Abstract
The NIH-funded CNTRICS initiative has coordinated efforts to promote the vertical translation of novel procognitive molecules from testing in mice, rats and non-human primates, to clinical efficacy in patients with schizophrenia. CNTRICS highlighted improving construct validation of tasks across species to increase the likelihood that the translation of a candidate molecule to humans will be successful. Other aspects of cross-species behaviors remain important however. This review describes cognitive tasks utilized across species, providing examples of differences and similarities of innate behavior between species, as well as convergent construct and predictive validity. Tests of attention, olfactory discrimination, reversal learning, and paired associate learning are discussed. Moreover, information on the practical implication of species differences in drug development research is also provided. The issues covered here will aid in task development and utilization across species as well as reinforcing the positive role preclinical research can have in developing procognitive treatments for psychiatric disorders.
Keywords: Species, differences, mice, rats, non-human primates, monkeys, human primates, CNTRICS, cognitive, attention, reversal learning, impulsivity
1.1. Introduction
CNTRICS is a NIH-funded initiative ultimately aimed at developing procognitive therapeutics for schizophrenia. In order to develop these treatments, it is understood that the drug discovery process requires testing putative treatments in animals first, prior to testing in humans. Moreover, the likelihood that an efficacious treatment in animals will be efficacious in humans is increased if the behavioral tasks used for these species examine the same cognitive construct, because it is reasoned that construct validity increases the involvement of common biological mechanisms across species. Hence, CNTRICS has identified specific cognitive constructs that require treatment in schizophrenia and have attempted to identify tasks that measure these specific constructs in animals and in man. While there exist examples of well-developed tasks with cross-species translational validity from mouse, to rat, to non-human primate (NHP), and to human primate, species differences in performance of otherwise identical cognitive tasks have been observed. The purpose of this review is to: 1) Provide a structure by which the cross-species translational validity of tasks can be assessed; 2) Give examples of a) divergent behavior between species despite similarities in testing protocols and b) convergent behavior in similar tasks – particularly from those chosen to represent specific cognitive constructs identified by CNTRICS; 3) Highlight the differences in techniques that may be utilized when developing tests for mice, rats, NHPs, and human primates; and 4) Comment on the practical implications of these technical differences for the drug discovery process.
1.2. Aspects of cross-species translational validity
There are numerous criteria that one can use to determine whether a task used to assess a cognitive function is similarly performed by laboratory animal species and humans. The primary interest of CNTRICS has been to develop tasks that measure a specific cognitive construct utilizing the same neurocircuitry between species. Evidence for such similarities is often referred to as construct validity. It is tempting to assume that a task is valid when tasks performed by humans activate certain structures that when lesioned in animals impair performance of a similar task. While this might indeed confer some construct validity, there are other aspects to validation that require examination because brain activations measured in humans may correlate with, but not causally mediate, performance, while lesions can sometimes exert non-specific effects on cognitive task performance. Within construct validity one can examine convergent and divergent validities (see below). Moreover, though construct validity is thought to confer predictive validity, the latter is the primary goal of the research. On the other hand, face validity, though often cited as important, can ultimately have limited impact on the cross-species translational validity of the task.
1.2.1 Construct validity
Most simply defined as the accuracy to which the test measures that which it is intended to measure (Geyer et al., 1999), construct validity is usually considered as the most important property to establish the cross-species translational validity of a test (Geyer et al., 1999; Lipska et al., 1995), but its establishment can be challenging and is – consequently - rare. Problems arise as conceptions about what a test is supposed to measure can change as scientific theories and theoretical constructs are modified. Thus, a task’s usefulness and hence its overall validity cannot be determined simply by the degree of construct validation that it has. The process of construct validation is extremely valuable in establishing the overall development, refinement and validity of the task. As new experimental and observational evidence accrues from both preclinical and clinical testing, the task can be refined and therefore enable more accurate predictions.
A test has convergent (or concurrent) and discriminant validity only in relation to other tests. Convergent validity is the degree to which a test correlates with other tests that attempt to measure the same construct (Taiminen et al., 2000). Discriminant validity is the degree to which a test measures aspects of a phenomenon that differ from other aspects that is assessed by other tests (Taiminen et al., 2000). Testing these aspects of validity of the tasks chosen by CNTRICS for each construct will be essential.
1.2.2. Predictive Validity
This aspect of validity concerns the ability of a test to make correct predictions about the clinical test of interest (Geyer and Braff, 1987; Geyer and Markou, 1995). Predictive validity is often used narrowly to refer only to the test’s ability to identify drugs that have therapeutic value in humans (referred to as pharmacological isomorphism, (Matthysse, 1986). This utilization is limited however, because it ignores other important aspects of a task that can be validated by making successful predictions (Ellenbroek and Cools, 2000). Predictive validation of the experimental preparation is also observed whereby variables have similar influences in the preclinical task and the clinical task and can enhance one’s understanding of the phenomenon. For example, a vigilance decrement – where attention wanes over time – is observed in numerous human continuous performance tests (Parasuraman, 1998; Riccio et al., 2002), and is also observed in mice performing the 5-choice continuous performance test (5C-CPT) (Young et al., 2009a; Young et al., 2011b). Another example is that in the intradimensional/extradimensional (ID/ED) shifting task in humans (Owen et al., 1991), more trials are required to complete the ED vs. ID shifts, which is also the case for the animal versions of the task (Birrell and Brown, 2000; Bissonette et al., 2008; Dias et al., 1996b; Young et al., 2009b).
1.2.3. Face validity
Refers to the phenomenological similarity between the task used in preclinical testing to that used in the clinic (Lieberman et al., 1997). Although face validity is an intuitively appealing criterion with which to validate tasks, appearing desirable (Lipska and Weinberger, 2000; Weiner et al., 1996), it a) is not actually necessary, b) can be misleading, and c) is difficult to defend rigorously. The latter proves most difficult as these tasks almost invariably involve subjective, arbitrary arguments that are not necessarily accepted by all investigators in the field (see (Lipska and Weinberger, 1995). Thus, while face validity may provide a heuristic starting point for the development of a cross-species task with translational validity, it cannot be used to establish the validity of the task.
1.3. Structure to assess cross-species translational validity
Various aspects of validity exist and can be described both in terms of the validity that a) animal tasks have for those used in man, and b) models of disease states. Translating a molecule from preclinical studies to clinical efficacy is of paramount importance however. Hence, it is possible to utilize tasks that have limited validity to what is being tested in man as long as the model predicts effective treatment. An example of such targeted drug discovery is apomorphine-induced disruption of prepulse inhibition in rats, the reversal of which reliably predicts the clinical efficacy of treatments for the positive symptoms of schizophrenia (Swerdlow et al, 1994). Latterly, it was discovered that this model was effective because it revealed the ability of the compound to block dopamine D2-family of receptors at levels sufficient to treat psychosis (Kapur et al, 2000). This model was only available however, because treatments were available at the time of its development and thus novel molecules could be compared with positive controls, resulting in treatments with similar mechanisms of action (pharmacological isomorphism). Given that no procognitive treatments exist for schizophrenia however, CNTRICS has proposed a more structured task valid approach in order to assess novel molecules as procognitives for schizophrenia. As such, the validity of disease models is not discussed in this review.
Examining the validity of a task across species is vital for assessing the suitability of novel molecules to treat schizophrenia. Because divergent performance levels can be observed between species performing similar tasks (see 2.1.) due to innate differences in the approach species take to such tasks, evidence for consistency of effects of the molecule across species in the same task – as opposed to evidence in one species only – would increase the confidence of a positive effect when translated to human clinical trials. Positive effects of a molecule across species would also be beneficial so that it can be tested in animal models of schizophrenia where some are more prevalent in mice (e.g. genetic models), compared with rats and NHPs (e.g. pharmacological models). Hence, convergent validation of a molecule could be generated using a range of models. Convergent validation of a molecule could also arise if the molecule improved of animals in tasks that reportedly assess the same cognitive domain (e.g. the 5C-CPT and the sustained attention task; see (Lustig et al., 2012), this issue). Hence, in these series of meetings CNTRICS has identified at least two tasks per cognitive domain in which a molecule could be tested for convergent validation.
Before such novel molecules are tested in various models however, the cognitive tasks utilized should have cross-species translational validity. An example of a task with some reliable validity is described below (reversal learning 3.2.) with examples where validation of this task across species has been conducted. In brief, the validity of reversal learning was established by demonstrating aspects of construct, predictive, and face validities across mice, rats, NHPs, and humans. Face validity was simply established by training subjects to learn the reinforcement contingencies between two stimuli which are then swapped once pre-established criterion is attained. Construct validity in this case has been established by the necessity of an intact orbitofrontal cortex to learn reversed contingencies, while unaffecting simple learning. Multiple aspects of predictive validity are observed for this task. For example, more trials are required to learn reversed contingencies as opposed to the number required to learn the initial relationship of these stimuli. Moreover, pharmacological predictive validity is supported whereby altered regulation of the dopamine D2-like receptor produces impairments in the ability to update behavior during reversed contingencies. Thus, by performing similar experimental manipulations across species, the validity of a task to assess a cognitive construct can be established.
2.1. Examples of tasks where differences between species’ performance have been observed
Identifying possible differences between species is not only important for bringing a drug from preclinical to clinical testing, but also when comparing results between preclinical species. For example, the importance of genetic influences to psychiatric diseases is becomingly increasingly apparent and the generation of transgenics that possess such mutations increasingly important. NIH first sponsored a Request For Applications and now a mainstream R21/R33 program announcement for researchers to develop and characterize novel mouse models that express human genes or human genetic elements that may be important to psychiatric disorders (PAR-08-158: Mouse Models Containing Human Alleles: Novel Tools to Study Brain Function). It is unsurprising that all awardees of these two mechanisms have utilized mice to generate these models because the vast majority of mutant rodent animals that have been created are mice and thus our knowledge on the behavioral effects of certain genes stem from our behavioral examination of these mice. In contrast, a great deal of our knowledge of pharmacological manipulation and lesion-induced alterations in behavior are performed in rats. Thus, pharmacological mouse models of cognitive disruption in schizophrenia are rare, while many mutant mouse models exist (Brigman et al., 2010; Young et al., 2011a). For these reasons, inherent, qualitative differences in performance of identical tasks in rats and mice may complicate translational research that depends upon integrated study of both species.
Investigation into the ramification of drug-/gene-/lesion-induced alterations in behavior relevant to diseases cannot take such a distinct line. Indeed, efforts are being made to develop rat mutant models, while more lesion and pharmacological studies in mice are being conducted. This discrepancy between study types by species remains however, and may be important when the innate behavior of these two species is compared in identical tasks. Despite the tasks utilizing operant training techniques, it is clear that the natural behaviors of the mice can impact performance and thus perhaps the effect of a novel molecule.
