Abstract
The dorsolateral reticular formation of the caudal medulla, the lateral tegmental field (LTF), participates in generating vomiting. LTF neurons exhibited complex responses to vestibular stimulation in decerebrate cats, indicating that they received converging inputs from a variety of labyrinthine receptors. Such a convergence pattern of vestibular inputs is appropriate for a brain region that participates in generating motion sickness. Since responses of brainstem neurons to vestibular stimulation can differ between decerebrate and conscious animals, the current study examined the effects of whole-body rotations in vertical planes on the activity of LTF neurons in conscious felines. Wobble stimuli, fixed-amplitude tilts, the direction of which moves around the animal at a constant speed, were used to determine the response vector orientation, and also to ascertain whether neurons had spatial–temporal convergence (STC) behavior (which is due to the convergence of vestibular inputs with different spatial and temporal properties). The proportion of LTF neurons with STC behavior in conscious animals (25 %) was similar to that in decerebrate cats. Far fewer neurons in other regions of the feline brainstem had STC behavior, confirming findings that many LTF neurons receive converging inputs from a variety of labyrinthine receptors. However, responses to vertical plane vestibular stimulation were considerably different in decerebrate and conscious felines for LTF neurons lacking STC behavior. In decerebrate cats, most LTF neurons had graviceptive responses to rotations, similar to those of otolith organ afferents. However, in conscious animals, the response properties were similar to those of semicircular canal afferents. These differences show that higher centers of the brain that are removed during decerebration regulate the labyrinthine inputs relayed to the LTF, either by gating connections in the brainstem or by conveying vestibular inputs directly to the region.
Keywords: Otolith organ, Semicircular canal, Vomiting, Sensorimotor integration
Introduction
Motion sickness is triggered by movement or perceived movement in the environment and in humans is characterized by the presence of nausea and vomiting, pallor, and cold sweating (Yates 2009). Typically, the malady occurs when multiple sensory inputs are present that provide contradictory information regarding body position in space, or when the inputs regarding body movement deviate from those previously experienced (Yates 2009). For example, “barbecue spit stimulation,” or rotation of a subject about the earth horizontal axis with the Z-axis aligned with the rotatory axis, often causes motion sickness, likely because the movement is uncommon (Guedry 1965; Correia and Guedry 1966). The critical inputs required for the generation of motion sickness come from the vestibular system, as evidenced by the observation that this condition cannot be induced by stimuli that are typically highly provocative in individuals with bilateral vestibular dysfunction (Cheung et al. 1990).
Although the physiologic conditions that produce motion sickness are well understood, little is known about the processing of vestibular inputs by the medullary circuitry that generates vomiting associated with motion sickness (Yates et al. 1998; Golding 2006). Emesis, including that induced by vestibular stimulation (Miller and Wilson 1983), can be evoked in decerebrate animals, indicating that the intrinsic circuitry that generates vomiting is located in the brainstem (Fukuda and Koga 1991; Miller et al. 1994). Studies utilizing brainstem transections have also shown that vomiting can still be elicited after all portions of the central nervous system are removed except for the medulla and spinal cord (Fukuda and Koga 1991; Miller et al. 1994). One region of the medulla that has been implicated in coordinating vomiting is the dorsolateral reticular formation of the caudal medulla, which is referred to as the lateral tegmental field (LTF) in felines (Berman 1968). Some investigators have classified the LTF as the brainstem’s “vomiting center” (Borison and Wang 1949; Wang and Borison 1951). Classical studies showed that stimulation within the LTF of emetic species could evoke vomiting (Borison and Wang 1949; Fukuda and Koga 1991, 1992), while lesions of this area abolished the capacity for vomiting (Wang and Borison 1951; Koga et al. 1998). More recent studies indicated that the LTF constitutes only a portion of the brainstem circuitry that elicits and coordinates vomiting, although it is evident that neurons within the LTF are key components of this extended emetic circuitry (Miller et al. 1994).
Anatomical studies have shown that the inferior and caudal medial vestibular nuclei project to the LTF, and experiments using electrical stimulation of vestibular afferents demonstrated that LTF neurons additionally receive labyrinthine signals through polysynaptic pathways (Yates et al. 1995). We recently obtained the first recordings of the activity of LTF neurons during whole-body rotations in vertical planes that activate vestibular afferents in the decerebrate cat preparation (Moy et al. 2012). Approximately half of the LTF neurons had graviceptive responses to vertical plane tilts, similar to those of otolith organ afferents, whereas the other half had much more complex responses that neither resembled those of otolith organ or semicircular canal afferents, or a simple summation of these responses. Nearly, one-third of LTF neurons in decerebrate cats exhibited spatiotemporal convergence (STC) behavior (Moy et al. 2012), a response pattern attributed to the convergence of inputs with different spatial and temporal properties (for instance, the convergence of inputs from semicircular canal afferents activated by roll rotations and otolith organ afferents activated by pitch rotations) (Baker et al. 1984; Schor et al. 1984; Schor and Angelaki 1992). Such a preponderance of neurons with complex responses to rotations in vertical planes has not been reported in studies of other regions in the feline brainstem or cerebellum that process labyrinthine inputs, including the vestibular nuclei (Kasper et al. 1988; Miller et al. 2008a), fastigial nucleus (Miller et al. 2008b), or medial medullary reticular formation (Bolton et al. 1992). These complex responses of LTF neurons likely reflect the convergence of diverse labyrinthine inputs, such that neurons exhibiting the responses would only attain a maximal firing rate during multifaceted head movements that activate a variety of vestibular afferents (Schor and Angelaki 1992). Furthermore, STC responses reflect a convergence of inputs that would not ordinarily be expected, since they have different spatial properties (i.e., are generated by different directions of head movement) and thus are appropriate for neurons that participate in generating motion sickness.
However, responses of brainstem neurons to vestibular stimulation can differ tremendously between decerebrate and conscious animals. For example, the activity of some vestibular nucleus neurons is silenced during saccadic eye movements in conscious animals, although there is no effect of eye movements on the activity of vestibular nucleus neurons in decerebrate cats (Scudder et al. 2002). In addition, neurons in a region of the medulla that regulates sympathetic nervous system activity, the rostral ventrolateral medulla (RVLM), are much more sensitive to vestibular inputs in decerebrate cats than in conscious animals (Destefino et al. 2011). In order to appreciate the role of the LTF in generating motion sickness, it is critical to compare the responses of LTF neurons to vestibular stimulation in conscious and decerebrate animals. In the current study, we tested the hypothesis that similar dynamics of responses of LTF neurons to vertical vestibular stimulation would be evident in both populations of animals. Furthermore, we hypothesized that a similar proportion of LTF neurons would display complex responses, such as STC behavior, in conscious felines as was discovered in the decerebrate preparation. To assure that data collected in this study could be accurately compared with those from decerebrate cats, the same apparatus was used to provide vertical vestibular stimulation in both studies.
