Abstract
BACKGROUND
Tramadol, a monoaminergic reuptake inhibitor, is hepatically metabolized to an opioid agonist (M1). This atypical analgesic is generally considered to have limited abuse liability. Recent reports of its abuse have increased in the U.S., leading to more stringent regulation in some states, but not nationally. The purpose of this study was to examine the relative abuse liability and reinforcing efficacy of tramadol in comparison to a high (oxycodone) and low efficacy (codeine) opioid agonist.
METHODS
Nine healthy, non-dependent prescription opioid abusers (6 male, 3 female) participated in this within-subject, randomized, double blind, placebo-controlled study. Participants completed 14 paired sessions (7 sample, 7 self-administration). During each sample session, an oral dose of tramadol (200, 400 mg), oxycodone (20, 40 mg), codeine (100, 200 mg) or placebo was administered, and a full array of abuse liability measures was collected. During self-administration sessions, volunteers were given the opportunity to work (via progressive ratio) for the sample dose or money.
RESULTS
All active doses were self-administered; placebo engendered no responding. The high doses of tramadol and oxycodone were readily self-administered (70%, 59% of available drug, respectively); lower doses and both codeine doses maintained intermediate levels of drug taking. All three drugs dose-dependently increased measures indicative of abuse liability, relative to placebo; however, the magnitude and time course of these and other pharmacodynamic effects varied qualitatively across drugs.
CONCLUSIONS
This study demonstrates that, like other mu opioids, higher doses of tramadol function as reinforcers in opioid abusers, providing new empirical data for regulatory evaluation.
Keywords: tramadol, o-desmethyltramadol (M1), human, self-administration, codeine, oxycodone
1. INTRODUCTION
Tramadol, marketed in the United States (U.S.) since 1995 to treat mild-to-moderate pain, is structurally similar to morphine and codeine but produces its analgesic effects through two mechanisms. It has minimal affinity for the µ-opioid receptor and inhibits the reuptake of serotonin and norepinephrine (Raffa et al., 1992; Desmeules et al., 1996). The active hepatic metabolite, o-desmethyltramadol or M1, is an opioid agonist with high relative intrinsic efficacy and moderate affinity for the µ-opioid receptor (Gillen et al., 2000; Raffa et al., 1992; Volpe et al., 2011).
Historically, tramadol has been considered to have limited abuse liability and was introduced in the U.S. as an unscheduled analgesic. Prior to its U.S. approval, tramadol was marketed in Europe for approximately 20 years with little evidence of abuse or diversion (Radbruch et al., 1996). Epidemiological studies conducted after its U.S. marketing indicated that tramadol misuse was rather low compared to hydrocodone or oxycodone (Cicero et al., 1999, 2005; Inciardi et al., 2006). Preclinical abuse liability assessments have generally supported its limited abuse potential, as tramadol produced modest rates of IV self-administration relative to prototypic opioids like morphine (O’Connor and Mead, 2010; Yanagita, 1978).
Early clinical studies also yielded no abuse liability signal for tramadol from experienced opioid users. Examination of intramuscular (IM: 75, 150, 300 mg) and intravenous (IV: 100, 200 mg) tramadol indicated that the lower IM doses (75, 150 mg) were placebo-like, while higher IM doses and IV doses produced self-reported global drug effects but did not produce miosis or increase abuse liability measures (Preston et al., 1991; Epstein et al., 2006). The acute effects of tramadol (100 and 300 mg; IM) were examined in methadone-maintained volunteers, and these doses did not produce agonist-like effects or precipitate withdrawal (Cami et al., 1994).
Epidemiological reports and surveillance studies have indicated that tramadol diversion, abuse and overdose have recently increased in the U.S. (Dart et al., 2011; Spiller et al., 2010; Watson et al., 2003; SAMHSA, 2006), leading several states (Kentucky, Arkansas, Wyoming and Tennessee) to change it to a more stringent category (Schedule IV), while it remains unscheduled nationally. Recent clinical research suggests that the abuse liability of tramadol may have been previously underestimated with respect to oral administration, as the earlier preclinical and clinical studies employed parenteral dosing. As production of the opioid-agonist metabolite, M1, is largely dependent on hepatic metabolism, concentrations of M1 are much higher after oral, relative to parenteral administration (Ardakani and Rouini, 2007; Enggaard et al., 2006; Poulsen et al., 1996; Campanero et al., 1999), likely resulting in greater opioid agonist effects after oral administration. Jasinski and colleagues (1993) evaluated oral tramadol (175, 350, 700 mg) in non-dependent, opioid-experienced users and reported higher doses of oral tramadol (350, 700 mg) produced miosis, increased ratings on abuse liability measures (e.g., drug liking, MBG scale of ARCI), and were identified as opioid-like on a pharmacological class questionnaire (Jasinski et al., 1993; Epstein et al., 2006). These effects were similar in magnitude to those produced by oral oxycodone (20, 40 mg) but with a delayed onset (Jasinski et al., 1993; Epstein et al., 2006). Higher (200, 400 mg), but not lower doses (50, 100 mg) of oral tramadol produced hydromorphone-like drug discrimination responding in opioid abusers; no doses produced effects on VAS measures associated with abuse liability (e.g., like drug effects, good drug effects; Duke et al., 2011). Opioid agonist effects have also been observed in naïve/light opioid users, whereby a therapeutic dose of oral tramadol (100 mg) increased scores on several subjective measures (e.g., like drug, want to take drug again) similar to oral morphine (Zacny, 2005). Further evidence of the opioid agonist action arises from reports that oral tramadol may suppress spontaneous opioid withdrawal (Lofwall et al., 2007; trend reported in Carroll et al., 2006). Naloxone challenge or cessation of chronic oral tramadol also leads to dose-dependent opioid-like withdrawal signs/symptoms (Lanier et al., 2010; Barsotti et al., 2003; Freye and Levy, 2000), although additional atypical withdrawal symptoms, such as anxiety, confusion and hallucinations, have been reported (Senay et al., 2003).
Given the dramatic increase in prescription opioid abuse in the U.S., along with emerging signals of tramadol abuse and overdose, further evaluation of the abuse liability of therapeutic and supratherapeutic doses of tramadol is warranted. The purpose of this study was to examine directly the relative abuse liability and reinforcing efficacy (measured via self-administration) of oral tramadol compared to oxycodone, a high efficacy µ-opioid agonist with known abuse liability, codeine, a moderate affinity µ-opioid agonist with relatively low abuse liability, and placebo in a cohort of non-dependent prescription opioid abusers.
2. METHODS
2.1 Participants
Participants were healthy, adult prescription opioid abusers who were not physically dependent on opioids. All volunteers were recruited by local advertisements and paid for participation. Participants completed an on-site evaluation, including an investigator interview, medical history and physical examination, ECG, blood chemistry and urinalysis. Volunteers were literate, English-speaking adults, ages 18–50. Individuals seeking treatment for substance abuse, successfully maintaining abstinence, or with significant medical problems (e.g., seizure disorders, asthma), serious psychiatric illness (e.g., schizophrenia), current physiological drug dependence or pregnancy were excluded. All participants reported illicit use of prescription opioids confirmed by urine drug testing. Participants were also required to provide an opioid negative urine sample in the absence of opioid withdrawal symptoms to exclude physiological opioid dependence. All participants provided sober, written informed consent prior to participation. This study was approved by the University of Kentucky (UK) Medical Institutional Review Board and a Certificate of Confidentiality was obtained from the National Institute on Drug Abuse. All study procedures were conducted in accordance with the Helsinki guidelines for ethical research.
2.2 Drugs
This study was conducted under an investigator-initiated Investigational New Drug Application from the Food and Drug Administration (#69,214). All study medications were stored and prepared by the UK Investigational Pharmacy. Oxycodone hydrochloride (Spectrum Chemical Manufacturing Corp., Gardena, CA), tramadol hydrochloride and codeine phosphate powders (both from Medisca, Plattsburgh, NY) were weighed and packed into uniformly appearing size 0 capsules (Health Care Logistics, Circleville, OH). Lactose (Mallinckrodt Chemical, Paris, KY) was used for the placebo condition and for filler in the active dose capsules.
