Abstract
The transcription factor NF-E2-related factor 2 (Nrf2) mediates transcription of antioxidant/cytoprotective genes by binding to the antioxidant response element (ARE) within DNA. Upregulation of these genes constitutes a pleiotropic cytoprotective-defense pathway which has been shown to produce neuroprotection in numerous models by decreasing lipid peroxidation (LP) as measured by the neurotoxic LP by-product 4-hyrdoxynonenal (4-HNE). As neuronal mitochondria have previously been shown to be susceptible to insult-induced LP-mediated oxidative damage, we sought to mechanistically investigate whether Nrf2-ARE activation in vivo could protect mitochondria from subsequent 4-HNE exposure ex vivo. Young adult male CF-1 mice were administered one of two known Nrf2-ARE activators as single I.P. doses – sulforaphane (SFP; 5.0 mg/kg) or carnosic acid (CA; 1.0mg/kg) – or their respective vehicles 48 hours prior to Ficoll isolation of rat cerebral cortical mitochondria. Purified mitochondria were then exposed ex vivo to 4-HNE for 15 minutes at 37°C which we showed to cause a concentration-related inhibition of mitochondrial respiration together with covalent binding of 4-HNE to mitochondrial proteins. We chose a 30 μM concentration of 4-HNE, which produced an approximate 50% inhibition of complex I or complex II-driven respiration, to assess whether prior in vivo the Nrf2-ARE activating compounds would increase the resistance of the isolated cortical mitochondria to 4-HNE's mito-toxic effects. Administration of either compound significantly increased (p< 0.05) expression of heme oxygenase-1 mRNA in cortical tissue 48 hours post-administration, verifying that both compounds were capable of inducing the Nrf2-ARE pathway. Moreover, the prior in vivo administration of sulforaphane (SFP) and carnosic acid (CA) significantly (p< 0.05) attenuated 4-HNE-induced inhibition of mitochondrial respiration for complex I while only carnosic acid acted to protect complex II. Furthermore, both CA and SFP significantly (p< 0.05) reduced the amount of 4-HNE bound to mitochondria proteins as determined by Western blot. These results demonstrate the capability of Nrf2-ARE induction in vivo to protect from 4-HNE toxicity to cortical mitochondria ex vivo. Ongoing studies will determine the therapeutic efficacy of Nrf2-ARE activators to attenuate traumatic brain injury induced pathophysiology.
Keywords: Nrf2, mitochondria, oxidative damage, lipid peroxidation, 4-hydroxy-2-nonenal
Introduction
Oxidative stress, and especially lipid peroxidation, is a deleterious component of many neurodegenerative disorders, often causing harmful downstream consequences that can result in cell death and dysfunction [1-3]. More specifically, previous work has demonstrated that free radical mediated lipid peroxidation (LP) plays a critical role in the acute pathophysiology of traumatic brain injury (TBI) [3, 4]. Lipid peroxidation involves the oxidation of polyunsaturated fatty acids (e.g., arachidonic, linoleic, and docosahexaenoic acids) in cells or membrane phospholipids at allylic carbons. Peroxidized polyunsaturated fatty acids subsequently undergo phospholipase-mediated hydrolysis and disruption of the membrane phospholipid architecture, and eventual loss of proper functioning phospholipid-dependent enzymes, ion channels, and structural proteins. However, as consequence to LP-induced membrane damage, peroxidized fatty acids eventually lead to aldehydic breakdown products, including 4-hydroxy-2-nonenal (4-HNE). The aldehyde 4-HNE is highly reactive with many cellular proteins, primarily via Schiff base and Michael adduct reactions with basic (e.g., lysine and histidine) and sulfhydryl (e.g. cysteine) containing amino acids. These reactions are capable of impairing the function of a variety of cellular proteins, which contributes to neurodegenerative processes [4, 5]. Sources of post-TBI reactive oxygen species (ROS) that contribute to toxic LP production include iron-dependent Fenton reactions, which result in hydroxyl radical production and peroxynitrite (PON)-derived free radicals including •OH, •NO2, and •CO3 [6-10]. Free radical mediated oxidative damage in acute CNS injury can result in protein oxidation and mitochondrial dysfunction, largely due to intrinsic propensity for mitochondria to produce ROS as a byproduct of the electron transport chain function [11]. In fact, previous work by our laboratory [12] and others has demonstrated that one major source of post-injury free radical production is the increased ROS leakage from injured brain mitochondria after injury [13-16].
Furthermore, previous work from our laboratory has shown that PON is able to directly inhibit mitochondrial function in the injured brain mitochondria and is associated with elevated 4-HNE [17]. Moreover, direct application of PON to normal mitochondria simulates the effects of in vivo TBI [18]. While LP can directly cause membrane destruction and likely impair mitochondrial function, we recently demonstrated that the LP-derived reactive aldehydes 4-HNE and acrolein can directly inhibit mitochondrial respiration in vitro in isolated brain and spinal cord mitochondria [19]. This is likely due to 4-HNE binding to critical proteins affecting mitochondrial function.
One of the most heavily investigated aspects of neurodegenerative processes, oxidative stress involves an imbalance in the ratio of harmful reactive oxygen/nitrogen species (ROS/RNS) and protective endogenous antioxidant defense enzymes [3, 12, 20]. An endogenous cytoprotective defense system exists to combat the basal and injury-induced imbalance in ROS/RNS and antioxidant/defense enzymes. This system is primarily under the control of the pleiotropic transcription factor NF-E2-related factor 2 (Nrf2) [21]. Nrf2 has been identified as the key mediator of this inducible cytoprotective response via its interaction with the genomic cis-acting enhancer region of defense genes known as the antioxidant response element (ARE) [22, 23]. Under normal conditions, Nrf2 is sequestered in the cytoplasm by the repressor protein Keap1 [22]. This binding interaction between Nrf2 and Keap1 facilitates the proteasomal degradation of Nrf2 by recruitment of a Cul3 ubiquitin ligase via the BTB domain of Keap1 [24]. Only under conditions of stress (e.g. oxidative stress, ER stress, injury, toxicity, etc.) is Nrf2 released from Keap1 by a proposed hinge-latch mechanism [25, 26]. This release allows for subsequent Nrf2 translocation into the nucleus where it heterodimerizes with small Maf proteins and binds to the ARE of cytoprotective genes [27], inducing transcription and consequent production of defense proteins.
Numerous studies in a multitude of different neurodegeneration paradigms have indicated that manipulation of the Nrf2-ARE pathway can dramatically attenuate multiple pathophysiological processes, including oxidative stress [28], mitochondrial dysfunction [29, 30], and inflammation [31, 32]. Moreover, recent work has demonstrated that this Nrf2-ARE defense response is inducible by a variety of small molecules, as demonstrated in several different in vitro [29, 33] and in vivo [34-39] paradigms. For example, it has recently been shown that the Nrf2-ARE pathway is involved after TBI [40, 41]. Specifically, the promising Nrf2-ARE activator sulforaphane (an isothiocyanate) has been shown to attenuate post-TBI pathophysiology, including blood-brain-barrier dysfunction [42], edema formation [43], and cognitive deficits [44]. Another impressive small molecule capable of inducing the Nrf2-ARE response is carnosic acid, previously shown to be a more potent activator of the ARE and to be protective in vivo in a cerebral ischemia paradigm [45]. Interestingly, the structures of the most potent Nrf2-ARE activators vary greatly, with some also possessing direct antioxidant (e.g. carnosic acid's phenolic ring structure) capacities [33, 45]. Accordingly, both sulforaphane and carnosic acid were compared in this study.