Methods and materials
Surgical procedures
Experiments were conducted on 3 female purpose-bred cats obtained from Liberty Research (Waverly, NY). The University of Pittsburgh’s Institutional Animal Care and Use Committee approved all procedures on animals.
After a period of acclimation for restraint, animals were instrumented for single-unit recordings from the brainstem, as in our recent studies (Miller et al. 2008a, b; Barman et al. 2011; Destefino et al. 2011). Animals were initially anesthetized using an intramuscular injection of ketamine (20 mg/kg) and acepromazine (0.2 mg/kg). Subsequently, an endotracheal tube and intravenous catheter were inserted. Anesthesia was maintained using 1–2 % isoflurane vaporized in O2, so that limb withdrawal reflexes were absent and heart rate was stable. A saline solution was infused intravenously to replace fluid loss during the surgery. A heating pad and heat lamp were used to maintain core temperature. A 1-cm craniotomy was made at the posterior aspect of the skull, and a David Kopf (David Kopf Instruments, Tujunga, CA) recording chamber was positioned in accordance with stereotaxic coordinates and attached to the skull adjacent to the craniotomy using Palacos bone cement (Zimmer, Warsaw, IN). Silver ball electrodes were placed beneath the skin for recording the electrocardiogram, and the leads from the electrodes were routed subcutaneously and soldered to a connector mounted on the skull. In addition, a fixation plate was attached to the skull and subsequently used for restraint of the head.
After surgery, animals received antibiotics (amoxicillin, two 50-mg oral doses per day) for 10 days. For 72 h after the surgery, analgesia was provided through transdermal delivery of fentanyl (25 μg/h; Janssen Pharmaceutical Products, Titusville, NJ). Animals recovered for several weeks following the surgery before recordings commenced, during which they were acclimated for head restraint by inserting a screw into the fixation plate mounted on the skull.
Recording procedures
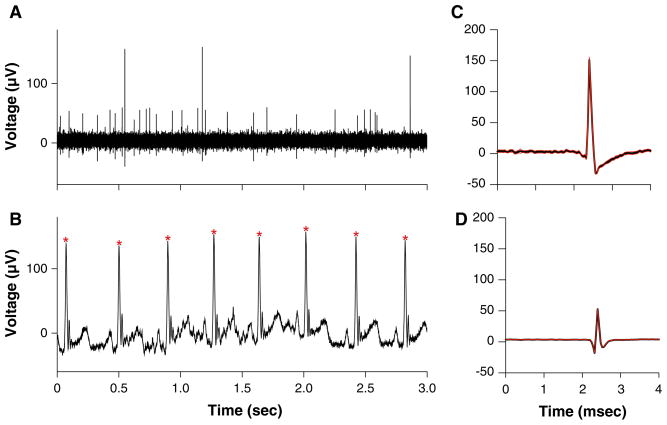
Animals were restrained during recordings as in our previous studies, with the head pitched down 30° to align the anterior and posterior semicircular canals vertically (Miller et al. 2008a, b; Barman et al. 2011; Destefino et al. 2011). The restraint device was placed on a hydraulic tilt table (NeuroKinetics, Pittsburgh, PA). A low light level was present during recordings, and the visual field rotated with the animal so that no visual cues regarding body position in space were available. During recording sessions, an x–y positioner (608-B, David Kopf) was attached to the recording chamber and used to maneuver a ~5-MΩ epoxy-insulated tungsten microelectrode (Frederick Haer, Bowdoin, ME), which was inserted through a 25-gauge guide tube into the cerebellum and lowered into the medulla using a hydraulic microdrive (model 650, David Kopf). Neuronal activity recorded using the microelectrode was amplified by a factor of 10,000, filtered with a bandpass of 300–10,000 Hz, and sampled at 25,000 Hz using a Micro1401 mk 2 data collection system and Spike2 version 6 software (Cambridge Electronic Design, Cambridge, UK). Neuronal activity was parsed in real time using a window discriminator to provide instantaneous information regarding the responses of units to rotations, but the final data analysis was conducted offline. All trials were subjected to analysis using the spike detection and sorting feature of the Spike2 software to confirm that individual units were parsed separately, as illustrated in Fig. 1. In addition, the electrocardiogram was sampled at 1,000 Hz and full-wave rectified by the Spike2 software to facilitate the detection of the R-wave, which was used to trigger averages of unit activity to ascertain whether it was synchronized to the cardiac cycle (see Fig. 1).
Fig. 1.
Example of data collected during experiments. a Activity recorded from the LTF using a microelectrode and sampled at 25,000 Hz. b Electrocardiogram, which was full-wave rectified and sampled at 1,000 Hz. Asterisks designate R-waves. c, d Average waveforms for two units that were isolated using spike sorting from the recording partially depicted in a (black lines), along with one standard deviation (red lines). c is the average of 38 waveforms, while d is the average of 878 waveforms
We examined the responses of units to the same battery of rotations utilized in the parallel study that characterized in decerebrate cats the responses of LTF neurons to vertical plane tilts (Moy et al. 2012). The “wobble” stimulus, a fixed-amplitude tilt, the direction of which moves around the animal at a constant speed (Schor et al. 1984), was used to determine whether a unit responded to stimulation of the vestibular endorgans. Clockwise wobble stimuli were generated by driving the pitch axis of the tilt table with a sine wave while simultaneously driving the roll axis with a cosine wave. During this stimulus, the animal’s body (viewed from above) appeared to wobble, taking in succession nose-up, left ear-down, nose-down, and right ear-down positions. When the signal to the pitch axis of the tilt table was inverted, the stimulus vector rotated in the counterclockwise direction. The wobble stimulus was employed for this study because it simultaneously activates both vertical semicircular canal and otolith afferents; only the endorgans associated with the horizontal semicircular canals were unaffected by the rotations. Furthermore, the wobble stimulus has the potential to elicit STC responses if a neuron receives inputs from both semicircular canal and otolith organ afferents that are not aligned spatially (Schor and Angelaki 1992). In addition, the combination of semicircular canal and otolith organ stimulation experienced during wobble deviates from that occurring during normal movements, such that the movement has the potential to generate motion sickness.