2.2. 5-choice serial reaction-time task (5CSRTT)
The 5CSRTT is a well developed test of sustained attention commonly used throughout the world. Originally devised for rats by Robbins and colleagues (Carli et al., 1983) it was not developed for mice until 16 years later (Humby et al., 1999). The large number of 5CSRTT studies performed in rats means a consistent pattern of performance that can be expected in trained rats, accuracy between 60–90%, omission levels below 10%, and premature responses typically above 20 in a session of 100 trials (Robbins, 2002), with some of the variability as a result of differences between strains, e.g. Long Evans vs. Wistar rats (Didriksen and Christensen, 1993), or Sprague Dawley vs. Lister Hooded rats (Mirza and Bright, 2001), which can impact drug effects on performance (Mirza and Bright, 2001; Wooters and Bardo, 2011). The consistency of these findings between studies within strain has lent great weight that the numerous 5CSRTT studies were measuring consistent aspects of performance. When studies began in mice however, it became clear that this species did not conform to this pattern of performance. Even between strains mice exhibit higher accuracy, typically 90–97%, higher omission levels, typically around 20% and above, and very few premature responses (Fletcher et al., 2007; Humby et al., 1999; Young et al., 2004), with DBA/2 mice consistently worse than C57BL/6 mice, which equaled 129/s mice (Humby et al., 1999; Loos et al., 2010; Patel et al., 2006; Pattij et al., 2007). The equality of performance between C57BL/6 and 129/s mice is of importance given that these strains are often used to create mutant mice. The consistent performance differences observed between rats and mice however, led Spratt et al (2000) to examine the strategy by which these two species perform the task in order to ascertain why their performance levels vary by species so consistently. Standard performance of these two species using a constant inter-trial interval (ITI) was ‘blank-trial’ challenged. This blank-trial challenge meant that on 1 out of 6 trials no target would appear during the period that a stimulus would ordinarily appear. Thus, if entrained to only respond to the stimuli lights, no response during this period would be recorded. During this time period, mice responded on average 15%, while rats responded 90% (Spratt et al., 2000). These data support the premise that rats use a temporally mediating strategy to perform the task during a constant ITI, while mice use the strategy to a lesser degree. These findings likely explain at least in part why rats exhibit differences in response measures compared to mice, e.g. if the rat does not see the location of the light when it is expected (timed) to appear, it will guess a location (leading to poorer accuracy and lower omissions) and an imperfect temporal ability may lead them to think stimuli appeared before the ITI was finished (increased premature responding). These studies highlight the differences in performance that can occur between species despite performing what is arguably the same task.
These species differences are important given that drug challenges may result in differing effects because of differing strategies utilized by each species as well as because of differing receptor selectivity between species. For example, amphetamine consistently increases premature responding in rats in the 5CSRTT (Robbins, 2002). In mice however, amphetamine does not always increase premature responding (Yan et al., 2011); (Loos et al., 2010). This could reflect the different reliance each species puts on a temporal mediating strategy because amphetamine speeds interval timing of both species (Balci et al., 2008; Body et al., 2009; Fowler et al., 2009). An alternate hypothesis is that while amphetamine is ~4 fold more selective at inhibiting the dopamine transporter (DAT) when compared to the norepinephrine transporter in rats, it is actually ~4–6 fold more selective for the NET in mice and in humans compared to the DAT. Because NET inhibition might act as a global inhibitory signal in tasks requiring reduced responding (Robinson et al., 2008), the selectivity of amphetamine for NET over DAT in mice could influence its lack of effects on premature responding. The need for DAT inhibition selectivity is supported by findings that GBR 12909, a more selective DAT vs. NET inhibitor reliably increases premature responding in mice, albeit in a simpler task (Young and Geyer, 2010). As yet there appears to be no clear distinction as to whether the differences of effects of amphetamine between mice and rats on premature responding is because of the putative greater use of a temporal strategy in rats or the more selective DAT inhibition effects of amphetamine on rats. These differences exist however and should be explicable if drugs developed in mice and rats are to be efficacious in humans.
5CSRTT performance can be further examined across species because a human version exists as a part of the Cambridge Neuropsychological Test Automated Battery. Indeed, it has been reported that patients with schizophrenia exhibit poorer performance in the task, albeit only as measured by a more varied reaction-time (Barnett et al., 2010), sometimes referred to as deficits in visual motor skills (Chouinard et al., 2007; Fagerlund et al., 2007). Differences in accuracy and omissions, while forming an important description of rodent performance, do not appear to be as relevant to human testing in the 5CSRTT. Thus, although differences in rodent species’ performance of the 5CSRTT may negatively impact the development of novel molecules to the clinical, the relevance of rodent 5CSRTT performance to clinical testing still needs to be established.
2.3. Olfactory Discrimination
Other examples of species differences when tested in the same task have been noted. For example, rats and mice can be trained to differentiate between olfactory cues using an olfactometer developed by Slotnick and colleagues (Bodyak and Slotnick, 1999; Slotnick, 2001; Slotnick and Risser, 1990). Discriminatory accuracy of rats (Bodyak and Slotnick, 1999) and mice (Abraham et al., 2004; Rinberg et al., 2006) are near perfect even when comparing no odor vs. 0.0001% ethyl acetate.
Performance of rats and mice are affected only when odor similarities are increased by mixing (or morphing) two odors (Abraham et al., 2004; Rinberg et al., 2006; Uchida and Mainen, 2003). Interestingly, rats and mice differ in their response to these mixed odors. Rats exhibit reduced accuracy but maintain reaction-time speed when the odors are mixed (Uchida and Mainen, 2003), while the reaction-time of mice slow yet accuracy is maintained in similar circumstances (Abraham et al., 2004; Rinberg et al., 2006). Remarkably, if rats are forced to increase their sampling time when mixed stimuli are presented their reaction-time subsequently slows and accuracy increases (Slotnick and Risser, 1990). Thus, the natural strategy of rats to olfactory stimuli appear to be reacting rapidly, but when forced to slow down they will utilize the extra information gathered to improve accuracy (Slotnick and Risser, 1990). In contrast, mice appear to utilize a strategy that is primed to withhold from responding and thus they naturally make use of more information to maintain accuracy (Bodyak and Slotnick, 1999).
These baseline strategy preferences – responsivity in rats and withholding in mice - are not dissimilar to those described by rats and mice performing the 5CSRTT. In a recently developed adaptation to the 5CSRTT, termed the 5-choice continuous performance test (5C-CPT) rodents are required to not only respond to target stimuli, but also to inhibit from responding to non-target stimuli. When training rodents in the task, mice can be trained to inhibit to non-target stimuli when the ratio of target to non-target trials is 5:1 (Young et al., 2009a). Rats could not learn at this ratio however, and required initial training with increased salience of the stimulus in which they were required to withhold from responding to – utilizing a 2 target to 1 non-target ratio (Barnes et al., 2011b). These findings support the supposition of increased tendency to respond in rats compared to mice. When trained in the 5C-CPT, the bias of responding can also be measured, and interestingly, rats and mice exhibit comparable bias levels (Barnes et al., 2011a, b; Young et al., 2009a; Young et al., 2011b). Thus, by emphasizing discriminated responding, it may be possible that rat and mouse behaviors become comparable.
2.4. Touchscreen testing
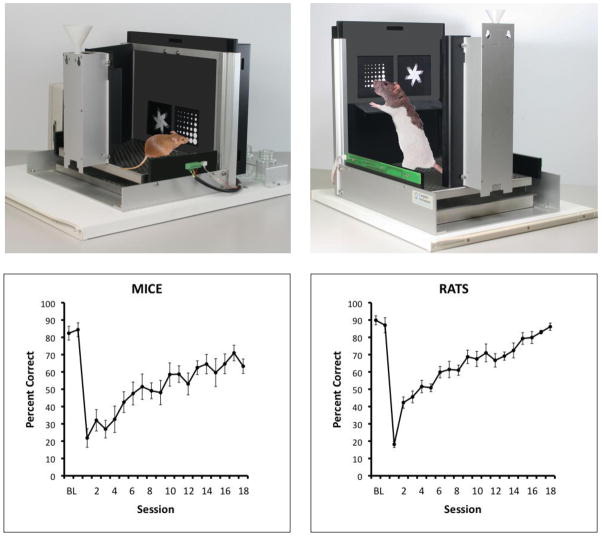
The 5CSRTT, 5C-CPT, and olfactometers are examples of apparatus which can be used in rats and mice providing opportunities to directly compare performance using stimuli from the same modalities. Another example of where this is possible is the touchscreen testing method, which uses a computer monitor to provide a visual display for stimuli to mice and rats which can record responses in the location stimuli appear (Bussey et al., 1994; Bussey et al., 2001). Hence, responses are made directly to stimuli via nose-pokes toward the screen (touching the screen is not required). Many tasks measuring a variety of aspects of cognition are possible using this apparatus (Bussey et al., 2011), which does not have a high motor demand allowing testing of rodents with even moderate motor impairments (Morton et al., 2006). The upper panels of Figure 1 illustrate mice and rats performing a pair-wise visual discrimination, in which one stimulus is correct and rewarded (the S+) and the other is incorrect and not rewarded (the S−). The lower panels of Figure 1 show the final performance levels of mice and rats following acquisition of the initial discrimination, and also their performance when the reward contingency is reversed, that is, when the stimulus that is the S+ during initial acquisition becomes the S−, and vice versa (see 3.2. below for species comparisons on reversal learning).
Figure 1.
The touchscreen testing apparatus and reversal learning curves for mice and rats. The upper panels show the most recent versions of the apparatus for mice (left) and rat (right). The lower panels show representative archival reversal learning curves for C57BL/6 mice (n=7) and Lister Hooded rats (n=7) obtained using this method. Data from two days of pre-reversal baseline (BL) performance are shown, followed by 18 sessions of reversal learning. The reversal curves for mouse and rat look very similar; note, however, that the number of trials per session differs for rats and mice (rat: 100 trials/session; mice: 30 trials/session). Mice are wild-type control animals from a study of M1 receptor function (Bartko et al., 2011); rats are sham lesion control animals from an unpublished study of prefrontal cortical function (data courtesy of Katie McAllister). Thus the rat and mouse groups were not compared in a controlled fashion nor were the data compared statistically; the comparison is for illustrative purposes only. Photographs of apparatus courtesy of Campden Instruments, Loughborough, U.K.
Advantages of the touchscreen method over other automated methods, e.g., standard operant chambers fitted with lights and levers, include the rich possibilities for stimulus manipulation. To continue with the example of pair-wise visual discrimination, McCarthy et al (2011) trained rats to discriminate two visual stimuli to high levels, and then ‘morphed’ (blended) the stimuli together (akin to mixing odors, described in 2.3.) to increase the perceptual similarity of the stimuli, thus bringing baseline performance down from ceiling. On this new baseline these authors were able to see not only large improvements in performance in wild-type rats given the anticholinesterase donepezil (Aricept), but were able to observe the synergistic cognitive enhancing effects of the combination of otherwise ineffective doses of donepezil and a putative add-on therapeutic compound, FK-962 (McCarthy et al., 2011). Similar examples can be found in mice; for example Bartko et al (2011a) found that mice with deletion of the M1 muscarinic receptor were unimpaired in the acquisition of a pair-wise shape discrimination. To challenge the animals further, mice were then tested on a more difficult pair of photographic stimuli and then, to provide an even more stringent test of the mouse’s perceptual and learning abilities, these photographic stimuli were morphed (blended) together to create an even more difficult discrimination (the difficulties of these discriminations were reflected in their rates of acquisition). Despite the absence of M1 receptors in these animals, they were not impaired on any of these challenges (although they did show abnormal responding in the 5CSRTT in the touchscreen). In this example, the results of these challenges supported the conclusion that the ability to perform perceptively difficult visual discriminations was unaffected by global M1 receptor deletion.
