Abstract
Purpose
Clinically validated biomarkers for anti-angiogenesis agents are not available. We have previously reported associations between candidate VEGFA SNPs and overall survival (OS) in E2100. The associations between tumor VEGFA amplification and outcome are evaluated here.
Patients and Methods
E2100 was a phase III trial comparing paclitaxel with or without bevacizumab for patients with metastatic breast cancer. Fluorescence in situ hybridization to assess gene amplification status for VEGFA was performed on paraffin embedded tumors from 363 patients in E2100. Evaluation for association between amplification status and outcomes was performed.
Results
ER+ or PR+ tumors were less likely to have VEGFA amplification compared with ER/PR-tumors (p=0.020). VEGFA amplification was associated with worse OS (20.2 vs. 25.3 months; p=0.013) in univariate analysis with a trend for worse OS in multivariate analysis (p=0.08). There was a significant interaction between VEGFA amplification, hormone-receptor status, and study arm. Patients with VEGFA amplification and triple negative breast cancers (TNBCs) or HER2 amplification had inferior OS (p=0.047); amplification did not affect OS for those who were ER+ or PR+ and HER2-. Those who received bevacizumab with VEGFA amplification had inferior PFS (p=0.010) and OS (p=0.042); no association was seen in the control arm. Test for interaction between study arm and VEGFA amplification with OS was not significant.
Conclusion
VEGFA amplification in univariate analysis was associated with poor outcomes; this was particularly prominent in HER2+ or TNBCs. Additional studies are necessary to confirm the trend for poor OS seen on multivariate analysis for patients treated with bevacizumab.
Keywords: Breast cancer, VEGF amplification, bevacizumab
Background
E2100 was a randomized, phase III trial in the first line setting for patients with metastatic breast cancer.1 This trial demonstrated a significant improvement in progression free survival (PFS) for the addition of bevacizumab to paclitaxel when compared to paclitaxel alone. These data led to the accelerated approval by the United States Food and Drug Administration (US FDA) for bevacizumab in this setting. Although subsequent first line trials such as AVADO and RIBBON-1 also met their primary endpoints for showing significantly improved PFS, the absolute improvements in median PFS were much more modest than in the original E2100 trial, and none of the trials demonstrated an improvement in overall survival (OS).2,3 Based on these more modest benefits in combination with a unique toxicity profile, the US FDA subsequently recommended rescinding approval for bevacizumab in this setting.4
One major drawback for the clinical use of bevacizumab (as well as other anti-angiogenic therapies) is the lack of a validated biomarker to predict which patients might be expected to gain the most benefit.5,6 Our group previously reported single nucleotide polymorphisms (SNPs) that predicted a genetic subgroup that derived substantial benefit in OS for those who received bevacizumab in E2100.7 These SNPs have been tested in other settings, and have been validated in some but not all studies.8–10 Somatic aberrancy also has great potential to serve as a prognostic or predictive marker.11 Gene amplification and deletion are common aberrancies and is the basis for one of the most successful predictive and prognostic biomarkers to date for breast cancer (i.e. HER2 amplification).12 Those with HER2 amplified tumors gain substantial benefit from therapies that target the HER2 protein, including trastuzumab,13–16 lapatinib,17 pertuzumab,18 and T-DM1.19 In this correlative study of E2100, we evaluate the ability for tumor amplification of the target gene of bevacizumab, VEGFA, to predict outcome.
Patients and Methods
Samples
In the E2100 parent trial there were 671 eligible patients with 641 disease progression events and 544 deaths as of May 1, 2009.1 Patients were randomized to paclitaxel with bevacizumab (Arm A) or paclitaxel alone (Arm B). The results from the parent trial have previously been reported. Paraffin embedded tumor blocks were available from 367 for assessment of VEGFA amplification by fluorescence in situ hybridization (FISH). In all cases, these blocks were derived from the patient’s primary tumor. Median follow-up for surviving patients was 59 months at the time of this analysis. All specimens were provided to the investigators of this trial in a de-identified manner. For VEGFA FISH, 178 samples were available from Arm A and 189 from Arm B. This retrospective correlative trial was approved by the Institutional Review Board at Indiana University and The North American Breast Cancer Group Correlative Sciences Committee.
VEGFA FISH
A VEGFA/centromere enumerization-6 (CEN-6) probe set was previously created and validated.20 The validation included a test of the DNA clones by restriction enzyme fragment measurements. The final product contained a BAC probe, RP11-710-L16, covering 183 KB including the VEGFA gene and flanking regions with a start position of 43633251 and an end position of 43817196 according to UCSC Genome Browser on Human Feb. 2009 (GRCh37/hg19) Assembly. Upstream the probe overlaps the entire MRPS18 gene (human mitochondrial ribosomal protein S18-2) and 20% of the RSPH9 gene; downstream there is overlap with approximately 67% of the AK097853 gene. The CEN-6 probe was labeled with fluorescein isothiocyanate (FITC) labeled peptide nucleic acid (PNA) oligonucleotides and the VEGFA probe labeled with Texas Red. Both the VEGFA and CEN-6 probes were tested on metaphase spreads to localize the signals to chromosome 6 and exclude cross-hybridization to other chromosomes. The concentration of Texas-Red VEGFA and FITC CEN-6 were fine-tuned to give well-balanced red and green signals when hybridized on human breast cancer tissue.