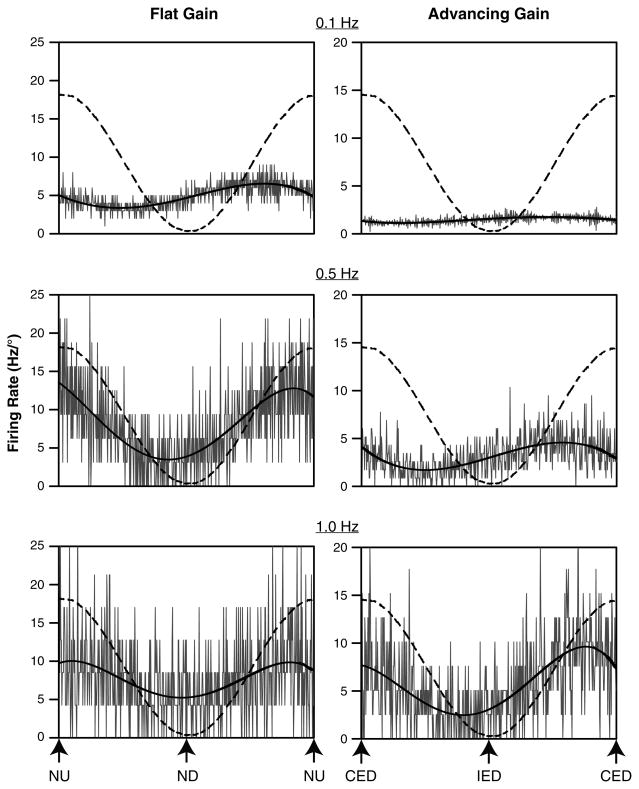
Wobble stimuli were routinely delivered at a frequency of 0.5 Hz and an amplitude of 5°, although 7.5° rotations were utilized if 5° tilts were ineffective. The response vector orientation, or the plane of tilt that elicited the greatest change in a neuron’s firing rate, was calculated from responses to clockwise (CW) and counterclockwise (CCW) wobble stimulation (Schor et al. 1984). Subsequently, rotations were delivered in the roll (longitudinal) and pitch (transverse) axes at 0.5 Hz, and the relative gains of responses to the two planes of tilts were employed to confirm the response vector orientation. Tilts were then delivered at or near the plane of the response vector orientation at 0.1–1 Hz to determine response dynamics.
Data analysis procedures
Neural activity recorded during rotations was binned (500 bins/cycle) and averaged over the sinusoidal stimulus period. A least squares minimization procedure (Schor et al. 1984) conducted using MATLAB (MathWorks, Natick, MA) software fit a sine wave to responses to rotations, and the amplitude of the sine wave (response gain) and phase shift of the sine wave from the stimulus (response phase) were calculated. As in our previous studies (Miller et al. 2008a, b; Barman et al. 2011; Destefino et al. 2011; Moy et al. 2012), three criteria were used to determine whether a neuron’s activity was significantly modulated by rotations in vertical planes: a signal-to-noise ratio (Schor et al. 1984) >0.5, consistency in responses from trial to trial, and lack of prominent components other than the first harmonic.
To determine whether a unit exhibited STC behavior, we compared the gains of responses to CW and CCW wobble stimulation. The larger response gain was used as the numerator, and the smaller gain served as the denominator, such that all values were ≥1. A neuron was classified as having STC behavior if relative gains of responses to CW and CCW wobble stimulation differed by more than a 2:1 ratio (Baker et al. 1984; Schor et al. 1984; Schor and Angelaki 1992; Moy et al. 2012). STC neurons exhibit disparate responses to different directions of wobble stimulation, because the misaligned inputs to the cell add during one direction of rotation but subtract when rotations are delivered in the opposite direction (Schor and Angelaki 1992). Data were tabulated, and statistical analyses were performed, using Prism 6 software (GraphPad Software, San Diego, CA). Pooled data are represented as means ± one standard error.
Histological procedures
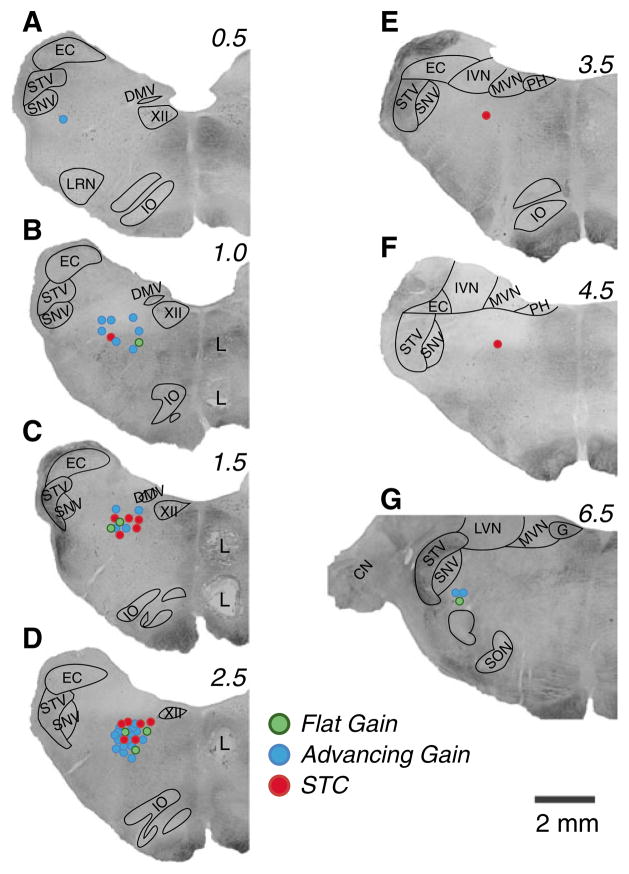
Following recordings in each animal, two to three lesions were created in the brainstem by passing a 100–150 μA current through a 0.5 MΩ electrode for 60 s. Cell death and gliosis at the sites of current delivery were permitted to occur for 5–7 days following the lesions, after which the animals were anesthetized and transcardially perfused with 10 % formalin as described in our previous manuscripts (Miller et al. 2008a, b; Barman et al. 2011; Destefino et al. 2011). A freezing microtome was used to cut the brainstem transversely at 50-μm thickness, and tissue sections were stained using thionine. Sections were photographed using a Nikon Eclipse E600 N photomicroscope equipped with a Spot RT monochrome digital camera (Diagnostic Instruments, Sterling Heights, MI). Montages of images were assembled using PTGui-Pro photostitching software (New House Internet Services B.V., Netherlands); examples of such montages are shown in Fig. 2. Panels C and D of Fig. 2 show two lesions that were placed 2 mm apart in the same track. Recording sites were reconstructed on the photomontages with reference to the locations of electrolytic lesions, the relative positions of electrode tracks, and microelectrode depths.
Fig. 2.
