Abstract
Purpose
The ataxia telangiectasia mutated (ATM) gene mediates detection and repair of DNA damage. We investigated associations between ATM polymorphisms and severe radiation-induced pneumonitis (RP).
Methods and Materials
We genotyped three potentially functional single nucleotide polymorphisms (SNPs) of ATM (rs1801516 [D1853N/5557G>A], rs189037 [−111G>A] and rs228590) in 362 patients with non-small cell lung cancer (NSCLC), who received definitive radio(chemo)therapy. The cumulative severe RP probabilities by genotypes were evaluated using the Kaplan-Meier analysis. The associations between severe RP risk and genotypes were assessed by both logistic regression analysis and Cox proportional hazard model with time to event considered.
Results
Of 362 patients with 82% of non-Hispanic whites, 56 (15.5%) experienced grade ≥ 3 RP. Patients carrying ATM rs189037 AG/GG or rs228590 TT/CT genotypes, or rs189037G/rs228590T/rs1801516G (G-T-G) haplotype had a lower risk of severe RP (rs189037: GG/AG vs. AA, adjusted hazard ratio [HR] = 0.49, 95% confidence interval [CI], 0.29–0.83, P = 0.009; rs228590: TT/CT vs. CC, HR=0.57, 95% CI, 0.33–0.97, P =0.036; haplotype: G-T-G vs. A-C-G, HR=0.52, 95% CI, 0.35–0.79, P =0.002). Such positive findings remained in non-Hispanic whites.
CONCLUSIONS
ATM polymorphisms may serve as biomarkers for susceptibility to severe RP in non-Hispanic whites. Large prospective studies are required to confirm our findings.
Keywords: Non–small cell lung cancer, radiation pneumonitis, single-nucleotide polymorphisms, ataxia telangiectasia mutated gene
INTRODUCTION
Lung cancer remains the most common cause of cancer-related deaths, with an estimated 160,340 deaths in the United States in 2012 (1). Non-small cell lung cancer (NSCLC) represents nearly 85% of all lung cancer cases, and radiation therapy is one of the essential therapeutic modalities in multidisciplinary management for locally advanced NSCLC. It has been demonstrated for many years that definite radiation dose should be no less than the biologic equivalent of 60 Gy in 1.8 Gy to 2.0 Gy fractions for locally advanced NSCLC patients (2). However, the vicinity of noncancerous “normal” tissues or structures will be inevitably involved, which can lead to a series of side effects or toxicities.
Radiation pneumonitis (RP), one of the most prevalent radiation-induced toxicities, has been identified as a major barrier for thoracic cancer patients to receive radiotherapy alone or concomitant radiochemotherapy. Clinically, about 10% to 20% of NSCLC patients worldwide have experienced severe RP (grade ≥ 3) when they undergo definite radiotherapy, which can compromise their life quality and even threat their lives (3–5). Therefore, the search of new molecular markers, which may help physicians to identify patients who are more susceptible to severe RP, has been a long-lasting effort of lung cancer treatment study.
Ataxia telangiectasia (A-T) syndrome, a rare autosomal recessive disorder, is caused by mutations in the ataxia telangiectasia mutated (ATM) gene. Functional experiments demonstrated that ATM acted as a central mediator of the radioprotective machinery in response to radiation therapy, participating in cellular stress responses, control of cell cycle checkpoints, repair of double-strand breaks (DSBs), and initiation of apoptosis (6). In vitro, cells acquired from individuals heterozygous for ATM demonstrated an intermediate radiosensitivity, compared with cells from normal subjects (7). In vivo, compared with the wild-type mice, ATM heterozygous ones were more susceptible to radiation-induced cataracts (8). Furthermore, recent experiments showed that down-regulation of ATM expression by RNA interference or antisense technology could enhance radiosensitivity of irradiated cells in vitro (9).
Our previous studies and others’ have demonstrated that single nucleotide polymorphisms (SNPs) may be associated with disease propensity by modifying gene functions, or they may be served as genetic predictors or adjacent disease-causing variants through association or linkage (5). However, to date and to our knowledge, there have been no studies addressing the role of ATM SNPs in RP risk in non-Hispanic white populations. We hypothesized that ATM polymorphisms could be biomarkers for predicting susceptibility to severe RP among NSCLC patients undergoing definitive radiation therapy. In the present study, we conducted a case-only study to evaluate associations between ATM polymorphisms and severe RP among NSCLC patients.
METHODS AND MATERIALS
Patients
The current study initially included 392 patients for whom DNA sample were available and who had both radiation dosimetric data and documented data on RP, from a dataset of 832 NSCLC patients treated with definitive radiation at a single institution between March 1998 and June 2009. After we excluded those who developed recurrent diseases or underwent surgical resection before radiotherapy, the final data pool for the RP analysis included 362 patients. The characteristics of patients, tumor, and treatment are described in Table 1. Common Terminology Criteria for Adverse Events version 3.0 was used to evaluate and grade RP. The guideline for RP evaluation, follow-up schedule and tests, clinical data gathering and radiation treatment planning have been described in previous publications (5). In accordance with our previous studies, the time to RP development was calculated from the start of radiation therapy, and patients not going through the end point were censored at the time of the final follow-up (5, 10). This study was approved by our institutional review board, and the Health Insurance Portability and Accountability Act (HIPAA) regulations were followed.