A tissue microarray with 93 human primary breast tumor core specimens was then evaluated by FISH for the presence of VEGFA gene amplification and deletion on formalin- fixed, paraffin-embedded tumors using a protocol similar to the manufacturer’s protocol for TOP2A FISH pharmDx™ Kit.21 Results were interpreted using a fluorescence microscope equipped with appropriate filters for the fluorophors. Cancer cells were located and then scored for total number of VEGFA and/CEN-6 signals. A ratio was calculated from the average number of signals for each probe. Normal cells in the analyzed tissue section served as an internal positive control of pretreatment and hybridization efficiency. Based on this validation array, a ratio <0.8 was considered deleted; a ratio ≥ 1.5 but < 2 was considered borderline amplified; and a ratio ≥ 2 was considered amplified.
All samples were scored by an experienced technologist using the TOP2A FISH scoring guidelines.21 The signals were preferably scored in three distinct tumor areas and totaled. The signals were scored in non-overlapping nuclei where bright and point-shaped signals of balanced size could be identified. Nuclei were scored until 60 red VEGFA signals were reached and then the green signals were scored in the same nuclei.22 A minimum of 6 nuclei were scored and a total of 60 nuclei were scored in samples at or near the cut-off (1.80–2.20 for amplification and 0.70–0.90 for deletion) or near the 1.5 ratio for borderline amplification. Reproducibility was tested in 17 samples with inter-observer concordance 88.2%; these were re-scored by a second evaluator who counted nuclei from 3 more tumor areas until 60 red signals were reached. The final ratio for each specimen was based on all scoring.
Statistical Design
Comparison of VEGFA amplification with PFS and OS
To optimize power, we combined those patients who had a tumor that was amplified and borderline amplified (this group will be referred to as amp/BA). Those with amp/BA status were compared to all other groups (normal + deleted). We evaluated the progression free survival (PFS) and overall survival (OS) for those with amp/BA status compared with those who did not in univariate analysis. Univariate analysis was conducted using log-rank test. We also performed multivariate analysis with Cox proportional hazard model using significant covariates from backward elimination stepwise regression. VEGFA amplification tests were formally evaluated using Kaplan Meier (KM) curves. These were also performed on subgroups based on estrogen receptor/progesterone receptor (ER/PR) status and arm of study. A formal test for interaction between arm of study and amplification status was also performed using the Cox regression model. Cox regression analyses and KM curves were conducted in R. A p-value less than 5% was considered statistically significant.
Results
Performance and frequency of VEGFA gene amplification in E2100
Of the 367 cases available for assessment of amplification for VEGFA by FISH, 324 had successful hybridization (success rate: 88.3%). 21 cases demonstrated VEGFA amplification, 31 had borderline amplification, 251 were VEGFA normal, and 21 had deletion; (Table 1). The characteristics and outcome of the subgroup of patients studied in this subgroup fared similarly to the parent trial (Supplemental Table 1 and 2).
Table 1.
Amplification by arm of trial and ER/PR/HER2 status
| n | Amplified + borderline amplified n (%) | Amplified (ratio ≥ 2) | Borderline amplified (ratio ≥ 1.5 but < 2) | Normal | Deleted (ratio < 0.8) | |
|---|---|---|---|---|---|---|
| All patients | 324 | 52 (16.0%) | 21 | 31 | 251 | 21 |
| Arm A | 157 | 24 (15.3%) | 6 | 18 | 120 | 13 |
| Arm B | 167 | 28 (16.8%) | 15 | 13 | 131 | 8 |
| ER+ or PR+/HER2− | 210 | 26 (12.4%) | 6 | 20 | 172 | 12 |
| TNBC | 100 | 23 (23.0%) | 13 | 10 | 69 | 8 |
| HER2+ | 6 | 3 (50.0%) | 2 | 1 | 2 | 1 |
Ratio= VEGF-A/CEN-6 signal ratio; TNBC=triple negative breast cancer; Arm A=paclitaxel with bevacizumab; Arm B= paclitaxel alone
VEGFA amplification by arm of E2100 and by Estrogen/Progesterone Receptor and HER2 status
VEGFA amplification status is summarized in Table 1. A total of 52 patients (16%) had amp/BA status. Amp/BA status was well balanced across arms in E2100 comprising 15.3% and 16.8% in arm A and arm B, respectively. There was less amplification in those who had ER and/or PR positive tumors (12.4%, n=26/210) compared with those who were ER/PR negative (23.0%, n=23/100; p= 0.020 by Fisher’s exact test). E2100 was predominately designed for HER2 negative patients, and thus only 6 patients in this correlative study had HER2 amplification; however 50.0% (n=3/6) of these patients demonstrated VEGFA amp/BA.
Association of VEGFA amplification with efficacy
On univariate analysis in the entire study population including both treatment arms, patients with tumor VEGFA amp/BA had significantly worse median PFS (7.8 months vs. 8.3 months; p=0.040) and median OS (20.2 months vs. 25.3 months; p=0.013) compared with those whose tumors did not exhibit amplification (Figure 1). Multivariate analysis was performed and included covariates that significantly impacted PFS (arm of study, ER/PR status, use of hormonal therapy) and OS (ER/PR status and use of hormonal therapy). On multivariate analysis, amp/BA status had no statistically significant impact on PFS (p=0.178) or OS (p=0.08). Because hormonal sensitivity and the arm of study nullified the significant results seen on univariate analysis, further evaluation was performed to see if there was an interaction between arm of study, ER/PR status, and amplification status.
