Abstract
Reading-impaired children have difficulty tapping to a beat. Here we tested whether this relationship between reading ability and synchronized tapping holds in typically-developing adolescents. We also hypothesized that tapping relates to two other abilities. First, since auditory-motor synchronization requires monitoring of the relationship between motor output and auditory input, we predicted that subjects better able to tap to the beat would perform better on attention tests. Second, since auditory-motor synchronization requires fine temporal precision within the auditory system for the extraction of a sound’s onset time, we predicted that subjects better able to tap to the beat would be less affected by backward masking, a measure of temporal precision within the auditory system. As predicted, tapping performance related to reading, attention, and backward masking. These results motivate future research investigating whether beat synchronization training can improve not only reading ability, but potentially executive function and basic auditory processing as well.
Keywords: reading, auditory perception, attention, rhythm
1. Introduction
Tapping to a beat is a seemingly simple task. In reality, though, it is a specialized, complex process that calls upon a wide-ranging network of auditory, motor, and prefrontal areas (Penhune, Zatorre, & Evans, 1998; Pollok, Gross, Muller, Aschersleben, & Schnitzler, 2005; Chen, Zatorre, & Penhune, 2006; Chen, Penhune, & Zatorre, 2008) and may be an ability limited to species capable of vocal learning (Patel, Iversen, Bregman, & Schulz, 2009). Although synchronized tapping requires fine motor control, it also places stringent demands on auditory processing, as listeners must accurately track the rhythm of the beat in order to reproduce it. This rhythmic tracking may rely on processes shared with language processing, as it has been proposed that temporal sampling of slow information within auditory signals is vital for syllable segmentation and, therefore, for the successful acquisition of reading skill (Goswami, 2011). Supporting this hypothesis, children and adults with reading disorders show greater variability when asked to tap along to a steady beat (Thomson, Fryer, Maltby, & Goswami, 2006; Thomson & Goswami, 2008; Corriveau & Goswami, 2009). This impairment may be related to their difficulty in tracking changes in the amplitude of the sound envelope, which is a cue to the onset time of speech sounds (Goswami et al., 2002; Muneaux, Ziegler, Truc, Thomson, & Goswami, 2004; Hamalainen, Leppanen, Torppa, Muller, & Lyytinen, 2005; Surányi et al., 2009; Goswami et al., 2010; Leong, Hamalainen, Soltzész, & Goswami, 2011).
If reading and rhythm tracking do share neural resources, one would expect tapping ability to relate to reading skill not only in reading-impaired populations, but in typically-developing subjects as well. We tested this hypothesis by measuring the ability of typically-developing adolescents to tap along to a metronomic beat. We hypothesized that tapping variability relates to reading ability.
It is known that auditory-motor synchronization relies heavily on the motor system (Penhune et al. 1998; Pollok et al., 2005; Chen et al., 2006; Chen et al., 2008), and that individual differences in tapping performance are linked to structural characteristics of motor areas such as white matter volume within frontal cortex (Ullén, Forsman, Blom, Karabanov, & Madison, 2008) and gray matter volume within the cerebellum (Steele, 2012), as well as brain activity within the basal ganglia and cerebellum (Steele & Penhune, 2010). However, the extent to which auditory-motor synchronization also relies upon the fidelity with which sound is represented in the auditory system is unknown. Synchronization to an auditory beat is more accurate than synchronization to a visual beat (Semjen & Ivry, 2001; Patel et al. 2005) and the basal ganglia are involved in synchronization to auditory but not visual stimuli (Witt et al. 2008); the fine temporal precision of the auditory system, therefore, may be vital for the production of accurate, consistent responses during auditory-motor synchronization. It is possible, therefore, that auditory-motor synchronization is affected by individual differences in the auditory system’s ability to extract the exact time of onset of a sound. If so, then less variable tapping performance should be linked to fine temporal precision within the auditory system. Thus, we predicted that tapping variability would also relate to backward masking thresholds, a measure of auditory temporal processing. To measure backward masking thresholds, a tone is presented, followed by a noise burst. The ability to detect soft tones despite the presence of the noise is an indication of fine temporal precision within the auditory system. Furthermore, backward masking thresholds may relate to speech processing, as it is thought that backward masking of consonants by the louder, longer subsequent vowel affects the perception of initial consonants in consonant-vowel syllables. We predicted that less variable tapping would be linked to easier detection of a target sound masked by a subsequent noise burst.
However, auditory-motor synchronization does not solely rely on accurate tracking of temporal rhythms by the auditory system and consistent motor responses. No matter how accurate the brain’s representation of the auditory rhythm and no matter how finely the motor system is able to control the output, slight discrepancies between the target rate and response rate will quickly lead to large asynchronies between tap and auditory stimulus. Successful tapping, therefore, also requires constant attending to the relationship between motor output and auditory input, as well as the appropriate adjustment of motor commands to bring the two in line. We predicted, therefore, that tapping variability would also be linked to attention, particularly sustained attention. To ensure that any relationships between tapping and perceptual and cognitive abilities found were not driven by general intelligence, we also gave participants an IQ test.
Backward masking thresholds (and, generally, temporal precision within the auditory system) have been linked to reading skill (McArthur & Hogben, 2001; Griffiths, Hill, Bailey, & Snowling, 2003; Montgomery, Morris, Sevcik, & Clarkson, 2005). Executive function and attention have also been linked to reading ability (Asbjornsen & Brynden, 1998; Booth, Boyle, & Steve, 2010; Foy & Mann, 2012). A relationship between tapping performance and measures of backward masking and attention would, therefore, provide a further basis for the link between tapping performance and reading ability, as it would suggest that auditory-motor synchronization calls upon a wide range of skills also known to be involved in reading.
