Abstract
New treatments for adults with acute lymphoblastic T-cell leukemia (T-ALL) are urgently needed, as the current rate of overall remission in these patients is only about 40 percent. We recently showed the potential therapeutic benefit of the pegylated-human-arginase I (peg-Arg I) in T-ALL. However, the mechanisms by which peg-Arg I induces an anti-T-ALL effect remained unknown. Our results show the induction of T-ALL cell apoptosis by peg-Arg I, which associated with a global arrest in protein synthesis and with the phosphorylation of the eukaryotic-translation-initiation factor 2 alpha (eIF2α). Inhibition of eIF2α phosphorylation in T-ALL cells prevented the apoptosis induced by peg-Arg I, whereas the expression of a phosphomimetic eIF2α form increased the sensibility of T-ALL cells to peg-Arg I. Phosphorylation of eIF2α by peg-Arg I was mediated through kinases PERK and GCN2 and down-regulation of phosphatase GADD34. GCN2 and decreased GADD34 promoted T-ALL cell apoptosis after treatment with peg-Arg I, whereas PERK had an unexpected anti-apoptotic role. Additional results showed that phospho-eIF2α signaling further increased the anti-leukemic effects induced by peg-Arg I in T-ALL-bearing mice. These results suggest the central role of phospho-eIF2α in the anti-T-ALL effects induced by peg-Arg I and support its study as a therapeutic target.
Keywords: pegylated arginase, arginine starvation, arginase, T-ALL
INTRODUCTION
Almost 5000 cases of acute lymphoblastic leukemia (ALL) are diagnosed annually in the United States. Two thirds of the ALL cases occur in children under the age of 15, making ALL the most common cancer in this age group.1 Approximately, 15% and 25% of the newly diagnosed ALL cases in children and adults, respectively, are T cell ALL (T-ALL). Advances in therapies have resulted in an overall complete remission rate of approximately 85% for childhood T-ALL.2 In contrast, the overall remission rate of adults with T-ALL continues to be approximately 40%.3 Furthermore, a subset of patients with T-ALL (20–25%) are completely refractory from the beginning of their treatment.4 Therefore, it is imperative to generate new therapies that alone or in combination with other treatments could potentially increase the percentages of complete responders, prolong the time of remission, or be used to treat the refractory T-ALL population.
We recently reported the therapeutic benefit of the depletion of the non-essential amino acid L-arginine in T-ALL through the use of a pegylated form of the human L-arginine-metabolizing enzyme arginase I (peg-Arg I).5 Peg-Arg I blocked T-ALL cell proliferation by arresting cell cycle progression, followed by the induction of malignant T cell apoptosis.5 The anti-leukemic effect induced by peg-Arg I in T-ALL cells was associated with a global decrease in de novo protein synthesis.5 However, the major mediators in the induction of T-ALL-cell apoptosis by peg-Arg I remain unknown.
Diverse stress signals including hypoxia, exposure to ultraviolet irradiation and nutrient starvation, among others, elicit in cells an integrated cellular response that is characterized by the phosphorylation of the eukaryotic-translation-initiation factor 2 alpha (eIF2α).6 Phosphorylated eIF2α (phospho-eIF2α) inhibits nucleotide exchange on the eIF2 complex, attenuating cellular translation of most mRNAs and reducing protein synthesis.6,7 Four different kinases, the double-stranded RNA-dependent protein kinase (PKR), the hemin-regulated inhibitor (HRI), the PKR-like endoplasmic reticulum-related kinase (PERK) and the general control nonrepressed 2 kinase (GCN2), phosphorylate eIF2α in response to different stress signals.8 Additionally, phospho-eIF2α levels are controlled by the expression of its phosphatases, which are formed by the growth arrest and DNA damage-inducible protein (GADD34, also named PPP1R15A) and the constitutive repressor of eIF2α phosphorylation (CReP, also named PPP1R15B), bound to one of several isoforms of protein phosphatase 1 (PPP1).9,10 GADD34 has been suggested to have a major role in the dephosphorylation of eIF2α during the recovery phase of stress responses, while CReP regulates dephosphorylation of eIF2α in unstressed cells.9,10
In this study, we aimed to determine the mechanisms by which peg-Arg I induces an anti-leukemic effect in T-ALL cells. Our results suggest that peg-Arg I triggers T-ALL cell apoptosis through the phosphorylation of eIF2α. Phosphorylation of eIF2α induced by peg-Arg I was mediated by the kinases GCN2 and PERK and by low expression of phosphatase GADD34. Furthermore, phospho-eIF2α signaling further increased the therapeutic effect induced by peg-Arg I in T-ALL-bearing mice. Altogether, the results suggest a central and novel role of phospho-eIF2α in the anti-T-ALL effects induced by peg-Arg I and strongly support the need to further study its effect as a therapeutic target in T-ALL.
MATERIALS AND METHODS
Cell lines, vectors and animals
To determine the effect of peg-Arg I on T-ALL cells, we used cell lines CCRF-CEM, Molt-4, H9, Loucy, Jurkat (ATCC, Manassas, VA, USA), HPB-ALL, KOPTK1, T-ALL1 and ALL-SIL (DSMZ Human and Animal Cell Lines Database, Braunschweig, Germany), which are heterogeneous in their mutations.11,12 Malignant T cell lines were maintained in RPMI-1640 (Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (Hyclone, Logan, UT, USA). To understand the role of the phospho-eIF2α in the effects induced by peg-Arg I in T-ALL cells in vitro, we used plasmids coding for: a wild type eIF2α (eIF2α-S51S), eIF2α-S51A, a dominant negative non-phosphorylable form of eIF2α in which serine 51 has been mutated to alanine, and eIF2α-S51D, a phosphomimetic form of eIF2α (serine to aspartate). These vectors were obtained from Dr David Ron (University of Cambridge) through Addgene (Cambridge, MA, USA) and subcloned into pE-C1 (Clontech, Mountain View, CA, USA). CCRF-CEM cells were transfected with the different eIF2α coding plasmids using Lipofectamine 2000 (Invitrogen), following the vendor’s protocol, and single clones selected in RPMI-1640 medium supplemented with 800 μg/ml geneticin (Invitrogen). Vectors coding for the dominant negative PERK-K618A (DN-PERK), the dominant negative GCN2-K618M (DN-GCN2),13 and the corresponding wild-type counterparts were provided by Dr David Ron (University of Cambridge) and Dr Ronald Wek (Indiana University School of Medicine). These cDNAs were sub-cloned into pBABE-puro and used to transfect 293 T cells. Viral supernatants were collected after 72 h, filtered and used to transduce CCRF-CEM cells. Specific clones were cultured in medium containing 500 μg/ml puromycin. For in vivo experiments, T-ALL cells were stably transduced with eIF2α-coding lentiviruses. For this, eIF2α-S51S, or eIF2α-S51D vectors were subcloned into pLenti6/V5-D-TOPO vector, which was then used to transfect 293FT packaging cells. Stably transduced CCRF-CEM cells were obtained after infection of the cells with the specific lentiviral particles, followed by selection of single cell clones in blasticidin (10 μg/ml). Six-weeks-old female non-obese-diabetic severe-combined-immuno-deficient NOD.CB17-Prkdcscid/J mice (NOD-Scid) (The Jackson Laboratory, Bar Harbor, ME, USA) were injected intravenously with 1 × 107 wild-type CCRF-CEM cells, or CCRF-CEM cells expressing eIF2α-S51S or eIF2α-S51D. Treatment of the T-ALL-bearing mice started on day 19 post-T-ALL injection, a time when they had about 2 × 104 cells/μl in blood and were still susceptible to treatments. Leukemic mice were continuously treated with peg-Arg I, as we have previously reported.5 As controls, mice were injected with phosphate-buffered saline (PBS), starting at day 19 post-T-ALL injection. All experiments using mice were approved by the LSU-IACUC.