2.3 Study Design
This 4-week inpatient study utilized a within-subject, randomized, double-blind, placebo controlled design and examined oral tramadol (200, 400 mg), oxycodone (20, 40 mg), codeine (100, 200 mg) and placebo. Volunteers resided at the Clinical Research Development and Operations Center, a closed inpatient hospital research unit, and participated in a total of 7 pairs of experimental sessions (14 sessions total): 7 Sample and 7 Self-Administration Sessions.
2.4 General Methods
Participants were trained on study procedures using a Macintosh Mini computer (Cupertino, CA) and were accompanied by a trained research assistant during each session. Participants received a caffeine-free diet and were provided a standardized, light breakfast 2 hr before experimental sessions. Smoking was permitted up to 30 min prior to the start of sessions. Ad lib smoking was permitted after sessions and on non-session days. Urine samples were collected each morning and tested for drugs of abuse; females were tested for pregnancy daily. Breath samples were obtained before each session and tested for alcohol.
2.4.1 Sample sessions
Sample sessions were 6.5 hours in length. At the beginning of Sample Sessions, participants were reminded to pay close attention to the drug effects, as they would be given the opportunity to earn some, none or all of the same drug dose the next day. An array of measures was collected prior to and at regular intervals after drug administration (see below).
2.4.2 Self-administration sessions
Self-administration sessions were 1.5 to 4.5 hours in length and were conducted 24 hr after each Sample Session. Selected safety measures were collected at baseline and at 0.5 hr intervals after drug administration. Participants were given a total of 7 opportunities (i.e., trials) to respond on a progressive ratio (PR) schedule to earn portions of the sample dose (in 1/7th increments) or a portion of money (a total of $21 available, in increments of $3). Participants responded on the PR schedule via clicks on a computer mouse. The response requirement successively increased across the 7 trials: 50, 250, 500, 1000, 1500, 2000 and 2500 responses, with a total of 7,800 responses necessary to earn all of the available drug or money over a maximum of 210 min. As each reinforcer operated under an independent PR schedule, responding for one reinforcer did not impact response requirements for the other reinforcer. When PR responding was completed, the participants received the amount of drug or money earned. Cash was delivered to the volunteer, but kept in a locked location until study completion. If drug was administered, participants were monitored and safety data collected for 3 hr.
2.4.3 Physiological Measures
Heart rate, blood pressure and oxygen saturation (Dinamap Non-Invasive Patient Monitor, GE Medical Systems, Tampa, FL) were collected every minute 30-min before and for 6 hr after sample drug administration. Respiration rate, expired end tidal CO2 (N-85 Capnograph, Nellcor, Boulder, CO) and pupil diameter measurements (PLR-200, NeurOptics, Irvine, CA) were measured at baseline, every 15 min after sample drug administration for the first 2.5 hr, and every 30 min for the remaining 3.5 hr.
2.4.4 Subjective and Observer-Rated Measures
Subjective effects measurements during Sample Sessions included a six-item Visual Analog Scale (VAS; Middleton et al., 2012), collected at baseline and in 15-minute intervals for the first 2.5 hours, then every 30 minutes for the remaining 3.5 hours; the Addiction Research Center Inventory (ARCI) short form (Martin et al., 1971), presented at baseline and 2 hours and 4 hours after drug administration; the Pharmacological Class Questionnaire (Jasinski et al., 1977), collected once, 6 hours post-dose; a drug street value measure, presented in 30-minute intervals after drug administration; and Participant-Rated Opioid Adjective Scale (Fraser et. al., 1961), presented at baseline and at 30-minute intervals post-dose. Trained research assistants rated signs of opioid agonist effects on the Observer-Rated Opioid Adjective Scale (Fraser et. al., 1961) at baseline at 30-minute intervals after drug administration. For further detail on these measures, please see Walsh et al. (2008) for full descriptions.
2.4.5 Performance and Ocular Measures
The Flicker-Fusion Task, an ocular measure that is sensitive to the visual perception-impairing effects of opioids (Walsh et al., 2008; Stoops et al., 2010) and a 90-second computerized version of the DSST (adopted from McLeod et al., 1982) were collected at baseline at 30-minute intervals after drug administration.
2.4.6 Statistical Analyses
All session measures were initially analyzed as raw time course data using a two-factor repeated measures model (drug condition, time) with an AR(1) covariance structure. However, physiological measures, collected minute-by-minute, were initially averaged across 15–30 min intervals corresponding to the subjective reporting intervals. Peak scores (either minimum or maximum) were analyzed using a one-factor model (drug condition). Time-to-peak effect (e.g., Tmin or Tmax) was calculated for individual subjects and dose conditions and was analyzed in a one-factor model. Dunnett post-hoc tests were performed to explore the time course of the drug effects and to clarify the effects of individual doses on peak score analyses (as compared to placebo). Tukey’s post-hoc tests were used to clarify differences between active dose conditions. Progressive ratio data (number of trials completed and breakpoint) were analyzed using a one-factor model (drug condition). Subjective data from self-administration sessions were not analyzed as volunteers received varying doses. All models were conducted with Proc Mixed in SAS 9.3 (Cary, NC) with significance at p ≤ .05.
3. RESULTS
3.1 Participants
Fourteen participants signed consent for study screening. Two did not meet drug use criteria, one was excluded for medical reasons, one declined due to work obligations and one was excluded because he stated that would only work for money and never choose drug in the self-administration task. A total of nine participants met the qualification criteria, were enrolled and completed the study. All were Caucasian (6 male, 3 female) with an average age of 33 (±2.6, SEM) years. Participants reported current illicit prescription opioid abuse of 9.4 (±1.3) days out of the past 30 days. All reported primarily using oxycodone products, with occasional reports of hydromorphone, hydrocodone and methadone use. None reported current heroin use or current tramadol use. Intranasal use was the most common preferred route (IN=8, PO=1); however, occasional use via IV and smoked routes was reported. Participants reported a history of 7.6 (±2.9) years of opioid abuse, and five subjects reported a prior history of opioid physical dependence. Eight were current cigarette smokers. In the month prior to study participation, one volunteer reported using cocaine (2 days), five reported alcohol use (~4 days) and three reported benzodiazepine use (~2 days).
3.2 Sample Sessions: Pharmacodynamic Outcomes
3.2.1 Physiological Measures
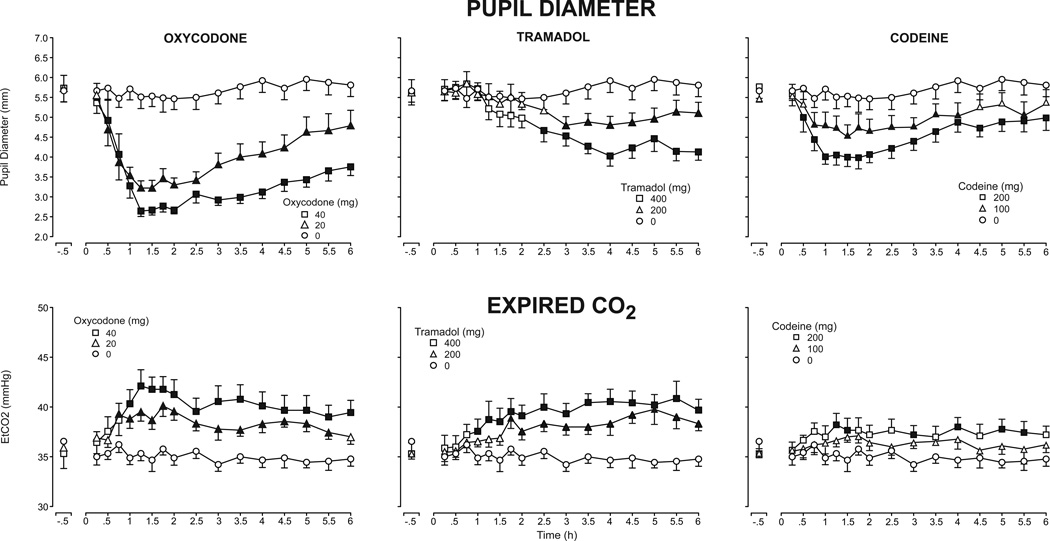
The time course profile of oxycodone, tramadol and codeine effects on pupil diameter are presented in the top panel of Fig. 1. Each of the active doses produced dose-dependent decreases in pupil diameter and peak miotic effects different from placebo (Table 1); however, the magnitude and time course of these effects differed across the three drugs. Oxycodone produced the greatest magnitude of effects, while both tramadol and codeine produced moderate miosis. The peak effects of oxycodone and codeine occurred between 1.1 and 2.4 hours (i.e., calculated tmin), while the peak miotic effects of tramadol occurred much later (calculated tmin = 4.3 to 4.4 hours) (p<.05). The effects of all three drugs were present through the end of the 6-hour session (p<.05), except with the low dose of codeine, which subsided by 4.5 hours.