2. Results
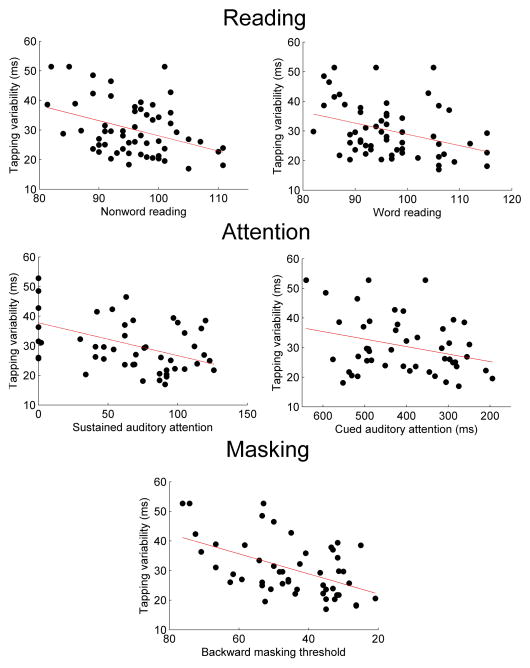
Pearson’s r-values for correlations between tapping performance and all behavioral measures are listed in Table 1. (p-values for all correlations are listed in tables S1, S2, S4, and S6 within Supplementary Information.) The composite tapping measure consisting of tapping variability in 2 Hz and 1.5 Hz paced conditions correlated with three of the four measures of reading: untimed nonword reading (Word Attack, r = −0.38, p = 0.0036), untimed word reading (Letter-Word ID, r = −0.35, p = 0.0067), and timed nonword reading (TOWRE Phonetic Decoding, r = −0.27, p = 0.038). Timed word reading showed only a weak trends towards being related to tapping performance (TOWRE Sight Reading, r = −0.18, p = 0.18). Composite paced tapping also related to backward masking threshold in both conditions (no-gap: r = 0.51, p = 0.00016, gap: r = 0.39, p = 0.0059). Composite paced tapping related to sustained attention in both the visual (−0.51, p = 0.00017) and auditory (r = −0.47, p = 0.00060) modalities but related to cued attention in only the auditory modality (r = 0.30, p = 0.038). For each significant relationship found, less variable tapping was linked to better performance. Scatterplots displaying selected relationships between tapping variability and attention, backward masking threshold, and reading ability are shown in Figure 1.
Table 1.
Pearson’s r-values for correlations between tapping variability and behavioral measures.
| Correlations with tapping variability (r-value) | Paced | Unpaced | ||||
|---|---|---|---|---|---|---|
| 2 Hz | 1.5 Hz | Compo | 2 Hz | 1.5 Hz | Compo | |
| WJIII Word Attack | −0.38 | −0.28 | −0.38 | −0.19 | −0.15 | −0.20 |
| WJIII Letter-Word ID | −0.35 | −0.27 | −0.35 | −0.00 | −0.10 | −0.08 |
| WJIII Basic Reading Composite | −0.38 | −0.3 | −0.39 | −0.08 | −0.14 | −0.15 |
| TOWRE Sight Word Efficiency | −0.20 | −0.12 | −0.18 | 0.22 | 0.00 | 0.1 |
| TOWRE Phonetic Decoding Efficiency | −0.39 | −0.14 | −0.27 | 0.03 | 0.04 | 0.02 |
| TOWRETotal Composite | −0.34 | −0.15 | −0.26 | 0.12 | 0.04 | 0.08 |
| Sustained Auditory Attention | −0.54 | −0.35 | −0.47 | −0.09 | −0.31 | −0.26 |
| Sustained Visual Attention | −0.49 | −0.45 | −0.51 | −0.25 | −0.37 | −0.39 |
| Auditory Cued Attention | 0.42 | 0.18 | 0.30 | −0.02 | 0.24 | 0.17 |
| Auditory Uncued Attention | 0.31 | 0.09 | 0.19 | −0.08 | 0.24 | 0.12 |
| Visual Cued Attention | 0.11 | 0.15 | 0.13 | −0.03 | 0.18 | 0.13 |
| Visual Uncued Attention | 0.07 | 0.04 | 0.08 | −0.09 | 0.24 | 0.17 |
| Backward masking, no gap | 0.44 | 0.46 | 0.51 | −0.06 | 0.18 | 0.07 |
| Backward masking, 50 ms gap | 0.42 | 0.32 | 0.39 | 0.08 | 0.23 | 0.21 |
| WASI performance IQ | −0.07 | 0.14 | 0.06 | −0.10 | 0.04 | −0.05 |
| WASI verballQ | −0.02 | 0.00 | −0.02 | −0.06 | 0.04 | −0.01 |
| WASI TotallQ | −0.16 | −0.17 | −0.19 | −0.08 | −0.02 | −0.07 |
Bolded entries are significant at p < 0.05. All significant relationships were in the direction of less variability in tapping correlating with better behavioral performance.
Figure 1.
The variability of subjects’ tapping to a beat (composite paced condition) correlates with performance on tests of reading (Woodcock-Johnson Test of Achievement), attention, and the ability to detect a stimulus in the presence of a masking sound (no gap condition). Each x-axis is arranged such that better performance is to the right.