2-choice discrimination and reversal is one of simplest of several tasks currently worked up for both mice and rats in the touchscreen apparatus. So far all of the tasks that have been initially developed in rats have been successfully adapted for mice, usually with minimal changes. These include quite complex tests of, e.g., object-location paired associate learning (rat: (Talpos et al., 2009); mouse: (Bartko et al., 2011a); CNTRICS long-term memory paper this issue) and spatial working memory and pattern separation (rat: (McTighe et al., 2009; Talpos et al., 2010); mouse: (Clelland et al., 2009; Creer et al., 2010); (Dudchenko et al., 2012) this issue) as well as simple tests of conditioning (rat: (Bussey et al., 1997); mouse: CNTRICS reinforcement learning paper this issue). Indeed some touschscreen tasks, such as the 5CSRTT, have been worked up in mice first (Romberg et al., 2011) and subsequently ported to rats. If one is pressed to comment on species differences in this method, a general observation is that variability is greater in the mouse than the rat, but such increased variability in mice is unlikely to be specific to touchscreen apparatus; indeed increased group sizes for mice, in order to combat this effect, have been suggested for behavioral testing in general (Crawley, 2007). Perhaps more interesting is a small difference in the apparatus required for mouse versus rat. In the original rat methods paper (Bussey et al., 1994), it was reported that the use of a “shelf” (fitted with springs so that the rat can’t jump up on it) was required to force rats to rear up and to make an effortful response to the screen, thus minimizing accidental and impulsive choices and increasing precise responding to the stimuli on the screen. Interestingly mice do not need the shelf. Perhaps consistent with more impulsive action of rats as observed in the 5CSRTT (see 2.2.), mice generally behave less impulsively in touchscreen apparati, stopping in front of the screen and sampling the stimuli on the screen before making a response. Thus, the temperament of the mouse seems to be well-suited to the touchscreen testing method – perhaps more so even than the rat.
3.1. Examples of tasks whereby consistency between species have been observed
A considerable amount of cross-species research has focused on aspects of executive control, which are defined as a set of processes that contribute to voluntary (top-down) modulation of behavior, affective reactions and thought processes. The rationale guiding these studies stems from the observation that a variety of forms of executive control impairment are present in most forms of major psychiatric disorders, including psychoses.
Executive control over behavior can be assessed using very simple tasks that require the flexible updating of responding when subjects are exposed to context or contingency shifts. For example, subjects can be trained to solve a discrimination problem, and once their behavior meets some pre-determined criteria for efficiency and accuracy, the associative rules that govern the discrimination can be swapped. This is referred to as reversal learning, and after the switch, subjects are usually thought of as recruiting executive control mechanisms to inhibit the initially trained responses in order to permit re-exploration of the rules and the adoption of a new, now-accurate response strategy. While the acquisition of the initial discrimination and the subsequent reversal both require the same set of motor responses, motivational states and associative learning abilities, reversal differs from acquisition in the additional need for inhibitory control mechanisms.
The simplicity of the design of most discrimination reversal learning studies has made them highly suitable for cross-species investigation, and there is remarkable consensus that common neural systems and molecular pathways determine reversal learning abilities in rodents, NHPs, and human primates.
3.2. Reversal Learning
Irrespective of species, most discrimination reversal learning studies involve three dimensions: a set of discriminative stimuli that the subject uses to guide response selection, a set of operant responses and an outcome. Many variants include making an approach or foraging response towards a reward-associated cue, which is presented in the context of distractor cues that predict reward omission as opposed to reward presentation. Alternatively, variants may include pressing one operandum in the presence of one stimulus and another operandum in the presence of a different one. In both cases, the idea is that – over initial training – the discriminative cues come to efficiently, and increasingly automatically, evoke a particular response (through associative learning mechanisms), such that updating behavior at reversal is challenging and requires effortful, top-down processes.
These procedures have been used with great success in mice, rats, NHPs, and human subjects to uncover the genetic, neural and pharmacological basis of executive control. In rodent models, the discriminative cues used are often olfactory (Birrell and Brown, 2000; Bissonette et al., 2008; Schoenbaum et al., 2002; Schoenbaum et al., 2003), but can also be spatial or visual (Brigman et al., 2010; Izquierdo et al., 2010; Laughlin et al., 2011a); the instrumental response is typically a foraging response or operandum manipulation and the outcome is a food reward. In NHPs, the discriminative cues are either 2-dimensional visual stimuli or objects (Arnsten et al., 1997; Butters et al., 1973; Clarke et al., 2005; Dias et al., 1996b; Ettlinger and Ridley, 1975; Groman et al., 2011; Izquierdo et al., 2007; Lee et al., 2007; Seu et al., 2008, 2009), and the response is contact with a touchscreen (see 2.4. for details) or a foraging response (moving the object to uncover the reward); the outcome is typically food. In humans, the stimuli are typically 2-dimensional visual stimuli on a touchscreen (Clark et al., 2004; Cools et al., 2002; Evers et al., 2005; Fellows and Farah, 2003, 2005; Ghahremani et al., 2010), the response is usually a touch to the screen and the outcome is typically social or abstract. Patients with schizophrenia take more trials to rule reverse in comparison to healthy subjects (Leeson et al., 2009). Touchscreens can also be used in rats and mice to investigate systems in these animals relevant to schizophrenia, such as the glutamatergic, dopaminergic, serotonergic, and cholinergic systems, prefrontal cortex and hippocampus (e.g., Barkus et al., 2011; Brigman et al., 2010; Chudasama and Robbins, 2003; Karlsson et al., 2009).
Additional procedural details of relevance include: the number of discriminative stimuli being learned, whether discriminanda are presented concurrently or sequentially and whether the associative rules are deterministic and probabilistic. One major difference is that human studies often use probabilistic feedback to slow down the rate of learning, while animal studies involve deterministic rules. That said, some animal studies are now including probabilistic feedback (Bari et al., 2010), while human studies are beginning to involve deterministic feedback (Ghahremani et al., 2010). Some reversal learning studies in rodents utilize signaled reversals, whereby the reversal of contingencies are clearly signaled (Abdul-Monim et al., 2003; Idris et al., 2010), unlike the seamless transition from one reward configuration to its reversal which occurs in human studies. Moreover, some studies subject animals to multiple reversals in a row (serial reversal learning), which becomes striatal-dependent as the animal ‘learns to reverse’ (Castane et al., 2010). Such differences between rodent and human reversal learning paradigms may limit the development of novel molecules for treating schizophrenia. Likewise, not all clinical studies include an assessment of reversal learning in patients, despite the evidence generated using preclinical studies. In fact, many studies do not report effects on specific cognitive domains, but instead only report composite scores. Such grouping of data limits the comparative analysis of the effects of a molecule on task performance between preclinical and clinical studies. Future clinical studies would benefit from utilizing tasks with animal equivalents where positive effects of the molecule have been observed and presenting resulting data on the primary outcome measures of that task.
Typical 2-choice discrimination reversal learning data can be evaluated for learning rate (behavioral change, post reversal, as a function of trial) and can be subjected to error analyses that indicate whether behavior is strictly perseverative (meaning that responding is rigidly following the initially trained rule) or is entropic (Clarke et al., 2004; Haluk and Floresco, 2009; Ridley et al., 1981a; Ridley et al., 1981b). In addition, clever behavioral experiments that include stimulus substitutions can be used to dissect the role for response perseveration and learned irrelevance to reversal learning (Clarke et al., 2007; Tait and Brown, 2007). These sorts of manipulations can be made across species to determine whether the reversal learning deficits caused by a variety of manipulations represent the same type of cognitive impairment, allowing for a deep interrogation of construct validity. Such analyses are not typically detailed in human clinical studies however, perhaps their incorporation would be useful in future studies for cross-species comparisons.
During touchscreen reversal learning procedures, both mice and rats show a similar profile of responding: when the reward contingency is reversed, initially animals perform well below chance level, after which they first progress back up to chance levels (it is during this stage that changes in perseveration to the previously rewarded stimulus can be observed) and then finally up to asymptotic performance levels. The reversal curves for mouse and rat look very similar; note, however, that the number of trials per session differs for rats and mice; indeed on the basis of trials to criterion performance, the mice appear to learn faster than rats. Such conclusions need to be made with caution however; the spacing (as opposed to “massing”) of trials can have a substantial impact on learning rates (Barnet et al., 1995). What can be concluded that experimenter time is about equivalent when running an experiment in either mice or rats.
Another potential difference between species may be apparent in the final stages of reversal learning: it appears that whereas rats reverse to levels similar to those they attained during initial acquisition, mice never seem to get back up to those same pre-reversal levels. This species-difference has been informally observed on a number of occasions (Bartko et al., 2011a). The reason for this difference may be that mice continue to sample the previously rewarded stimulus, which might be thought of as consistent with a more impoverished prefrontal cortical function in mice. However without further head-to-head comparisons of mice and rats such ideas must remain speculative.
Irrespective of putative species differences and all the procedural details described above, there is overwhelming convergent construct validity that orbital regions of the frontal lobe contribute in a behaviorally specific manner to reversal learning (meaning it is does not mediate aspects of performance shared with the initial discrimination acquisition). Lesions of the orbitofrontal cortex impair reversal learning in mice (Bissonette et al., 2008), rats (Birrell and Brown, 2000; Boulougouris et al., 2007; Boulougouris and Robbins, 2008; McAlonan and Brown, 2003; Schoenbaum et al., 2002; Schoenbaum and Shaham, 2007; Tait and Brown, 2007), and NHPs (Butters et al., 1973; Dias et al., 1996a; Iversen and Mishkin, 1970; Izquierdo and Murray, 2004; Izquierdo et al., 2004; Rudebeck and Murray, 2008, 2011).
Even more striking is the convergent predictive evidence across species that dopamine D2-like receptor mechanisms contribute to reversal learning. In mice, knockout of the dopamine D2 receptor gene impairs reversal learning (De Steno and Schmauss, 2009; Kruzich and Grandy, 2004; Kruzich et al., 2006). In rats and non-human and human primates, pharmacological regulation of the D2 receptor produces impairments in the ability to update behavior during reversal (Boulougouris et al., 2008; Haluk and Floresco, 2009; Lee et al., 2007; Mehta et al., 2001; Ridley et al., 1981b; Smith et al., 1999). Moreover, heritable variation in dopamine D2 receptor complement in brain predicts individual differences in reversal learning in mice, monkeys and humans (Groman et al., 2011; Jocham et al., 2009; Laughlin et al., 2011b). While these data certainly do not implicate D2 receptor-dependent dopamine transmission as the only, or even most crucial, molecular regulator of executive control, it does indicate that cross-species investigations can be remarkably effective at identifying common molecular and neural mechanisms mediating a cognitive construct of interest.
What is lacking in some of the mechanistic studies described above is the use of behavioral manipulations to determine whether lesions or pharmacological substances produce identical changes in the construct of interest across species. The use of 3, as opposed to 2, discriminanda, and the employment of stimulus substitution schemes, will both permit a much more robust analysis of executive control abilities across different laboratory models and species. In addition, the use of identical sensory modalities and testing apparati (such as visual stimuli on a touchscreen, see 2.4.), as well as learning criterion, may be an important avenue for future comparative research. Each of these developments make it is possible to directly dissociate perseverative from random responses (e.g., in a 2-choice test, there is only one error, and its nature must be inferred, not directly assessed), and it becomes experimentally straightforward to conduct tests that identify whether a particular manipulation distinctly modulates behavioral flexibility, influences learned irrelevance or creates a disorganized pattern of choice that all affects overall reversal performance (e.g., in touchscreen tests it is possible to determine whether a manipulation only affects performance when a previously trained stimulus is present and supports perseverative responding). Thus, although there is evidence for many similarities of reversal learning performance across species, the refinement of tasks to assess this construct would aid data interpretation and the translational development of novel molecules across species.
3.3. Paired Associates Learning
Another example of a paradigm that has been successfully utilized across species is the visuo-spatial paired-associates learning (vsPAL) task. The vsPAL task requires subjects to associate a set of objects with particular spatial locations on a trial-by-trial basis, and involves the proper functioning of the temporal lobes, and in particular the hippocampi (Owen et al., 1995) (Murray et al., 1993) (Wood et al., 2002) (Talpos et al., 2009). VsPAL has been successfully established and used in humans, macaques and marmosets for decades, and more recently has been translated into rats and mice (Bartko et al., 2011b; Owen et al., 1995; Spinelli et al., 2005; Taffe et al., 2002; Talpos et al., 2009). The use of the touchscreen based computerized system across species has provided consistency in the presentation of stimuli and mode of responses and enables the effective translation of this test across species.
