Abstract
Two new agents based upon the structure of the clinically active prodrug laromustine were synthesized. These agents, 2-(2-chloroethyl)-N-methyl-1,2-bis(methylsulfonyl)-N-nitrosohydrazinecarboxamide (1) and N-(2-chloroethyl)-2-methyl-1,2-bis(methylsulfonyl)-N-nitrosohydrazinecarboxamide (2), were designed to retain the potent chloroethylating and DNA cross-linking functions of laromustine, and gain the ability to methylate DNA at the O-6 position of guanine, while lacking the carbamoylating activity of laromustine. The methylating arm was introduced with the intent of depleting the DNA repair protein O6-alkylguanine-DNA alkyltransferase (AGT). Compound 1 is markedly more cytotoxic than laromustine in both AGT minus EMT6 mouse mammary carcinoma cells and high AGT expressing DU145 human prostate carcinoma cells. DNA cross-linking studies indicated that its cross-linking efficiency is nearly identical to its predicted active decomposition product, 1,2-bis(methylsulfonyl)-1-(2-chloroethyl)hydrazine (90CE), which is also produced by laromustine. AGT ablation studies in DU145 cells demonstrated that 1 can efficiently deplete AGT. Studies assaying methanol and 2-chloroethanol production as a consequence of the methylation and chloroethylation of water by 1 and 2 confirmed their ability to function as methylating and chloroethylating agents and provided insights into the superior activity of 1.
Keywords: chloroethylating; O6-alkylguanine-DNA alkyltransferase; 1, 2-bis(sulfonyl)hydrazines; methylating; laromustine; dual function
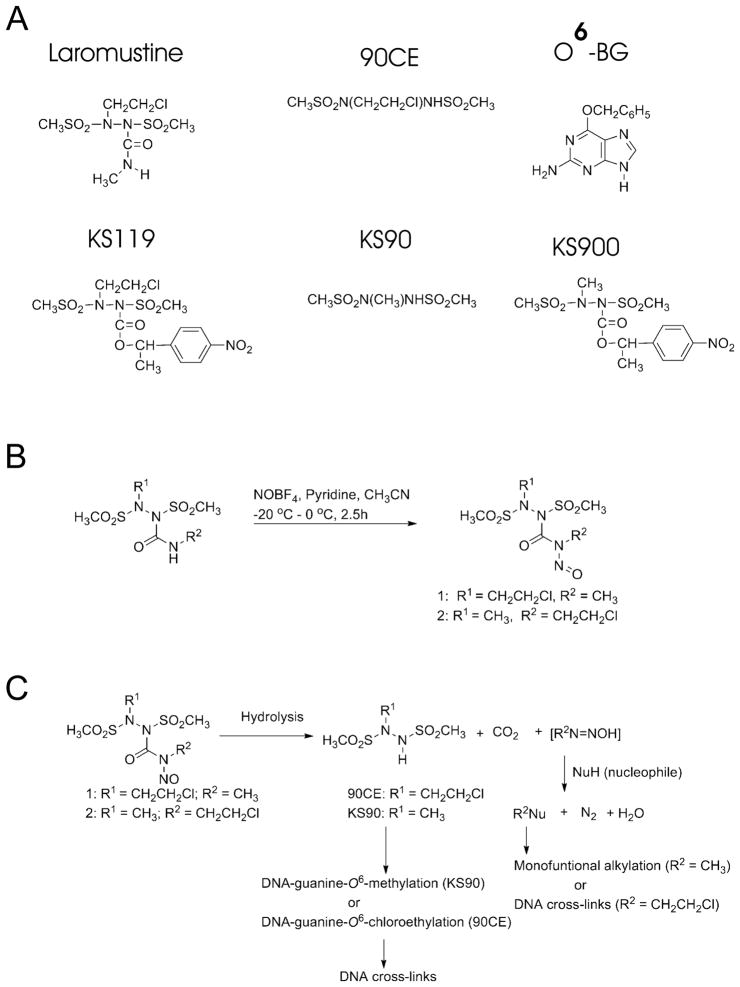
This laboratory has worked to design and synthesize novel alkylating agents that target the O-6 position of guanine in DNA. These agents include the globally activated clinically active chloroethylating prodrug, laromustine (also called onrigin, cloretazine, 101M and VNP40101M),1–5 the hypoxia targeted chloroethylator, KS119,6–8 and the hypoxia targeted methylator, KS9009 (Fig. 1A). The efficacy of these agents against tumors is impaired by the presence of the DNA repair protein O6-alkylguanine-DNA alkyltransferase (AGT, also abbreviated MGMT), which stoichiometrically removes an alkyl group from the O-6 position of guanine by transferring it to cysteine 145 within the protein, inactivating itself in the process.10–12 AGT is also the primary source of resistance to the clinically used O-6 modifying anticancer drugs BCNU, temozolomide and dacarbazine.
Figure 1.
Panel A: Schematic representation of the compounds employed. Panel B: Synthesis scheme for key compounds.15 Panel C: Schematic of the proposed routes of decomposition that result in the formation of therapeutic alkylating species for compound 1, and compound 2.
Laromustine, which exhibited substantial efficacy against acute myeloid leukemia (AML) in phase II clinical trials,4 is a dual function agent which both chloroethylates and carbamoylates cellular nucleophiles. There is some evidence that carbamoylation can marginally attenuate AGT activity,13,14 perhaps partially explaining some of laromustine’s efficacy against AGT expressing cells compared to agents lacking carbamoylating activity.2 We have also developed another dual function alkylator, 1, based upon the structure of laromustine, that both chloroethylates and methylates the O-6 position of guanine in DNA, with the intent of enhancing the ability of this agent to kill tumor cells, by more efficiently impairing AGT activity. Herein, we demonstrate that this agent is significantly more potent than laromustine and can ablate AGT in cultured tumor cells. An analog of 1, compound 2, in which the positions of the critical groups have been switched, was substantially less cytotoxic than 1 (Fig. 1B and 1C).
