Abstract
This study examined the longitudinal relationship between health-related quality of life (HR-QOL) and subjective and objective sleep quality in 166 women with newly diagnosed stage I-III breast cancer who were scheduled to receive ≥4 cycles of adjuvant/neoadjuvant chemotherapy. HR-QOL was assessed with the Medical Outcomes Study-Short Form Physical Component Scale and Mental Component Scale scores. Subjective sleep was assessed with the Pittsburgh Sleep Quality Index (PSQI); objective sleep was measured with actigraphy. Data were collected before starting chemotherapy and during the last week of cycle 4 of chemotherapy. Patients reported poor HR-QOL and poor sleep quality before and during chemotherapy. Short sleep time and long naps were recorded at both time points. The Mental Component score was related to reports of poor sleep but not to recorded sleep, worse Physical Component scores were associated with reports of poor sleep and less recorded nap time, suggesting sleep plays an important role in cancer patients’ HR-QOL.
As cancer treatment has improved, survival has stopped being the sole end point of treatment with improved quality of life becoming a vital outcome for cancer survivors. Due to its broad coverage of individual’s feelings, beliefs and perceptions, there is a lack of consensus on the exact definition of quality of life (Siddiqui, Kachnic, & Movsas, 2006; Soni & Cella, 2002). However, health-related quality of life (HR-QOL) is defined as a combination of health status, functional status and quality of life (Guyatt, Feeny, & Patrick, 1993). While there are different types of measurements for quality of life in cancer patients (Berger, Sankaranarayanan, & Watanabe-Galloway, 2007; Soni & Cella, 2002), the Medical Outcomes Study-Short Form (SF-36) is one frequently used. The SF-36 measures HR-QOL, and a Physical Component Scale (PCS) score and a Mental Component Scale (MCS) score are usually generated and reported from the SF-36 (Ware, Kosinski, & Gandek, 2002).
Increasingly cancer patients and their health care providers are becoming concerned with maintaining HR-QOL as it frequently decreases after diagnosis or treatment (Pockaj et al., 2009; Trentham-Dietz et al., 2008). In addition, HR-QOL has been found to be a predictor of survival in patients with head and neck, lung or colorectal cancer (Efficace et al., 2006; Karvonen-Gutierrez et al., 2008; Maione et al., 2009). Therefore, identifying which factors specifically contribute to poorer HR-QOL in cancer patients and exploring possible ways to improve HR-QOL, have become very important therapeutic goals (Siddiqui, Kachnic, & Movsas, 2006; Soni & Cella, 2002).
Studies show that poor HR-QOL is associated with multiple factors in cancer patients, including sleep disturbances, fatigue, pain, anxiety, and depression (Frick, Tyroller, & Panzer, 2007; Redeker, Lev, & Ruggiero, 2000; Visser & Smets, 1998). However, only a handful of studies have examined the relationship between sleep disturbances and HR-QOL and sleep in those studies was often examined only as part of a symptom cluster (Dodd, Cho, Cooper, & Miaskowski, 2010; Miaskowski et al., 2006; Pud et al., 2008). A 2007 review of methodological approaches to the study of sleep disturbances and HR-QOL in cancer patients concluded that while the changes of HR-QOL and sleep over time have been studied, the relationship between HR-QOL and sleep remained unclear (Berger, Sankaranarayanan, & Watanabe-Galloway, 2007). Limitations of most studies include a single-item subjective measurement of sleep and cross-sectional rather than longitudinal data collection (Berger, Sankaranarayanan, & Watanabe-Galloway, 2007), and a single-item measure of sleep is not effective in identifying sleep problems in cancer patients (Gooneratne et al., 2007). A few studies investigating sleep and HR-QOL in cancer patients have been published since the above review (Dodd, Cho, Cooper, & Miaskowski, 2010; Eyigor, Eyigor, & Uslu, 2010; Lis, Gupta, & Grutsch, 2008; Pud et al., 2008; Sandadi et al., 2011), but most used questionnaires to measure sleep quality and thus were still limited by the subjective nature of the measurements, not focusing on HR-QOL-sleep relationship, and cross-sectional designs. Therefore, longitudinal studies examining the specific relationship between HR-QOL and sleep disturbances, especially between HR-QOL and comprehensively and objectively measured sleep are needed in cancer patients.
To our knowledge, only one study with a small sample of lung cancer patients (n=29) used actigraphy and the SF-36 to examine the relationship of quality of life and sleep in cancer patients. No significant correlations were identified, except for a negative association between the physical component score of the SF-36 and sleep-log-documented sleep time (Le Guen Y. et al., 2007). Prior findings from our laboratory showed that HR-QOL was associated with sleep quality, fatigue and depressive symptoms before chemotherapy (Ancoli-Israel et al., 2006). In the current study, we examined the longitudinal relationship between HR-QOL (as measured by the SF-36) and subjectively (questionnaire) and objectively (actigraph) measured sleep quality in breast cancer patients before the start of the first cycle of chemotherapy and at the end of four cycles of treatment (C4LW).
