Summary
Cancer vaccines have now demonstrated clinical efficacy, but immune modulatory mechanisms that prevent autoimmunity limit their effectiveness. Systemic administration of mAbs targeting immune modulatory receptors CTLA-4 and glucocorticoid-induced TNFR-related protein (GITR) on Treg cells and effector T cells augments anti-tumor immunity both experimentally and clinically, but can induce life-threatening autoimmunity. We hypothesized that local delivery of anti-CTLA-4 and anti-GITR mAbs to the sites where T cells and tumor antigen-loaded DC vaccines interact would enhance the induction of anti-tumor immunity while avoiding autoimmunity. To achieve this goal, DCs transfected with mRNA encoding the H and L chains of anti-mouse CTLA-4 and GITR mAbs were co-administered with tumor antigen mRNA-transfected DCs. We observed enhanced induction of anti-tumor immunity and significantly improved survival in melanoma-bearing mice, without signs of autoimmunity. In human in vitro assays, we demonstrated that DCs transfected with mRNA encoding humanized anti-CTLA-4 mAb and mRNA encoding a soluble human GITR-L fusion protein enhance the induction of anti-tumor CTLs in response to DCs transfected with mRNAs encoding either melanoma or breast cancer antigens. Based on these results, this approach of using local delivery of immune modulators to enhance vaccine-induced immunity is currently being evaluated in a Phase I clinical cancer immunotherapy trial.
Keywords: Dendritic Cell, Cancer, Immunotherapy, Tumor Immunology
Introduction
APC-based cancer immunotherapy has demonstrated clinical activity [1]; however, immune and tumor responses remain modest, suggesting the need for new strategies to enhance anti-tumor immunity. The induction of antigen-specific T cell-mediated immunity with vaccines is regulated by both stimulatory and inhibitory receptor-mediated signals [2]. When APCs such as DCs are exposed to Ag, they process and present Ag-derived peptides to T cells and provide co-stimulatory signals through CD80 and CD86, which engage the T cell activating receptor CD28. CTLA-4 (CD152), upregulated on T-cells for 2–3 days after activation, also binds CD80 and CD86 and mediates immune homeostasis and induction of tolerance to self antigens [3]. Because CTLA-4 appears to undermine T-cell activation, a number of studies have evaluated the effect of CTLA-4 blockade on anti-tumor immunity in murine models. Many studies have demonstrated that administering mAbs targeting such immune-modulating receptors on T cells can enhance vaccine-induced tumor immunity, suggesting that such an approach might improve the efficacy of clinical cancer vaccines [4, 5]. The combination of antagonistic anti-CTLA-4 mAbs and vaccination with cytokine-modified melanoma cells has synergistic effects in mice and humans[6, 7]. Several clinical trials have shown that anti-CTLA-4 mAb administration augments clinical anti-tumor responses [4], and in a recent trial, improves survival in patients with metastatic melanoma[8].
GITR, expressed on Tregs and activated T cells, also appears to regulate the induction of immune responses [9]. Murine in vivo studies using an agonistic mAb to bind GITR have demonstrated enhanced antitumor immunity against poorly immunogenic tumors[10, 11]. Co-administered anti-GITR and anti-CTLA-4 mAbs in mice led to eradication of tumors [11–13]. These data suggest that modulation of GITR and/or CTLA-4 function represents a promising adjunct to cancer immunotherapy, rendering effector T cells resistant to suppression and abrogating the ability of Tregs to suppress anti-tumor immune responses[14, 15]. However, systemic administration of anti-CTLA-4 mAbs to cancer patients has been associated with severe, life-threatening autoimmunity [8, 16]. Autoimmunity has also been induced in mice by systemic administration of anti-GITR mAbs.
To overcome these limitations, we have developed a novel approach to target delivery of such immune modulators, using DCs transfected with immune modulator-encoding mRNA, to sites where anti-tumor T cells are induced. We have previously reported that co-administration of DCs transfected with mRNA encoding the H and L chains of an agonistic anti-GITR mAb or soluble GITR-L enhances the induction of anti-tumor immunity in response to vaccination with tumor associated Ag (TAA) mRNA-transfected DCs in a murine melanoma model and improves survival in tumor-bearing mice, while avoiding the induction of autoimmunity observed with systemic administration of anti-GITR mAb. [17
In our present study, we first evaluated the effect of combined immune modulation of both CTLA-4 and GITR using DCs transfected with mRNA encoding the H and L chains of anti-CTLA-4 and anti-GITR mAbs in a murine melanoma immunotherapy model. In preparation for translating this experimental approach to the clinic to enhance the response to DC-based cancer immunotherapy, we generated mRNAs encoding humanized H and L chains of an anti-human CTLA-4 mAb as well as a human soluble GITR-L fusion protein and demonstrated that DCs transfected with these mRNAs secrete functional immune modulating proteins that bind CTLA-4 and GITR, respectively. We then demonstrated the immune-enhancing effect of DCs transfected with these mRNAs in two in vitro human immunotherapy models in which CTLs were induced in response to DCs loaded with either melanoma or breast TAAs using mRNA-transfected DCs.