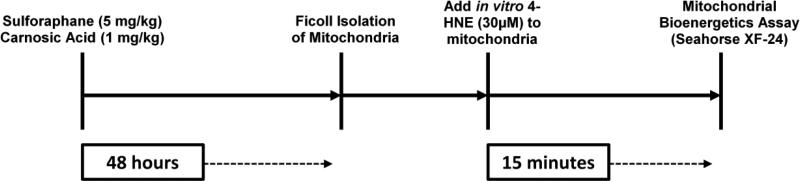
While previous research has extensively implicated the importance of mitochondria in the pathogenesis of numerous neurodegenerative processes, very little is known with regard to Nrf2-ARE's potential effects on mitochondrial bioenergetics post-insult. Recent work by Greco and colleagues [46] found that a single administration of sulforaphane to naïve animals 40 hours prior to mitochondrial isolation could provide resistance to mitochondrial permeability transition pore formation via Nrf2-ARE mediated defenses. Therefore, the purpose of the current study was to compare the capabilities of sulforaphane (SFP) and carnosic acid (CA) to attenuate 4-HNE induced mitochondrial dysfunction in an ex vivo paradigm using brain mitochondria isolated from naïve animals 48 hours after drug administration (see experimental design time line in Fig. 1). It was hypothesized that both compounds would be capable of attenuating the 4-HNE induced inhibition; however, carnosic acid would be more efficacious in regard to its mitochondrial protection.
Figure 1.
Experimental timeline of the current study. Naïve mice were administered either sulforaphane or carnosic acid (or respective vehicles) 48 hours prior to isolation of cortical mitochondria. A 30 μM dose of 4-HNE was applied in vitro to challenge the mitochondria. Mitochondrial bioenergetics were then assessed on the Seahorse XF-24 extracellular flux analyzer (Seahorse Biosciences, North Billerica, MA, USA).
Materials & methods
Animals
The experiments described in this study were completed using isolated mitochondria from naïve young, adult (8 weeks old) male CF-1 mice (Charles River Labs, Portage, MI). Animals received ad libitum access to food and water. The protocols described herein were approved by the University of Kentucky Institutional Animal Care and Use Committee, in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
Chemicals
Sodium pyruvate, malate, rotenone, and carbonyl cyanide p-rifluoromethoxyphenylhydrazone (FCCP), were obtained from Sigma-Aldrich (St. Louis, MO). Oligomycin was obtained from Biomol, USA. 4-hydroxy 2-nonenal (4-HNE) was purchased from EMD Chemicals Inc. (Merck KGaA, Darmstadt, Germany). Chemicals were stored at -20°C as stock solutions. Working solutions for each bioenergetics experiment were always prepared fresh by creating appropriate dilutions in respiration buffer. All materials and reagents for the XF-24 assays were obtained from Seahorse Biosciences (North Billerica, MA, USA).
Isolation of Ficoll-purified mitochondria
Cortical brain mitochondria were extracted as previously described [18]. Briefly, mice were decapitated and the brain rapidly removed. Cortical regions were dissected out in an ice-cold Petri dish containing isolation buffer (1mM EGTA, 215mM mannitol, 75mM sucrose, 0.1% BSA, and 20mM HEPES adjusted to a pH of 7.2 with KOH). Brain tissue was homogenized using Potter-Elvehjem homogenizers containing ice-cold isolation buffer. The tissue homogenates were centrifuged twice at 1300g for 3 minutes in an Eppendorf microcentrifuge at 4°C to remove cellular debris and nuclei, and the supernatant was further centrifuged at 13,000g for 10 minutes. The resulting crude mitochondrial pellet was subjected to nitrogen decompression to release synaptic mitochondria, using a nitrogen cell disruption bomb, at 4°C under a pressure of 1200 psi for 10 minutes. After nitrogen disruption, the mitochondrial pellet was resuspended in isolation buffer and layered on top of a discontinuous Ficoll gradient (7.5% and 10%), and centrifuged at 100,000g for 30 minutes. The mitochondrial pellets at the bottom were transferred to microcentrifuge tubes, topped off with isolation buffer without EGTA, and centrifuged at 10,000g for 10 minutes at 4°C to yield a tighter pellet. The final mitochondrial pellet was resuspended in 25-50 microliters of isolation buffer without EGTA to yield a concentration of approximately 10 mg/ml. The final protein concentration was determined using a BCA protein assay kit measuring absorbance at 562nm using a BioTek Synergy HT plate reader (Winooski, VT). Following isolation of purified mitochondria, 30μM of 4-HNE was applied to each mitochondrial aliquot and incubated for 15 minutes at 37°C. These samples were then analyzed for mitochondrial bioenergetics on the Seahorse XF-24 extracellular flux analyzer instrument (Seahorse Bioscience, North Billerica, MA, USA).
Preparation and calibration of Seahorse XF-24 sensor cartridge sample plate
A Seahorse Bioscience XF24 extracellular flux analyzer was used to measure mitochondrial bioenergetics in intact isolated mitochondria as previously described [47]. The XF-24 creates a transient, 7μl chamber in specialized microplates that allows for the determination of oxygen and proton concentrations in real time. The day before the experiment, 1.0 ml of XF Calibrant solution (Seahorse Bioscience) was added to each well of a 24 well dual-analyte sensor cartridge (Seahorse Bioscience). The sensor cartridge was placed back on the 24 well calibration plate and put in a 37°C incubator without CO2 (Seahorse Bioscience) overnight. The day of the experiment, the injection ports on the sensor cartridge were pre-loaded with the appropriate mitochondrial substrates or inhibitors at 10x concentrations. Once the sensor cartridge was loaded with all of the experimental reagents it was placed into the Seahorse XF-24 extracellular flux analyzer for automated calibration. During the sensor calibration, isolated mitochondria were then seeded in 50μl volume of isolation buffer containing 2.5 μg, 5.0 μg, or 10.0 μg of protein (determined by BCA method) per well in XF-24 V7 cell culture microplates. Following the centrifugation of the plates at 2000 rpm for 4 minutes at 4°C, 450μl of respiration buffer (215mM mannitol, 75mM sucrose, 0.1% BSA, 20mM HEPES, 2mM MgCl, 2.5mM KH2PO4 at pH 7.2) at 37°C was gently added to each well for a final volume of 500μl per well at the beginning of the experiment. Plates were immediately placed into the calibrated Seahorse XF-24 extracellular flux analyzer for mitochondrial bioenergetics analysis.
Seahorse XF-24 assay protocol for isolated mitochondria bioenergetics
The following protocol was utilized for the analysis of bioenergetic function in purified mitochondria using the Seahorse Biosciences XF-24 extracellular flux analyzer as previously described [47]. Briefly, pyruvate plus malate plus ADP, oligomycin, FCCP, and rotenone plus succinate were injected sequentially through ports A, B, C, and D, respectively, in the Seahorse Flux Pak cartridges to yield final concentrations of 5.0 mM (pyruvate), 2.5 mM (malate), 1.0 mM (ADP), 1.0 μg/ml (oligomycin), 4.0 μM (FCCP) and 10.0 mM (succinate) plus 100.0 nM (rotenone), respectively.