METHOD
Participants
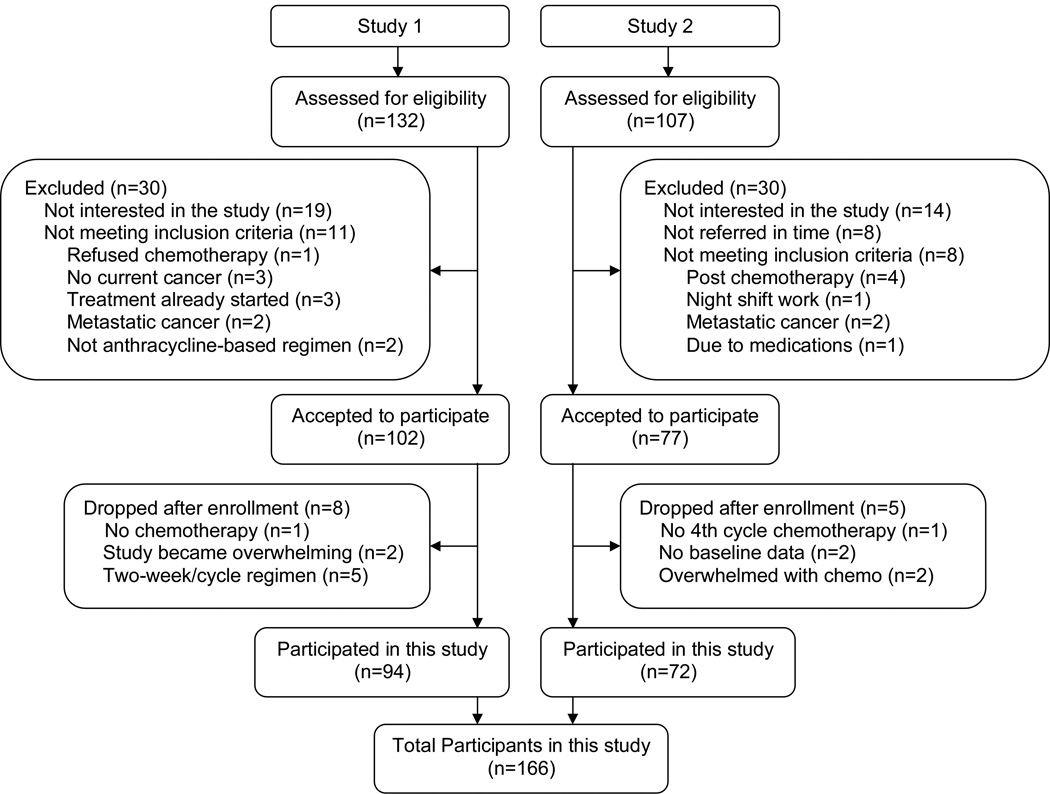
Participants were from two completed studies of women with breast cancer undergoing chemotherapy. The first study focused on fatigue, sleep and circadian rhythms (Study 1), while the second focused on chemotherapy related cognitive impairments (Study 2). Both studies had similar protocols and were conducted among women with newly diagnosed stage I-III breast cancer scheduled to receive at least four cycles of adjuvant or neoadjuvant anthracycline-based chemotherapy. Ninety-four women from Study 1 and 72 women from Study 2 met inclusion criterion (see below for inclusion criteria and Figure 1 for the Screening and Enrollment processes). Study 1 data were collected between 2000 and 2005 and as recommended at that time, women in Study 1 all received 3-week chemotherapy cycles. Study 2 data were collected between 2005 and 2010, at which point the recommended treatment regimen had changed to a 2-week cycle; therefore 38 (63%) women in Study 2 received a 2-week cycle regimen and 22 (37%) received a 3-week cycle regimen of chemotherapy. The different length of treatment cycles was tested and controlled as confounders. There were no significant differences between the two samples for age, race, body mass index (BMI), education level, marital status, annual household income, menopausal status, use of other medications, cancer stage, surgery type, chemotherapy regimen, HR-QOL, or subjective and objective sleep quality. Therefore, the two samples were merged and a total of 166 women were included in this analysis. Detailed demographic and disease characteristics of the women are listed in Table 1.
Figure 1. Screening and Enrollment Flowchart.
CONSORT diagram showing the flow of participants.
Table 1.
Demographic, disease and treatment characteristics of the participants (n=166)
| Variable | Value | |
|---|---|---|
| Age (years) | ||
| Mean (SD) | 51.3 (9.6) | |
| Range | 31 – 80 | |
| BMI (kg/m2) | ||
| Mean (SD) | 28.2 (7.0) | |
| Range | 17.4 – 61.9 | |
| Race [n (%)] | ||
| Caucasian | 133 (80.1) | |
| Non-Caucasian | 33 (19.9) | |
| Education [n (%)] | ||
| Some or completed high school | 29 (17.5) | |
| Some college | 53 (31.9) | |
| Completed college and above | 84 (50.6) | |
| Marital status [n (%)] | ||
| Never married | 13 (7.8) | |
| Divorced/separated/widowed | 38 (22.9) | |
| Married | 115 (69.3) | |
| Household annual income [n (%)] | ||
| ≤ $30,000 | 25 (15.1) | |
| > $30,000 | 119 (71.7) | |
| Refused to answer | 22 (13.2) | |
| Menopausal status [n (%)] | ||
| Baseline | pre-menopause | 65 (41.7) |
| peri-menopause | 16 (10.3) | |
| post-menopause | 52 (33.3) | |
| hysterectomy | 23 (14.7) | |
| Not available | 10 | |
| Cycle 4 Week 3 | pre-menopause | 7 (4.9) |
| peri-menopause | 28 (19.6) | |
| post-menopause | 85 (59.4) | |
| hysterectomy | 23 (16.1) | |
| Not available | 23 | |
| Cancer stage [n (%)] | ||
| Stage I | 40 (27.8) | |
| Stage II | 66 (45.8) | |
| Stage III | 38 (26.4) | |
| Not available | 22 | |
| Surgery type | ||
| Lumpectomy | 60 (41.7) | |
| Mastectomy | 65 (45.1) | |
| Double mastectomy | 8 (5.6) | |
| No surgery before Chemotherapy | 11 (7.6) | |
| Not available | 22 | |
| Chemotherapy regimen [n (%)] | ||
| AC | 38 (26.4) | |
| AC + docetaxel | 34 (23.6) | |
| AC + paclitaxel | 45 (31.2) | |
| AC + fluorouracil | 4 (2.8) | |
| Other | 23 (16.0) | |
| Not available | 22 | |
| Chemotherapy cycle length [n (%)] | ||
| 3-week | 116 (75.3) | |
| 2-week | 38 (24.7) | |
| Not available | 12 | |
Note: AC = Doxorubicin + Cyclophosphamide, ECF = Epirubicin + Cytoxan + Fluorouracil
Pregnant women, those undergoing bone marrow transplants, and those with metastatic breast cancer, with confounding underlying medical illnesses, with significant pre-existing anemia or with other physical or psychological impairments were excluded from both studies. The University of California Committee on Protection of Human Subjects and the UCSD Rebecca and John Moores Cancer Center’s Protocol Review and Monitoring Committee approved both studies, and an informed consent was obtained from each woman at the beginning of her participation in the study.
