Abstract
Biting flies are economically important, blood-feeding pests of medical and veterinary significance. Chemosensory-based biting fly behaviors, such as host/nutrient source localization and ovipositional site selection, are intriguing targets for the development of supplemental control strategies. In an effort to expand our understanding of biting fly chemosensory pathways, transcripts encoding the highly conserved insect odorant co-receptor (Orco) were isolated from two representative biting fly species, the stable fly (Scal\Orco) and the horn fly (Hirr\Orco). Orco forms a complex with an odor-specific odorant receptor to form an odor-gated ion channel. The biting fly transcripts were predicted to encode proteins with 87% – 94% amino acid similarity to published insect Orco sequences and were detected in various immature stages as well as in adult structures associated with olfaction, i.e. antennae and maxillary palps, and gustation, i.e. proboscis. Further, the relevant proteins were immunolocalized to specific antennal sensilla using anti-serum raised against a peptide sequence conserved between the two fly species. Results from this study provide a basis for functional evaluation of repellent/attractant effects on as yet uncharacterized stable fly and horn fly conventional odorant receptors.
Introduction
Stable flies, Stomoxys calcitrans L., and horn flies, Haematobia irritans L. are significant livestock pests that are problematic in confined (Campbell et al., 1987; Wieman et al., 1992) and rangeland operations (Kunz et al., 1984; Byford et al., 1992; Campbell et al., 2001; Broce et al., 2005). Their economic impact on the U.S. cattle industry is astounding, impacting cattle production at $1 – $2.2 billion annually (Cupp et al., 1998; Taylor et al., 2012). Both biting fly species primarily parasitize livestock, yet exhibit different associations with their host and utilize different substrates for development of the immature stages. The horn fly spends the majority of its time somewhat permanently associated with its bovine host, feeding 10 – 40 times per day and leaving only periodically to oviposit in fresh manure pats or to migrate short distances (Campbell, 2006). The stable fly tends to perch on resting sites where it ‘ranges’ for a host (Gibson & Torr, 1999), takes 1 – 2 bloodmeals per day, and spends comparably less time on-animal (Foil & Hogsette, 1994). The stable fly is also a much more cosmopolitan pest with evidence of feeding on humans (Newson, 1977; Koehler & Kaufman, 2006), dogs (Pitzer et al., 2011), and pelicans (Johnson et al., 2010) and of using sugar as an alternative energy resource (Jones et al., 1985; Jones et al., 1992; Taylor & Berkebile, 2008). Immature horn fly development requires fresh cattle manure, as it provides an optimal moisture level and bacterial community on which immature development can succeed (Kuramochi, 2000; Perotti et al., 2001); stable fly larval habitats are more varied, comprised of decaying organic matter, such as spilled hay, mixed with moisture from host urine and manure or rain that enables persistence of a microbe-rich community (Romero et al., 2006; Talley et al., 2009). Sugar cane debris (Koller et al., 2009) and biosolid cakes at wastewater treatment facilities (Doud et al., 2012) are also substrates in which immature stable fly development has been observed.
Olfaction plays a critical role in biting fly host localization (Gibson & Torr, 1999; Birkett, et al., 2004; Jeanbourqin & Guerin, 2007b; Oyarzún et al., 2009) and ovipositional site selection (Perotti et al., 2001; Romero et al., 2006; Jeanbourquin & Guerin, 2007a; Tangtrakulwanich et al., 2011), and the biological/behavioral differences between the stable fly and the horn fly may result in their displaying a varied repertoire of chemosensory gene products. Insect olfaction has been studied extensively in several model dipteran and lepidopteran species (Jacquin-Joly and Merlin, 2004; Dahanukar et al., 2005; Pelosi et al., 2006). The conversion of a volatile to a nervous impulse directed to the insect brain center begins with porous sensilla (hairs) associated with the cuticle of olfactory organs, i.e. antennae, maxillary palps. Each sensillum houses between one and four specialized olfactory sensory neurons (OSNs), the dendrites of which extend into the sensillar lymph. Upon exposure to an odor plume, odorant binding proteins present in the sensillar lymph are believed to bind a hydrophobic volatile and shuttle it to ligand/odorant-selective odorant receptors (Or) bound to dendritic membranes. An individual OSN typically expresses a single Or (one-neuron, one receptor; Couto et al., 2005) although there are known exceptions to this relationship (Fishilevich & Vosshall, 2005; Goldman et al., 2005). Once the ligand/odorant is bound, a cascade of events is initiated that leads to nervous activity, but the mechanism by which this occurs remains unclear. An elegant set of studies by Sato et al (2008) and Wicher et al (2008) redefined our understanding of insect Ors as ligand-gated ion channels. A functional channel is comprised of an Or that heterodimerizes with Orco, an odorant co-receptor that is highly conserved in Insecta (Krieger et al., 2003) and is absolutely critical for Or stability (Benton et al., 2006). A newly described class of chemosensory receptors, members of the ionotropic receptor family, has recently been described in Drosophila (Benton et al., 2006). Interestingly, the receptors are expressed in a subset of OSNs that are distinct from OSNs expressing Or-Orco (Benton et al., 2009), underscoring the complexity of insect odor recognition pathways.
Manipulation of insect olfaction for the development of control technologies based on insect behavior modification (Bohbot & Dickens, 2012) would be useful in an integrated pest management program and is desirable for biting fly pests, especially given the increasing development of insecticide resistance in horn flies (reviewed by Oyarzún et al., 2008) and the low level of adult stable fly control achieved using insecticides due to the minimal time they spend feeding on their host. Such examples include modulation of the Or-Orco complex, one mechanism of action for the insect repellent DEET (Ditzen et al., 2008; Pellegrino et al., 2011), and activation or inhibition of Orco (Jones et al., 2012; Chen & Luetje, 2012). The biochemical pathway regulating chemosensation in the stable fly and the horn fly warrants further study to improve our understanding of the biting fly response to attractants/repellents and their utility for enhancing pest control. While Olafson et al. (2010) identified a number of genes with a putative role in stable fly chemosensation, including two unique odorant receptors, no published reports are available for such genes in the horn fly. In this study, Orco was isolated from the stable fly and the horn fly and its temporal and spatial expression pattern as well as its localization to OSNs within antennal sensilla was described.
Results and discussion
Isolation of Orco orthologues from Muscidae
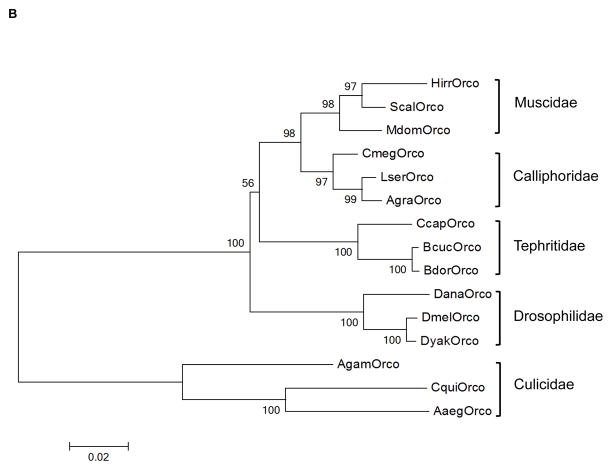
Degenerate-primed PCR was used to isolate 660 bp and 672 bp fragments from the stable fly and the horn fly, respectively that demonstrated 89% nucleotide identity to each other and 82–83% nucleotide sequence identity to Orco from two calliphorid species, Chrysomya megacephala (F.) and C. rufifacies (Macquart). These fragments enabled isolation of full-length Orco cDNA sequences from the stable fly and horn fly that encoded 478 aa and 485 aa proteins, respectively, both of which are predicted to contain seven transmembrane (TM) domains, a signature of odorant receptor sequences (Fig. 1A). Further, the coding sequences displayed 87 – 94% amino acid identity to Orco from Drosophila melanogaster Meigen, Bactrocera cucurbitae Coquillet, and C. rufifacies, and phylogenetic comparison of dipteran Orco revealed the muscid sequences shared a common node with Orco from calliphorid fly species (Fig. 1B). As a result, the muscid transcripts were named Scal\Orco and Hirr\Orco and the corresponding predicted amino acid sequences were designated ScalOrco and HirrOrco, respectively. Analysis of membrane topology using the TopPred, TMHMM, and TMPred algorithms indicated the biting fly Orco sequences have an intracellular N-terminus and an extracellular C-terminus, an organization similar to what has been experimentally confirmed for Drosophila Ors (Benton et al., 2006; Lundin et al., 2007). HirrOrco is seven amino acids (254SAALLPN260) longer than ScalOrco and these residues are located in the predicted intracellular loop connecting TM4 and TM5 (Fig. 1A), a region thought to be important for intracellular transport (Benton et al., 2006). Conservation of residues within the C-terminus (predicted TM6–TM7) has been observed for conventional Drosophila Ors and insect Orco sequences, and Benton et al. (2006) demonstrated that the loop connecting TM6 and TM7 is part of a region where Or and Orco interact. The predicted TM6 and TM7 of both ScalOrco and HirrOrco display a high level of sequence conservation with other insect Orcos, including the tyrosine residue in TM7 (Y478 in D. melanogaster) that is important for successful Or-Orco interactions in vivo (Nakagawa et al. 2012). Sequence conservation within the C-terminal region of ScalOrco and HirrOrco may translate to functional conservation, suggesting they may be able to dimerize with ligand/odorant-selective Ors.