Figure 1.
Mean pupil diameter (top panel) and expired CO2 (bottom panel) after administration of oxycodone (left column), tramadol (middle column) and codeine (right column) as a function of time following drug administration across the 6-hour session (n=9, ±1 SEM). Time course analysis revealed a significant effect of dose on pupil diameter (F[6,48]=14.79, p<.001), and Dunnett post-hoc tests indicated both doses of oxycodone and the high doses of tramadol and codeine decreased pupil diameter relative to placebo (p<.05). A main effect of dose was also detected on expired CO2 concentrations (F[6,48]=7.98, p<.001), with a significant effect of both oxycodone and tramadol doses and 200 mg codeine on expired CO2, relative to placebo (Dunnett post-hoc, p<.05). Filled symbols indicate means that were significantly different from placebo at a particular time point (Dunnett post-hoc, p<.05).
Table 1.
Mean peak values of measures for which a significant drug effect was detected.
| Outcome Measure | F (6,48) | PLC | OXY 20 | OXY 40 | TRAM 200 | TRAM 400 | CODE 100 | CODE 200 |
|---|---|---|---|---|---|---|---|---|
| Physiological Effects | ||||||||
| Expired CO2 | 7.0 | 38.1 (0.7) | 42.2 (0.8) | 44.1 (1.4) | 41.3 (1.3) | 42.9 (1.6) | 39.3 (0.6) | 40.2 (1.0) |
| Pupil Diameter | 34.5 | 5.2 (0.3) | 3.0 (0.2) | 2.5 (0.1) | 4.5 (0.2) | 3.8 (0.2) | 4.3 (0.3) | 3.7 (0.2) |
| Respiration Rate | 3.2 | 13.9 (0.8) | 12.4 (0.6) | 11.9 (0.7) | 12.3 (0.5) | 12.3 (0.5) | 13.7 (0.7) | 12.4 (0.8) |
| Subjective Effects | ||||||||
| Visual Analog Scales | ||||||||
| Drug Effect | 10.7 | 0.1 (0.1) | 42.1 (8.8) | 48.6 (6.6) | 26.2 (8.1) | 39.9 (6.3) | 16.9 (5.2) | 42.1 (9.8) |
| High | 9.7 | 0.0 (0.0) | 40.8 (9.6) | 47.7 (7.0) | 23.6 (8.8) | 40.2 (6.7) | 15.9 (5.3) | 36.2 (10.2) |
| Good Drug Effect | 10.0 | 0.0 (0.0) | 42.2 (8.9) | 48.1 (6.1) | 24.7 (8.8) | 35.7 (8.2) | 15.3 (5.2) | 36.7 (9.8) |
| Like Drug Effect | 9.0 | 0.0 (0.0) | 48.4 (9.2) | 53.6 (7.3) | 26.2 (10.0) | 36.9 (9.0) | 15.1 (5.2) | 37.9 (10.6) |
| Bad Drug Effect | 5.9 | 0.0 (0.0) | 0.7 (0.7) | 4.1 (3.1) | 2.9 (2.4) | 22.2 (6.9) | 2.2 (1.3) | 17.2 (6.5) |
| Street Value | 6.7 | 0.2 (0.2) | 20.0 (7.2) | 19.9 (4.2) | 12.3 (6.1) | 15.4 (4.4) | 8.3 (4.2) | 17.8 (5.5) |
| Participant Side Effects | ||||||||
| Agonist Sub-Scale | 5.6 | 4.9 (0.9) | 13.1 (2.1) | 13.6 (2.1) | 9.8 (1.8) | 11.8 (1.9) | 8.1 (1.5) | 12.1 (2.5) |
| Itchy Skin | 9.1 | 0.0 (0.0) | 1.2 (0.2) | 1.8 (0.2) | 0.7 (0.2) | 1.1 (0.3) | 0.9 (0.2) | 2.3 (0.5) |
| Turning Stomach | 3.3 | 0.2 (0.1) | 0.1 (0.1) | 0.4 (0.2) | 0.4 (0.2) | 1.2 (0.3) | 0.3 (0.2) | 0.8 (0.4) |
| Nodding | 4.6 | 0.0 (0.0) | 1.0 (0.4) | 1.0 (0.3) | 0.4 (0.2) | 1.0 (0.4) | 0.1 (0.1) | 0.7 (0.3) |
| Relaxed | 3.9 | 0.9 (0.3) | 2.2 (0.3) | 2.1 (0.3) | 1.7 (0.3) | 1.9 (0.3) | 1.2 (0.3) | 1.9 (0.4) |
| Talkative | 2.8 | 0.1 (0.1) | 1.0 (0.5) | 1.2 (0.4) | 0.8 (0.3) | 0.7 (0.4) | 0.4 (0.3) | 1.0 (0.4) |
| Drive | 2.6 | 0.3 (0.2) | 1.3 (0.5) | 0.4 (0.3) | 0.8 (0.3) | 0.6 (0.3) | 0.7 (0.3) | 0.6 (0.3) |
| Carefree | 2.6 | 0.4 (0.2) | 1.6 (0.5) | 1.1 (0.4) | 0.7 (0.3) | 1.0 (0.5) | 0.6 (0.3) | 0.8 (0.4) |
| Yawning | 2.4 | 0.9 (0.3) | 0.2 (0.1) | 0.4 (0.3) | 0.6 (0.3) | 0.2 (0.1) | 0.3 (0.2) | 0.3 (0.2) |
| Sick to Stomach | 2.4 | 0.0 (0.0) | 0.1 (0.1) | 0.6 (0.3) | 0.3 (0.2) | 0.9 (0.4) | 0.3 (0.2) | 0.4 (0.2) |
| Observer-Rated Effects | ||||||||
| Observer Adjectives | ||||||||
| Agonist Sub-Scale | 13.8 | 5.0 (0.4) | 10.2 (1.4) | 13.4 (1.0) | 7.6 (1.1) | 9.4 (1.1) | 7.1 (0.6) | 11.0 (1.1) |
| Itchy Skin | 8.8 | 0.0 (0.0) | 0.7 (0.2) | 1.6 (0.3) | 0.1 (0.1) | 0.7 (0.2) | 0.3 (0.2) | 1.8 (0.4) |
| Nodding | 2.8 | 0.0 (0.0) | 0.6 (0.2) | 1.1 (0.5) | 0.4 (0.3) | 0.7 (0.3) | 0.0 (0.0) | 0.4 (0.3) |
| Talkative | 4.5 | 0.6 (0.2) | 1.7 (0.4) | 1.8 (0.3) | 1.0 (0.2) | 1.2 (0.3) | 1.3 (0.3) | 1.7 (0.2) |
| Heavy | 3.0 | 0.1 (0.1) | 0.8 (0.2) | 1.1 (0.3) | 0.4 (0.2) | 0.7 (0.2) | 0.2 (0.1) | 0.7 (0.2) |
| Ocular Task | ||||||||
| Flicker Fusion #1 | 8.9 | 36.5 (1.2) | 33.1 (1.2) | 33.1 (1.2) | 34.7 (1.4) | 32.7 (1.9) | 35.8 (1.5) | 33.9 (1.5) |
| Flicker Fusion #2 | 6.9 | 35.9 (1.5) | 33.0 (1.3) | 33.0 (1.3) | 34.9 (1.6) | 31.9 (2.2) | 35.4 (1.5) | 33.1 (1.7) |
All measures were analyzed as peak maximum score, with the exception of pupil diameter, respiration rate and both Flicker Fusion means (which are trough or minimum scores). Values are mean peak scores and standard error of the mean for placebo (PLC), oxycodone (OXY), tramadol (TRAM) and codeine (CODE) (numbers next to drug abbreviations are doses expressed in mg). Bolded values indicate the mean is significantly different from the corresponding placebo value (p < 0.5, Dunnett post-hoc).