Results
Co-administration of DCs transfected with mRNA encoding anti-GITR and anti-CTLA-4 mAbs enhances the induction of protective anti-tumor immunity in a murine melanoma model
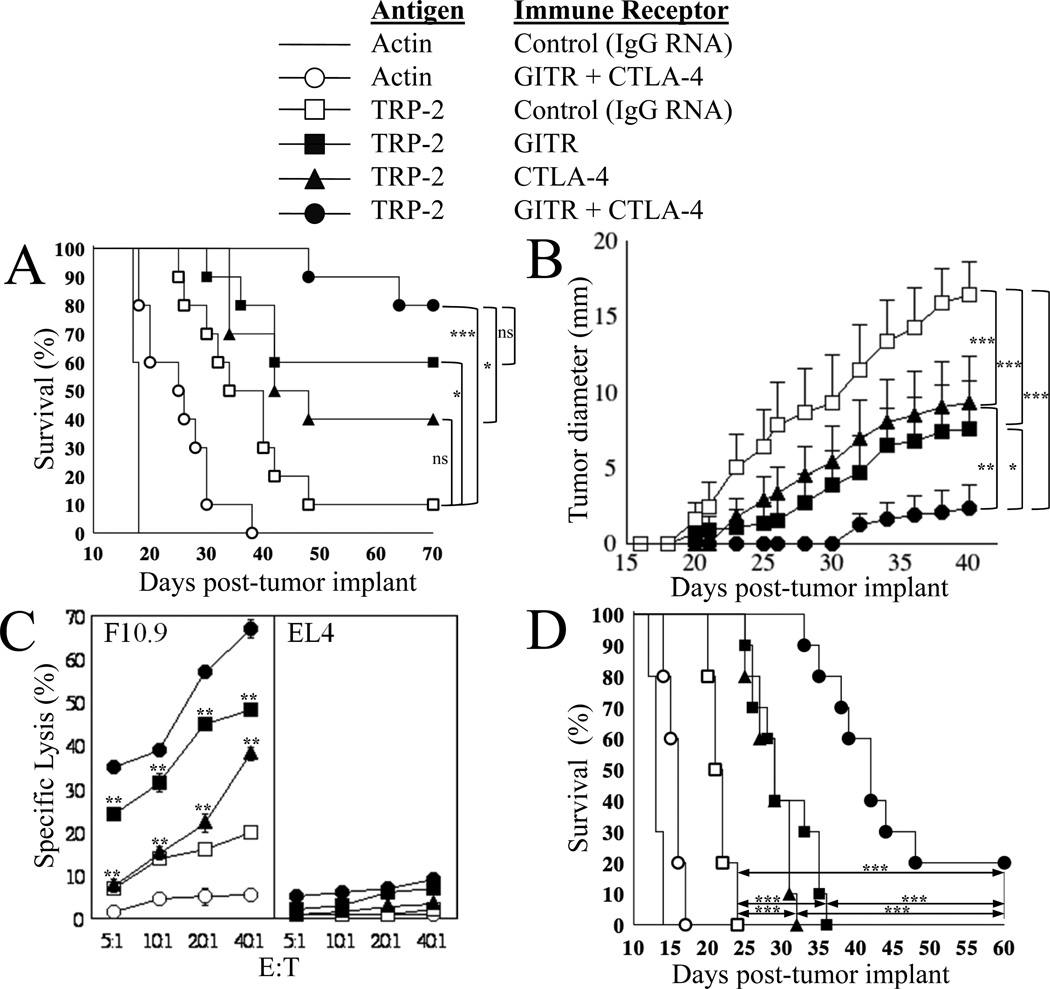
We previously demonstrated that in tumor-bearing mice vaccinated with DCs transfected with mRNA encoding the tumor antigen tyrosine related protein-2 (TRP-2), the co-administration of DCs transfected with mRNA encoding the H and L chains of an anti-GITR mAb significantly prolonged survival, while avoiding the induction of autoimmunity seen with systemic administration of anti-GITR mAb [17]. Using the same model, in the current study we evaluated the effect of co-administration of DCs transfected with mRNA encoding an anti-CTLA-4 mAb alone and of co-administration of DCs transfected with mRNA encoding both anti-GITR and anti-CTLA-4 mAbs on the induction of anti-tumor immunity in response to vaccination with DCs transfected with mRNA encoding the TAA TRP-2. As shown in Figure 1A, in control mice vaccinated with actin mRNA-transfected DCs, the co-administration of anti-GITR and anti-CTLA-4 mAb-encoding mRNA-transfected DCs minimally prolonged survival, suggesting that these immune modulator mRNA-transfected DCs may, to some degree, augment anti-tumor immunity in these animals even in the absence of antigen-specific vaccination. In tumor-bearing mice vaccinated with DCs transfected with mRNA encoding TRP-2, survival was significantly prolonged when DCs transfected with either anti-GITR mAb-encoding mRNA or anti-CTLA-4 mAb-encoding mRNA were co-administered. Survival was further, although not significantly, prolonged when a combination of both anti-GITR mAb mRNA-transfected and anti-CTLA-4 mAb mRNA-transfected DCs were co-administered, with 80% of these mice being tumor free at 120 days. No signs of autoimmunity were observed in any vaccinated mice. Importantly, when tumor diameter was measured as a function of time after tumor implantation, the co-administration of anti-CTLA-4 mAb mRNA-transfected and anti-GITR mAb mRNA-transfected DCs led to a significant reduction in tumor growth compared with co-administration of either immune modulator mRNA-transfected DC population alone (Figure 1B). These data support the hypothesis that local delivery of immune modulators enhances cancer vaccine efficacy.
