Abstract
Butyrylcholinesterase (BChE) is the leading pretreatment candidate against exposure to organophosphates (OPs), which pose an ever increasing public and military health. Since respiratory failure is the primary cause of death following acute OP poisoning, an inhaled BChE therapeutic could prove highly efficacious in preventing acute toxicity as well as the associated delayed neuropathy. To address this, studies have been performed in mice and macaques using Chinese Hamster Ovary cells (CHO)-derived recombinant (r) BChE delivered by the pulmonary route, to examine whether the deposition of both macaque (Ma) and human (Hu) rBChE administered as aerosols (aer) favored the creation and retention of an efficient protective “pulmonary bioshield” that could scavenge incoming (inhaled) OPs in situ thereby preventing entry into the circulation and inhibition of plasma BChE and AChE on red blood cells (RBC-AChE) and in cholinergic synapses. In contrast to parenteral delivery of rBChE, which currently requires posttranslational modification for good plasma stability, an unmodified aer-rBChE pretreatment given 1–40 hr prior to >1 LD50 of aer-paraoxon (Px) was able to prevent inhibition of circulating cholinesterase in a dose-dependent manner. These studies are the first to show protection by rBChE against a pesticide such as paraoxon when delivered directly into the lung and bode well for the use of a non-invasive and consumer friendly method of rHuBChE delivery as a human treatment to counteract OP toxicity.
Keywords: butyrylcholinesterase, aerosol delivery, paraoxon, protection, monkey model
1. Introduction
Organophosphates (OPs) such as nerve agents and pesticides cause toxicity by inhibiting the activity of acetylcholinesterase (AChE) in neuromuscular junctions (1–2). Because of this critical targeting of cholinesterases, any efficacious therapeutic candidate for preventing/treating OP poisoning will be a molecule that can bind and competitively scavenge OPs before they reach their physiological target. Plasma-derived BChE currently represents the most advanced pretreatment for protection against OP exposure (3–5) which could potentially take the form of threats to military personnel and first responders, agricultural workers and potentially civilians in the case of deliberate contamination of the environment and critical water supplies. The efficacy of BChE prophylaxis, in terms of survivability and prevention of cognitive impairment, has been clearly demonstrated in rodents and macaques against multiple LD50s of nerve agents (3,5,6). However because of limited availability and the cost of plasma-derived HuBChE, focus has switched to the development of a recombinant (r) BChE counter-measure. To date, rBChE has been successfully produced in goat milk, mammalian cells and plants (7–9). However, all forms exhibit poor plasma stability presumably due to host cell-specific glycosylation (10) and must undergo post-translational modification to increase their circulatory retention times following parenteral i.m. (intramuscular), s.c. (subcutaneous) and i.v. (intravenous) delivery (7–9). In this context, while PEG-ylation of rBChE molecules improves their pharmacokinetic (PK) parameters e.g. AUC, Cmax and MRT similar to that of the plasma-derived form (10), it also increases the cost and may limit delivery options.
Another challenge to optimal delivery results from the 1:1 stoichiometry between enzyme and OP which necessitates a large rBChE pretreatment dose that must be efficiently transported into the blood and circulate at high concentrations sufficient to scavenge OPs to a level below their median lethal dose within one blood-circulation-time to prevent toxicity (11). In previous animal PK and protection studies in non-human primates, minipigs and guinea pigs (GP)(5,6,12), plasma-derived BChE pretreatments of 8.5–30 mg/kg have usually been administered i.m. with reproducible results. However, im and sc injections into macaques of large tetrameric PEG-rBChE molecules, resulted in variable T max following i.m. injections while Cmax poorly correlated with dose following s.c. injections (13) due to delayed transport from the injection site.
To optimize the scavenging efficacy of large rBChE molecules, an alternate delivery approach has been developed using an aerosolized form of rBChE (aer-rBChE). This takes advantage of the fact that (i) inhalation of vapours and particles is the predominant form of exposure to insecticides and G-type nerve agents and serves as a major means of intoxication because of rapid accesses of the OP to the blood; (ii) inhaled rBChE molecules will be retained in the lung; being too large to transit the lung endothelium; and (iii) levels of rBChE can be easily maintained in the lungs in a user-friendly way by maintenance “puffs”. Thus, in this scenario, BChE, delivered as an aerosol could coat the airways of the lungs forming a “pulmomary bioshield” that can scavenge incoming (inhaled) OPs in situ thereby preventing both their entry into the systemic circulation and their inhibition of RBC-AChE and plasma BChE.