The time course profile of end tidal CO2 (EtCO2) concentrations is displayed in the bottom panel of Fig. 1. Peak EtCO2 concentrations were significantly increased after administration of both oxycodone doses and the high tramadol dose, relative to placebo (Table 1), while codeine produced modest effects, with the time course analyses indicating a significant effect of codeine 200 mg (p=.049). Oxycodone and tramadol produced effects of similar magnitude; however, the peak effects of tramadol (between 3.6 and 4 hours; i.e., calculated Tmax) occurred much later than those of oxycodone (1.4 – 1.6 hours), with the high doses of oxycodone and tramadol producing significantly different time course effects (p<.05). The effects of oxycodone and tramadol persisted through the end of the session, except for oxycodone (20 mg), which did not differ from placebo by 6 hours (p>.05).
None of the active doses produced significant effects on oxygen saturation, heart rate or systolic or diastolic blood pressure. Time course analyses indicated that respiratory rate was significantly decreased by both doses of oxycodone only.
3.2.2 Ocular and performance measures
Significant peak opioid effects were also detected on the Flicker Fusion task, such that both oxycodone doses and the high dose of tramadol decreased thresholds for flicker detection and the high doses of oxycodone and tramadol decreased thresholds for fusion detection, relative to placebo (Table 1). No differences in DSST rate or accuracy were observed.
3.2.3 Subjective Effects
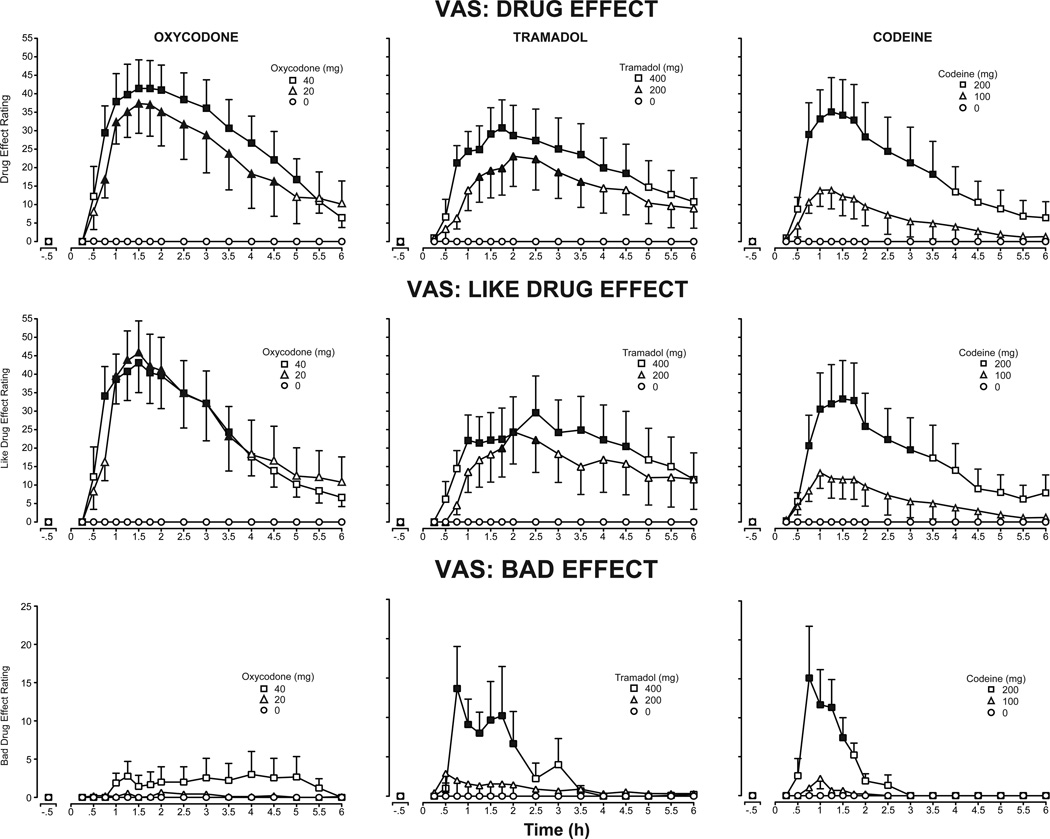
Figure 2 presents time course effects for ratings of the visual analog questions “Do you feel any drug effect?” (top panel), “How much do you like the drug?” (middle panel) and “Does the drug have any bad effects?” (bottom panel). All active doses, except for codeine (100 mg), increased peak ratings of Drug Effect and Like Drug Effects, relative to placebo (Table 1). The Tmax of each drug occurred at approximately similar times (between 0.87 and 1.6 hours post-dose); however, the magnitude of the effects differed across drugs, with oxycodone and codeine (200 mg) producing the highest scores. Comparable peak ratings of Bad Drug Effects occurred after the high doses of tramadol and codeine (Table 1), while oxycodone produced minimal effects. Tramadol effects occurred between 0.75 to 2 hours post-dose, and codeine effects were present between 0.75 to 1.5 hours post-dose.
Figure 2.
Mean VAS ratings of the subjective measures Drug Effect (top panel), Like Drug Effect (middle panel) and Bad Drug Effect (bottom panel) after administration of oxycodone (left column), tramadol (middle column) and codeine (right column) as a function of time following drug administration across the 6-hour session (n=9, ±1 SEM). Time course analysis indicated a main effect of dose on VAS Drug Effect (F[6,48]=5.6, p<.001), Like Drug Effect (F[6,48]=4.8, p<.001) and Bad Drug Effect (F[6,48]=3.8, p<.004). Dunnett post-hoc tests indicated both doses of oxycodone increased ratings of Drug Effect and Like Drug Effect, while the high dose of tramadol increased ratings of Bad Drug Effect, relative to placebo (p<.05). Filled symbols indicate means that were significantly different from placebo at a particular time point (Dunnett post-hoc, p<.05).
A main peak effect of dose was also detected for several agonist-like subjective effects, including the VAS items Good Drug Effect and High, and the Opioid Agonist Scales for both the Participant and Observer-Rated Adjectives (Table 1).
3.2.4 Drug Identification
All volunteers identified oxycodone and 400 mg tramadol as opioid agonists on the Drug Identification Questionnaire. All volunteers also correctly identified placebo. The remaining doses were also primarily identified as opioid agonists, with a few exceptions where volunteers identified the doses as placebo (200 mg tramadol: n=2, 100 mg codeine: n=2; 200 mg codeine: n=1).
3.3 Drug Self-Administration Outcomes
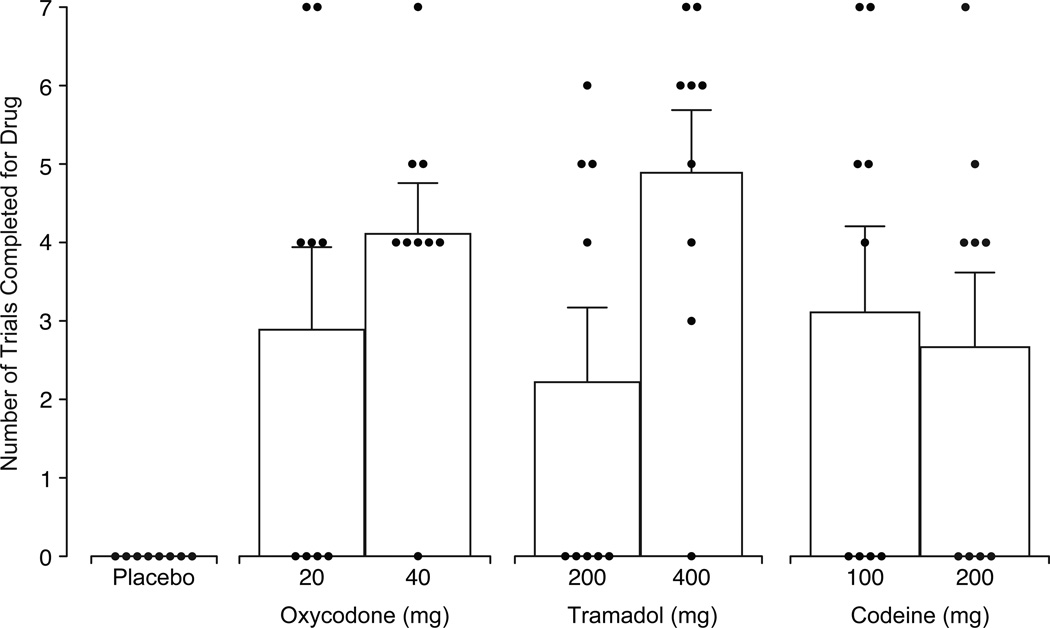
Figure 3 displays the number of trials completed on the Progressive Ratio task for drug responding as a function of test dose. All active doses were self-administered, while placebo engendered no responding. Both doses of oxycodone functioned as reinforcers, as 100 mg oxycodone maintained moderate levels of self-administration, while 200 mg was reliably self-administered, and both were significantly different from placebo (20 mg: p=.014, 40 mg: p=.001). The mean amount of oxycodone self-administered was 8 mg (±3 mg) and 24 mg (±4 mg) in the 20 and 40 mg conditions. Tramadol also functioned as a dose-dependent reinforcer; the low dose did not differ significantly from placebo but the high dose was readily self-administered (p <.001). Participants self-administered a mean of 63 mg (±27 mg) of tramadol in the 200 mg condition and 279 mg (±46 mg) of tramadol in the 400 mg condition. Codeine maintained moderate levels of self-administration, but the effects were not dose-dependent. Both codeine doses functioned as reinforcers (100 mg: p=.007; 200 mg: p= .027), with responding comparable to oxycodone 20 mg. Participants self-administered a mean of 44 mg (±16 mg) and 76 mg (±27 mg) of codeine in the 100 and 200 mg conditions, respectively.