Measures
Health Related Quality of life (HR-QOL)
HR-QOL was assessed with the Medical Outcomes Study Short Form (SF-36) health survey. The SF-36 health status survey is a generic 36-item health status instrument with eight subscales measuring eight domains of health: physical functioning, role limitations because of physical problems, bodily pain, general health perceptions, vitality, social functioning, role limitations because of emotional problems, and mental health (Ware, Kosinski, & Gandek, 2002). The SF-36 has no questions related to sleep or fatigue. SF-36 is a commonly used measurement of health-related QOL (HR-QOL) (Coons, Keininger, & Hays, 2000). Each subscale is scored on a range from 0 to 100, with lower scores indicating worse HR-QOL. Norm-based Physical Component Scale (PCS) and Mental Component Scale (MCS) scores are calculated from these eight subscales. Three subscales contribute primarily to the PCS scale (physical functioning, bodily pain, role limitations because of physical problems), two subscales contribute primarily to the MCS scale (mental health and role limitations because of emotional problems), and the remaining three subscales contribute substantially to both summary scales (vitality, social functioning, and general health perceptions). Both PCS and MCS have a mean of 50 and a standard deviation of 10 in the 1998 general U.S. population, with a score below 50 indicating that the HR-QOL is below the average (Ware & Kosinski, 2002).
Subjective sleep quality
Subjective sleep quality was assessed with the Pittsburgh Sleep Quality Index (PSQI) (Buysse et al., 1991; Buysse, Reynolds, Monk, Berman, & Kupfer, 1989). PSQI is a 19-item questionnaire which rates patients’ reports of sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleep medication and daytime dysfunction. The total PSQI scores can range from 0–21 with high scores reflecting poor sleep quality. A total score above 5 is considered poor sleep, while a cut-off score of 8 was suggested to indicate poor sleep in clinical populations (Beck, Schwartz, Towsley G, Dudley, & Barsevick, 2004; Carpenter & Andrykowski, 1998).
Objective sleep quality
Objective sleep quality was measured with the Actillume (Ambulatory Monitoring Inc, Ardsley, New York) or Actiwatch-Light (Philips Respironics Mini Mitter, Bend, OR) actigraphs. The Actillume actigraph is a small device approximately 1x3x6cm in size, it contains a piezoelectric linear accelerometer (sensitive to 0.003 g and above), a log-linear photometric transducer (sensitive from <0.01 lux to >100,000 lux), a microprocessor, 32K RAM memory, and associated circuitry. The Actiwatch-Light is a watch-like device approximately 1×2.5×5 cm in size. It also has a piezoelectric accelerometer (sensitive to <0.01 g force), and its luminance sensor has a spectral sensitivity approximating that of the human eye (sensitive from 0.1 to 150,000 lux). The Actiwatch-Light has a 64K on-board memory and associated circuit. A one minute epoch was used for both actigraphs. Once collected, data were downloaded onto a desktop computer and hand-edited with additional information from a sleep log completed by the participant’s recording of time to bed, time up in the morning, nap time and other information needed for editing the actigraphy data. The Action-4 software package for Actillume and Actiware 5 software for Actiwatch-L were used to score sleep and wake. All women in Study 1 and 14 women in Study 2 wore the Actillume, and the other 58 women in Study 2 wore the Actiwatch-Light. To establish equivalency between the two devices, a validation study in 8 volunteers was conducted with both devices worn concurrently on the same wrist for 72-hours. The Actillume-derived SUMACT (summary activity) count and the Actiwatch-Light-derived activity count data, as well as the software-scored sleep/wake data based on the two types of activity count were highly correlated (both r >0.85), and therefore deemed equivalent for the purpose of this study. Actigraphy has been validated and shown to be reliable in recording sleep and wake in multiple studies (Ancoli-Israel, et al., 2003; Lichstein et al., 2006).
Total sleep time (TST), total wake time from time to bed to final awakening in the morning (TWT), and total nap time (NAPTIME) were calculated. Naps were defined as any 10 or more minutes of consecutive actigraphic sleep during the hours between final up time and bedtime.
Procedure
Detailed procedural information for Study 1 can be found in (Liu et al. (2005). Briefly, after consent forms were signed, medical records were abstracted for medical history and current medication use. Study 2 followed similar procedures. Only data collected at baseline and during the last week of cycle 4 (C4LW) are reported in this study.
Starting on the first day of each data collection time point, women wore an actigraph for 3 consecutive days (72 hours) and completed a daily sleep log used for editing actigraphy data. For each woman, actigraphy was recording on the same day at each time point. The day chosen was based on the day of the chemotherapy administration. While the ideal recording time for an actigraph is generally one week, due to potential subject burden, the minimum of three days suggested by the AASM practice parameters for actigraphy was used (Ancoli-Israel et al., 2003).
Data analysis
Descriptive statistics (mean and standard deviation) were calculated for all outcomes at both time points. One-sample T tests were performed for PCS and MCS scores in comparison with the U.S. norms. Pearson correlation analyses were performed for PCS and MCS scores and sleep parameters. A mixed model analysis (Diggle, Liang, & Zeger, 1994) was used to test the significance of possible confounding factors, to examine changes in HR-QOL and sleep (subjective and objective) over the course of chemotherapy, and to examine the longitudinal relationship between HR-QOL and sleep parameters. This modeling approach accounts for correlations in repeated measures within a subject, and also allows for partially missing data. A random intercept was included in each mixed model to account for subject-specific effects.
In order to identify potential confounding factors, the following mixed models were developed: HR-QOL or sleep parameters were the response variables and demographic, disease or treatment characteristics were the main effect. Variables with p<0.1 were determined to be confounders, and were adjusted for in subsequent analyses. Changes in HR-QOL or sleep (subjective and objective) over time were examined with mixed models separately; chemotherapy week (time) was modeled as a fixed effect and confounding factors were controlled in each model.
Finally, a set of mixed models was developed to explore the longitudinal relationship between HR-QOL (outcome) and sleep parameters (predictors). In those mixed models, total PSQI scores or objective sleep variables (TST, TWT, or NAPTIME) was the response variable, PCS or MCS scores was the main effect, and the sleep parameter was included as a random effect, thereby allowing for subject-specific slope terms for sleep parameters in the model. These mixed models were adjusted with chemotherapy week (time) and confounding demographic, disease and treatment characteristic variables. Adjusted regression coefficients (β-value) with standard errors and associated p-values are presented.
All analyses were performed using version 9.2 of SAS (SAS Institute Inc. 2008). All statistical tests with p-values <0.05 are reported as statistically significant.