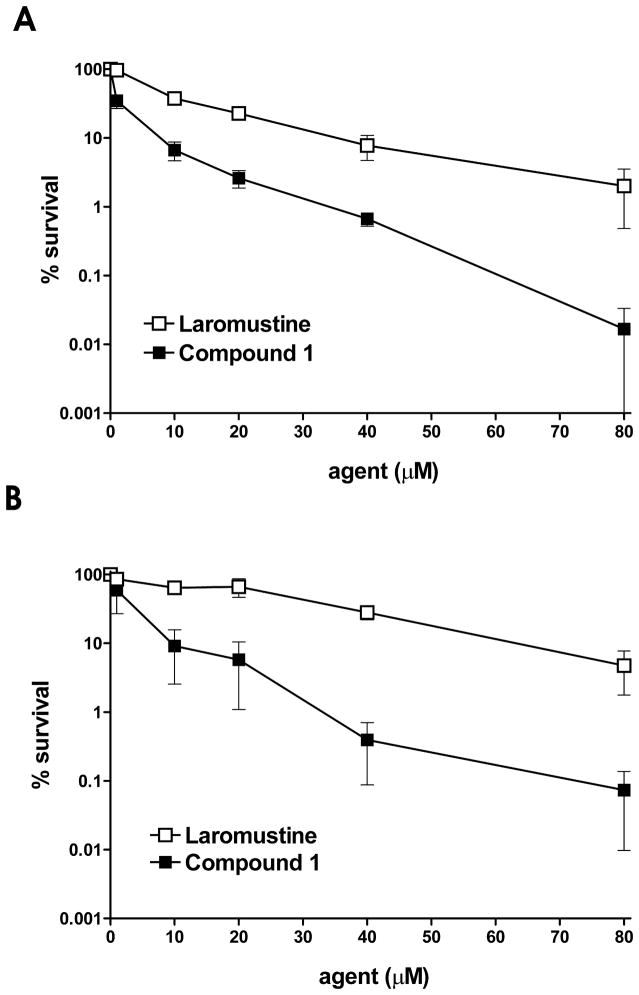
The dual function agent 1, which is expected to both methylate and chloroethylate DNA at the O-6 position of guanine, was tested for cytotoxicity against EMT6 murine mammary carcinoma cells that do not express AGT.14,16,17 Compound 1 was compared to the clinically tested agent laromustine, which chloroethylates DNA at the O-6 position of guanine and also produces methyl isocyanate, a carbamoylating agent. As shown in Figure 2, compound 1 was more cytotoxic (LC50 0.7 μM) over the entire concentration range than laromustine alone (LC50 8.3 μM). This included both short term (4 h; Fig. 2A) and long term (24 h; Fig. 2B) exposure of the EMT6 cell line. Compound 1 is surprisingly toxic for this class of agents, being ~ 12-fold more cytotoxic than laromustine and nearly equal to the intracellular targeted agent KS119 (0.5 μM), which previous studies from our laboratory indicated could even kill cell lines that grossly over-expressed the resistance protein AGT.8 In contrast, 1,2-bis(methylsulfonyl)-1-methylhydrazine (KS90), an agent possessing methylating only activity exhibited no cytotoxicity at 50 μM against EMT6 cells (the maximum concentration tested)9 and has an IC50 of ~ 3000 μM (unpublished observation). It should be noted that murine cells (i.e. EMT6) are relatively insensitive to methylating agents, and human DU145 cells are mismatch repair (MMR) deficient and so are also relatively methylation tolerant.18
Figure 2.
Cell survival experiments using AGT negative EMT6 cells treated with laromustine or 1 under aerobic conditions for 4h (panel A) or 24h (panel B). EMT6 cells were exposed to incremental concentrations of laromustine (□), or compound 1 (■) and cell survival was determined using a clonogenic assay.17 The Y axis gives cell survival in percent; the X axis gives the concentration of the agent employed. All experimental points represent at least 3 independent determinations ± SEM.
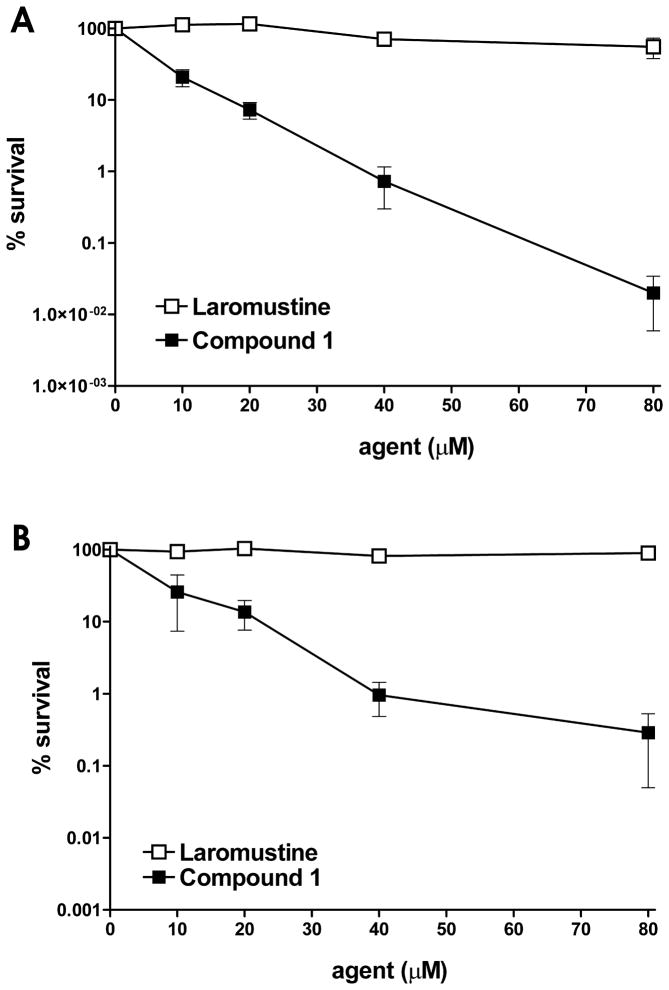
In tests against DU145 human prostate carcinoma cells, which naturally express high levels of AGT (42,000 molecules/cell),16 1 was >12-fold more cytotoxic (LC50 ~ 6.3 μM) to AGT high expressing human DU145 cells than laromustine (LC50 > 80 μM) at both 4 h and 24 h exposure times (Fig. 3A and B), while the methylating agent KS90 produced an LC50 value of ~ 1600 μM.9 The high sensitivity of DU145 cells to 1 despite the presence of high levels of protective AGT suggests that the resistance conferred by AGT was strongly attenuated by exposure to compound 1. This high cytotoxicity is likely the result of the ability of the methylating component of 1 to ablate AGT activity, thereby sensitizing the cells to the more cytotoxic chloroethylating DNA cross-linking component. While DU145 and EMT6 are both relatively insensitive to methylating agents given as single agents, it is still likely that the methylating activity of 1 makes a contribution to the overall cytotoxicity of this agent directly, (independent of any AGT ablating effect) at least in some part.