Quantitative RT-PCR analysis
Quantitative real-time PCR (qRT-PCR) was employed to determine mRNA levels of the Nrf2-ARE mediated gene target heme oxygenase-1 (HO-1). Briefly, 48 hours after administration of sulforaphane or carnosic acid, mice received an overdose of sodium pentobarbital (200.0 mg/kg I.P.). The cortical tissue was then rapidly dissected out on an ice-chilled stage and immediately transferred to a RNAlater® solution (Ambion Inc.) for 24 hours at 4°C to prevent RNase activity and sample degradation. Samples were then placed in a -80°C freezer for storage until further analysis. To isolate total RNA, tissue samples were homogenized in TRIzol® reagent (Ambion Inc.) according to manufacturer specifications. Isolated total RNA was precipitated out using isopropanol, washed with ethanol, and then decontaminated of residual genomic DNA by DNase I treatment per manufacturer specifications. Total RNA concentrations were determined using a Nanodrop®, with 260/280 ratios of 1.8-2.2 considered acceptable. Purified total RNA (1.0 μg) was then reverse transcribed to acquire complementary total DNA (cDNA). Final cDNA samples were then used for quantitative real-time PCR assay. In this study, qRT-PCR was performed using the StepOne-Plus real-Time PCR System (Applied Biosystems; CA, USA) in conjunction with Taqman® primer-probe reagent-based chemistry. Commercial, inventoried Taqman® gene expression assays consisting of a gene specific set of primers and a fluorogenic internal probe were used (Applied Biosystems; CA, USA). The mouse GAPDH endogenous control was used for normalization purposes of target gene analysis as previously validated in our laboratory. PCR reactions were run in triplicate in a 96 well format using a standard (~2.5 hours) amplification protocol. Each reaction well of the plate contained a total of 25 μl per reaction. The PCR reaction for the specific target gene (HO-1) contained 10.0 μl of 1:10 diluted total cDNA and a total of 15.0 μl of a Taqman® PCR master mix and gene specific primers and probe. The PCR reaction for GAPDH gene expression assay contained 2.0 μl of 1:10 diluted total cDNA and 23.0 μl of a TaqMan® PCR master mix and control gene primers and probe. Following PCR reaction, the resulting amplification curves were then further analyzed by the established ΔΔCt method wherein GAPDH was used as the reference gene and samples from naïve mice were used as the control group. Relative expression was then analyzed as percent change from the naïve control group.
Western blot analysis
Western blotting technique was employed as previously described [48] – with some modifications – to detect 4-HNE adducts of mitochondrial proteins. Briefly, following isolation of purified mitochondria, 30 μM of 4-HNE was applied to each mitochondrial aliquot and incubated for 15 minutes at 37°C (as with above described mitochondrial bioenergetics experiments). After incubation, samples were spun down and frozen at -80°C until further analysis. An aliquot of each protein sample (15 μg for 4-HNE blots) were separated on an SDS–PAGE precast gel (12% Bis-Tris w/v acrylamide; Criterion XT, Bio-Rad) using a XT-MES running buffer system and then transferred to nitrocellulose membranes using a semi-dry electro-transferring unit at 15 V for 30 minutes. Preliminary experiments established protein concentration curves in order to ensure that quantified bands were in the linear range. Membranes were incubated for 1 hour at room temperature in 5% milk/TBS blocking solution. The membranes were then incubated overnight at 4°C in blocking solution with 0.5 mM Tween-20 (TBST) containing the appropriate dilution of primary antibody (1:200). A mouse monoclonal primary antibody was used for detecting 4-HNE bands (Japan Institute for Control of Aging, JaICA, Japan). A goat anti-mouse secondary antibody (2 hour incubation at room temperature) conjugated to an infrared dye (1:5000, IRdye800CW, Rockland) was used for detection of the primary labeled bands. Dry membranes were imaged and quantified using Odyssey Infra Red Imaging System (Li-Cor). All bands ranging from 250 kD to 50 kD were quantified for each lane, representing the smear of 4-HNE labeled proteins for each sample. This was then analyzed as percent of control samples.
Drug treatments
The pharmacological compounds used in this study include sulforaphane (SFP) and carnosic acid (CA), both previously shown to be potent activators of the Nrf2-ARE pathway [38, 45]. R, S-SFP (LKT Labs, Minnesota, USA) was administered I.P. at 5.0 mg/kg in a 90% PBS/10% corn oil vehicle. CA (Sigma, USA) was administered I.P. at 1.0 mg/kg in a 10% Ethanol/ 90% PBS vehicle. The volumes administered of each compound I.P. did not exceed 0.3 ml. Drugs (or their respective vehicles) were administered 48 hours prior to tissue collection or mitochondrial isolation and subsequent in vitro application of 4-HNE. The dose of SFP was chosen based upon its demonstrated effects in a rat TBI model [44]. The dose of CA was chosen based upon previous studies with this compound in a rat acute focal stroke model [45].
Statistical analysis
Data are presented as group means +/- standard deviation (SD) and were analyzed using GraphPad PRISM version 5.0 (San Diego, CA, USA). Both mitochondrial bioenergetics and immunoblot quantification data were analyzed by appropriately designed ANOVAs followed by Student Newman-Keuls (SNK) post-hoc tests as appropriate. Assessment of HO-1 mRNA levels by quantitative PCR was analyzed by one-tailed, unpaired Student's t-test. A p value of <0.05 was considered significant for all analyses.
Results
Baseline bioenergetics of isolated brain mitochondria
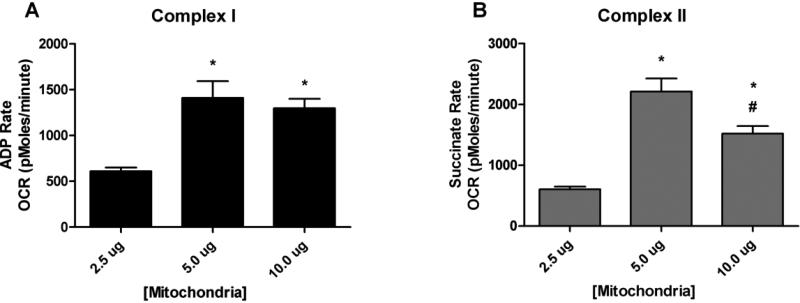
To assess cellular bioenergetics of intact isolated mitochondria, extracellular flux analysis was used to determine oxygen consumption rate (OCR). In the first series of experiments, 2.5, 5.0, and 10.0 μg of mitochondria were utilized to obtain measurable complex-I (ADP rate) and complex-II (succinate rate) oxygen consumption rates (OCR) as shown in Figs. 2A and 2B. Complex-I OCR showed a proportional response with increasing mitochondrial protein. After standardizing the mitochondrial protein curve, subsequent experiments utilized 5.0 μg of mitochondrial protein to study 4-HNE induced mitochondrial dysfunction.
Figure 2.
Mitochondrial bioenergetics: Assay Optimization. Mitochondrial respiration measurements of cortical mitochondria from young adult male CF-1 mice. Analysis revealed a significant increase in oxygen consumption (OCR) for 5.0 μg of mitochondria for both Complex I and II as compared to 2.5 μg of mitochondria protein. One-way ANOVA followed by Student Newman-Keuls post-hoc test. * = p<.05. Error bars represent +/- SD.
Bioenergetic effects of 4-HNE and a protective role for Nrf2-ARE activators
We previously reported that mitochondria isolated from the spinal cord and brain tissue of naïve rats were metabolically intact and well-coupled using a Clark-type oxygen electrode, exhibiting a respiratory control ratio (RCR; ratio of state III to state IV respiration) above 5.0 [18, 49]. We also previously reported on the effects of the lipid peroxidation aldehyde byproduct, 4-hydroxy-2-nonenal (4-HNE), to produce mitochondrial bioenergetic dysfunction in isolated brain mitochondria in vitro using a Clark-type oxygen electrode [19]. We now report on the detrimental effects of 4-HNE on isolated brain mitochondria using a very sensitive and high-resolution extracellular flux method developed by Seahorse Biosciences (North Billerica, MA, USA).