Pegylation of human recombinant arginase I
O-(2-(N-Succinimidyloxycarbonyl)-ethyl)-O′-methylpolyethylene-glycol (PEG) 5000 mw (Sigma-Aldrich, St Louis, MO, USA) was covalently attached to the human recombinant arginase I (AbboMax, San Jose, CA, USA) in a 50:1 molar ratio for 2.5 h, as described by Cheng et al.14 To determine the efficiency of the procedure, peg-Arg I, recombinant arginase I, and PEG 5000 mw were electrophoresed in 10% Tris-Glycine gels (Invitrogen) and gels stained using GelCode Blue Stain Solution (Thermo Scientific, Waltham, MA, USA). The molecular weight of the peg-Arg I ranged between 150–225 kD, while the native unpegylated human recombinant arginase I was 36 kD. Pegylation of arginase I was confirmed by staining of the gels in glutaraldehyde-based stain solution, finding the presence of the peg-Arg I between 150–225 kD and the PEG 5000 mw around 5 kD. The specific activity of the native arginase and the peg-Arg I was about 400 IU/mg protein. One international unit of arginase I is defined as the amount of enzyme that can produce 1 μmol urea/minute at 30 °C, pH 8.5.
Metabolic Labeling
35S-Methionine metabolic labeling was performed as previously described by Muaddi et al.15 with minor modifications. Briefly, the different T-ALL cell lines were seeded overnight in 12-well plates at 1 × 105 cells/well, and subjected to peg-Arg I treatment for 24 h. Following the peg-Arg I treatment, T-ALL cells were washed twice in PBS and incubated 15 min in methionine-free medium, after which they were pulsed with 30 μCi/ml 35S-methionine (Perkin Elmer Life Sciences, Boston, MA, USA) for additional 90 min. Then, T-ALL cells were washed twice with ice-cold PBS and resuspended in protein lysys buffer.5 Radioactivity was determined by liquid scintillation counting in 1 μg of protein extracts, previously quantified using BCA protein Assay kit (Pierce Biotechnology, Inc., Rockford, IL, USA).
Immunocytofluorescence
Cytospin slides were prepared from CD5+ CCRF-CEM cells previously isolated from NOD-Scid mice using magnetic beads.5 For immunostaining, the cells were fixed and permeablized with PBS buffer containing 0.02% Triton X-100 and 4% formaldehyde. Fixed cells were washed three times in PBS and blocked in 5% BSA for 1 h at 37 °C. Phospho-eIF2α was detected using rabbit anti-pS51-eIF2α antibody (Epitomics, Burlingame, CA, USA) followed by FITC-conjugated goat anti-rabbit secondary antibody (Molecular Probes Inc. Eugene, OR, USA). Then, nuclei were stained using 4′-6-diamidino-2-phenylindole. The images were visualized with a Nikon Eclipse E400 upright fluorescence microscope equipped with EXI aqua camera (Qimaging photometrics, Surrey, BC, Canada), motorized Z-axis, and SlideBook5 acquisition/deconvolution software (Intelligent Imaging Innovations Inc., Santa Monica, CA, USA). A series of three-dimensional images of each individual picture were deconvoluted to one two-dimensional picture and resolved by adjusting the signal cut-off to near maximal intensity to increase resolution. Quantification of eIF2α (number of voxel per cell) was performed by utilizing Mask analysis included in SlideBook5 software according to manufacturer recommendation (Intelligent Imaging Innovations Inc.)
Western blot
Thirty micrograms of cell lysates, collected as described,16 were electrophoresed in 4–20% Tris-Glycine gels, transferred to PVDF membranes, and immunobloted with specific antibodies against phospho-eIF2α, phospho-GCN2, HRI (all from Epitomics), eIF2α (Biosource, Carlsbad, CA, USA), activating transcription factor 4 (Atf4), GADD34 (all from Santa Cruz Biotechnologies, Santa Cruz, CA, USA), CReP (Abcam, Cambridge, MA, USA), cleaved poly-ADP-ribose polymerase (PARP), GCN2, PERK, PPP1 α, β, γ, phospho-p70S6, p70S6, phospho-eIF4E binding protein (4E-BP), phospho-eukaryotic elongation factor 2 (eEF2) (all from Cell Signaling Technologies, Danvers, MA, USA), PARP, PKR (from Becton Dickinson-Transduction, San Jose, CA, USA) and Actin (Sigma). Membrane-bound immune complexes were detected by using ECL western blotting detection system (GE healthcare life sciences, Pittsburgh, PA, USA) and scanned in a densitometer GS-710 (Bio-Rad, Hercules, CA, USA).