Clinically, deficits in vsPAL performance have been demonstrated to occur with advanced age (Robbins et al., 1994; Rabbitt and Lowe, 2000) and in specific diseases. In particular, patients with mild cognitive impairment show impaired performance in vsPAL that has been shown to accurately predict later conversion to Alzheimer’s disease (Blackwell et al., 2004; Sahakian et al., 1988; Swainson et al., 2001; Barnett et al., 2005). Similarly, first episode and chronic schizophrenic patients show impaired paired-associates learning (Wood et al., 2002; Barnett et al., 2005). Possibly a deficit in PAL in these patients may be due to reduced gray matter in temporal lobe structures as shown by MRI in at-risk prodromal patients prior to first psychotic episode (Pantelis et al., 2003). Neuronal loss in key brain structures such as the hippocampus in AD patients is also a hallmark pathology of the disease progression (Selkoe, 1999) that may underlie the observed cognitive impairments in vsPAL.
3.3.1. PAL Testing and Training
In humans and NHPs, vsPAL requires subjects to learn to associate a specific stimulus with a particular location on a trial-by-trial basis and the cognitive demand of the task increases as the number of stimuli-locations presented increases. Varying the number of stimulus-locations within a session allows experimenters to assess task performance at different memory loads (Taffe et al., 2002). NHPs typically have up to 4 stimuli/4 locations as their most difficult trials, whereas humans may have up to 6–8 sample stimuli locations in the most challenging trials (Fowler et al., 1997; Swainson et al., 2001; Taffe et al., 2002).
Typically in the human test, 6 white boxes appear around the periphery of the computer screen and in turn each one “opens up” to reveal a particular stimulus until all 6 stimulus-location pairings have been shown independently (sample phase). The subject is then shown one of the sample stimuli in the center of the screen and must match the stimulus to the box (location) in which it was originally presented (choice phase). Subjects go through each of the sample stimuli until they have responded to each of them. In the human version, subjects may or may not be rewarded for correct response. In addition, subjects are given task instructions either verbally or in written format prior to testing, thus minimal training is necessary.
In the NHP version of vsPAL, extensive training is necessary prior to testing. Since NHPs must acquire the rules of the task through a trial-and-error approach (i.e., without verbal instructions as for humans), modifications have been made to the task many times to facilitate training. For example, during training responding to the sample stimuli can be rewarded to help shape this behavior and modifications can be removed once the subjects are properly trained. However, in NHPs it should be noted that touch responses to the sample stimuli are required as a measure of having attended to these stimuli. A failure to touch the sample stimuli means the trial does not proceed to the test phase (and potential reward). In the CANTAB vsPAL, stimulus-locations are randomized (9 locations; 20 stimuli) so that stimulus-location pairs are not memorized. Once animals are trained to a particular criterion, the performance generally remains stable, which is imperative for chronic or cross-over study designs.
In the rodent version of vsPAL, animals learn to associate a particular stimulus with a particular location over a lengthy training period. The mouse version of the task was modeled after the rat version, so many of the same approaches were used to translate between species. Animals must learn which location is associated with each stimulus. Two stimuli are presented at once in three possible locations (i.e., 6 possible stimulus-location options), with one stimulus being in the correct location (S+) and the other stimulus in an incorrect location (S−). The animal is required to select the correct stimulus-location to receive a food reward (Bartko et al., 2011b; Talpos et al., 2009). The animals are given a fixed number of trials (mouse: 36 trials; rat: 72-trials) within each session after performing at a set criteria consistently during the training phase. Memory load remains constant throughout the test in the rodent version of vsPAL. Given the length of time and number of trials across sessions rodents are given to associate pairs in comparison to the within session changes of humans and monkeys, it may be that the former assesses long-term association formation while the latter assesses recent association formation. Comparative studies comparing rodents and primates have not been investigated as yet and so this issue remains unanswered, future studies using this paradigm may yield insight on this matter.
3.3.2. Pharmacology
A better understanding of the neurochemical receptor systems underlying the vsPAL task is emerging as more pharmacological based studies are being conducted and reported. Drugs that traditionally impair cognition such as the muscarinic antagonist scopolamine, also are reported to impair accuracy in PAL in rhesus macaques (Taffe et al., 2002) and mice (Bartko et al., 2011b), and scopolamine produced marginal impairments in healthy volunteers (Robbins et al., 1997). Intra-hippocampally injected scopolamine or mecamylamine into the rat showed no effect on vsPAL performance however, suggesting that the hippocampus may not be involved in scopolamine-induced deficits following systemic administration (Talpos et al., 2009). Possibly broader disruptions in other cognitive functions, such as attention, following systemic injections of scopolamine in other species may explain the discrepancy in results from the rat studies. Interestingly, impairment of vsPAL performance with NMDA receptor antagonists (e.g., ketamine, MK-801) was observed in rat, as well as rhesus monkeys following intra-hippocampal or systemic injections, respectively (Taffe et al., 2002; Talpos et al., 2009). Although complementary drug studies have not been confirmed in humans to date, deficits in PAL performance have been reported in pre-psychotic individuals (Bartok et al., 2005), in first-episode schizophrenic patients (Barnett et al., 2005), and in patients with a long-standing diagnosis of schizophrenia (Wood et al., 2002), suggesting impaired NMDA receptor function could effect visuospatial learning. Interestingly, one report from (Stip et al., 2005) indicated greater sensitivity (vsPAL task performance deficit) in distinguishing patients with schizophrenia, as compared to patients diagnosed with schizoaffective disorder.
Direct and indirect cholinergic agonists have been assessed for pro-cognitive properties in vsPAL. Nicotine administered to rhesus macaques improves accuracy in the PAL model (Katner et al., 2004), whereas cholinesterase inhibitors, which are the most widely prescribed therapeutic agents for Alzheimer’s disease have yielded mixed results. Tetrahydroaminoacridine was reported not to improve vsPAL performance (Riekkinen et al., 1998) in AD patients following a single administration; however, phenserine was reported to significantly enhance PAL performance in an AD patient population over a 12-week period in a placebo-controlled phase II study (Greig et al., 2005). These results are consistent with the clinical literature for the cholinesterase inhibitors in that chronic dosing is required for therapeutic benefit. More recently, donepezil has been reported to facilitate vsPAL in mice (Bartko et al., 2011b).
The catecholamine systems have also been investigated for their role in mediating the cognitive properties of visual spatial learning and memory. In particular, the dopaminergic system is involved in mediating complex cognitive processes and impairments to this neurochemical system prevalent in many CNS disorders (e.g., Parkinson’s disease, attention deficit/hyperactivity disorder). Specifically, antagonism of dopamine D2-like receptors with raclopride, but not of the D1-like receptors with SCH23390, impaired vsPAL performance in rhesus macaques (Von Huben et al., 2006). Noradrenergic alpha2 receptor agonists clonidine and guanfacine improved vsPAL performance in healthy volunteers (Jakala et al., 1999), consistent with the role of this system in arousal, vigilance and attention. However, it should be noted that clonidine administration also has been reported to have no effect or to even impair vsPAL performance depending on different experimental conditions used (e.g., verbal versus non-verbal stimuli, dose; Coull et al., 1995; Coull et al., 1997; Frith et al., 1985). It would be useful to investigate the involvement of the noradrenergic system or dopamine-D2-like receptors to vsPAL in other species under similar conditions.
4.1. Practical implications for the drug discovery process
The development of novel molecules for the treatment of cognitive impairments in schizophrenia remains an important unmet medical need. The effective translation of preclinical research findings into proof of concept clinical trials remains a challenge and contributes to the high attrition rate of molecules entering into human populations (Kola and Landis, 2004). Focusing our efforts on the construct validity of particular tests and improving our understanding of the strengths and limitations of these models across species will improve the success of the discovery and development process.
Selecting the appropriate species for the key questions at hand is critical for successful translation. Rodents are particularly good for early PK-PD assessments for lead identification/lead optimization stages of drug discovery, as models can be developed to give relatively quick feedback for determining structural activity relationships. Mice in particular are ideal for the study of genetic determinations and they have been used for years to overexpress, knockout or mutate particular genes of interest; although, transgenic rat models are beginning to gain attention recently. Some challenges may arise with using mice exclusively for characterization of novel compounds due to difficulty in translating mouse pharmacology data into rat, NHP or human, for some of the reasons described in 2.1.1. (e.g., different strategies in 5CSRTT), as well as due to different pharmacokinetic properties (e.g., rapid metabolism). For example, nicotine is used commonly in animal models as a pro-cognitive agent, yet the half-life (t1/2) of the compound varies widely depending on the species (e.g., mouse: t1/2 7–10 minutes (Petersen et al., 1984); rat: t1/2 60 minutes in rat (Miller et al., 1977); monkey: t1/2 100–200 minutes (Seaton et al., 1991); human: t1/2 95–227 minutes (Benowitz and Jacob, 1994), a factor that can influence results from tests taking longer to complete as drug levels may not be optimal for appropriate assessment (e.g. ASST testing can take 3 – 6 hours). Furthermore, one must take into account the preciousness of the species being tested. For example, novel molecules may be tested in well-trained rodents up to six or seven times prior to a novel cohort being trained. In contrast, NHPs will be tested with novel molecules for numerous years given the ethical considerations of constantly training a new cohort after every treatment. While many steps are taken to ensure the performance of the animals stabilize prior to testing – with positive controls included to ensure drug-induced effects can be observed – such considerations must be taken into account when evaluating the utility of each species for each stage in drug discovery.
Whereas rodents are used to assess cognitive function especially in early stages of drug discovery process, the use of NHPs provides the ability to investigate complex behavioral processes that are closely aligned with human function for both lead optimization, as well as into clinical development stages of investigation The utility of using NHPs in the drug discovery process is of growing importance with the traditionally poor translation between preclinical and clinical studies, and costly failures in clinical development creating a “valley of death” for promising CNS-active molecules. Although within-subjects designs often are necessary in NHP studies due to lengthy training periods of some tasks (e.g., 4–6 months) and costs associated with maintaining colonies of trained animals, the translatability of the resulting data from NHPs can provide an invaluable link between data generated in rodents and human studies. An example of the utility of NHPs for drug discovery purposes can be highlighted by the successful registration of guanfacine for ADHD, which was extensively characterized in NHP cognition tasks with a focus on prefrontal cortical function in which it improved performance at orally administered doses that are identical to those used in patients (for review see Gamo and Arnsten, 2011). Proper modulation of the catecholaminergic system in the prefrontal cortex of the ADHD brain underlies the efficacy of these molecules, which were ideally investigated in a NHP species that has benefitted from a more advanced cortical system. The similarities in anatomy, neuronal circuitry, neurochemistry, functional and cognitive abilities exhibited between NHPs and humans provides for an ideal approach to investigate higher cortical-mediated behaviors in particular, and allows for direct comparisons of cognitive function to be made using the same endpoints in both species (e.g., CANTAB). Overall, the ability to iterate the characterization of a novel molecule from rodent to NHP to human strengthens the understanding of the mechanism of action as well as the identification of endpoints and patient populations most sensitive to a potential new therapy.
5.1. Conclusions
In order to facilitate vertical translation during the drug discovery process, it will be important for the cognitive testing that occurs in preclinical species is consistent with the cognitive testing that occurs in humans. Consistency is required in terms of the cognitive construct being assessed, the neurobiology underlying that construct, and the pharmacological effects of the treatment on that construct. In this review we have described some of the underlying differences and similarities across species in a variety of cognitive paradigms, from mice and rats to NHPs and humans.
