Introduction
Worldwide regional disparities in the occurrence of prostate cancer (PCa) have been attributed in part to the effect of diet, with Asian men having up to a six fold decreased incidence over their western counterparts [1]. Epidemiologic observations, however, have been inconclusive about which specific dietary components contribute to PCa [2–5]. A logical extension is that if diet can affect the development of cancer, it can also influence the growth of an established tumor, and indeed this has been demonstrated in animal studies [6–9]. While traditionally a low fat diet was thought to be optimal, we previously hypothesized that a reduced carbohydrate diet was likewise beneficial [10]. In a prior study we found that mice consuming a no-carbohydrate ketogenic diet (NCKD) had increased overall survival and slower tumor growth, compared to mice on a western diet, a benefit not observed in mice consuming a low fat diet [11]. These findings were then replicated in a similar xenograft model with the finding that a low-fat diet was also beneficial [12]. The favorable effect in the NCKD mice was thought to be from decreased signaling of the insulin/ insulin-like growth factor (IGF) axis, a pathway integral in the progression of PCa [8, 10, 13–18].
These provocative results were further explored by another group with diets not completely devoid of carbohydrates, but rather with lower proportions (such as might be reasonably tolerated in human subjects) which have also been shown to delay tumor growth in a prostate xenograft model [19]. However, in that prior study a low-carbohydrate diet was not directly compared to a NCKD. To address this, we recently tested low-carbohydrate diets (10 and 20% carbohydrate kcals, respectively) vs. a NCKD in a mouse xenograft model and found all carbohydrate restricted diets had similar effects on tumor growth, overall survival, and IGF axis signaling, suggesting that low carbohydrate diets may be as effective as a NCKD [20].
While these dietary changes appear to be promising for slowing tumor growth, the best established therapy for stunting PCa involves chemical or surgical castration. The deleterious effects of androgen deprivation on weight, diabetes and cardiovascular health make dietary decisions under this circumstance of particular interest [21–23]. Thus we sought to explore the effect of low- and no-carbohydrate diets on tumor growth and survival as an adjunct to androgen deprivation therapy in a PCa xenograft model.
Materials and Methods
Cell Culture
LAPC-4 human prostate cancer cells were a generous gift from Dr. William Aronson, UCLA School of Medicine. Cells were maintained in Iscove’s modified medium with 10% Fetal Bovine Serum and supplemented with synthetic androgen R1881 at 1 nM. Cells were grown in 5% CO2 at 37°C and harvested by trypsinization at 70–80% confluence in log phase growth.
Animal Studies
After obtaining approval from the Duke University Institutional Animal Care and Use Committee, 175 male 4–6 week-old SCID (CB.17 scid/scid) mice were obtained from Harlan Sprague Dawley Inc (Indianapolis, IN). Animals were acclimated on an ad libitum western diet (35% fat, 49% carbohydrates, 16% protein kcals) for 4 days, then injected in the right flank subcutaneously with 1 × 105 LAPC-4 tumor cells in 0.1 mL of Matrigel (Becton-Dickinson, Franklin Lakes, NJ). After 14 days all mice were singly housed for precise recording of calories consumed, and all but 10 mice were castrated. The 10 non-castrated mice were fed an ad libitum western diet for the remainder of the study and were used as a control. Of the 165 mice who were castrated, 15 died within 1–2 days following this procedure and prior to randomization. The remaining 150 mice were kept on an ad libitum western diet for an additional 2 days and were then randomized as follows: 38 on a western diet (WD), 37 on a no carbohydrate ketogenic diet (NCKD, 84% fat, 0% carbohydrate, 16% protein kcals), 38 on a 10% carbohydrate diet (10% carbohydrate, 74% fat, 16% protein kcals), and 37 on a 20% carbohydrate diet (20% carbohydrate, 64% fat, 16% protein kcals) (Figure 1). Diets were prepared and sterilized (irradiated) by Purina TestDiet (St. Louis, MO).
Figure 1.