Figure 1. Effect of local immune receptor modulation on anti-tumor immunity in response to tumor-specific immunization in a B16/F10.9 melanoma immunotherapy model.
(A) 7–8-week old C57BL/6 mice (10/group) were implanted with 2.5x104 B16/F10.9 tumor cells s.c. in the flank region, then vaccinated 2 days later. The Figure key depicts the antigen used and the immune receptor targeted in each group. For experiments testing the combination of TRP-2 tumor antigen mRNA-transfected DCs and mAb-encoding mRNA-transfected DCs, mice were immunized s.c. at the base of each ear pinna with 1x105 DCs for each DC group in 50 µl PBS/ear pinna. Total number of DCs per mouse was 4x105 for groups testing 2 combinations and 6x105 DCs per mouse for groups testing 3 combinations. Starting on day 10, tumor growth was monitored every other day by measuring the appearance of palpable (5–6 mm diameter) tumors and mice were sacrificed once the tumor diameter reached 20 mm. All surviving mice were tumor-free at day 120 with no evidence of autoimmunity, at which time they were sacrificed. Comparisons between groups at day 70 were performed using the log-rank test (Mantel-Haenszel test), *p<0.05, ***p≤0.001, ns=non-significant.
Median survival day: TRP-2+IgG: 37; TRP-2+anti-CTLA-4: 45; actin+anti-GITR+anti-CTLA-4: 25.5 and actin+IgG: 18. All other groups, median: undefined.
(B) In these same groups of mice, average tumor size ± SEM versus days post-tumor implant is displayed. Tumor growth curves over time were compared using one-way ANOVA for repeated measures (p<0.0001) with Bonferroni multiple comparison post-test to compare the four groups indicated, *p≤0.05, **p≤0.01, ***p≤0.001.
(C) 7–8-week old C57BL/6 mice (2/group) were implanted with 2.5x104 B16/F10.9 tumor and immunized as indicated in (A). Cells were harvested from the draining auricular lymph nodes 10-days post-immunization and the non-adherent cells were stimulated with DCs transfected with TRP-2 mRNA. Induction of TRP-2-specific CTLs was determined 5-days post-restimulation using a standard cytotoxicity assay as described in Materials and Methods. F10.9 and EL4 cells were used as targets. The standard deviation reflects variation between triplicate wells. ** p<0.01 as compared to TRP-2+anti-GITR+anti-CTLA-4 group (filled circles) using paired two-tailed Student’s t test.
(D) 7–8-week old C57BL/6 mice (10/group) were implanted with 3.0x104 B16/F10.9 tumor cells s.c. in the flank region. Mice were vaccinated once 7-days after tumor implantation, at which time 3mm diameter s.c. tumor deposits were palpable in all mice. DCs were transfected with the mRNA combinations as indicated in the Figure key. Mice were immunized s.c. at the base of each ear pinna with 1.5x105 DCs in 50 µl PBS/ear pinna. Total number of DCs per mouse was 3x105 for all groups tested. Starting on day 8, tumor growth was monitored and recorded when tumor size was 5mm and above. Mice were sacrificed once the tumor diameter reached 20 mm (used to determine percent survival). All surviving mice were tumor-free at day 60 with no evidence of autoimmunity, at which time they were sacrificed. Comparisons between groups on day 50 were performed using the log-rank test (Mantel-Haenszel test), ***p≤0.0001.
Median survival day: TRP-2+IgG: 21.5; TRP-2+GITR-L: 29; TRP-2+anti-CTLA-4: 29; TRP-2+GITR-L+anti-CTLA-4: 42; actin+GITR-L+anti-CTLA-4: 16 and actin+IgG: 13.
In order to study the mechanism of the antitumor activity of locally delivered immune modulators, we depleted selected T cell subsets before immunization and observed that the protective anti-tumor immune response induced by vaccination with TRP-2 mRNA-transfected DCs was completely abrogated by depletion of either CD4+ or CD8+ T cells (Supplemental Figure 1), indicating that both CD4+ and CD8+ T cells are responsible for the antitumor activity of this vaccination strategy.
In mice vaccinated with TRP-2 mRNA-transfected DCs co-administered with DCs transfected with control IgG mRNA, depletion of Treys prior to vaccination minimally improved survival (Supplemental Figure 1A). However, the co-administration of DCs transfected with either anti-GITR mAb mRNA (Supplemental Figure 1B) or anti-CTLA-4 mAb mRNA (Supplemental Figure 1C) prolonged survival to a much greater extent than did Treg depletion. Furthermore, Treg depletion did not add to the effect achieved with anti-GITR or anti-CTLA-4 mAb-secreting DCs. In our opinion, this suggests that our approach to GITR and CTLA-4 modulation not only overcomes Treg-mediated immune suppression but also has additional effects on the effector T cells responsible for enhanced tumor immunity.