mRNA silencing in CCRF-CEM cells
Silencing of the different genes was performed, as previously reported,5 using specific sh-RNA coding plasmids from SABiosciences (Qiagen, Valencia, CA, USA). Briefly, shRNA-coding sequences 5′-GGTCCTGAGTGCA TCTAATGT-3′ (KH58340N, plasmid 3, sh-GCN2 #1) or 5′-GCAGCACAATGGA ATCATCTT-3′ (KH58340N, plasmid 1, sh-GCN2 #2) were used to silence GCN2, shRNA-coding sequences 5′-CAACCATTGTGCTAATAAACT-3′ (KH10874N, plasmid 1, sh-PERK #1) or 5′-AGGCCTGATTCTATTTGAATT-3′ (KH10874N, plasmid 4, sh-PERK #2) were used for silencing of PERK, shRNA-coding sequence 5′-GAAGGCAGTTAGTCCTTTATT-3′ (KH01327N, plasmid 2, sh-PKR) was used for silencing of PKR, shRNA-coding sequence 5′-CGGTGAAGTACA CCACCAATT-3′ (KH20406N, plasmid 4, sh-HRI) was used for silencing of HRI, and shRNA-coding sequence 5′-CCCTGCAAGTGCTTTCTTGAA-3′ (KH02081N, plasmid 4, sh-GADD34) was used for silencing of GADD34. As controls, cells were transfected with sh-mock coding plasmids, also from SABiosciences. Single specific clones were obtained after transfection and selection of cells in 800 μg/ml geneticin-containing RPMI.
Apoptosis assay
Expression of annexin V was tested using the annexin V-FITC Apoptosis Detection Kit (BD Biosciences).17 The results are expressed as the percentage of annexin V+ cells. To test the mitochondrial membrane potential, CCRF-CEM cells cultured with or without peg-Arg I were labeled with 3,3′-diethyloxacarbocyanine-iodide (DiOC2(3)), using the MitoProbe DiOC2(3) Assay Kit (Molecular Probes-Invitrogen), and analyzed by flow cytometry.
Isolation of human CD5+ cells
Spleens from CCRF-CEM T-ALL tumor-bearing mice treated with peg-Arg I or PBS were isolated after 30 days of T-ALL injection. Splenocytes were labeled with anti-human CD5-biotin antibodies (BD Biosciences), followed by the isolation of biotin-labeled cells using anti-biotin magnetic beads separation kit (Miltenyi Biotech, Auburn, CA, USA). Human T-ALL CD5+ cells purity ranged between 95–99% as tested by flow cytometry.
Statistical analysis
Results were analyzed in SAS v9.1 using a mixed models approach to repeated measures settings and inequality of variances. No data transformations were attempted. Comparisons of means were carried out by either the Dunnet or Tukey procedures to account for multiple testing. Survival functions were estimated by the Kaplan–Meier method and they were compared with the log-rank or Renyi tests.
RESULTS
Peg-Arg I induces apoptosis and phosphorylation of eIF2α in T-ALL cells
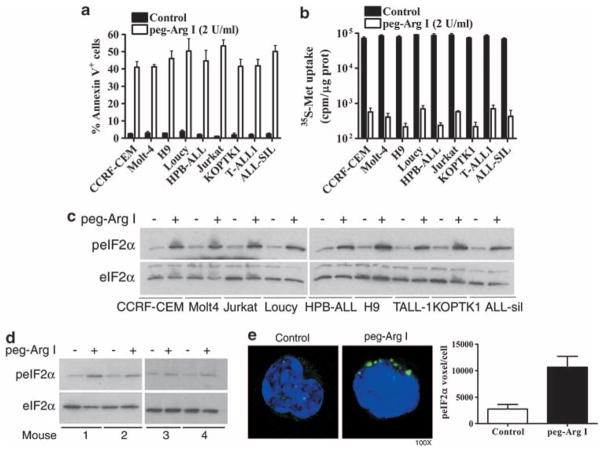
Our previous results suggested that treatment with peg-Arg I induced apoptosis and phosphorylation of eIF2α in the T-ALL cell line CCRF-CEM.5 To ensure that these results were not limited to CCRF-CEM cells, we repeated the experiment using a panel of different T-ALL cell lines including CCRF-CEM, Molt-4, H9, Loucy, HPB-ALL, Jurkat, KOPTK1, T-ALL-1 and ALL-Sil. An increased expression of the apoptosis marker annexin V was found in all the T-ALL cell lines treated with peg-Arg I, but not in PBS treated cells (Figure 1a). Furthermore, the anti-leukemic effect induced by peg-Arg I in T-ALL cells correlated with a global decrease in de novo protein synthesis (Figure 1b) and an increased phosphorylation of eIF2α (Figure 1c). Although the translation of most mRNAs is inhibited during stress, there is induction of a limited number of proteins, such Atf4, that control the cell fate during stress.6 Accordingly, we found a similar kinetic of induction of phospho-eIF2α and Atf4 expression in CCRF-CEM cells cultured in the presence of peg-Arg I (Supplementary Figures 1A and B).
Figure 1.
Peg-Arg I induces apoptosis, an arrested translation, and phosphorylation of eIF2α in T-ALL cells. (a) Malignant T cell lines (1 × 105) were cultured for 48 h in the presence or the absence of peg-Arg I (2 IU/ml) or PBS (control). Then, the levels of the apoptosis marker annexin V were determined by flow cytometry. (b) T-ALL cell lines were treated with peg-Arg I for 24 h, after which de novo translation was tested using 35S-Metionine (35S-Met) incorporation, as described in the methods. (c) Malignant T cell lines (1 × 105/ml) were cultured for 8 h in the presence or the absence of peg-Arg I (2 IU/ml), after which phospho-eIF2α levels were detected by western blot. (d–e). A representative experiment showing the expression of phosphorylated and total eIF2α by western blot (d) and phospho-eIF2α by cytofluorescence (e) in CD5+ cells sorted from individual mice bearing CCRF-CEM cells for 30 days and treated with PBS (n = 6) or 0.5 mg per mouse peg-Arg I (n = 7) for 4 h. The experiment was repeated a minimum of 3 times obtaining similar results.
We also tested whether peg-Arg I induced phospho-eIF2α in T-ALL cells in vivo. NOD-Scid mice bearing CCRF-CEM cells for 30 days received one injection of peg-Arg I, and 4 h later, the splenic human CD5+ T-ALL cells were sorted and tested for phospho-eIF2α levels by immunoblot and cytofluorescence. An increased expression of phospho-eIF2α was detected in sorted CCRF-CEM cells from mice treated with peg-Arg I, but not from those mice treated with PBS (Figures 1d–e).