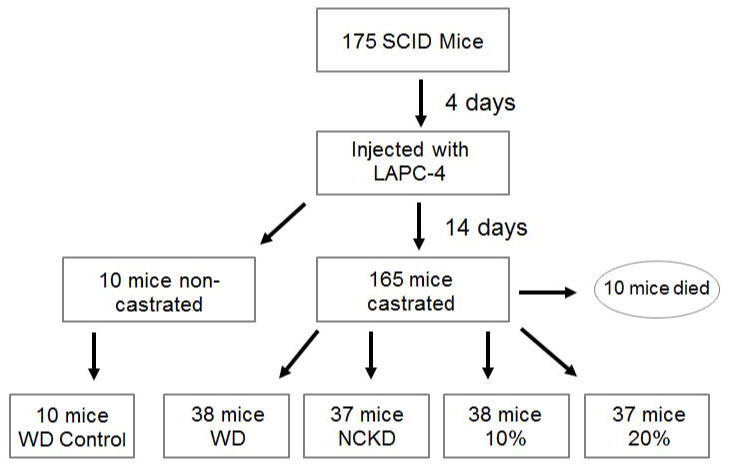
Flowchart to randomization
The mice in the castrated Western diet group were designated the reference group in a modified paired-feeding protocol and were fed ad libitum. NCKD mice have been observed to overeat and gain weight relative to a low-fat diet when fed ad libitum, whereas they lose weight if fed isocalorically. Based on prior studies from our group, it was determined that the NCKD mice should be fed approximately 10% extra calories relative to an ad libitum Western diet, while the 10% and 20% carbohydrate groups should be fed approximately 7.5% extra calories20. All mice were weighed and fed 3 times per week. Caloric intake for the castrated Western diet group was determined at each feeding and used to calculate the amount of feed to be given to the other 3 groups. Food intake among the carbohydrate restricted groups was then titrated to maintain equal body weights. Tumor dimensions were measured twice a week with calipers starting on day 12 post randomization. The volume was determined according to the formula width x length x height x 0.5236 [24].
Four days prior to randomization/castration and then again 4 weeks post randomization (both post-tumor injection) the mice underwent a facial vein bleed. Blood glucose was measured using the Accu-Check Active glucometer (Roche Diagnostics, Indianapolis, IN). Urine was collected during the same sessions by applying gentle suprapubic pressure to measure urinary ketones using Ketostix semi-quantitative urine strips (Bayer Corporation, Elkhart, IN). Animals were euthanized using a lethal dose of pentobarbital when tumors reached 1,000 mm3 or when the health of the animal appeared compromised per Duke institutional criteria (e.g. severe weight loss, failure to thrive, ruffled fur, lethargy, etc.). Serum was obtained via cardiac puncture. Tumor samples were cut in half and either snap-frozen at −80°C, or preserved in a 10% formalin solution. Serum from the median surviving 8 mice from each experimental group (total 32 mice) were assayed for murine levels of insulin, IGF-1, and insulin-like growth factor binding protein (IGFBP)-3 using mouse-specific enzyme-linked immunoassays (ELISA) (Millipore Corp., Billerica, MA; R&D Systems, Inc., Minneapolis, MN).
Statistical Analysis
The primary end-point was survival, defined as time from randomization to sacrifice, which was examined using a log-rank test. We also compared survival among the groups using a Cox proportional hazards model. Graphically, survival was represented using a Kaplan-Meier survival curve. Comparisons among groups in calories consumed, body weights, tumor volumes, IGF-1 levels, IGFBP-3 levels, insulin levels, serum glucose and ketone levels were determined using the Kruskal-Wallis test. All statistical analyses were performed using STATA 10.0 (Stata Corp., College Station, TX) with p ≤ 0.05 considered statistically significant.
Results
There was minimal variability in mouse weights among groups throughout the study (Figure 2). Considering the first 96 days, after which the small number of remaining mice precluded a meaningful comparison, a statistically significant difference in weight was reached on days 19–33 and 79. Caloric intake was tightly regulated (Figure 3) according to the modified paired-feeding protocol. Serum glucose levels were similar (p = 0.10) prior to randomization; however, at the time of sacrifice serum glucose levels were significantly higher in the Western diet group compared to the carbohydrate restricted group (p <0.001). Ketone production was negligible in all mice at pre-randomization and at sacrifice (data not shown).
Figure 2.