Figure 1.
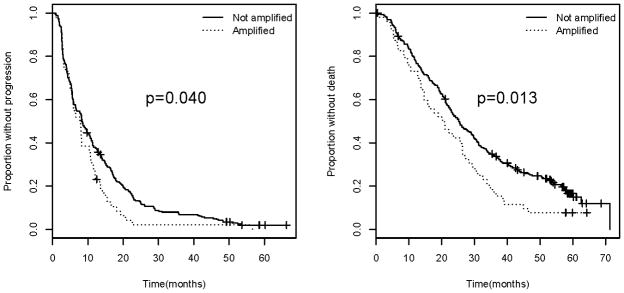
Progression free survival (left) and overall survival (right) for all patients comparing those with VEGFA amplification/borderline amplification versus those without.
In addition to having a lower likelihood of having VEGFA amp/BA, those with ER or PR positive tumors did not experience inferior outcome (PFS; p=0.418 and OS; p=0.321) whether amplified or not (Figure 2). Patients with HER2 positive tumors were not analyzed separately for association with outcome due to insufficient numbers, but were combined with those with TNBC as prior data have demonstrated higher expression of VEGFA in both subtypes compared with those who have hormone-receptor positive tumors. When combining the subtypes that displayed a higher frequency of VEGFA amp/BA (HER2 positive and triple negative populations), those with amp/BA had a trend for inferior PFS with the curves separating late (p=0.092) and a statistically significantly inferior OS (p=0.047; Figure 2). The improvement in OS was no longer statistically significant when excluding those patients with HER2+ tumors (p=0.143).
Figure 2.
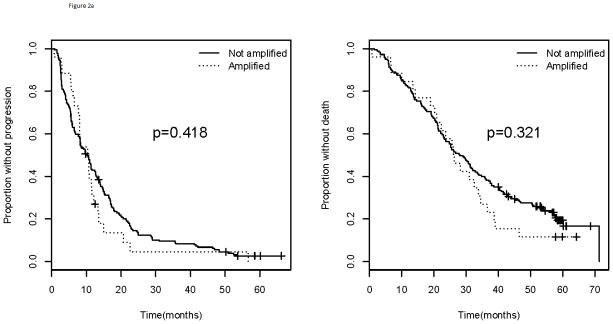
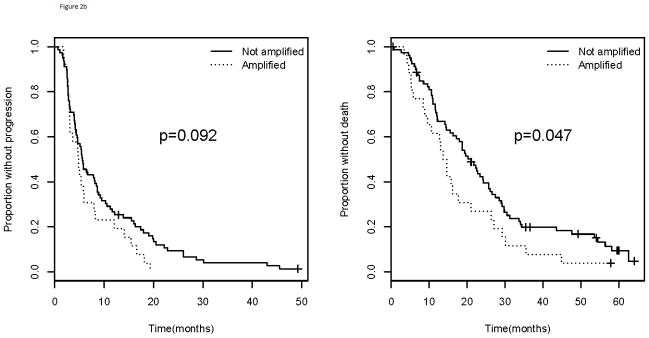
Progression free survival (left) and overall survival (right) for a). ER or PR positive and b). TNBC and HER2 positive patients. Comparison of those with VEGFA amplification/borderline amplification versus those without. TNBC= triple negative breast cancer
When considering treatment arm, patients in Arm A (bevacizumab and paclitaxel) of E2100 had a statistically significant inferior median PFS for those with amp/BA status (10.5 months) compared with those who do not (11.3 months; p=0.010) (Figure 3). These patients also had inferior median OS for those with amp/BA status (21.0 months) when compared to those without (25.6 months; p=0.042). Those in Arm B (paclitaxel alone) did not have differential PFS (p=0.676) or OS (p=0.123) based on amplification status (Figure 3). All patients in arm B, however, fared worse than those on arm A with a median PFS of 5.6 months and a median OS of 23.7 months.
Figure 3.