Table 1.
Demographics, clinical covariates and their association with severe RP (grade ≥3) in NSCLC patients who received definitive radiation therapy
| Parameter | Patient No. (%) | Crude |
P* | Adjusted |
P† | ||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | ||||
| Sex | |||||||
| Male | 199 (55.0) | 1.00 | |||||
| Female | 163 (45.0) | 0.83 | 0.49–1.42 | 0.501 | 0.79 | 0.46–1.38 | 0.414 |
| Age (years) | |||||||
| <65 | 181 (50.0) | 1.00 | |||||
| ≥65 | 181 (50.0) | 0.96 | 0.57–1.63 | 0.886 | 0.99 | 0.57–1.73 | 0.114 |
| KPS | |||||||
| <80 | 74 (20.4) | 1.00 | |||||
| ≥80 | 288 (79.6) | 0.62 | 0.34–1.12 | 0.111 | 0.56 | 0.30–1.02 | 0.059 |
| Race | |||||||
| White | 297 (82.0) | 1.00 | |||||
| Black | 64 (17.7) | 0.99 | 0.50–1.97 | 0.984 | 0.80 | 0.40–1.61 | 0.557 |
| Other† | 1 ( 0.3) | ||||||
| Histology | |||||||
| Adenocar | 137 (37.8) | 1.00 | |||||
| Squamous | 127 (35.1) | 0.99 | 0.53–1.85 | 0.981 | 0.96 | 0.50–1.88 | 0.913 |
| Other | 98 (27.1) | 1.08 | 0.56–2.06 | 0.825 | 1.07 | 0.55–2.09 | 0.843 |
| Disease Stage | |||||||
| I, II | 54 (14.9) | 1.00 | |||||
| III, IV | 307 (84.8) | 0.82 | 0.42–1.63 | 0.580 | 0.54 | 0.25–1.16 | 0.114 |
| missing | 1 ( 0.3) | ||||||
| Chemotherapy | |||||||
| No | 32 ( 8.8) | 1.00 | |||||
| Yes | 329 (90.9) | 1.29 | 0.47–3.55 | 0.629 | 1.08 | 0.36–3.23 | 0.887 |
| missing | 1 ( 0.3) | ||||||
| Smoking status | |||||||
| Never | 32 ( 8.8) | 1.00 | |||||
| Ever | 330 (91.2) | 1.18 | 0.43–3.27 | 0.746 | 1.09 | 0.38–3.16 | 0.868 |
| Radiation dose | |||||||
| <66 Gy | 173 (47.8) | 1.00 | |||||
| ≥66 Gy | 189 (52.2) | 1.00 | 0.59–1.69 | 0.999 | 1.12 | 0.65–1.95 | 0.686 |
| MLD | |||||||
| <19.0 Gy | 181 (50.0) | 1.00 | |||||
| ≥19.0 Gy | 181 (50.0) | 3.12 | 1.73–5.64 | <0.001 | 3.89 | 2.04–7.41 | <0.001 |
Abbreviations ATM = ataxia telangiectasia mutated gene; RP= radiation pneumonitis; NSCLC = non–small cell lung cancer HR = hazard ratio; CI = confidence interval.
P values were calculated by Cox proportional model using univariate analysis.
P values were calculated with adjustment for sex, age, kps, race, histology, disease stage, radiation dose, chemotherapy history, smoke history and mean lung dose.
SNPs Selection and Genotyping Methods
Using the National Center for Biotechnology Information SNP database (http://www.ncbi.nlm.nih.gov/projects/SNP), Hapmap database (http://www.hapmap.org/, Rel 27) and SNP Function Prediction tool (http://snpinfo.niehs.nih.gov/snpfunc.htm), we selected three ATM SNPs (rs189037G>A, rs228590C>T, and rs1801516G>A), following at least two of the three criteria: (1) the minor allele frequency was more than 5% in Caucasians, (2) the variant was located in the promoter region or coding region of the gene, and (3) previously reported to be associated with lung cancer. Among these three SNPs, the change of rs189037 G to A may result in a transcriptional inhibitor-binding site in the ATM promoter and thus affect mRNA expression (11). D1853N (5557G>A, rs1801516) can cause a missense change, whereas rs228590C>T, located in intron 1 of the gene, is predicted to have an impact on the binding of some transcription factors. Though it was reported that rs4987886, rs4987889, rs1800058, and rs1800889 might play some roles in the radiation side effects, we did not choose them in this investigation, because their minor allele frequencies were just close to 0.05 in Caucasians. In addition, we found that rs189037 and rs228590 are in high LD (D’ = 0.95; r2 = 0.87), but rs189037 and rs1801516 are not (D’=0.86; r2 =0.12) (data not shown). Therefore, their haplotypes may be informative as well.