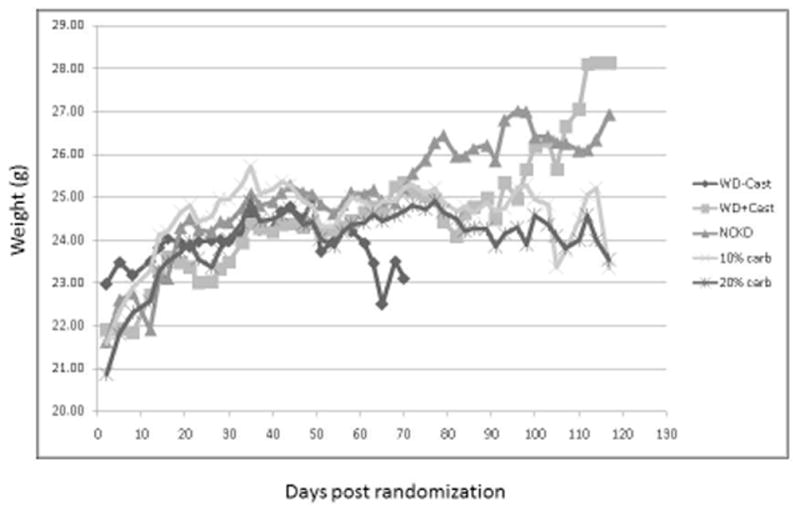
Mean body weights following randomization
Figure 3.
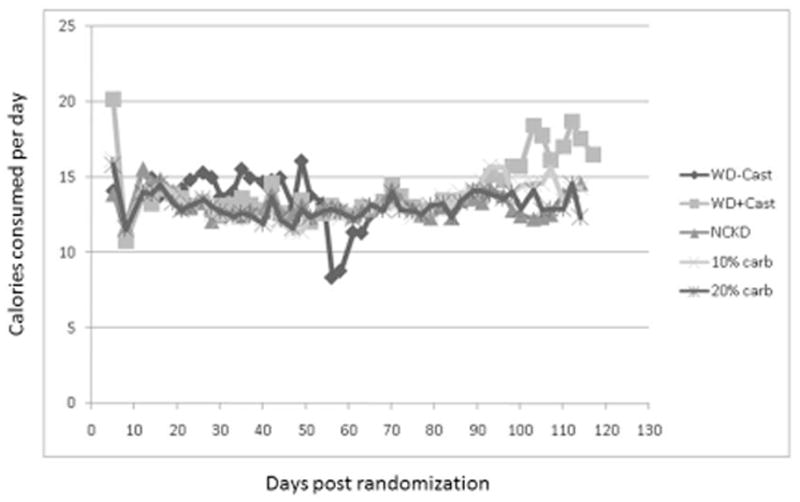
Mean caloric consumption following randomization
As expected, the non-castrated mice experienced accelerated tumor growth (Figure 4) and short survival. Of the castrated mice, a significant difference in median tumor size among groups occurred on days 26, 40, 43, 54, 64, 68, and 71. In general, mice fed a western diet had the shortest survival, while those on a 20% carbohydrate diet had the slowest tumor growth and thus the subsequent longest survival (p = 0.046). Comparing survival among carbohydrate restricted groups, showed no significant difference among the three groups (p = 0.51). When mice in the NCKD, 10% and 20% carbohydrate diet groups were pooled there was a non-significant trend for improved survival compared to castrated western diet mice (p = 0.11, Figure 5). In the Cox model, we did not find a significant association when comparing survival of the pooled carbohydrate restricted diets versus western diet (95% CI 0.53 – 1.10, p=0.15).
Figure 4.
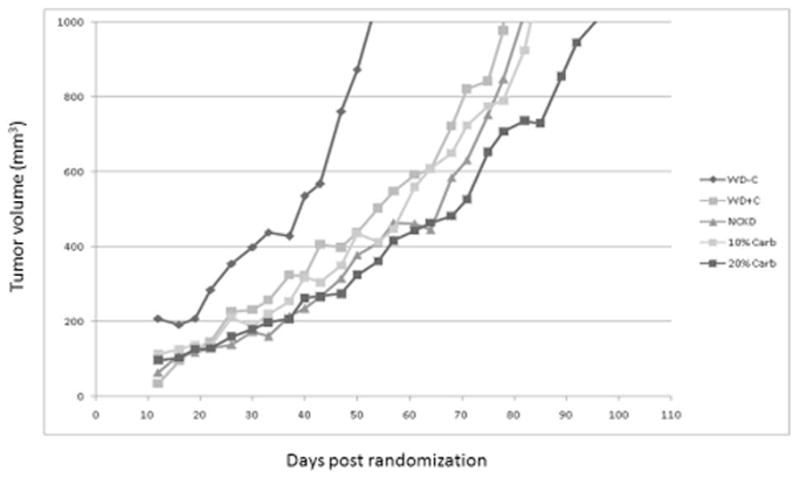
Median tumor volumes
Figure 5.