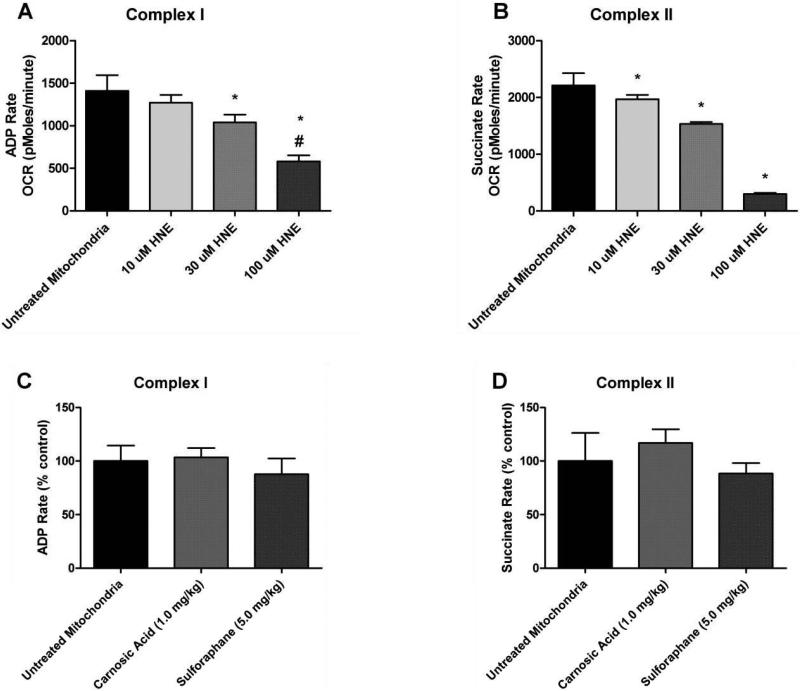
Following Ficoll isolation of purified cortical mitochondria, a dose of 4-HNE (10 μM, 30 μM, or 100 μM) was applied to each mitochondrial aliquot (controls received no 4-HNE) and incubated for 15 minutes at 37°C. These samples were then assayed for mitochondrial bioenergetics on the Seahorse XF-24 instrument. Complex I (ADP rate using pyruvate plus malate substrate) and complex II (succinate substrate)-driven oxygen consumption rates (OCR, pmoles O2/min) were assessed after exposure to 4-HNE. The measurement of complex-I (ADP rate) using XF-24 analyzer is equivalent to state-III rate measured by pyruvate plus malate as substrates for complex-I activity using a Clark-type oxygen electrode. As shown in Figs. 3A and 3B, HNE decreased complex-I (ADP rate) and complex-II (succinate rate) oxygen consumption rates (OCR) significantly (p<0.05) in a concentration-dependent manner. A significant impairment was observed treated with increasing concentrations of 30 μM and 100 μM of 4-HNE. As shown in Fig. 3A, 30 μM 4-HNE significantly decreased complex-I function to approximately 37% in the presence of pyruvate plus malate and ADP (p<0.05 compared to untreated mitochondria). Exposure of isolated mitochondria to higher concentrations of 100 μM HNE resulted in an even greater decrease in OCR (see Figs. 3A and 3B). Interestingly, there were no differences observed in complex-I (ADP rate) or complex-II (succinate rate) basal mitochondrial OCR as shown in Figs. 3C & 3D from mitochondria isolated from animals injected with either SFP or CA but without application of 4-HNE to the mitochondria. This suggests SFP and CA are not altering basal mitochondrial function (e.g. respiration); however, they may still be capable of attenuating the 4-HNE induced impairment in respiration. We selected the 30 μM dose of 4-HNE for further testing of the comparative protective effects of SFP and CA on isolated brain mitochondria.
Figure 3.
Mitochondrial bioenergetics: 4-HNE concentration curve and controls. Top: Mitochondrial respiration measurements of cortical mitochondria from young adult male CF-1 mice. Analysis revealed that both 30 μM and 100 μM of 4-HNE significantly decreased Complex I driven respiration whereas all three tested concentrations reduced Complex II driven respiration. One-way ANOVA followed by Student Newman-Keuls post-hoc test. * = p<.05. Error bars represent +/- SD. Bottom: Sulforaphane and Carnosic Acid Do Not Affect Basal Respiration. Mitochondrial respiration measurements of cortical mitochondria from young adult male CF-1 mice treated with either sulforaphane (SFP) or carnosic acid (CA) in vivo 48 hours prior to isolation of mitochondria. Analysis revealed that SFP and CA did not affect basal mitochondrial oxygen consumption in the absence of extrinsic application of 4-HNE. One-way ANOVA followed by Student Newman-Keuls post-hoc test. * = p<.05. Error bars represent +/- SD.
Nrf2-ARE activators SFP and CA increase HO-1 mRNA levels in cortical tissue
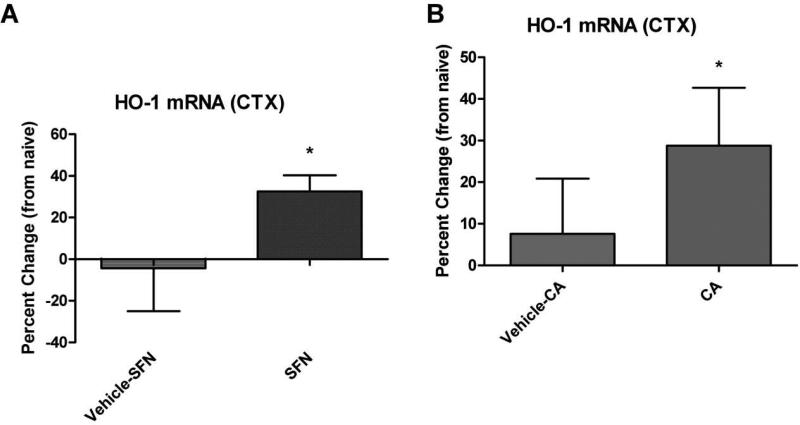
Cortical tissue samples were collected from naïve, SFP treated, CA treated (or respective vehicle controls) animals 48 hours after administration of the compounds. Quantitative real-time PCR (qRT-PCR) was then employed to determine mRNA levels of the Nrf2-ARE mediated gene target heme oxygenase-1 (HO-1). The data indicate that SFP significantly (p<.05) increased HO-1 mRNA levels by nearly 37% compared to vehicle control (Fig. 4A). Similarly, our results demonstrate that administration of CA significantly (p<.05) elevated HO-1 mRNA levels by 21% compared to vehicle control (Fig. 4B). These findings are consistent with previously published work stating that these doses of sulforaphane and carnosic acid are effective inducers of the Nrf2-ARE pathway.
Figure 4.
Nrf2-ARE activators SFN and CA increase HO-1 mRNA levels in cortical tissue. Samples collected from naïve, SFN treated, CA treated (or vehicle controls) animals 48 hours after administration of the compounds. Quantitative PCR (qRT-PCR) was used to determine mRNA levels of the Nrf2-ARE mediated gene target heme oxygenase-1 (HO-1). The data indicate that SFN significantly (p<.05) increased HO-1 mRNA levels 37% compared to vehicle control (Fig. 4A). The data also demonstrate that administration of CA significantly (p<.05) elevated HO-1 mRNA levels 21% compared to vehicle control (Fig. 4B). One-tailed, unpaired Student's t-test. * = p<.05. Error bars represent +/- SD.