Phosphorylation of eIF2α has a central role in the induction of T-ALL cell apoptosis by peg-Arg I
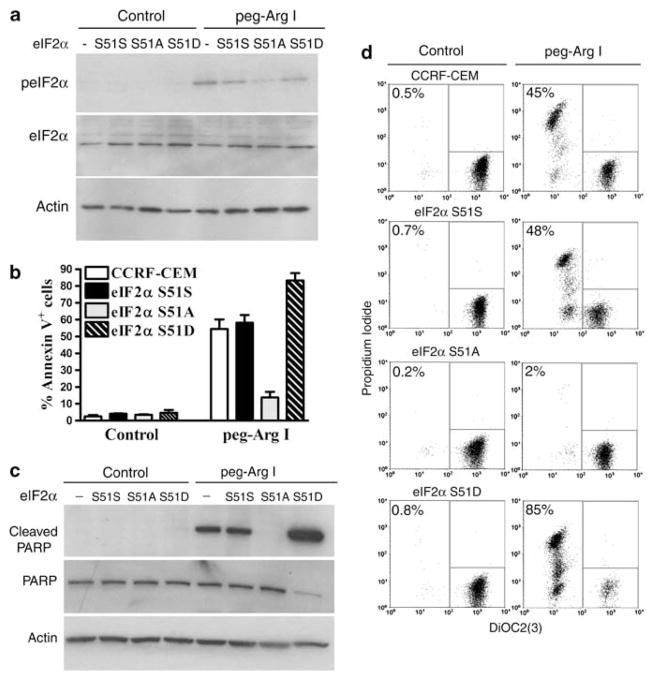
To understand the role of phospho-eIF2α in the effects induced by peg-Arg I in T-ALL cells, we stably transfected CCRF-CEM cells with plasmids coding for: (1) a wild-type eIF2α (eIF2α-S51S), (2) a dominant negative non-phosphorylable form of eIF2α in which serine 51 has been mutated to alanine (eIF2α-S51A), or (3) a phosphomimetic eIF2α form (serine to aspartate) (eIF2α-S51D). These vectors compete with the wild-type eIF2α that still exists in the T-ALL cells. As expected, eIF2α-S51A CCRF-CEM cells treated with peg-Arg I had a lower phosphorylation of eIF2α, as compared with wild-type, eIF2α-S51S, and eIF2α-S51D CCRF-CEM cells (Figure 2a). Furthermore, eIF2α-S51A CCRF-CEM cells treated with peg-Arg I showed a decreased expression of the apoptosis markers annexin V, cleaved poly-ADP-ribose polymerase, and impaired mitochondrial membrane potential, as compared with treated untransfected cells or those expressing the control-eIF2α-S51S vector (Figures 2b–d and Supplementary Figure 2A) (P<0.0001). Conversely, T-ALL cells carrying the phosphomimetic eIF2α-S51D form displayed a higher susceptibility to peg-Arg I (Figures 2b–d, Supplementary Figure 2A) (P<0.0001). These differences could not be explained by a general predisposition to cell death, as a similar induction of apoptosis was found in all the eIF2α-transfected cells treated with Staurosporine, an eIF2α-independent-inducer of apoptosis (Supplementary Figure 2B). Altogether the results suggest that phosphorylation of eIF2α has a central role in the induction of T-ALL cell apoptosis after peg-Arg I treatment.
Figure 2.
Phosphorylation of eIF2α is a central mediator in the induction of T-ALL cell apoptosis by peg-Arg I. (a) Phospho-eIF2α expression in untransfected CCRF-CEM cells (−), and CCRF-CEM cells stably transfected with eIF2α-S51S, eIF2α-S51 A or eIF2α-S51D, and treated with peg-Arg I for 8 h. (b–d) Expression of the apoptosis markers annexin V (b), cleaved poly-ADP-ribose polymerase (PARP) (c), and DiOC2(3)/propidium iodide (d) in cells from a cultured with peg-Arg I for 72 h. The experiments were repeated a minimum of three times obtaining similar results.
The arrest in protein synthesis induced by peg-Arg I is not exclusively dependent on phospho-eIF2α
Although phospho-eIF2α plays a major role in the induction of T-ALL cell apoptosis by peg-Arg I, we found a similar inhibition in the global de novo translation in all the eIF2α transfected CCRF-CEM cells cultured with peg-Arg I (Supplementary Figure 3A). However, eIF2α-S51A-expressing cells maintained protein synthesis after treatment with thapsigargin, an inducer of endoplasmic reticulum (ER) stress (Supplementary Figure 3B). To further characterize the additional pathways by which peg-Arg I could arrest de novo protein synthesis in T-ALL cells, we tested other potential regulatory pathways such as the mammalian target of rapamycin and eEF2. Decreased phosphorylation of the mammalian target of rapamycin substrates p70S6 and 4EBP1 and increased phosphorylation and therefore inactivation of eEF2 were found in CCRF-CEM cells treated with peg-Arg I (Supplementary Figure 3C). These results suggest that the arrest in global protein synthesis induced by peg-Arg I in T-ALL cells can be the result of the inhibition of several pathways promoting translation.