The efficacy of antidotes against OP poisoning cannot be investigated in humans for ethical reasons and will most likely be approved using the Animal rule. To examine the efficacy of pulmonary delivery, mice and macaques were administered different doses of aerosolized PEG-rMaBChE and rHuBChE at 1–40 hr prior to 1–2 LD50 aer-paraoxon (Px) and blood cholinersterase activity was monitored at various times thereafter as a measure of protection. The results demonstrate good dose-dependent protection against aer-Px toxicity by both PEG-ylated and unmodified forms of rHuBChE and rMaBChE without immunogenicity.
2. Methods and Materials
Animal studies were conducted in compliance with the Animal Welfare Act and other federal statutes and regulations stated in the Guide for Guide for the Care and Use of Laboratory Animals (NRC Publication, 1996). Procedures with animals received prior approval by Institutional Animal Care and Use Committees at Bioqual (mice studies) and the Johns Hopkins University School of Medicine (macaque studies) and were performed at Bioqual, MD and at the JHU Research Animal Resources facilities MD, both of which are fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care, International.
2.1. Chemicals
Paraoxon (99.1% pure) was obtained from ChemService Inc. (West Chester, PA), diluted in sterile water to obtain a stock solution of 1mg/ml and maintained at RT protected from light. Inoculation doses were prepared 12 to 24 hours prior to use in 1ml in PBS pH 6.8. Handling of Px and loading of syringes were performed in a biological safety cabinet.
2.2 Production and purification of rMaBChE and rHuBChE
CHO-derived tetrameric rMaBChE (25mg/kg) and rHuBChE (>80mg/kg) were produced in CHO-K1 cells were purified using procainamide sepharose chromatography as described (9) and stored at 4°C or in a lyophilized form at −20°C. The purified enzyme exhibits good stability at RT.
Assays for plasma BChE and RBC-AChE activity were performed as described previously (9) using a modified Ellman assay (14). Briefly, whole blood samples (20 ul) from mice and macaques were first diluted in 180 ul of water (1/10 diln) and tested for plasma BChE and AChE activity. In the latter assay, 20uM ethopropazine was used as a BChE-specific inhibitor and 1mM ATC (SigmaAldrich) as substrate. The specific activity of MaBChE (900 U/mg) is hig h-er than HuBChE (700 U/mg). Background levels of mice ranged from 0.515–1.528U/ml (AChE) and 1.034–1.784 (BChE) and in macaques from 2.5–6.5 U/ml (AChE) and 3.7–8.2 (BChE).
2.3 Efficacy of aerosolized BChE in mice
Mice were anesthetized by i.p. injection of ketamine (100ug/g) and xylazine (16ug/g) and suspended from their upper incisors at a 45° incline. Fifty μl aliquots containing known doses of PEG-rMaBChE or Px diluted in PBS were introduced into the distal part of the oropharynx by a pipette (15). With the nose gently clamped closed with forceps, the mouse aspirated the liquid, which was dispersed as an aerosol and deposited in the lungs. Ten BALB/c mice/group (Jackson Labs, ME) received different doses of aer-Px to determine which caused ~50% inhibition of RBC-AChE. PEG-rMaBChE (100 or 200U) was administered by aspiration 24 hr prior to Px to assess protection. Mice were tail bled before and 0.5, 1, 2, 6, 24, 48 and 72 hr after administration of Px.