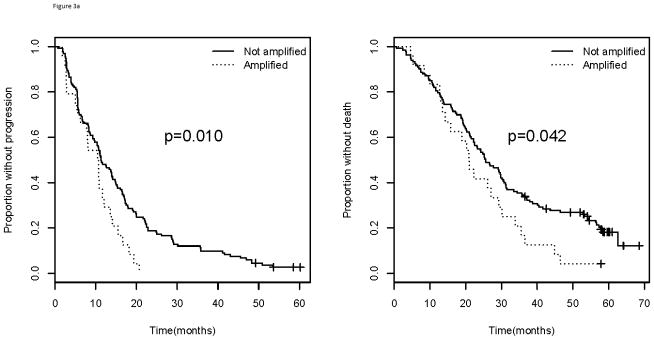
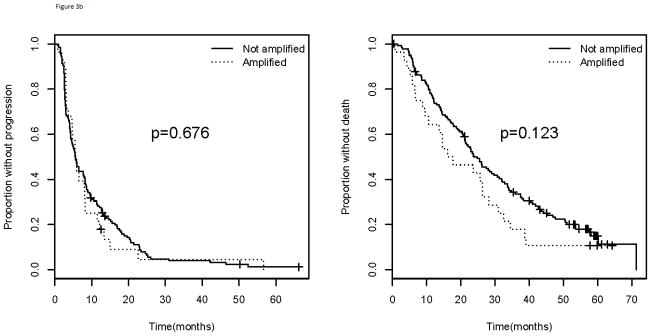
Progression free survival (left) and overall survival (right) for patients in a). Arm A and b). Arm B of E2100. Comparison of those with VEGFA amplification/borderline amplification versus those without. Arm A= paclitaxel and bevacizumab; Arm B= paclitaxel alone
As stated above, patients with amp/BA status do worse compared with those who do not have amp/BA status. When specifically considering those patients in this poor prognosis group (amp/BA), there is no difference in median PFS (10.5 vs. 5.7 months; p=0.438) or median OS (21.0 vs. 16.9 months; p=0.973) for those receiving bevacizumab (Arm A) compared with placebo (Arm B) suggesting bevacizumab has no effect in this subgroup (Figure 4). For those patients who are not amplified, however, there is a significant improvement in median PFS for those who received bevacizumab (Arm A) compared with those who did not in Arm B (11.3 vs. 5.5 months; p=6.29×10−5). This improvement in PFS did not translate into an improvement in median OS (25.6 vs. 24.8 months; p=0.472). When considering both the arm of study and amplification status simultaneously, those patients who did not have amp/BA status and who received bevacizumab had the best PFS (p= 8.55×10−6; Figure 5). There was no corresponding OS improvement, however, seen for those who were not amp/BA and received bevacizumab and paclitaxel (p=0.136). A formal test for interaction between amplification and arm of the trial was not significant for PFS (p=0.220) or OS (p=0.690).
Figure 4.
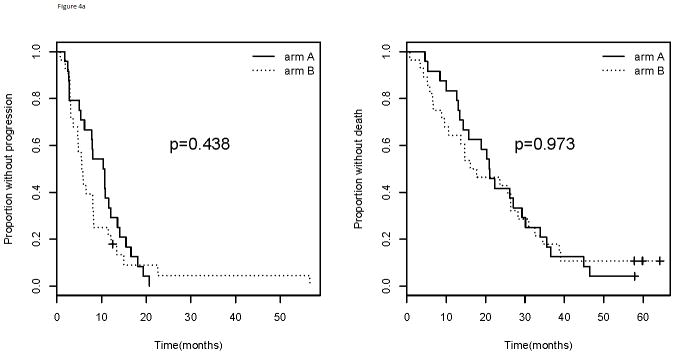
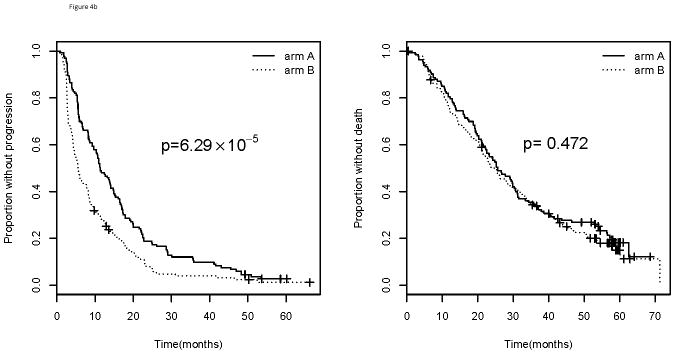
Progression free survival (left) and overall survival (right) by arm of E2100 for those with a). Amplified/borderline amplified and b). not-amplified/borderline amplified tumor status.
Figure 5.
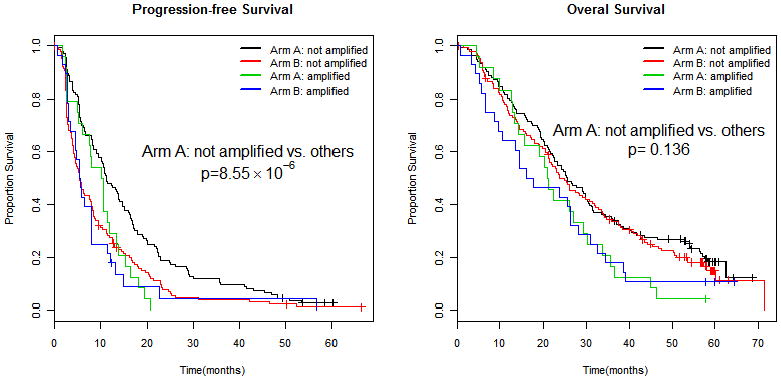
Progression free survival (left) and overall survival (right) by VEGFA amplification status and arm of therapy.