Genomic DNA was extracted from peripheral blood leukocytes by a Blood Mini Kit (Qiagen, Valencia, CA), following the manufacturer’s instructions. The genotyping was performed by the polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) method. The sequences of primers, restriction enzymes and PCR conditions for each SNP are shown in Supplementary Table 1. About 10% of the samples were selected randomly for repeated genotyping, and no discrepancies were observed.
Statistical Analysis
Patients were categorized according to their genotyping results. Chi-square test was used to test for differences in distributions between the observed and those expected by the Hardy-Weinberg equilibrium. The associations between severe RP risk and genotypes were assessed by both logistic regression analysis and Cox proportional hazard model with time to event considered. Kaplan-Meier analysis was performed to assess the effect of different genotypes on cumulative probability of radiation pneumonitis. Individual haplotypes were generated using SAS PROC HAPLOTYPE. A P value of 0.05 or less for a given test was considered statistically significant. All statistical analyses were performed using the SAS software (version 9.2; SAS Institute, Cary, NC). Finally, with the rpart package in S-PLUSVersion 8.0.4 (TIBCO, Palo Alto, CA, USA), we also performed classification and regression tree (CART) analysis to determine higher-order interactions between clinical factors and genetic variants.
RESULTS
Patient Characteristics and RP (grade ≥ 3) risk
Table 1 shows baseline characteristics of the 362 patients. The dataset consisted of 199 men and 163 women, with a median age of 65 years (range, 35–88 years). Of all these patients, 297 were whites, among whom 88.2% were self-reported non Hispanic Caucasians, 85% of patients had stage III/IV diseases according to the 6th edition of the AJCC stage grouping criteria (12), and 91.1% received a combination of chemotherapy and radiotherapy. The median radiation dose received by patients wa 66 Gy (range, 50.0–87.5 Gy) with a median mean lung dose (MLD) of 19.0 Gy (range, 2.7–30.6 Gy). The median follow-up time was 20.6 months (range, 1.0 to 157.6 months). The overall incidence of severe RP was 15.5%, and the median occurrence time for severe RP was 3.6 months (95% CI, 2.1–10.1 months). We evaluated the association between clinic-pathologic characteristics and RP (grades ≥3, ≥2 and ≥1) risk. Table 1 showed that only mean lung dose (MLD) was associated with statistically significant increased risk of grade ≥3 RP in both univariate and multivariate Cox proportional hazard analyses in this study population (HR for MLD ≥ 19.0 Gy vs. MLD < 19.0 Gy, 3.12, 95% CI, 1.73–5.64, P <0.001; adjusted HR =3.89, 95% CI=2.04–7.41, P<0.001). In addition, disease stage, chemotherapy history, smoke history and mean lung dose (MLD) were all significantly associated with grade ≥ 1 RP risk in the univariate analysis (Supplementary Table 2).
Supplementary Table 3 listed genotype distributions of all studied SNPs. The frequency distribution of ATM rs1801516 genotypes was in agreement with published data from the National Center for Biotechnology Information’s SNP database of nucleotide sequence variation (dbSNP; http://www.ncbi.nlm.nih.gov/projects/SNP), which shows a relatively high minor allele frequency among Caucasian populations but no or very low frequencies in Chinese (HCB), Japanese (JPT), and Yoruba (YRI) populations. More importantly, genotype distributions of these three SNPs (ATM rs18011516, rs228590 and rs189037) were all consistent with the Hardy-Weinberg equilibrium in our study population.