Role of kinases GCN2 and PERK in the phosphorylation of eIF2α in T-ALL cells by peg-Arg I
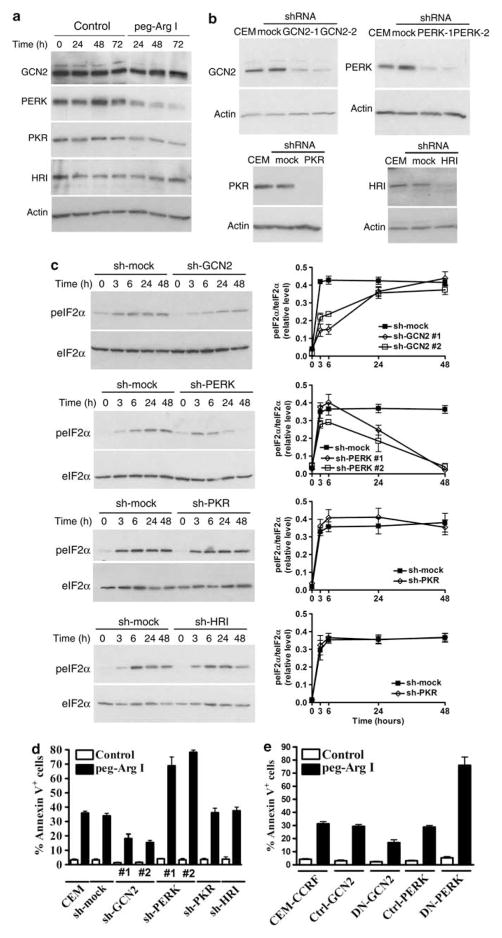
Four different kinases, PKR, HRI, PERK, and GCN2, have been identified to phosphorylate eIF2α in response to stress.8 A constitutive expression of all the eIF2α kinases was found in CCRF-CEM cells cultured in normal media (Figure 3a). In addition, peg-Arg I induced the phosphorylation of GCN2 as early as 1 h (Supplementary Figure 4A) and impaired the expression of PERK after 48 h of culture (Figure 3a). To determine the role of the eIF2α kinases in the phosphorylation of eIF2α induced by peg-Arg I, we silenced their expression in CCRF-CEM cells using transfection with plasmids coding for specific shRNA (Figure 3b). Silencing of the expression of PKR and HRI in CCRF-CEM cells did not prevent the phosphorylation of eIF2α induced by peg-Arg I (Figure 3c). Conversely, silencing of GCN2 in T-ALL cells prevented the early phosphorylation of eIF2α induced by peg-Arg I, while silencing of PERK prevented the eIF2α phosphorylation at later time points (Figure 3c). These results were reproduced using a second set of specific sh-RNA-coding sequences for GCN2 (sh-GCN2 #2) and for PERK (sh-PERK #2) (Figure 3c, Supplementary Figure 4B), suggesting a specific role of these kinases in the phosphorylation of eIF2α by peg-Arg I. Then, we tested the role of the different eIF2α kinases in the T-ALL cell apoptosis induced by peg-Arg I. Silencing of PKR and HRI did not alter the induction of apoptosis by peg-Arg I. In contrast, a significant decrease in the induction of T-ALL apoptosis was found in the GCN2-silenced T-ALL cells treated with peg-Arg I (Figure 3d and Supplementary Figure 4C) (P<0.0001). Surprisingly, silencing of PERK in CCRF-CEM cells led to a higher induction of apoptosis after treatment with peg-Arg I (P<0.0001) (Figure 3d and Supplementary Figure 4C). To confirm the role of PERK and GCN2 in the apoptosis induced by peg-Arg I, we transduced CCRF-CEM cells with vectors coding for DN-GCN2 and DN-PERK. Similar to the sh-RNA experiments, a decreased induction of cellular apoptosis (Figure 3e) and a profound prevention of eIF2α phosphorylation (Supplementary Figure 4D) was found in DN-GCN2-expressing cells, but not in control cells, after treatment with peg-Arg I. Moreover, a high rate of apoptosis and a significant prevention of the phosphorylation of eIF2α, especially at 48 h, was observed in DN-PERK-expressing T-ALL cells treated with peg-Arg I. These results suggest the role of GCN2 and PERK in the phosphorylation of eIF2α by peg-Arg I, and the opposite role of these kinases in the peg-Arg I-induced T-ALL cell apoptosis.
Figure 3.
Role of eIF2α kinases in the phosphorylation of eIF2α and the cellular apoptosis induced by peg-Arg I. (a) Expression of eIF2α kinases was detected by western blot in CCRF-CEM cells treated with peg-Arg I (2 IU/ml). (b) Stably silencing of the expression of the specific eIF2α kinases was achieved after transfection of CCRF-CEM cells with plasmids coding for specific sh-RNA or sh-controls and further selection of single clones in medium supplemented with 800 μg/ml Geneticin. (c) Cells from (b) were treated with peg-Arg I (2 IU/ml) and the levels of phospho-eIF2α (3–48 h) were detected by western blot (left column) and phospho-eIF2α/eIF2α densitometric values graphed (right column). (d–e) CCRF-CEM cells previously silenced for GCN2, PERK, PKR, or HRI (d), or expressing DN-GCN or DN-PERK (e) were treated with peg-Arg I (2 IU/ml) for 48 h, after which the levels annexin V were measured by flow cytometry. Experiments were repeated a minimum of three times obtaining similar results.
Role of eIF2α dephosphorylation in the induction of T-ALL cell apoptosis by peg-Arg I
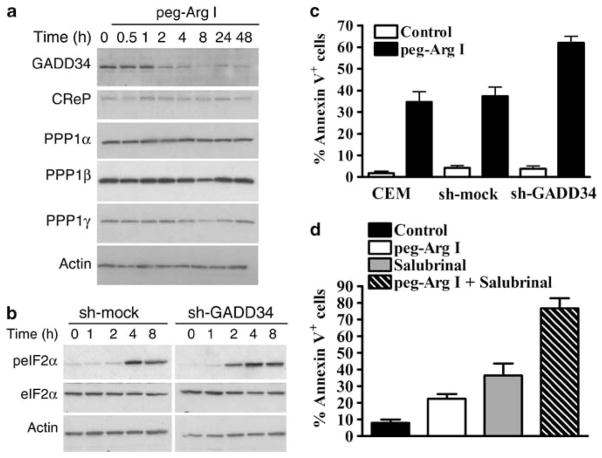
The regulation of the dephosphorylation of eIF2α is mediated by the expression of GADD34 and CReP, which bind to one of several isoforms of PPP1.18,9,10 We found the expression of all the components of the eIF2α phosphatase in CCRF-CEM cells cultured in normal media (Figure 4a). However, treatment with peg-Arg I induced a specific down-regulation of GADD34 as early as 2 h, without affecting the other components of the eIF2α phosphatase complex (Figure 4a). To further understand the effect of the GADD34 inhibition in the induction of T-ALL apoptosis, we silence GADD34 expression in CCRF-CEM cells (Supplementary Figure 5A). GADD34-silenced T-ALL cells showed an earlier phosphorylation of eIF2α and were highly susceptible to the apoptosis induced by peg-Arg I, as compared with sh-control cells (Figures 4b–c, Supplementary Figure 5B). Then, we tested the effect in T-ALL cells of salubrinal, a selective inhibitor of GADD34.19 Similar to the effects induced by peg-Arg I, T-ALL cells cultured with salubrinal had an increased expression of phospho-eIF2α, a dose-dependent inhibition in T-ALL cell proliferation, and an increased rate of T-ALL cell apoptosis (Supplementary Figure 5C–E). Furthermore, combination of salubrinal and peg-Arg I synergistically induced T-ALL cell apoptosis in vitro (Figure 4d). Altogether, these results suggest the role of the inhibition of GADD34 in the T-ALL cell apoptosis induced by peg-Arg I, and suggest a potential therapeutic opportunity in T-ALL using the combination of peg-Arg I and inhibitors of eIF2α phosphatases.