Discussion
In E2100, paclitaxel and bevacizumab demonstrated an improvement over paclitaxel alone in measurement of PFS but not OS.1 Subsequent first line trials testing bevacizumab in breast cancer, including a meta-analysis of all trials, also demonstrated significantly improved PFS but not median OS. 2,3,23 Despite consistent improvement in PFS observed when bevacizumab is added to chemotherapy, the absolute improvement varied with the type of chemotherapy it was partnered with, with the greatest benefit when partnered with paclitaxel.24 Based upon the results of the AVADO and RIBBON-1 trials, the accelerated approval initially granted by the US FDA was rescinded25, whereas the European Medicine Agency (EMA) granted approval in combination with paclitaxel as first line therapy. Bevacizumab therefore remains approved for the treatment of metastatic breast in multiple countries throughout the world, based largely on the results of the E2100 trial. In addition, anti-VEGF therapy with bevacizumab and other agents remains important for other tumor types.26–31 Predictive biomarkers that identify which patients derive benefit and toxicity from anti-angiogenic agents are needed.32
Amplification of HER2 is a well-studied prognostic and predictive biomarker for patients with breast cancer and represents one of the gold-standards of clinical applicability.12 Here we tested for amplification of the target gene for bevacizumab, VEGFA, using FISH. The samples from E2100 were derived from paraffin embedded tissue from the primary tumor. In this study, we demonstrate that testing archived tumor blocks for VEGFA amplification by FISH is feasible. Previously we demonstrated that VEGF expression (determined by immunohistochemistry) did not correlate with outcome in E2100.7 From a technical standpoint, amplification is easier to quantify compared with expression.7,33 From a biological standpoint, it is likely that genomic amplification is less dynamic over time in response to changes in the microenvironment compared with protein expression. For example, VEGFA expression is highly variable in response to hypoxia and thus may represent a less reliable surrogate to describe the potential range of VEGFA influence at various time points in the life of a metastatic tumor. 34,35 However, it is critical to remember that bevacizumab targets the VEGFA protein. The variable nature of protein expression is a problem exacerbated in a metastatic trial (like E2100) where the primary tumor being assessed is often far removed in time from the initiation of treatment in the metastatic setting. This issue may also be relevant to the VEGFA gene, but data are not available.
While the majority of tumors have similar HER2 amplification status when comparing the primary tumor with a metachronous metastasis, several studies have demonstrated that a clinically relevant fraction (as high as 15%) does change.36–38 Since all samples evaluated in this correlative study included the primary tumor, any biological change that took place at the time of metastasis cannot be accounted for here. Another inherent limitation of this correlative study, like many others, is that samples are not available from all cases of the parent trial. This limits statistical power and can also introduce an unintended confounder if the subgroup evaluated does not reflect the characteristics of the population and outcome in the parent trial. This latter concern is somewhat dampened as this subgroup closely mirrors the parent trial in terms of important characteristics and outcome.
Amplification of the VEGFA gene has previously been shown to serve as a poor prognostic marker in patients with colorectal cancer and osteosarcoma.39,40 As far as we are aware, the utility for VEGFA amplification as a predictive biomarker for anti-VEGF therapy has not been previously reported in a phase III trial. In this correlative study from E2100, we demonstrate those patients with VEGFA amplification and borderline amplification (together as a group) do worse than those without amplification in univariate analysis and thus this may represent a prognostic marker as seen in other tumor types. The significant inferiority seen in those with amplification was no longer significant in multivariate analysis which included ER positivity as a significant variable. There appears, then, to be an important biological interaction with VEGFA amplification and ER expression. Tumors that were hormone sensitive were almost two times less likely than those who were ER negative to have VEGFA amplification. ER positive patients also had lower frequency of VEGFA amplification than HER2 positive patients, but the number in the latter subgroup was too small to formally compare. Higher VEGFA expression has previously been seen in HER2 positive and triple negative breast cancers when compared to ER positive tumors; further validating this biological correlation.41–45 In addition to lower frequency of VEGFA amplification, the implication of this amplification in the ER positive subgroup appears to be different as well. In the ER positive population, prognosis does not appear to be adversely impacted by amplification. Those with triple negative breast cancer or HER2 positivity, however, do appear to have outcome adversely impacted by amplification. While the effect of VEGFA amplification on HER2 positive tumors (as a unique group) was not evaluated here due to small numbers, the biology would suggest it to be a provocative area of future investigation.
In this study, those patients that had VEGFA amplification did worse in univariate analysis and appeared to have no incremental benefit from bevacizumab. While the pathophysiology is not elucidated, this may simply represent a scenario where the amplification “overwhelms” the ability to successfully block VEGFA. Conversely, a significant benefit for bevacizumab in PFS was maintained in those who did not have amplification. Similar to the results of the parent trial, this effect did not carry over to a benefit in OS. The test for interaction between treatment arm and amplification was not statistically significant and thus VEGFA amplification cannot be formally considered a predictive marker for bevacizumab based on these data. However, the difference in PFS was statistically and clinically substantial when comparing those who did not have amplification but did receive bevacizumab against those who were either amplified or did not receive the anti-VEGFA blockade. It does beg the question as to whether the PFS could have been more robust in other data sets (e.g. AVADO and RIBBON-1) if the subgroups with VEGFA amplification were excluded. Further, would the enrichment of this population also translate into improved OS with greater numbers and more statistical power (e.g. the first line meta-analysis), or does the biology of this drug dictate that an improvement in OS is simply not possible for this disease type and setting?
We have previously demonstrated that host derived genetic variation (i.e. single nucleotide polymorphisms; SNPs) correlated with improved OS for patients that received bevacizumab in E2100.7 In that study, two genotypes (VEGFA -2578AA and -1154AA) had superior OS in the bevacizumab containing arm. Combining germline (SNPs) and somatic (tumor specific) variability to create a predictive signature is a potentially complex approach but may be necessary to uncover the most accurate biomarker. Specifically, is there a genetic subgroup (based on SNPs) that is able to overcome the poor prognostic effect seen in those with VEGFA amplified tumors while receiving bevacizumab? Alternatively, are those who do not have tumor VEGFA amplification and who have the good SNP profile destined to experience a meaningful improvement in OS? Recently, a prospective trial has begun enrolment (the MERIDIAN trial) with the goal of prospectively evaluating outcome using a biomarker-guided approach with baseline plasma VEGFA levels. The result from this study may further inform biomarker-driven trials like MERIDIAN. Further, the interaction between the host (i.e. SNPs) and the tumor represents the entire picture of variability and this interaction warrants further evaluation in larger data sets.