Figure 3.
Mean number of trials completed for drug as a function of dose condition, with small circles representing the number of trials completed by individual subjects (n=9, ±1 SEM). A main effect of dose (F[6,48]=5.7, p<.001) was detected and Dunnett post-hoc tests indicated all doses except the low dose of tramadol were significantly different from placebo (p <.05).
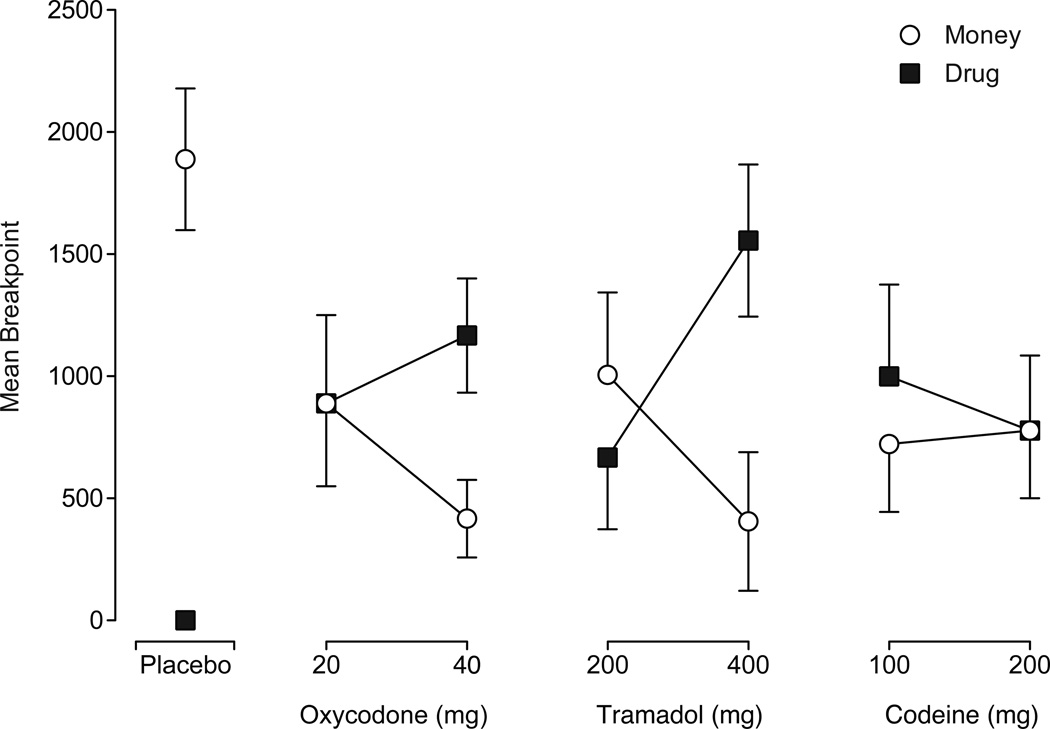
Figure 4 presents drug and money breakpoints generated under each condition. Drug breakpoints were the greatest under 400 mg tramadol (p<.001), followed by 40 mg oxycodone (p=.002), 100 mg codeine (p=.008), and 20 mg oxycodone (p=.022). Moderate drug breakpoints were achieved for the remaining active doses, but none differed significantly from placebo. Money breakpoints were greatest when placebo was available and lowest when the high doses of oxycodone and tramadol were available. Moderate levels of responding occurred under all other dose conditions. All money breakpoints were significantly different from placebo (p<.05).
Figure 4.
Mean breakpoints for drug and money as a function of dose condition, with filled squares representing mean drug breakpoints and open circles representing mean money breakpoints (n=9, ±1 SEM). A main effect of dose (F[6,48]=5.2, p<.001) was detected on drug breakpoints and all breakpoints except those under the low dose of tramadol and the high dose of codeine were significantly different from placebo (Dunnett post-hoc, p <.05). A main effect of dose was also detected on money breakpoints (F[6,48]=4.8, p<.001) and all money breakpoints were significantly different from placebo (Dunnett post-hoc, p<.05).
4. DISCUSSION
This study examined the subjective, physiological and reinforcing effects of oral tramadol in comparison to oral oxycodone, codeine and placebo in prescription opioid abusers. Collectively, all three agents produced opioid-like effects on numerous outcomes (miosis, positive subjective effects and opioid agonist signs and symptoms); however, the relative magnitude, time course, and composite profile of effects differed considerably among the drugs. Interestingly, all active drugs were self-administered to varying degrees, indicating that each acts as a reinforcer. Placebo was never self-administered. All of the medication conditions were well tolerated, and no serious adverse events occurred.
All active doses produced dose-related miosis; however, the time-to-peak effect (Tmax) was substantially later for tramadol (i.e., 4 hours) than for oxycodone or codeine (i.e., 1 – 2.4 hours). Moreover, the duration of tramadol-induced miosis was briefer (3 – 3.5 hours) compared to oxycodone and codeine (5 – 5.5 hours). Maximum miosis, as a proxy for intrinsic activity, differed among the drugs with oxycodone > codeine = tramadol. The delayed onset and sub-maximal miosis with tramadol may be attributable to its mixed pharmacological profile. A recent report suggests that tramadol produces opposing pupillary actions of mydriasis (monoaminergic effects of the parent drug) and miosis (opioid effects of M1; Stoops et al., 2012). Here, miosis emerged at 2.5 hours, coinciding with the Tmax of M1 (2.3 hours) as opposed to tramadol itself (~1.5 hours) (Ardakani and Rouini, 2007). As both tramadol and M1 have relatively long half-lives (7 and 8 hours, respectively) (Ardakani and Rouini, 2007), the parent drug may act to prevent maximal M1 miosis.
Both doses of tramadol and oxycodone (and the high dose of codeine only modestly) decreased respiratory function, as measured by EtCO2 concentrations and did so to a comparable extent. As with miosis, the Tmax for tramadol (3.5 – 4 hr) on EtCO2 was later than with oxycodone (1 – 1.5 hr) and consistent with the time-action of M1. Previous studies have reported that parenteral tramadol did not significantly reduce respiratory function (Tarkkila et al., 1997; Mildh et al., 1999; Warren et. al., 2000); thus, these findings demonstrating respiratory depression at therapeutic and supratherapeutic oral doses have implications for safety and drug overdose risk.