There was no relationship between tapping performance and uncued attention in either modality. No tapping measure related to two-scale WASI IQ, confirming that relationships between tapping performance and linguistic, cognitive, and perceptual skills were not driven by differences in general intelligence. The composite measure for the unpaced condition related only to performance on the test of sustained visual attention; thus, it is specifically the ability to synchronize to a concurrently presented beat, rather than simply motor coordination, or the ability to imagine a beat, that relates to reading, attention, and auditory temporal processing.
Performance on attention and backward masking tasks was related to reading ability (Table 2). Untimed nonword reading was correlated with sustained attention in both the auditory (r = 0.28, p = 0.046) and visual (r = 0.35, p = 0.013) modalities. Timed reading also correlated with auditory (r = 0.33, p = 0.019) and visual (r = 0.38, p = 0.0069) sustained attention. However, cued and uncued attention tests from the IMAP battery did not significantly correlate with any reading measure. Backward masking threshold in the no-gap condition correlated with untimed nonword reading (r = −0.43, p = 0.0019), untimed word reading (r = −0.39, p = 0.0048), and timed reading (r = −0.46, p = 0.00082), but the less perceptually demanding 50-ms-gap condition was not significantly correlated with any reading measure.
Table 2.
Pearson’s r-values for correlations between reading ability and measures of attention and backward masking thresholds.
| Correlations with reading (r-value) | WJIIWA | WJIII LWID | WJIII BR | TOWRE S | TOWRE P | TOWRET |
|---|---|---|---|---|---|---|
| Sustained Auditory Attention | 0.28 | 0.09 | 0.20 | 0.31 | 0.29 | 0.33 |
| Sustained Visual Attention | 0.35 | 0.20 | 0.30 | 0.41 | 0.27 | 0.38 |
| Auditory Cued Attention | −0.15 | −0.05 | −0.11 | −0.17 | −0.17 | −0.19 |
| Auditory Uncued Attention | −0.26 | −0.10 | −0.19 | −0.23 | −0.22 | −0.25 |
| Visual Cued Attention | −0.00 | 0.09 | 0.04 | −0.10 | 0.02 | −0.06 |
| Visual Uncued Attention | −0.18 | 0.03 | −0.07 | −0.20 | −0.15 | −0.20 |
| Backward masking, no gap | −0.43 | −0.39 | −0.44 | −0.32 | −0.48 | −0.46 |
| Backward masking, 50 ms gap | −0.14 | −0.11 | −0.14 | 0.06 | −0.18 | −0.055 |
| WASI performance IQ | 0.24 | 0.45 | 0.39 | 0.12 | 0.15 | 0.15 |
| WASI verballQ | 0.11 | 0.54 | 0.38 | 0.41 | 0.14 | 0.32 |
| WASI TotallQ | 0.33 | 0.64 | 0.53 | 0.38 | 0.25 | 0.37 |
Bolded entries are significant at p < 0.05. Bolded entries are significant at p < 0.05. All significant relationships were in the direction of less variability in tapping correlating with better behavioral performance.
To determine whether the relationships between tapping and backward masking and between tapping and reading ability were entirely driven by an influence of attention on all three abilities, these relationships were re-assessed via partial correlations controlling for variance in sustained auditory attention. This procedure preserved the relationships between tapping and the WJIII composite untimed reading score (r = −0.30, p = 0.041) and between tapping and backward masking threshold in the no-gap condition (r = 0.34, p = 0.019), but rendered insignificant relationships between tapping and the TOWRE composite reading score (r = −0.10, p = 0.49) and between tapping and backward masking threshold in the 50-ms gap condition (r = 0.25, p = 0.093). Similarly, after partialling out variance in sustained auditory attention, the relationship between backward masking threshold in the no-gap condition and both reading measures remained significant (WJIII, r = −0.38, p = 0.0089; TOWRE, r = −0.38, p = 0.0077), but the relationship between backward masking threshold in the 50-ms gap condition and both reading measures did not reach significance (WJIII, r = −0.06, p = 0.71; TOWRE, r = 0.08, p = 0.62).
3. Discussion
We asked adolescent subjects to synchronize to a metronomic beat. We found that variability in tapping to a beat correlated with performance on tests of reading, attention, and auditory temporal precision. Their ability to tap to a remembered beat, however, did not correlate with these measures. Moreover, IQ did not correlate with tapping variability. These relationships between tapping variability and reading, attention, and perception, therefore, reflect not general intelligence or purely motor skills but the variety of perceptual and cognitive processes on which auditory-motor synchronization draws.
The finding that auditory-motor synchronization ability correlates with reading skill in a normal-developing population lends support to the idea that reading and the perception of rhythm rely on shared processes. Synchronized tapping may rely heavily on rhythmic tracking within the auditory system, such that successful fine temporal representation of rhythmic patterns is a necessary precursor for reproduction of and synchronization to these patterns. Supporting this idea is the finding that auditory-motor synchronization ability is linked to the ability to perceive the rate of increase in amplitude marking the onsets of sounds, or “rise time” (Thomson et al. 2006). Reading ability has also been linked to the perception of more complex, musical rhythmic sequences (Anvari, Trainor, Woodside, & Levy, 2002; Dellatolas, Watier, Le Normand, Lubart, & Chevrie-Muller, 2009; Huss, Verney, Fosker, Mead, & Goswami, 2010; Moritz, Yampolsky, Papadelis, Thomson, & Wolf, 2012), the perception of rise time (Goswami et al., 2002; Muneaux et al., 2004; Hamalainen et al., 2005; Surányi et al., 2009; Goswami et al., 2010; Leong et al., 2011), and neural tracking of the amplitude “envelope” of speech (Abrams, Nicol, Zecker, & Kraus, 2009). The ability to represent slow temporal information may, therefore, underlie both reading ability (Goswami, 2011) and auditory-motor synchronization. For both the timed and untimed reading measures, tapping performance at the 2 Hz rate more strongly related to reading ability than tapping performance at the 1.5 Hz rate; in fact, the relationship between tapping at 1.5 Hz and timed reading did not reach significance. Tapping at 2 Hz may relate more strongly to reading performance because it is closer to the average rate of production of stressed syllables, which falls around 2 Hz (Goswami 2011).