Figure 1.
Sequence conservation of ScalOrco and HirrOrco. (A) Amino acid sequence alignment of ScalOrco and HirrOrco to protein sequences from related dipterans. Identical residues are shaded, and the predicted transmembrane domains TM1—TM7 are identified by dashed bars. Location of four known introns in the partial Scal\Orco genomic sequence are identified as ↓1–4; the location of intron 1 (↓1) is shared by the horn fly. Asterisks indicate the 14 amino acid peptide used to generate polyclonal antibodies and # indicates the conserved tyrosine (Y) residue in TM7 (Y478 in D. melanogaster). (B) Phylogenetic analysis of dipteran Orco sequences. The neighbor-joining tree was constructed with Orco sequences from the indicated dipteran species, and bootstrap values are based on 1000 replicates with support values >80% displayed on nodes of the tree. Abbreviations and relevant accession numbers are as follows: Aaeg (Aedes aegypti, EAT42706); Agam (Anopheles gambiae, XP_312379); Agra (Aldrichina graham, ADN88092); Bcuc (Bactrocera curcurbitae, ADK97803); Bdor (Bactrocera dorsalis, ACC86853); Ccap (Ceratitis capitata, AAX14775); Cmeg (Chrysomya megacephala, AEA30004); Cqui (Culex quinquefasciatus; ABB29301); Dana (Drosophila ananassae, XP_001953343); Dmel (Drosophila melanogaster, AAT71306); Dyak (Drosophila yakuba, XP_002096053); Hirr (Haematobia irritans, ACF21678); Lser (Lucilia sericata, AEA30005); Mdom (Musca domestica, AFH96944); Scal (Stomoxys calcitrans; ACF21677).
Immunolocalization of ScalOrco and HirrOrco to antennal sensilla
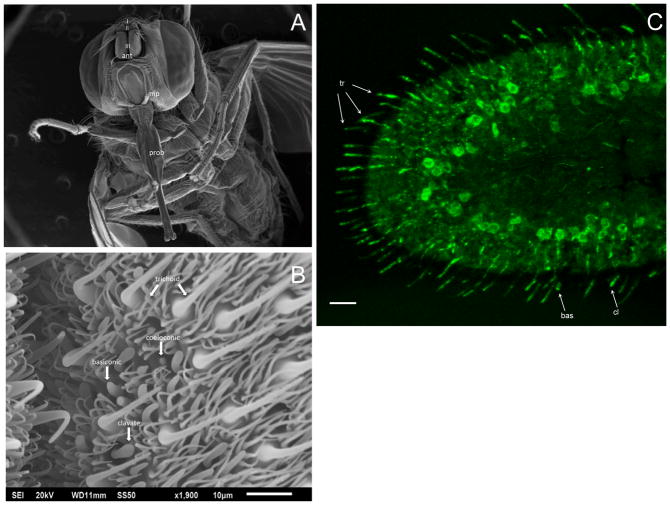
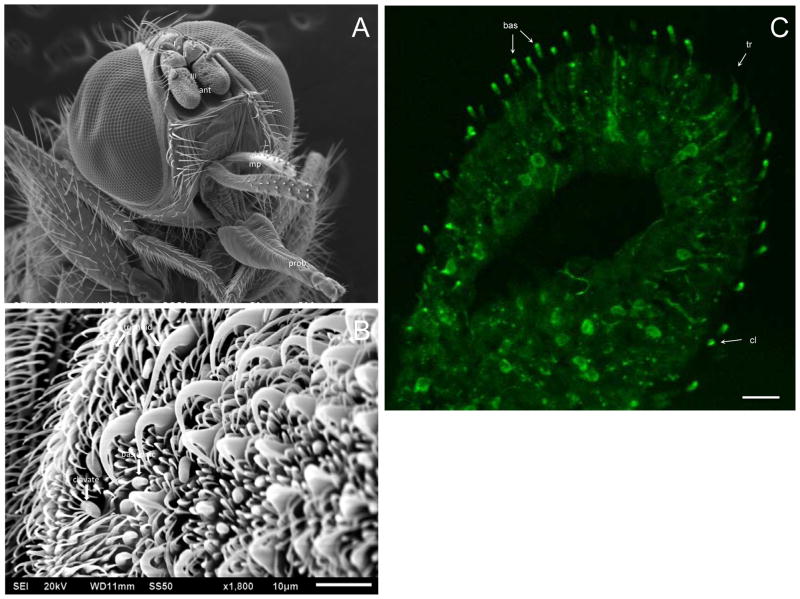
Stable fly and horn fly antennae are comprised of a scape, pedicel, and flagellum, and antennal sensilla are predominantly located in the flagellum (Figs. 2A and 3A; Lewis, 1971; White and Bay, 1980; Tangtrakulwanich et al., 2011). In order to localize the expression of Orco, affinity purified polyclonal antibodies were generated against a 14 amino acid synthetic peptide conserved between ScalOrco and HirrOrco (Fig. 1A), and the antibody was used in immunohistochemical evaluation of head cryosections containing antennae from the stable fly and horn fly. Antibody binding was detected in numerous distinct cell bodies within the respective flagella, as well as in the dendritic extension of a number of OSNs that project into antennal sensilla (Figs. 2C and 3C), supporting Orco expression in a majority of antennal OSNs. It was difficult to identify an optical plane that could be used to trace the labeling from the cell body along the dendritic extension within a specific sensillum, but it was apparent that basiconic, clavate and long trichoid type hairs (sensilla) were labeled in stable fly preparations (Figs. 2B, 2C; J.J. Zhu, personal communication). SEM observations by both Lewis (1971) and Tangtrakulwanich et al. (2011) confirm pores on the surface of basiconic and clavate antennal sensilla, supporting their role in olfaction; however, the authors differed in their assessment of trichoid sensilla with Tangtrakulwanich et al. (2011) assigning them a mechano-receptor function and Lewis (1971) an olfactory function. Anti-Orco labeling of stable fly trichoid sensilla observed in this study supports an olfactory role, but single-sensillum recordings are desirable to clarify their classification. In horn flies (Fig. 3C), antibody labeling was observed in multiporous thick-walled, thin-walled Ia/Ib, and clavate sensilla, as described by White and Bay (1980). Based on their properties, i.e. length, cuticle thickness, base diameter, these sensilla correspond to what is described in the stable fly as long trichoid, basiconic and clavate sensilla, respectively (Fig. 3B). Insect trichoid sensilla with an olfactory function have been described for mosquitos (Ghaninia et al., 2007; Hill et al., 2009), D. melanogaster (Clyne et al., 1997), and the migratory locust (Locusta migratoria L.; Cui et al., 2011), supporting the results observed. Specificity of antibody labeling was assessed by incubating antennal cryosections with pre-immune serum or with polyclonal antibodies in the presence of the peptide used to generate the anti-serum. In both cases, no distinct cell or sensillum labeling was observed although there did appear to be some general background (data not shown). Sections were also incubated with the fluorophore-conjugated secondary antibody in the absence of anti-serum; no labeling or background was observed in these sections.