Locations of units in the LTF that responded to vertical vestibular stimulation. Locations are plotted on montages of micrographs of histological sections from one of the animals; numbers above each montage designate distance in millimeters rostral to the obex. Different color symbols indicate whether units had STC behavior (red circles) or response gains that increased more (advancing gain, blue circles) or less (flat gain, green circles) than fivefold per stimulus decade. Lesions (L) placed 2 mm apart in one track to permit reconstruction of unit locations are evident. CN cochlear nucleus, DMV dorsal motor nucleus of the vagus, EC external cuneate, G genu of the facial nerve, IO inferior olivary nucleus, IVN inferior vestibular nucleus, LRN lateral reticular nucleus, LVN lateral vestibular nucleus, MVN medial vestibular nucleus, PH prepositus hypoglossi, SNV spinal trigeminal nucleus, SON superior olivary nucleus, STV spinal trigeminal tract, XII hypoglossal nucleus
Results
Data were collected from 57 neurons whose activity was modulated by vertical plane tilts and whose locations were histologically confirmed in the LTF; the locations of these units are shown in Fig. 2. Most of the neurons were studied in two animals, with approximately equal numbers of cells examined in each. On average, the LTF neurons that responded to vertical tilts were positioned 2.5 ± 0.2 mm rostral to the obex. We triggered averages of the activity of 37 of these units from the R-wave of the ECG, as described in our previous studies (Barman et al. 2011; Destefino et al. 2011). In rare cases (2/37 units) where this initial analysis indicated a possibility of a weak relationship between unit activity and the cardiac cycle, an ANOVA procedure was used to compare maximal and minimal firing rates during different phases of the cycle. In no case did we find a trial where a cardiac-related firing pattern could be confirmed (P > 0.1). None of the animals exhibited any overt symptoms of motion sickness such as retching or vomiting during the course of the experiments, although we did not monitor for the presence of prodromal symptoms such as excessive salivation. The lack of such symptoms could be related to the motion sickness susceptibility of the particular animals used in these experiments, or the fact that the wobble stimuli lasted only for a period of seconds to minutes before being discontinued, which may not have been sufficient to elicit the malady.
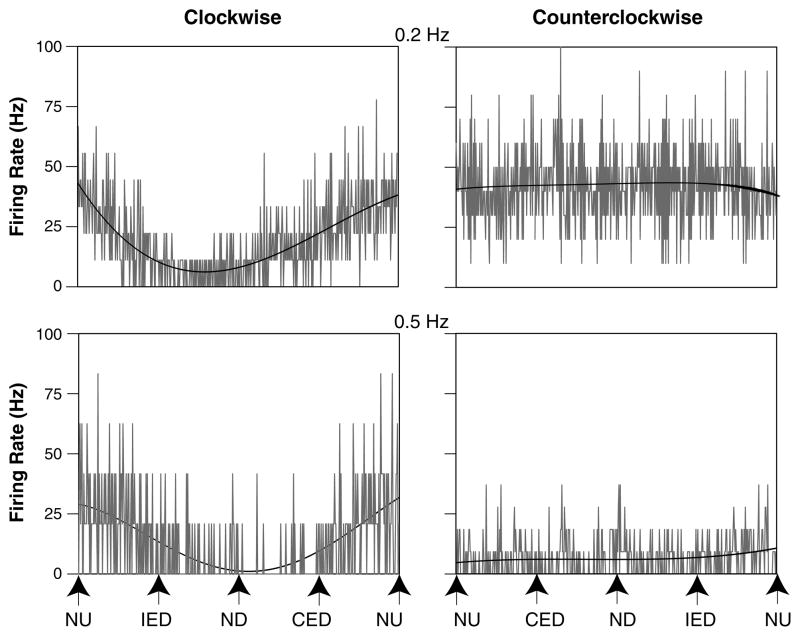
Fourteen of the 57 LTF neurons (25 %) whose activity was modulated during vertical plane tilts were classified as having STC behavior. Figure 3 illustrates STC responses of an LTF neuron in a conscious cat, whose activity was robustly modulated by CW wobble rotations but not CCW rotations. The proportion of LTF neurons with STC behavior observed in the two animals that provided the majority of data for the study was nearly identical: 25 and 26 % of the population.
Fig. 3.
Averaged responses of an STC neuron whose activity was modulated by only one direction of wobble stimulation. Each panel shows unit activity, with a superimposed sine wave fit to the response. Stimulus amplitudes were 7.5° at 0.2 Hz and 5° at 0.5 Hz. The following number of sweeps was averaged to generate each trace: 0.2 Hz CW, 9; 0.2 Hz CCW, 10; 0.5 Hz CW, 12; 0.5 Hz CCW, 27. The signal-to-noise ratios for each response were as follows: 0.2 Hz CW, 1.2; 0.2 Hz CCW, 0.1; 0.5 Hz CW, 0.9; 0.5 Hz CCW, 0.1. CED contralateral ear-down roll, IED ipsilateral ear-down roll, ND nose-down pitch, NU nose-up pitch
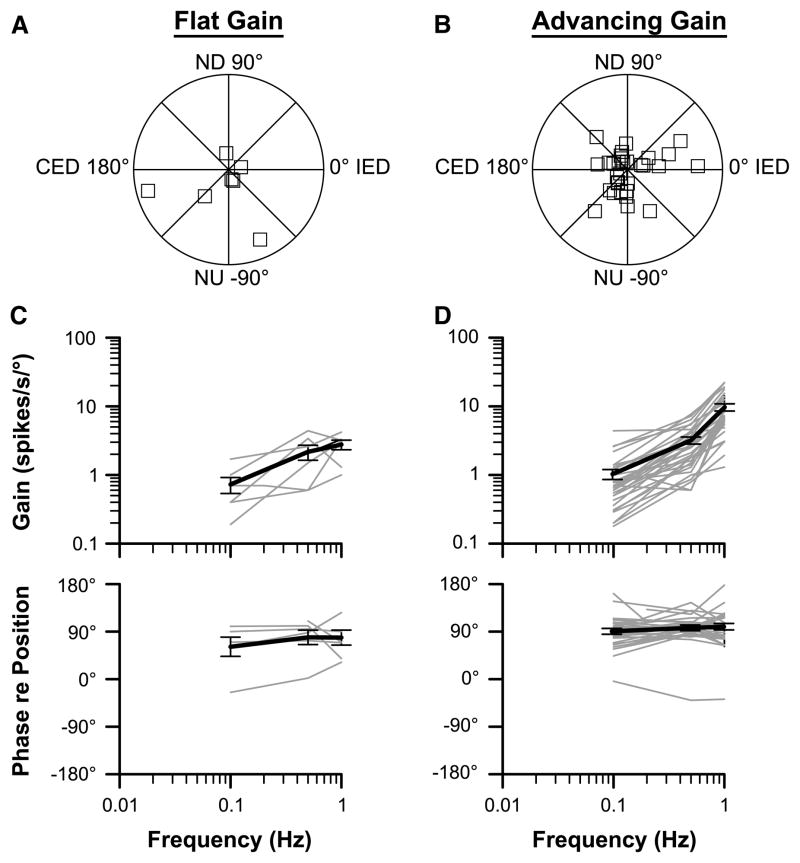
The majority of the neurons had responses to CW and CCW wobble stimuli that were nearly equivalent in gain. Response vector orientations and response dynamics were determined for these units and are shown in Fig. 4. Figure 5 illustrates the responses of two neurons to single-plane sinusoidal rotations at different frequencies. As in our previous study of the responses of LTF neurons to vertical plane rotations in decerebrate cats (Moy et al. 2012), we divided the neurons into two categories, based on whether the increase in response gain per stimulus decade was greater or less than fivefold. The former units (with large gain increases) were classified as “advancing gain” neurons, whereas the latter were classified as “flat gain” neurons. The response gains for flat gain neurons increased by an average factor of 3.6 ± 0.4 per stimulus decade, whereas the response gains for advancing gain neurons increased by an average factor of 11.6 ± 1.0. Advancing gain neurons (n = 31) were more prevalent than flat gain neurons (n = 7); five of the LTF neurons in our sample were not classified, as they were lost before responses to a decade of stimulus frequencies were recorded. The response phases for the majority of neurons of both populations were near stimulus velocity (90° advanced from stimulus position) across the range of stimulus frequencies examined.