The relationship between backward masking threshold and synchronized tapping abilities suggests that fine temporal precision within the auditory system may be necessary for rhythm tracking. The speech sound segregation necessary for the development of phonological awareness may also rely on temporal precision, as both specific language impairment (Wright et al., 1997; McArthur & Hogben, 2001; Marler & Champlin, 2005) and dyslexia (Griffiths et al., 2003; Montgomery et al., 2005) have been linked to elevated backward masking thresholds. Similarly, we found that elevated backward masking thresholds were linked to poorer reading ability. Given that the relationship between backward masking thresholds and tapping ability was stronger than the relationship between tapping ability and reading, a common factor contributing to the relationship between tapping performance and reading ability may be their shared reliance on accurate neural timing mechanisms for processing auditory input. The relationship between tapping ability and sustained attention was found in both auditory and visual modalities. Researchers have previously shown that tapping to beat is more variable in children with ADHD whether presented in the visual modality also (Rubia, Noorloos, Smith, Gunning, & Sergeant, 2003) or in the auditory and visual modalities simultaneously (Ben-Pazi, Shaley, Gross-Tsur, & Bergman, 2006), and that variability when tapping to a visual beat is related to attention in a normal-developing population (Birkett and Talcott 2012). Our results show that, in a normally developing population, synchronized tapping relates to sustained attention regardless of the domain in which stimuli are presented. Synchronized tapping may, therefore, draw upon domain-general executive process, perhaps because successful tapping requires the performer to constantly revise his or her actions to minimize the discrepancy between motor output and auditory input. Sustained attention in both modalities also correlated with reading ability, replicating previous findings linking sustained attention to reading ability van der Sluis et al. (2007) or, more generally, relating executive function to reading ability (Asbjornsen & Brynden, 1998, Booth et al., 2010; Foy & Mann, 2012). Given that the relationship between sustained attention and tapping ability was stronger than the relationship between tapping ability and reading, a third potential factor underlying the link between synchronized tapping and reading is a shared reliance on executive function and attention. Sustained attention also related to tapping ability in the unpaced condition, suggesting that the relationship between sustained attention and tapping ability is not driven by error correction, but instead by variation in the ability to sustain a consistent tapping tempo over time. This relationship was found only for the 1.5 Hz condition, perhaps because sustaining a constant tapping rate is more difficult at slower tempos.
A relationship between tapping ability and short-term cued attention was found for the auditory but not visual modality. This may reflect individual differences in the extent to which knowledge about the time of onset of a future event facilitates processing within the auditory system. In humans the presence of temporal regularity in auditory stimuli has been shown to result in enhanced late cortical responses to sound (i.e. N2, P3) and diminished early cortical responses to sound (i.e. P1, N1) (Lange, 2009; Rimmele, Jolsvai, & Sussman, 2011), while in nonhuman animals it has been shown that stimuli presented at expected times result in increased firing rates in a number of cortical areas, including auditory cortex (Jaramillo & Zador, 2011). Some subjects may be better able to take advantage of an expectation about the onset of an incoming sound, potentially increasing temporal resolution and facilitating rhythm tracking. The domain-specificity of this relationship between tapping ability and cued attention lends support to the idea that time estimation calls upon different processes in the visual and auditory domains, an idea also supported by the fact that synchronization to an auditory beat is more accurate than synchronization to a visual beat (Semjen & Ivry, 2001; Patel et al. 2005). The relationship between cued auditory attention and tapping ability was specific to the 2 Hz tapping condition; a similar relationship was found for the 1.5 Hz condition but did not reach significance. This specificity could indicate that subjects are able to benefit more from temporal expectation at the 0.5 seconds inter-stimulus interval; a 0.667 second delay may be long enough to render temporal expectation less accurate and less useful.
We find that cued attention (in either modality) is not related to reading performance. The lack of this relationship would seem to contradict the findings of Facoetti et al. (2005), who argue that children with dyslexia have slower responses to both visual and auditory cues. Specifically, they found that, when performing a visual detection task, children with dyslexia were unable to benefit from cues that preceded the target by 100 ms, but normal-developing children were able to benefit from these cues. Cues preceding the target by 250 ms, on the other hand, led to enhanced performance in both subject groups. In the IMAP cued attention tasks used in the current paper, the cue preceded the target by between 500 and 1000 ms. This time window exceeds by a substantial amount the threshold necessary for attentional shifting even in children with dyslexia. Our findings are, therefore, consistent with Facoetti’s suggestion that dyslexia is linked to a slowing of attentional shifting, as subjects were given so much time to focus their attention that rapid attentional shifting was not called for.
Given that attention relates to backward masking, tapping performance, and reading, it is possible that the relationship between tapping and these behavioral measures is driven to some degree by a shared influence of attention ability. To test this, we re-examined relationships between tapping, reading, and backward masking threshold while partialling out auditory attention ability. The relationship between tapping ability and untimed reading remained significant, while the relationship between tapping ability and timed reading did not reach significance. This suggests that a shared reliance on attentional resources may be driving the relationship between timing reading and tapping ability, but that attention does not entirely account for the overlap in neural resources between tapping and untimed reading. The relationship between tapping ability and backward masking threshold in the easier no-gap condition remained significant, while the relationship between tapping ability and backward masking threshold in the 50-ms gap condition did not reach significance. Thus, a shared reliance on attention is partially, but not wholly, responsible for the relationship between tapping and temporal precision as well.