Radiation pneumonitis and ATM genotypes
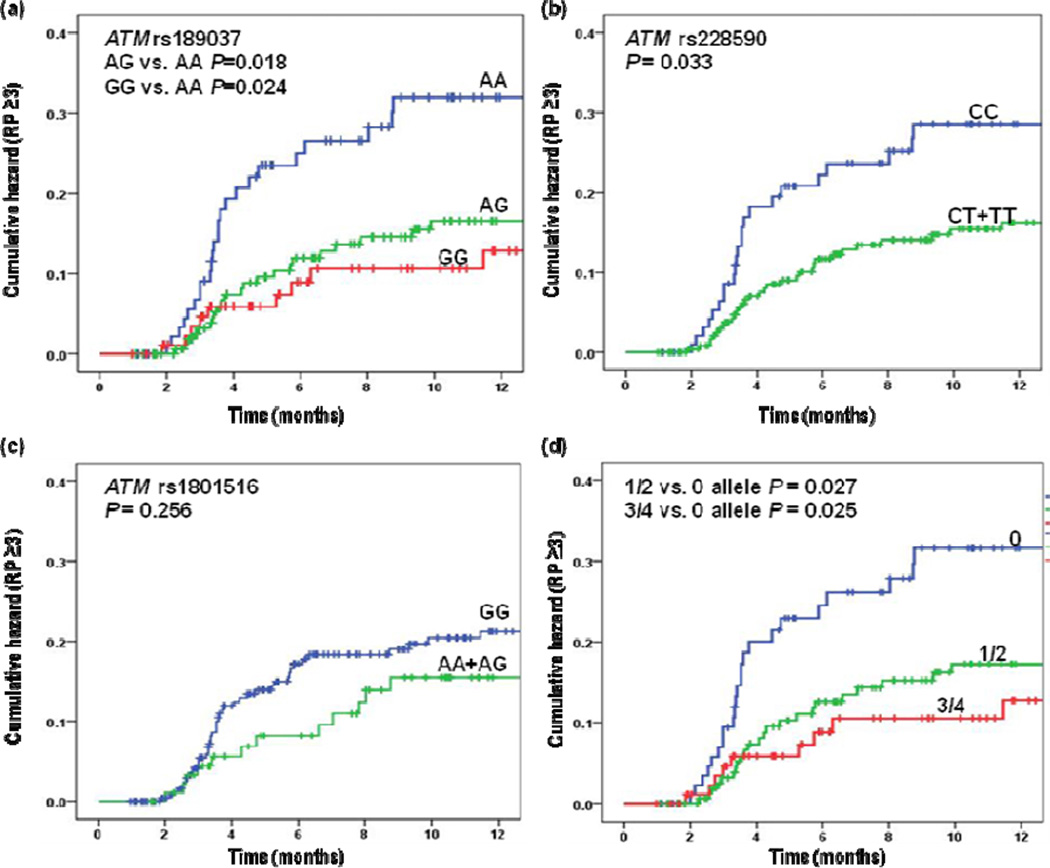
The univariate and multivariate analyses of the associations between ATM SNPs and severe RP using the Cox proportional hazards model are summarized in Table 2. Both ATM rs189037 AG/GG and rs228950 CT/TT genotypes were significantly associated with reduced hazards of severe RP in the univariate analyses (HR for AG/GG VS. AA 0.47; 95% CI, 0.28–0.80; P = 0.006; and HR for CT/TT VS. CC 0.56; 95% CI, 0.33–0.96; P = 0.033). Moreover, there were trend in the effects both for having the increasing number of ATM rs189037 G and rs228590 T alleles with decreasing HR (P= 0.011 and P= 0.013, respectively). This effect was virtually unchanged after adjustment for potential confounding factors for RP, including disease stage, smoking status, chemotherapy history and MLD as used in previous investigations. In addition, we observed that patients with an accumulating number of the combined protective G or T alleles demonstrated a tendency towards decreasing hazards of severe RP in both univariate and multivariate analyses, compared with those without protective genotypes (Ptrend = 0.015 and 0.014, respectively, Table 2). Figure 1 plots the cumulative probability of severe RP as a function of time since radiation therapy began by the selected SNPs. Additionally, rs189037 and rs228590 were in strong linkage disequilibrium (D’ = 0.95; r2 = 0.87). We further found that compared with the A-C-G haplotype, the most common G-T-G haplotype was associated with significantly decreased risk of severe RP in both univariate and multivariate models (adjusted HR =0.52; 95% CI =0.35–0.79, P = 0.002) (Table 3).
Table 2.
Univariate and multivariate analyses of associations between ATM genotypes and severe RP (grade ≥ 3) in NSCLC patients who received definitive radiation therapy
| Genotypes | Patient No. | Event | Crude |
P* | Adjusted |
P† | ||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | |||||
| ATM rs1801516: 5557G>A | ||||||||
| GG | 265 | 44 | 1.00 | 1.00 | ||||
| AG | 92 | 12 | 072 | 0.38–1.36 | 0.314 | 0.80 | 0.42–1.53 | 0.507 |
| AA | 4 | 0 | N/A | N/A | ||||
| Trend test | P=0.213 | P=0.327 | ||||||
| AA+AG | 96 | 12 | 0.69 | 0.37–1.31 | 0.256 | 0.76 | 0.40–1.45 | 0.406 |
| ATM rs228590:C>T | ||||||||
| CC | 102 | 23 | 1.00 | 1.00 | ||||
| CT | 174 | 26 | 0.66 | 0.38–1.15 | 0.142 | 0.68 | 0.39–1.19 | 0.172 |
| TT | 85 | 7 | 0.36 | 0.16–0.85 | 0.019 | 0.35 | 0.15–0.82 | 0.016 |
| Trend test | P=0.013 | P=0.012 | ||||||
| CT+TT | 259 | 33 | 0.56 | 0.33–0.96 | 0.033 | 0.57 | 0.33–0.97 | 0.036 |
| ATM rs189037 :-111G>A | ||||||||
| AA | 98 | 24 | 1.00 | 1.00 | ||||
| AG | 171 | 22 | 0.50 | 0.28–0.88 | 0.018 | 0.53 | 0.30–0.95 | 0.033 |
| GG | 92 | 10 | 0.43 | 0.21–0.90 | 0.024 | 0.42 | 0.20–0.88 | 0.021 |
| Trend test | P=0.011 | P=0.012 | ||||||
| AG+GG | 263 | 32 | 0.47 | 0.28–0.80 | 0.006 | 0.49 | 0.29–0.83 | 0.009 |
| rs189037-111 G+ rs228590 T‡ | ||||||||
| 0 | 94 | 23 | 1.00 | 1.00 | ||||
| 1/2 | 172 | 23 | 0.52 | 0.29–0.93 | 0.027 | 0.55 | 0.31–0.98 | 0.042 |
| 3/4 | 94 | 10 | 0.43 | 0.20–0.90 | 0.025 | 0.42 | 0.20–0.89 | 0.023 |
| Trend test | P=0.015 | P=0.014 | ||||||
Abbreviations ATM = ataxia telangiectasia mutated gene; RP= radiation pneumonitis; NSCLC = non–small cell lung cancer HR = hazard ratio; CI = confidence interval.