The induction of anti-tumor CTL activity detected in lymphocytes harvested from lymph nodes draining the sites of DC vaccination correlated with the observed improvement in survival. As shown in Figure 1C, the highest CTL activity against the B16/F10.9 melanoma cell line was induced when a combination of anti-GITR mRNA-transfected and anti-CTLA-4 mRNA-transfected DCs was co-administered with DCs transfected with mRNA encoding TRP-2. Importantly, non-specific lytic activity, as assessed using EL4 tumor cell targets, was not significantly elevated in mice receiving co-administration of DCs transfected with mRNA encoding either or both of these immune modulatory mAbs.
We next evaluated our approach using a more stringent murine melanoma model in which mice were vaccinated 7 days after melanoma implantation. Additionally, in this experiment, DCs were co-transfected with a combination of TAA and immune modulatory mRNAs. To avoid heterologous associations between H and L chains of anti-CTLA-4 and anti-GITR mAbs in the mRNA-transfected DCs, instead of using anti-GITR mAb mRNA, DCs were transfected with mRNA encoding a soluble GITR-L fusion protein. In our prior study, we found that DCs transfected with either anti-GITR mAb mRNA or mRNA encoding soluble GITR-L had equivalent effects [17]. As shown in Figure 1D, a single vaccination with DCs co-transfected with TRP-2 mRNA, anti-CTLA-4 mAb mRNA, and mRNA encoding soluble GITR-L significantly prolonged survival in this stringent model. This prolongation was statistically significant when compared to vaccination with DCs transfected with either TRP-2 plus anti-CTLA-4 mAb mRNAs or TRP-2 plus soluble GITR-L mRNAs.
Human monocyte-derived DCs transfected with mRNA encoding either a humanized anti-CTLA-4 mAb or a soluble GITR-L fusion protein secrete functional protein
Our next goal was to generate mRNAs encoding human immune modulators that could be used for human studies. We therefore cloned the H and L chains of an anti-human CTLA-4 mAb and replaced the murine C regions with those of human IgG. We were unable to identify a source for an anti-human GITR mAb-producing hybridoma to use in our experiments using human cells (and potentially for future clinical use), but we previously demonstrated that the immune-modulating effects of DCs transfected with mRNA encoding a soluble murine GITR-L fusion protein construct were comparable to those observed with DCs transfected with mRNA encoding the H and L chains of an anti-GITR mAb [17]. Therefore, we constructed a gene encoding a soluble human GITR-L fusion protein.
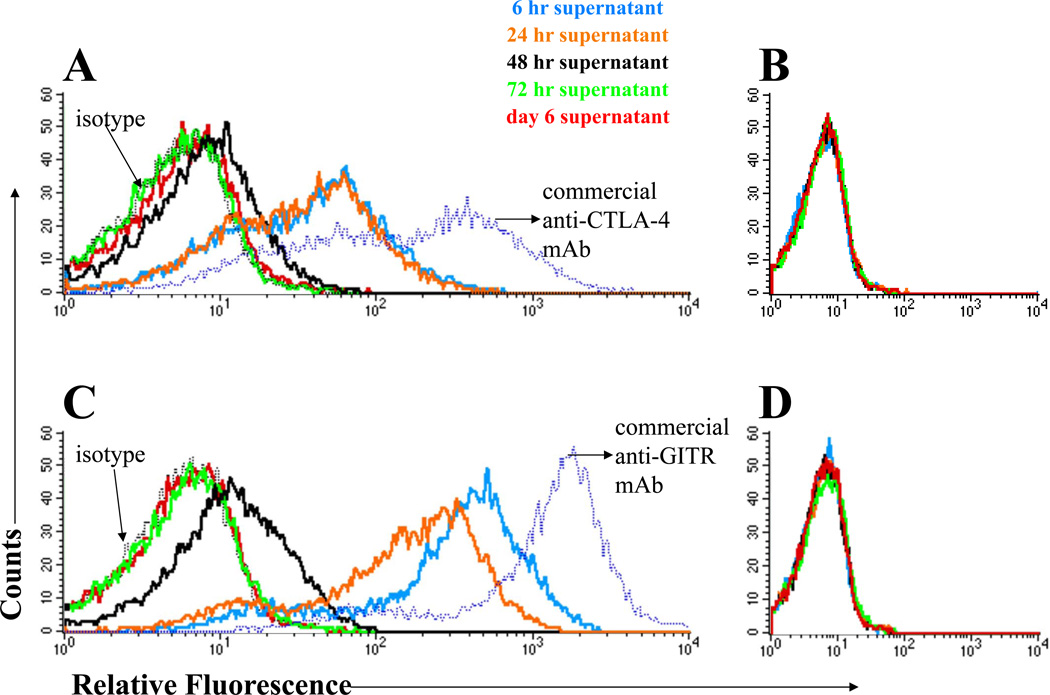
We then evaluated the time course of immune modulator secretion by human DCs after transfection with mRNA encoding these immune modulators. As shown in Figure 2, human monocyte-derived DCs transfected with mRNA encoding either the H and L chains of humanized anti-CTLA-4 (Panel A) or soluble GITR-L fusion protein (Panel C) secrete proteins that specifically bind to cells expressing their respective targets. In these assays, a sample of culture medium was collected at 6 h, but for all remaining time points, the entire supernatant was collected and replaced with fresh medium. Thus, for both anti-CTLA-4 mAb and soluble GITR-L, most of the secretion of immune modulator occurred within the first 6 h after mRNA transfection, with additional secretion continuing during the period from 24–48 h after mRNA transfection.