Fig. 4.
Characteristics of responses of LTF neurons to tilts in vertical planes. Data are segregated based on whether the response gain for a particular unit increased more (advancing gain) or less (flat gain) than fivefold per stimulus decade. a, b Polar plots showing response vector orientations and gains of LTF units. Response vector orientations were determined using wobble stimuli delivered at 0.5 Hz. The maximal radius of each plot designates a response gain of 10 spikes/s/°. The response vector orientations were plotted using a head-centered coordinate system, with 0° corresponding to ipsilateral ear-down (IED) roll tilt, 90° corresponding to nose-down (ND) pitch, 180° corresponding to contralateral ear-down (CED) roll, and −90° corresponding to nose-up (NU) pitch. c, d Bode plots illustrating the dynamic properties of responses of LTF neurons to rotations in a fixed plane at multiple frequencies. Response gain and phase were plotted with respect to stimulus position. Thin gray lines show data for individual neurons; thick dark lines indicate average values. Error bars designate one SEM
Fig. 5.
Averaged responses of two neurons to sinusoidal tilts at frequencies of 0.1–1 Hz. The stimulus amplitudes were 10° at 0.1 Hz, 5° at 0.5 Hz, and 2.5° at 1 Hz. Because smaller tilt amplitudes were used for higher-frequency rotations, firing rate is expressed in Hz/° to facilitate comparisons. In each panel, a dashed line shows tilt table position, and a dark line is a sine wave fit to the response. The response vector orientation for the unit whose data are provided in the left column was near the pitch plane, and the response gain increased approximately threefold as the stimulus frequency advanced from 0.1 to 1.0 Hz. The response vector orientation for the unit whose data are shown on the right was near the roll plane, and the response gain increased approximately sevenfold as the stimulus frequency advanced from 0.1 to 1.0 Hz. CED contralateral ear-down roll, IED ipsilateral ear-down roll, ND nose-down pitch, NU nose-up pitch
The response vector orientations for flat and advancing gain LTF neurons are indicated in Fig. 4, panels A and B. Seventeen of the 43 LTF units (40 %; including the 5 units that were lost before response dynamics could be completely characterized) had response vector orientations closer to the roll plane than the pitch plane; nine of these cells were excited by contralateral ear-down tilt, and eight were excited by ipsilateral ear-down rotations. However, the majority of the LTF neurons (26/43, 60 %) had response vector orientations closer to the pitch plane than the roll plane, with 18 of the 26 units being excited by nose-up rotations.
Comparisons of responses of LTF neurons to vertical plane tilts in conscious and decerebrate animals
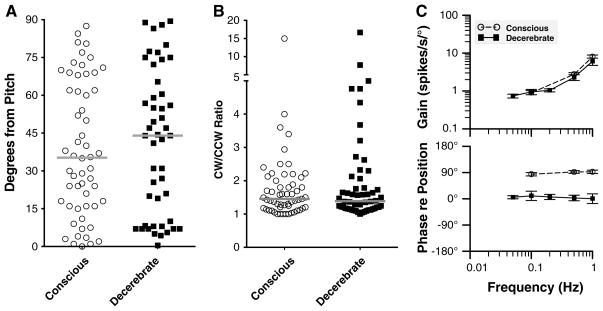
Figure 6 compares the responses of LTF neurons to vertical plane tilts characterized in this study with those recently described in decerebrate cats (Moy et al. 2012). As noted above, 60 % of LTF neurons in conscious animals had response vector orientations closer to the pitch plane than the roll plane, with a preponderance of those units being excited by nose-up rotations. In decerebrate cats, about half (53 %) of LTF neurons had response vector orientations that were closer to the pitch than the roll plane, with 60 % of these units being excited by nose-down pitch. However, a chi-square test failed to reveal that the major axis of tilt that excited LTF neurons (ipsilateral ear down, contralateral ear down, nose up, nose down) differed between the two populations (p = 0.15). Figure 6a compares the axis of the response vector orientation for LTF neurons in conscious and decerebrate animals. For this analysis, the axis of the response vector orientation was expressed in degrees from the pitch axis, such that a value of 90° indicates a response vector orientation aligned with the roll plane. The median value for LTF units in conscious animals was 35° from the pitch axis, whereas the median value for decerebrate animals was 44° from the pitch axis. These values were not shown to be significantly different using a Mann–Whitney test (p = 0.6).
Fig. 6.
Comparisons of the responses of LTF neurons to vertical plane tilts in conscious animals (obtained in this study) with those collected in decerebrate animals (from Moy et al. 2012). Open circles and filled squares, respectively, show data from conscious and decerebrate animals. a Comparison of the axis of the response vector orientation in the two preparations. The axis direction is indicated relative to the pitch plane, such that a value of 90° indicates a response vector orientation aligned with the roll plane. Gray bars designate median values. b Comparison of the relative gains of responses to CW and CCW wobble stimuli delivered at 0.5 Hz for each neuron. To facilitate comparisons, the larger gain was always used as the numerator, and the smaller as the denominator, so the ratio for each unit was ≥1. Gray bars designate median values. c Average Bode plots for all neurons. Response gain and phase were plotted with respect to stimulus position. Error bars designate one SEM
Figure 6b compares the relative gains of responses to wobble stimuli delivered in the CW and CCW directions. The median ratios for conscious and decerebrate animals were almost identical (1.5 vs. 1.4, respectively) and were not shown to be significantly different using a Mann–Whitney test (p = 0.97). Accordingly, the fraction of LTF neurons classified as having STC behavior was similar in the two populations: 14/57 units in conscious animals and 17/55 in decerebrate cats.