2.4. Delivery of liquid aerosols into monkeys by microsprayer
The method used was similar to that described (16). Rhesus macaques were initially given ketamine hydrochloride (10–15 mg/kg i.m.) and moved to the surgery where an endotracheal tube was inserted while the animal was maintained under anesthesia with isoflurane/oxygen. EKG leads and a pulse-oximeter sensor were attached to allow monitoring of heart rate and rhythm; respiratory rate and blood oxygen saturation. Since the animals were too small to permit placement of an endotracheal tube of sufficient size (4 mm ID) for passage of a 3 mm OD pediatric bronchoscope, the procedure was modified to allow use of a rigid microsprayer (Model 1A–1B, Penn Century Inc., PA). The IA–1B MicroSprayer® delivers a plume of aerosol (MMD of 25–30 μm) in a closed system and utilizes a 19-gauge, stainless steel tube and is operated by a specially made 1-ml plastic syringe. In early studies a customized version with a large 30 ml reservoir was used. Prior to endotracheal tube insertion, the rigid microsprayer was with a piece of tape around the proximal shaft of the microsprayer to permit its passage down the inserted ET tube to a point which allow optimal aerosolization of the material. Animals were restrained in dorsal recumbency with the head slightly higher than the tail and hot water heating pads beneath in conjunction with a “Baer” hugger warm air blanket, to maintain normal body temperature. Deposition of the aer-rMaBChE following delivery by microsprayer was examined using a dose of 9 mg/kg rMaBChE admixed with the radioisotope 99m technetium (Tc) and scanned using gamma scintigraphy (16).
2.5. Efficacy of aer- PEG-rBChE and aer-rBChE in monkeys
For the macaque studies, Px was delivered by microsprayer in one ml of PBS at RT. After inoculation, the microsprayer was rinsed with 0.1N of NaOH, followed by 70% alcohol and distilled water. Residues were mixed with 0.1N NaOH and autoclaved. For protection studies, PEG-rMaBChE and unmodified forms of rMaBChE and rHuBChE at various doses were administered by microsprayer 1–40 hr prior to aerPx. For high doses multiple syringes were used. Sixteen monkeys (2/group), ranging in weight from 2.7 to 5.6 kg have been studied. Before enzyme and aer-Px and at 0.5, 1, 2, 4, 8, 24, 48, 72 hr after aer-Px administration, 20ul blood samples from the peripheral vein were diluted in 180 ul water and kept at 4°C until assayed for AChE and BChE activity.
2. RESULTS
3.1. Determination of aer-Px dose required to inhibit ~50% of RBC-AChE in mice
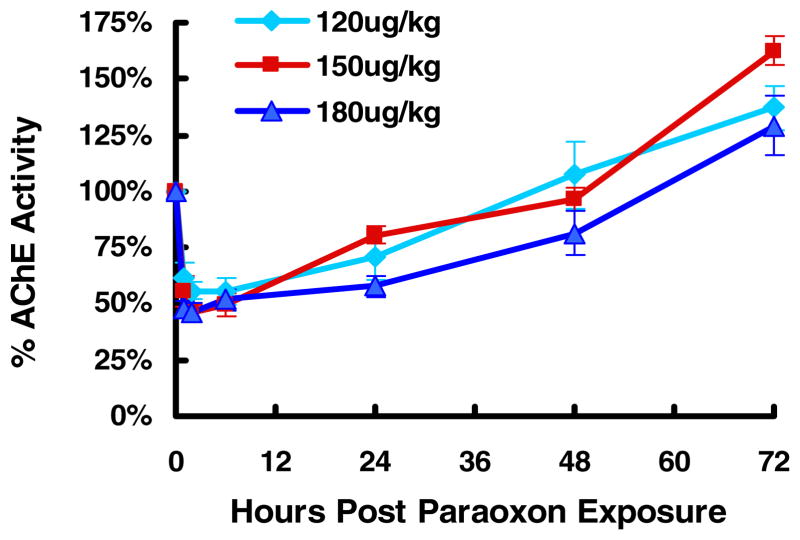
The LD50 of Px in mice is 760 ug/kg orally and 270 – 800 ug/kg sc (17) although no information is available for inhalation exposure. In rat studies, a single acute sc injection of 0.09, 0.12, or 0.19 mg/kg Px, representing 40%, 52% and 83% LD50 respectively, did not produce signs of cholinergic hyperactivity and less than 20% AChE in the brain or diaphragm (18). Based on these results, a dose of 200ug/kg of aer-Px was initially used to assess inhibition kinetics of RBC-AChE and plasma BChE in mice. Since this dose resulted in 7/10 deaths, doses of 120 ug/kg, 150 ug/kg, 180 ug/kg were examined and revealed that 150–180 ug/kg achieved the required ~50% inhibition of AChE without inducing respiratory failure (Fig. 1). At these aer-Px levels, AChE inhibition in mice were always higher (peak 54%) than those observed with BChE (20–30% at the same dose (not shown). In addition, recovery of the AChE levels usually exhibited a positive feedback reaching 125–160 % of baseline at 72 hr; an effect which may be anesthesia related (19).