Figure 3.
DU145 AGT expressing cell survival following treatment with laromustine or 1. DU145 cells were exposed to graded amounts of laromustine (□), or compound 1 (■) for 4 h (panel A) or 24 h (panel B) and cell survival was measured by a clonogenic assay17. The Y axis indicates the percent survival; the X axis indicates the concentration of the agent employed. All experimental points represent at least 3 independent determinations ± SEM.
The unique potency of 1 led us to synthesize a new agent (compound 2) to determine if the cytotoxicity of 1 could be further enhanced. Compound 2 is identical to 1 except that the locations of the two alkylating moieties have been switched. In theory, if one considers a single molecule, the change would result in a reversal of the order in which the methylating and the chloroethylating species are released. We therefore examined the half-lives of these agents spectrophotometrically at pH 7.4 at 37 °C and those of the key secondary alkylating agents they generate (Table 1).19 The half lives of 1 and 2 under these conditions were relatively brief, approximately 10 and 13 min, respectively, compared to laromustine, which is approximately 1 h.20 The secondary alkylating component released from compound 1 is the chloroethylating component 90CE (T1/2 ~ 0.6 min), and from compound 2 is the methylating component KS90 (T1/2 ~ 2.8 min), which are both 1,2-bis(sulfonyl)-1-alkylhydrazines with relatively short T1/2 values. In contrast, the primary alkylation reactions which accompany the generation of 90CE from compound 1, and KS90 from compound 2, are slower with T1/2 values of ~10 and 13 min, respectively and therefore represent the rate determining step. Thus, a pronounced switch in the order of alkylation will not be seen within a population of molecules.
Table 1.
Half-lives of 1,2-bis(methylsulfonyl)-1-(alkyl)hydrazines (50 μM) at pH 7.4 and 37°C in 1 mM Potassium Phosphate Buffer.a
| compound | T1/2 (min)b |
|---|---|
| 90CE | 0.55 ± 0.05 |
| KS90 | 2.80 ± 0.08 |
| 1 | 10.04 ± 0.08 |
| 2 | 13.38 ± 0.13 |
As previously described.19
Values are the result of at least three independent determinations ± SEM.
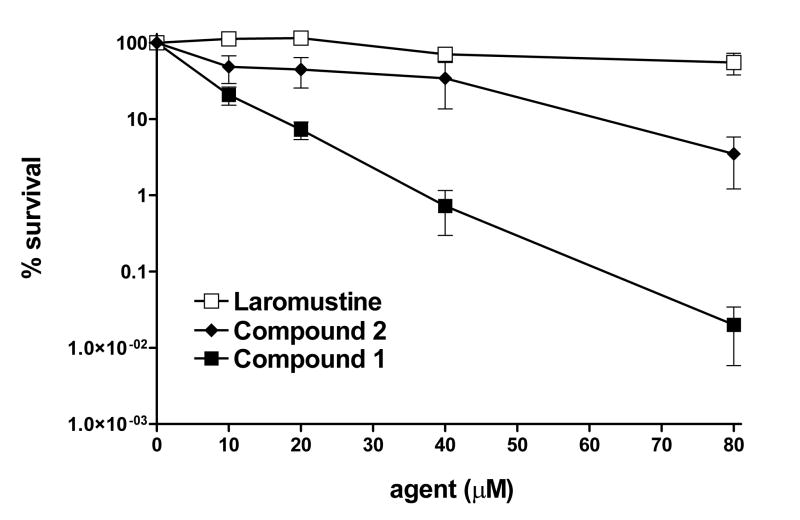
A mixture of chloroethylating and methylating reactions will occur largely simultaneously with both 1 and 2. However, during the initial phase of decomposition the primary alkylating species, derived from the moiety attached to the hydrazine N-1, will transiently predominate. Compound 2 was tested using cytotoxicity assays against AGT expressing DU145 cells. As shown in Figure 4, compound 2 was somewhat more cytotoxic to DU145 cells than laromustine, but substantially less active than 1. These studies suggest that the chemical architecture of 1 may be optimal for the delivery of cytotoxic lesions to tumor cells.
Figure 4.
DU145 cell survival following treatment with various agents. DU145 cells were exposed to incremental amounts of laromustine (□), compound 2 (◆) or compound 1 (■) for 4 h prior to plating for clonogenic analysis17. The Y axis indicates the percent survival; the X axis indicates the concentration of the agent employed. All experimental points represent at least 3 independent determinations ± SEM.