Nrf2-ARE activators significantly reduce 4-HNE induced impairment of mitochondrial bioenergetics
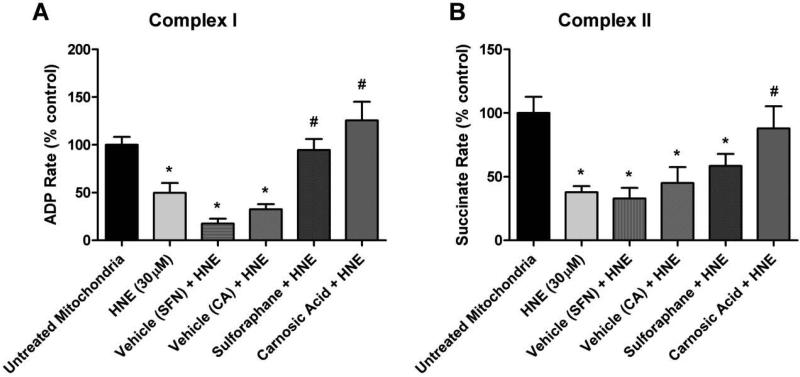
Animals injected with SFP or CA did not display altered basal mitochondrial respiration rates (see Figs. 3C and 3D). Once this was established, mitochondrial respiration measurements of cortical mitochondria isolated from young adult male CF-1 mice treated with either SFP or CA in vivo 48 hours prior to isolation were conducted. Analysis revealed that in vivo administration of both SFP and CA significantly (p<.05) attenuated 4-HNE induced impairment in mitochondrial oxygen consumption for Complex I driven respiration (Fig. 5A). However, only administration of CA was able to significantly (p<.05) attenuate 4-HNE induced reduction in oxygen consumption for Complex II driven respiration (Fig. 5B) as compared to the 4-HNE 30 μM group. The differential effect of CA versus SFP is likely partially due to CA being a more potent activator of the Nrf2-ARE pathway as previously described [45]. Collectively, these data represent novel evidence that Nrf2-ARE activators are capable of directly attenuating mitochondrial dysfunction induced by reactive aldehydes (e.g. 4-HNE) produced by oxidative damage.
Figure 5.
Administration of SFP and CA in vivo attenuates 4-HNE induced impairment in mitochondrial respiration. Mitochondrial respiration measurements of cortical mitochondria from young adult male CF-1 mice treated with either sulforaphane (SFP) or carnosic acid (CA) in vivo 48 hours prior to isolation of mitochondria. Analysis revealed that in vivo administration of both SFP and CA significantly attenuated 4-HNE induced reduction in mitochondrial oxygen consumption for Complex I driven respiration (Fig. 5A). However, only administration of CA was able to significantly attenuate 4-HNE induced reduction in oxygen consumption for Complex II driven respiration (Fig. 5B) as compared to the 4-HNE 30 μM group. One-way ANOVA followed by Student Newman-Keuls post-hoc test. * = p<.05. Error bars represent +/- SD.
Nrf2-ARE activators significantly reduce 4-HNE bound mitochondrial protein
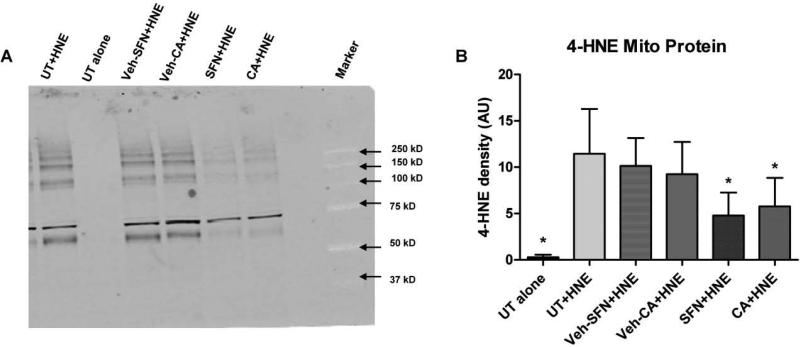
Western Blot analysis was performed to determine whether SFP or CA in vivo pre-treatment could reduce 4-HNE bound mitochondrial protein. After isolation of purified mitochondria, 30 μM of 4-HNE was applied to each mitochondrial aliquot (controls received no 4-HNE) and incubated for 15 minutes at 37°C (similar to above described mitochondrial bioenergetics experiments). A representative immunoblot demonstrates reduced presence of 4-HNE bound proteins in mitochondrial samples (Fig. 6A). Quantitative analysis of immunoblots revealed that both SFP and CA significantly (p<.05) reduced 4-HNE bound mitochondrial protein as compared to the UT plus HNE group (Fig. 6B). This suggests the mechanism by which the Nrf2-ARE activators SFP and CA are attenuating the 4-HNE induced inhibition of mitochondrial respiration likely involves a decrease in 4-HNE adducts to mitochondrial proteins. This is an important proof of principle demonstration that suggests the cytoprotective antioxidant defenses mediated by Nrf2-ARE can directly antagonize the reactive aldehydes such as 4-HNE that are produced via free-radical induced oxidative damage.
Figure 6.
Administration of SFP and CA reduces 4-HNE bound mitochondrial protein. Western Blot analysis was performed to determine whether SFP or CA could reduce 4-HNE bound mitochondrial protein. Representative immunoblot demonstrating reduced presence of 4-HNE bound proteins in mitochondrial samples (Fig. 6A). Quantitative analysis of immunoblots revealed that both SFP and CA significantly (p<.05) reduced 4-HNE bound mitochondrial protein as compared to the untreated (UT) plus HNE group (Fig. 6B). One-way ANOVA followed by Student Newman-Keuls post-hoc test. * = p<.05. Error bars represent +/- SD.
Discussion
Previous work from our laboratory has shown that the reactive nitrogen species peroxynitrite (PON) is able to directly inhibit mitochondrial function in the injured brain mitochondria and is associated with elevated 4-HNE [17]. Moreover, direct application of PON to normal mitochondria simulates the effects of in vivo TBI [18, 19]. While the process of lipid peroxidation can directly cause membrane destruction and likely impair mitochondrial function, we also recently demonstrated that the LP-derived reactive aldehydes 4-HNE and acrolein can directly inhibit mitochondrial respiration in vitro in isolated brain and spinal cord mitochondria [19]. These findings suggest that both lipid peroxidation and its toxic byproducts (reactive aldehydes) play a deleterious role in the secondary injury cascade post-TBI, primarily by impairing mitochondrial function.
Extensive evidence in different neurodegeneration paradigms indicates that manipulation of the Nrf2-ARE pathway can dramatically attenuate multiple pathophysiological processes, including oxidative stress [28], mitochondrial dysfunction [29, 30], and inflammation [31, 32]. Moreover, recent work has demonstrated that this Nrf2-ARE defense response is inducible by a variety of small molecules, as demonstrated in several different in vitro [29, 33] and in vivo [34-39] paradigms. Specifically, the promising Nrf2-ARE activator SFP (an isothiocyanate) has been shown to attenuate post-TBI pathophysiology, including blood-brain-barrier dysfunction [42], edema formation [43], and cognitive deficits [44]. Another impressive small molecule capable of inducing the Nrf2-ARE response is CA, previously shown to be a more potent effective activator of the ARE and to be protective in vivo in a cerebral ischemia paradigm [45]. Interestingly, the structures of the most potent Nrf2-ARE activators vary greatly, with some also possessing direct antioxidant (e.g. CA's phenolic ring structure) capacities [33, 45].