RESULTS
As summarized in Table 1, the mean age of the 166 women was 51.3 years, 80% were Caucasian, 69% were married, 51% had at least completed-college education, 72% reported an annual income of more than $30,000, 60% had a BMI ≥25 and 31% were >30. Twenty-five percent of the women received a 2-week cycle regimen of chemotherapy, 84% percent were treated with doxorubicin and cyclophosphamide [AC], or AC plus fluorouracil, AC plus docetaxel, or AC plus paclitaxel, the rest were either treated with cyclophosphamide, epirubicin and fluorouracil [CEF]), or their therapy was indicated as ‘other’ regimen.
The following variables listed in Table 1 were tested as potential confounders in relation to HR-QOL and sleep: age, BMI, race, education, income, marital status, menopausal status, use of different medications, cancer stage, surgery type, adjuvant or neoadjuvant treatment, chemotherapy regimen, and cycle length of chemotherapy. According to the STRAW criteria (Soules et al., 2001), menopausal status was defined as pre-menopause, peri-menopause and post-menopause; due to this particular study sample, one extra group, hysterectomy (surgical menopause), was also included as a type of menopausal status.
In addition to chemotherapy, patients used other medications to treat other symptoms (such as analgesics, antacids, antidepressants, antihypertensives, insulin, laxatives, diuretics, stimulants, and vitamins). Sedating medications, including antihistamines, minor tranquilizers, major tranquilizers, over the counter hypnotics and sedative hypnotics, were categorized as sleeping medications. Medications identified as confounders are listed in Table 2.
Table 2.
Uses of medications for other symptoms (n=166)
| Medications | n (%) | |
|---|---|---|
| Sleeping medications | ||
| Baseline | yes | 70 (42.7) |
| no | 94 (57.3) | |
| not available | 2 | |
| Cycle 4 week 3 | yes | 59 (46.5) |
| no | 68 (53.5) | |
| not available | 39 | |
| Analgesics | ||
| Baseline | yes | 111 (68.1) |
| no | 52 (31.9) | |
| not available | 3 | |
| Cycle 4 week 3 | yes | 46 (38.0) |
| no | 75 (62.0) | |
| not available | 45 | |
| Antacids | ||
| Baseline | yes | 42 (25.8) |
| no | 121 (74.2) | |
| not available | 3 | |
| Cycle 4 week 3 | yes | 53 (43.8) |
| no | 68 (56.2) | |
| not available | 45 | |
| Antihypertensives | ||
| Baseline | yes | 18 (11.1) |
| no | 144 (88.9) | |
| not available | 4 | |
| Cycle 4 week 3 | yes | 11 (8.3) |
| no | 111 (91.7) | |
| not available | 45 | |
| Laxatives | ||
| Baseline | yes | 31 (19.1) |
| no | 131 (80.9) | |
| not available | 4 | |
| Cycle 4 week 3 | yes | 30 (24.8) |
| no | 91 (75.2) | |
| not available | 36 | |
| Diuretics | ||
| Baseline | yes | 12 (7.4) |
| no | 150 (92.6) | |
| not available | 4 | |
| Cycle 4 week 3 | yes | 8 (6.6) |
| no | 113 (93.4) | |
| not available | 36 | |
| Antidepressants | ||
| Baseline | yes | 32 (19.6) |
| no | 131 (80.4) | |
| not available | 3 | |
| Cycle 4 week 3 | yes | 22 (18.2) |
| no | 99 (81.8) | |
| not available | 45 | |
| Insulin | ||
| Baseline | yes | 9 (5.6) |
| no | 153 (94.4) | |
| not available | 4 | |
| Cycle 4 week 3 | yes | 6 (5.0) |
| no | 115 (95.0) | |
| not available | 45 | |
| Vitamins | ||
| Baseline | yes | 112 (68.7) |
| no | 51 (31.3) | |
| not available | 3 | |
| Cycle 4 week 3 | yes | 71 (58.7) |
| no | 50 (41.3) | |
| not available | 45 | |
Note: sleeping medications included antihistamines, minor tranquilizers, major tranquilizers, OTC hypnotics and sedative hypnotics.
At the p<0.1 level, confounders for lower PCS scores were use of antacids (p=0.004), annual household income <$30,000 (p=0.01), use of sleeping medications (p=0.02), not completed college (p=0.03), use of insulin (p=0.05), higher BMI (p=0.06), 2-week cycle chemotherapy (p=0.07) and pre- or peri-menopause status (p=0.09); confounders for lower MCS score were use of antidepressant (p=0.04) and antihypertensives (0.04), not married (p=0.05), younger age (p=0.07), and use of antacids (0.09); confounders for higher total PSQI score were use of sleeping medications (p=0.006), not married (p=0.03), 2-week cycle chemotherapy (p=0.04), use of laxatives (p=0.04) and analgesics (p=0.07), higher BMI (p=0.07) and not completed college (p=0.07); confounders for shorter TST were higher BMI (0.004), 2-week cycle chemotherapy (p=0.01), non-Caucasians (p=0.03) and use of antidepressants (p=0.07); confounders for longer TWT were non-Caucasians (p=0.003), 2-week cycle chemotherapy (p=0.01) and higher BMI (p=0.02); confounders for longer NAPTIME were use of diuretics (p=0.001), antacids (p=0.07) and antidepressants (p=0.07), and higher cancer stage (p=0.09). These confounding factors were adjusted accordingly in the mixed models.
HR-QOL
The mean PCS and MCS scores were both below 50 at both time points, and one-sample T tests revealed that both scores were significantly below U.S. norms (Ware & Kosinski, 2002) at both time points (all p’s <0.0001); however, the changes of PCS and MCS scores from baseline to the C4LW chemotherapy did not reach statistical significance (PCS: 43.3±9.8 vs. 41.2±8.9; MCS: 46.5±10.8 vs. 44.4±12.4; both p’s>0.05) after controlling for confounders, suggesting that the women had poor HR-QOL before the start of chemotherapy and HR-QOL continued to be poor during treatment.
Subjective sleep quality
Total and subscale scores of PSQI before treatment and at C4LW are listed in Table 3. Compared to baseline (65%), more women had a total PSQI score above 5 (74%) at C4LW, while the number with a score above 8 remained relatively constant (39% baseline vs. 38% at C4LW). From baseline to C4LW, total PSQI score and Sleep Efficiency and Daytime Dysfunction subscales score rose significantly after controlling for confounders (all p’s<0.05); there were no significant changes in scores of the other 5 PSQI subscales. PSQI results suggest that women reported poor sleep quality before the start of treatment and reported even worse sleep quality after 4 cycles of chemotherapy.