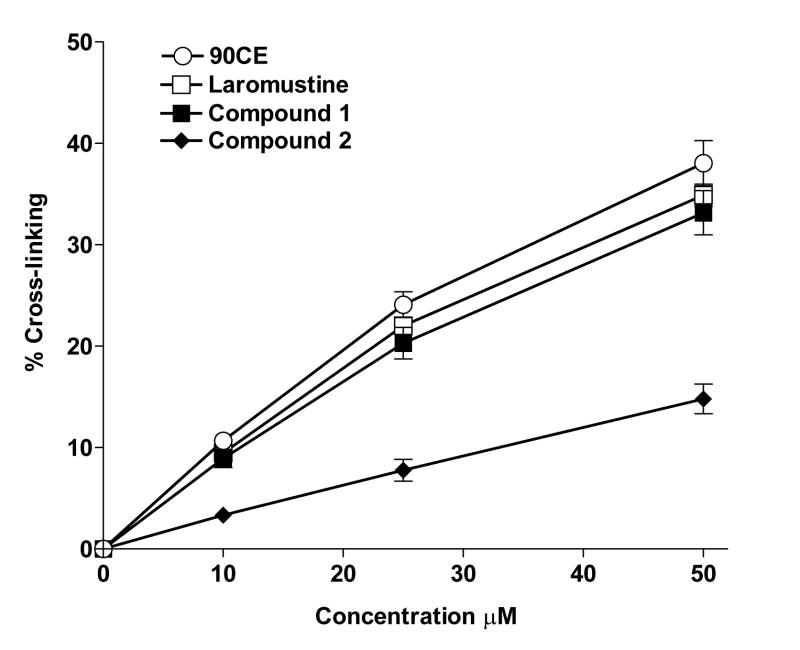
The capacity of 1, 2 and laromustine to cross-link DNA through chloroethylation of guanine was tested using the H33258 fluorescent dye binding rapid renaturation assay20,21 and was compared to that of 90CE (Fig. 1A). 90CE is the active chloroethylating product responsible for the cross-linking activity of laromustine and is the anticipated chloroethylating/cross-linking agent generated during the decomposition of 1. However, the chloroethylating activity of 2, unlike that of compound 1, arises from the nitrosourea-like primary alkylating moiety attached to the hydrazine N-1 and does not involve 90CE formation. The rank order of DNA cross-linking activity under the test conditions employed was found to be 90CE >laromustine≥1 > 2 (Fig. 5). At all of the concentrations tested, 1 cross-linked DNA almost as efficiently as the equivalent 90CE or laromustine concentrations. This is of interest because it suggests that the presence of the methylating component in 1 contributes substantially to its potent cytotoxicity compared to laromustine, presumably by ablation of the resistance protein AGT.
Figure 5.
Cross-linking levels were determined using the fluorescent H33258 dye binding rapid DNA renaturation assay for various agents. Samples of L1210 leukemia cell DNA were treated with 90CE (○), the active cross-linking agent produced by laromustine, laromustine (□), compound 1 (■) or compound 2 (◆) over a range of concentrations and given sufficient to time to fully react (at least four times their respective half-life values). The level of cross-linking was then determined as described20, 21. The Y axis indicates the percentage of DNA molecules containing one or more cross-links per molecule. All experimental points represent at least 3 independent determinations ± SEM.
Previous studies from this laboratory have demonstrated that the chloroethylating agents 90CE and laromustine do not substantially ablate AGT activity at normal cytotoxic concentrations compared to methylating agents, even though AGT expression imparts significant resistance to these agents.14 Possible reasons include a lower reactivity (~ 0.18-fold) towards the DNA guanine O-6 position compared to analogous methylating agents,14 a greater reactivity with water, the substantially greater toxicity of a cross-link which requires fewer lesions to kill, and the reduced preference for O6-chloroethyl lesions by AGT itself.14,22 In contrast, methylating agents which alkylate the O-6 position of guanine in DNA, are highly effective at ablating AGT activity.14, 23
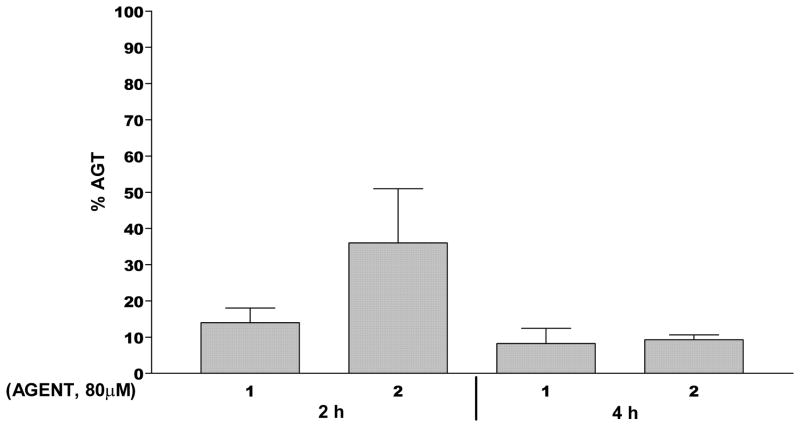
Compounds 1 and 2 were evaluated for their ability to ablate AGT levels in DU145 prostate carcinoma cells. As shown in Figure 6, 1 (80 μM) decreased AGT levels by over 80% in DU145 human prostate carcinoma cells after 2 h of treatment and by over 90% after 4 h of exposure. Compound 2, however, was almost four-fold less effective at ablating AGT at 2 h than 1, although it did narrow the gap in activity after 4 h of exposure. These differences in AGT ablation are larger than expected but may be partially explained by a number of important differences between 1 and 2. These include: a) differences in the relative yields of both types of alkylating moiety between 1 and 2, in particular the lower yield of the more efficiently AGT ablating methylating species with 2 (see below); b) potential differences in the guanine O-6 preference of the methylating species produced; c) the shorter T1/2 of 1 compared to 2 (see later) together with the release sequence of the alkylating moieties of 1 and 2, i.e., the methylating species initially predominating with 1, while the chloroethylating species initially predominate with 2, with both predicting a more rapid initial ablation of AGT by 1 than by 2. A more rapid and complete ablation of protective AGT would be expected to cause greater sensitization to the more toxic chloroethyl lesion.