Supplementary Material
Statement of Translational Relevance.
The use of bevacizumab for breast cancer has been a highly controversial topic. This agent, based on non-specific implementation in the clinical trial E2100, received accelerated approval by the US-FDA. Based on the less impressive improvements in progression free survival in subsequent trials (AVADO and RIBBON-I) and the lack of overall survival in all trials coupled with a unique toxicity profile, the approval was rescinded. Interestingly, despite the same data, the European Union has maintained approval. Despite the controversy, most would agree that identification of a successful biomarker for bevacizumab to establish which subgroup might obtain the greatest benefit would be of highest importance. Prior studies in breast cancer have demonstrated that amplification of the therapeutics’ target gene can serve as an excellent predictive marker (i.e. HER2 and trastuzumab). In this study we set out to evaluate the role of VEGF-A amplification as a biomarker for bevacizumab in E2100.
Acknowledgments
FINANCIAL SUPPORT: This study was coordinated by the Eastern Cooperative Oncology Group (Robert L. Comis, M.D., Chair) and supported in part by Public Health Service Grants CA23318, CA66636, CA21115, CA49883, CA16116, CA32102, CA20319, CA14958, CA77651, CA12027, CA25224 and from the National Cancer Institute, National Institutes of Health and the Department of Health and Human Services. The science was also supported by an R-01 CA155311-01A1 (Bryan P. Schneider; Principal Investigator). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute. The procurement and preparation of samples was supported by the Breast Cancer Research Foundation.
Footnotes
CONFLICT OF INTEREST STATEMENT:
Bryan P. Schneider is on the advisory board of Genentech (compensated). Kathy D. Miller has received honoraria and research funding from Genentech. Joseph A. Sparano has received consulting fees from Genentech. Sven Müller holds a team leader position at Dako-Denmark.
References
- 1.Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357(26):2666–76. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 2.Robert NJ, Dieras V, Glaspy J, Brufsky AM, Bondarenko I, Lipatov ON, et al. RIBBON-1: randomized, double-blind, placebo-controlled, phase III trial of chemotherapy with or without bevacizumab for first-line treatment of human epidermal growth factor receptor 2-negative, locally recurrent or metastatic breast cancer. Journal of Clinical Oncology. 2011;29(10):1252–60. doi: 10.1200/JCO.2010.28.0982. [DOI] [PubMed] [Google Scholar]
- 3.Miles DW, Chan A, Dirix LY, Cortes J, Pivot X, Tomczak P, et al. Phase III study of bevacizumab plus docetaxel compared with placebo plus docetaxel for the first-line treatment of human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clinic Oncol. 2010;28(20):3239–47. doi: 10.1200/JCO.2008.21.6457. [DOI] [PubMed] [Google Scholar]
- 4.Carpenter D, Kesselheim AS, Joffe S. Reputation and precedent in the bevacizumab decision. New England Journal of Medicine. 2011;365(2):e3. doi: 10.1056/NEJMp1107201. [DOI] [PubMed] [Google Scholar]
- 5.Jubb AM, Harris AL. Biomarkers to predict the clinical efficacy of bevacizumab in cancer. Lancet Oncology. 2010;11(12):1172–83. doi: 10.1016/S1470-2045(10)70232-1. [DOI] [PubMed] [Google Scholar]
- 6.Schneider BP, Sledge GW., Jr Anti-vascular endothelial growth factor therapy for breast cancer: can we pick the winners? J Clin Oncol. 2011;29(18):2444–7. doi: 10.1200/JCO.2011.34.9266. [DOI] [PubMed] [Google Scholar]
- 7.Schneider BP, Wang M, Radovich M, Sledge GW, Jr, Badve S, Thor A, et al. Association of vascular endothelial growth factor and vascular endothelial growth factor receptor-2 genetic polymorphisms with outcome in a trial of paclitaxel compared with paclitaxel plus bevacizumab in advanced breast cancer: ECOG 2100. J Clin Oncol. 2008;26(28):4672–8. doi: 10.1200/JCO.2008.16.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Formica V, Palmirotta R, Del Monte G, Savonarola A, Ludovici G, De Marchis ML, et al. Predictive value of VEGF gene polymorphisms for metastatic colorectal cancer patients receiving first-line treatment including fluorouracil, irinotecan, and bevacizumab. International Journal of Colorectal Disease. 2011;26(2):143–51. doi: 10.1007/s00384-010-1108-1. [DOI] [PubMed] [Google Scholar]