Figure 2. Secretion of immune modulators by DCs transfected with mRNA encoding humanized anti-CTLA-4 mAb or human GITR-L fusion proteins.
DCs were transfected with mRNA encoding humanized anti-CTLA-4 mAb or human GITR-L fusion protein. 106 cells were electroporated with 10 µg of anti-CTLA-4-encoding H chain mRNA and 5 µg of L chain mRNA or 10 µg of GITR-L/Fc fusion protein mRNA. The supernatant was harvested at the time-points indicated i and concentrated by centrifugation through a 50 kD cut-off filter (65–70-fold concentrated). A supernatant sample was collected at 6 h, then at each subsequent time-point, all of the supernatant was harvested and fresh medium containing GMCSF and IL-4 was added to the mRNA-transfected DCs. The concentrate was incubated with CHO cells expressing CTLA-4 (CHO-CTLA-4) or GITR (CHO-GITR). Binding of anti-CTLA-4 mAb and GITR-L fusion proteins in the supernatant to the CHO cells was determined by staining with biotinylated anti-human IgG followed by streptavidin-APCs. The cells were also stained with a commercially available antibody as a positive control. Cells were analyzed on a FACScalibur flow cytometer. (A) Supernatant from DCs transfected with anti-CTLA-4 mAb-encoding mRNA incubated with CHO-CTLA-4 cells. (B) Supernatant from DCs transfected with anti-CTLA-4 mAb-encoding mRNA incubated with cells that do not express CTLA-4. (C) Supernatant from DCs transfected with GITR-L fusion protein-encoding mRNA incubated with CHO-GITR cells. (D) Supernatant from DCs transfected with GITR-L fusion protein-encoding mRNA incubated with cells that do not express GITR. The results shown are representative of xxx independent experiments.
Melanoma antigen-specific immune responses induced by human monocyte-derived DCs transfected with mRNA encoding melanoma TAAs are enhanced by DCs transfected with mRNA encoding soluble immune modulators
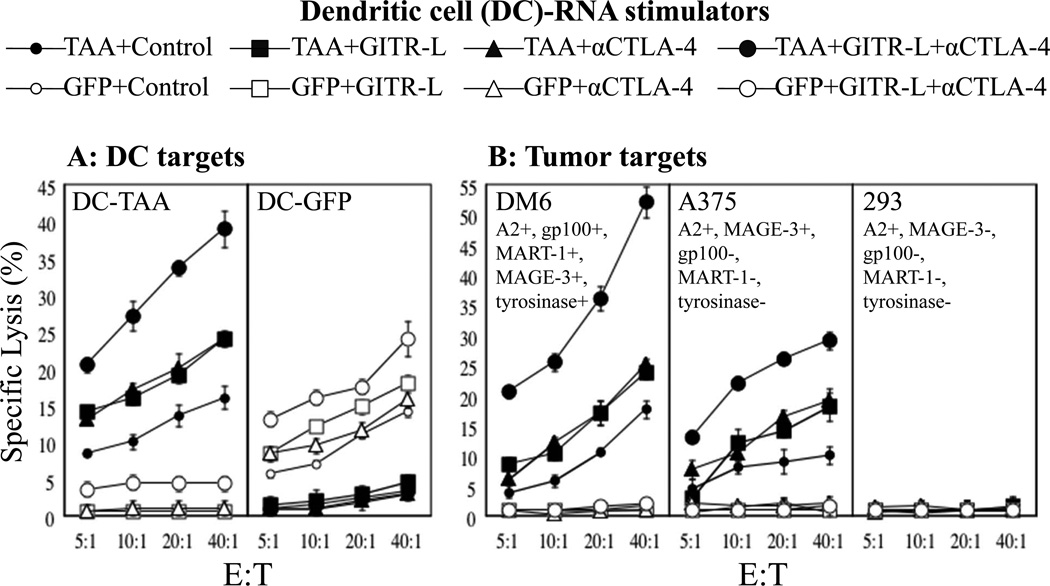
Using cells from HLA-A2+ human donors, we next evaluated the ability of DCs transfected with mRNA encoding soluble GITR-L and anti-CTLA-4 mAb to augment the induction of anti-melanoma CTL in response to DCs transfected with mRNA encoding four defined melanoma TAAs (MART, tyrosinase, MAGE-3, and gp100). As shown in Figure 3, panel A, DCs transfected with either TAA mRNA or GFP mRNA stimulated the induction of antigen specific CTL, as assessed by using mRNA-transfected autologous DCs as targets. Co-incubation of autologous T cells with DCs transfected with mRNA encoding either soluble GITR-L fusion protein or anti-CTLA-4 mAb alone enhanced the induction of melanoma TAA-specific CTL activity, while co-incubation with DCs transfected with mRNA encoding both of these immune modulators demonstrated a further enhancement of antigen-specific CTL induction, without an increase in non-specific background CTL activity.