P values were calculated by Cox proportional model using univariate analysis.
P values were calculated with adjustment for disease stage, chemotherapy history, smoke history and mean lung dose.
0 (1, 2, 3, 4) indicates the number of combined G or T alleles in individual patients.
Fig. 1.
Cumulative probability of Grade ≥ 3 radiation pneumonitis in 362 patients with non–small cell lung cancer as a function of time from the start of radiation therapy by genotypes: (a) ATM rs189037 AG vs. AA and GG vs. AA, (b) ATM rs228590 CT+TT vs. CC, (c) ATM rs1801516 AG+AA vs. GG, (d) ATM rs189037 G and ATM rs228590 T combined alleles, RP = radiation pneumonitis.
Table 3.
Univariate and multivariate analyses of associations between ATM haplotyes and RP (grade ≥ 3) in NSCLC patients who received definitive radiation therapy
| ATM haplotype | Patient No. | Event | Crude |
P* | Adjusted |
P† | ||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | |||||
| A-C-G | 132 | 29 | 1.00 | 1.00 | ||||
| G-T-G | 166 | 19 | 0.58 | 0.39–0.86 | 0.007 | 0.52 | 0.35–0.79 | 0.002 |
| A-C-A | 48 | 6 | 0.57 | 0.44–0.72 | 0.954 | 0.57 | 0.57–2.08 | 0.800 |
| others | 13 | 2 | 0.68 | 0.71–1.93 | 0.547 | 0.73 | 0.50–3.93 | 0.518 |
Abbreviations: ATM = ataxia telangiectasia mutated gene; RP= radiation pneumonitis; NSCLC = non–small cell lung cancer HR = hazard ratio; CI = confidence interval.
P values were calculated by Cox proportional model using univariate analysis.
P values were calculated with adjustment for disease stage, chemotherapy history, smoke history and mean lung dose.
The above associations were further analyzed in non-Hispanic Caucasians only (n=262), and similar results (Supplementary Table 4) were observed, although the significance level was not sustained for ATM rs228590 in the multivariate Cox analyses, likely due to the reduced sample size.
Association between ATM SNPs and severe RP (grade ≥ 3) versus lower RP (grade 0–2)
We further assessed the impact of ATM polymorphisms on susceptibility to severe RP versus lower RP (grade 0–2) by using a logistic regression model. Compared with the rs189037 AA genotype, the risk of severe RP in patients with rs189037 AG/GG genotypes decreased to less than one half (adjusted HR =0.45; 95% CI =0.25–0.84, P = 0.011). Similar results were observed in patients with the rs228590 TT genotype, compared with patients carrying the rs228590 AA genotype (Table 4).
Table 4.
Associations of ATM genotypes between lower and severe RP groups in NSCLC patients who received definitive radiation therapy
| Genotypes | Lower RP | Severe RP | Crude |
P* | Adjusted |
P† | ||
|---|---|---|---|---|---|---|---|---|
| group(0–2) | group(≥3) | OR | 95% CI | OR | 95% CI | |||
| rs1801516 | ||||||||
| GG | 221 | 44 | 1.00 | 1.00 | ||||
| AG | 80 | 12 | 075 | 0.38–1.50 | 0.420 | 0.81 | 0.40–1.66 | 0.572 |
| AA | 4 | 0 | N/A | N/A | ||||
| AA+AG | 84 | 12 | 0.72 | 0.36–1.43 | 0.343 | 0.76 | 0.37–1.55 | 0.451 |
| rs228590 | ||||||||
| CC | 79 | 23 | 1.00 | 1.00 | ||||
| CT | 148 | 26 | 0.60 | 0.32–1.13 | 0.113 | 0.64 | 0.33–1.21 | 0.170 |
| TT | 78 | 7 | 0.31 | 0.13–0.76 | 0.011 | 0.31 | 0.12–0.77 | 0.012 |
| CT+TT | 226 | 33 | 0.50 | 0.28–0.91 | 0.022 | 0.52 | 0.28–0.95 | 0.035 |
| rs189037 | ||||||||
| AA | 74 | 24 | 1.00 | 1.00 | ||||
| AG | 149 | 22 | 0.46 | 0.24–0.87 | 0.016 | 0.50 | 0.26–0.96 | 0.038 |
| GG | 82 | 10 | 0.38 | 0.17–0.84 | 0.017 | 0.38 | 0.17–0.87 | 0.022 |
| AG+GG | 231 | 32 | 0.43 | 0.24–0.77 | 0.005 | 0.45 | 0.25–0.84 | 0.011 |
| rs189037 G+ rs228590 T‡ | ||||||||
| 0 | 71 | 23 | 1.00 | 1.00 | ||||
| 1/2 | 149 | 23 | 0.48 | 0.25–0.91 | 0.024 | 0.50 | 0.26–0.98 | 0.043 |
| 3/4 | 84 | 10 | 0.37 | 0.16–0.82 | 0.015 | 0.37 | 0.16–0.85 | 0.019 |
Abbreviations: ATM= ataxia telangiectasia mutated gene; RP = radiation pneumonitis; NSCLC = non–small cell lung cancer OR = odds ratio; CI = confidence interval.