Table 3.
PSQI total and subscale scores before and after chemotherapy [mean (SD)]
| Total * | Sleep quality |
Sleep latency |
Sleep duration |
Sleep efficiency * |
Sleep disturbance |
Sleep medication |
Daytime dysfunction ** |
|
|---|---|---|---|---|---|---|---|---|
| Baseline | 7.3 (3.7) | 1.2 (0.8) | 1.0 (1.0) | 0.8 (0.8) | 0.9 (1.1) | 1.5 (0.6) | 1.0 (1.3) | 0.8 (0.6) |
| C4LW | 8.0 (3.9) | 1.2 (0.8) | 1.1 (0.9) | 0.8 (0.8) | 1.1 (1.2) | 1.6 (0.6) | 1.1 (1.3) | 1.0 (0.7) |
Note: Between two time points
p<0.05,
p<0.001, adjusted for BMI, marital status, college degree, use of laxatives, analgesics and sleep medications, and 2-week cycle of chemotherapy.
C4LW = last week of cycle 4 chemotherapy. PSQI = Pittsburgh Sleep Quality Index, higher score indicates poorer sleep quality.
Objective sleep parameters
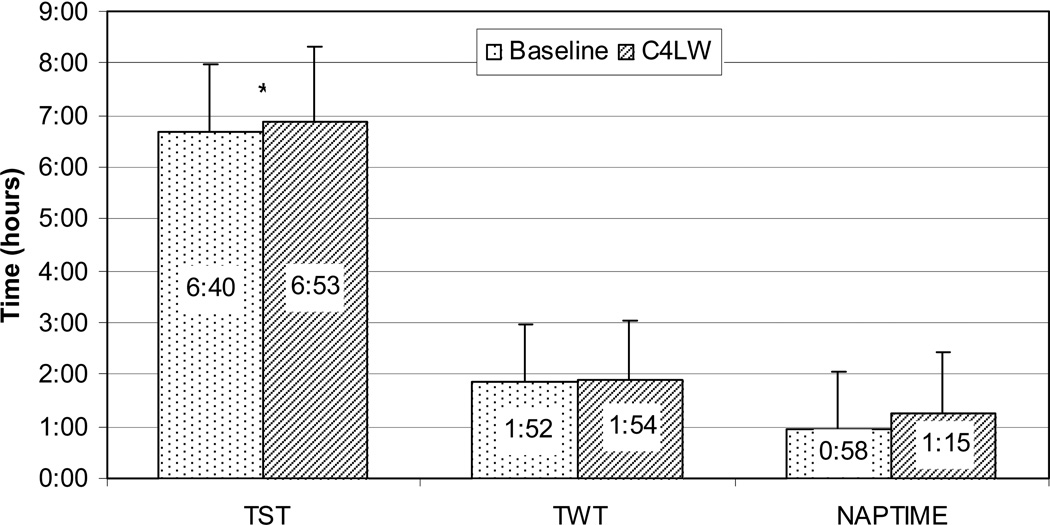
As seen in Figure 2, compared to baseline, women were sleepier at night and continued being sleepy during the day after C4LW. Controlling for confounders, TST increased significantly from baseline to C4LW (p=0.034), although mean TST was below 7 hours at both time points. There were no significant changes in TWT or NAPTIME after controlling for confounders (both p’s>0.2), but women were awake for about two hours at night and napped for close to one hour at both time points. More women slept >7 hours per night (43% at baseline vs. 49% at C4LW) and more were napping >1 hour per day at C4LW (46%) compared to baseline (39%).
Figure 2. Objective Sleep Before and During Chemotherapy.
Objective sleep (TST, TWT and NAPTIME) before (Baseline) and during the last week of cycle 4 chemotherapy (C4LW). Note: Compared to baseline, after 4 cycles of chemotherapy, TST (nighttime total sleep time, hour:minute) increased significantly (6:40±1:18 vs. 6:53±1:23, p=0.03) after controlling for confounders (time, BMI, race, chemotherapy cycle length, and use of antihypertensives) but remained under 7 hours at both time points. There were no significant changes in TWT (nighttime total wake time, hour:minute, 1:52±1:6 vs. 1:54±1:8, p=0.5) or NAPTIME (daytime total nap time, hour:minute, 0:58±1:4 vs. 1:15±1:8, p=0.2), but both remained relatively high after adjusting for confounders (TWT: adjusted for time, BMI, race and chemotherapy cycle length; NAPTIME: adjusted for time, cancer stage, and use of antacids, antidepressants and diuretics).
Associations between HR-QOL and sleep
As shown in Table 4, lower PCS and MCS scores at baseline and C4LW were significantly associated with higher total PSQI scores and higher scores on most of the PSQI subscales. Lower PCS scores were also associated with longer naptime at both time points (both p’s<0.05), while MCS was not significantly correlated with any of the objective sleep parameters.
Table 4.
Correlation coefficients between HR-QOL and subjective and objective sleep measures
| Sleep parameters | PCS | MCS | ||
|---|---|---|---|---|
| Baseline | C4LW | Baseline | C4LW | |
| PSQI Total score | −0.263 ** | −0.347 *** | −0.400 *** | −0.426 *** |
| Sleep quality | −0.278 ** | −0.226 ** | −0.314 *** | −0.377 *** |
| Sleep latency | −0.078 | −0.129 | −0.251 ** | −0.211 * |
| Sleep duration | −0.065 | −0.105 | −0.221 ** | −0.112 |
| Sleep efficiency | −0.127 | −0.113 | −0.224 ** | −0.211 * |
| Sleep disturbance | −0.310 ** | −0.445 *** | −0.116 | −0.250 ** |
| Sleep medication | −0.115 | −0.196 * | −0.140 | −0.220 * |
| Daytime dysfunction | −0.179 * | −0.401 *** | −0.506 *** | −0.476 *** |
| Actigraphy | ||||
| Total sleep time | 0.131 | −0.010 | 0.123 | 0.027 |
| Nighttime total wake time | −0.100 | −0.155 | −0.052 | −0.059 |
| Total nap time | −0.173 * | −0.216 * | −0.027 | −0.105 |
Note:
p<0.5,
p<0.01,
p<0.0001.