Figure 2.
Localization of ScalOrco in S. calcitrans female antenna. Green fluorescence represents anti-Orco detected using an Alexa Fluor 488-conjugated secondary antibody. (A) SEM of a stable fly head depicting the three antennal (ant) segments (I: scape, II: pedicel, III: flagellum), the maxillary palp (mp), and the proboscis (prob). (B) A region of the flagellum identifying trichoid, basiconic, clavate, and coeloconic sensilla, as described by Lewis (1971) and Tangtrakulwanich et al. (2011). (C) ScalOrco labeling of nerve cell bodies and dendrites within specific sensilla. Different sensillar types were labeled by anti-Orco, namely basiconic (bas), clavate (cl) and trichoid (tr) sensilla. Scale bar represents 20μm.
Figure 3.
Localization of HirrOrco in H. irritans male antenna. Green fluorescence represents anti-Orco detected using an Alexa Fluor 488-conjugated secondary antibody. (A) SEM of a horn fly head depicting the three antennal (ant) segments (I: scape, II: pedicel, III: flagellum), the maxillary palp (mp), and the proboscis (prob). (B) A region of the flagellum identifying trichoid, basiconic, and clavate sensilla, as described by White and Bay (1980). (C) HirrOrco labeling of nerve cell bodies and dendrites within specific antennal sensilla. Different sensillar types were labeled by anti-Orco, namely basiconic (bas), clavate (cl) and trichoid (tr) sensilla. Scale bar represents 20μm.
Temporal and spatial expression pattern of Scal\Orco and Hirr\Orco
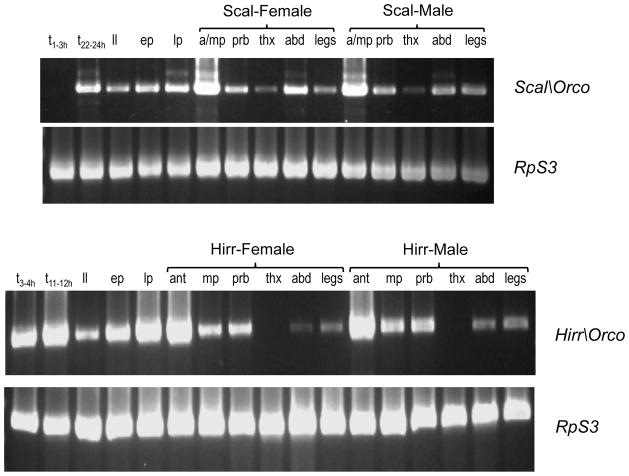
Using non-quantitative RT-PCR (Fig. 4), a robust Scal\Orco amplification product was detected in pooled antennae/maxillary palps from female and male adults. The transcript was also detected relatively less abundantly in proboscides, thoraces, abdomens and legs of both adult sexes. Scal\Orco was detected in most of the immature stages, i.e. 22–24 h embryos, 3rd instar larvae, early (pre-tanning) puparia, and late pharate adults, but no transcript was detected in newly laid (1–3 h) embryos. Conversely, Hirr\Orco was expressed in all immature stages evaluated, displaying a relative increase in abundance over time, as evidenced by increasing intensity of the product through the pupal stages (Fig. 4). Hirr\Orco was detected most robustly in adult antennae of both sexes and less so in proboscides and maxillary palps. Since horn fly maxillary palps are large compared with those of the stable fly (Fig. 2), they were not pooled with antennae for expression analysis. Hirr\Orco expression was relatively faint in abdomens and legs and apparently absent from the thorax of both sexes.
Figure 4.
Temporal and spatial expression patterns of Scal\Orco (A) and Hirr\Orco (B). Temporal expression was evaluated using template synthesized separately from newly laid embryos (t1–3h or t3–4h), pre-hatching embryos (t22–24h or t11–12h), 3rd instar larvae (ll), pre-tanning/’early’ puparia (ep) and late pharate adults (lp; puparia 2 days prior to adult eclosion). Spatial expression was analyzed from female or male pooled antennae-maxillary palp (a-mp; stable fly) or antennae (ant) and maxillary palps (mp) isolated separately (horn fly), proboscis (prb), thorax (thx), abdomen (abd), and legs. Expression of biting fly ribosomal protein S3 (RpS3) was used as a positive control for presence of template.
In Drosophila, Orco is detected in larvae but not in early pupae, and this is coincident with a transition from the larval sensory system to the adult sensory system (Stocker 2008; Larsson et al., 2004). In contrast, there was no apparent decrease in biting fly Orco expression during the early (pre-tanning) pupal stages, but a relative decrease in expression for both transcripts was observed in 3rd instar larvae with subsequent elevated expression during the early and late pupal stages. Detection in the majority of immature stages evaluated suggests the importance of Orco throughout biting fly development.
The Orco expression pattern observed in biting fly adults supports its role in olfaction, especially given its detection in antennae and maxillary palps that are classic insect olfactory organs. Apparently equivalent Orco expression in female and male antennae was not unexpected since the different types of antennal sensilla are equally represented between the sexes of the stable fly and the horn fly (Lewis, 1971; White and Bay, 1980; Tangtrakulwanich et al., 2011). Contact chemoreceptive hairs (taste hairs) have been described on the labellum and tarsi of stable flies (Adams et al., 1965; Adams and Forgash, 1966; van der Wolk et al., 1984), supporting a gustatory function for these structures; however, detection of Scal\Orco and Hirr\Orco in proboscides and legs suggests either these structure also have a role in olfaction or Orco may have an additional, non-olfactory role. Orco detection in legs has been reported in several mosquito species (Pitts et al., 2004; Melo et al., 2004; Xia and Zweibel, 2006), female fig wasps (Lu et al., 2009), the blowfly (L. sericata; Wang et al., 2012), and L. migratoria; Yang et al., 2012), yet its expression is absent in legs from the oriental fruit fly (Zheng et al., 2012) and several lepidopterans (Malpel et al., 2008; Wu et al., 2012) further indicating that Orco may have a broader chemosensory role, as suggested by Vosshall et al. (2000) and Krieger et al. (2003). While Orco expression in proboscides of several mosquitos was attributed to the presence of labial olfactory sensilla (Pitts et al., 2004; Melo et al., 2004; Xia and Zwiebel, 2006; Kwon et al., 2006), comparable sensilla were not observed in head cryosections containing the proboscis of stable flies or horn flies. This does not rule out their existence, though, and additional studies using an electrolabellogram may shed some light on the results obtained here. Detection of Scal\Orco in stable fly male and female thoraces was unexpected, especially since Orco is not typically detected in this tissue (Pitts, et al., 2004; Wang et al., 2012; Wu et al., 2012; Zheng et al., 2012) and appears to be absent in thoraces of male and female horn flies. To evaluate whether Orco protein could be detected in these tissues, the insoluble protein fraction was isolated from heads and thoraces of adult stable flies and horn flies for use in immunoblotting with the anti-Orco antibody. Interestingly, protein was detected in heads and thoraces of both fly species (Fig. S1) supporting ScalOrco and HirrOrco expression in the thorax and suggesting that the inability to detect Hirr\Orco by RT-PCR may be a result of assay sensitivity. Expression of Orco in stable fly thoraces may be an adaptive advantage that enables them to parasitize a broader host range or utilize more varied ovipositional substrates, similar to what has been proposed for fig wasps (Lu et al., 2009).