Figure 4.
Inhibition of GADD34 accelerates eIF2α phosphorylation and further increases cellular apoptosis induced by peg-Arg I. (a) CCRF-CEM cells were cultured in medium with or without peg-Arg I (2 IU/ml) and the expression of eIF2α phosphatase components was tested by immunoblotting. (b) Silencing of GADD34 in CCRF-CEM cells induces an earlier phosphorylation of eIF2α after treatment with peg-Arg I. (c) CCRF-CEM cells stably transfected with plasmids coding for sh-GADD34 or sh-mock were treated with peg-Arg I for 24 h, after which annexin V expression was detected using flow cytometry. (d) Expression of annexin V in CCRF-CEM cells treated for 24 h with peg-Arg I (2 IU/ml) and/or salubrinal (20 μM). Values are from 3 similar experiments.
Phospho-eIF2α signaling further increase the anti-leukemic effect induced by peg-Arg I
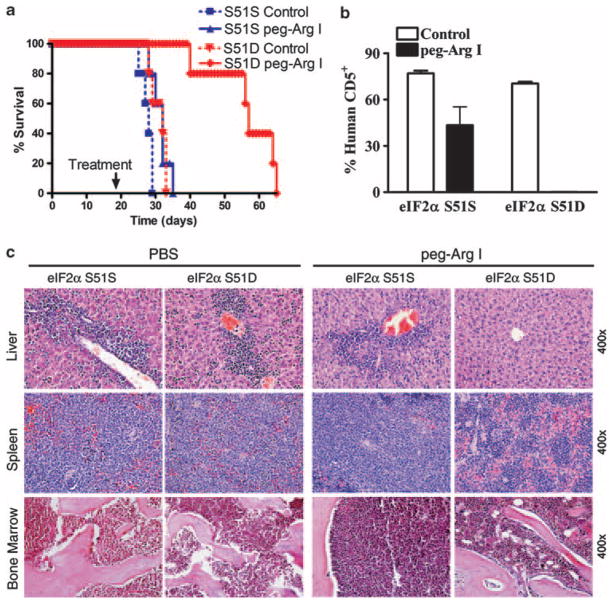
We previously found that monotherapy with peg-Arg I led to heterogeneous anti-leukemic responses in NOD-Scid mice bearing CCRF-CEM T-ALL cells, which are not reflected in a clear extension of survival.5 To test whether phospho-eIF2α pathways further increase the anti-T-ALL therapeutic effect induced by peg-Arg I in vivo, we treated NOD-Scid mice harboring CCRF-CEM cells transduced with lentivirus particles coding for eIF2α-S51S or eIF2α-S51D. Monotherapy with peg-Arg I significantly extended the survival of mice injected with CCRF-CEM cells carrying the phosphomimetic form of eIF2α, but not in those bearing S51S-CCRF-CEM cells (Figure 5a) (P<0.0001) or untransduced CCRF-CEM cells (data not shown).5 In addition, a dramatic prevention in the accumulation of human CD5+ malignant T cells in the spleen occurred in peg-Arg I-treated mice harboring the eIF2α-S51D cells, as compared with treated mice carrying S51S-CCRF-CEM cells (Figure 5b) (P<0.0001). Accordingly, histopathology experiments showed a lower accumulation of T-ALL cells in the liver, spleen and bone marrow of eIF2α-S51D-bearing mice treated with peg-Arg I, as compared with those treated mice expressing eIF2α-S51S (Figure 5c). These results were not caused by a lower engraftment of eIF2α-S51D cells, as a similar survival and accumulation of malignant T cells occurred in mice bearing eIF2α-S51S- and eIF2α-S51D-expressing CCRF-CEM cells after treatment with PBS (Figures 5a–c). These results suggest that phospho-eIF2α signaling enhances the anti-leukemic effect induced by peg-Arg I in vivo.
Figure 5.
Phospho-eIF2α sensitizes T-ALL to the anti-leukemic effect of peg-Arg I in vivo. (a) Survival of mice bearing CCRF-CEM cells stably transduced with eIF2α-S51S or eIF2α-S51D and that were treated with PBS (Control) (n = 10) or peg-Arg I (n = 10). Peg-Arg I extended the survival of mice injected with T-ALL cells expressing a phosphomimetic form of eIF2α. (b) Percentage of human CD5+ cells was established in the spleens after 30 days of the injection of CCRF-CEM cells expressing eIF2α-S51S or eIF2α-S51D. Mice were treated with 0.5 mg/mouse peg-Arg I (n = 10) or PBS (n = 10) twice a week starting on day 19 post-T-ALL injection, as described in the methods. (c) A representative hematoxylin and eosin staining histopathology from liver, spleen and bone marrow from b.
DISCUSSION
Over the past 40 years refinements in the chemotherapy regimens and supportive care have lead to a great success in the treatments of children with T-ALL. However, the generation of successful therapies for adult patients with T-ALL has been less rewarding. The overall complete remission rate of adult T-ALL continues to be approximately 40%. Therefore, it is imperative to generate new therapies that alone, or in combination with traditional treatments, can increase the cure rate of adult T-ALL. The use of asparagine-metabolizing enzyme asparaginase as part of the standard treatment for T-ALL patients for the last 50 years have suggested that limitation of amino acids can be used as a therapeutic approach in T-ALL. Based on this, we recently studied the effect peg-Arg I in T-ALL.5 A similar strategy of L-arginine depletion has been used by other groups to treat melanoma and liver carcinoma.20,14,21 Our previous results showed that peg-Arg I blocked T-ALL cell proliferation in vitro by arresting cell cycle progression, followed by the induction of malignant T cell apoptosis.5 Interestingly, the effects induced by peg-Arg I did not affect the anti-neoplastic activity of normal T cells, suggesting an anti T-ALL-specific effect.22 However, the mediators of the anti-T-ALL effect induced by peg-Arg I remained unknown.