Figure 6.
AGT ablation by 1 and 2. DU145 human prostate carcinoma cells were treated for 2 h or 4 h with 80 μM of 1, or 2 then the AGT levels were determined by binding to [benzene-3H] O6-benzylguanine16. The Y axis indicates the percent AGT remaining; the X axis indicates the agent employed. All experimental points represent at least 2–3 independent determinations ± SEM.
Model studies were conducted using a novel assay developed in this laboratory and to be published in detail elsewhere, to assess both the chloroethylating and methylating capacities of 1, 2, 90CE and laromustine based upon their ability to alkylate water to generate 2-chloroethanol and methanol.24,25 Water present at a concentration of ~ 55 M traps the vast majority of the reactive alkylating electrophiles generated. Compound 1 generated greater yields of methanol and 2-chloroethanol than 2, supporting the concept that it produces considerable quantities of both methylating and chloroethylating species (Table 2). The yield of 2-chloroethanol from 1 was approximately twice that of 2, indicating that it possessed substantially greater chloroethylating activity. The yields of 2-chloroethanol corresponded well with the levels of cross-linking determined for each of the four tested agents (Table 2 and Fig. 5). The smaller methanol yield of 2 compared to 1 would be expected to result in a smaller potentiation of its lesser chloroethylating activity (Table 2). The much greater cytotoxicity seen with 1 compared to laromustine despite the fact that laromustine generates somewhat greater quantities of 2-chloroethanol (resulting from the highly cytotoxic chloroethylating species) highlights the cytotoxicity potentiating activity of co-methylation.
Table 2.
2-Chloroethanol and methanol yields a
| Compound | 2-Chloroethanol (μM) | Methanol (μM) |
|---|---|---|
| Methanol | - b | 100 ± 1.9 |
| 2-Chloroethanol | 100 ± 1.7c | - |
| Compound 1 | 62.8 ± 2.3 | 91.4 ± 2.6 |
| Compound 2 | 33.3 ± 1.0 | 77.0 ± 1.8 |
| 90CE | 88.3 ± 0.9 | - |
| Laromustine | 86.1 ± 1.4 | - |
2-Chloroethanol and methanol yields were determined as described.26
Indicates none detected.
Values are the result of at least three independent determinations ± SE.
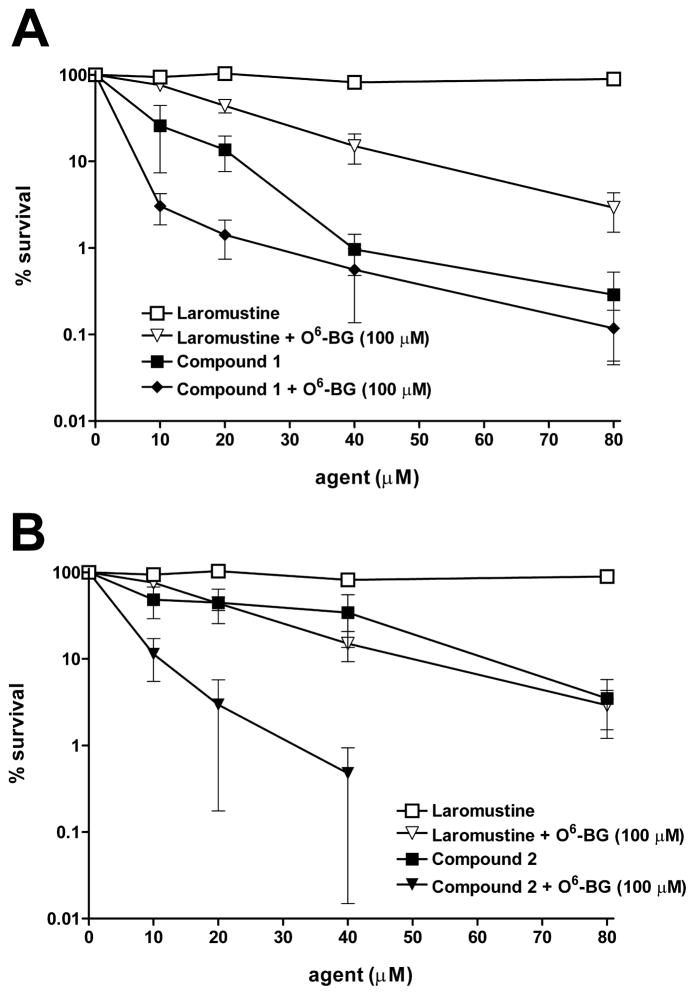
Laromustine and 1 were tested for increased cytotoxity after pretreatment with the known AGT inhibitor O6-BG in DU145 cells expressing 42,000 AGT molecules per cell. Cells were treated with high dose (100 μM) O6-BG for 2 h prior to exposure to graded concentrations of laromustine or 1. As shown in Figure 7A, pretreatment with O6-BG sensitized DU145 cells to laromustine and also to a lesser extent to compound 1. This finding is consistent with the AGT ablation studies which indicated that 1 did not completely deplete AGT in DU145 cells, leaving a window for O6-BG to exert an effect. Compound 1 could transiently pre-ablate AGT activity to some extent during the relatively short methylation predominance phase of its decomposition, but this short-term advantage would not be expected to have a major contribution to the overall AGT ablation effect. DU145 cells were sensitized to a greater extent to 2 by pretreatment with 100 μM O6-BG (Fig. 7B) than to 1; this is consistent with the measured lower ability of 2 compared with 1 to ablate AGT activity leaving a larger margin for inhibitory assistance.
Figure 7.