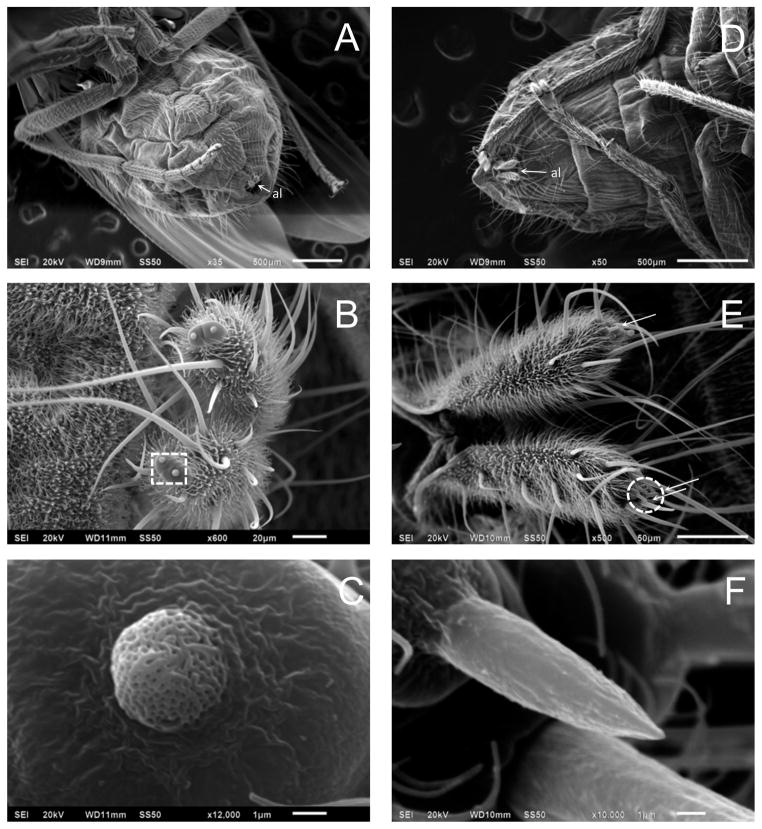
Detection of Hirr\Orco in the female abdomen corresponds with previous descriptions by Bay et al. (1996) of porous sensory pegs located on the anal leaflets at the terminus of the female horn fly ovipositor (Figs. 5B, 5C); the pores indicate the sensilla have an olfactory function. The anal leaflets also contain numerous, long sensilla that are believed to be mechano-receptors (Bay et al., 1996). SEM analysis of the female stable fly ovipositor revealed similar long, grooved sensilla on each anal leaflet that may have a mechanosensory role; these sensilla surround a distinct, short (~ 8.8 μm) pointed sensillum situated on an elevated base (Figs. 5E, 5F) that is located at a position corresponding to the described horn fly sensory pegs. Scal\Orco expression was detected by RT-PCR in dissected ovipositors as well as abdomens with the ovipositors removed (Fig. S2); this suggested that the pointed sensillum may have a chemosensory role but also indicated the possibility of other Scal\Orco –expressing chemosensory sensilla on the abdomen. The presence of abdominal olfactory sensilla was recently described for the midge, Culicoides imicola (Sollai et al., 2010), but has not been investigated for biting flies. Several calliphorid fly species also have similar sensory pegs on their anal leaflets (Wallis, 1962; Rice, 1976; Chaiwong et al., 2008), emphasizing the importance of olfactory cues to oviposition/ovipositional site selection. Both the stable fly and the horn fly rely on a microbe-rich environment for successful development of the immature stages (Lysyk et al., 1999; Romero et al., 2006, Talley et al., 2009) and may choose a site for oviposition based on the volatiles emitted by the various bacterial species occupying a potential substrate; presence of olfactory sensilla on the abdomen and external genitalia would serve to enhance detection of these olfactory cues.
Figure 5.
Scanning electron micrograph (SEM) of the horn fly and stable fly ovipositor. (A) Ventral view of an adult female horn fly with extended terminus showing the paired anal leaflets (al). (B) Horn fly anal leaflets showing the paired multiparous sensory pegs. (C) Higher magnification of the sensory peg [region denoted by a square in (B)]. (D) Ventral view of an adult female stable fly with extended terminus showing the paired anal leaflets (al). (E) Stable fly anal leaflets with pointed sensilla identified by arrows (see text). (F) Higher magnification of the pointed sensillum [region denoted by a circle in (E)].
Collectively, the sequence conservation of ScalOrco and HirrOrco with published insect Orcos, the predicted size and presence of transmembrane domains, and the expression and labeling of ScalOrco and HirrOrco in tissues known to have a role in chemosensation supports classification of these biting fly sequences as members of the Orco gene family (Vosshall and Hansson, 2011). These findings enable future in vitro functional characterization of conventional biting fly Ors, providing a means to screen volatile attractant/repellent compounds that may form the basis of supplemental pest control alternatives (Justice et al., 2003; van der Goes van Naters & Carlson, 2006).
Experimental procedures
Insect Rearing and Tissue Collection
Stable fly and horn fly specimens used in all experiments were obtained from colonized strains maintained by the Knipling-Bushland U.S. Livestock Insects Research Laboratory (Kerrville, TX) at 27 °C, 60% RH, and a photoperiod of 12:12 [L:D] h. Staged embryos were collected representing newly laid and pre-hatching timepoints (1–3 h and 22–24 h for the stable fly; 3–4 h and 11–12h for the horn fly). Third instar larvae were collected from both species, as well as newly pupariated (pre-tanning) and late pharate adults (puparia 2d prior to adult emergence). All collected immature stages were frozen on dry ice prior to storage at −80 °C. Antennae, maxillary palps, proboscides, thoraces, abdomens and legs of adult females and males (0 – 2d, fed) were dissected from specimens frozen on dry ice, and the tissues were stored separately in RNAlater®-ICE (Life Technologies, Grand Island, NY) at −20 °C prior to further manipulation.
RNA Isolation and cDNA Synthesis
Frozen samples of the various immature stages were macerated in TRI Reagent® (Sigma, St. Louis, MO) using a liquid nitrogen-cooled Kontes PELLET PESTLE® (Kimble Chase, Vineland, NJ). Adult samples in RNAlater®-ICE were centrifuged to collect the tissue, the storage buffer discarded, and the tissues macerated in TRI Reagent® using a liquid nitrogen-cooled Kontes PELLET PESTLE® (Kimble Chase). Subsequent to homogenization, BCP (3-bromopropylchloride; Molecular Research Center, Cincinnati, OH) was used to aid in phase separation and the RNAs were purified on silica matrix spin columns (RNeasy® Mini Kit; Qiagen, Valencia, CA). All total RNAs were DNase-treated using the TURBO DNA-free™ system (Life Technologies) following the manufacturer’s protocol. First-strand cDNAs were synthesized using equal amounts of total RNA from each source (1 μg), 2.5 μM anchored oligo dT primer (5′-T(20)VN -3′), and 0.5 mM dNTP mix denatured at 65 °C for 5 min and then combined with 1 x First Strand Buffer (Life Technologies), 5 mM DTT, 40 U RNaseOUT™ (Life Technologies), and 200 U SuperScript™ III Reverse Transcriptase (Life Technologies) in a total volume of 20 μl. The cDNAs were synthesized at 50 °C for 1.5 h.
Degenerate Primed PCR
Degenerate primers were designed based on the amino acid sequences LIFACE (5′-GYTIATHTTYGCITGYGARC-3′) and CQQCQK (5′-GCYTTYTGRCAYTGYTGRCA-3′), which are highly conserved among characterized insect Orco sequences. Oligo-d T(20)VN -primed first strand cDNAs synthesized from unfed adult female stable fly and horn fly were used as template for amplification with the following cycling conditions: 94 °C, 2 mins; 19 cycles of: 94 °C, 30 s; 55 °C, 40 s; 68 °C, 1 min with a decrease in annealing temperature of 0.5 °C per cycle; and 19 cycles of: 94 °C, 30 s; 45 °C, 40 s; 68 °C, 1 min with a final extension at 68 °C for 3 mins. Reactions (50μl) consisted of 40 mM Tricine-KOH, 15 mM potassium acetate, 3.5 mM magnesium acetate, 3.75 μg/ml BSA, 0.005 % Tween 20, 0.005 % Nonidet-P40, 0.2 mM dNTPs (Life Technologies), 100 picomoles each primer, and 1x Advantage® 2 Polymerase Mix (BD Clontech, Mountain View, CA). Amplified products of the expected size were cloned using the pCR®4-TOPO® TA system (Life Technologies) in TOP10 E. coli cells. Transformed cells were plated on LB agar supplemented with kanamycin (50 mg/ml), and plasmid DNA was isolated from fifty isolates using the QIAprep® Spin Miniprep Kit (Qiagen). Plasmid template was used in cycle sequencing with BigDye™ version 3.1 chemistry (Life Technologies), and reactions were analyzed on an ABI3130xl Genetic Analyzer (Life Technologies).