While previous research has extensively implicated the importance of mitochondria in the pathogenesis of neurodegenerative disorders, very little is known with regard to Nrf2-ARE's potential effects on mitochondrial bioenergetics post-insult. Recent work by Greco and colleagues [46] found that a single administration of SFP to naïve animals 40 hours prior to mitochondrial isolation could provide resistance to mitochondrial permeability transition pore formation via Nrf2-ARE mediated defenses. Thus, the purpose of the current study was to compare the capabilities of SFP and CA to attenuate 4-HNE induced mitochondrial dysfunction in an ex vivo paradigm using brain mitochondria isolated from naïve animals 48 hours after drug administration. Here, we demonstrate direct mitochondria protective effects of two known Nrf2-ARE activators, SFP and CA, in a unique and relevant ex vivo paradigm. The differential effects of CA versus SFP seen in the current study are likely at least partially due to CA being a more efficacious activator of the Nrf2-ARE pathway as previously described [45]. In the injured brain, however, it may be critical which cell-types exhibit increased Nrf2-ARE activity as it was recently shown that astrocytes activate the pathway to higher degree and confer indirect neuroprotection to neurons [30].
Collectively, the data presented in the current study represent novel evidence that Nrf2-ARE activators are capable of directly attenuating mitochondrial respiratory dysfunction induced by reactive aldehydes (e.g. 4-HNE) produced by oxidative damage. Moreover, these data suggest that the likely mechanism by which the Nrf2-ARE activators SFP and CA are attenuating the 4-HNE-induced inhibition of mitochondrial respiration involves a reduction of 4-HNE available to form covalent adducts with mitochondrial proteins. This could occur by multiple mechanisms, each of which is inducible by the Nrf2-ARE pathway's upregulation of numerous critical detoxifiers of reactive aldehydes. These include, as a first possibility, the glutathione antioxidant system in which glutathione-S-transferase (GST) catalyzes the formation of a glutathione-4-HNE adduct which is thereby rendered unable to bind to mitochondrial proteins [50]. The second mechanism involves a Nrf2-induced increase in either oxidation of 4-HNE to an alcohol via aldehyde dehydroxygenase or reduction to a carboxylic acid via alcohol dehydrogenase. Thirdly, Nrf2 has been shown to upregulate the levels of another antioxidant enzyme, NAD(P)H-dependent alkenal/one oxidoreductase (AO), which reduces the double bond between α and β carbons of 4-HNE rendering it unreactive with proteins [51, 52]. These three possibilities for how pharmacological activation of Nrf2-ARE might protect mitochondrial complex I and II respiratory function from impairment by 4-HNE application are highlighted in Figure 7. Thus, this is critically important mechanistic “proof of principle” evidence that suggests the vast cytoprotective defenses mediated by Nrf2-ARE can directly antagonize the toxic reactive aldehydes produced via free-radical induced lipid peroxidation in mitochondria. This thereby suggests that pharmacological Nrf2-ARE manipulation – specifically via the potent activator CA – may be a strong therapeutic approach for neurodegenerative disorders and warrants continued investigation.
Figure 7.
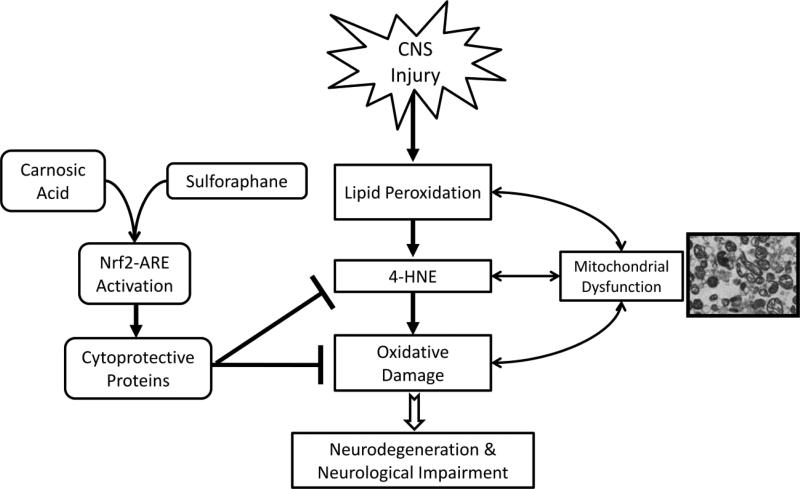
Schematic Representation of Nrf2-ARE role in CNS injury. This scheme outlines the potential protective role that the Nrf2-ARE pathway may play following acute CNS injury. Cytoprotective gene expression mediated by Nrf2-ARE represents a diverse, pleiotropic battery that may attenuate multiple facets of the acute secondary injury cascade, including mitochondrial dysfunction. Administration of pharmacological activators of this pathway – such as SFP and CA – causes subsequent upregulation of cytoprotective defenses. These protective proteins are capable of attenuating the oxidative damage that ensures following CNS injury. Reduction in oxidative damage post-injury may mitigate or even prevent consequent neurodegeneration and neurological impairment.
In conclusion, the impact of acute and chronic neurodegenerative disorders on society is devastating and hence it is imperative to discover and translate rational therapies for the clinical treatment of these growing epidemics. In particular, oxidative damage has been identified as one of the key pathological processes underlying the secondary damage following TBI. However, although numerous antioxidant-based therapies have been investigated, none have proven effective in clinical trials for TBI [4]. The Nrf2-ARE pathway may provide a more comprehensive, pleiotropic approach to attenuate oxidative damage after TBI and other acute CNS injuries. To that end, the current study provides evidence that Nrf2-ARE has potential for attenuating mitochondrial dysfunction in a pathologically relevant ex vivo paradigm. However, further work is needed to better define the role of the Nrf2-ARE pathway following TBI and to determine whether amplifying this pathway has useful neuroprotective potential.
Highlights.