Figure 1.
Percentage inhibition of AChE at the indicated doses of aer-Px in mice.
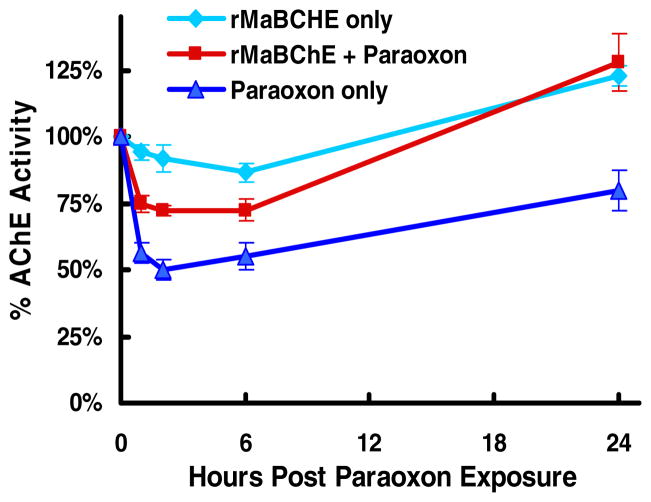
Protection studies were performed to assess the ability of an aer-PEG-rMaBChE pretreatment to scavenge aer-Px in situ in the lung and prevent inhibition of circulating RBC-AChE and plasma BChE. To achieve this, mice were administered aer-PEG-rMaBChE at 10 mg/kg, 6.7mg/kg and 3.3 mg/kg of enzyme 24 hr before 180ug/kg of aer-PX and assessed for blood cholinesterase activity. Fig. 2 indicates that the 10mg/kg (180U) dose resulted in a 24% peak AChE inhibition, which quickly recovered to background levels by 24hr, compared to a 50% inhibition with aer-Px alone; representing 50% protection. Doses of 6.7 and 3.3 mg/kg aer-BChE resulted in a linear relationship between percent protection and dose (not shown). The ability of PEG-rBChE to protect circulating BChE was also dose dependent but less clear because of the low inhibition of BChE by Px in mice.
Figure 2.
Ability of aer-PEG-rBChE to protect against aer-Px given 24 hr later. (A) AChE activity in mice given 10 mg/kg of aer-PEG-rMaBChE prior to aer-Px (180 ug/kg), aer-PEG-rMaBChE only or Px only.
3.3. Efficacy of aer-rMaBChE to prevent AChE and BChE inhibition by aer-Px in macaques
In monkeys, 15 ug/kg of aer-Px caused peak inhibition of AChE (63.3%) and BChE (65%) one hr after delivery and was used as the challenge dose in all macaque protection studies; 12 ug/kg of aer-Px giving 47% inhibition. No loss in rBChE activity was observed following delivery using a microsprayer (not shown). To assess protection, macaques received increasing doses of aer-PEG-rMaBChE, ranging from 4.8 mg/kg to 7.8 mg/kg one hr prior to 15 ug/kg aer-Px and monitored for AChE and BChE activity in the blood. Fig. 3 indicates that AChE and BChE were both inhibited equally by aer-Px, with increasing pretreatment doses resulting in similar protection of each enzyme and a linear relationship between the level of protection and dose of the aer-BChE pretreatment (Fig. 4). To study whether modification of rBChE was required for retention in the lungs, two monkeys were administered unmodified rMaBChE at 9 mg/kg which result in almost 100% AChE activity (upper blue lines)(Fig. 3) indicating that, unlike parenteral delivery, PEGylation is not required for lung delivery. In all protection studies, a small transient inhibition was observed early following aer-Px and is under investigation.
Figure 3.