We used as our measure of tapping performance the standard deviation of inter-tap times, following previous work examining relationships between tapping behavior and reading (Thomson, Fryer, Maltby, & Goswami, 2006; Thomson & Goswami, 2008; Corriveau & Goswami, 2009). Thus, we were able to directly compare our results to those of this previous work, replicating in a normal-developing population a relationship between tapping and reading that had previously only been shown when comparing language-impaired and normal-developing children. However, one disadvantage of using this measure is that beat synchronization is a complex task, containing several component processes that are not separated by the use of a simple tapping task and a variability measure. Successfully tapping to a beat involves extraction of the sequence of inter-stimulus times, internal representation of the beat, motor implementation of the beat, and error correction; it is unclear which of these components are contributing to the variability measure and, therefore, the relationships between tapping and attention, temporal precision, and reading.
The fact that tapping variability in the paced condition relates to reading and backward masking while tapping variability in the unpaced condition does not suggests that differences in error correction may account for some variation in reading ability and temporal precision. To test this hypothesis, future work could examine the relationships between reading ability and error correction during beat synchronization by introducing minor perturbations into isochronous signals and examining subjects’ ability to recover from these perturbations. These perturbations could take the form of either tempo changes, requiring correction of the period of the subject’s tapping, or phase changes, requiring correction of the phase of the subject’s tapping. Dissociating individual differences in these two different kinds of error correction could provide further information about the exact source of the overlap between tapping and reading ability, as phase correction and period correction have been shown to engage different neural circuits (Repp 2005, Schwartze et al. 2011), and period correction, but not phase correction, calls upon attentional resources (Repp and Keller 2004).
We performed a large number of bivariate correlations which, if accounted for via a strict Bonferroni correction, would render many of our findings insignificant. However, the number of significant relationships which we find is much greater than would be expected according to chance. Table 1, for example, contains 102 correlations. At p = 0.05, one would expect around 5 relationships purely by chance, only have of which (2–3) would be in the direction that ascribes less variable tapping to better performance. Instead we found more than ten times that number, 31. It is extremely unlikely that this pattern is due simply to chance; for example, if each of 31 relationships was randomly selected to be either positive or negative, the chance that all 31 would be in the same direction is less than 1×10−8.
The link between auditory-motor synchronization and reading in a normal population reported here motivates future work on the impact of rhythmic training on reading skill. Musical training with a strong emphasis on rhythmic abilities and metronome practice, for example, may facilitate the acquisition of reading skill. Moreover, given that synchronized tapping also relates to measures of basic auditory processing and executive function, the potential benefits of rhythmic training may be even greater than has been previously supposed. Beat synchronization training could potentially lead to increases not only in reading skill, but in basic auditory function, sustained attention, and cognitive flexibility.
4. Methods
4.1 Participants
58 subjects, 31 female, were recruited for this study. All subjects were students in high schools within the Chicago metropolitan area ranging in age from 14.2 to 17.4 years (mean 15.2, standard deviation 0.754). Subjects had air-conduction pure-tone hearing thresholds <= 20 dB SPL from 125 to 8000 Hz, normal auditory brainstem responses to 80 dB SPL 100 μs click stimuli presented at 31.1 Hz, two-scale IQ scores of above 85 on the Wechsler Abbreviated Scale of Intelligence, and no history of learning impairment or neurological disorder.
4. 2 Behavioral testing
4.2.1 Synchronized tapping
To assess auditory-motor synchronization ability participants were tested on a tapping test developed in-house that was modeled after Thomson et al. (2006). A snare drum sound was isochronously presented over speakers to the subjects, who were asked to tap along to the beat on a NanoPad2 tapping pad (Korg, Tokyo, Japan). Two different conditions were presented: “paced” and “unpaced”. Each condition began with 20 practice trials during which data was not collected to give the subject ample time to begin synchronizing to the beat. During the “paced” condition this practice session was immediately followed, with no break, by 20 more sound presentations, during which time the subject’s taps were recorded. This condition measured the subject’s ability to synchronize movement to an auditory beat. During the “unpaced” condition, the practice session was followed by a period of silence equivalent to 20 stimulus presentations, during which time the subject was asked to continue tapping as if the sound were still present. This condition measured the subject’s ability to produce a steady beat at a particular rate without needing to synchronize to an auditory stimulus; thus, this condition primarily indexes motor coordination ability. Each condition was run three times, with stimuli presented at inter-onset intervals (IOIs) of 667 ms and 500 ms (1.5 and 2 Hz, respectively). To assess synchronized tapping ability, the variability of tapping performance for each condition and rate was measured by calculating the standard deviation of inter-tap intervals. For both the paced and unpaced conditions we calculated a composite score by averaging performance at the 667 ms and 500 ms IOIs.
4.2.2 Reading
Reading ability was assessed using the Test of Word Reading Efficiency (TOWRE) (Torgeson, Wagner, & Rashotte, 1999), which consists of Sight Reading Efficiency (word reading) and Phonetic Decoding Efficiency (nonword reading) subtests which are combined to form a Total Composite score. Reading ability was also assessed with the Word Attack and Letter-Word ID subtests of the Woodcock Johnson Tests of Achievement (Woodcock, McGre, & Mather, 2001), which were combined to form a Basic Reading composite score.. The TOWRE is a timed test that asks subjects to read aloud lists of words and nonwords. The Word Attack and Letter-Word ID subtests ask subjects to read aloud lists of nonwords and words, respectively. Neither subtest is timed. Scores on both tests are age-normed.