CNS injury causes oxidative damage and production of toxic aldehydes, such as 4-HNE
Mitochondria are susceptible to 4-HNE, which inhibits respiration
Nrf2-ARE mediates the antioxidant defense enzymes
Nrf2-ARE activators sulforaphane and carnosic acid attenuate 4-HNE mito-toxicity
Acknowledgements
This work was supported by grants NIH-NIDA 1T32 DA022738, NIH-NINDS 2P30 NS051220-01 and funds from the Kentucky Spinal Cord & Head Injury Research Trust.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Hall ED. Mechanisms of Secondary CNS Injury. In: Palme JD, editor. Neurosurgery 96: Manual of Neurosurgery. Churchill Livingstone; New York: 1996. pp. 505–510. [Google Scholar]
- 2.Kochanek PM, Clark SB, Jenkins LW. TBI: Pathobiology. In: Zasler ND, Katz DI, Zafonte RD, editors. Brain Injury Medicine. Demos Medical; New York: 2007. pp. 81–96. [Google Scholar]
- 3.Hall ED, Braughler JM. Free radicals in CNS injury. Res Publ Assoc Res Nerv Ment Dis. 1993;71:81–105. [PubMed] [Google Scholar]
- 4.Hall ED, Vaishnav RA, Mustafa AG. Antioxidant therapies for traumatic brain injury. Neurotherapeutics. 2010;7:51–61. doi: 10.1016/j.nurt.2009.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bosken JM, Wang JA, Hall ED. Assessments of Oxidative Damage and Lipid Peroxidation After TBI/SCI. In: Chen J, Xu XC, Xu XM, Zheng JH, editors. Animal Models of Neurological Injuries II: Injury and Mechanistic Assessments. Humana Press; New York: 2012. pp. 347–375. [Google Scholar]
- 6.Deng Y, Thompson BM, Gao X, Hall ED. Temporal relationship of peroxynitrite-induced oxidative damage, calpain-mediated cytoskeletal degradation and neurodegeneration after traumatic brain injury. Experimental neurology. 2007;205:154–165. doi: 10.1016/j.expneurol.2007.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Denicola A, Radi R. Peroxynitrite and drug-dependent toxicity. Toxicology. 2005;208:273–288. doi: 10.1016/j.tox.2004.11.023. [DOI] [PubMed] [Google Scholar]
- 8.Radi R. Nitric oxide, oxidants, and protein tyrosine nitration. Proc Natl Acad Sci U S A. 2004;101:4003–4008. doi: 10.1073/pnas.0307446101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Radi R, Beckman JS, Bush KM, Freeman BA. Peroxynitrite-induced membrane lipid peroxidation: the cytotoxic potential of superoxide and nitric oxide. Archives of biochemistry and biophysics. 1991a;288:481–487. doi: 10.1016/0003-9861(91)90224-7. [DOI] [PubMed] [Google Scholar]
- 10.Radi R, Beckman JS, Bush KM, Freeman BA. Peroxynitrite oxidation of sulfhydryls. The cytotoxic potential of superoxide and nitric oxide. J Biol Chem. 1991b;266:4244–4250. [PubMed] [Google Scholar]
- 11.Pandya JD, Pauly JR, Nukala VN, Sebastian AH, Day KM, Korde AS, Maragos WF, Hall ED, Sullivan PG. Post-Injury Administration of Mitochondrial Uncouplers Increases Tissue Sparing and Improves Behavioral Outcome following Traumatic Brain Injury in Rodents. J Neurotrauma. 2007;24:798–811. doi: 10.1089/neu.2006.3673. [DOI] [PubMed] [Google Scholar]
- 12.Singh IN, Sullivan PG, Deng Y, Mbye LH, Hall ED. Time course of post-traumatic mitochondrial oxidative damage and dysfunction in a mouse model of focal traumatic brain injury: implications for neuroprotective therapy. J Cereb Blood Flow Metab. 2006b;26:1407–1418. doi: 10.1038/sj.jcbfm.9600297. [DOI] [PubMed] [Google Scholar]
- 13.Azbill RD, Mu X, Bruce-Keller AJ, Mattson MP, Springer JE. Impaired mitochondrial function, oxidative stress and altered antioxidant enzyme activities following traumatic spinal cord injury. Brain research. 1997;765:283–290. doi: 10.1016/s0006-8993(97)00573-8. [DOI] [PubMed] [Google Scholar]
- 14.Matsushita M, Xiong G. Projections from the cervical enlargement to the cerebellar nuclei in the rat, studied by anterograde axonal tracing. J Comp Neurol. 1997;377:251–261. doi: 10.1002/(sici)1096-9861(19970113)377:2<251::aid-cne7>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 15.Sullivan PG, Bruce-Keller AJ, Rabchevsky AG, Christakos S, Clair DK, Mattson MP, Scheff SW. Exacerbation of damage and altered NF-kappaB activation in mice lacking tumor necrosis factor receptors after traumatic brain injury. J Neurosci. 1999a;19:6248–6256. doi: 10.1523/JNEUROSCI.19-15-06248.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sullivan PG, Thompson MB, Scheff SW. Cyclosporin A attenuates acute mitochondrial dysfunction following traumatic brain injury. Exp Neurol. 1999b;160:226–234. doi: 10.1006/exnr.1999.7197. [DOI] [PubMed] [Google Scholar]
- 17.Singh IN, Sullivan PG, Deng Y, Mbye LH, Hall ED. Time course of post-traumatic mitochondrial oxidative damage and dysfunction in a mouse model of focal traumatic brain injury: implications for neuroprotective therapy. J Cereb Blood Flow Metab. 2006a;26:1407–1418. doi: 10.1038/sj.jcbfm.9600297. [DOI] [PubMed] [Google Scholar]
- 18.Singh IN, Sullivan PG, Hall ED. Peroxynitrite-mediated oxidative damage to brain mitochondria: Protective effects of peroxynitrite scavengers. Journal of neuroscience research. 2007;85:2216–2223. doi: 10.1002/jnr.21360. [DOI] [PubMed] [Google Scholar]
- 19.Vaishnav RA, Singh IN, Miller DM, Hall ED. Lipid peroxidation-derived reactive aldehydes directly and differentially impair spinal cord and brain mitochondrial function. Journal of neurotrauma. 2010;27:1311–1320. doi: 10.1089/neu.2009.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hall ED, McCall JM, Means ED. Therapeutic potential of the lazaroids (21-aminosteroids) in acute central nervous system trauma, ischemia and subarachnoid hemorrhage. Adv Pharmacol. 1994;28:221–268. doi: 10.1016/s1054-3589(08)60497-4. [DOI] [PubMed] [Google Scholar]
- 21.Chan JY, Han XL, Kan YW. Isolation of cDNA encoding the human NF-E2 protein. Proc Natl Acad Sci U S A. 1993;90:11366–11370. doi: 10.1073/pnas.90.23.11366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD, Yamamoto M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999;13:76–86. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moi P, Chan K, Asunis I, Cao A, Kan YW. Isolation of NF-E2-related factor 2 (Nrf2), a NF-E2-like basic leucine zipper transcriptional activator that binds to the tandem NF-E2/AP1 repeat of the beta-globin locus control region. Proc Natl Acad Sci U S A. 1994;91:9926–9930. doi: 10.1073/pnas.91.21.9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kobayashi A, Kang MI, Okawa H, Ohtsuji M, Zenke Y, Chiba T, Igarashi K, Yamamoto M. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol Cell Biol. 2004;24:7130–7139. doi: 10.1128/MCB.24.16.7130-7139.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kobayashi A, Kang MI, Watai Y, Tong KI, Shibata T, Uchida K, Yamamoto M. Oxidative and electrophilic stresses activate Nrf2 through inhibition of ubiquitination activity of Keap1. Mol Cell Biol. 2006;26:221–229. doi: 10.1128/MCB.26.1.221-229.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tong KI, Kobayashi A, Katsuoka F, Yamamoto M. Two-site substrate recognition model for the Keap1-Nrf2 system: a hinge and latch mechanism. Biol Chem. 2006;387:1311–1320. doi: 10.1515/BC.2006.164. [DOI] [PubMed] [Google Scholar]