Figure 6c compares the average response dynamics for LTF neurons in conscious and decerebrate felines. Although a larger fraction of LTF neurons had flat gains in decerebrate animals, a two-way ANOVA analysis (factors were animal preparation and stimulus frequency) did not reveal that response gains were significantly different in the two populations. However, response phases were highly different in each group of animals. In conscious animals, response phases were typically near stimulus velocity at all stimulus frequencies (see Fig. 4), whereas in decerebrate cats, the response phases were generally either near stimulus position or they lagged stimulus position. Consequently, a two-way ANOVA analysis (factors were animal preparation and stimulus frequency) coupled with Tukey’s multiple comparisons test revealed that response phases were significantly different in conscious and decerebrate cats at all frequencies where sufficient data were available for the analysis (0.1, 0.5, and 1 Hz, p < 0.001).
Discussion
The main goal of the present study was to determine whether LTF neurons in conscious felines had the same complex responses to vertical plane tilts recently described in decerebrate cats (Moy et al. 2012). In both preparations, LTF neurons with STC behavior, as defined by responses to CW and CCW wobble stimulation that differed >2:1 in gain (Schor and Angelaki 1992), were prevalent: 25 and 31 % of the vestibular-responsive cells in conscious and decerebrate animals, respectively. This fraction of neurons with STC behavior is much higher than reported for other areas of the feline brainstem and cerebellum that process vestibular inputs. For example, Miller et al. reported that just six of 85 neurons (7 %) in the caudal regions of the vestibular nuclei (Miller et al. 2008a) that project to the LTF (Yates et al. 1995) and two of the 47 neurons (4 %) in the cerebellar fastigial nucleus (Miller et al. 2008b) had responses to CW and CCW wobble rotations that differed more than 2:1 in gain. Since STC behavior is attributed to the convergence of vestibular inputs with different spatial and temporal properties (Baker et al. 1984; Schor et al. 1984; Schor and Angelaki 1992), this response characteristic is appropriate for a brain region that participates in generating vomiting related to motion sickness, which occurs when inputs reflecting body position in space deviate from those expected based on experience (Reason 1978). Consequently, the current data collected in conscious cats in combination with previous findings from decerebrate felines (Moy et al. 2012) support the notion that the LTF participates in generating at least some of the symptoms associated with motion sickness.
Despite the presence of neurons with STC behavior in the LTF of both decerebrate and conscious animals, other characteristics of the responses of LTF neurons to vertical vestibular stimulation differed in the two preparations. In conscious cats, response dynamics were similar to those of semicircular canal afferents for the large majority of LTF neurons without STC behavior: Response gains increased approximately tenfold per stimulus decade, while response phases were near stimulus velocity (Fernandez and Goldberg 1971; Anderson et al. 1978). In contrast, most LTF neurons lacking STC responses in decerebrate animals exhibited graviceptive responses (Moy et al. 2012) to vertical plane tilts, similar to those of otolith organ afferents (Fernandez and Goldberg 1976; Anderson et al. 1978): relatively flat response gains across stimulus frequencies, and response phases that were synchronized with stimulus position or lagged stimulus position.
These dissimilarities show that a different complement of labyrinthine inputs reaches the LTF in conscious and decerebrate cats. One possibility is that graviceptive signals relayed through projections from the vestibular nuclei to the LTF (Yates et al. 1995) are gated-out by higher centers in conscious animals and thus are more evident in decerebrate cats in which the gating mechanism has been removed. Such gating is evident in the transmission of vestibular signals to the RVLM, since many RVLM neurons exhibited graviceptive responses to whole-body rotations in decerebrate cats, but RVLM neurons were relatively insensitive to labyrinthine inputs in conscious animals (Destefino et al. 2011). Another possibility is that descending pathways that are removed during decerebration play a direct role in shaping the responses of LTF neurons to vertical plane tilts. The latter possibility is feasible, since the sensory conflict that induces motion sickness typically entails a mismatch between visual and vestibular signals regarding body position in space (Reason 1978). Consequently, LTF neurons that participate in generating motion sickness-related vomiting must receive visual information from higher centers. It is certainly possible that visual and vestibular inputs are integrated together upstream from the brainstem and that the processed signal is relayed to the LTF.
Vomiting is only one of the signs and symptoms associated with motion sickness; additional characteristics in humans include nausea, decreases in skin and gastrointestinal blood flow, and cold sweating (Money 1970). The role that the LTF plays in generating these features of motion sickness is currently unknown. A fraction of neurons in the LTF participate in regulating sympathetic nerve activity and blood pressure and thus could mediate the alterations in blood flow that accompany motion sickness (Barman and Gebber 1987; Orer et al. 1999, 2004 Barman et al. 2000). None of the neurons in our sample had overt cardiac-related activity, a hallmark feature of presympathetic neurons that regulate blood flow, but there is some evidence that such activity is suppressed in conscious animals (Barman et al. 2011). It is also feasible that our methodology was not sensitive enough to detect subtle cardiac-related discharges or that LTF neurons that regulate blood flow are segregated from those that coordinate vomiting. Further studies will thus be needed to ascertain which of the signs and symptoms associated with motion sickness are mediated by the LTF.
The parabrachial nucleus, which is reciprocally connected with the LTF (Herbert et al. 1990), has been postulated to serve as a key region in generating the affective components of motion sickness (Balaban 1996, 1999). The responses of both parabrachial nucleus (Suzuki et al. 2012) and LTF units (Moy et al. 2012) to vestibular stimulation were altered when the emetic compound copper sulfate was introduced into the stomach, indicating that neurons in both of these regions integrate multiple emetic signals. However, the relative contributions of these two areas and others, such as nucleus tractus solitarius (Sugiyama et al. 2011), to processing vestibular inputs that trigger motion sickness are yet to be determined. Deciphering these contributions will require additional studies that entail the use of anti-dromic stimulation to map the projections of the neurons whose responses to rotations are characterized. In addition, it would be informative to examine the responses of LTF neurons to conflicting visual and vestibular inputs regarding body position in space, as such signals constitute the most common trigger for motion sickness.