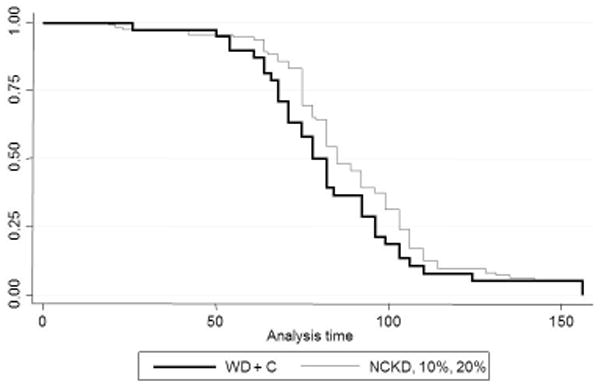
Kaplan-Meier survival curve WD + castration versus pooled low-carbohydrate diets
Examination of the hormonal axis revealed that the levels of insulin, IGF-1, and IGFBP-3 among groups were not significantly different prior to randomization (data not shown). Similarly, no significant differences were found among all groups at sacrifice (Table 1).
Table 1.
Hormonal axis at time of sacrifice
| WD − C | WD + C | NCKD | 10% | 20% | P value | |
|---|---|---|---|---|---|---|
| IGF-1 (ng/ml) | 608 ± 100 | 548 ± 101 | 614 ± 136 | 743 ± 386 | 652 ± 154 | 0.07 |
| IGFBP-3 (ng/ml) | 704 ± 109 | 609 ± 339 | 627 ± 192 | 673 ± 167 | 711 ± 139 | 0.10 |
| Insulin (ng/ml) | 2.3 ± 1.6 | 2.2 ± 1.6 | 3.7 ± 2.5 | 3.5 ± 1.9 | 2.7 ± 3.8 | 0.43 |
Discussion
Epidemiologic data highlights regional differences in the worldwide incidence of PCa, with lower rates in non-Western countries, particularly among Asian men. Environmental factors likely contribute to the risk, and in some areas where PCa is being diagnosed with greater frequency there has been a concurrent rise in the rate of diabetes and colorectal cancers [1,4,10]. This implies a role for nutritional factors, particularly an evolution to higher proportions of animal fats and proteins and other “Western” style dietary constituents including refined carbohydrates and excess calories in general.
While the effects of these changes are myriad, a noted consequence is an elevation in the levels of serum insulin and IGF-1. These hormones appear to be associated with an increased risk of PCa in population-based studies[10,13,25–26]. Additionally, IGF-1 is primarily bound to IGFBP-3, and thus as the levels of the binding protein fluctuate the amount of bioavailable hormone and resultant PCa risk change [16]. Animal models have confirmed the relationship between higher IGF-1 and PCa growth [6, 8, 11–12, 18, 20], likely mediated in part through the activation of AKT, VEGF, and other pro-tumorigenesis pathways [18–19].
This observation has lead to attempts at influencing PCa tumor growth through alterations in diet. Although low fat diets have been shown to be beneficial for this purpose [6–9], animal models suggest carbohydrate restricted diets are at least as effective: insulin and IGF-1 levels may be decreased while raising IGFBP-3 levels to a greater degree than that achieved by restricting fat intake [16, 27–28]. Additionally, in mice fed high carbohydrate diets activation of the insulin axis occurs in concert with the expression of a greater number of insulin receptors, an increase in activated AKT, and promotion of tumor growth [19].
In the present study, no difference in tumor growth, IGF-axis, and survival was found among mice fed 3 different carbohydrate restricted diet, varying in proportion from 0% to 20%. In fact, those consuming a 20% carbohydrate diet had the slowest tumor growth, which was significantly slower than mice fed a western diet. This suggests that alternative mechanisms may be involved in providing the beneficial effects observed with carbohydrate restriction. In a previous study in non-castrate mice, restriction of carbohydrates at differing levels (NCKD, 10%, and 20%) provided similar survival benefit among groups; however, among the mice fed a 20% carbohydrate diet insulin levels were significantly lowered while a trend was noted for higher IGF-1 levels. IGFBP-3 levels among all groups were similar [20]. Although the trends in this prior study did not reach statistical significance, we noted a similar distribution in insulin, IGF-1 and IGFBP-3 levels in our castrate mice.