Stress conditions including nutrient starvation, hypoxia and UV exposure elicit an integrated cellular response that leads to a global decrease in protein synthesis and to the expression of a limited number of proteins, such as Atf4, that eventually control the cell fate during stress.6 The induction of this small group of proteins is mediated by mRNA elements known as internal ribosome entry sequences that can direct translation without the need of mRNA-capping.23 In models of stress induced by unfolded protein responses, both the arrest in protein synthesis and the induction of stress-related proteins are dependent on the phosphorylation of eIF2α.8 Phospho-eIF2α inhibits global translation by reducing the dissociation rate of eIF2B, which blocks the access of methionyl tRNA into the translation initiation complex.24 Our results show that treatment of a panel of T-ALL cell lines with peg-Arg I led to apoptosis, a global decrease in de novo translation, and an increased expression of phospho-eIF2α and its downstream target Atf4. However, although the inhibition of eIF2α phosphorylation in T-ALL cells prevented the induction of apoptosis by peg-Arg I, we still found a similar decrease in global translation in S51A-eIF2α-transfected cells cultured with peg-Arg I. A decrease in the mammalian target of rapamycin signaling and inactivation of eEF225 were detected in T-ALL cells treated with peg-Arg I. Therefore, it is possible that the decrease in de novo protein synthesis observed in T-ALL cells treated with peg-Arg I is the result of the inactivation of different pathways regulating translation. This is different from the typical ER stress in which translational inhibition is exclusively dependent on phospho-eIF2α.24
Phosphorylation of eIF2α in cells is the result of the activation of stress-related kinases PKR, HRI, PERK and GCN2, and the low expression of eIF2α phosphatases GADD34 and CReP bound to different PPP1. Our results suggest a major role of kinases GCN2 and PERK, and phosphatase member GADD34 in the increased phosphorylation of eIF2α observed in T-ALL cells treated with peg-Arg I. GCN2 activation and low expression of GADD34 regulated the phosphorylation of eIF2α by peg-Arg I at initial stages (within first 6 h), while PERK controlled peg-Arg I-induced phosphorylation of eIF2α at later time points. Activation of GCN2 has been reported after nutrient starvation, while PERK is activated during ER stress.8 Therefore, the initial phosphorylation of eIF2α could be the result of pathways sensing L-arginine starvation, while the late eIF2α phosphorylation by unfolded protein responses. Interestingly, we found that GCN2 and decreased GADD34 have a pro-apoptotic role in response to peg-Arg I, whereas PERK had an unexpected cytoprotective effect. These results suggest a potential key role of the early phosphorylation of eIF2α by GCN2 and low GADD34 in the final induction of apoptosis by peg-Arg I. In accordance, T-ALL cells expressing eIF2α-S51A had a decreased early phosphorylation of eIF2α and were highly resistant to the apoptosis induced by peg-Arg I. An important question is whether the effect of PERK on the phosphorylation of eIF2α and its anti-apoptotic role after peg-Arg I treatment are related. It is possible that the anti-apoptotic effect induced by PERK can be independent of its role on the phosphorylation of eIF2α. Another possibility is that the late phosphorylation of eIF2α through PERK could induce anti-apoptotic effects, which would suggest a differential effect of phospho-eIF2α on T-ALL survival depending on its kinetics. Ongoing studies using the inducible expression of eIF2α-S51A, eIF2α kinases and phosphatases will enable the characterization of the effect of phopho-eIF2α kinetics in the peg-Arg I-induced T-ALL cell apoptosis.
Although peg-Arg I monotherapy induces increased levels of T-ALL cell apoptosis in vitro, we did not find a significant extension of the survival in eIF2α-S51S or wild-type T-ALL-bearing mice treated with peg-Arg I.5 These results correlated with a higher phosphorylation of eIF2α in T-ALL cells treated with peg-Arg I in vitro, as compared with T-ALL cells isolated from peg-Arg I-treated mice (Figure 1). Possible reasons for this lower phosphorylation of eIF2α in T-ALL cells in vivo include a higher depletion of L-arginine in vitro as compared with in vivo, a protection/feeding effect induced by cells present in the leukemic microenvironment (stroma, myeloid cells), and the ability of T-ALL cells in vivo to synthesize limited amounts of L-arginine from citrulline, among others. Therefore, it is possible that the in vivo effect induced by peg-Arg I in T-ALL cells will depend on the level of eIF2α phosphorylation. In fact, monotherapy with peg-Arg I significantly extended the survival and prevented the accumulation of malignant cells in mice injected with T-ALL cells carrying the phosphomimetic form of eIF2α. In addition, combination of peg-Arg I and salubrinal, an inhibitor of eIF2α dephosphorylation, synergistically induced T-ALL cell apoptosis. A similar synergistic effect was found in multiple myeloma cells treated with ER stress inducer bortezomib and salubrinal.26 Altogether the results suggest that the combination of peg-Arg I and treatments that maintain eIF2α phosphorylation could potentially represent a novel therapy for T-ALL.
In conclusion, the results suggest a central and novel role of phospho-eIF2α in the anti-T-ALL effects induced by peg-Arg I, and strongly support the need to further study its use as a potential therapeutic target in T-ALL. The continuation of this research will help design new, safe and effective approaches to test the use of peg-Arg I as a new therapy for the treatment of T-ALL patients.