In summary, the present study confirmed the results of previous experiments in decerebrate cats (Moy et al. 2012) showing that an appreciable fraction of LTF neurons has complex responses to rotations in vertical planes, particularly STC behavior. These complex responses likely reflect the convergence of inputs from semicircular canal and otolith organ afferents reflecting head motion in different directions, which could only be produced during complex head movements beyond the normal behavioral repertoire. Consequently, these response properties are consistent with the LTF participating in generating motion sickness, in alignment with classical studies showing that this region is the “vomiting center.” Additional studies are thus warranted to more fully characterize how conflicting sensory signals reflecting body position in space are integrated by neurons in this region.
Acknowledgments
The authors thank Lucy Cotter for technical assistance. Funding was provided by Grant R01-DC003732 from the National Institutes of Health (USA).
Contributor Information
Andrew A. McCall, Department of Otolaryngology, Eye and Ear Institute, University of Pittsburgh, Room 519, Pittsburgh, PA 15213, USA
Jennifer D. Moy, Department of Otolaryngology, Eye and Ear Institute, University of Pittsburgh, Room 519, Pittsburgh, PA 15213, USA
William M. DeMayo, Department of Otolaryngology, Eye and Ear Institute, University of Pittsburgh, Room 519, Pittsburgh, PA 15213, USA, Department of Neuroscience, University of Pittsburgh, Pittsburgh, PA 15213, USA
Sonya R. Puterbaugh, Department of Otolaryngology, Eye and Ear Institute, University of Pittsburgh, Room 519, Pittsburgh, PA 15213, USA
Daniel J. Miller, Department of Otolaryngology, Eye and Ear Institute, University of Pittsburgh, Room 519, Pittsburgh, PA 15213, USA, Department of Neuroscience, University of Pittsburgh, Pittsburgh, PA 15213, USA
Michael F. Catanzaro, Department of Otolaryngology, Eye and Ear Institute, University of Pittsburgh, Room 519, Pittsburgh, PA 15213, USA, Department of Neuroscience, University of Pittsburgh, Pittsburgh, PA 15213, USA
Bill J. Yates, Email: byates@pitt.edu, Department of Otolaryngology, Eye and Ear Institute, University of Pittsburgh, Room 519, Pittsburgh, PA 15213, USA, Department of Neuroscience, University of Pittsburgh, Pittsburgh, PA 15213, USA
References
- Anderson JH, Blanks RHI, Precht W. Response characteristics of semicircular canal and otolith systems in the cat. I. Dynamic responses of primary vestibular fibers. Exp Brain Res. 1978;32:491–507. doi: 10.1007/BF00239549. [DOI] [PubMed] [Google Scholar]
- Baker J, Goldberg J, Hermann G, Peterson B. Spatial and temporal response properties of secondary neurons that receive convergent input in vestibular nuclei of alert cats. Brain Res. 1984;294:138–143. doi: 10.1016/0006-8993(84)91318-0. [DOI] [PubMed] [Google Scholar]
- Balaban CD. Vestibular nucleus projections to the parabrachial nucleus in rabbits: implications for vestibular influences on the autonomic nervous system. Exp Brain Res. 1996;108:367–381. doi: 10.1007/BF00227260. [DOI] [PubMed] [Google Scholar]
- Balaban CD. Vestibular autonomic regulation (including motion sickness and the mechanism of vomiting) Curr Opin Neurol. 1999;12:29–33. doi: 10.1097/00019052-199902000-00005. [DOI] [PubMed] [Google Scholar]
- Barman SM, Gebber GL. Lateral tegmental field neurons of cat medulla: a source of basal activity of ventrolateral medullospinal sympathoexcitatory neurons. J Neurophysiol. 1987;57:1410–1424. doi: 10.1152/jn.1987.57.5.1410. [DOI] [PubMed] [Google Scholar]
- Barman SM, Gebber GL, Orer HS. Medullary lateral tegmental field: an important source of basal sympathetic nerve discharge in the cat. Am J Physiol Regul Integr Comp Physiol. 2000;278:R995–R1004. doi: 10.1152/ajpregu.2000.278.4.R995. [DOI] [PubMed] [Google Scholar]
- Barman SM, Sugiyama Y, Suzuki T, Cotter LA, DeStefino VJ, Reighard DA, Cass SP, Yates BJ. Rhythmic activity of neurons in the rostral ventrolateral medulla of conscious cats: effect of removal of vestibular inputs. Am J Physiol Regul Integr Comp Physiol. 2011;301:R937–R946. doi: 10.1152/ajpregu.00265.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman AI. The brain stem of the cat. University of Wisconsin Press; Madison: 1968. [Google Scholar]
- Bolton PS, Goto T, Schor RH, Wilson VJ, Yamagata Y, Yates BJ. Response of pontomedullary reticulospinal neurons to vestibular stimuli in vertical planes. Role in vertical vestibulospinal reflexes of the decerebrate cat. J Neurophysiol. 1992;67:639–647. doi: 10.1152/jn.1992.67.3.639. [DOI] [PubMed] [Google Scholar]
- Borison HL, Wang SC. Functional localization of central coordinating mechanism for emesis in cat. J Neurophysiol. 1949;12:305–313. doi: 10.1152/jn.1949.12.5.305. [DOI] [PubMed] [Google Scholar]
- Cheung BS, Money KE, Jacobs I. Motion sickness susceptibility and aerobic fitness: a longitudinal study. Aviation Space Environ Med. 1990;61:201–204. [PubMed] [Google Scholar]
- Correia MJ, Guedry FE., Jr Modification of vestibular responses as a function of rate of rotation about an Earth-horizontal axis. Acta Otolaryngol. 1966;62:297–308. doi: 10.3109/00016486609119575. [DOI] [PubMed] [Google Scholar]
- DeStefino VJ, Reighard DA, Sugiyama Y, Suzuki T, Cotter LA, Larson MG, Gandhi NJ, Barman SM, Yates BJ. Responses of neurons in the rostral ventrolateral medulla (RVLM) to whole-body rotations: comparisons in decerebrate and conscious cats. J Appl Physiol. 2011;110:1699–1707. doi: 10.1152/japplphysiol.00180.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez C, Goldberg JM. Physiology of peripheral neurons innervating semicircular canals of the squirrel monkey. II. Response to sinusoidal stimulation and dynamics of peripheral vestibular system. J Neurophysiol. 1971;34:661–675. doi: 10.1152/jn.1971.34.4.661. [DOI] [PubMed] [Google Scholar]