All active doses (except for low dose codeine, which was placebo-like) significantly increased ratings on subjective measures associated with abuse liability, such as “high,” “liking” for the drug, and street value estimates. Both doses of oxycodone, 400 mg tramadol, and 200 mg codeine increased µ-opioid specific measures (e.g., itchy skin, Participant- and Observer-Rated Opioid Agonist Subscale). The time course was similar to those presented in Fig. 2, with a Tmax of 1–2 hours. The magnitude of effects was typically greatest for oxycodone, followed by the high doses of tramadol and codeine, which were comparable (see Table 1); these findings are consistent with prior studies (Jasinski et al., 1993; Epstein et al., 2006). Codeine (100 mg) produced minimal subjective effects, consistent with studies reporting on low (60–120 mg) codeine doses (Kim et al., 2002; Oyler et al., 2000; Stacher et al., 1987), while 200 mg was active and more similar to 40 mg oxycodone.
In the present study, self-administration of oxycodone and tramadol was dose dependent (see Fig. 3). Two prior studies have examined oxycodone self-administration in non-dependent subjects, with one reporting dose dependent drug-taking for intranasal oxycodone (14, 28 mg; Middleton et al., 2012) and the other reporting oral self-administration (15 and 30 mg) only in the presence of a painful stimulus (i.e., cold pressor), which was not dose-dependent (Comer et al., 2010). This is the first human study directly examining the reinforcing effects of oral tramadol, and the results indicate that tramadol exhibits reinforcing efficacy comparable to oxycodone at supratherapeutic doses. Eight volunteers self-administered drug in the high dose tramadol and oxycodone conditions, identifying these doses as opioid agonists, while one, who reported nausea after tramadol and vomited after oxycodone, did not work for either. Low dose tramadol failed to significantly increase self-administration, with five volunteers working for none and four working for 4/7th-6/7th of the dose. Breakpoints for 400 mg tramadol were the highest observed in the study, higher even than for oxycodone 40 mg. However, 400 mg tramadol produced lower subjective ratings on many abuse liability measures compared to oxycodone 40 mg. There are two possible explanations for these findings. Firstly, tramadol also increased ratings of “bad drug effects,” and these included nausea, flushing and, in one case, vomiting, leading to a composite profile of both positive and negative effects. Secondly, it is possible that the 6-hr collection of subjective measures did not fully capture the time action profile given the reported half-lives of 7 and 8 hours for tramadol and M1, respectively. In several instances, participants reported that opioid-like effects either intensified and/or reemerged after session termination (particularly after cigarette smoking); these latter effects, while unmeasured, may have contributed to the higher rates of self-administration.
While codeine misuse and abuse has been well documented (McAvoy et al., 2011; Peters et al., 2007; Romach et al., 2000; Schuster et al., 1971), few human laboratory studies have examined its abuse liability profile. This study found that 100 and 200 mg oral codeine were self-administered, but with breakpoints lower than those for the high doses of oxycodone and tramadol. Interestingly, low dose codeine appeared placebo-like on subjective measures and yet was self-administered to a comparable extent as the higher dose. The high dose of codeine increased subjective measures associated with abuse liability but also increased ratings of Bad Effects and produced transient and mild hives, itchy skin, flushing and nausea on some occasions. These findings may account for the failure of high dose codeine to engender greater self-administration. Overall, codeine displayed less pharmacological activity on physiological measures, such as miosis and increased expired CO2, compared to oxycodone, consistent with prior studies reporting lower intrinsic efficacy for codeine at the µ-opioid receptor compared to full mu agonists (Sudheer et al., 2007; Walker and Zacny, 1998; Volpe et al., 2011). It is possible that individual differences in P450 2D6 activity could decrease codeine effects by decreasing its metabolism to morphine (Kirchheiner et al., 2007; Crews et al., 2006). Although no genotyping was conducted, it seems unlikely that this was a mediating factor, as tramadol is o-demethylated to M1 through this same pathway (Wu et al., 2002; Grond and Sablotzki, 2004) and exhibited robust activity, particularly with miosis (a measure which functions as a biomarker for 2D6 function after tramadol administration; Matouskova et al., 2011).
Overall, this study demonstrates that oral tramadol has reinforcing efficacy in prescription opioid abusers under double blind experimental conditions, confirming its abuse potential in this population. Prior to its marketing in the U.S., clinical abuse liability studies as well as surveillance studies conducted in Germany suggested limited evidence of tramadol abuse, particularly by the parenteral route; however, more recent reports of tramadol abuse, diversion and overdose have emerged from several countries, including the U.S. (Brinker et al., 2002; Skipper et al., 2004; Marquardt et al., 2005; Tjäderborn et al., 2007). With the present epidemic of opioid abuse in the U.S., there are more individuals misusing opioids as a class of compounds and tramadol prescribing is increasing (e.g., currently in the top 25 most prescribed medications in the U.S.; IMS, 2012), and is often prescribed as an alternative to full mu agonists (particularly in circumstances where drug misuse and drug seeking are suspected, e.g., emergency department prescribing). Given these circumstances, along with the fact that similar agents have been recently approved (e.g., tapentadol, which interestingly is registered as Schedule II in the U.S.), it is important to expand our understanding of the relative abuse and safety risks associated with atypical opioid analgesic compounds. As tramadol remains unscheduled nationally by the U.S., but has been scheduled by a few states, these data may provide empirical evidence for regulatory reconsideration.
Acknowledgements
We thank the staff at the University of Kentucky (UK) Center on Drug and Alcohol Research and Center for Human Behavioral Science, particularly Pamela A. Henderson and Victoria A. Vessels for their technical expertise, Drs. Stephen Sitzlar and Jeffery Carrico at the UK Investigational Pharmacy for preparing the study medications and the nursing staff at the UK Center for Clinical and Translational Science (CCTS) for providing patient care.
Role of funding source: Grants from National Institute on Drug Abuse (R01DA016718 [SLW] and T32 DA 007304 [SB]) and the National Center for Research Resources UL1RR033173 (UK CCTS) provided support for this project. These institutes had no role in the study design, collection, analysis or interpretation of the data, writing of the report or in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors: Shanna Babalonis directly supervised the conduct of the study, interviewed and consented the participants, directed the statistical analyses and wrote the manuscript. Michelle Lofwall conducted medical interviews and physical examinations, reviewed laboratory results, provided medical coverage and edited the manuscript. Paul Nuzzo trained the staff, provided technical support services, supervised daily operations and conducted the statistical analyses. Anthony Siegel conducted daily medical exams while participants resided as inpatients and provided medical coverage. Sharon Walsh designed the study, supervised the conduct of the study and statistical analyses, and assisted with writing the manuscript.
Conflict of interest
Shanna Babalonis, Michelle Lofwall, Paul Nuzzo, Anthony Siegel, and Sharon Walsh have no conflicts of interest to report.