Figure 3. Effect of DCs transfected with soluble GITR-L fusion protein mRNA and anti-CTLA-4 mAb mRNA on melanoma antigen-specific CTL responses.
DCs transfected with TAA mRNA (combination of MART-1, gp100, MAGE-3 and tyrosinase mRNAs) or GFP mRNA, and either control (actin) mRNA, GITR-L mRNA, humanized anti-CTLA-4 mAb (α-CTLA-4) mRNA, or GITR-L mRNA+α-CTLA-4 mRNA were used to stimulate autologous T cells weekly 2 times followed by a CTL assay. Induction of TAA-specific CTLs was measured using mRNA-transfected DCs and tumor targets. This experiment was performed three times, using cells from different donors, each with similar results. Data show mean +/−SD?. (A) DC targets: Targets were autologous DCs transfected with mRNA encoding TAA’s MART-1, gp100, MAGE-3 and tyrosinase (DC-TAA) or transfected with GFP (DC-GFP). (B) Tumor cell targets: Because the donor for these experiments was HLA-A2+, CTL activity was assessed against the HLA-A2+ melanoma cell lines DM6 and A375, and the HLA-A2+ tumor cell line 293 that does not express TAA’s MART-1, gp100, MAGE-3 or tyrosinase.
CTL activity against melanoma cells was also assessed. As shown in Figure 3 panel B, co-incubation with DCs transfected with mRNA encoding both GITR-L and anti-CTLA-4 mAb markedly enhanced the induction of melanoma TAA-specific CTL activity against DM6, an HLA-A2+ melanoma cell line which expresses all four melanoma TAAs used in these experiments, and against A375, an HLA-A2+ melanoma cell line which expresses only MAGE-3. No lytic activity was observed against the HLA-A2+ 293 cell line which does not express any of the four melanoma TAAs, suggesting that non-specific autoimmune responses were not induced in these in vitro experiments.
Breast cancer antigen-specific immune responses induced by human monocyte-derived DCs transfected with mRNA encoding breast cancer TAAs are enhanced by DCs transfected with mRNA encoding soluble immune modulators
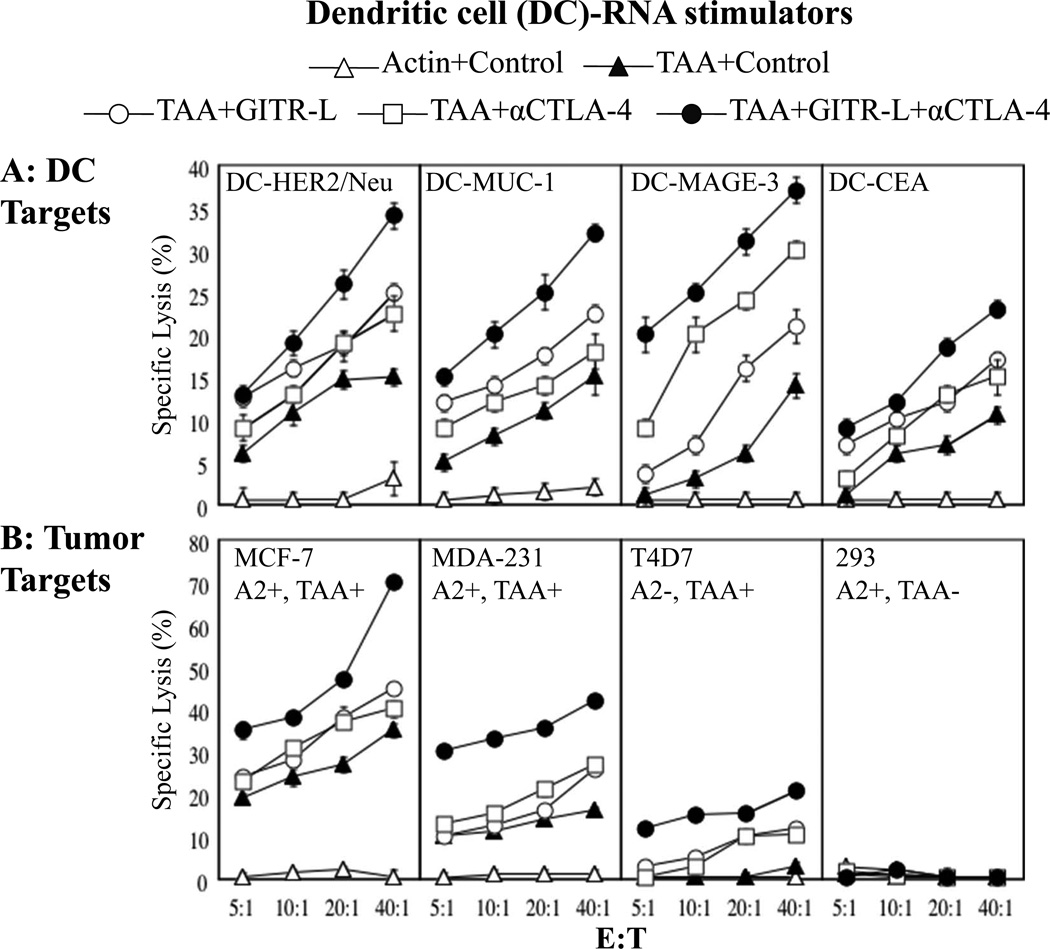
Again using cells from HLA-A2+ human donors in vitro, we evaluated the ability of DCs transfected with mRNA encoding soluble GITR-L and anti-CTLA-4 mAb to augment the induction of antigen-specific CTL in response to DCs transfected with mRNA encoding four defined breast cancer TAAs (CEA, MUC1, MAGE-3, and HER2/neu). As shown in Figure 4, panel A, DCs transfected with a combination of mRNAs encoding all four breast cancer TAAs stimulated the induction