C4LW = last week of cycle 4 chemotherapy. PCS = Physical Component Scale of SF-36, higher score indicates better HR-QOL. MCS = Mental Component Scale of SF-36, higher score indicates better HR-QOL. PSQI = Pittsburgh Sleep Quality Index, higher score indicates poorer sleep quality.
Associations between changes of HR-QOL and changes in sleep
As shown in Table 5, mixed-model results revealed that changes in PCS scores were negatively associated with changes in total PSQI scores and NAPTIME. Changes in MCS scores were also negatively associated with changes in total PSQI scores. Specifically, every increase of 1 point of total PSQI score was associated with a decrease of 0.5 point of PCS score and a decrease of 1.1 points of MCS score; every increase of 1 hour of nap time was associated with a decrease of 1.3 points of total PCS score. There were no associations between changes in PCS score and objective nighttime sleep (TST and TWT) or between MCS score and objective sleep parameters (TST, TWT and NAPTIME). When only those with less than 7 hours TST at baseline (n=90) were examined, change in PCS was negatively associated with change in TWT (t=2.2, p=0.03), indicating that women reported lower PCS scores if they slept less than 7 hours before chemotherapy and had more TWT at night.
Table 5.
Mixed model results with PCS or MCS as the response variable and parameter of subjective or objective sleep as the main effect
| SF-36 | Sleep parameters | Mixed model results |
||
|---|---|---|---|---|
| Adj. β-value | Standard Error |
p-value | ||
| PCS scorea | Total PSQI score | −0.498 | 0.164 | 0.0032 |
| TST | 0.377 | 0.496 | 0.45 | |
| TWT | −0.823 | 0.545 | 0.13 | |
| NAPTIME | −1.276 | 0.542 | 0.021 | |
| MCS score b | Total PSQI score | −1.06 | 0. 188 | <0.0001 |
| TST | 0.560 | 0.577 | 0.33 | |
| TWT | −0.473 | 0.655 | 0.47 | |
| NAPTIME | −0.578 | 0.621 | 0.35 | |
Note:
adjusted for time, BMI, education level, household income, menopausal status, chemotherapy cycle length, and use of sleeping medications, antacids and insulin.
adjusted for time, age, marital status, and use of antacids, antidepressants and antihypertensives. SF-36 = Medical Outcomes Study Short Form. PCS = Physical Component Scale of SF-36, higher score indicates better HR-QOL. MCS = Mental Component Scale of SF-36, higher score indicates better HR-QOL. PSQI = Pittsburgh Sleep Quality Index, higher score indicates poorer sleep quality. TST = Nighttime total sleep time; TWT = Nighttime total wake time; NAPTIME = Daytime total nap time.
DISCUSSION
The results of this study showed that HR-QOL was poor before chemotherapy (as previously reported by Ancoli-Israel et al., 2006), and did not worsen over time while self-reported sleep quality was poor before (Ancoli-Israel et al., 2006), and got worse after chemotherapy, with more women scoring above cut-off for good sleep. Objectively, the women were sleepier after chemotherapy but continued to spend long periods awake at night and to nap during the day. In addition, the self-reported variables of HR-QOL and sleep quality were consistently related to each other at both time points and both changed for the worse in synchrony over time. However, HR-QOL and recorded sleep changes were generally not related to each other and did not change synchronously over time except for PCS and total nap time.
In the current study, in addition to subjective sleep data, objective measures of sleep were also collected. PCS was negatively correlated with total nap time at both time points, indicating that more naptime was associated with worse physical HR-QOL. There was however, no relationship between other objective nighttime sleep parameters and HR-QOL although total sleep time was less than 7 hours and total wake time was almost 2 hours at both time points. When we examined only those women with less than 7 hours of sleep at baseline, the changes in physical HR-QOL were negatively associated with changes in nighttime total wake time, with those staying awake longer at night reporting worse physical HR-QOL. These results suggest that a significant relationship between low physical HR-QOL and poor objective sleep might exist in patients with shorter sleep time, but this hypothesis needs to be tested in larger samples.
In addition to the significant relationship between HR-QOL and subjective sleep quality, the unique finding of this study was that the worse physical HR-QOL was correlated with longer naps. To our knowledge, this is the first study to report a relationship between HR-QOL and naptime in breast cancer patients undergoing chemotherapy. Adequate napping, e.g., no more than 30 minutes per day, has been shown to have multiple health benefits, but frequent and longer napping may lead to adverse long-term health outcomes (Dhand & Sohal, 2006). In this study, almost half the women napped more than 1 hour at both study time points. Although TST increased during C4LW compared to baseline, the mean TST was still below 7 hours at C4LW. This shorter TST and longer napping time may indicate poor daytime sleep habits and lower sleep quality. On the other hand, excessive napping might lead to reduced physical activity during the day. Thus, it is not surprising that the physical component of HR-QOL was associated with total nap time in this group of women. This phenomenon might also be explained by our previous findings which showed that more fatigue was associated with longer nap time in women with breast cancer undergoing chemotherapy (Liu et al., in press), since fatigue is one of the most important contributors to low HR-QOL (Bower et al., 2000; Curt, 2000).
Another interesting finding of this study was that the mental component of HR-QOL was not associated with objective sleep parameters. A possible explanation for this result is that actigraphy measures objective sleep quality while mental HR-QOL is based on subjective feelings. In the same manner that actigraph-measured sleep is associated more with results from polysomnography than with subjectively-reported sleep in sleep diaries (Ancoli-Israel et al., 2003), it is not surprising that these objective sleep parameters were not related to mental HR-QOL. As discussed above, subjectively-measured sleep quality (PSQI) was significantly associated with subjectively-reported mental HR-QOL.
As summarized by Berger et al. (2007), although quality of life in cancer patients has been extensively studied, the relationship between quality of life and sleep was not the primary aim of those studies, and the direct relationship between quality of life and sleep has rarely been explored. A few studies explored the relationship between quality of life and subjective sleep (Eyigor, Eyigor, & Uslu, 2010; Gooneratne et al., 2007; Lis, Gupta, & Grutsch, 2008; Sandadi et al., 2011) in patients with different types of cancer, and all found a relationship between lower quality of life and reports of poor sleep quality or sleep disturbance. The significant relationships between HR-QOL and total PSQI scores found in this study confirm the above findings.