O6- Benzylguanine sensitization studies in DU145 cells. DU145 human prostate carcinoma cells which express a relatively high level of AGT (42,000 molecules/cell) were pretreated for 2 h with the potent AGT inhibitor O6-benzylguanine then treated with the indicated agents. Following treatment, cells were plated in six well plates to determine cell survival by a clonogenic assay17. Panel A: laromustine alone (□); laromustine plus 100 μM O6-BG (▽); compound 1 alone (■) and compound 1 plus 100 μM O6-BG (◆). Panel B: laromustine alone (□); laromustine plus 100 μM O6-BG (▽); compound 2 alone (■); compound 2 plus 100 μM O6-BG (▼). The Y axis indicates the percent survival; the X axis indicates the concentration of the agent employed. All experimental points represent at least 3 independent determinations ± SEM.
Laromustine is a dual function agent in that it releases two classes of reactive intermediates, a series of chloroethylating species and methyl isocyanate, a carbamoylating species.20 While the chloroethylating species that alkylate the O-6 position of guanine result in the formation of highly cytotoxic cross-links, the contribution of the carbamoylating component to the anticancer activity of laromustine is not well defined. There is some evidence that methyl isocyanate can inactivate AGT, 14 is involved in the inhibition of thioredoxin reductase,27 and can inhibit the polymerase activity of Pol β in in vitro assays.28 The inhibition of the polymerase activity of Pol β is particularly interesting in that it has the potential to impact base excision repair which is frequently involved in the repair of DNA alkylation damage. A similar situation occurs with the chloroethyl nitrosoureas which also produce a range of chloroethylating species and a carbamoylating species (2-chloroethyl isocyanate in the case of the nitrosourea BCNU). Some of the chloroethyl nitrosourea derived chloroethylating species are likely to be identical to those generated by the chloroethylating sulfonyl hydrazines, but both classes are in addition capable of producing their own unique chloroethylating species.20 Variation in the electrophilic species generated, their relative yields, and their relative preferences for the guanine O-6 position result in differences in the cross-link yields between these two classes of agents. Typically, the chloroethylating sulfonyl hydrazines give higher yields of chloroethylation reactions and cross-links, and cellular resistance to these agents has a higher AGT dependency than the chloroethyl nitrosoureas.14,16,20
The nature and yield of the chloroethylating and methylating species generated from 1 and 2 depend upon the location of the chloroethyl and methyl groups and upon the mechanism of activation of each of these analogs. In 1, the chloroethylating species is generated by base-catalyzed activation of 90CE; whereas, in 2, the chloroethylating species results from the fragmentation of the N-nitrosourea like moiety (Figure 1). In contrast, the methylating species results from the N-alkyl-N-nitroso component of 1, and from the sulfonylhydrazine component of 2. These features inevitably result in significant differences in the cytotoxic properties of these agents. Of the analogs examined, 1 was the most cytotoxic, displaying substantially greater toxicity in AGT expressing and non-expressing cells than either laromustine or 2.
The cytotoxic effects of methylation and 2-chloroethylation at guanine O-6 differ in important respects. O6-Methylguanine is rapidly titrated by AGT and O6-methylguanine lesions only persist and result in cytotoxic actions if the number of methylations exceed the number of AGT molecules.14 If unrepaired, methylation lethality appears to be due to the “mismatched repair machinery”, initiating apoptosis due to failed repair.29 In the absence of functional mismatch repair the guanine O-6 methylations lead to point mutations which require a substantial number to be lethal. In contrast, the O6-chloroethylguanine lesion has a limited AGT window of repair because it undergoes a spontaneous rearrangement to produce the highly lethal cross-link which then cannot be repaired by AGT.30 It is therefore likely that the efficient delivery of two different DNA guanine O-6 alkyl lesions by 1 accounts for its superior cytotoxicity compared to laromustine in AGT deficient cell lines. If similar secondary non-AGT dependent repair mechanism(s) are involved in the repair of both O-6 lesion types, competition for these repair mechanism(s) may allow more time for O-6 chloroethylations to progress to highly lethal cross-links. In cell lines with high AGT activity, which are normally very resistant to agents such as laromustine, the profoundly greater cytotoxicity seen with 1 compared to laromustine (Fig. 3) appears to be largely a consequence of AGT ablation, as most if not all of the O6-methylguanine lesions generated will rapidly eliminate protective AGT. Thus, the addition of an efficient AGT titrating methylating component to laromustine, while eliminating its weakly AGT attenuating carbamoylating activity, markedly enhanced its cytotoxicity. Interestingly, compound, 2, which also generated both a methylating and chloroethylating species, while being more cytotoxic than laromustine, was not comparably cytotoxic to 1, emphasizing the importance of the relative locations of the alkylating groups. Earlier work in our laboratory has clearly demonstrated that 90CE gives a much higher yield of cross-links than the nitrosoureas BCNU or CCNU.20 Therefore, we expected that the chloroethylating species generated by 1 would give a higher yield of cross-links than those from 2, in keeping with the relative preferences of these classes of chloroethylating agents for the guanine O-6 position. In addition, the greater absolute yields of methylating species in the case of 1, as measured by methanol yield, should result in superior sensitization by AGT ablation. AGT ablation studies support these contentions since 1 showed greater depletion of AGT activity at 2 h than 2. Furthermore, it is possible that the faster and early predominant release of the methylating species in the case of 1 may more efficiently sensitize cells to the more cytotoxic chloroethylating species by depleting AGT without initially competing with the chloroethyl lesions to the same extent, as would be in the case of 2. Thus, it is likely that all of these effects contribute to the cytotoxic superiority of 1 over 2. Cross-linking studies comparing 1 to laromustine indicate that the enhanced toxicity observed with 1 is not the result of a direct increase in the yield of cross-linking species over that seen with laromustine and strongly support the idea of toxicity enhancement as a consequence of a dual function agent whose primary action sensitizes cells to its secondary action.