P values were calculated by Logistic regression model using univariate analysis.
P values were calculated with adjustment for disease stage, chemotherapy history, smoke history and mean lung dose.
0 (1, 2, 3, 4) indicates the number of combined G or T alleles in individual patients.
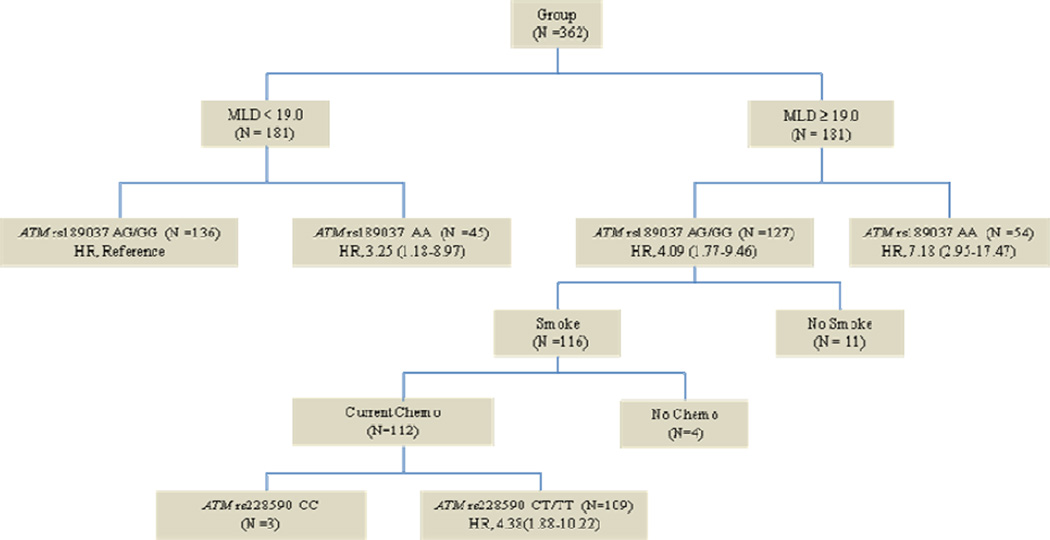
Potential interactions in the RP risk (the CART analysis)
We performed the CART analysis of the clinicopathologic variables (e.g., disease stage, smoking status, MLD, and chemotherapy history), and the two SNPs that were significantly associated with severe RP risk in the analyses described earlier (Figure. 2). We observed that the first split was MLD, which indicates that MLD was the strongest predictor for severe RP among all factors considered in the analysis. Further analysis showed that compared with patients carrying rs189037 AG/GG genotypes with MLD <19.0 Gy, patients carrying the rs189037 AA genotype with MLD ≥19.0 Gy had the highest risk of severe RP (HR = 7.18, 95% CI 2.95–17.47, P <0.001).
Fig. 2.
Classification and regresion tree analysis for predicators of grade≥3 radiation pneumonitis in 362 patients with non-small cell lung cancer. ATM = ataxia telangiectasia mutated gene; HR = hazard ratio; MLD = mean lung dose; Chemo = chemotherapy.
DISCUSSION
To the best of our knowledge, this is the first study to investigate associations between potentially functional SNPs of ATM and the risk of severe RP among NSCLC patients in an American population, mostly non-Hispanic whites. Our data indicated that ATM variants (e.g. rs189037 [−111G] and rs228590T) independently and jointly had a substantial impact on the risk of severe RP in NSCLC patients treated with radiotherapy as well as the consistent effect of the rs189037G/rs228590T/rs1801516G (G-T-G) haplotype, although ATM D1853N (rs1801516) alone, one of the commonly studied ATM variants in cancer risk and in susceptibility to adverse radiation effects among Caucasian populations, was not associated with severe RP. In contrast, we did not find an association between ATM SNPs and the risk of grade 1–2 RP in this mostly non-Hispanic white population, as did two previous studies in Chinese Han populations (11, 13). However, the incidence of severe RP (grade ≥3) in our study patient population was 15.5%, and the median occurrence time for severe RP was 3.6 months, which are similar to those reported in previous studies (5). In addition, the finding that MLD was the strongest predictor of severe RP was also confirmed in our current study with a much larger sample size.