Androgen deprivation therapy in humans has been shown to increase the risk of development of diabetes and perhaps cardiovascular disease, and stroke. This risk is coupled with increases in percent body fat, weight, and body mass index, as well as with a concurrent decrease in percentage of lean body mass [21–22]. These changes highlight how the effects of dietary modification are of increased importance in this patient population. Although some have suggested that the food constituents that increase risk for heart disease are different than those that confer PCa risk, the data in human epidemiologic studies are conflicting, likely as a result of the difficulty of attempting to objectively categorize diets [4–5].
Diet and exercise interventions in humans appear to have benefit with regards to slowing tumor cell growth and influencing the insulin/IGF-1 hormonal axis [1, 13, 15]. In PCa castrate xenograft models, restricting dietary fats appears to be beneficial, although the type of fat (saturated versus unsaturated) likely makes a difference [8, 29]. In our study we demonstrate that carbohydrate restriction likewise may have a benefit, though the effects were modest compared to the effects of castration. Interestingly, the best results were achieved for the likely most palatable diet in humans, with a 20% carbohydrate restriction that is similar to that targeted by the popular Atkins diet during the maintenance phase.
We acknowledge that these are preliminary results from a single xenograft model and require more general validation. However, we have previously shown that a NCKD slows tumor growth across different models [11, 12]. As such, we hypothesize that the lack of a dose-effect among low-carbohydrate diets and the overall benefit of low-carbohydrate diet are generalizable, though this requires formal testing in future studies. Based upon these data, we are currently conducting a phase II trial in humans examining if an Atkins diet can minimize the metabolic consequences of androgen deprivation and if positive, future studies will test whether this can impact cancer control [30].
Conclusions
Carbohydrate restriction provided a benefit to slowing PCa tumor growth compared to a western diet in castrate mice. Our data suggest that diets achievable in humans (e.g., 20% carbohydrate restriction) may play a role in PCa management. Human studies are underway.
Acknowledgments
Supported by the Department of Veterans Affairs; Division of Urology, Department of Surgery, Duke University; National Institutes of Health Grant NIH R01 CA131235.
References
- 1.Baade PD, Youlden DR, Krnjacki LJ. International epidemiology of prostate cancer: geographical distribution and secular trends. Mol Nutr Food Res. 2009;53(2):171–174. doi: 10.1002/mnfr.200700511. [DOI] [PubMed] [Google Scholar]
- 2.Gann PH, Hennekens CH, Sacks FM, Grodstein F, Giovannucci EL, Stampfer MJ. Prospective study of plasma fatty acids and risk of prostate cancer. J Natl Cancer Inst. 1994;86(4):281–286. doi: 10.1093/jnci/86.4.281. [DOI] [PubMed] [Google Scholar]
- 3.Kolonel LN, Nomura AM, Cooney RV. Dietary fat and prostate cancer: current status. J Natl Cancer Inst. 1999;91(5):414–428. doi: 10.1093/jnci/91.5.414. [DOI] [PubMed] [Google Scholar]
- 4.Walker M, Aronson KJ, King W, Wilson JW, Fan W, Heaton JP, MacNeily A, Nickel JC, Morales A. Dietary patterns and risk of prostate cancer in Ontario, Canada. Int J Cancer. 2005;116(4):592–598. doi: 10.1002/ijc.21112. [DOI] [PubMed] [Google Scholar]
- 5.Wu K, Hu FB, Willett WC, Giovannucci E. Dietary patterns and risk of prostate cancer in U.S men. Cancer Epidemiol Biomarkers Prev. 2006;15(1):167–171. doi: 10.1158/1055-9965.EPI-05-0100. [DOI] [PubMed] [Google Scholar]