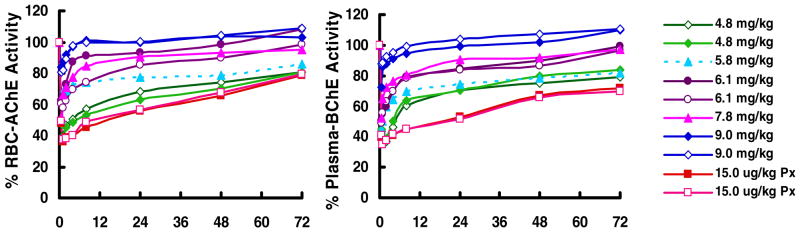
Protection by aer-PEG-rMaBChE (4.8–7.8mg/kg) and rMaBChE (9mg/kg) against aer-Px (15ug/kg) given one hour later, as measured by RBC-AChE activity (left) and plasma BChE activity (right). Inhibition by aer-Px alone (peak 63% at 1 hr) is shown by the lower, dashed orange lines.
Figure 4.
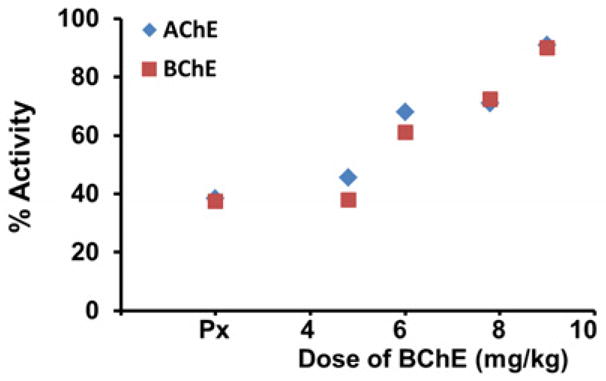
Dose response relationship between aer- rMaBChE and protection in macaques. (Px: no BChE)
3.4. Lung distribution of aer-rMaBchE using gamma scintigraphy
Aerosol particle size, aerosol velocity, inspiratory flow rate and the nature of the airways are major determinants of aerosol deposition in lungs. To examine the deposition of rBChE following delivery by microsprayer, 9 mg/kg rMaBChE was admixed with 99mtechnetium and delivered to the lung with a microsprayer (16). The results demonstrate that, although microsprayer particles are large (~26um), the generated aerosol coated the surfaces of large and small airways of both lungs (Fig. 5); this generalized distribution being consistent with the good protection observed. Dark areas within the lungs are assumed to represent high concentrations of the radioisotope in the larger conducting airways.
Figure 5.

Example of distribution of rMaBChE (9 mg/kg) admixed with 99mtechnetium in lungs of one of the rhesus macaques that was studied using a microsprayer.
3.4. Efficacy of aer-rHuBChE to prevent AChE and BChE inhibition by Px in macaques
Once it was established that (i) rMaBChE was not immunogenic in homologous macaques (ii) a good dose response relationship existed and (iii) PEGylation was not required for protection (Fig. 3), CHO-derived aer-rHuBChE (8.5–9 mg/kg) was tested both for its ability to protect against aer-Px and the duration of the protection. Similar to aer-rMaBChE, unmodified aer-rHuBChE at 8.5–9 mg/kg given 1,16, 24 and 40 hr prior to 15 ug/kg of Px almost fully protected with only small changes in inhibition as the time between pretreatment and OP increased (Fig. 6).
Figure 6.
Protection by prophylactic aer-rHuBChE (8–10 mg/kg) against aer-Px (15ug/kg) given 1, 16, 24 and 40 hr later, as measured by RBC-AChE and plasma BChE activity. Inhibition by aer-Px alone (peak 63% at 1 hr) is shown by the lower, dashed orange lines.
4. DISCUSSION
Although exposure to OPs can occur by ingestion or skin absorption, inhalation of airborne powders, droplets or vapours serves as the major means of intoxication because of the rapid access of OPs to the blood. Respiratory exposure may be increased with mist- or fog-size particles when high pressure, ultra-low volume application or fogging equipment is used. An efficient pre-exposure rBChE pulmonary therapeutic could be delivered rapidly before known OP release to prevent both acute toxicity and delayed neurological symptoms. Other formulations intended to remain in the lung following delivery include measles vaccines to induce local mucosal immunity, and gene therapy vectors delivered to the lung in patients with cystic fibrosis (16).