of antigen-specific CTL activity against each of the four TAAs, as assessed using autologous DCs transfected with individual breast cancer TAA-encoding mRNAs as targets. Co-incubation with DCs transfected with mRNA encoding either GITR-L fusion protein or anti-CTLA-4 mAb alone enhanced the induction of antigen-specific CTL activity, while co-incubation with DCs transfected with mRNA encoding both of these immune modulators demonstrated an additive enhancement of tumor antigen-specific CTL induction, without an increase in non-specific background CTL activity.
Figure 4. Effect of DCs transfected with soluble GITR-L protein mRNA and anti-CTLA-4 mAb mRNA on breast tumor antigen-specific CTL responses.
DCs transfected with TAA mRNA (combination of CEA, MUC-1, MAGE-3 and HER2/neu mRNAs) or GFP mRNA, and either control (actin) mRNA, GITR-L mRNA, humanized anti-CTLA-4 mAb (α-CTLA-4) mRNA, or GITR-L mRNA+α-CTLA-4 mRNA were used to stimulate autologous T cells weekly 2 times followed by a CTL assay. Induction of TAA-specific CTLs was measured using DCs and tumor targets. This experiment was performed twice, using cells from different donors, both with similar results. Data show mean +/−SD?. (A) Targets are DCs transfected with mRNA encoding the breast TAAs, HER2/neu, MUC-1, MAGE-3 or CEA. (B) Because the donor for these experiments was HLA-A2+, CTL activity was assessed against the HLA-A2+ breast cancer cell line MCF7 and MDA-231, the HLA-A2− breast cancer cell line T47D, and the HLA-A2+ tumor cell line 293 that does not express TAAs CEA, MUC-1, MAGE-3 or HER2/neu.
CTL activity against cultured breast cancer cells was also assessed. As shown in Figure 4B, co-incubation with DCs transfected with mRNA encoding both GITR-L and anti-CTLA-4 mAb further enhanced the induction of CTL activity against HLA-A2+ MCF-7 cells (which express CEA, HER2/neu and MUC1), while stimulating no lytic activity against the HLA-A2+ 293 cell line (that does not express any of the four breast cancer TAAs used in this study), suggesting that non-specific autoimmune responses were not induced. In this experiment, activity against HLA-A2- T47D breast cancer cells (which express breast TAAs MAGE-3 and MUC1), may be due to TAA-derived peptides expressed in the context of non-A2 class I molecules possibly shared by the cell donor and the T47D cells, as expression of HLA alleles other than A2 was not assessed in these experiments.