- 27.Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- 28.Johnson JA, Johnson DA, Kraft AD, Calkins MJ, Jakel RJ, Vargas MR, Chen PC. The Nrf2-ARE pathway: an indicator and modulator of oxidative stress in neurodegeneration. Ann N Y Acad Sci. 2008;1147:61–69. doi: 10.1196/annals.1427.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Calkins MJ, Jakel RJ, Johnson DA, Chan K, Kan YW, Johnson JA. Protection from mitochondrial complex II inhibition in vitro and in vivo by Nrf2-mediated transcription. Proc Natl Acad Sci U S A. 2005;102:244–249. doi: 10.1073/pnas.0408487101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Calkins MJ, Vargas MR, Johnson DA, Johnson JA. Astrocyte-specific overexpression of Nrf2 protects striatal neurons from mitochondrial complex II inhibition. Toxicol Sci. 2010;115:557–568. doi: 10.1093/toxsci/kfq072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Innamorato NG, Rojo AI, Garcia-Yague AJ, Yamamoto M, de Ceballos ML, Cuadrado A. The transcription factor Nrf2 is a therapeutic target against brain inflammation. J Immunol. 2008;181:680–689. doi: 10.4049/jimmunol.181.1.680. [DOI] [PubMed] [Google Scholar]
- 32.Rojo AI, Innamorato NG, Martin-Moreno AM, De Ceballos ML, Yamamoto M, Cuadrado A. Nrf2 regulates microglial dynamics and neuroinflammation in experimental Parkinson's disease. Glia. 2010;58:588–598. doi: 10.1002/glia.20947. [DOI] [PubMed] [Google Scholar]
- 33.Li J, Johnson D, Calkins M, Wright L, Svendsen C, Johnson J. Stabilization of Nrf2 by tBHQ confers protection against oxidative stress-induced cell death in human neural stem cells. Toxicol Sci. 2005;83:313–328. doi: 10.1093/toxsci/kfi027. [DOI] [PubMed] [Google Scholar]
- 34.Innamorato NG, Jazwa A, Rojo AI, Garcia C, Fernandez-Ruiz J, Grochot-Przeczek A, Stachurska A, Jozkowicz A, Dulak J, Cuadrado A. Different susceptibility to the Parkinson's toxin MPTP in mice lacking the redox master regulator Nrf2 or its target gene heme oxygenase-1. PLoS One. 2010;5:e11838. doi: 10.1371/journal.pone.0011838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kraft AD, Resch JM, Johnson DA, Johnson JA. Activation of the Nrf2-ARE pathway in muscle and spinal cord during ALS-like pathology in mice expressing mutant SOD1. Exp Neurol. 2007;207:107–117. doi: 10.1016/j.expneurol.2007.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Son TG, Camandola S, Arumugam TV, Cutler RG, Telljohann RS, Mughal MR, Moore TA, Luo W, Yu QS, Johnson DA, Johnson JA, Greig NH, Mattson MP. Plumbagin, a novel Nrf2/ARE activator, protects against cerebral ischemia. J Neurochem. 2010;112:1316–1326. doi: 10.1111/j.1471-4159.2009.06552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vargas MR, Johnson DA, Sirkis DW, Messing A, Johnson JA. Nrf2 activation in astrocytes protects against neurodegeneration in mouse models of familial amyotrophic lateral sclerosis. J Neurosci. 2008;28:13574–13581. doi: 10.1523/JNEUROSCI.4099-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao J, Kobori N, Aronowski J, Dash PK. Sulforaphane reduces infarct volume following focal cerebral ischemia in rodents. Neurosci Lett. 2006;393:108–112. doi: 10.1016/j.neulet.2005.09.065. [DOI] [PubMed] [Google Scholar]
- 39.Zhao X, Sun G, Zhang J, Strong R, Dash PK, Kan YW, Grotta JC, Aronowski J. Transcription factor Nrf2 protects the brain from damage produced by intracerebral hemorrhage. Stroke. 2007b;38:3280–3286. doi: 10.1161/STROKEAHA.107.486506. [DOI] [PubMed] [Google Scholar]
- 40.Jin W, Wang H, Ji Y, Zhu L, Yan W, Qiao L, Yin H. Genetic ablation of Nrf2 enhances susceptibility to acute lung injury after traumatic brain injury in mice. Exp Biol Med (Maywood) 2009a;234:181–189. doi: 10.3181/0807-RM-232. [DOI] [PubMed] [Google Scholar]
- 41.Jin W, Wang H, Yan W, Zhu L, Hu Z, Ding Y, Tang K. Role of Nrf2 in protection against traumatic brain injury in mice. J Neurotrauma. 2009b;26:131–139. doi: 10.1089/neu.2008.0655. [DOI] [PubMed] [Google Scholar]
- 42.Zhao J, Moore AN, Redell JB, Dash PK. Enhancing expression of Nrf2-driven genes protects the blood brain barrier after brain injury. J Neurosci. 2007a;27:10240–10248. doi: 10.1523/JNEUROSCI.1683-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao J, Moore AN, Clifton GL, Dash PK. Sulforaphane enhances aquaporin-4 expression and decreases cerebral edema following traumatic brain injury. J Neurosci Res. 2005;82:499–506. doi: 10.1002/jnr.20649. [DOI] [PubMed] [Google Scholar]
- 44.Dash PK, Zhao J, Orsi SA, Zhang M, Moore AN. Sulforaphane improves cognitive function administered following traumatic brain injury. Neurosci Lett. 2009;460:103–107. doi: 10.1016/j.neulet.2009.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Satoh T, Kosaka K, Itoh K, Kobayashi A, Yamamoto M, Shimojo Y, Kitajima C, Cui J, Kamins J, Okamoto S, Izumi M, Shirasawa T, Lipton SA. Carnosic acid, a catechol-type electrophilic compound, protects neurons both in vitro and in vivo through activation of the Keap1/Nrf2 pathway via S-alkylation of targeted cysteines on Keap1. J Neurochem. 2008;104:1116–1131. doi: 10.1111/j.1471-4159.2007.05039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Greco T, Shafer J, Fiskum G. Sulforaphane inhibits mitochondrial permeability transition and oxidative stress. Free radical biology & medicine. 2011;51:2164–2171. doi: 10.1016/j.freeradbiomed.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sauerbeck A, Pandya J, Singh I, Bittman K, Readnower R, Bing G, Sullivan P. Analysis of regional brain mitochondrial bioenergetics and susceptibility to mitochondrial inhibition utilizing a microplate based system. Journal of Neuroscience Methods. 2011;198:36–43. doi: 10.1016/j.jneumeth.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mustafa AG, Singh IN, Wang J, Carrico KM, Hall ED. Mitochondrial protection after traumatic brain injury by scavenging lipid peroxyl radicals. Journal of Neurochemistry. 2010;114:271–280. doi: 10.1111/j.1471-4159.2010.06749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xiong Y, Hall ED. Pharmacological evidence for a role of peroxynitrite in the pathophysiology of spinal cord injury. Exp. Neurol. 2009;216:105–114. doi: 10.1016/j.expneurol.2008.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pettazzoni P, Ciamporcero E, Medana C, Pizzimenti S, Dal Bello F, Giacomo Minero V, Toaldo C, Minelli R, Uchida K, Umberto Dianzani M, Pili R, Barrera G. Nuclear factor erythroid 2-related factor-2 activity controls 4-hydroxynonenal metabolism and activity in prostate cancer cells. Free Radical Biology and Medicine. 2011;51:8, 1610–1618. doi: 10.1016/j.freeradbiomed.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 51.Dick RA, Kwak MK, Sutter TR, Kensler TW. Antioxidative function and substrate specificity of NAD(P)H-dependent alkenal/one oxidoreductase. A new role for leukotriene B4 12-hydroxydehydrogenase/15-oxoprostaglandin 13-reductase. J. Biol. Chem. 2001;276(44):40803–40810. doi: 10.1074/jbc.M105487200. [DOI] [PubMed] [Google Scholar]
- 52.Yu X, Egner PA, Wakabayashi J, Wakabayashi N, Yamamoto M, Kensler TW. Nrf2-mediated induction of cytoprotective enzymes by 15-deoxy-Delta12,14-prostaglandin J2 is attenuated by alkenal/one oxidoreductase. J. Biol. Chem. 2006;281:26245–26252. doi: 10.1074/jbc.M604620200. [DOI] [PubMed] [Google Scholar]