Although AGT protects tumor cells against chemotherapeutic agents that alkylate the O-6 position of DNA guanine, it is also a potential source of vulnerability if it can be selectively depleted in cancers while being spared in normal tissue. Current efforts to ablate AGT have employed global depletion agents such as O6-BG, which also deplete AGT in normal tissue, with the result being that little net therapeutic benefit is realized in patients because normal tissue is also sensitized to the alkylating agent.31–33 These findings underscore the importance of therapeutically generating, or at least maintaining, any pre-existing tumor AGT deficit with respect to normal tissue. The selective sensitization/destruction of tumors through AGT ablation and AGT deficit exploitation strategies34,35 would be substantially enhanced by the availability of clinical agents that selectively alkylate at the O-6 position of guanine, thereby reducing non-specific and non-therapeutic toxicities. This preferential destruction would be particularly efficacious if the O-6 position of the guanine alkylating agent also exhibited tumor selectivity. Hence, one of our long term goals is the development of highly cytotoxic guanine O6 specific dual function agents, with short half lives such as 1, that are incorporated into a targeted platform such as that employed by KS9009 and KS119.7,8 More optimal segregation/pre-release of the AGT ablating methylating activity can be engineered into molecules by making the primary methylation event occur with a significantly shorter half-life than the secondary chloroethylation event. Highly cytotoxic dual function (methylating and chloroethylating) agents, or combinations of single agents that emulate these two functions, especially if sequentially delivered, may be highly useful candidates as targeted prodrugs. The approximate 10-fold increase in overall potency is likely to be a major advantage when using tumor activated prodrugs since the quantity of agent selectively delivered to the tumor is limited.
Acknowledgments
This work was supported in part by U.S. Public Health Service Grants CA122112, and CA129186 from the National Cancer Institute, and a Grant from the National Foundation for Cancer Research.
Abbreviations
- AGT
O6-alkylguanine-DNA alkyltransferase
- Compound 1
2-(2-chloroethyl)-N-methyl-1,2-bis(methylsulfonyl)-N-nitrosohydrazinecarboxamide
- Compound 2
N-(2-chloroethyl)-2-methyl-1,2-bis(methylsulfonyl)-N-nitrosohydrazinecarboxamide
- 90CE
1,2-bis(methylsulfonyl)-1-(2-chloroethyl)hydrazine
- laromustine
1,2-bis(methylsulfonyl)-1-(2-chloroethyl)-2-(methylaminocarbonyl)hydrazine
- O6-BG
O6-benzylguanine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.Shyam K, Penketh PG, Loomis RH, Rose WC, Sartorelli AC. J Med Chem. 1996;39:796. doi: 10.1021/jm9505021. [DOI] [PubMed] [Google Scholar]
- 2.Finch RA, Shyam K, Penketh PG, Sartorelli AC. Cancer Res. 2001;61:3033. [PubMed] [Google Scholar]
- 3.Giles F, Thomas D, Garcia-Manero G, Faderl S, Cortes J, Verstovsek S, Ferrajoli A, Jeha S, Beran M, Koller C, Andreeff M, Cahill A, Clairmont C, Sznol M, Kantarjian H. Clin Cancer Res. 2004;10:2908. doi: 10.1158/1078-0432.ccr-03-0738. [DOI] [PubMed] [Google Scholar]
- 4.Giles F, Vey N, DeAngelo D, Seiter K, Stock W, Stuart R, Boskovic D, Pigneux A, Tallman M, Brandwein J, Kell J, Robak T, Staib P, Thomas X, Cahill A, Albitar M, O’Brien S. Blood. 2009;114:4027. doi: 10.1182/blood-2009-06-229351. [DOI] [PubMed] [Google Scholar]
- 5.Schiller G, O’Brien S, Pigneux A, DeAngelo DJ, Vey N, Kell J, Solomon S, Stuart RK, Karsten V, Cahill AL, Albitar MX, Giles FJ. J Clin Oncol. 2010;28:815. doi: 10.1200/JCO.2009.24.2008. [DOI] [PubMed] [Google Scholar]
- 6.Shyam K, Penketh PG, Shapiro M, Belcourt MF, Loomis RH, Rockwell S, Sartorelli AC. J Med Chem. 1999;42:941. doi: 10.1021/jm9805891. [DOI] [PubMed] [Google Scholar]
- 7.Seow HA, Penketh PG, Shyam K, Rockwell S, Sartorelli AC. Proc Natl Acad Sci. 2005;102:9282. doi: 10.1073/pnas.0409013102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baumann RP, Penketh PG, Ishiguro K, Shyam K, Zhu YL, Sartorelli AC. Biochem Pharmacol. 2010;79:1553. doi: 10.1016/j.bcp.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baumann RP, Ishiguro K, Penketh PG, Shyam K, Zhu R, Sartorelli AC. Biochem Pharmacol. 2011;81:1201. doi: 10.1016/j.bcp.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brent TP, Remack JS. Nucleic Acids Res. 1988;16:6779. doi: 10.1093/nar/16.14.6779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pegg AE. Cancer Res. 1990;50:6119. [PubMed] [Google Scholar]
- 12.Pegg AE. Mutat Res. 2000;462:83. doi: 10.1016/s1383-5742(00)00017-x. [DOI] [PubMed] [Google Scholar]
- 13.Penketh PG, Shyam K, Baumann RP, Remack JS, Brent TP, Sartorelli AC. Cancer Chemother Pharmacol. 2004;53:279. doi: 10.1007/s00280-003-0740-7. [DOI] [PubMed] [Google Scholar]
- 14.Ishiguro K, Zhu YL, Shyam K, Penketh PG, Baumann RP, Sartorelli AC. Biochem Pharmacol. 2010;80:1317. doi: 10.1016/j.bcp.2010.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.2-(2-Chloroethyl)-N-methyl-1,2-bis(methylsulfonyl)-N-nitrosohydrazinecarboxamide (1) was synthesized by stirring 1,2-bis(methylsulfonyl)-1-(2-chloroethyl)-2-(methylaminocarbony)hydrazine (laromustine, 0.92 g, 3 mmol) and anhydrous pyridine (0.47 g, 6 mmol) in acetonitrile (5 ml) at −20 °C under a nitrogen atmosphere, nitrosonium tetrafluoroborate (0.70 g, 6 mmol) was added in one portion. The mixture was stirred at 0 °C for an additional 2 h. After removal of the solvent, the residue was purified by chromatography (dichloromethane). N-(2-Chloroethyl)-2-methyl-1,2-bis(methylsulfonyl)-N-nitrosohydrazinecarboxamide (2) was synthesized using a similar procedure by treating N-(2-chloroethyl)-2-methyl-1,2-bis(methylsulfonyl)hydrazinecarboxamide with nitrosonium tetrafluoroborate.