REFERENCES
- Ardakani YH, Rouini MR. Pharmacokinetics of tramadol and its three main metabolites in healthy male and female volunteers. Biopharm. Drug Dispos. 2007;28:527–534. doi: 10.1002/bdd.584. [DOI] [PubMed] [Google Scholar]
- Barsotti CE, Mycyk MB, Reyes J. Withdrawal syndrome from tramadol hydrochloride. Am. J. Emerg. Med. 2003;21:87–88. doi: 10.1053/ajem.2003.50039. [DOI] [PubMed] [Google Scholar]
- Brinker A, Bonnel RA, Beitz J. Abuse, dependence, or withdrawal associated with tramadol. Am. J. Psychiatry. 2002;159:881. doi: 10.1176/appi.ajp.159.5.881. author reply 881–882. [DOI] [PubMed] [Google Scholar]
- Cami J, Lamas X, Farre M. Acute effects of tramadol in methadone-maintained volunteers. Drugs. 1994;47(Suppl. 1):39–43. doi: 10.2165/00003495-199400471-00007. [DOI] [PubMed] [Google Scholar]
- Campanero MA, Calahorra B, Valle M, Troconiz IF, Honorato J. Enantiomeric separation of tramadol and its active metabolite in human plasma by chiral high-performance liquid chromatography: application to pharmacokinetic studies. Chirality. 1999;11:272–279. doi: 10.1002/(SICI)1520-636X(1999)11:4<272::AID-CHIR3>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Carroll CP, Walsh SL, Bigelow GE, Strain EC, Preston KL. Assessment of agonist and antagonist effects of tramadol in opioid-dependent humans. Exp. Clin. Psychopharmacol. 2006;14:109–120. doi: 10.1037/1064-1297.14.2.109. [DOI] [PubMed] [Google Scholar]
- Cicero TJ, Adams EH, Geller A, Inciardi JA, Munoz A, Schnoll SH, Senay EC, Woody GE. A postmarketing surveillance program to monitor ultram (tramadol hydrochloride) abuse in the United States. Drug Alcohol Depend. 1999;57:7–22. doi: 10.1016/s0376-8716(99)00041-1. [DOI] [PubMed] [Google Scholar]
- Cicero TJ, Inciardi JA, Adams EH, Geller A, Senay EC, Woody GE, Munoz A. Rates of abuse of tramadol remain unchanged with the introduction of new branded and generic products: results of an abuse monitoring system, 1994–2004. Pharmacoepidemiol. Drug Saf. 2005;14:851–859. doi: 10.1002/pds.1113. [DOI] [PubMed] [Google Scholar]
- Comer SD, Sullivan MA, Vosburg SK, Kowalczyk WJ, Houser J. Abuse liability of oxycodone as a function of pain and drug use history. Drug Alcohol Depend. 2010;109:130–138. doi: 10.1016/j.drugalcdep.2009.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews KR, Gaedigk A, Dunnenberger HM, Klein TE, Shen DD, Callaghan JT, Kharasch ED, Skaar TC. Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines for codeine therapy in the context of cytochrome P450 2D6 (CYP2D6) genotype. Clin. Pharmacol. Ther. 2012;91:321–326. doi: 10.1038/clpt.2011.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dart RC, Dasgupta N, Bailey JE, Spiller HA. Interpreting poison center call volume associated with tramadol. Ann. Pharmacother. 2011;45:424. doi: 10.1345/aph.1P064b. [DOI] [PubMed] [Google Scholar]
- Desmeules JA, Piguet V, Collart L, Dayer P. Contribution of monoaminergic modulation to the analgesic effect of tramadol. Br. J. Clin. Pharmacol. 1996;41:7–12. doi: 10.1111/j.1365-2125.1996.tb00152.x. [DOI] [PubMed] [Google Scholar]
- Duke AN, Bigelow GE, Lanier RK, Strain EC. Discriminative stimulus effects of tramadol in humans. J. Pharmacol. Exp. Ther. 2011;338:255–262. doi: 10.1124/jpet.111.181131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enggaard TP, Poulsen L, Arendt-Nielsen L, Brosen K, Ossig J, Sindrup SH. The analgesic effect of tramadol after intravenous injection in healthy volunteers in relation to CYP2D6. Anesth. Analg. 2006;102:146–150. doi: 10.1213/01.ane.0000189613.61910.32. [DOI] [PubMed] [Google Scholar]
- Epstein DH, Preston KL, Jasinski DR. Abuse liability, behavioral pharmacology, and physical-dependence potential of opioids in humans and laboratory animals: lessons from tramadol. Biol. Psychol. 2006;73:90–99. doi: 10.1016/j.biopsycho.2006.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser HF, Van Horn GD, Martin WR, Wolbach AB, Isbell H. Methods for evaluating addiction liability. (A) "attitude" of opiate addicts toward opiate-like drugs. (B) a short-term "direct" addiction test. J. Pharmacol. Exp. Ther. 1961;133:371–387. [PubMed] [Google Scholar]
- Freye E, Levy J. Acute abstinence syndrome following abrupt cessation of long-term use of tramadol (ultram): a case study. Eur. J. Pain. 2000;4:307–311. doi: 10.1053/eujp.2000.0187. [DOI] [PubMed] [Google Scholar]
- Gillen C, Haurand M, Kobelt DJ, Wnendt S. Affinity, potency and efficacy of tramadol and its metabolites at the cloned human mu-opioid receptor. Naunyn Schmiedebergs Arch. Pharmacol. 2000;362:116–121. doi: 10.1007/s002100000266. [DOI] [PubMed] [Google Scholar]
- Grond S, Sablotzki A. Clinical pharmacology of tramadol. Clin. Pharmacokinet. 2004;43:879–923. doi: 10.2165/00003088-200443130-00004. [DOI] [PubMed] [Google Scholar]
- IMS Health. National Prescription Audit, Top 25 Pharmaceutical Products by Dispensed Prescriptions in the US Market. 2012 http://www.imshealth.com.
- Inciardi JA, Cicero TJ, Munoz A, Adams EH, Geller A, Senay EC, Woody GE. The diversion of ultram, ultracet, and generic tramadol HCL. J. Addict. Dis. 2006;25:53–58. doi: 10.1300/J069v25n02_08. [DOI] [PubMed] [Google Scholar]
- Jasinski DR, Griffith JD, Pevnick JS, Gorodetzky C, Cone E, Kay D. Washington, DC: National Research Council, National Academy of Sciences; 1977. Progress Report from the Clinical Pharmacology Section of the NIDA Addiction Research Center, 39th Annual Meeting, The Committee on the Problems of Drug Dependence; pp. 133–168. [Google Scholar]
- Jasinski DR, Preston KL, Sullivan JT, Testa MP. Abuse potential of oral tramadol. NIDA Research Monograph. 1993;132:103. [Google Scholar]
- Kim I, Barnes AJ, Oyler JM, Schepers R, Joseph RE, Jr, Cone EJ, Lafko D, Moolchan ET, Huestis MA. Plasma and oral fluid pharmacokinetics and pharmacodynamics after oral codeine administration. Clin. Chem. 2002;48:1486–1496. [PubMed] [Google Scholar]
- Kirchheiner J, Schmidt H, Tzvetkov M, Keulen JT, Lotsch J, Roots I, Brockmoller J. Pharmacokinetics of codeine and its metabolite morphine in ultra-rapid metabolizers due to CYP2D6 duplication. Pharmacogenomics J. 2007;7:257–265. doi: 10.1038/sj.tpj.6500406. [DOI] [PubMed] [Google Scholar]
- Lanier RK, Lofwall MR, Mintzer MZ, Bigelow GE, Strain EC. Physical dependence potential of daily tramadol dosing in humans. Psychopharmacology (Berl.) 2010;211:457–466. doi: 10.1007/s00213-010-1919-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lofwall MR, Walsh SL, Bigelow GE, Strain EC. Modest opioid withdrawal suppression efficacy of oral tramadol in humans. Psychopharmacology (Berl.) 2007;194:381–393. doi: 10.1007/s00213-007-0847-3. [DOI] [PubMed] [Google Scholar]
- Marquardt KA, Alsop JA, Albertson TE. Tramadol exposures reported to statewide poison control system. Ann. Pharmacother. 2005;39:1039–1044. doi: 10.1345/aph.1E577. [DOI] [PubMed] [Google Scholar]
- Martin WR, Sloan BS, Sapira JD, Jasinski DR. Physiologic, subjective and behavioral effects of amphetamine, methamphetamine, ephedrine, phenmetrazine and methylphenidate in man. Clin. Pharmacol. Ther. 1971;12:245–258. doi: 10.1002/cpt1971122part1245. [DOI] [PubMed] [Google Scholar]
- Matouskova O, Slanar O, Chytil L, Perlik F. Pupillometry in healthy volunteers as a biomarker of tramadol efficacy. J. Clin. Pharm. Ther. 2011;36:513–517. doi: 10.1111/j.1365-2710.2010.01203.x. [DOI] [PubMed] [Google Scholar]
- McAvoy BR, Dobbin MD, Tobin CL. Over-the-counter codeine analgesic misuse and harm: characteristics of cases in Australia and New Zealand. N.Z. Med. J. 2011;124:29–33. [PubMed] [Google Scholar]