- 9.Koutras AK, Antonacopoulou AG, Eleftheraki AG, Dimitrakopoulos FI, Koumarianou A, Varthalitis I, et al. Vascular endothelial growth factor polymorphisms and clinical outcome in colorectal cancer patients treated with irinotecan-based chemotherapy and bevacizumab. Pharmacogenomics J. 2011 Aug 16;2011 doi: 10.1038/tpj.2011.37. [DOI] [PubMed] [Google Scholar]
- 10.Miles D, De Haas S, Romieu G, Chan A, Dirix L, Cortes J, et al. Polymorphism analysis in the avado randomised phase iii trial of first-line bevacizumab combined with docetaxel in her2-negative metastatic breast cancer. ECCO16-EMSO36. 2011:Abstract 1.423. [Google Scholar]
- 11.La Thangue NB, Kerr DJ. Predictive biomarkers: a paradigm shift towards personalized cancer medicine. Nature Reviews Clinical Oncology. 2011;8(10):587–96. doi: 10.1038/nrclinonc.2011.121. [DOI] [PubMed] [Google Scholar]
- 12.Hudis CA. Trastuzumab--mechanism of action and use in clinical practice. New England Journal of Medicine. 2007;357(1):39–51. doi: 10.1056/NEJMra043186. [DOI] [PubMed] [Google Scholar]
- 13.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. New England Journal of Medicine. 2011;353(16):1659–72. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 14.Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE, Jr, Davidson NE, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. New England Journal of Medicine. 2005;353(16):1673–84. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 15.Perez EA, Romond EH, Suman VJ, Jeong J-H, Davidson NE, Geyer CE, Jr, et al. Four-year follow-up of trastuzumab plus adjuvant chemotherapy for operable human epidermal growth factor receptor 2-positive breast cancer: joint analysis of data from NCCTGN9831 and NSABP B-31. Journal of Clinical Oncology. 2011;29(25):3366–73. doi: 10.1200/JCO.2011.35.0868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Slamon D, Eiermann W, Robert N, Pienkowski T, Martin M, Press M, et al. Adjuvant trastuzumab in HER2-positive breast cancer. New England Journal of Medicine. 2011;365(14):1273–83. doi: 10.1056/NEJMoa0910383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geyer CE, Forster J, Lindquist D, Chan S, Romieu CG, Pienkowski T, et al. Lapatinib plus Capecitabine for HER2-Positive Advanced Breast Cancer. New England Journal of Medicine. 2006;355(26):2733–43. doi: 10.1056/NEJMoa064320. [DOI] [PubMed] [Google Scholar]
- 18.Baselga J, Cortés J, Kim S-B, Im S-A, Hegg R, Im Y-H, et al. Pertuzumab plus Trastuzumab plus Docetaxel for Metastatic Breast Cancer. New England Journal of Medicine. 2012;366(2):109–19. doi: 10.1056/NEJMoa1113216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blackwell KL, Miles D, Gianni L, Krop IE, Welslau M, Baselga J, et al. Primary results from EMILIA, a phase III study of trastuzumab emtansine (T-DM1) versus capecitabine (X) and lapatinib (L) in HER2-positive locally advanced or metastatic breast cancer (MBC) previously treated with trastuzumab (T) and a taxane. J Clin Oncol. 2012;30(Suppl):abstr LBA1. [Google Scholar]
- 20.Schneider BP, Radovich M, Hancock BA, Kassem N, Vance GH, Sledge GW, Jr, et al. VEGFA amplification/deletion in human breast tumors. J Clin Oncol. 2010;28(suppl):abstr e21017. [Google Scholar]
- 21.DAKO. TOP2A FISH pharmDx Kit. 2008 http://www.dako.com/us/ar42/p223972/prod_products.htm.
- 22.Olsen KE, Knudsen H, Rasmussen BB, Balslev E, Knoop A, Ejlertsen B, et al. Amplification of HER2 and TOP2A and deletion of TOP2A genes in breast cancer investigated by new FISH probes. Acta Oncologica. 2004;43(1):35–42. doi: 10.1080/02841860310019007. [DOI] [PubMed] [Google Scholar]
- 23.O’Shaughnessy J, Miles D, Gray RJ, Dieras V, Perez EA, Zon R, et al. A meta-analysis of overall survival data from three randomized trials of bevacizumab (BV) and first-line chemotherapy as treatment for patients with metastatic breast cancer (MBC) J Clin Oncol. 2010;28(15s suppl):abstr 1005. [Google Scholar]
- 24.Burstein HJ. Bevacizumab for advanced breast cancer: all tied up with a RIBBON? Journal of Clinical Oncology. 2011;29(10):1232–5. doi: 10.1200/JCO.2010.33.2684. [DOI] [PubMed] [Google Scholar]
- 25.FDA NEWS RELEASE: FDA Commissioner announces Avastin decision: Drug not shown to be safe and effective in breast cancer patients. 2011 http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm280536.htm.