4.2.3 Attention
Attention was assessed using the Integrated Visual and Auditory Continuous Performance Test (IVA) (Sandford & Turner, 2000) and the Institute for Hearing Research Multicentre Battery of Auditory Processing (IMAP) (Barry, Ferguson, & Moore, 2010). The IVA is a test of sustained attention lasting over twenty minutes. Subjects watch a monitor and listen over headphones, and are asked to press a mouse button whenever they either hear or see a “1”. Subjects are asked not to respond after hearing or seeing the number “2”. At some points during the test target stimuli are more common than distractors, while at other points distractors are more common than target stimuli. The test generates composite scores for visual and auditory sustained attention separately, based on the subject’s reaction times and error rates. Higher scores are indicative of better attention.
The IMAP is a battery containing a variety of tests, including tests of cued auditory and visual attention. For the cued auditory attention test, subjects are presented with stimuli via headphones connected to a laptop computer and are instructed to press a button whenever they hear a target sound (a pure tone presented at 80 dB SPL). Some of the target sound presentations are preceded by a “siren” sound presented at 70 dB SPL. This cue always precedes the target sound by 0.5 to 1 seconds and, thus, informs the participant about the approximate time when the target sound will be presented. For the test of visual attention, the subject is asked to watch a cartoon character on the laptop screen and to press a button whenever they see the character raise his arms. Some of the target presentations are preceded by the presentation of a visual cue: the character’s shirt changes color. This cue always precedes the target by 0.5 to 1 seconds. These tests generate separate reaction time scores for the trials preceded by the cue and those trials not preceded by the cue. Both attention tests are preceded by shortened five-trial practice tests identical to the actual test, except that the subject is given feedback whenever a response is not produced within a short window or a response is produced to the cue rather than to the target. Subjects are given the opportunity to repeat the practice session if they answer more than two-fifths of the trials incorrectly. Thus, the IVA is a measure of sustained attention over a 20-minute period, while the IMAP tests attention over only a few minutes.
4.2.4 Backward masking
Temporal processing was assessed using two subtests from the IMAP testing battery: backward masking and backward masking with a 50-ms gap. These tests were conducted using a laptop computer connected to a button-box with three large colored buttons. Three cartoon characters were displayed on the screen. During each trial, each cartoon character, one at a time, opened its mouth; this display was accompanied by the presentation of a sound. All three sounds were noise bursts (bandpass noise with a center frequency of 1000 Hz, a width of 800 Hz, a duration of 300 ms, and a fixed spectrum level of 30 dB). One of the three sounds also contained a target stimulus (a pure tone with a frequency of 1000 Hz and duration of 20 ms). In the no-gap condition, the noise burst began as soon as the pure tone ceased. In the 50-ms gap condition, the noise burst began 50 ms after the pure tone ceased; in this condition the noise burst should interfere less with detection of the target tone and the task should, therefore, be easier. The subject was told that one of the sounds would be different from the other two, and was asked to press the button corresponding to the cartoon character that made the different sound. The signal-to-noise threshold at which the subject was able to detect the target tone was determined via a one-up, two down adaptive staircase procedure (i.e., the intensity of the target tone was lowered if the subject answered correctly twice in a row, and raised if the subject answered incorrectly once, while the intensity of the masking noise was unaltered). Lower thresholds indicate less masking of the tone by the noise burst and better temporal processing. The backward masking tests were preceded by a practice test in which the target tone was always presented at 90 dB SPL. Four out of five correct responses were required before the subject was allowed to advance past the practice test.
4.2.5 IQ
IQ was measured using the Matrix Reasoning and Vocabulary subsets of the Wechsler Abbreviated Scale of Intelligence (Woerner & Overstreet, 1999). Scores on these subtests were combined to form a two-scale measure of general intelligence.
4.3 Analysis
Relationships between between the reading, backward masking, and attention measures and tapping variability in both paced and unpaced conditions using the 2 Hz, 1.5 Hz, and composite tapping measures were assessed using Pearson’s correlations. Furthermore, to confirm the relationship between attention and backward masking abilities and reading ability, Pearson’s correlations were run between the attention and backward masking measures and the reading measures. Prior to analysis, outliers for each variable were brought to within 2 standard deviations of the mean.
Supplementary Material
Subjects were asked to tap along to a metronomic beat.
Subjects whose taps were less variable performed better on tests of reading and attention.
Tap variability was also inversely related to temporal precision within the auditory system.
