião AM, Ferré S, Cunha RA, Ribeiro JA. GDNF control of the glutamatergic cortico-striatal pathway requires tonic activation of adenosine A receptors. J Neurochem. 2009;108:1208–19. doi: 10.1111/j.1471-4159.2009.05876.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes CA, Vaz SH, Ribeiro JA, Sebastião AM. Glial cell line-derived neurotrophic factor (GDNF) enhances dopamine release from striatal nerve endings in an adenosine A2A receptor-dependent manner. Brain Res. 2006;1113:129–36. doi: 10.1016/j.brainres.2006.07.025. [DOI] [PubMed] [Google Scholar]
- Hauser J, Rudolph U, Keist R, Möhler H, Feldon J, Yee BK. Hippocampal alpha5 subunit-containing GABAA receptors modulate the expression of prepulse inhibition. Mol Psychiatry. 2005;10:201–7. doi: 10.1038/sj.mp.4001554. [DOI] [PubMed] [Google Scholar]
- Huang ZL, Qu WM, Eguchi N, Chen JF, Schwarzschild MA, Fredholm BB, Urade Y, Hayaishi O. Adenosine A2A, but not A1, receptors mediate the arousal effect of caffeine. Nat Neurosci. 2005;8:858–9. doi: 10.1038/nn1491. [DOI] [PubMed] [Google Scholar]
- Iwasato T, Nomura R, Ando R, Ikeda T, Tanaka M, Itohara S. Dorsal telencephalon-specific expression of Cre recombinase in PAC transgenic mice. Genesis. 2004;38:130–8. doi: 10.1002/gene.20009. [DOI] [PubMed] [Google Scholar]
- Jacobson KA, Gao ZG. Adenosine receptors as therapeutic targets. Nat Rev Drug Discov. 2006;5:247–64. doi: 10.1038/nrd1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson KA, Nikodijevic O, Padgett W, Gallo-Rodriguez C, Maillard M, Daly JW. 8-(3-chlorostyryl)caffeine is a selective A2-adenosine antagonist in vitro and in vivo. FEBS Lett. 1993;323:141–44. doi: 10.1016/0014-5793(93)81466-d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson LR, Le Doux JE. The anatomy of fear: microcircuits of the lateral amygdala. In: Gorman JM, editor. Fear and Anxiety: The Benefits of Translational Research. APPA Press; Washington D.C: 2004. pp. 227–50. [Google Scholar]
- Kuzmin A, Johansson B, Gimenez L, Ogren SO, Fredholm BB. Combination of adenosine A1 and A2A receptor blocking agents induces caffeine-like locomotor stimulation in mice. Eur Neuropsychopharmacol. 2006;16:129–36. doi: 10.1016/j.euroneuro.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Lara DR, Dall’Igna OP, Ghisolfi ES, Brunstein MG. Involvement of adenosine in the neurobiology of schizophrenia and its therapeutic implications. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:617–29. doi: 10.1016/j.pnpbp.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Lazarus M, Shen HY, Cherasse Y, Qu WM, Huang ZL, Bass CE, Winsky-Sommerer R, Semba K, Fredholm BB, Boison D, Hayaishi O, Urade Y, Chen JF. Arousal effect of caffeine depends on adenosine A2A receptors in the shell of the nucleus accumbens. J Neurosci. 2011;31:10067–75. doi: 10.1523/JNEUROSCI.6730-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarus M, Shen HY, Cherasse Y, Qu WM, Huang ZL, Bass CE, Winsky-Sommerer R, Semba K, Fredholm BB, Boison D, Hayaishi O, Urade Y, Chen JF. Arousal effect of caffeine depends on adenosine A2A receptors in the shell of the nucleus accumbens. J Neurosci. 2011;31:10067–75. doi: 10.1523/JNEUROSCI.6730-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Doux JE, Iwata J, Cicchetti P, Reis DJ. Different projections of the central amygdaloid nucleus mediate autonomic and Behavioral correlates of conditioned fear. J Neurosci. 1988;8:2517–29. doi: 10.1523/JNEUROSCI.08-07-02517.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Doux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–84. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Li T, Ren G, Lusardi T, Wilz A, Lan JQ, Iwasato T, Itohara S, Simon RP, Boison D. Adenosine kinase is a target for the prediction and prevention of epileptogenesis in mice. J Clin Invest. 2008;118:571–82. doi: 10.1172/JCI33737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S. Neurobiology of Pavlovian fear conditioning. Annu Rev Neurosci. 2001;24:897–931. doi: 10.1146/annurev.neuro.24.1.897. [DOI] [PubMed] [Google Scholar]