- 26.Aghajanian C, Blank SV, Goff BA, Judson PL, Teneriello MG, Husain A, et al. OCEANS: A Randomized, Double-Blind, Placebo-Controlled Phase III Trial of Chemotherapy With or Without Bevacizumab in Patients With Platinum-Sensitive Recurrent Epithelial Ovarian, Primary Peritoneal, or Fallopian Tube Cancer. Journal of Clinical Oncology. 2012;30(17):2039–45. doi: 10.1200/JCO.2012.42.0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burger RA, Brady MF, Bookman MA, Fleming GF, Monk BJ, Huang H, et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. New England Journal of Medicine. 2011;365(26):2473–83. doi: 10.1056/NEJMoa1104390. [DOI] [PubMed] [Google Scholar]
- 28.Feldman DR, Baum MS, Ginsberg MS, Hassoun H, Flombaum CD, Velasco S, et al. Phase I trial of bevacizumab plus escalated doses of sunitinib in patients with metastatic renal cell carcinoma. Journal of Clinical Oncology. 2009;27(9):1432–9. doi: 10.1200/JCO.2008.19.0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. New England Journal of Medicine. 2004;350(23):2335–42. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 30.Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer.[Erratum appears in N Engl J Med 2007 Jan 18;356(3):318] New England Journal of Medicine. 2006;355(24):2542–50. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 31.Yang JC, Haworth L, Sherry RM, Hwu P, Schwartzentruber DJ, Topalian SL, et al. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. New England Journal of Medicine. 2003;349(5):427–34. doi: 10.1056/NEJMoa021491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jain RK, Duda DG, Willett CG, Sahani DV, Zhu AX, Loeffler JS, et al. Biomarkers of response and resistance to antiangiogenic therapy [Review] [81 refs] Nature Reviews Clinical Oncology. 2009;6(6):327–38. doi: 10.1038/nrclinonc.2009.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Theodosiou Z, Kasampalidis IN, Livanos G, Zervakis M, Pitas I, Lyroudia K. Automated analysis of FISH and immunohistochemistry images: a review. Cytometry Part A: The Journal of the International Society for Analytical Cytology. 2007 Jul;71(7):439–50. doi: 10.1002/cyto.a.20409. [DOI] [PubMed] [Google Scholar]
- 34.Goel S, Duda DG, Xu L, Munn LL, Boucher Y, Fukumura D, et al. Normalization of the vasculature for treatment of cancer and other diseases. Physiological Reviews. 2011;91(3):1071–121. doi: 10.1152/physrev.00038.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsuzuki Y, Fukumura D, Oosthuyse B, Koike C, Carmeliet P, Jain RK. Vascular endothelial growth factor (VEGF) modulation by targeting hypoxia-inducible factor-1alpha--> hypoxia response element--> VEGF cascade differentially regulates vascular response and growth rate in tumors. Cancer Research. 2000;60(22):6248–52. [PubMed] [Google Scholar]
- 36.Curigliano G, Bagnardi V, Viale G, Fumagalli L, Rotmensz N, Aurilio G, et al. Should liver metastases of breast cancer be biopsied to improve treatment choice? Annals of Oncology. 2011;22(10):2227–33. doi: 10.1093/annonc/mdq751. [DOI] [PubMed] [Google Scholar]
- 37.Fabi A, Di Benedetto A, Metro G, Perracchio L, Nistico C, Di Filippo F, et al. HER2 protein and gene variation between primary and metastatic breast cancer: significance and impact on patient care. Clinical Cancer Research. 2011;17(7):2055–64. doi: 10.1158/1078-0432.CCR-10-1920. [DOI] [PubMed] [Google Scholar]
- 38.Karlsson E, Lindström LS, Wilking U, Skoog L, Johansson U, Bergh J. Discordance in hormone receptor status in breast cancer during tumor progression. J Clin Oncol. 2010;28(15s suppl):abstr 1009. [Google Scholar]
- 39.Vlajnic T, Andreozzi MC, Schneider S, Tornillo L, Karamitopoulou E, Lugli A, et al. VEGFA gene locus (6p12) amplification identifies a small but highly aggressive subgroup of colorectal cancer [corrected] patients.[Erratum appears in Mod Pathol 2011 Nov;24(11):1530] Modern Pathology. 2011;24(10):1404–12. doi: 10.1038/modpathol.2011.96. [DOI] [PubMed] [Google Scholar]
- 40.Yang J, Jubb AM, Pike L, Buffa FM, Turley H, Baban D, et al. The histone demethylase JMJD2B is regulated by estrogen receptor alpha and hypoxia, and is a key mediator of estrogen induced growth. Cancer Research. 2010;70(16):6456–66. doi: 10.1158/0008-5472.CAN-10-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fuckar D, Dekanic A, Stifter S, Mustac E, Krstulja M, Dobrila F, et al. VEGF expression is associated with negative estrogen receptor status in patients with breast cancer. International Journal of Surgical Pathology. 2006;14(1):49–55. doi: 10.1177/106689690601400109. [DOI] [PubMed] [Google Scholar]
- 42.Ghosh S, Zerkowski MP, Abu-Khalaf MM, Camp RL, Rimm DL, Chung GG. Quantitative Image Analysis of VEGF Expression on a Breast Cancer Tissue Microarray. J Clin Oncol. 2005;23(16s):abstr: 9534. [Google Scholar]
- 43.Konecny GE, Meng YG, Untch M, Wang H-J, Bauerfeind I, Epstein M, et al. Association between HER-2/neu and vascular endothelial growth factor expression predicts clinical outcome in primary breast cancer patients. Clinical Cancer Research. 2004;10(5):1706–16. doi: 10.1158/1078-0432.ccr-0951-3. [DOI] [PubMed] [Google Scholar]
- 44.Linderholm B, Bergqvist J, Hellborg H, Johansson U, Linderholm M, von Schoultz E, et al. Shorter survival-times following adjuvant endocrine therapy in oestrogen- and progesterone-receptor positive breast cancer overexpressing HER2 and/or with an increased expression of vascular endothelial growth factor. Medical Oncology. 2009;26(4):480–90. doi: 10.1007/s12032-008-9157-9. [DOI] [PubMed] [Google Scholar]
- 45.Linderholm B, Grankvist K, Wilking N, Johansson M, Tavelin B, Henriksson R. Correlation of vascular endothelial growth factor content with recurrences, survival, and first relapse site in primary node-positive breast carcinoma after adjuvant treatment. Journal of Clinical Oncology. 2000;18(7):1423–31. doi: 10.1200/JCO.2000.18.7.1423. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