In Initial protection studies in mice, an aer-PEG-rMaBChE pretreatment 24 hr before aer-Px reduced AChE inhibition 50% compared with aer-Px alone. Similar studies in larger monkeys using aer-rMaBChE (both PEG-ylated and unmodified) as pretreatments, clearly indicated that such a bioscavenger “pulmonary shield” was highly effective in neutralizing aer-Px preventing its egress into the blood and its inhibition of both AChE and BChE. These homologous macaque studies demonstrated very good correlation between protection and dose (Fig. 3) with no immunogenicity (not shown). Later macaque studies using rHuBChE focused on the efficacy of 8.5–9 mg/kg doses to protect as the period between pretreatment and aer-Px exposure was extended from 1hr to 40 hr and demonstrated that AChE and BChE activity was only slightly decreased with peak inhibition at 40hr still <20%. The observation that rBChE is well retained in the lung is consistent with both those in mice where an average of 30–40% of the aer-PEG-rMaBChE deposited in the lung still remained at 72hr (not shown) and with the long half-life of native BChE and PEG-rMaBChE (8–12 days) following parenteral injection (20–21).
Paraoxon is the active metabolite of the inactive parathion which has been responsible for many accidental poisoning and deaths (22) and was recently phased out of use in the US. It was chosen for these studies because it inhibits AChE, BChE and carboxylesterase (18), has a relatively low LD50 and is stabile in aqueous solution. The intensity and duration of symptoms of OP exposure are determined by the agent used and route of challenge. In this context, while previous animal protection studies have mostly administered OP challenges by sc injection to ensure administration of known doses, inhalation of vapours or particles is the predominant route of exposure to insecticides and G-type nerve agents and is therefore highly relevant. In the present studies, 150–180 ug/kg (mice) and 15 ug/kg (macaque) of aer-Px were used as challenge doses since these were shown to inhibit cholinesterase by ~50–60% without respiratory toxicity. The ~10-fold difference in dose required to elicit similar inhibition between mice (which also contain carboxylesterase) and macaques is consistent with the findings of Maxwell (23). While the exact LD50 of aer-Px is not known, 15ug/kg aer-Px in macaques resulted in 63% AChE inhibition respectively and is estimated to be >1.0 LD50. By comparison, 93% and 91% changes in AChE activity were observed in baboons 4 hr following 2 LD50 of soman and sarin vapour respectively (24) and 3.8–4.2 ug/kg of inhaled sarin in humans was required to inhibit RBC-AChE by 50% (25).
The unexpected transient AChE and BChE inhibition observed in the first hour following aer-Px was inversely related to protection and may be due to (i) transfer of Px on macaque RBC into the in vitro AChE assays and/or (ii) cholinesterase inhibition of due to the isofluorane anaesthesia.
In summary, current treatment of respiratory OP toxicity consists of prompt i.v. atropine and oximes. Inhaled rHuBChE will provide a noninvasive, user-friendly method of delivery for protection against chemical warfare agents, pesticide intoxication as well as cocaine overdose. Since 40% RBC-AChE inhibition produces no clinical symptoms (25,26), it is predicted that protection can be achieved with lower rBChE doses, at higher OP doses and for longer periods following pretreatment; consistent with 7.5 mg/kg shown to protect minipigs against lethal sarin vapour (13).
KAZAN MANUSCRIPT HIGHLIGHTS.
Recombinant human and macaque butyrylcholinesterase (rBChE) were produced
Aerosolized (aer) rBChE was tested for protection against aer-paraoxon (Px)
PEGylated and unmodified rBChE protected both protected equally
Protection lasted at least 40 hours.