In 5CSRTT testing it is evident that rats rely more on a temporal mediating strategy to readily perform the task than do mice. This reliance may underlie the reliability of amphetamine-induced increases of premature responses in rats not seen with mice. Alternatively, this discrepancy of amphetamine effects between rats and mice could result from the greater selectivity of amphetamine on norepinephrine transporters compared with dopamine transporters in rats while the opposite is true for mice (and humans). With the availability of the human 5CSRTT, the relevance of these data to human testing can be established. Irrespective of the underlying cause however, it is evident that there are some differences between species in terms of impulsivity measures from the 5CSRTT. Likewise, it is apparent that mice are less impulsive to stimuli they should inhibit from responding to when compared with rats, observed across numerous types of apparatus. These differences could relate to the natural behavior of the two species, where rats interact with their environment more as a predator species, while mice are predated upon by many more species, including rats. When required to inhibit prelearned responses in order to learn new reward contingencies in reversal learning, mice and rats exhibit comparable behaviors. Moreover, there is evidence of consistent neurobiological underpinnings such as orbitofrontal or dopamine D2-like receptor mediation of reversal learning across species including humans and NHPs.
The availability of translatable cognitive test batteries that are based on comparable platforms (e.g., touchscreen apparatus) allows testing in a number of behaviors to occur similarly across species, including reversal learning and vsPAL. Whereas this approach has many strengths for successful translation between species, it also highlights one of the fundamental differences between cognitive tests developed for rodents and NHPs, namely the lengthy training time, which is not needed for testing in humans given the option of verbal instructions. Extended training in some paradigms, such as the vsPAL, may result in different constructs being assessed between these species. Detailing differences between species will prove as useful in the long run as detailing consistency during experiments. Another important point regarding species differences is the numerous behavioral differences observed between strains within the same species. Some studies report pharmacological effects in some strains within a species (e.g., mouse) that are absent in others. While these effects may be a result of poorer performance of some strains allowing for improvements to be seen (ceiling effects) differences between strains highlight the difficulty of expecting perfect symmetry across species.
Developing novel molecules for treating cognitive dysfunction in patients with schizophrenia will require developing evidence for targeting the mechanism and testing the molecule in tasks with cross-species translational validity such as reversal learning and vsPAL. While some refinement may be needed in these tasks, more is also required from clinical studies. Care should be taken that similar tasks should be used in clinical studies. For example, many clinical trials continue to use standardized paper and pen tests that unfortunately have little relevance to cognitive testing in preclinical species (rodents, NHPs). Designing clinical tests using tasks that can be performed in animals will also be extremely useful in future (e.g., CANTAB). Furthermore, evidence for procogntiive effects of novel molecules can readily be generated in normal animals, avoiding difficulties of the need to model schizophrenia. Using early Phase I testing of the molecule in healthy volunteers offers the opportunity to confirm positive effects of the compound in ‘normal’ subjects prior to more expensive testing in patients in Phase II and III trials. Thus, more can be done across all levels of testing to ensure the molecule being investigated will exhibit comparable effects across species.
Despite a lack of perfect symmetry of behavior between species, even between rodents, it is clear that the construct underlying behaviors exhibit remarkable consistency across species in comparable cognitive tests. Such consistency can, and should, be used in the future to develop molecules that will treat cognitive dysfunction in neuropsychiatric disorders.
Highlights.
Cross-species translation is vital for the development of cognition enhancers
Ordinarily arguments are made for translatability (preclinical to clinic)
Differences in cognition between species are discussed.
From tests of attention, to reversal and paired associates learning
Practical implications of species differences on drug development also provided
Acknowledgments
The authors would like to acknowledge the support of everyone from the CNTRICS initiative. JWY receives support from NIH R21MH091571. TJB receives support from the Innovative Medicine Initiative Joint Undertaking under grant agreement n° 115008 of which resources are composed of EFPIA in-kind contribution and financial contribution from the European Union’s Seventh Framework Programme (FP7/2007–2013). TJB consults for Campden Instruments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
7.1. References
- Abdul-Monim Z, Reynolds GP, Neill JC. The atypical antipsychotic ziprasidone, but not haloperidol, improves phencyclidine-induced cognitive deficits in a reversal learning task in the rat. J Psychopharmacol. 2003;17:57–65. doi: 10.1177/0269881103017001700. [DOI] [PubMed] [Google Scholar]
- Abraham NM, Spors H, Carleton A, Margrie TW, Kuner T, Schaefer AT. Maintaining accuracy at the expense of speed: stimulus similarity defines odor discrimination time in mice. Neuron. 2004;44:865–876. doi: 10.1016/j.neuron.2004.11.017. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Lin CH, Van Dyck CH, Stanhope KJ. The effects of 5-HT3 receptor antagonists on cognitive performance in aged monkeys. Neurobiol Aging. 1997;18:21–28. doi: 10.1016/s0197-4580(96)00162-5. [DOI] [PubMed] [Google Scholar]
- Balci F, Ludvig EA, Gibson JM, Allen BD, Frank KM, Kapustinski BJ, Fedolak TE, Brunner D. Pharmacological manipulations of interval timing using the peak procedure in male C3H mice. Psychopharmacology (Berl) 2008;201:67–80. doi: 10.1007/s00213-008-1248-y. [DOI] [PubMed] [Google Scholar]
- Bari A, Theobald DE, Caprioli D, Mar AC, Aidoo-Micah A, Dalley JW, Robbins TW. Serotonin modulates sensitivity to reward and negative feedback in a probabilistic reversal learning task in rats. Neuropsychopharmacology. 2010;35:1290–1301. doi: 10.1038/npp.2009.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkus C, Feyder M, Graybeal C, Wright T, Wiedholz L, Izquierdo A, Kiselycznyk C, Schmitt W, Sanderson DJ, Rawlins JN, Saksida LM, Bussey TJ, Sprengel R, Bannerman D, Holmes A. Do GluA1 knockout mice exhibit behavioral abnormalities relevant to the negative or cognitive symptoms of schizophrenia and schizoaffective disorder? Neuropharmacology. 2011 doi: 10.1016/j.neuropharm.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes SA, Young JW, Neill JC. D(1) receptor activation improves vigilance in rats as measured by the 5-choice continuous performance test. Psychopharmacology (Berl) 2011a doi: 10.1007/s00213-011-2460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes SA, Young JW, Neill JC. Rats tested after a washout period from sub-chronic PCP administration exhibited impaired performance in the 5-Choice Continuous Performance Test (5C-CPT) when the attentional load was increased. Neuropharmacology. 2011b doi: 10.1016/j.neuropharm.2011.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnet RA, Graheme NJ, Miller RR. Trial spacing effects in Pavlovian conditioning: A role for local context. Animal Learning and Behavior. 1995;23:340–348. [Google Scholar]
- Barnett JH, Robbins TW, Leeson VC, Sahakian BJ, Joyce EM, Blackwell AD. Assessing cognitive function in clinical trials of schizophrenia. Neurosci Biobehav Rev. 2010;34:1161–1177. doi: 10.1016/j.neubiorev.2010.01.012. [DOI] [PubMed] [Google Scholar]
- Barnett JH, Sahakian BJ, Werners U, Hill KE, Brazil R, Gallagher O, Bullmore ET, Jones PB. Visuospatial learning and executive function are independently impaired in first-episode psychosis. Psychol Med. 2005;35:1031–1041. doi: 10.1017/s0033291704004301. [DOI] [PubMed] [Google Scholar]
- Bartko SJ, Romberg C, White B, Wess J, Bussey TJ, Saksida LM. Intact attentional processing but abnormal responding in M1 muscarinic receptor-deficient mice using an automated touchscreen method. Neuropharmacology. 2011a;61:1366–1378. doi: 10.1016/j.neuropharm.2011.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartko SJ, Vendrell I, Saksida LM, Bussey TJ. A computer-automated touchscreen paired-associates learning (PAL) task for mice: impairments following administration of scopolamine or dicyclomine and improvements following donepezil. Psychopharmacology (Berl) 2011b;214:537–548. doi: 10.1007/s00213-010-2050-1. [DOI] [PubMed] [Google Scholar]
- Bartok E, Berecz R, Glaub T, Degrell I. Cognitive functions in prepsychotic patients. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:621–625. doi: 10.1016/j.pnpbp.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Jacob P., 3rd Metabolism of nicotine to cotinine studied by a dual stable isotope method. Clin Pharmacol Ther. 1994;56:483–493. doi: 10.1038/clpt.1994.169. [DOI] [PubMed] [Google Scholar]
- Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci. 2000;20:4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissonette GB, Martins GJ, Franz TM, Harper ES, Schoenbaum G, Powell EM. Double dissociation of the effects of medial and orbital prefrontal cortical lesions on attentional and affective shifts in mice. J Neurosci. 2008;28:11124–11130. doi: 10.1523/JNEUROSCI.2820-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell AD, Sahakian BJ, Vesey R, Semple JM, Robbins TW, Hodges JR. Detecting dementia: novel neuropsychological markers of preclinical Alzheimer’s disease. Dement Geriatr Cogn Disord. 2004;17:42–48. doi: 10.1159/000074081. [DOI] [PubMed] [Google Scholar]
- Body S, Cheung TH, Hampson CL, den Boon FS, Bezzina G, Fone KC, Bradshaw CM, Szabadi E. Attenuation of the effects of d-amphetamine on interval timing behavior by central 5-hydroxytryptamine depletion. Psychopharmacology (Berl) 2009;203:547–559. doi: 10.1007/s00213-008-1400-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodyak N, Slotnick B. Performance of mice in an automated olfactometer: odor detection, discrimination and odor memory. Chem Senses. 1999;24:637–645. doi: 10.1093/chemse/24.6.637. [DOI] [PubMed] [Google Scholar]
- Boulougouris V, Castane A, Robbins TW. Dopamine D2/D3 receptor agonist quinpirole impairs spatial reversal learning in rats: investigation of D3 receptor involvement in persistent behavior. Psychopharmacology (Berl) 2008 doi: 10.1007/s00213-008-1341-2. [DOI] [PubMed] [Google Scholar]