- Meyer U, Chang DL, Feldon J, Yee BK. Expression of the CS- and US-pre-exposure effects in the conditioned taste aversion paradigm and their abolition following systemic amphetamine treatment in C57BL6/J mice. Neuropsychopharmacology. 2004;29:2140–8. doi: 10.1038/sj.npp.1300522. [DOI] [PubMed] [Google Scholar]
- Muhia AM, Willadt S, Yee BK, Feldon J, Paterna JC, Schwendener S, Vogt K, Kennedy MB, Knuesel I. Molecular and behavioral changes associated with adult hippocampus-specific SynGAP1 knockout. Learn Mem. 2012;19:268–81. doi: 10.1101/lm.026351.112. [DOI] [PubMed] [Google Scholar]
- Parkinson JA, Robbins TW, Everitt BJ. Dissociable roles of the central and basolateral amygdala in appetitive emotional learning. Eur J Neurosci. 2000;12:405–13. doi: 10.1046/j.1460-9568.2000.00960.x. [DOI] [PubMed] [Google Scholar]
- Pietropaolo S, Mintz M, Feldon J, Yee BK. The Behavioral sequela following the prevention of home-cage grid-climbing activity in C57BL/6 mice. Behav Neurosci. 2007;121:345–55. doi: 10.1037/0735-7044.121.2.345. [DOI] [PubMed] [Google Scholar]
- Popoli P, Gimenez-Llort L, Pezzola A, Reggio R, Martinez E, Fuxe K, Ferre S. Adenosine A1 receptor blockade selectively potentiates the motor effects induced by dopamine D1 receptor stimulation in rodents. Neurosci Lett. 1996;218:209–213. doi: 10.1016/s0304-3940(96)13143-8. [DOI] [PubMed] [Google Scholar]
- Quarta D, Ferré S, Solinas M, You ZB, Hockemeyer J, Popoli P, Goldberg SR. Opposite modulatory roles for adenosine A1 and A2A receptors on glutamate and dopamine release in the shell of the nucleus accumbens. Effects of chronic caffeine exposure. J Neurochem. 2004;88:1151–8. doi: 10.1046/j.1471-4159.2003.02245.x. [DOI] [PubMed] [Google Scholar]
- Reilly S, Bornovalova MA. Conditioned taste aversion and amygdala lesions in the rat: a critical review. Neurosci Biobehav Rev. 2005;29:1067–88. doi: 10.1016/j.neubiorev.2005.03.025. [DOI] [PubMed] [Google Scholar]
- Ribeiro JA, Sebastião AM, de Mendonça A. Adenosine receptors in the nervous system: pathophysiological implications. Prog Neurobiol. 2003;68:377–92. doi: 10.1016/s0301-0082(02)00155-7. [DOI] [PubMed] [Google Scholar]
- Richmond MA, Murphy CA, Pouzet B, Schmid P, Rawlins JNP, Feldon J. A computer controlled analysis of freezing. Behavior J Neurosci Methods. 1998;86:91–9. doi: 10.1016/s0165-0270(98)00150-2. [DOI] [PubMed] [Google Scholar]
- Sebastião AM, Ribeiro JA. Adenosine receptors and the central nervous system. Handb Exp Pharmacol. 2009;193:471–534. doi: 10.1007/978-3-540-89615-9_16. [DOI] [PubMed] [Google Scholar]
- Shen HY, Coelho JE, Ohtsuka N, Canas PM, Day Y-J, Huang Q-Y, Rebola N, Yu L, Boison D, Cunha RA, Linden J, Tsien JZ, Chen JF. A critical role of the adenosine A2A receptor in extra-striatal neurons in modulating psychomotor activity as revealed by opposite phenotypes of striatum- and forebrain-A2A receptor knockouts. J Neurosci. 2008a;28:2970–5. doi: 10.1523/JNEUROSCI.5255-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen HY, Li T, Abreu R, Fredholm BB, Boison D, Chen JF. Selective inactivation of adenosine A2A receptors in striatal neurons abolishes motor and arousal effects of caffeine. Soc Neurosci Abst. 2008b:384.385. [Google Scholar]