A few studies have tested different intervention strategies to improve HR-QOL in cancer patients. In the Berger et al. (2007) review, 10 intervention studies were identified and several intervention strategies were studied, such as pharmacology, exercise, massage and cognitive-behavior therapy (CBT), however, findings were inconclusive, and almost all of the intervention studies were conducted in cancer survivors after completion of cancer treatment. The significant associations between HR-QOL and subjective and objective sleep quality revealed in this study, together with the suggestive effects from those intervention studies in cancer survivors, suggest that HR-QOL may be improved in women with breast cancer by improving sleep quality and optimization of sleep and napping behaviors. This hypothesis needs to be tested in well-designed controlled intervention studies.
Strengths of this study included objective as well as subjective measures of sleep and the longitudinal design. Along with its strengths, this study also had some limitations. Data were collected after surgery but before chemotherapy, so it is unknown if the relationship between HR-QOL and sleep also exists after diagnosis of cancer but before surgery. Data were obtained from women with breast cancer and from women with relatively higher education levels and higher household annual income, so conclusions cannot be extended to men, to patients with different socioeconomic status, or to women with different cancers. Although data generated by two types of actigraph were highly correlated in our validation study (data not reported), two types of actigraphs from different manufacturers may still potentially affect the results. Even with great effort to acquire diagnosis and treatment information for all participants, some data were still missing for a portion of women, mostly from Study 2.
In summary, this study revealed that HR-QOL was low and sleep quality was poor in breast cancer patients prior to chemotherapy, that HR-QOL remained unchanged but continued to be low after chemotherapy while sleep quality got worse. This decreased HR-QOL was significantly associated with both subjectively-reported poor sleep quality and objectively-measured nap time, and changes in one were associated with changes in the other. These data suggest that clinicians need to pay more attention to sleep in women with breast cancer undergoing treatment as it is possible that improving sleep may also improve quality of life. Studies are needed to examine if improving sleep quality and maladaptive sleep behaviors using intervention strategies such as CBT-I, might also improve HR-QOL in cancer patients. Intervention studies with well-chosen comparisons are needed and should be initiated early in the course of cancer treatments. Long-term follow-ups after the completion of treatment are especially warranted.
Acknowledgements
This study was supported by NCI CA112035, UL1RR031980, NIH M01 RR00827, and the UCSD Stein Institute for Research on Aging and the Department of Veterans Affairs Center of Excellence for Stress and Mental Health (CESAMH).
References
- Ancoli-Israel S, Cole R, Alessi CA, Chambers M, Moorcroft WH, Pollak C. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26:342–392. doi: 10.1093/sleep/26.3.342. [DOI] [PubMed] [Google Scholar]
- Ancoli-Israel S, Liu L, Marler M, Parker BA, Jones V, Robins Sadler G, et al. Fatigue, sleep and circadian rhythms prior to chemotherapy for breast cancer. Support. Care Cancer. 2006;14:201–209. doi: 10.1007/s00520-005-0861-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck SL, Schwartz AL, Towsley G, Dudley W, Barsevick A. Psychometric evaluation of the Pittsburgh Sleep Quality Index in cancer patients. J Pain Symptom Manage. 2004;27:140–148. doi: 10.1016/j.jpainsymman.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Berger AM, Sankaranarayanan J, Watanabe-Galloway S. Current methodological approaches to the study of sleep disturbances and quality of life in adults with cancer: a systematic review. Psychooncology. 2007;16:401–420. doi: 10.1002/pon.1079. [DOI] [PubMed] [Google Scholar]
- Bower JE, Ganz PA, Desmond KA, Rowland JH, Meyerowitz BE, Belin TR. Fatigue in breast cancer survivors: occurrence, correlates, and impact on quality of life. J. Clin. Oncol. 2000;18:743–753. doi: 10.1200/JCO.2000.18.4.743. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CFI, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CRI, Monk TH, Hoch CC, Yeager AL, Kupfer DJ. Quantification of subjective sleep quality in healthy elderly men and women using the Pittsburgh Sleep Quality Index (PSQI) Sleep. 1991;14(4):331–338. [PubMed] [Google Scholar]
- Carpenter JS, Andrykowski MA. Psychometric evaluation of the Pittsburgh Sleep Quality Index. J Psychosom Res. 1998;45:5–13. doi: 10.1016/s0022-3999(97)00298-5. [DOI] [PubMed] [Google Scholar]
- Coons SJ, Keininger DL, Hays RD. A comparative review of generic quality-of-life instruments. Pharmacoeconomics. 2000;17:13–35. doi: 10.2165/00019053-200017010-00002. [DOI] [PubMed] [Google Scholar]
- Curt GA. Impact of fatigue on quality of life in oncology patients. Semin. Hematol. 2000;37:14–17. doi: 10.1016/s0037-1963(00)90063-5. [DOI] [PubMed] [Google Scholar]
- Dhand R, Sohal H. Good sleep, bad sleep! The role of daytime naps in healthy adults. Curr. Opin. Pulm. Med. 2006;12:379–382. doi: 10.1097/01.mcp.0000245703.92311.d0. [DOI] [PubMed] [Google Scholar]
- Diggle PJ, Liang KY, Zeger SL. Analysis of Longitudinal Data. New York, NY: Oxford University Press; 1994. [Google Scholar]
- Dodd MJ, Cho MH, Cooper BA, Miaskowski C. The effect of symptom clusters on functional status and quality of life in women with breast cancer. Eur J Oncol. Nurs. 2010;14:101–110. doi: 10.1016/j.ejon.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efficace F, Bottomley A, Coens C, Van Steen K, Conroy T, Schoffski P, et al. Does a patient’s self-reported health-related quality of life predict survival beyond key biomedical data in advanced colorectal cancer? Eur J Cancer. 2006;43:42–49. doi: 10.1016/j.ejca.2005.07.025. [DOI] [PubMed] [Google Scholar]