- 16.Ishiguro K, Shyam K, Penketh PG, Sartorelli A. Anal Biochem. 2008;383:44. doi: 10.1016/j.ab.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baumann RP, Seow HA, Shyam K, Penketh PG, Sartorelli AC. Oncology Res. 2005;15:313. doi: 10.3727/096504005776404553. [DOI] [PubMed] [Google Scholar]
- 18.Chen Y, Wang J, Fraig MM, Metcalf J, Turner WR, Bissada NK, Watson DK, Schweinfest CW. Cancer Res. 2001;61:4112. [PubMed] [Google Scholar]
- 19.Penketh PG, Shyam K, Patton CL, Sartorelli AC. Anal Biochem. 1996;238:46. doi: 10.1006/abio.1996.0248. [DOI] [PubMed] [Google Scholar]
- 20.Penketh PG, Shyam K, Sartorelli AC. Biochem Pharmacol. 2000;59:283. doi: 10.1016/s0006-2952(99)00328-7. [DOI] [PubMed] [Google Scholar]
- 21.Penketh PG, Baumann RP, Ishiguro K, Shyam K, Seow HA, Sartorelli AC. Leuk Res. 2008;32:1546. doi: 10.1016/j.leukres.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morimoto K, Dolan ME, Scicchitano D, Pegg AE. Carcinogenesis. 1985;6:1027. doi: 10.1093/carcin/6.7.1027. [DOI] [PubMed] [Google Scholar]
- 23.Gerson SL. Nat Rev Cancer. 2004;4:296. doi: 10.1038/nrc1319. [DOI] [PubMed] [Google Scholar]
- 24.Sahm H, Schütte H, Kula MR. Methods Enzymol. 1982;89:424. doi: 10.1016/s0076-6879(82)89074-5. [DOI] [PubMed] [Google Scholar]
- 25.Couderc R, Baratti J. Agric Biol Chem. 1980;44:2279. [Google Scholar]
- 26.Agents (100 μM) were reacted with H2O at 40°C for at least 1 h in 5 mM Tris/0.25 mM EDTA buffer (pH 7.4); alcohol oxidase (AO) was added (10 units/ml) at 40°C for 30 min. then samples were quenched with 500 μl of a 0.4% solution of 2,4-dinitrophenylhydrazine (2,4-DNPH) in acetonitrile. Fifty μl of 1 M perchloric acid was added to catalyze the formation of the hydrazone at 40°C for 5 min. and mixtures were analyzed by HPLC using a 5 micron 220 × 4.6 mm C-18 reverse phase column (Applied Biosystems Carlsbad, CA, USA RP-18) by elution with 52.5% acetonitrile, 47.5% 0.03 M KH2PO4, 1.0 mM NaN3, pH 5.4. at 370 nm using a Beckman 168 UV/vis detector. The methanol and 2-chloroethanol derived hydrazone products elute at 11.1 and 16.3 min, respectively. The compounds were quantified with calibration curves generated using authentic alcohols, the limit of alcohol detection being < 1 μM.
- 27.Rice KP, Klinkerch EJ, Gerber SA, Schleicher TR, Kraus TJ, Buros CM. Mol Cell Biochem. 2012 doi: 10.1007/s11010-012-1411-y. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frederick AM, Davis ML, Rice KP. Biochem Biophys Res Commun. 2009;378:419. doi: 10.1016/j.bbrc.2008.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.D’Atri S, Tentor L, Lacal PM, Graziani G, Pagani E, Benincasa E, Zambruno G, Bonmassar E, Jiricny J. Mol Pharmacol. 1998;54:334. doi: 10.1124/mol.54.2.334. [DOI] [PubMed] [Google Scholar]
- 30.Ludlum DB. Cancer Invest. 1997;15:588. doi: 10.3109/07357909709047601. [DOI] [PubMed] [Google Scholar]
- 31.Schilsky RL, Dolan ME, Bertucci D, Ewesuedo RB, Vogelzang NJ, Mani S, Wilson LR, Ratain MJ. Clin Cancer Res. 2000;6:3025. [PubMed] [Google Scholar]
- 32.Kaina B, Margison GP, Christmann M. Cell Mol Life Sci. 2010;67:3663. doi: 10.1007/s00018-010-0491-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rizzieri D, LoRusso S, Tse W, Khan K, Advani A, Moore J, Karsten V, Cahill A, Gerson SL. Clin Lymphoma Myeloma Leuk. 2010;10:211. doi: 10.3816/CLML.2010.n.033. [DOI] [PubMed] [Google Scholar]
- 34.Zhu R, Seow HA, Baumann RP, Ishiguro K, Penketh PG, Shyam K, Sartorelli AC. Bioorg Med Chem Lett. 2012;22:6242. doi: 10.1016/j.bmcl.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu R, Liu MC, Luo MZ, Penketh PG, Baumann RP, Shyam K, Sartorelli AC. J Med Chem. 2011;54:7720. doi: 10.1021/jm201115f. [DOI] [PMC free article] [PubMed] [Google Scholar]