- Boulougouris V, Dalley JW, Robbins TW. Effects of orbitofrontal, infralimbic and prelimbic cortical lesions on serial spatial reversal learning in the rat. Behav Brain Res. 2007;179:219–228. doi: 10.1016/j.bbr.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Boulougouris V, Robbins TW. Pre-surgical training ameliorates orbitofrontal-mediated impairments in spatial reversal learning. Behav Brain Res. 2008 doi: 10.1016/j.bbr.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Brigman JL, Mathur P, Harvey-White J, Izquierdo A, Saksida LM, Bussey TJ, Fox S, Deneris E, Murphy DL, Holmes A. Pharmacological or genetic inactivation of the serotonin transporter improves reversal learning in mice. Cereb Cortex. 2010;20:1955–1963. doi: 10.1093/cercor/bhp266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussey TJ, Everitt BJ, Robbins TW. Dissociable effects of cingulate and medial frontal cortex lesions on stimulus-reward learning using a novel Pavlovian autoshaping procedure for the rat: implications for the neurobiology of emotion. Behav Neurosci. 1997;111:908–919. doi: 10.1037//0735-7044.111.5.908. [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Holmes A, Lyon L, Mar AC, McAllister KA, Nithianantharajah J, Oomen CA, Saksida LM. New translational assays for preclinical modelling of cognition in schizophrenia: The touchscreen testing method for mice and rats. Neuropharmacology. 2011 doi: 10.1016/j.neuropharm.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussey TJ, Muir JL, Robbins TW. A novel automated touchscreen procedure for assessing learning in the rat using computer graphic stimuli. Neuroscience Research Communications. 1994;15:103–110. [Google Scholar]
- Bussey TJ, Saksida LM, Rothblat LA. Discrimination of computer-graphic stimuli by mice: a method for the behavioral characterization of transgenic and gene-knockout models. Behav Neurosci. 2001;115:957–960. doi: 10.1037//0735-7044.115.4.957. [DOI] [PubMed] [Google Scholar]
- Butters N, Butter C, Rosen J, Stein D. Behavioral effects of sequential and one-stage ablations of orbital prefrontal cortex in the monkey. Exp Neurol. 1973;39:204–214. doi: 10.1016/0014-4886(73)90223-9. [DOI] [PubMed] [Google Scholar]
- Carli M, Robbins TW, Evenden JL, Everitt BJ. Effects of lesions to ascending noradrenergic neurones on performance of a 5-choice serial reaction task in rats; implications for theories of dorsal noradrenergic bundle function based on selective attention and arousal. Behav Brain Res. 1983;9:361–380. doi: 10.1016/0166-4328(83)90138-9. [DOI] [PubMed] [Google Scholar]
- Castane A, Theobald DE, Robbins TW. Selective lesions of the dorsomedial striatum impair serial spatial reversal learning in rats. Behav Brain Res. 2010;210:74–83. doi: 10.1016/j.bbr.2010.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouinard S, Stip E, Poulin J, Melun JP, Godbout R, Guillem F, Cohen H. Rivastigmine treatment as an add-on to antipsychotics in patients with schizophrenia and cognitive deficits. Curr Med Res Opin. 2007;23:575–583. doi: 10.1185/030079906X167372. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Robbins TW. Dissociable contributions of the orbitofrontal and infralimbic cortex to pavlovian autoshaping and discrimination reversal learning: further evidence for the functional heterogeneity of the rodent frontal cortex. J Neurosci. 2003;23:8771–8780. doi: 10.1523/JNEUROSCI.23-25-08771.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark L, Cools R, Robbins TW. The neuropsychology of ventral prefrontal cortex: decision-making and reversal learning. Brain Cogn. 2004;55:41–53. doi: 10.1016/S0278-2626(03)00284-7. [DOI] [PubMed] [Google Scholar]
- Clarke HF, Dalley JW, Crofts HS, Robbins TW, Roberts AC. Cognitive inflexibility after prefrontal serotonin depletion. Science. 2004;304:878–880. doi: 10.1126/science.1094987. [DOI] [PubMed] [Google Scholar]
- Clarke HF, Walker SC, Crofts HS, Dalley JW, Robbins TW, Roberts AC. Prefrontal serotonin depletion affects reversal learning but not attentional set shifting. J Neurosci. 2005;25:532–538. doi: 10.1523/JNEUROSCI.3690-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke HF, Walker SC, Dalley JW, Robbins TW, Roberts AC. Cognitive inflexibility after prefrontal serotonin depletion is behaviorally and neurochemically specific. Cereb Cortex. 2007;17:18–27. doi: 10.1093/cercor/bhj120. [DOI] [PubMed] [Google Scholar]
- Clelland CD, Choi M, Romberg C, Clemenson GD, Jr, Fragniere A, Tyers P, Jessberger S, Saksida LM, Barker RA, Gage FH, Bussey TJ. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009;325:210–213. doi: 10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R, Clark L, Owen AM, Robbins TW. Defining the neural mechanisms of probabilistic reversal learning using event-related functional magnetic resonance imaging. J Neurosci. 2002;22:4563–4567. doi: 10.1523/JNEUROSCI.22-11-04563.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coull JT, Frith CD, Dolan RJ, Frackowiak RS, Grasby PM. The neural correlates of the noradrenergic modulation of human attention, arousal and learning. Eur J Neurosci. 1997;9:589–598. doi: 10.1111/j.1460-9568.1997.tb01635.x. [DOI] [PubMed] [Google Scholar]
- Coull JT, Middleton HC, Robbins TW, Sahakian BJ. Clonidine and diazepam have differential effects on tests of attention and learning. Psychopharmacology (Berl) 1995;120:322–332. doi: 10.1007/BF02311180. [DOI] [PubMed] [Google Scholar]
- Crawley J. What’s wrong with my mouse. Wily-Liss; New York: 2007. [Google Scholar]
- Creer DJ, Romberg C, Saksida LM, van Praag H, Bussey TJ. Running enhances spatial pattern separation in mice. Proc Natl Acad Sci U S A. 2010;107:2367–2372. doi: 10.1073/pnas.0911725107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Steno DA, Schmauss C. A role for dopamine D2 receptors in reversal learning. Neuroscience. 2009;162:118–127. doi: 10.1016/j.neuroscience.2009.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias R, Robbins TW, Roberts AC. Dissociation in prefrontal cortex of affective and attentional shifts. Nature. 1996a;380:69–72. doi: 10.1038/380069a0. [DOI] [PubMed] [Google Scholar]
- Dias R, Robbins TW, Roberts AC. Primate analogue of the Wisconsin Card Sorting Test: effects of excitotoxic lesions of the prefrontal cortex in the marmoset. Behav Neurosci. 1996b;110:872–886. doi: 10.1037//0735-7044.110.5.872. [DOI] [PubMed] [Google Scholar]
- Didriksen M, Christensen AV. Differences in performance in three strains of rats in a 5-choice serial reaction time task. Pharmacol Toxicol. 1993;72:66–68. doi: 10.1111/j.1600-0773.1993.tb01341.x. [DOI] [PubMed] [Google Scholar]
- Dudchenko PA, Talpos J, Young J, Baxter MG. Animal models of working memory: A review of tasks that might be used in screening drug treatments for the memory impairments found in schizophrenia. Neurosci Biobehav Rev. 2012 doi: 10.1016/j.neubiorev.2012.03.003. [DOI] [PubMed] [Google Scholar]
- Ellenbroek BA, Cools AR. Animal models for the negative symptoms of schizophrenia. Behav Pharmacol. 2000;11:223–233. doi: 10.1097/00008877-200006000-00006. [DOI] [PubMed] [Google Scholar]
- Ettlinger G, Ridley RM. Visual discrimination in the monkey: distinguishing the incorrect response. Neuropsychologia. 1975;13:111–113. doi: 10.1016/0028-3932(75)90055-x. [DOI] [PubMed] [Google Scholar]
- Evers EA, Cools R, Clark L, van der Veen FM, Jolles J, Sahakian BJ, Robbins TW. Serotonergic modulation of prefrontal cortex during negative feedback in probabilistic reversal learning. Neuropsychopharmacology. 2005;30:1138–1147. doi: 10.1038/sj.npp.1300663. [DOI] [PubMed] [Google Scholar]
- Fagerlund B, Soholm B, Fink-Jensen A, Lublin H, Glenthoj BY. Effects of donepezil adjunctive treatment to ziprasidone on cognitive deficits in schizophrenia: a double-blind, placebo-controlled study. Clin Neuropharmacol. 2007;30:3–12. doi: 10.1097/01.WNF.0000240940.67241.F6. [DOI] [PubMed] [Google Scholar]
- Fellows LK, Farah MJ. Ventromedial frontal cortex mediates affective shifting in humans: evidence from a reversal learning paradigm. Brain. 2003;126:1830–1837. doi: 10.1093/brain/awg180. [DOI] [PubMed] [Google Scholar]
- Fellows LK, Farah MJ. Different underlying impairments in decision-making following ventromedial and dorsolateral frontal lobe damage in humans. Cereb Cortex. 2005;15:58–63. doi: 10.1093/cercor/bhh108. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Tampakeras M, Sinyard J, Higgins GA. Opposing effects of 5-HT(2A) and 5-HT(2C) receptor antagonists in the rat and mouse on premature responding in the five-choice serial reaction time test. Psychopharmacology (Berl) 2007;195:223–234. doi: 10.1007/s00213-007-0891-z. [DOI] [PubMed] [Google Scholar]
- Fowler KS, Saling MM, Conway EL, Semple JM, Louis WJ. Computerized neuropsychological tests in the early detection of dementia: prospective findings. J Int Neuropsychol Soc. 1997;3:139–146. [PubMed] [Google Scholar]
- Fowler SC, Pinkston J, Vorontsova E. Timing and space usage are disrupted by amphetamine in rats maintained on DRL 24-s and DRL 72-s schedules of reinforcement. Psychopharmacology (Berl) 2009;204:213–225. doi: 10.1007/s00213-008-1451-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith CD, Dowdy J, Ferrier IN, Crow TJ. Selective impairment of paired associate learning after administration of a centrally-acting adrenergic agonist (clonidine) Psychopharmacology (Berl) 1985;87:490–493. doi: 10.1007/BF00432519. [DOI] [PubMed] [Google Scholar]
- Gamo NJ, Arnsten AF. Molecular modulation of prefrontal cortex: rational development of treatments for psychiatric disorders. Behav Neurosci. 2011;125:282–296. doi: 10.1037/a0023165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer MA, Braff D, Swerdlow NR. Startle-response measures of information processing in animals. In: Haug M, Whalen RE, editors. Animal models of human emotion & cognition. APA Books; Washington D.C: 1999. pp. 103–116. [Google Scholar]
- Geyer MA, Braff DL. Startle habituation and sensorimotor gating in schizophrenia and related animal models. Schizophr Bull. 1987;13:643–668. doi: 10.1093/schbul/13.4.643. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Markou A. Animal models of psychiatric disorders. In: Bloom FE, Kupfer D, editors. Psychopharmacology: The fourth generation of progress. Raven Press; New York: 1995. pp. 787–798. [Google Scholar]
- Ghahremani DG, Monterosso J, Jentsch JD, Bilder RM, Poldrack RA. Neural components underlying behavioral flexibility in human reversal learning. Cereb Cortex. 2010;20:1843–1852. doi: 10.1093/cercor/bhp247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greig NH, Sambamurti K, Yu QS, Brossi A, Bruinsma GB, Lahiri DK. An overview of phenserine tartrate, a novel acetylcholinesterase inhibitor for the treatment of Alzheimer’s disease. Curr Alzheimer Res. 2005;2:281–290. doi: 10.2174/1567205054367829. [DOI] [PubMed] [Google Scholar]