- Shen HY, Lusardi TA, Williams-Karnesky RL, Lan JQ, Poulsen DJ, Boison D. Adenosine kinase determines the degree of brain injury after ischemic stroke in mice. J Cereb Blood Flow Metab. 2011;31:1648–59. doi: 10.1038/jcbfm.2011.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen HY, Singer P, Lytle N, Wei CJ, Lan JQ, Williams-Karnesky RL, Chen JF, Yee BK, Boison D. Adenosine augmentation ameliorates psychotic and cognitive endophenotypes of schizophrenia. J Clin Invest. 2012;122:2567–77. doi: 10.1172/JCI62378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer P, McGarrity S, Shen HY, Boison D, Yee BK. Working memory and the homeostatic control of brain adenosine by adenosine kinase. Neuroscience. 2012;213:81–92. doi: 10.1016/j.neuroscience.2012.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinas M, Ferré S, You ZB, Karcz-Kubicha M, Popoli P, Goldberg SR. Caffeine Induces Dopamine and Glutamate Release in the Shell of the Nucleus Accumbens. J Neurosci. 2002;22:6321–4. doi: 10.1523/JNEUROSCI.22-15-06321.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studer FE, Fedele DE, Marowsky A, Schwerdel C, Wernli K, Vogt K, Fritschy JM, Boison D. Shift of adenosine kinase expression from neurons to astrocytes during postnatal development suggests dual functionality of the enzyme. Neuroscience. 2006;142:125–37. doi: 10.1016/j.neuroscience.2006.06.016. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Le Moine C, Fisone G, Fredholm BB. Distribution, biochemistry and function of striatal adenosine A2A receptors. Prog Neurobiol. 1999a;59:355–96. doi: 10.1016/s0301-0082(99)00011-8. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Nomikos GG, Fredholm BB. The stimulatory action and the development of tolerance to caffeine is associated with alterations in gene expression in specific brain regions. J Neurosci. 1999b;19:4011–22. doi: 10.1523/JNEUROSCI.19-10-04011.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theofilas P, Brar S, Stewart KA, Shen HY, Sandau US, Poulsen D, Boison D. Adenosine kinase as a target for therapeutic antisense strategies in epilepsy. Epilepsia. 2011;52:589–601. doi: 10.1111/j.1528-1167.2010.02947.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei CJ, Singer P, Coelho J, Boison D, Feldon J, Yee BK, Chen JF. Selective inactivation of adenosine A(2A) receptors in striatal neurons enhances working memory and reversal learning. Learn Mem. 2011;18:459–74. doi: 10.1101/lm.2136011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werka T. Involvement of the lateral and dorsolateral amygdala in conditioned stimulus modality dependent two-way avoidance performance in rats. Acta neurobiol exp. 1998:55131–47. doi: 10.55782/ane-1998-1267. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Shimura T, Sako N, Yasoshima Y, Sakai N. Neural substrates for conditioned taste aversion in the rat. Behav Brain Res. 1994;65:123–37. doi: 10.1016/0166-4328(94)90097-3. [DOI] [PubMed] [Google Scholar]
- Yee BK, Balic E, Singer P, Schwerdel C, Grampp T, Gabernet L, Knuesel I, Benke D, Feldon J, Möhler H, Boison D. Disruption of glycine transporter 1 restricted to forebrain neurons is associated with a procognitive and antipsychotic phenotypic profile. J Neurosci. 2006;26:3169–81. doi: 10.1523/JNEUROSCI.5120-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee BK, Hauser J, Dolgov VV, Keist R, Möhler H, Rudolph U, Feldon J. GABA receptors containing the alpha5 subunit mediate the trace effect in aversive and appetitive conditioning and extinction of conditioned fear. Eur J Neurosci. 2004;20:1928–36. doi: 10.1111/j.1460-9568.2004.03642.x. [DOI] [PubMed] [Google Scholar]
- Yee BK, Singer P, Chen JF, Feldon J, Boison D. Transgenic overexpression of adenosine kinase in brain leads to multiple learning impairments and altered sensitivity to psychomimetic drugs. Eur J Neurosci. 2007;26:3237–52. doi: 10.1111/j.1460-9568.2007.05897.x. [DOI] [PubMed] [Google Scholar]