Isolation of full-length Orco transcripts from Scal and Hirr (RACE)
RACE-Ready cDNAs were synthesized separately utilizing stable fly and horn fly unfed adult female total RNA as template in the SMART™ RACE cDNA Amplification Kit (BD Clontech) following the manufacturer’s protocol. The 5′ and 3′ RACE for each template was conducted using an Scal or Hirr Orco-specific primer in a primary round of cycling followed by a nested reaction using a transcript-specific nested primer, both in combination with relevant commercial adapter primers UPM (primary) or NUPM (nested). Primers used in 5′ and 3′ stable fly RACE reactions were ScalOr83b-Rev4 (primary 5′; 5′-GCCCAAGTAGCCGATCAC-3′), ScalOr83b-Rev5 (nested 5′; 5′-TTGGTTGCCTGGTACGC-3′), ScalOr83b-Fwd1 (primary 3′; 5′-CAGTTGC AGCATTTGAAGGG-3′), and ScalOr83b-Fwd2 (nested 3′; 5′-CATAATGAAACCTCTGATGGAG CTG-3′). Primers used in 5′ and 3′ horn fly RACE reactions were HiOr83b-Rev1 (primary 5′; 5′-GCCCAAGTAGCCGATCAC-3′), HiOr83b-Rev2 (nested 5′; 5′-AGTAAACGCATAGACATCCAC ACC-3′), HiOr83b-Fwd1 (primary 3′; 5′-AGCATCACTGGACACCTACCG -3′) and HiOr83b-Fwd2 (nested 3′; 5′-ACCTAATTCGGCTGCCTT -3′). Fragments of interest were cloned and sequenced as described above. A 5.8 kb stable fly genomic sequence comprising exons that are spliced to encode amino acids 1 – 342 of ScalOrco was obtained by primer walking using the Genome Walker system (Clontech). The sequence comprises four exons and four introns, the last intron of which was greater than 4 kb in length. The genomic sequence downstream of this region was not obtained. Further, primer pair HirrOr83b F5/R4 was used to amplify horn fly genomic DNA revealing the presence of a 208 bp intron, the location of which is shared with intron 1 of the Scal\Orco partial genomic sequence. This data supported use of selected primers for gene expression studies that could assist in distinguishing the presence of genomic DNA contamination, if present.
Semi-quantitative evaluation of ScalOrco and HirrOrco expression
First-strand cDNAs (equivalent to the amount synthesized from 20ng total RNA) were used as template in 20 μl RT-PCR reactions consisting of 40 mM Tricine-KOH, 15 mM potassium acetate, 3.5 mM magnesium acetate, 3.75 μg/ml BSA, 0.005 % Tween 20, 0.005 % Nonidet-P40, 0.2 mM dNTPs (Life Technologies), 5 picomoles each primer, and 1x Advantage® 2 Polymerase Mix (BD Clontech). Cycling conditions consisted of: 95 °C, 2 mins; 35 cycles of 95 °C, 30 s; 65 °C, 30 s; 68 °C, 30 s with final extension at 68 °C, 2 mins. Primer combinations used were ScOr83b Fwd4/ScOr83b R7 (expected size: 1090 bp) and HiOr83b-ATG/HiOr83b-Rev2 (expected size: 1173 bp); these were designed to span introns, providing a means to detect genomic DNA contamination if present. The RpS3 gene was amplified as an internal control for the presence of template using primer pair RpS3 Fwd/Rev (Tannealing: 63 °C) for both the stable fly and horn fly samples. Primer sequences used were as follows: ScalOr83b-Fwd4: 5′-GGTGCAGTTCGCCTTGA - 3′; ScalOr83b-R7: 5′-CAGCTTCCATGACCGATG - 3′; HiOr83b-ATG: 5′ –ATGACGAGTATGCAACCCACC - 3′; HiOr83b-Rev2: 5′-AGTAAACGCATA GACATCCACACC - 3′; RpS3-Fwd: 5′-CGTTCTCTGCGAGTCGTATG - 3′; RpS3-Rev: 5′-TTAATGAGCAGCAGCTTCATCA - 3′. A ‘no template’ control (water only) was included in all RT-PCR amplification experiments.
Antibody Production and Immunohistochemistry
A 15 amino acid peptide (CKSELINNEEKEPVN), located within the predicted intracellular loop connecting TM4 and TM5, was synthesized, conjugated to keyhole limpet hemocyanin (KLH), and used to generate sequence-specific, peptide-affinity purified polyclonal antibodies in rabbits (Genscript, Piscataway NJ).
Adult stable flies and horn flies (0 – 2 d old, fed) were fixed for 30 mins in 4% paraformaldehyde in PBS with 0.1% Triton X (PBSTx), washed with PBS for a total of 30 mins (10 mins/wash), and transferred to 25% sucrose overnight. Flies that sank to the bottom of the container were loaded onto a Fly Collar (Genessee Scientific, San Diego, CA) to properly orient the heads. The heads were embedded in Tissue-Tek O.C.T. embedding medium (Sakura Finetek, Torrance, CA), and blocks were wrapped in aluminum foil and stored at −20 °C prior to sectioning. Head cryosections (10 – 14 μm) obtained using a Leica CM1800 cryostat (Leica Microsystems, Buffalo Grove, IL) were thaw-mounted on Polysine® microscope slides (Erie Scientific, Portsmouth, NH) and air-dried at room temperature for 30 mins before either using immediately or storing at −20 °C. Slides were incubated in fixative (4% paraformaldehyde in PBSTx) for 30 mins, rinsed with PBSTx for a total of 30 mins (10 mins/wash), and blocked for 1 h at room temperature in blocking solution (PBSTx with 5% normal donkey serum). Primary anti-sera was diluted 1:2500 in blocking solution, dispensed onto sections and incubated at 4 °C overnight. Slides were washed in PBSTx for a total of 30 mins (10 mins/wash) and incubated at room temperature for 2 h in donkey anti-rabbit Alexa 488 antibody (Life Technologies) diluted 1:1000 in blocking solution. Slides were washed for a total of 30 mins in PBSTx (10 mins/wash) and coverslips were mounted in 80% glycerol. Sections were visualized on an LSM 510 Meta confocal microscope (Zeiss, Thornwood, NY), and Adobe Photoshop Elements version 10 was used to adjust image brightness/contrast.
Sequence Analyses and Sequence Accession Numbers
Insect Orco amino acid sequences were aligned using Clustal W (Larkin et al., 2007), and a neighbor-joining tree (unrooted) was calculated with 1000 bootstrap replicates using the MEGA-5 program (Tamura et al., 2007). The publicly available TopPred (Claros and von Heijne, 1994; http://mobyle.pastuer.fr), TMHMM server version 2.0 (Krogh et al., 2001; http://www.cbs.dtu.dk/services/TMHMM/), and TMpred (Hofmann & Stoffel, 1993; http://ch.embnet.org/software/TMPRED_form.html) programs were used to predict locations of transmembrane domains within Scal and HirrOrco. The GenBank accession numbers for the genes described in this study are: Scal\Orco transcript: EU622914, Scal\Orco partial genomic sequence: JX996042 Hirr\Orco transcript: EU622915, Hirr\Orco partial genomic sequence: JX996043.
Scanning Electron Microscopy
Whole adult stable fly and horn fly specimens (female and male, fed) were incubated in fixative (4% formaldehydye, 1% gluteraldehyde) for at least 24 h, after which they were rinsed in 0.1 M phosphate buffer and incubated in 1% Zetterquist’s Osmium for 30 mins. The fly specimens were rinsed in Zetterquist’s buffer for 2 mins and dehydrated in a series of ethanol washes: 70% for 45 mins, 95% for 45 mins, and 100% for 40 mins. The specimens were then immersed in hexamethyldisilizane for 5 mins, air dried in a dessicator, mounted on aluminum stubs and sputter coated with gold palladium. Specimens were observed using a JSM-6610LV scanning electron microscope (JEOL Ltd., Tokyo, Japan) at the Pathology Electron Microscopy Facility, University of Texas Health Science Center (San Antonio, TX).