- 6.Kobayashi N, Barnard RJ, Said J, Hong-Gonzalez J, Corman DM, Ku M, Doan NB, Gui D, Elashoff D, Cohen P, Aronson WJ. Effect of low-fat diet on development of prostate cancer and Akt phosphorylation in the Hi-Myc transgenic mouse model. Cancer Res. 2008;688(8):3066–3073. doi: 10.1158/0008-5472.CAN-07-5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ngo TH, Barnard RJ, Anton T, Tran C, Elashoff D, Heber D, Freedland SJ, Aronson WJ. Effect of isocaloric low-fat diet on prostate cancer xenograft progression to androgen independence. Cancer Res. 2004;64(4):1252–1254. doi: 10.1158/0008-5472.can-03-3830. [DOI] [PubMed] [Google Scholar]
- 8.Ngo TH, Barnard RJ, Cohen P, Freedland S, Tran C, deGregorio F, Elshimali YI, Heber D, Aronson WJ. Effect of isocaloric low-fat diet on human LAPC-4 prostate cancer xenografts in severe combined immunodeficient mice and the insulin-like growth factor axis. Clin Cancer Res. 2003;9(7):2734–2743. [PubMed] [Google Scholar]
- 9.Wang Y, Corr JG, Thaler HT, Tao Y, Fair WR, Heston WD. Decreased growth of established human prostate LNCaP tumors in nude mice fed a low-fat diet. J Natl Cancer Inst. 1995;87(19):1456–1462. doi: 10.1093/jnci/87.19.1456. [DOI] [PubMed] [Google Scholar]
- 10.Mavropoulos JC, Isaacs WB, Pizzo SV, Freedland SJ. Is there a role for a low-carbohydrate ketogenic diet in the management of prostate cancer? Urology. 2006;68(1):15–18. doi: 10.1016/j.urology.2006.03.073. [DOI] [PubMed] [Google Scholar]
- 11.Freedland SJ, Mavropoulos J, Wang A, Darshan M, Demark-Wahnefried W, Aronson WJ, Cohen P, Hwang D, Peterson B, Fields T, Pizzo SV, Isaacs WB. Carbohydrate restriction, prostate cancer growth, and the insulin-like growth factor axis. Prostate. 2008;68(1):11–19. doi: 10.1002/pros.20683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mavropoulos JC, Buschemeyer WC, 3rd, Tewari AK, Rokhfeld D, Pollak M, Zhao Y, Febbo PG, Cohen P, Hwang D, Devi G, Demark-Wahnefried W, Westman EC, Peterson BL, Pizzo SV, Freedland SJ. The effects of varying dietary carbohydrate and fat content on survival in a murine LNCaP prostate cancer xenograft model. Cancer Prev Res (Phila) 2009;2(6):557–565. doi: 10.1158/1940-6207.CAPR-08-0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barnard RJ, Ngo TH, Leung PS, Aronson WJ, Golding LA. Alow-fat diet and/or strenuous exercise alters the IGF axis in vivo and reduces prostate tumor cell growth in vitro. Prostate. 2003;56(3):201–206. doi: 10.1002/pros.10251. [DOI] [PubMed] [Google Scholar]
- 14.Dewell A, Weidner G, Sumner MD, Barnard RJ, Marlin RO, Daubenmier JJ, Chi C, Carroll PR, Ornish D. Relationship of dietary protein and soy isoflavones to serum IGF-1 and IGF binding proteins in the Prostate Cancer Lifestyle Trial. Nutr Cancer. 2007;58(1):35–42. doi: 10.1080/01635580701308034. [DOI] [PubMed] [Google Scholar]
- 15.Giovannucci E, Pollak M, Liu Y, Platz EA, Majeed N, Rimm EB, Willett WC. Nutritional predictors of insulin-like growth factor I and their relationships to cancer in men. Cancer Epidemiol Biomarkers Prev. 2003;12(2):84–89. [PubMed] [Google Scholar]
- 16.Gunnell D, Oliver SE, Peters TJ, Donovan JL, Persad R, Maynard M, Gillatt D, Pearce A, Hamdy FC, Neal DE, Holly JM. Are diet-prostate cancer associations mediated by the IGF axis? Across-sectional analysis of diet, IGF-I and IGFBP-3 in healthy middle-aged men. Br J Cancer. 2003;88(11):1682–1686. doi: 10.1038/sj.bjc.6600946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ngo TH, Barnard RJ, Tymchuk CN, Cohen P, Aronson WJ. Effect of diet and exercise on serum insulin, IGF-I, and IGFBP-1 levels and growth of LNCaP cells in vitro (United States) Cancer Causes Control. 2002;13(10):929–935. doi: 10.1023/a:1021911517010. [DOI] [PubMed] [Google Scholar]