These results motivate research on cognitive and perceptual effects of synchronization training.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrams D, Nicol T, Zecker S, Kraus N. Abnormal cortical processing of the syllable rate of speech in poor readers. Journal of Neuroscience. 2009;29:7686–7693. doi: 10.1523/JNEUROSCI.5242-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anvari S, Trainor L, Woodside J, Levy B. Relations among musical skills, phonological processing, and early reading ability in preschool children. J Experimental Child Psychology. 2002;83:111–130. doi: 10.1016/s0022-0965(02)00124-8. [DOI] [PubMed] [Google Scholar]
- Asbjørnsen A, Brynden M. Auditory attentional shifts in reading-disabled students: quantification of attentional effectiveness by the Attentional Shift Index. Neuropsychologia. 1998;36:143–148. doi: 10.1016/s0028-3932(97)00090-0. [DOI] [PubMed] [Google Scholar]
- Barry J, Ferguson M, Moore D. Making sense of listening: the IMAP test battery. J Vis Esp. 2010:44. doi: 10.3791/2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Pazi H, Shalev R, Gross-Tsur V, Bergman H. Age and medication effects on rhythmic responses in ADHD: Possible oscillatory mechanisms? Neuropsychologia. 2006;44:412–416. doi: 10.1016/j.neuropsychologia.2005.05.022. [DOI] [PubMed] [Google Scholar]
- Birkett E, Talcott J. Interval timing in children: effects of auditory and visual pacing stimuli and relationships with reading and attention variables. PLoS ONE. 2012;7:e42820. doi: 10.1371/journal.pone.0042820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth J, Boyle J, Steve K. Do tasks make a difference? Accounting for heterogeneity of performance of children with reading difficulties on tasks of executive function: findings from a meta-analysis. British Journal of Developmental Psychology. 2010;28:133–176. doi: 10.1348/026151009x485432. [DOI] [PubMed] [Google Scholar]
- Chen J, Zatorre R, Penhune V. Interactions between auditory and dorsal premotor cortex during synchronization to musical rhythms. NeuroImage. 2006;32:1771–1781. doi: 10.1016/j.neuroimage.2006.04.207. [DOI] [PubMed] [Google Scholar]
- Chen J, Penhune V, Zatorre R. Moving on time: brain network for auditory-motor synchronization is modulated by rhythm complexity and musical training. Journal of Cognitive Neuroscience. 2008;20:226–239. doi: 10.1162/jocn.2008.20018. [DOI] [PubMed] [Google Scholar]
- Corriveau K, Goswami U. Rhythmic motor entrainment in children with speech and language impairments: tapping to the beat. Cortex. 2009;45:119–130. doi: 10.1016/j.cortex.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Dellatolas G, Watier L, Le Normand M, Lubart T, Chevrie-Muller C. Rhythm reproduction in kindergarten, reading performance at second grade, and developmental dyslexia theories. Archives of Clinical Neuropsychology. 2009;24:555–563. doi: 10.1093/arclin/acp044. [DOI] [PubMed] [Google Scholar]
- Facoetti A, Lorusso M, Cattaneo C, Galli R, Molteni M. Visual and auditory attentional capture are both sluggish in children with developmental dyslexia. Acta Neurobiologiae Experimentalis. 2005;65:61–72. doi: 10.55782/ane-2005-1540. [DOI] [PubMed] [Google Scholar]
- Foy J, Mann V. Executive function and early reading skills. Reading and Writing. 2012 doi: 10.1007/s11145–012–9376–5. [DOI] [Google Scholar]
- Goswami U, Thomson J, Richardson U, Stainthorp R, Hughes D, Rosen S, Scott S. Amplitude envelope onsets and developmental dyslexia: a new hypothesis. PNAS. 2002;99:10911–10916. doi: 10.1073/pnas.122368599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goswami U, Wang H, Cruz A, Fosker T, Mead N, Huss M. Language-universal sensory deficits in developmental dyslexia: english, spanish, and chinese. Journal of Cognitive Neuroscience. 2010;23:325–337. doi: 10.1162/jocn.2010.21453. [DOI] [PubMed] [Google Scholar]
- Goswami U. A temporal sampling framework for developmental dyslexia. Trends in Cognitive Sciences. 2011;15:3–10. doi: 10.1016/j.tics.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Griffiths Y, Hill N, Bailey P, Snowling M. Auditory temporal order discrimination and backward recognition masking in adults with dyslexia. Journal of Speech, Language, and Hearing Research. 2003;46:1352–1366. doi: 10.1044/1092-4388(2003/105). [DOI] [PubMed] [Google Scholar]
- Hamalainen J, Leppanen P, Torppa M, Muller K, Lyytinen H. Detection of sound rise time by adults with dyslexia. Brain and Language. 2005;94:32–42. doi: 10.1016/j.bandl.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Huss M, Verney J, Fosker T, Mead N, Goswami U. Music, rhythm, rise time perception and developmental dyslexia: perception of musical meter predicts reading and phonology. Cortex. 2011;47:674–689. doi: 10.1016/j.cortex.2010.07.010. [DOI] [PubMed] [Google Scholar]
- Jaramillo S, Zador A. The auditory cortex mediates the perceptual effects of acoustic temporal expectation. Nature Neuroscience. 2011;14:246–251. doi: 10.1038/nn.2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange K. Brain correlates of early auditory processing are attenuated by expectations for time and pitch. Brain and Cognition. 2009;69:127–137. doi: 10.1016/j.bandc.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Marler J, Champlin C. Sensory processing of backward-masking signals in children with language-learning impairment as assessed with the auditory brainstem response. Journal of Speech, Language, and Hearing Research. 2005;48:183–203. doi: 10.1044/1092-4388(2005/014). [DOI] [PubMed] [Google Scholar]
- McArthur G, Hogben J. Auditory backward recognition masking in children with a specific language impairment and children with a specific reading disability. JASA. 2001;109:1092–1100. doi: 10.1121/1.1338559. [DOI] [PubMed] [Google Scholar]