Supplementary Material
Acknowledgments
The author greatly appreciates Greta Buckmeier for her continued superb technical assistance; Matthew Waldon for fly colony maintenance and ensuring specimen availability throughout the study; Dr. Jerry Zhu for discussions regarding biting fly sensilla; Lauren Chesnut and Barbara Hunter at the Pathology Department of UT Health Science Center for mounting the fly specimens used in SEM and for providing access to the microscope used to capture images; Darci Burchers for assisting with confocal microscopy [supported by grants from the National Center for Research Resources (5 G12RR-13646-12) and the National Institute on Minority Health and Health Disparities (G12MD007591) from the National Institutes of Health]; and Drs. Chris Geden, Kevin Temeyer, and Don Thomas for reviewing an earlier draft of the manuscript. This paper reports the results of research only. Mention of a commercial or proprietary product in this paper does not constitute an endorsement by the U.S. Department of Agriculture. USDA is an equal opportunity provider and employer. This work was supported by the USDA-Agricultural Research Service, National Program 104, Project # 6205-32000-033-00.
References
- Adams JR, Forgash AJ. The location of the contact chemoreceptors of the stable fly, Stomoxys calcitrans (Diptera: Muscidae) Ann Entomol Soc Am. 1966;59:133–141. doi: 10.1093/aesa/58.6.909. [DOI] [PubMed] [Google Scholar]
- Adams JR, Holbert PE, Forgash AJ. Electron microscopy of the contact chemoreceptors of the stable fly, Stomoxys calcitrans (Diptera: Muscidae) Ann Entomol Soc Am. 1965;58:909–17. doi: 10.1093/aesa/58.6.909. [DOI] [PubMed] [Google Scholar]
- Bay DE, Meola SM, White SL. Scanning electron microscopy of the ovipositor of the horn fly. Southwestern Entomologist. 1996;21:337–339. [Google Scholar]
- Benton R, Sachse S, Michnick SW, Vosshall LB. Atypical membrane topology and heteromeric function of Drosophila odorant receptors in vivo. PLoS Biol. 2006;4:e20. doi: 10.1371/journal.pbio.0040020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton R, Vannice KS, Gomez-Diaz C, Vosshall LB. Variant ionotropic glutamate receptors as chemosensory receptors in Drosophila. Cell. 2009;136:149–162. doi: 10.1016/j.cell.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkett MA, Agelopoulos N, Jensen KM, Jespersen JB, Pickett JA, Prijs HJ, Thomas G, Trapman JJ, Wadhams LJ, Woodcock CM. The role of volatile semiochemicals in mediating host location and selection by nuisance and disease-transmitting cattle flies. Med Vet Entomol. 2004;18:313–22. doi: 10.1111/j.0269-283X.2004.00528.x. [DOI] [PubMed] [Google Scholar]
- Bohbot JD, Dickens JC. Odorant receptor modulation: ternary paradigm for mode of action of insect repellents. Neuropharmacology. 2012;62:2086–95. doi: 10.1016/j.neuropharm.2012.01.004. [DOI] [PubMed] [Google Scholar]
- Broce AB, Hogsette J, Paisley S. Winter feeding sites of hay in round bales as major developmental sites of Stomoxys calcitrans (Diptera: Muscidae) in pastures in spring and summer. J Econ Entomol. 2005;98:2307–12. doi: 10.1603/0022-0493-98.6.2307. [DOI] [PubMed] [Google Scholar]
- Byford RL, Craig ME, Crosby BL. A review of ectoparasites and their effect on cattle production. J Anim Sci. 1992;70:597–602. doi: 10.2527/1992.702597x. [DOI] [PubMed] [Google Scholar]
- Campbell JB. Horn fly control on cattle. University of Nebraska Lincoln Extension Publication. 2006;Publication G1180 [Google Scholar]
- Campbell JB, Berry IL, Boxler DJ, Davis RL, Clanton DC, Deutscher GH. Effects of stable flies (Diptera: Muscidae) on weight gain and feed efficiency of feedlot cattle. J Econ Entomol. 1987;80:117–9. doi: 10.1093/jee/80.1.117. [DOI] [PubMed] [Google Scholar]
- Campbell JB, Skoda SR, Berkebile DR, Boxler DJ, Thomas GD, Adams DC, Davis R. Effects of stable flies (Diptera: Muscidae) on weight gains of grazing yearling cattle. J Econ Entomol. 2001;94:780–3. doi: 10.1603/0022-0493-94.3.780. [DOI] [PubMed] [Google Scholar]
- Chaiwong T, Sukontason K, Olson JK, Kurahashi H, Chaithong U, Sukontason KL. Fine structure of the reproductive system of Chrysomya megacephala (Diptera: Calliphoridae): the external sexual organ. Parasitol Res. 2008;102:973–80. doi: 10.1007/s00436-007-0863-6. [DOI] [PubMed] [Google Scholar]
- Chen S, Luetje CW. Identification of new agonists and antagonists of the insect odorant receptor co-receptor subunit. PLoS ONE. 2012;7:e36784. doi: 10.1371/journal.pone.0036784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claros MG, von Heijne G. TopPred II: an improved software for membrane protein structure predictions. Comput Appl Biosci. 1994;10:685–6. doi: 10.1093/bioinformatics/10.6.685. [DOI] [PubMed] [Google Scholar]
- Clyne P, Grant A, O’Connell R, Carlson JR. Odorant response of individual sensilla on the Drosophila antenna. Invert Neurosci. 1997;3:127–35. doi: 10.1007/BF02480367. [DOI] [PubMed] [Google Scholar]
- Couto A, Alenius M, Dickson BJ. Molecular, anatomical, and functional organization of the Drosophila olfactory system. Curr Biol. 2005;15:1535–47. doi: 10.1016/j.cub.2005.07.034. [DOI] [PubMed] [Google Scholar]
- Cui X, Wu C, Zhang L. Electrophysiological response patterns of 16 olfactory neurons from the trichoid sensilla to odorant from fecal volatiles in the locust, locusta migratoria manilensis. Arch Insect Biochem Physiol. 2011;77:45–57. doi: 10.1002/arch.20420. [DOI] [PubMed] [Google Scholar]
- Cupp EW, Cupp MS, Ribeiro JM, Kunz SE. Blood-feeding strategy of Haematobia irritans (Diptera: Muscidae) J Med Entomol. 1998;35:591–5. doi: 10.1093/jmedent/35.4.591. [DOI] [PubMed] [Google Scholar]
- Dahanukar A, Hallem EA, Carlson JR. Insect chemoreception. Curr Opin Neurobiol. 2005;15:423–30. doi: 10.1016/j.conb.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Ditzen M, Pellegrino M, Vosshall LB. Insect odorant receptors are molecular targets of the insect repellent DEET. Science. 2008;319:1838–42. doi: 10.1126/science.1153121. [DOI] [PubMed] [Google Scholar]
- Doud CW, Taylor DB, Zurek L. Dewatered sewage biosolids provide a productive larval habitat for stable flies and house flies (Diptera: Muscidae) J Med Entomol. 2012;49:286–92. doi: 10.1603/me11158. [DOI] [PubMed] [Google Scholar]
- Fishilevich E, Vosshall LB. Genetic and functional subdivision of the Drosophila antennal lobe. Curr Biol. 2005;15:1548–53. doi: 10.1016/j.cub.2005.07.066. [DOI] [PubMed] [Google Scholar]
- Foil LD, Hogsette JA. Biology and control of tabanids, stable flies and horn flies. Rev Sci Tech. 1994;13:1125–58. doi: 10.20506/rst.13.4.821. [DOI] [PubMed] [Google Scholar]
- Ghaninia M, Ignell R, Hansson BS. Functional classification and central nervous projections of olfactory receptor neurons housed in antennal trichoid sensilla of female yellow fever mosquitoes, Aedes aegypti. Eur J Neurosci. 2007;26:1611–23. doi: 10.1111/j.1460-9568.2007.05786.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson G, Torr SJ. Visual and olfactory responses of haematophagous Diptera to host stimuli. Med Vet Entomol. 1999;13:2–23. doi: 10.1046/j.1365-2915.1999.00163.x. [DOI] [PubMed] [Google Scholar]