Our findings are biologically plausible. Some studies have observed that genetic variations possibly accounted for approximately 80% to 90% of the individual variation in susceptibility to normal tissue toxicities (14). During the past several years, it has been demonstrated that ATM IVS22–77T>C and IVS48 + 238C>G heterozygous genotypes are associated with a decreased risk of adverse radiotherapy response and that ATM codon 1853 Asn/Asp and Asn/Asn genotypes had a significant effect on the risk of grade 3 fibrosis in breast cancer patients treated with radiotherapy (15). In addition, the ATM rs1800057 missense variant appeared to predict high toxicity in prostate cancer patients treated with brachytherapy (16). In the current study, we found that among non-Hispanic white subjects, severe RP was less likely to occur in NSCLC patients carrying ATM rs189037 AG/GG and rs228950 CT/TT genotypes than in those carrying the ATM rs189037 AA and rs228950 CC genotypes, respectively. Furthermore, we observed that patients with rs189037G/rs228590T/rs1801516G (G-T-G) haplotype had a lower risk of severe RP, compared with patients with the A-C-G haplotype.
However, we could not validate the findings reported in Chinese Han populations that ATM variants had significant associations with the risk of grade ≥2 RP. The discrepancy between our non-Hispanic white and Chinese Han studies could result from three aspects. Firstly, we noticed that the variant allele frequency patterns of ATM rs189037 varied greatly among these two ethnic patient cohorts. It has been well established that different genetic backgrounds between different ethnic populations at least in part account for the differences in susceptibility to various diseases and toxicities including RP (17). Another possible explanation for the discrepancy is that fewer patients developed grade ≥2 RP (17.4%) and severe RP (5.5%) in the Chinese study. (11, 13), compared with 42.5% and 15.5% in the present study, respectively, which is likely due to lower MLD (below 15 Gy) used in that study. Thirdly, there was evidence that severe RP was less dependent on dose-volume and not associated with significant clinical or tumor-related factors compared with grade 1–2 RP, and the distinct characteristics of patients with severe RP suggested that different mechanisms might be involved in the development of severe RP; therefore, it is very important to note that the genotypes that best improve the predictive value of RP will be endpoint dependent (18).
Besides the positive results in ATM rs189037 and rs228590, the present study did not support an association between ATM D1853N (rs1801516) variant allele and the risk of severe RP. Although some investigations suggested that ATM D1853N variant could be a good predictor for subcutaneous fibrosis in patients treated with breast conserving radiotherapy (15), other studies showed that ATM D1853N had no significant effects on early adverse skin reactions. The diversity of these two genetic risk profiles suggest that different molecular and cellular events may account for acute and late adverse radiation response (19). Unlike radiation-induced lung fibrosis, which usually occurs 6 months after the completion of radiation treatments, RP may take place 6 to 16 weeks after radiotherapy and is characterized by inflammation and interstitial pneumonia. Furthermore, the mouse model for radiation-induced lung response also showed that different phases of lung injury (alveolitis, fibrosing alveolitis and fibrosis) were dictated by different loci (20). Due to different mechanisms involved in acute and late adverse radiation response in the lung, it is biologically plausible that ATM D1853N variant may be associated with the risk of radiation pulmonary fibrosis but not with the risk of severe RP. However our results require further mechanistic investigation and validation by larger studies.
Through CART analysis, we identified that MLD remained to be the most crucial risk factor for severe RP development. We then found that patients carrying the rs189037 AA genotype with MLD ≥19.0 Gy had over seven times greater risk for development of severe RP than patients carrying rs189037 AG/GG genotypes with MLD <19.0 Gy, which suggests that clinically such a high-risk group should be closely monitored or some active intervention should be considered, if such a drastic finding will be validated in future studies.
Several limitations need to be noted in the current study. Firstly, we were unable to unravel exact molecular mechanisms by which ATM SNPs are involved in RP. Secondly, we used the common candidate SNP method, which does not include all representative SNPs in the entire gene. It is possible that some important SNPs but with a low frequency may have been missed or that the observed associations may have resulted from genetic linkage with other untyped SNPs. Thirdly, as an exploratory study with a limited study power, the P values in the current study were not adjusted for multiple tests. Therefore, our findings are considered preliminary.
In summary, we found that ATM variants (e.g. rs189037 and rs228590) may independently and jointly affect the development of severe RP in non-Hispanic white NSCLC patients treated with radiotherapy. Further large prospective studies are essential to confirm our findings.
Supplementary Material
Summary.