- Montgomery C, Morris R, Sevcik R, Clarkson M. Auditory backward masking deficits in children with reading disabilities. Brain and Language. 2005;95:450–456. doi: 10.1016/j.bandl.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Moritz C, Yampolsky S, Papadelis G, Thomson J, Wolf M. Links between early rhythm skills, musical training, and phonological awareness. Reading and Writing. 2012 doi: 10.1007/s11145–012–9389–0. [DOI] [Google Scholar]
- Muneaux M, Ziegler J, Truc C, Thomson J, Goswami U. Deficits in beat perception and dyslexia: evidence from French. NeuroReport. 2004;15:1–5. doi: 10.1097/01.wnr.0000127459.31232.c4. [DOI] [PubMed] [Google Scholar]
- Patel A, Iversen J, Chen Y. The influence of metricality and modality on synchronization with a beat. Exp Brain Res. 2005;163:226–238. doi: 10.1007/s00221-004-2159-8. [DOI] [PubMed] [Google Scholar]
- Schwartze M, Keller P, Patel A, Kotz S. The impact of basal ganglia lesions on sensorimotor synchronization, spontaneous motor tempo, and the detection of tempo changes. Behavioral Brain Research. 2011;216:685–691. doi: 10.1016/j.bbr.2010.09.015. [DOI] [PubMed] [Google Scholar]
- Patel A, Iversen J, Bregman M, Schulz I. Experimental evidence for synchronization to a musical beat in a nonhuman animal. Current Biology. 2009;19:1–4. doi: 10.1016/j.cub.2009.03.038. [DOI] [PubMed] [Google Scholar]
- Penhune V, Zatorre R, Evans A. Cerebellar contributions to motor timing: a PET study of auditory and visual rhythm reproduction. Journal of Cognitive Neuroscience. 1998;10:752–765. doi: 10.1162/089892998563149. [DOI] [PubMed] [Google Scholar]
- Pollok B, Gross J, Muller K, Aschersleben G, Schnitzler A. The cerebral oscillatory network associated with auditorily paced finger movements. NeuroImage. 2005;24:646–655. doi: 10.1016/j.neuroimage.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Repp B. Sensorimotor synchronization: A review of the tapping literature. Psychonomic Bulletin and Review. 2005;12:969–922. doi: 10.3758/bf03206433. [DOI] [PubMed] [Google Scholar]
- Repp B, Keller P. Adaptation to tempo changes in sensorimotor synchronization: Effects of intention, attention, and awareness. The Quarterly Journal of Experimental Psychology. 2004;57:499–521. doi: 10.1080/02724980343000369. [DOI] [PubMed] [Google Scholar]
- Rimmele J, Jolsvai H, Sussman E. Auditory target detection is affected by implicit temporal and spatial expectations. Journal of Cognitive Neuroscience. 2011;23:1136–1147. doi: 10.1162/jocn.2010.21437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K, Noorloos J, Smith A, Gunning B, Sergeant J. Motor timing deficits in community and clinical boys with hyperactive behavior: the effect of methylphenidate on motor timing. Journal of Abnormal Child Psychology. 2003;31:301–313. doi: 10.1023/a:1023233630774. [DOI] [PubMed] [Google Scholar]
- Sandford J, Turner A. Integrated visual and auditory continuous performance test manual. Richmond, VA: Brain Train; 2000. [Google Scholar]
- Semjen A, Ivry R. The coupled oscillator model of between-hand coordination in alternate-hand tapping: a reappraisal. Journal of Experimental Psychology: Human Perception and Performance. 2001;27:251–265. doi: 10.1037//0096-1523.27.2.251. [DOI] [PubMed] [Google Scholar]
- Steele C, Penhune V. Specific increases within global decreases: a functional magnetic resonance imaging investigation of five days of motor sequence learning. The Journal of Neuroscience. 2010;30:8332–8341. doi: 10.1523/JNEUROSCI.5569-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele C. Dissertation. Concordia University; Monreal, Quebec, Canada: 2012. The relationship between brain structure, motor performance, and early musical training. [Google Scholar]
- Surányi Z, Csépe V, Richardson U, Thomson J, Honbolygó F, Goswami U. Sensitivity to rhythmic parameters in dyslexic children: a comparison of Hungarian and English. Reading and Writing. 2009;22:41–56. [Google Scholar]
- Thomson J, Fryer B, Maltby J, Goswami U. Auditory and motor rhythm awareness in adults with dyslexia. Journal of Research in Reading. 2006;29:334–348. [Google Scholar]
- Thomson J, Goswami U. Rhythmic processing in children with developmental dyslexia: auditory and motor rhythms link to reading and spelling. Journal of Physiology. 2008;102:120–129. doi: 10.1016/j.jphysparis.2008.03.007. [DOI] [PubMed] [Google Scholar]
- Torgeson J, Wagner R, Rashotte C. Test of Word Reading Efficiency. Austin, TX: Pro-Ed; 1999. [Google Scholar]
- Ullén F, Forsman L, Blom O, Karabanov A, Madison G. Intelligence and variability in a simple timing task share neural substrates in the prefrontal white matter. The Journal of Neuroscience. 2008;28:4238–4243. doi: 10.1523/JNEUROSCI.0825-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Sluis S, de Jong P, van der Leij A. Executive functioning in children, and its relations with reasoning, reading, and arithmetic. Intelligence. 2007;35:427–449. [Google Scholar]
- Witt S, Meyerand M, Laird A. Functional neuroimaging correlates of finger-tapping task variations: An ALE meta-analysis. Neuroimage. 2008;42:343–356. doi: 10.1016/j.neuroimage.2008.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woerner C, Overstreet K. Wechsler Abbreviated Scale of Intelligence (WASI) San Antonio, TX: The Psychological Corporation; 1999. [Google Scholar]
- Woodcock R, McGre K, Mather N. Woodcock-Johnson Psycho-Educational Battery. 3. Itasca, IL: Riverside; 2001. [Google Scholar]
- Wright B, Lombardino L, King W, Puranik C, Leonard C, Merzenich M. Deficits in auditory temporal and spectral resolution in language-impaired children. Nature. 1997;387:176–178. doi: 10.1038/387176a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.