- 18.Powolny AA, Wang S, Carlton PS, Hoot DR, Clinton SK. Interrelationships between dietary restriction, the IGF-I axis, and expression of vascular endothelial growth factor by prostate adenocarcinoma in rats. Mol Carcinog. 2008;47(6):458–465. doi: 10.1002/mc.20403. [DOI] [PubMed] [Google Scholar]
- 19.Venkateswaran V, Haddad AQ, Fleshner NE, Fan R, Sugar LM, Nam R, Klotz LH, Pollak M. Association of diet-induced hyperinsulinemia with accelerated growth of prostate cancer (LNCaP) xenografts. J Natl Cancer Inst. 2007;99(23):1793–1800. doi: 10.1093/jnci/djm231. [DOI] [PubMed] [Google Scholar]
- 20.Masko EM, Thomas JA, 2nd, Antonelli JA, Lloyd JC, Phillips TE, Poulton SH, Dewhirst MW, Pizzo SV, Freedland SJ. Low-carbohydrate diets and prostate cancer: how low is “low enough”? Cancer Prev Res (Phila) 2010;3(9):1124–1131. doi: 10.1158/1940-6207.CAPR-10-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haseen F, Murray LJ, Cardwell CR, O’Sullivan JM, Cantwell MM. The effect of androgen deprivation therapy on body composition in men with prostate cancer: systematic review and meta-analysis. J Cancer Surviv. 2010;4(2):128–139. doi: 10.1007/s11764-009-0114-1. [DOI] [PubMed] [Google Scholar]
- 22.Keating NL, O’Malley AJ, Freedland SJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy: observational study of veterans with prostate cancer. J Natl Cancer Inst. 2010;102(1):39–46. doi: 10.1093/jnci/djp404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Freedland SJ, Eastham J, Shore N. Androgen deprivation therapy and estrogen deficiency induced adverse effects in the treatment of prostate cancer. Prostate Cancer Prostatic Dis. 2009;12(4):333–338. doi: 10.1038/pcan.2009.35. [DOI] [PubMed] [Google Scholar]
- 24.Thomas JA, 2nd, Antonelli JA, Lloyd JC, Masko EM, Poulton SH, Phillips TE, Pollak M, Freedland SJ. Effect of intermittent fasting on prostate cancer tumor growth in a mouse model. Prostate Cancer Prostatic Dis. 2010;13(4):350–355. doi: 10.1038/pcan.2010.24. [DOI] [PubMed] [Google Scholar]
- 25.Albanes D, Weinstein SJ, Wright ME, Männistö S, Limburg PJ, Snyder K, Virtamo J. Serum insulin, glucose, indices of insulin resistance, and risk of prostate cancer. J Natl Cancer Inst. 2009;101(18):1272–1279. doi: 10.1093/jnci/djp260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chan JM, Stampfer MJ, Giovannucci E, Gann PH, Ma J, Wilkinson P, Hennekens CH, Pollak M. Plasma insulin-like growth factor-I and prostate cancer risk: a prospective study. Science. 1998;279(5350):563–566. doi: 10.1126/science.279.5350.563. [DOI] [PubMed] [Google Scholar]
- 27.Bidoli E, Talamini R, Bosetti C, Negri E, Maruzzi D, Montella M, Franceschi S, La Vecchia C. Macronutrients, fatty acids, cholesterol and prostate cancer risk. Ann Oncol. 2005;16(1):152–157. doi: 10.1093/annonc/mdi010. [DOI] [PubMed] [Google Scholar]
- 28.Meckling KA, O’Sullivan C, Saari D. Comparison of a low-fat diet to a low-carbohydrate diet on weight loss, body composition, and risk factors for diabetes and cardiovascular disease in free-living, overweight men and women. J Clin Endocrinol Metab. 2004;89(6):2717–2723. doi: 10.1210/jc.2003-031606. [DOI] [PubMed] [Google Scholar]
- 29.Lloyd JC, Antonelli JA, Phillips TE, Masko EM, Thomas JA, Poulton SH, Pollak M, Freedland SJ. Effect of isocaloric low fat diet on prostate cancer xenograft progression in a hormone deprivation model. J Urol. 2010;183(4):1619–1624. doi: 10.1016/j.juro.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. [accessed on: December 18, 2011]; http://www.clinicaltrials.gov/ct2/show/NCT00932672?term=prostate+cancer+AND+diet&state1=NA%3AUS%3ANC&rank=2.