ATM is a master regulator mediating DNA damage detection and repair. In this study, the authors tested the hypothesis that ATM polymorphisms are biomarkers for susceptibility to severe RP in 362 non-small cell lung cancer patients undergoing definitive radiation therapy. Our data showed that patients carrying the ATM rs189037 varian AA genotype had high risk of developing severe RP, particularly those receiving MLD ≥19.0 Gy. Such patients should be closely monitored for clinical management.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest Notification: The authors declare no conflicts of interest regarding the work presented here.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Perez CA, Pajak TF, Rubin P, et al. Long-term observations of the patterns of failure in patients with unresectable non-oat cell carcinoma of the lung treated with definitive radiotherapy. Report by the Radiation Therapy Oncology Group. Cancer. 1987;59:1874–1881. doi: 10.1002/1097-0142(19870601)59:11<1874::aid-cncr2820591106>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 3.Shi A, Zhu G, Wu H, et al. Analysis of clinical and dosimetric factors associated with severe acute radiation pneumonitis in patients with locally advanced non-small cell lung cancer treated with concurrent chemotherapy and intensity-modulated radiotherapy. Radiat Oncol. 2010;5:35. doi: 10.1186/1748-717X-5-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kimura T, Togami T, Takashima H, et al. Radiation pneumonitis in patients with lung and mediastinal tumours: a retrospective study of risk factors focused on pulmonary emphysema. Br J Radiol. 2012;85:135–141. doi: 10.1259/bjr/32629867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yuan X, Liao Z, Liu Z, et al. Single nucleotide polymorphism at rs1982073:T869C of the TGFbeta 1 gene is associated with the risk of radiation pneumonitis in patients with non-small-cell lung cancer treated with definitive radiotherapy. J Clin Oncol. 2009;27:3370–3378. doi: 10.1200/JCO.2008.20.6763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bouska A, Lushnikova T, Plaza S, et al. Mdm2 promotes genetic instability and transformation independent of p53. Mol Cell Biol. 2008;28:4862–4874. doi: 10.1128/MCB.01584-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weeks DE, Paterson MC, Lange K, et al. Assessment of chronic gamma radiosensitivity as an in vitro assay for heterozygote identification of ataxia-telangiectasia. Radiat Res. 1991;128:90–99. [PubMed] [Google Scholar]
- 8.Worgul BV, Smilenov L, Brenner DJ, et al. Atm heterozygous mice are more sensitive to radiation-induced cataracts than are their wild-type counterparts. Proc Natl Acad Sci U S A. 2002;99:9836–9839. doi: 10.1073/pnas.162349699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang SC, Wu CC, Wei YY, et al. Inactivation of ataxia telangiectasia mutated gene can increase intracellular reactive oxygen species levels and alter radiation-induced cell death pathways in human glioma cells. Int J Radiat Biol. 2011;87:432–442. doi: 10.3109/09553002.2011.538128. [DOI] [PubMed] [Google Scholar]
- 10.Yin M, Liao Z, Liu Z, et al. Genetic variants of the nonhomologous end joining gene LIG4 and severe radiation pneumonitis in nonsmall cell lung cancer patients treated with definitive radiotherapy. Cancer. 2012;118:528–535. doi: 10.1002/cncr.26214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang L, Yang M, Bi N, et al. ATM polymorphisms are associated with risk of radiation-induced pneumonitis. Int J Radiat Oncol Biol Phys. 2010;77:1360–1368. doi: 10.1016/j.ijrobp.2009.07.1675. [DOI] [PubMed] [Google Scholar]
- 12.Greene FL. The American Joint Committee on Cancer: updating the strategies in cancer staging. Bull Am Coll Surg. 2002;87:13–15. [PubMed] [Google Scholar]
- 13.Yang M, Zhang L, Bi N, et al. Association of P53 and ATM polymorphisms with risk of radiation-induced pneumonitis in lung cancer patients treated with radiotherapy. Int J Radiat Oncol Biol Phys. 2011;79:1402–1407. doi: 10.1016/j.ijrobp.2009.12.042. [DOI] [PubMed] [Google Scholar]
- 14.Ho AY, Atencio DP, Peters S, et al. Genetic predictors of adverse radiotherapy effects: the Gene-PARE project. Int J Radiat Oncol Biol Phys. 2006;65:646–655. doi: 10.1016/j.ijrobp.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 15.Andreassen CN, Overgaard J, Alsner J, et al. ATM sequence variants and risk of radiation-induced subcutaneous fibrosis after postmastectomy radiotherapy. Int J Radiat Oncol Biol Phys. 2006;64:776–783. doi: 10.1016/j.ijrobp.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 16.Pugh TJ, Keyes M, Barclay L, et al. Sequence variant discovery in DNA repair genes from radiosensitive and radiotolerant prostate brachytherapy patients. Clin Cancer Res. 2009;15:5008–5016. doi: 10.1158/1078-0432.CCR-08-3357. [DOI] [PubMed] [Google Scholar]
- 17.Wang L, Bi N. TGF-beta1 gene polymorphisms for anticipating radiation-induced pneumonitis in non-small-cell lung cancer: different ethnic association. J Clin Oncol. 2010;28:e621–e622. doi: 10.1200/JCO.2010.31.0458. [DOI] [PubMed] [Google Scholar]
- 18.Takeda A, Ohashi T, Kunieda E, et al. Comparison of clinical, tumour-related and dosimetric factors in grade 0–1, grade 2 and grade 3 radiation pneumonitis after stereotactic body radiotherapy for lung tumours. Br J Radiol. 2012;85:636–642. doi: 10.1259/bjr/71635286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raabe A, Derda K, Reuther S, et al. Association of single nucleotide polymorphisms in the genes ATM, GSTP1, SOD2, TGFB1, XPD and XRCC1 with risk of severe erythema after breast conserving radiotherapy. Radiat Oncol. 2012;7:65. doi: 10.1186/1748-717X-7-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haston CK, Begin M, Dorion G, et al. Distinct loci influence radiation-induced alveolitis from fibrosing alveolitis in the mouse. Cancer Res. 2007;67:10796–10803. doi: 10.1158/0008-5472.CAN-07-2733. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.