Protection studies were performed in macaque and mouse models
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Millard CB, Kryger G, Ordentlich A, Greenblatt HM, Harel M, Raves ML, Segall Y, Barak D, Shafferman A, Silman I, Sussman JL. Crystal structures of aged phosphonylated acetylcholinesterase: nerve agent reaction products at the atomic level. Biochemistry. 1999;38:7032–7039. doi: 10.1021/bi982678l. [DOI] [PubMed] [Google Scholar]
- 2.Rosenberry TL, Mallender WD, Thomas PJ, Szegletes T. A steric blockade model for inhibition of acetylcholinesterase by peripheral site ligands and substrate. Chem Biol Interact. 1999:119–120. 85–97. doi: 10.1016/s0009-2797(99)00017-4. [DOI] [PubMed] [Google Scholar]
- 3.Doctor BP, Maxwell DM, Ashani Y, Saxena A, Gordon PK. In: New approaches to Medical Protection against Chemical Warfare Nerve Agents. Somani SM, Romano JA, editors. CRC Press; New York: 2001. pp. 191–214. [Google Scholar]
- 4.Lenz DE, Broomfield CA, Maxwell DM, Cerasoli DM. In: Nerve Agent Bioscavengers: Protection against High- and Low- Dose Organophosphorus Exposure. Somani SM, Romano JA, editors. CRC Press; New York: 2001. pp. 215–243. [Google Scholar]
- 5.Lenz DE, Maxwell DM, Koplovitz I, Clark CR, Capacio BR, Cerasoli DM, Federko JM, Luo C, Saxena A, Doctor BP, Olson C. Protection against soman or VX poisoning by human butyrylcholinesterase in guinea pigs and cynomolgus monkeys. Chem Biol Interact. 2005;157–158:205–210. doi: 10.1016/j.cbi.2005.10.025. [DOI] [PubMed] [Google Scholar]
- 6.Saxena A, Sun W, Fedorko JM, Koplovitz I, Doctor BP. Prophylaxis with human serum butyrylcholinesterase protects guinea pigs exposed to multiple lethal doses of soman or VX. Biochem Pharmacol. 2011;81:164–169. doi: 10.1016/j.bcp.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 7.Geyer BC, Kannan L, Garnaud PE, Broomfield CA, Cadieux CL, Cherni I, Hodgins SM, Kasten SA, Kelley K, Kilbourne J, Oliver ZP, Otto TC, Puffenberger I, Reeves TE, Robbins N, 2nd, Woods RR, Soreq H, Lenz DE, Cerasoli DM, Mor TS. Plant-derived human butyrylcholinesterase, but not an organophosphorous-compound hydrolyzing variant thereof, protects rodents against nerve agents. Proc Natl Acad Sci U S A. 2010;107:20251–20256. doi: 10.1073/pnas.1009021107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang YJ, Huang Y, Baldassarre H, Wang B, Lazaris A, Leduc M, Bilodeau AS, Bellemare A, Côté M, Herskovits P, Touati M, Turcotte C, Valeanu L, Lemée N, Wilgus H, Bégin I, Bhatia B, Rao K, Neveu N, Brochu E, Pierson J, Hockley DK, Cerasoli DM, Lenz D, Karatzas CN, Langermann S. Recombinant human butyrylcholinesterase from milk of transgenic animals to protect against organophosphate poisoning. Proc Natl Acad Sci USA. 2007;104:13603–13608. doi: 10.1073/pnas.0702756104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosenberg YJ, Saxena A, Sun W, Jiang X, Chilukuri N, Luo C, Doctor BP, Lee KD. Demonstration of in vivo stability and lack of immunogenicity of a polyethyleneglycol-conjugated recombinant CHO-derived butyrylcholinesterase bioscavenger using a homologous macaque model. Chem Biol Interact. 2010;187:279–286. doi: 10.1016/j.cbi.2010.02.042. [DOI] [PubMed] [Google Scholar]
- 10.Cohen, Kronman C, Chitlaru T, Ordentlich A, Velan B, Shafferman A. Effect of chemical modification of recombinant human acetylcholinesterase by polyethylene glycol on its circulatory longevity. Biochem J. 2001;357:795–802. doi: 10.1042/0264-6021:3570795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ashani Y, Pistinner S. Estimation of the upper limit of human butyrylcholinesterase dose required for protection against organophosphates toxicity: a mathematically based toxicokinetic model. Toxicol Sci. 2004;77:358–67. doi: 10.1093/toxsci/kfh012. [DOI] [PubMed] [Google Scholar]