- Goldman AL, Van der Goes van Naters W, Lessing D, Warr CG, Carlson JR. Coexpression of two functional odor receptors in one neuron. Neuron. 2005;45:661–6. doi: 10.1016/j.neuron.2005.01.025. [DOI] [PubMed] [Google Scholar]
- Hill SR, Hansson BS, Ignell R. Characterization of antennal trichoid sensilla from female southern house mosquito, Culex quinquefasciatus Say. Chem Senses. 2009;34:231–52. doi: 10.1093/chemse/bjn080. [DOI] [PubMed] [Google Scholar]
- Hofmann K, Stoffel W. TMbase - a database of membrane spanning protein segments. Biological Chemistry Hoppe-Seyler. 1993;374:166. [Google Scholar]
- Jacquin-Joly E, Merlin C. Insect olfactory receptors: contributions of molecular biology to chemical ecology. J Chem Ecol. 2004;30:2359–97. doi: 10.1007/s10886-004-7941-3. [DOI] [PubMed] [Google Scholar]
- Jeanbourquin P, Guerin PM. Chemostimuli implicated in selection of oviposition substrates by the stable fly Stomoxys calcitrans. Med Vet Entomol. 2007a;21:209–16. doi: 10.1111/j.1365-2915.2007.00685.x. [DOI] [PubMed] [Google Scholar]
- Jeanbourquin P, Guerin PM. Sensory and behavioural responses of the stable fly Stomoxys calcitrans to rumen volatiles. Med Vet Entomol. 2007b;21:217–24. doi: 10.1111/j.1365-2915.2007.00686.x. [DOI] [PubMed] [Google Scholar]
- Johnson G, Panella N, Hale K, Komar N. Detection of West Nile virus in stable flies (Diptera: Muscidae) parasitizing juvenile American white pelicans. J Med Entomol. 2010;47:1205–11. doi: 10.1603/me10002. [DOI] [PubMed] [Google Scholar]
- Jones CJ, Hogsette JA, Patterson RS, Milne DE. Effects of natural saccharide and pollen extract feeding on stable fly (Diptera:Muscidae) longevity. Environ Entomol. 1985;14:223–227. [Google Scholar]
- Jones CJ, Milne DE, Patterson RS, Schreiber ET, Milio JA. Nectar feeding by Stomoxys calcitrans (Diptera: Muscidae): effects on reproduction and survival. Environ Entomol. 1992;21:141–147. [Google Scholar]
- Jones PL, Pask GM, Romaine IM, Taylor RW, Reid PR, Waterson AG, Sulikowski GA, Zwiebel LJ. Allosteric antagonism of insect odorant receptor ion channels. PLoS ONE. 2012;7:e30304. doi: 10.1371/journal.pone.0030304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justice RW, Biessmann H, Walter MF, Dimitratos SD, Woods DF. Genomics spawns novel approaches to mosquito control. Bioessays. 2003;25:1011–20. doi: 10.1002/bies.10331. [DOI] [PubMed] [Google Scholar]
- Koehler PG, Kaufman PE. Stable fly (dog fly) control. University of Florida, Institute of Food and Agricultural Sciences Extension Service. 2006;Document ENY-267:1–4. [Google Scholar]
- Koller WW, Catto JB, Bianchin I, Soares CO, Paiva F, Tavares LE, Graciolli G. Surtos da Mosca-dos-estabulos, Stomoxys calcitrans, em Mato Grosso do Sul: Novo problema para as cadeias produtivas da carne e sucroalcooleira? (Documentos 175) Empresa Brasileira de Pesquisa Agropecuaria, Embrapa Gado de Corte. 2009:1–31. [Google Scholar]
- Krieger J, Klink O, Mohl C, Raming K, Breer H. A candidate olfactory receptor subtype highly conserved across different insect orders. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2003;189:519–26. doi: 10.1007/s00359-003-0427-x. [DOI] [PubMed] [Google Scholar]
- Krogh A, Larsson B, von Heijne G, Sonnhammer EL. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J Mol Biol. 2001;305:567–80. doi: 10.1006/jmbi.2000.4315. [DOI] [PubMed] [Google Scholar]
- Kunz SE, Miller JA, Sims PL, Meyerhoffer DC. Economics of controlling horn flies (Diptera: Muscidae) in range cattle management. J Econ Entomol. 1984;77:657–660. [Google Scholar]
- Kuramochi K. Survival, ovarian development and bloodmeal size for the horn fly Haematobia irritans irritans reared in vitro. Med Vet Entomol. 2000;14:201–6. doi: 10.1046/j.1365-2915.2000.00224.x. [DOI] [PubMed] [Google Scholar]
- Kwon HW, Lu T, Rutzler M, Zwiebel LJ. Olfactory responses in a gustatory organ of the malaria vector mosquito Anopheles gambiae. Proc Natl Acad Sci U S A. 2006;103:13526–31. doi: 10.1073/pnas.0601107103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–8. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- Larsson MC, Domingos AI, Jones WD, Chiappe ME, Amrein H, Vosshall LB. Or83b encodes a broadly expressed odorant receptor essential for Drosophila olfaction. Neuron. 2004;43:703–14. doi: 10.1016/j.neuron.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Lewis CT. Superficial sense organs of the antennae of the fly, Stomoxys calcitrans. J Insect Physiol. 1971;17:449–461. [Google Scholar]
- Lu B, Wang N, Xiao J, Xu Y, Murphy RW, Huang D. Expression and evolutionary divergence of the non-conventional olfactory receptor in four species of fig wasp associated with one species of fig. BMC Evol Biol. 2009;9:43. doi: 10.1186/1471-2148-9-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundin C, Kall L, Kreher SA, Kapp K, Sonnhammer EL, Carlson JR, Heijne G, Nilsson I. Membrane topology of the Drosophila OR83b odorant receptor. FEBS Lett. 2007;581:5601–4. doi: 10.1016/j.febslet.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lysyk TJ, Kalischuk-Tymensen L, Selinger LB, Lancaster RC, Wever L, Cheng KJ. Rearing stable fly larvae (Diptera: Muscidae) on an egg yolk medium. J Med Entomol. 1999;36:382–8. doi: 10.1093/jmedent/36.3.382. [DOI] [PubMed] [Google Scholar]
- Malpel S, Merlin C, Francois MC, Jacquin-Joly E. Molecular identification and characterization of two new Lepidoptera chemoreceptors belonging to the Drosophila melanogaster OR83b family. Insect Mol Biol. 2008;17:587–96. doi: 10.1111/j.1365-2583.2008.00830.x. [DOI] [PubMed] [Google Scholar]
- Melo AC, Rutzler M, Pitts RJ, Zwiebel LJ. Identification of a chemosensory receptor from the yellow fever mosquito, Aedes aegypti, that is highly conserved and expressed in olfactory and gustatory organs. Chem Senses. 2004;29:403–10. doi: 10.1093/chemse/bjh041. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Pellegrino M, Sato K, Vosshall LB, Touhara K. Amino acid residues contributing to function of the heteromeric insect olfactory receptor complex. PLoS ONE. 2012;7:e32372. doi: 10.1371/journal.pone.0032372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newson HD. Arthropod problems in recreation areas. Annu Rev Entomol. 1977;22:333–53. doi: 10.1146/annurev.en.22.010177.002001. [DOI] [PubMed] [Google Scholar]
- Olafson PU, Lohmeyer KH, Dowd SE. Analysis of expressed sequence tags from a significant livestock pest, the stable fly (Stomoxys calcitrans), identifies transcripts with a putative role in chemosensation and sex determination. Arch Insect Biochem Physiol. 2010;74:179–204. doi: 10.1002/arch.20372. [DOI] [PubMed] [Google Scholar]