- Groman SM, Lee B, London ED, Mandelkern MA, James AS, Feiler K, Rivera R, Dahlbom M, Sossi V, Vandervoort E, Jentsch JD. Dorsal Striatal D2-Like Receptor Availability Covaries with Sensitivity to Positive Reinforcement during Discrimination Learning. J Neurosci. 2011;31:7291–7299. doi: 10.1523/JNEUROSCI.0363-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haluk DM, Floresco SB. Ventral striatal dopamine modulation of different forms of behavioral flexibility. Neuropsychopharmacology. 2009;34:2041–2052. doi: 10.1038/npp.2009.21. [DOI] [PubMed] [Google Scholar]
- Humby T, Laird FM, Davies W, Wilkinson LS. Visuospatial attentional functioning in mice: interactions between cholinergic manipulations and genotype. Eur J Neurosci. 1999;11:2813–2823. doi: 10.1046/j.1460-9568.1999.00701.x. [DOI] [PubMed] [Google Scholar]
- Idris N, Neill J, Grayson B, Bang-Andersen B, Witten LM, Brennum LT, Arnt J. Sertindole improves sub-chronic PCP-induced reversal learning and episodic memory deficits in rodents: involvement of 5-HT(6) and 5-HT (2A) receptor mechanisms. Psychopharmacology (Berl) 2010;208:23–36. doi: 10.1007/s00213-009-1702-5. [DOI] [PubMed] [Google Scholar]
- Iversen SD, Mishkin M. Perseverative interference in monkeys following selective lesions of the inferior prefrontal convexity. Exp Brain Res. 1970;11:376–386. doi: 10.1007/BF00237911. [DOI] [PubMed] [Google Scholar]
- Izquierdo A, Belcher AM, Scott L, Cazares VA, Chen J, O’Dell SJ, Malvaez M, Wu T, Marshall JF. Reversal-specific learning impairments after a binge regimen of methamphetamine in rats: possible involvement of striatal dopamine. Neuropsychopharmacology. 2010;35:505–514. doi: 10.1038/npp.2009.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo A, Murray EA. Combined unilateral lesions of the amygdala and orbital prefrontal cortex impair affective processing in rhesus monkeys. J Neurophysiol. 2004;91:2023–2039. doi: 10.1152/jn.00968.2003. [DOI] [PubMed] [Google Scholar]
- Izquierdo A, Newman TK, Higley JD, Murray EA. Genetic modulation of cognitive flexibility and socioemotional behavior in rhesus monkeys. Proc Natl Acad Sci U S A. 2007;104:14128–14133. doi: 10.1073/pnas.0706583104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo A, Suda RK, Murray EA. Bilateral orbital prefrontal cortex lesions in rhesus monkeys disrupt choices guided by both reward value and reward contingency. J Neurosci. 2004;24:7540–7548. doi: 10.1523/JNEUROSCI.1921-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakala P, Sirvio J, Riekkinen M, Koivisto E, Kejonen K, Vanhanen M, Riekkinen P., Jr Guanfacine and clonidine, alpha 2-agonists, improve paired associates learning, but not delayed matching to sample, in humans. Neuropsychopharmacology. 1999;20:119–130. doi: 10.1016/S0893-133X(98)00055-4. [DOI] [PubMed] [Google Scholar]
- Jocham G, Klein TA, Neumann J, von Cramon DY, Reuter M, Ullsperger M. Dopamine DRD2 polymorphism alters reversal learning and associated neural activity. J Neurosci. 2009;29:3695–3704. doi: 10.1523/JNEUROSCI.5195-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson RM, Tanaka K, Saksida LM, Bussey TJ, Heilig M, Holmes A. Assessment of glutamate transporter GLAST (EAAT1)-deficient mice for phenotypes relevant to the negative and executive/cognitive symptoms of schizophrenia. Neuropsychopharmacology. 2009;34:1578–1589. doi: 10.1038/npp.2008.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katner SN, Davis SA, Kirsten AJ, Taffe MA. Effects of nicotine and mecamylamine on cognition in rhesus monkeys. Psychopharmacology (Berl) 2004;175:225–240. doi: 10.1007/s00213-004-1804-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kola I, Landis J. Can the pharmaceutical industry reduce attrition rates? Nat Rev Drug Discov. 2004;3:711–715. doi: 10.1038/nrd1470. [DOI] [PubMed] [Google Scholar]
- Kruzich PJ, Grandy DK. Dopamine D2 receptors mediate two-odor discrimination and reversal learning in C57BL/6 mice. BMC neuroscience. 2004;5:12. doi: 10.1186/1471-2202-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruzich PJ, Mitchell SH, Younkin A, Grandy DK. Dopamine D2 receptors mediate reversal learning in male C57BL/6J mice. Cogn Affect Behav Neurosci. 2006;6:86–90. doi: 10.3758/cabn.6.1.86. [DOI] [PubMed] [Google Scholar]
- Laughlin RE, Grant TL, Williams RW, Jentsch JD. Genetic dissection of behavioral flexibility: reversal learning in mice. Biol Psychiatry. 2011a;69:1109–1116. doi: 10.1016/j.biopsych.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughlin RE, Grant TL, Williams RW, Jentsch JD. Genetic Dissection of Behavioral Flexibility: Reversal Learning in Mice. Biol Psychiatry. 2011b doi: 10.1016/j.biopsych.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B, Groman S, London ED, Jentsch JD. Dopamine D2/D3 receptors play a specific role in the reversal of a learned visual discrimination in monkeys. Neuropsychopharmacology. 2007;32:2125–2134. doi: 10.1038/sj.npp.1301337. [DOI] [PubMed] [Google Scholar]
- Leeson VC, Robbins TW, Matheson E, Hutton SB, Ron MA, Barnes TR, Joyce EM. Discrimination learning, reversal, and set-shifting in first-episode schizophrenia: stability over six years and specific associations with medication type and disorganization syndrome. Biol Psychiatry. 2009;66:586–593. doi: 10.1016/j.biopsych.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman JA, Sheitman BB, Kinon BJ. Neurochemical sensitization in the pathophysiology of schizophrenia: deficits and dysfunction in neuronal regulation and plasticity. Neuropsychopharmacology. 1997;17:205–229. doi: 10.1016/S0893-133X(97)00045-6. [DOI] [PubMed] [Google Scholar]
- Lipska BK, Swerdlow NR, Geyer MA, Jaskiw GE, Braff DL, Weinberger DR. Neonatal excitotoxic hippocampal damage in rats causes post-pubertal changes in prepulse inhibition of startle and its disruption by apomorphine. Psychopharmacology (Berl) 1995;122:35–43. doi: 10.1007/BF02246439. [DOI] [PubMed] [Google Scholar]
- Lipska BK, Weinberger DR. Genetic variation in vulnerability to the behavioral effects of neonatal hippocampal damage in rats. Proc Natl Acad Sci U S A. 1995;92:8906–8910. doi: 10.1073/pnas.92.19.8906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipska BK, Weinberger DR. To model a psychiatric disorder in animals: schizophrenia as a reality test. Neuropsychopharmacology. 2000;23:223–239. doi: 10.1016/S0893-133X(00)00137-8. [DOI] [PubMed] [Google Scholar]
- Loos M, Staal J, Schoffelmeer AN, Smit AB, Spijker S, Pattij T. Inhibitory control and response latency differences between C57BL/6J and DBA/2J mice in a Go/No-Go and 5-choice serial reaction time task and strain-specific responsivity to amphetamine. Behav Brain Res. 2010;214:216–224. doi: 10.1016/j.bbr.2010.05.027. [DOI] [PubMed] [Google Scholar]
- Lustig C, Kozak R, Sarter M, Young JW, Robbins TW. CNTRICS final animal model task selection: Control of attention. Neurosci Biobehav Rev. 2012 doi: 10.1016/j.neubiorev.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthysse S. Animal models in psychiatric research. Prog Brain Res. 1986;65:259–270. doi: 10.1016/S0079-6123(08)60655-X. [DOI] [PubMed] [Google Scholar]
- McAlonan K, Brown VJ. Orbital prefrontal cortex mediates reversal learning and not attentional set shifting in the rat. Behav Brain Res. 2003;146:97–103. doi: 10.1016/j.bbr.2003.09.019. [DOI] [PubMed] [Google Scholar]
- McCarthy AD, Owens IJ, Bansal AT, McTighe SM, Bussey TJ, Saksida LM. FK962 and donepezil act synergistically to improve cognition in rats: potential as an add-on therapy for Alzheimer’s disease. Pharmacol Biochem Behav. 2011;98:76–80. doi: 10.1016/j.pbb.2010.11.019. [DOI] [PubMed] [Google Scholar]
- McTighe SM, Mar AC, Romberg C, Bussey TJ, Saksida LM. A new touchscreen test of pattern separation: effect of hippocampal lesions. Neuroreport. 2009;20:881–885. doi: 10.1097/WNR.0b013e32832c5eb2. [DOI] [PubMed] [Google Scholar]
- Mehta MA, Swainson R, Ogilvie AD, Sahakian J, Robbins TW. Improved short-term spatial memory but impaired reversal learning following the dopamine D(2) agonist bromocriptine in human volunteers. Psychopharmacology (Berl) 2001;159:10–20. doi: 10.1007/s002130100851. [DOI] [PubMed] [Google Scholar]
- Miller RP, Rotenberg KS, Adir J. Effect of dose on the pharmacokinetics of intravenous nicotine in the rat. Drug Metab Dispos. 1977;5:436–443. [PubMed] [Google Scholar]
- Mirza NR, Bright JL. Nicotine-induced enhancements in the five-choice serial reaction time task in rats are strain-dependent. Psychopharmacology (Berl) 2001;154:8–12. doi: 10.1007/s002130000605. [DOI] [PubMed] [Google Scholar]
- Morton AJ, Skillings E, Bussey TJ, Saksida LM. Measuring cognitive deficits in disabled mice using an automated interactive touchscreen system. Nat Methods. 2006;3:767. doi: 10.1038/nmeth1006-767. [DOI] [PubMed] [Google Scholar]
- Murray EA, Gaffan D, Mishkin M. Neural substrates of visual stimulus-stimulus association in rhesus monkeys. J Neurosci. 1993;13:4549–4561. doi: 10.1523/JNEUROSCI.13-10-04549.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen AM, Roberts AC, Polkey CE, Sahakian BJ, Robbins TW. Extra-dimensional versus intra-dimensional set shifting performance following frontal lobe excisions, temporal lobe excisions or amygdalo-hippocampectomy in man. Neuropsychologia. 1991;29:993–1006. doi: 10.1016/0028-3932(91)90063-e. [DOI] [PubMed] [Google Scholar]
- Owen AM, Sahakian BJ, Semple J, Polkey CE, Robbins TW. Visuo-spatial short-term recognition memory and learning after temporal lobe excisions, frontal lobe excisions or amygdalo-hippocampectomy in man. Neuropsychologia. 1995;33:1–24. doi: 10.1016/0028-3932(94)00098-a. [DOI] [PubMed] [Google Scholar]
- Pantelis C, Velakoulis D, McGorry PD, Wood SJ, Suckling J, Phillips LJ, Yung AR, Bullmore ET, Brewer W, Soulsby B, Desmond P, McGuire PK. Neuroanatomical abnormalities before and after onset of psychosis: a cross-sectional and longitudinal MRI comparison. Lancet. 2003;361:281–288. doi: 10.1016/S0140-6736(03)12323-9. [DOI] [PubMed] [Google Scholar]
- Parasuraman R. The attentive brain. MIT Press; Cambridge: 1998. [Google Scholar]
- Patel S, Stolerman IP, Asherson P, Sluyter F. Attentional performance of C57BL/6 and DBA/2 mice in the 5-choice serial reaction time task. Behav Brain Res. 2006;170:197–203. doi: 10.1016/j.bbr.2006.02.019. [DOI] [PubMed] [Google Scholar]
- Pattij T, Janssen MC, Loos M, Smit AB, Schoffelmeer AN, van Gaalen MM. Strain specificity and cholinergic modulation of visuospatial attention in three inbred mouse strains. Genes Brain Behav. 2007;6:579–587. doi: 10.1111/j.1601-183X.2006.00284.x. [DOI] [PubMed] [Google Scholar]
- Petersen DR, Norris KJ, Thompson JA. A comparative study of the disposition of nicotine and its metabolites in three inbred strains of mice. Drug Metab Dispos. 1984;12:725–731. [PubMed] [Google Scholar]
- Riccio CA, Reynolds CR, Lowe P, Moore JJ. The continuous performance test: a window on the neural substrates for attention? Arch Clin Neuropsychol. 2002;17:235–272. [PubMed] [Google Scholar]