- 12.Saxena A, Sun W, Fedorko JM, Koplovitz I, Doctor BP. Pretreatment with human serum butyrylcholinesterase alone prevents cardiac abnormalities, seizures, and death in Göttingen minipigs exposed to sarin vapor. Biochem Pharmacol. 2011;82:1984–1993. doi: 10.1016/j.bcp.2011.09.019. [DOI] [PubMed] [Google Scholar]
- 13.Rosenberg YJ, Jiang X, Mao L, Hernandez-Abanto S, Lee K. Development of a prophylactic butyrylcholinesterase bioscavenger to protect against insecticide toxicity using a homologous macaque model. In: Soloneski S, Larramendy M, editors. Insecticides Basic and Others Applications. InTech Press; Croatia: 2012. pp. 79–100. [Google Scholar]
- 14.Ellman GL, Ellman KD, Courtney V, Andres, Feather-Stone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 15.McGrath-Morrow S, Laube B, Tzou SC, Cho C, Cleary J, Kimura H, Rose NR, Caturegli P. IL-12 overexpression in mice as a model for Sjögren lung disease. Am J Physiol Lung Cell Mol Physiol. 2006;291:837–846. doi: 10.1152/ajplung.00134.2006. [DOI] [PubMed] [Google Scholar]
- 16.Beck SE, Laube BL, Barberena CI, Fischer AC, Adams RJ, Chesnut K, Flotte TR, Guggino WB. Deposition and expression of aerosolized rAAV vectors in the lungs of Rhesus macaques. Mol Ther. 2002;4:546–554. doi: 10.1006/mthe.2002.0698. [DOI] [PubMed] [Google Scholar]
- 17.Levine ES. Prepared for the US Army Medical Research Institute of Chemical Defense under Contract No GS-23F-8006H. 2006. Nerve Agent Simulants: Can They Be Used as Substitutes for Nerve Agents in biomedical Research? [Google Scholar]
- 18.Milatovic D, Dettbarn WD. Modification of acetylcholinesterase during adaptation to chronic, subacute paraoxon application in rat. Toxicol Appl Pharmacol. 1996;136:20–28. doi: 10.1006/taap.1996.0003. [DOI] [PubMed] [Google Scholar]
- 19.Dorandeu F, Foquin A, Briot R, Delacour C, Denis J, Alonso A, Froment MT, Renault F, Lallement G, Masson P. An unexpected plasma cholinesterase activity rebound after challenge with a high dose of the nerve agent VX. Toxicology. 2008;248:151–157. doi: 10.1016/j.tox.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 20.Jenkins T, Balinsky D, Patient DW. Cholinesterase in plasma: first reported absence in the Bantu; half-life determination. Science. 1967;156:1748–1750. doi: 10.1126/science.156.3783.1748. [DOI] [PubMed] [Google Scholar]
- 21.Rosenberg, Luo C, Ashani Y, Doctor BP, Fischer R, Wolfe G, Saxena A. Pharmacokinetics and immunologic consequences of exposing macaques to purified homologous butyrylcholinesterase. Life Sci. 2002;72:125–134. doi: 10.1016/s0024-3205(02)02203-8. [DOI] [PubMed] [Google Scholar]
- 22.Lotti M, Moretto A. Organophosphate-induced delayed polyneuropathy. Toxicol Rev. 2005;24:37–49. doi: 10.2165/00139709-200524010-00003. [DOI] [PubMed] [Google Scholar]
- 23.Maxwell DM, Brecht KM, Koplovitz I, Sweeney RE. Acetylcholinesterase inhibition: does it explain the toxicity of organophosphorus compounds? Arch Toxicol. 2006;80:756–760. doi: 10.1007/s00204-006-0120-2. [DOI] [PubMed] [Google Scholar]
- 24.Anzueto RA, deLemos, Seidenfeld J, Moore G, Hamil H, Johnson D, Jenkinson SG. Acute inhalation toxicity of soman and sarin in baboons. Fundam Appl Toxicol. 1990;14:676–687. doi: 10.1016/0272-0590(90)90293-s. [DOI] [PubMed] [Google Scholar]
- 25.Oberst FW, Crook JW, Christensen MK, Cresthull P, Koon WS, Freeman G. Chemical Corps Research and Development Command, DTIC AD226805. Army Chemical Center Md; 1959. Inhaled GB retention studies in man at rest and during activity. [Google Scholar]
- 26.Freeman S. Rapid treatment of mass casualties. J R Army Med Corps. 1952;98:345–348. [PubMed] [Google Scholar]