Supplementary Material
Acknowledgments
We thank Daniel Nguyen, BS for his technical assistance in some of the experiments. This work was supported in part by NIH-NCRR (COBRE) grants P20RR021970 and 1R21CA162133 to PCR.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supplementary Information accompanies the paper on the Leukemia website (http://www.nature.com/leu)
References
- 1.Inaba H, Bhojwani D, Pauley JL, Pei D, Cheng C, Metzger ML, et al. Combination chemotherapy with clofarabine, cyclophosphamide, and etoposide in children with refractory or relapsed haematological malignancies. Br J Haematol. 2012;156:275–279. doi: 10.1111/j.1365-2141.2011.08847.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pui CH, Sandlund JT, Pei D, Campana D, Rivera GK, Ribeiro RC, et al. Improved outcome for children with acute lymphoblastic leukemia: results of Total Therapy Study XIIIB at St Jude Children’s Research Hospital. Blood. 2004;104:2690–2696. doi: 10.1182/blood-2004-04-1616. [DOI] [PubMed] [Google Scholar]
- 3.Tanosaki R, Tobinai K. Adult T-cell leukemia-lymphoma: current treatment strategies and novel immunological approaches. Expert Rev Hematol. 2010;3:743–753. doi: 10.1586/ehm.10.73. [DOI] [PubMed] [Google Scholar]
- 4.Laport GF, Larson RA. Treatment of adult acute lymphoblastic leukemia. Semin Oncol. 1997;24:70–82. [PubMed] [Google Scholar]
- 5.Hernandez CP, Morrow K, Lopez-Barcons LA, Zabaleta J, Sierra R, Velasco C, et al. Pegylated arginase I: a potential therapeutic approach in T-ALL. Blood. 2010;115:5214–5221. doi: 10.1182/blood-2009-12-258822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holcik M, Sonenberg N. Translational control in stress and apoptosis. Nat Rev Mol Cell Biol. 2005;6:318–327. doi: 10.1038/nrm1618. [DOI] [PubMed] [Google Scholar]
- 7.Woo CW, Cui D, Arellano J, Dorweiler B, Harding H, Fitzgerald KA, et al. Adaptive suppression of the ATF4-CHOP branch of the unfolded protein response by toll-like receptor signalling. Nat Cell Biol. 2009;11:1473–1480. doi: 10.1038/ncb1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blander JM, Amsen D. Immunology. amino acid addiction. Science. 2009;324:1282–1283. doi: 10.1126/science.1175678. [DOI] [PubMed] [Google Scholar]
- 9.Jousse C, Oyadomari S, Novoa I, Lu P, Zhang Y, Harding HP, et al. Inhibition of a constitutive translation initiation factor 2alpha phosphatase, CReP, promotes survival of stressed cells. J Cell Biol. 2003;163:767–775. doi: 10.1083/jcb.200308075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Novoa I, Zeng H, Harding HP, Ron D. Feedback inhibition of the unfolded protein response by GADD34-mediated dephosphorylation of eIF2alpha. J Cell Biol. 2001;153:1011–1022. doi: 10.1083/jcb.153.5.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan SM, Weng AP, Tibshirani R, Aster JC, Utz PJ. Notch signals positively regulate activity of the mTOR pathway in T-cell acute lymphoblastic leukemia. Blood. 2007;110:278–286. doi: 10.1182/blood-2006-08-039883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weng AP, Ferrando AA, Lee W, Morris JP, Silverman LB, Sanchez-Irizarry C, et al. Activating mutations of NOTCH1 in human T cell acute lymphoblastic leukemia. Science. 2004;306:269–271. doi: 10.1126/science.1102160. [DOI] [PubMed] [Google Scholar]
- 13.Sood R, Porter AC, Olsen DA, Cavener DR, Wek RC. A mammalian homologue of GCN2 protein kinase important for translational control by phosphorylation of eukaryotic initiation factor-2alpha. Genetics. 2000;154:787–801. doi: 10.1093/genetics/154.2.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng PN, Lam TL, Lam WM, Tsui SM, Cheng AW, Lo WH, et al. Pegylated recombinant human arginase (rhArg-peg5,000mw) inhibits the in vitro and in vivo proliferation of human hepatocellular carcinoma through arginine depletion. Cancer Res. 2007;67:309–317. doi: 10.1158/0008-5472.CAN-06-1945. [DOI] [PubMed] [Google Scholar]
- 15.Muaddi H, Majumder M, Peidis P, Papadakis AI, Holcik M, Scheuner D, et al. Phosphorylation of eIF2alpha at serine 51 is an important determinant of cell survival and adaptation to glucose deficiency. Mol Biol Cell. 2010;21:3220–3231. doi: 10.1091/mbc.E10-01-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodriguez PC, Quiceno DG, Ochoa AC. L-arginine availability regulates T-lymphocyte cell-cycle progression. Blood. 2007;109:1568–1573. doi: 10.1182/blood-2006-06-031856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodriguez PC, Ernstoff MS, Hernandez C, Atkins M, Zabaleta J, Sierra R, et al. Arginase I-producing myeloid-derived suppressor cells in renal cell carcinoma are a subpopulation of activated granulocytes. Cancer Res. 2009;69:1553–1560. doi: 10.1158/0008-5472.CAN-08-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jousse C, Averous J, Bruhat A, Carraro V, Mordier S, Fafournoux P. Amino acids as regulators of gene expression: molecular mechanisms. Biochem Biophys Res Commun. 2004;313:447–452. doi: 10.1016/j.bbrc.2003.07.020. [DOI] [PubMed] [Google Scholar]
- 19.Boyce M, Bryant KF, Jousse C, Long K, Harding HP, Scheuner D, et al. A selective inhibitor of eIF2alpha dephosphorylation protects cells from ER stress. Science. 2005;307:935–939. doi: 10.1126/science.1101902. [DOI] [PubMed] [Google Scholar]
- 20.Izzo F, Marra P, Beneduce G, Castello G, Vallone P, De RV, et al. Pegylated arginine deiminase treatment of patients with unresectable hepatocellular carcinoma: results from phase I/II studies. J Clin Oncol. 2004;22:1815–1822. doi: 10.1200/JCO.2004.11.120. [DOI] [PubMed] [Google Scholar]
- 21.Ascierto PA, Scala S, Castello G, Daponte A, Simeone E, Ottaiano A, et al. Pegylated arginine deiminase treatment of patients with metastatic melanoma: results from phase I and II studies. J Clin Oncol. 2005;23:7660–7668. doi: 10.1200/JCO.2005.02.0933. [DOI] [PubMed] [Google Scholar]
- 22.Highfill SL, Rodriguez PC, Zhou Q, Goetz CA, Koehn BH, Veenstra R, et al. Bone marrow myeloid-derived suppressor cells (MDSCs) inhibit graft-versus-host disease (GVHD) via an arginase-1-dependent mechanism that is up-regulated by interleukin-13. Blood. 2010;116:5738–5747. doi: 10.1182/blood-2010-06-287839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hellen CU, Sarnow P. Internal ribosome entry sites in eukaryotic mRNA molecules. Genes Dev. 2001;15:1593–1612. doi: 10.1101/gad.891101. [DOI] [PubMed] [Google Scholar]
- 24.Schneider R, Agol VI, Andino R, Bayard F, Cavener DR, Chappell SA, et al. New ways of initiating translation in eukaryotes. Mol Cell Biol. 2001;21:8238–8246. doi: 10.1128/MCB.21.23.8238-8246.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Talvas J, Obled A, Fafournoux P, Mordier S. Regulation of protein synthesis by leucine starvation involves distinct mechanisms in mouse C2C12 myoblasts and myotubes. J Nutr. 2006;136:1466–1471. doi: 10.1093/jn/136.6.1466. [DOI] [PubMed] [Google Scholar]
- 26.Schewe DM, guirre-Ghiso JA. Inhibition of eIF2alpha dephosphorylation maximizes bortezomib efficiency and eliminates quiescent multiple myeloma cells surviving proteasome inhibitor therapy. Cancer Res. 2009;69:1545–1552. doi: 10.1158/0008-5472.CAN-08-3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.