- Ridley RM, Baker HF, Haystead TA. Perseverative behaviour after amphetamine; dissociation of response tendency from reward association. Psychopharmacology (Berl) 1981a;75:283–286. doi: 10.1007/BF00432439. [DOI] [PubMed] [Google Scholar]
- Ridley RM, Haystead TA, Baker HF. An analysis of visual object reversal learning in the marmoset after amphetamine and haloperidol. Pharmacol Biochem Behav. 1981b;14:345–351. doi: 10.1016/0091-3057(81)90401-9. [DOI] [PubMed] [Google Scholar]
- Riekkinen M, Soininen H, Riekkinen P, Sr, Kuikka J, Laakso M, Helkala EL, Partanen J, Riekkinen P., Jr Tetrahydroaminoacridine improves the recency effect in Alzheimer’s disease. Neuroscience. 1998;83:471–479. doi: 10.1016/s0306-4522(97)00400-4. [DOI] [PubMed] [Google Scholar]
- Rinberg D, Koulakov A, Gelperin A. Speed-accuracy tradeoff in olfaction. Neuron. 2006;51:351–358. doi: 10.1016/j.neuron.2006.07.013. [DOI] [PubMed] [Google Scholar]
- Robbins TW. The 5-choice serial reaction time task: behavioural pharmacology and functional neurochemistry. Psychopharmacology (Berl) 2002;163:362–380. doi: 10.1007/s00213-002-1154-7. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Semple J, Kumar R, Truman MI, Shorter J, Ferraro A, Fox B, McKay G, Matthews K. Effects of scopolamine on delayed-matching-to-sample and paired associates tests of visual memory and learning in human subjects: comparison with diazepam and implications for dementia. Psychopharmacology (Berl) 1997;134:95–106. doi: 10.1007/s002130050430. [DOI] [PubMed] [Google Scholar]
- Robinson ES, Eagle DM, Mar AC, Bari A, Banerjee G, Jiang X, Dalley JW, Robbins TW. Similar effects of the selective noradrenaline reuptake inhibitor atomoxetine on three distinct forms of impulsivity in the rat. Neuropsychopharmacology. 2008;33:1028–1037. doi: 10.1038/sj.npp.1301487. [DOI] [PubMed] [Google Scholar]
- Romberg C, Mattson MP, Mughal MR, Bussey TJ, Saksida LM. Impaired attention in the 3xTgAD mouse model of Alzheimer’s disease: rescue by donepezil (Aricept) J Neurosci. 2011;31:3500–3507. doi: 10.1523/JNEUROSCI.5242-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudebeck PH, Murray EA. Amygdala and orbitofrontal cortex lesions differentially influence choices during object reversal learning. J Neurosci. 2008;28:8338–8343. doi: 10.1523/JNEUROSCI.2272-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudebeck PH, Murray EA. Dissociable effects of subtotal lesions within the macaque orbital prefrontal cortex on reward-guided behavior. J Neurosci. 2011;31:10569–10578. doi: 10.1523/JNEUROSCI.0091-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahakian BJ, Morris RG, Evenden JL, Heald A, Levy R, Philpot M, Robbins TW. A comparative study of visuospatial memory and learning in Alzheimer-type dementia and Parkinson’s disease. Brain. 1988;111(Pt 3):695–718. doi: 10.1093/brain/111.3.695. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Nugent SL, Saddoris MP, Setlow B. Orbitofrontal lesions in rats impair reversal but not acquisition of go, no-go odor discriminations. Neuroreport. 2002;13:885–890. doi: 10.1097/00001756-200205070-00030. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Setlow B, Nugent SL, Saddoris MP, Gallagher M. Lesions of orbitofrontal cortex and basolateral amygdala complex disrupt acquisition of odor-guided discriminations and reversals. Learn Mem. 2003;10:129–140. doi: 10.1101/lm.55203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Shaham Y. The Role of Orbitofrontal Cortex in Drug Addiction: A Review of Preclinical Studies. Biol Psychiatry. 2007 doi: 10.1016/j.biopsych.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaton M, Kyerematen GA, Morgan M, Jeszenka EV, Vesell ES. Nicotine metabolism in stumptailed macaques, Macaca arctoides. Drug Metab Dispos. 1991;19:946–954. [PubMed] [Google Scholar]
- Selkoe DJ. Translating cell biology into therapeutic advances in Alzheimer’s disease. Nature. 1999;399:A23–31. doi: 10.1038/399a023. [DOI] [PubMed] [Google Scholar]
- Seu E, Lang A, Rivera RJ, Jentsch JD. Inhibition of the norepinephrine transporter improves behavioral flexibility in rats and monkeys. Psychopharmacology (Berl) 2008 doi: 10.1007/s00213-008-1250-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seu E, Lang A, Rivera RJ, Jentsch JD. Inhibition of the norepinephrine transporter improves behavioral flexibility in rats and monkeys. Psychopharmacology (Berl) 2009;202:505–519. doi: 10.1007/s00213-008-1250-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotnick B. Animal cognition and the rat olfactory system. Trends Cogn Sci. 2001;5:216–222. doi: 10.1016/s1364-6613(00)01625-9. [DOI] [PubMed] [Google Scholar]
- Slotnick BM, Risser JM. Odor memory and odor learning in rats with lesions of the lateral olfactory tract and mediodorsal thalamic nucleus. Brain Res. 1990;529:23–29. doi: 10.1016/0006-8993(90)90807-n. [DOI] [PubMed] [Google Scholar]
- Smith AG, Neill JC, Costall B. The dopamine D3/D2 receptor agonist 7-OH-DPAT induces cognitive impairment in the marmoset. Pharmacol Biochem Behav. 1999;63:201–211. doi: 10.1016/s0091-3057(98)00230-5. [DOI] [PubMed] [Google Scholar]
- Spinelli S, Ballard T, Gatti-McArthur S, Richards GJ, Kapps M, Woltering T, Wichmann J, Stadler H, Feldon J, Pryce CR. Effects of the mGluR2/3 agonist LY354740 on computerized tasks of attention and working memory in marmoset monkeys. Psychopharmacology (Berl) 2005;179:292–302. doi: 10.1007/s00213-004-2126-x. [DOI] [PubMed] [Google Scholar]
- Spratt C, Marston HM, Sharkey J, Kelly JS. Comparison of rat and mouse attentional performance in the 5-choice serial reaction task. BNA. 2000;17:P49.03. [Google Scholar]
- Stip E, Sepehry AA, Prouteau A, Briand C, Nicole L, Lalonde P, Lesage A. Cognitive discernible factors between schizophrenia and schizoaffective disorder. Brain Cogn. 2005;59:292–295. doi: 10.1016/j.bandc.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Swainson R, Hodges JR, Galton CJ, Semple J, Michael A, Dunn BD, Iddon JL, Robbins TW, Sahakian BJ. Early detection and differential diagnosis of Alzheimer’s disease and depression with neuropsychological tasks. Dement Geriatr Cogn Disord. 2001;12:265–280. doi: 10.1159/000051269. [DOI] [PubMed] [Google Scholar]
- Taffe MA, Weed MR, Gutierrez T, Davis SA, Gold LH. Differential muscarinic and NMDA contributions to visuo-spatial paired-associate learning in rhesus monkeys. Psychopharmacology (Berl) 2002;160:253–262. doi: 10.1007/s00213-001-0954-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taiminen T, Jaaskelainen S, Ilonen T, Meyer H, Karlsson H, Lauerma H, Leinonen KM, Wallenius E, Kaljonen A, Salokangas RK. Habituation of the blink reflex in first-episode schizophrenia, psychotic depression and non-psychotic depression. Schizophr Res. 2000;44:69–79. doi: 10.1016/s0920-9964(99)00140-1. [DOI] [PubMed] [Google Scholar]
- Tait DS, Brown VJ. Difficulty Overcoming Learned Non-reward during Reversal Learning in Rats with Ibotenic Acid Lesions of Orbital Prefrontal Cortex. Ann N Y Acad Sci. 2007;1121:407–420. doi: 10.1196/annals.1401.010. [DOI] [PubMed] [Google Scholar]
- Talpos JC, McTighe SM, Dias R, Saksida LM, Bussey TJ. Trial-unique, delayed nonmatching-to-location (TUNL): a novel, highly hippocampus-dependent automated touchscreen test of location memory and pattern separation. Neurobiol Learn Mem. 2010;94:341–352. doi: 10.1016/j.nlm.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talpos JC, Winters BD, Dias R, Saksida LM, Bussey TJ. A novel touchscreen-automated paired-associate learning (PAL) task sensitive to pharmacological manipulation of the hippocampus: a translational rodent model of cognitive impairments in neurodegenerative disease. Psychopharmacology (Berl) 2009;205:157–168. doi: 10.1007/s00213-009-1526-3. [DOI] [PubMed] [Google Scholar]
- Uchida N, Mainen ZF. Speed and accuracy of olfactory discrimination in the rat. Nat Neurosci. 2003;6:1224–1229. doi: 10.1038/nn1142. [DOI] [PubMed] [Google Scholar]
- Von Huben SN, Davis SA, Lay CC, Katner SN, Crean RD, Taffe MA. Differential contributions of dopaminergic D1- and D2-like receptors to cognitive function in rhesus monkeys. Psychopharmacology (Berl) 2006;188:586–596. doi: 10.1007/s00213-006-0347-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner I, Gal G, Rawlins JN, Feldon J. Differential involvement of the shell and core subterritories of the nucleus accumbens in latent inhibition and amphetamine-induced activity. Behav Brain Res. 1996;81:123–133. doi: 10.1016/s0166-4328(96)00051-4. [DOI] [PubMed] [Google Scholar]
- Wood SJ, Proffitt T, Mahony K, Smith DJ, Buchanan JA, Brewer W, Stuart GW, Velakoulis D, McGorry PD, Pantelis C. Visuospatial memory and learning in first-episode schizophreniform psychosis and established schizophrenia: a functional correlate of hippocampal pathology? Psychol Med. 2002;32:429–438. doi: 10.1017/s0033291702005275. [DOI] [PubMed] [Google Scholar]
- Wooters TE, Bardo MT. Methylphenidate and fluphenazine, but not amphetamine, differentially affect impulsive choice in Spontaneously Hypertensive, Wistar-Kyoto and Sprague-Dawley rats. Brain Res. 2011;1396:45–53. doi: 10.1016/j.brainres.2011.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan TC, Dudley JA, Weir RK, Grabowska EM, Pena-Oliver Y, Ripley TL, Hunt SP, Stephens DN, Stanford SC. Performance deficits of NK1 receptor knockout mice in the 5-choice serial reaction-time task: effects of d-amphetamine, stress and time of day. PLoS One. 2011;6:e17586. doi: 10.1371/journal.pone.0017586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Finlayson K, Spratt C, Marston HM, Crawford N, Kelly JS, Sharkey J. Nicotine improves sustained attention in mice: evidence for involvement of the alpha7 nicotinic acetylcholine receptor. Neuropsychopharmacology. 2004;29:891–900. doi: 10.1038/sj.npp.1300393. [DOI] [PubMed] [Google Scholar]
- Young JW, Geyer MA. Action of Modafinil-Increased Motivation Via the Dopamine Transporter Inhibition and D1 Receptors? Biol Psychiatry. 2010;67:784–787. doi: 10.1016/j.biopsych.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Light GA, Marston HM, Sharp R, Geyer MA. The 5-choice continuous performance test: evidence for a translational test of vigilance for mice. PLoS ONE. 2009a;4:e4227. doi: 10.1371/journal.pone.0004227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Powell SB, Geyer MA. Mouse pharmacological models of cognitive disruption relevant to schizophrenia. Neuropharmacology. 2011a doi: 10.1016/j.neuropharm.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Powell SB, Risbrough V, Marston HM, Geyer MA. Using the MATRICS to guide development of a preclinical cognitive test battery for research in schizophrenia. Pharmacol Ther. 2009b;122:150–202. doi: 10.1016/j.pharmthera.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Powell SB, Scott CN, Zhou X, Geyer MA. The effect of reduced dopamine D4 receptor expression in the 5-choice continuous performance task: Separating response inhibition from premature responding. Behav Brain Res. 2011b;222:183–192. doi: 10.1016/j.bbr.2011.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]