- Eyigor S, Eyigor C, Uslu R. Assessment of pain, fatigue, sleep and quality of life (QoL) in elderly hospitalized cancer patients. Arch Gerontol. Geriatr. 2010;51:e57–e61. doi: 10.1016/j.archger.2009.11.018. [DOI] [PubMed] [Google Scholar]
- Frick E, Tyroller M, Panzer M. Anxiety, depression and quality of life of cancer patients undergoing radiation therapy: a cross-sectional study in a community hospital outpatient centre. Eur J Cancer Care (Engl) 2007;16:130–136. doi: 10.1111/j.1365-2354.2006.00720.x. [DOI] [PubMed] [Google Scholar]
- Gooneratne NS, Dean GE, Rogers AE, Nkwuo JE, Coyne JC, Kaiser LR. Sleep and quality of life in long-term lung cancer survivors. Lung Cancer. 2007;58:403–410. doi: 10.1016/j.lungcan.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyatt G, Feeny D, Patrick D. Measuring health-related quality of life. Ann Intern Med. 1993;118:622–629. doi: 10.7326/0003-4819-118-8-199304150-00009. [DOI] [PubMed] [Google Scholar]
- Karvonen-Gutierrez CA, Ronis DL, Fowler KE, Terrell JE, Gruber SB, Duffy SA. Quality of life scores predict survival among patients with head and neck cancer. J Clin Oncol. 2008;26:2754–2760. doi: 10.1200/JCO.2007.12.9510. [DOI] [PubMed] [Google Scholar]
- Le Guen Y, Gagnadoux F, Hureaux J, Jeanfaivre T, Meslier N, Racineux JL, et al. Sleep disturbances and impaired daytime functioning in outpatients with newly diagnosed lung cancer. Lung Cancer. 2007;58:139–143. doi: 10.1016/j.lungcan.2007.05.021. [DOI] [PubMed] [Google Scholar]
- Lichstein KL, Stone KC, Donaldson J, Nau SD, Soeffing JP, Murray D, et al. Actigraphy validation with insomnia. Sleep. 2006;29:232–239. [PubMed] [Google Scholar]
- Lis CG, Gupta D, Grutsch JF. The relationship between insomnia and patient satisfaction with quality of life in cancer. Support. Care Cancer. 2008;16:261–266. doi: 10.1007/s00520-007-0314-z. [DOI] [PubMed] [Google Scholar]
- Liu L, Marler M, Parker BA, Jones V, Johnson S, Cohen-Zion M, et al. The relationship between fatigue and light exposure during chemotherapy. Support. Care Cancer. 2005;13:1010–1017. doi: 10.1007/s00520-005-0824-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Rissling M, Natajaran L, Fiorentino L, Mills PJ, Dimsdale JE, et al. The Longitudinal Relationship between Fatigue and Sleep in Breast Cancer Patients Undergoing Chemotherapy. Sleep. doi: 10.5665/sleep.1630. (in press). http://www.journalsleep.org/AcceptedPapers/SP-170-11.pdf. [DOI] [PMC free article] [PubMed]
- Maione P, Perrone F, Gallo C, Manzione L, Piantedosi F, Barbera S, et al. Pretreatment quality of life and functional status assessment significantly predict survival of elderly patients with advanced non-small-cell lung cancer receiving chemotherapy: a prognostic analysis of the multicenter Italian lung cancer in the elderly study. J Clin Oncol. 2009;23:6865–6872. doi: 10.1200/JCO.2005.02.527. [DOI] [PubMed] [Google Scholar]
- Miaskowski C, Cooper BA, Paul SM, Dodd M, Lee K, Aouizerat BE, et al. Subgroups of patients with cancer with different symptom experiences and quality-of-life outcomes: a cluster analysis. Oncol Nurs Forum. 2006;33:E79–E89. doi: 10.1188/06.ONF.E79-E89. [DOI] [PubMed] [Google Scholar]
- Pockaj BA, Degnim AC, Boughey JC, Gray RJ, McLaughlin SA, Dueck AC, et al. Quality of life after breast cancer surgery: What have we learned and where should we go next. J Surg. Oncol. 2009;99:447–455. doi: 10.1002/jso.21151. [DOI] [PubMed] [Google Scholar]
- Pud D, Ben Am S, Cooper BA, Aouizerat BE, Cohen D, Radiano R, et al. The symptom experience of oncology outpatients has a different impact on quality-of-life outcomes. J Pain Symptom Manage. 2008;35:162–170. doi: 10.1016/j.jpainsymman.2007.03.010. [DOI] [PubMed] [Google Scholar]
- Redeker NS, Lev EL, Ruggiero J. Insomnia, fatigue, anxiety, depression, and quality of life of cancer patients undergoing chemotherapy. Sch Inq. Nurs. Pract. 2000;14:275–290. [PubMed] [Google Scholar]
- Sandadi S, Frasure HE, Broderick MJ, Waggoner SE, Miller JA, von GV. The effect of sleep disturbance on quality of life in women with ovarian cancer. Gynecol. Oncol. 2011 Aug 17; doi: 10.1016/j.ygyno.2011.07.028. 2011, [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Siddiqui F, Kachnic LA, Movsas B. Quality-of-life outcomes in oncology. Hematol Oncol Clin North Am. 2006;20:165–185. doi: 10.1016/j.hoc.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Soni MK, Cella D. Quality of life and symptom measures in oncology: an overview. Am J Manag Care. 2002;8:S560–S573. [PubMed] [Google Scholar]
- Soules MR, Sherman S, Parrott E, Rebar R, Santoro N, Utian W, et al. Executive summary: Stages of Reproductive Aging Workshop (STRAW) Park City, Utah, July, 2001. Menopause. 2001;8:402–407. doi: 10.1097/00042192-200111000-00004. [DOI] [PubMed] [Google Scholar]
- Trentham-Dietz A, Sprague BL, Klein R, Klein BE, Cruickshanks KJ, Fryback DG, et al. Health-related quality of life before and after a breast cancer diagnosis. Breast Cancer Res Treat. 2008;109:379–387. doi: 10.1007/s10549-007-9653-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser MRM, Smets EMA. Fatigue, depression and quality of life in cancer patients: how are they related? Support. Care Cancer. 1998;6:101–108. doi: 10.1007/s005200050142. [DOI] [PubMed] [Google Scholar]
- Ware JE, Kosinski M. SF-36 Physical & Mental Health Summary Scales: A Manual for User's of Version 1. Lincoln, RI: QualityMetric Incorporated; 2002. [Google Scholar]
- Ware JE, Kosinski M, Gandek B. SF-36 Health Survey: Manual & Interpretation Guide. Lincoln, RI: QualityMetric Incorporated; 2002. [Google Scholar]