- Fernandez C, Goldberg JM. Physiology of peripheral neurons innervating otolith organs of the squirrel monkey. III. Response dynamics. J Neurophysiol. 1976;39:996–1008. doi: 10.1152/jn.1976.39.5.996. [DOI] [PubMed] [Google Scholar]
- Fukuda H, Koga T. The Botzinger complex as the pattern generator for retching and vomiting in the dog. Neurosci Res. 1991;12:471–485. doi: 10.1016/s0168-0102(09)80001-1. [DOI] [PubMed] [Google Scholar]
- Fukuda H, Koga T. Non-respiratory neurons in the Botzinger complex exhibiting appropriate firing patterns to generate the emetic act in dogs. Neurosci Res. 1992;14:180–194. doi: 10.1016/0168-0102(92)90079-r. [DOI] [PubMed] [Google Scholar]
- Golding JF. Motion sickness susceptibility. Auton Neurosci. 2006;129:67–76. doi: 10.1016/j.autneu.2006.07.019. [DOI] [PubMed] [Google Scholar]
- Guedry FE., Jr Orientation of the rotation-axis relative to gravity: its influence on nystagmus and the sensation of rotation. Acta Otolaryngol. 1965;60:30–48. doi: 10.3109/00016486509126986. [DOI] [PubMed] [Google Scholar]
- Herbert H, Moga MM, Saper CB. Connections of the parabrachial nucleus with the nucleus of the solitary tract and the medullary reticular formation in the rat. J Comp Neurol. 1990;293:540–580. doi: 10.1002/cne.902930404. [DOI] [PubMed] [Google Scholar]
- Kasper J, Schor RH, Wilson VJ. Response of vestibular neurons to head rotations in vertical planes. I. Response to vestibular stimulation. J Neurophysiol. 1988;60:1753–1764. doi: 10.1152/jn.1988.60.5.1753. [DOI] [PubMed] [Google Scholar]
- Koga T, Qu R, Fukuda H. The central pattern generator for vomiting may exist in the reticular area dorsomedial to the retrofacial nucleus in dogs. Exp Brain Res. 1998;118:139–147. doi: 10.1007/s002210050265. [DOI] [PubMed] [Google Scholar]
- Miller AD, Wilson VJ. Vestibular-induced vomiting after vesti-bulocerebellar lesions. Brain Behav Evol. 1983;23:26–31. doi: 10.1159/000121484. [DOI] [PubMed] [Google Scholar]
- Miller AD, Nonaka S, Jakus J. Brain areas essential or non-essential for emesis. Brain Res. 1994;647:255–264. doi: 10.1016/0006-8993(94)91325-0. [DOI] [PubMed] [Google Scholar]
- Miller DM, Cotter LA, Gandhi NJ, Schor RH, Cass SP, Huff NO, Raj SG, Shulman JA, Yates BJ. Responses of caudal vestibular nucleus neurons of conscious cats to rotations in vertical planes, before and after a bilateral vestibular neurectomy. Exp Brain Res. 2008a;188:175–186. doi: 10.1007/s00221-008-1359-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DM, Cotter LA, Gandhi NJ, Schor RH, Huff NO, Raj SG, Shulman JA, Yates BJ. Responses of rostral fastigial nucleus neurons of conscious cats to rotations in vertical planes. Neuroscience. 2008b;155:317–325. doi: 10.1016/j.neuroscience.2008.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Money KE. Motion sickness. Physiol Rev. 1970;50:1–39. doi: 10.1152/physrev.1970.50.1.1. [DOI] [PubMed] [Google Scholar]
- Moy JD, Miller DJ, Catanzaro MF, Boyle BM, Ogburn SW, Cotter LA, Yates BJ, McCall AA. Responses of neurons in the caudal medullary lateral tegmental field to visceral inputs and vestibular stimulation in vertical planes. Am J Physiol Regul Integr Comp Physiol. 2012;303:R929–R940. doi: 10.1152/ajpregu.00356.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orer HS, Barman SM, Gebber GL, Sykes SM. Medullary lateral tegmental field: an important synaptic relay in the baroreceptor reflex pathway of the cat. Am J Physiol. 1999;277:R1462–R1475. doi: 10.1152/ajpregu.1999.277.5.R1462. [DOI] [PubMed] [Google Scholar]
- Orer HS, Gebber GL, Phillips SW, Barman SM. Role of the medullary lateral tegmental field in reflex-mediated sympathoexcitation in cats. Am J Physiol Regul Integr Comp Physiol. 2004;286:R451–R464. doi: 10.1152/ajpregu.00569.2003. [DOI] [PubMed] [Google Scholar]
- Reason JT. Motion sickness adaptation: a neural mismatch model. J R Soc Med. 1978;71:819–829. doi: 10.1177/014107687807101109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schor RH, Angelaki DE. The algebra of neural response vectors. Ann New York Acad Sci. 1992;656:190–204. doi: 10.1111/j.1749-6632.1992.tb25209.x. [DOI] [PubMed] [Google Scholar]
- Schor RH, Miller AD, Tomko DL. Responses to head tilt in cat central vestibular neurons. I. Direction of maximum sensitivity. J Neurophysiol. 1984;51:136–146. doi: 10.1152/jn.1984.51.1.136. [DOI] [PubMed] [Google Scholar]
- Scudder CA, Kaneko CS, Fuchs AF. The brainstem burst generator for saccadic eye movements: a modern synthesis. Exp Brain Res. 2002;142:439–462. doi: 10.1007/s00221-001-0912-9. [DOI] [PubMed] [Google Scholar]
- Sugiyama Y, Suzuki T, Destefino VJ, Yates BJ. Integrative responses of neurons in nucleus tractus solitarius to visceral afferent stimulation and vestibular stimulation in vertical planes. Am J Physiol Regul Integr Comp Physiol. 2011;301:R1380–R1390. doi: 10.1152/ajpregu.00361.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Sugiyama Y, Yates BJ. Integrative responses of neurons in parabrachial nuclei to a neurogenic gastrointestinal stimulus and vestibular stimulation in vertical planes. Am J Physiol Regul Integr Comp Physiol. 2012;302:R965–R975. doi: 10.1152/ajpregu.00680.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SC, Borison HL. The vomiting center; its destruction by radon implantation in dog medulla oblongata. Am J Physiol. 1951;166:712–717. doi: 10.1152/ajplegacy.1951.166.3.712. [DOI] [PubMed] [Google Scholar]
- Yates BJ. In: Motion sickness. Binder MD, Hirokawa N, Windhorst U, editors. Encyclopedia of neuroscience; Springer, Heidelberg: 2009. pp. 2410–2413. [Google Scholar]
- Yates BJ, Balaban CD, Miller AD, Endo K, Yamaguchi Y. Vestibular inputs to the lateral tegmental field of the cat: potential role in autonomic control. Brain Res. 1995;689:197–206. doi: 10.1016/0006-8993(95)00569-c. [DOI] [PubMed] [Google Scholar]
- Yates BJ, Miller AD, Lucot JB. Physiological basis and pharmacology of motion sickness: an update. Brain Res Bull. 1998;47:395–406. doi: 10.1016/s0361-9230(98)00092-6. [DOI] [PubMed] [Google Scholar]