- Oyarzún MP, Palma R, Alberti E, Hormazabal E, Pardo F, Birkett MA, Quiroz A. Olfactory response of Haematobia irritans (Diptera: Muscidae) to cattle-derived volatile compounds. J Med Entomol. 2009;46:1320–6. doi: 10.1603/033.046.0610. [DOI] [PubMed] [Google Scholar]
- Oyarzún MP, Quiroz A, Birkett MA. Insecticide resistance in the horn fly: alternative control strategies. Med Vet Entomol. 2008;22:188–202. doi: 10.1111/j.1365-2915.2008.00733.x. [DOI] [PubMed] [Google Scholar]
- Pellegrino M, Steinbach N, Stensmyr MC, Hansson BS, Vosshall LB. A natural polymorphism alters odour and DEET sensitivity in an insect odorant receptor. Nature. 2011;478:511–4. doi: 10.1038/nature10438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelosi P, Zhou JJ, Ban LP, Calvello M. Soluble proteins in insect chemical communication. Cell Mol Life Sci. 2006;63:1658–76. doi: 10.1007/s00018-005-5607-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perotti MA, Lysyk TJ, Kalischuk-Tymensen LD, Yanke LJ, Selinger LB. Growth and survival of immature Heamatobia irritans (Diptera; Muscidae) is influenced by bacteria isolated from cattle manure and conspecific larvae. J Med Entomol. 2001;38:180–7. doi: 10.1603/0022-2585-38.2.180. [DOI] [PubMed] [Google Scholar]
- Pitts RJ, Fox AN, Zwiebel LJ. A highly conserved candidate chemoreceptor expressed in both olfactory and gustatory tissues in the malaria vector Anopheles gambiae. Proc Natl Acad Sci U S A. 2004;101:5058–63. doi: 10.1073/pnas.0308146101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitzer JB, Kaufman PE, Tenbroeck SH, Maruniak JE. Host blood meal identification by multiplex polymerase chain reaction for dispersal evidence of stable flies (Diptera:Muscidae) between livestock facilities. J Med Entomol. 2011;48:53–60. doi: 10.1603/me10123. [DOI] [PubMed] [Google Scholar]
- Rice M. Contact chemoreceptors on the ovipositor of Lucilia cuprina (Wied.), the Australian sheep blowfly. Australian Journal of Zoology. 1976;24:353–360. [Google Scholar]
- Romero A, Broce A, Zurek L. Role of bacteria in the oviposition behaviour and larval development of stable flies. Med Vet Entomol. 2006;20:115–21. doi: 10.1111/j.1365-2915.2006.00602.x. [DOI] [PubMed] [Google Scholar]
- Sato K, Pellegrino M, Nakagawa T, Nakagawa T, Vosshall LB, Touhara K. Insect olfactory receptors are heteromeric ligand-gated ion channels. Nature. 2008;452:1002–6. doi: 10.1038/nature06850. [DOI] [PubMed] [Google Scholar]
- Sollai G, Solari P, Loy F, Masala C, Crnjar R, Liscia A. Morpho-functional identification of abdominal olfactory receptors in the midge Culicoides imicola. J Comp Physiol A. 2010;196:817–824. doi: 10.1007/s00359-010-0561-1. [DOI] [PubMed] [Google Scholar]
- Stocker RF. Design of the larval chemosensory system. Adv Exp Med Biol. 2008;628:69–81. doi: 10.1007/978-0-387-78261-4_5. [DOI] [PubMed] [Google Scholar]
- Talley J, Broce A, Zurek L. Characterization of stable fly (Diptera: Muscidae) larval developmental habitat at round hay bale feeding sites. J Med Entomol. 2009;46:1310–9. doi: 10.1603/033.046.0609. [DOI] [PubMed] [Google Scholar]
- Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol. 2007;24:1596–9. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- Tangtrakulwanich K, Chen H, Baxendale F, Brewer G, Zhu JJ. Characterization of olfactory sensilla of Stomoxys calcitrans and electrophysiological responses to odorant compounds associated with hosts and oviposition media. Med Vet Entomol. 2011;25:327–36. doi: 10.1111/j.1365-2915.2011.00946.x. [DOI] [PubMed] [Google Scholar]
- Taylor DB, Berkebile DR. Sugar feeding in adult stable flies. Environ Entomol. 2008;37:625–9. doi: 10.1603/0046-225x(2008)37[625:sfiasf]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Taylor DB, Moon RD, Mark DR. Economic impact of stable flies (Diptera: Muscidae) on dairy and beef cattle production. J Med Entomol. 2012;49:198–209. doi: 10.1603/me10050. [DOI] [PubMed] [Google Scholar]
- van der Goes van Naters W, Carlson JR. Insects as chemosensors of humans and crops. Nature. 2006;444:302–7. doi: 10.1038/nature05403. [DOI] [PubMed] [Google Scholar]
- van der Wolk FM, Koerten HK, van der Starre H. The external morphology of contact-chemoreceptive hairs of flies and the motility of the tips of these hairs. Journal of Morphology. 1984;180:37–54. doi: 10.1002/jmor.1051800106. [DOI] [PubMed] [Google Scholar]
- Vosshall LB, Hansson BS. A unified nomenclature system for the insect olfactory coreceptor. Chem Senses. 2011;36:497–8. doi: 10.1093/chemse/bjr022. [DOI] [PubMed] [Google Scholar]
- Vosshall LB, Wong AM, Axel R. An olfactory sensory map in the fly brain. Cell. 2000;102:147–59. doi: 10.1016/s0092-8674(00)00021-0. [DOI] [PubMed] [Google Scholar]
- Wallis DI. Olfactory stimuli and oviposition in the blowfly, Phormia regina Meigen. The Journal of Experimental Biology. 1962;39:603–615. [Google Scholar]
- Wang X, Zhong M, Wen J, Cai J, Jiang H, Liu Y, Aly SM, Xiong F. Molecular characterization and expression pattern of an odorant receptor from the myiasis-causing blowfly, Lucilia sericata (Diptera: Calliphoridae) Parasitol Res. 2012;110:843–51. doi: 10.1007/s00436-011-2563-5. [DOI] [PubMed] [Google Scholar]
- White SL, Bay DE. Antennal olfactory sensilla of the horn fly, Haematobia irritans irritans (L.) (Diptera: Muscidae) Journal of the Kansas Entomological Society. 1980;53:641–652. [Google Scholar]
- Wicher D, Schafer R, Bauernfeind R, Stensmyr MC, Heller R, Heinemann SH, Hansson BS. Drosophila odorant receptors are both ligand-gated and cyclic-nucleotide-activated cation channels. Nature. 2008;452:1007–11. doi: 10.1038/nature06861. [DOI] [PubMed] [Google Scholar]
- Wieman GA, Campbell JB, Deshazer JA, Berry IL. Effects of stable flies (Diptera: Muscidae) and heat stress on weight gain and feed efficiency of feeder cattle. J Econ Entomol. 1992;85:1835–42. doi: 10.1093/jee/85.5.1835. [DOI] [PubMed] [Google Scholar]
- Wu Z-N, Chen X, Du Y-J, Zhou J-J, ZhuGe Q-C. Molecular identification and characterization of the Orco orthologue of Spodoptera litura. Insect Science. 2012 doi: 10.1111/j.1744-7917.2011.01483.x. [DOI] [PubMed] [Google Scholar]
- Xia Y, Zwiebel LJ. Identification and characterization of an odorant receptor from the West Nile virus mosquito, Culex quinquefasciatus. Insect Biochem Mol Biol. 2006;36:169–76. doi: 10.1016/j.ibmb.2005.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Krieger J, Zhang L, Breer H. The olfactory co-receptor Orco from the migratory locust (Locusta migratoria) and the desert locust (Schistocerca gregaria): identification and expression pattern. Int J Biol Sci. 2012;8:159–70. doi: 10.7150/ijbs.8.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Zhu C, Peng T, Zhang H. Odorant receptor co-receptor Orco is upregulated by methyl eugenol in male Bactrocera dorsalis (Diptera: Tephritidae) J Insect Physiol. 2012;58:1122–7. doi: 10.1016/j.jinsphys.2012.05.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.