- McLeod DR, Griffiths RR, Bigelow GE, Yingling JE. An automated version of the digit symbol substitution test (DSST) Behav. Res. Methods Instrum. 1982;14:463–466. [Google Scholar]
- Middleton LS, Lofwall MR, Nuzzo PA, Siegel AJ, Walsh SL. Intranasal oxycodone self-administration in non-dependent opioid abusers. Exp. Clin. Psychopharmacol. 2012;20:310–317. doi: 10.1037/a0028327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mildh LH, Leino KA, Kirvela OA. Effects of tramadol and meperidine on respiration, plasma catecholamine concentrations, and hemodynamics. J. Clin. Anesth. 1999;11:310–316. doi: 10.1016/s0952-8180(99)00047-1. [DOI] [PubMed] [Google Scholar]
- O'Connor EC, Mead AN. Tramadol acts as a weak reinforcer in the rat self-administration model, consistent with its low abuse liability in humans. Pharmacol. Biochem. Behav. 2010;96:279–286. doi: 10.1016/j.pbb.2010.05.018. [DOI] [PubMed] [Google Scholar]
- Oyler JM, Cone EJ, Joseph RE, Jr, Huestis MA. Identification of hydrocodone in human urine following controlled codeine administration. J. Anal. Toxicol. 2000;24:530–535. doi: 10.1093/jat/24.7.530. [DOI] [PubMed] [Google Scholar]
- Peters RJ, Jr, Williams M, Ross MW, Atkinson J, Yacoubian GS., Jr Codeine cough syrup use among African-American crack cocaine users. J. Psychoactive Drugs. 2007;39:97–102. doi: 10.1080/02791072.2007.10399868. [DOI] [PubMed] [Google Scholar]
- Poulsen L, Arendt-Nielsen L, Brosen K, Sindrup SH. The hypoalgesic effect of tramadol in relation to CYP2D6. Clin. Pharmacol. Ther. 1996;60:636–644. doi: 10.1016/S0009-9236(96)90211-8. [DOI] [PubMed] [Google Scholar]
- Preston KL, Jasinski DR, Testa M. Abuse potential and pharmacological comparison of tramadol and morphine. Drug Alcohol Depend. 1991;27:7–17. doi: 10.1016/0376-8716(91)90081-9. [DOI] [PubMed] [Google Scholar]
- Radbruch L, Grond S, Lehmann KA. A risk-benefit assessment of tramadol in the management of pain. Drug Saf. 1996;15:8–29. doi: 10.2165/00002018-199615010-00002. [DOI] [PubMed] [Google Scholar]
- Raffa RB, Friderichs E, Reimann W, Shank RP, Codd EE, Vaught JL. Opioid and nonopioid components independently contribute to the mechanism of action of tramadol, an 'atypical' opioid analgesic. J. Pharmacol. Exp. Ther. 1992;260:275–285. [PubMed] [Google Scholar]
- Romach MK, Otton SV, Somer G, Tyndale RF, Sellers EM. Cytochrome P450 2D6 and treatment of codeine dependence. J. Clin. Psychopharmacol. 2000;20:43–45. doi: 10.1097/00004714-200002000-00008. [DOI] [PubMed] [Google Scholar]
- SAMHSA. Results from the 2006 National Survey on Drug Use and Health: National Findings. Rockville, MD: Substance Abuse and Mental Health Administration; 2007. [Google Scholar]
- Schuster CR, Smith BB, Jaffe JH. Drug abuse in heroin users. An experimental study of self-administration of methadone, codeine, and pentazocine. Arch. Gen. Psychiatry. 1971;24:359–362. doi: 10.1001/archpsyc.1971.01750100069010. [DOI] [PubMed] [Google Scholar]
- Senay EC, Adams EH, Geller A, Inciardi JA, Munoz A, Schnoll SH, Woody GE, Cicero TJ. Physical dependence on Ultram (tramadol hydrochloride): both opioid-like and atypical withdrawal symptoms occur. Drug Alcohol Depend. 2003;69:233–241. doi: 10.1016/s0376-8716(02)00321-6. [DOI] [PubMed] [Google Scholar]
- Skipper GE, Fletcher C, Rocha-Judd R, Brase D. Tramadol abuse and dependence among physicians. JAMA. 2004;292:1818–1819. doi: 10.1001/jama.292.15.1818-b. [DOI] [PubMed] [Google Scholar]
- Spiller HA, Scaglione JM, Aleguas A, Foster H, Durback-Morris L, Scharman EJ, Baker SD. Effect of scheduling tramadol as a controlled substance on poison center exposures to tramadol. Ann. Pharmacother. 2010;44:1016–1021. doi: 10.1345/aph.1P064. [DOI] [PubMed] [Google Scholar]
- Stacher G, Steinringer H, Schneider S, Mittelbach G, Gaupmann G, Abatzi TA, Stacher-Janotta G. Effects of graded oral doses of a new 5-hydroxytryptamine/noradrenaline uptake inhibitor (Ro 15-8081) in comparison with 60 mg codeine and placebo on experimentally induced pain and side effect profile in healthy men. Br. J. Clin. Pharmacol. 1987;24:627–635. doi: 10.1111/j.1365-2125.1987.tb03222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoops WW, Hatton KW, Lofwall MR, Nuzzo PA, Walsh SL. Intravenous oxycodone, hydrocodone, and morphine in recreational opioid users: abuse potential and relative potencies. Psychopharmacology (Berl.) 2010;212:193–203. doi: 10.1007/s00213-010-1942-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoops WW, Lofwall MR, Nuzzo PA, Craig LB, Siegel AJ, Walsh SL. Pharmacodynamic profile of tramadol in humans: Influence of naltrexone pretreatment. Psychopharmacology (Berl.) in press. doi: 10.1007/s00213-012-2739-4. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudheer PS, Logan SW, Terblanche C, Ateleanu B, Hall JE. Comparison of the analgesic efficacy and respiratory effects of morphine, tramadol and codeine after craniotomy. Anaesthesia. 2007;62:555–560. doi: 10.1111/j.1365-2044.2007.05038.x. [DOI] [PubMed] [Google Scholar]
- Tarkkila P, Tuominen M, Lindgren L. Comparison of respiratory effects of tramadol and oxycodone. J. Clin. Anesth. 1997;9:582–585. doi: 10.1016/s0952-8180(97)00147-5. [DOI] [PubMed] [Google Scholar]
- Tjaderborn M, Jonsson AK, Hagg S, Ahlner J. Fatal unintentional intoxications with tramadol during 1995–2005. Forensic Sci. Int. 2007;173:107–111. doi: 10.1016/j.forsciint.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Volpe DA, McMahon Tobin GA, Mellon RD, Katki AG, Parker RJ, Colatsky T, Kropp TJ, Verbois SL. Uniform assessment and ranking of opioid mu receptor binding constants for selected opioid drugs. Regul. Toxicol. Pharmacol. 2011;59:385–390. doi: 10.1016/j.yrtph.2010.12.007. [DOI] [PubMed] [Google Scholar]
- Walker DJ, Zacny JP. Subjective, psychomotor, and analgesic effects of oral codeine and morphine in healthy volunteers. Psychopharmacology (Berl.) 1998;140:191–201. doi: 10.1007/s002130050757. [DOI] [PubMed] [Google Scholar]
- Walsh SL, Nuzzo PA, Lofwall MR, Holtman JR., Jr The relative abuse liability of oral oxycodone, hydrocodone and hydromorphone assessed in prescription opioid abusers. Drug Alcohol Depend. 2008;98:191–202. doi: 10.1016/j.drugalcdep.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren PM, Taylor JH, Nicholson KE, Wraith PK, Drummond GB. Influence of tramadol on the ventilatory response to hypoxia in humans. BrJAnaesth. 2000;85:211–216. doi: 10.1093/bja/85.2.211. [DOI] [PubMed] [Google Scholar]
- Watson WA, Litovitz TL, Rodgers GC, Jr, Klein-Schwartz W, Youniss J, Rose SR, Borys D, May ME. 2002 annual report of the American Association of Poison Control Centers Toxic Exposure Surveillance System. Am. J. Emerg. Med. 2003;21:353–421. doi: 10.1016/s0735-6757(03)00088-3. [DOI] [PubMed] [Google Scholar]
- Wu WN, McKown LA, Liao S. Metabolism of the analgesic drug ULTRAM (tramadol hydrochloride) in humans: API-MS and MS/MS characterization of metabolites. Xenobiotica. 2002;32:411–425. doi: 10.1080/00498250110113230. [DOI] [PubMed] [Google Scholar]
- Yanagita T. Drug dependence potential of 1-(m-methoxyphenyl)-2-dimethylaminomethyl)-cyclohexan-1-ol hydrochloride (tramadol) tested in monkeys. Arzneimittelforschung. 1978;28:158–163. [PubMed] [Google Scholar]
- Zacny JP. Profiling the subjective, psychomotor, and physiological effects of tramadol in recreational drug users. Drug Alcohol Depend. 2005;80:273–278. doi: 10.1016/j.drugalcdep.2005.05.007. [DOI] [PubMed] [Google Scholar]