Secretion of soluble immune modulators by mRNA-transfected DCs inhibits the induction of Tregs and Treg-mediated suppression
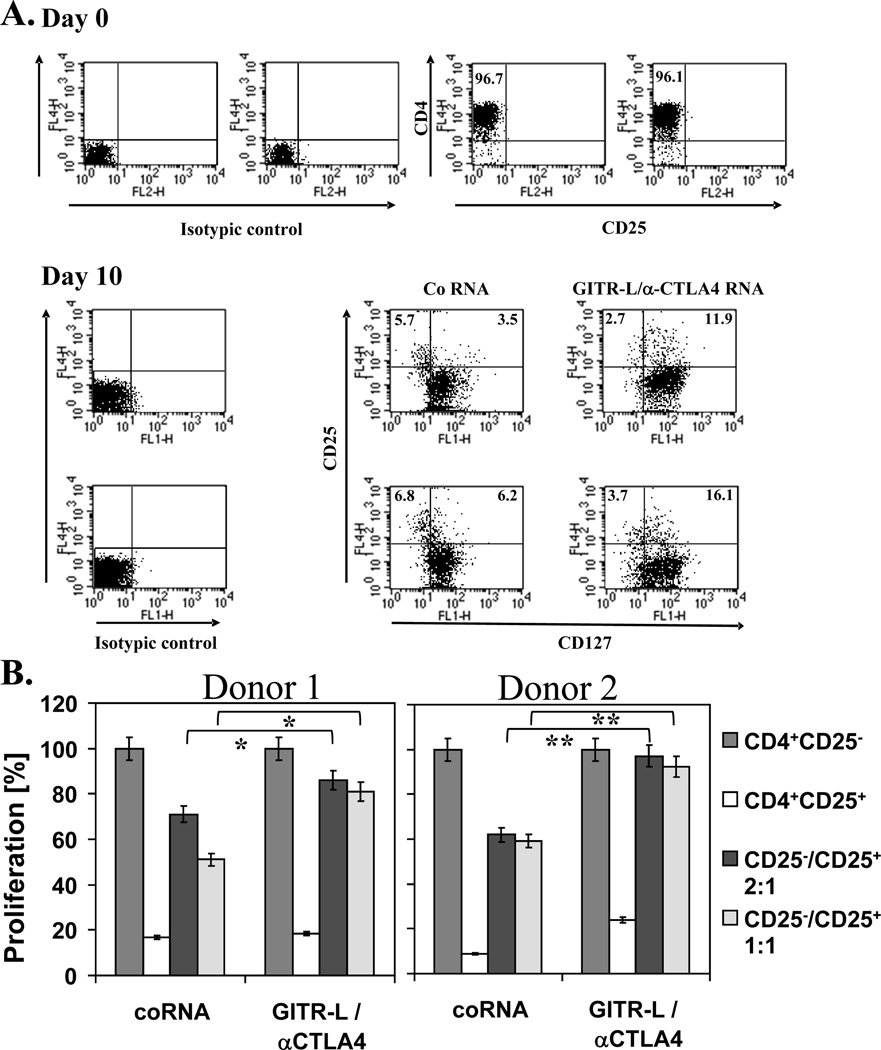
We next assessed the ability of human monocyte-derived DCs transfected with mRNA encoding these two immune modulators to inhibit the induction of Tregs in vitro. As shown in Figure 5A, the number of CD25+ CD127low Tregs induced when naïve CD4+ CD25-T cells were stimulated with cytokine and PGE2-matured autologous DCs was markedly reduced when the DCs were transfected with mRNA encoding both anti-CTLA-4 mAb and soluble GITR-L. DCs transfected with mRNA encoding either immune modulator alone did not significantly reduce the induction of CD25+ CD127low Tregs (data not shown). Similar results were obtained using cells from two donors when flow cytometric staining for CD25 and Foxp3 was used to assess Treg induction (data not shown).
Figure 5. Effect of DCs transfected with mRNA encoding anti-CTLA-4 mAb and soluble GITR-L protein on the induction of Treg cells in DC-T cell co-cultures and the suppressive function of induced CD4+CD25+ cells.
(A) CD4+CD25- T cells were isolated as described in Materials and Methods and cultured with mRNA-transfected matured DCs. After 10 days of culture, the induction of CD25+CD127low Treg cells was assessed by flow cytometry. This experiment was performed using cells from 2 donors, as indicated. (B) CD4+CD25+ T cells isolated from co-cultures of CD4+CD25- T cells with autologous DCs transfected with the indicated mRNAs were co-cultured with autologous CD4+CD25- T cells in the presence of anti-CD3 and anti-CD28 mAbs. After 4 days, cell proliferation was assessed using the WST-1 reagent, with absorbance at 450 nm reflecting the proliferative response. This experiment was performed using cells from 2 different donors, as indicated. Data show mean +/−SD?. Comparisons were made using the Student t-test, *p<0.05, **p<0.01.
To corroborate the results obtained by flow cytometric analysis of the DC-CD4 T-cell co-cultures, we isolated the induced CD4+CD25+ T cells and determined their suppressive function in proliferation assays. These isolated CD4+CD25+ T cells were cultured with autologous CD4+CD25- cells and proliferation was determined after 4 days. As can be seen in Figure 5B, CD4+CD25+ T cells induced by DCs transfected with mRNA encoding both anti-CTLA-4 mAb and GITR-L exhibited a significantly reduced capacity to inhibit CD4+CD25- T-cell proliferation compared to CD4+CD25+ T cells that had been induced in the presence of control mRNA-transfected DCs.
Discussion
Cancer immunotherapy is now demonstrating promising clinical activity [1]; however, major improvements in clinical outcome will likely require strategies to abrogate tumor-induced and normal host immunomodulatory mechanisms that tend to dampen the induction of T and B cell responses capable of eradicating established malignancies. Amongst the candidate target molecules are CTLA-4 and GITR.
GITR is constitutively expressed on Tregs but it is also expressed at low levels on multiple immune cells including naïve T cells, natural killer (NK) cells and B cells, on which it is upregulated upon activation [9, 15, 18–20]. Treatment of mice with an agonistic anti-GITR mAb alone enhances anti-viral and anti-tumor immune responses [10, 11, 20–24]. When combined with immunization against melanoma-related antigens, we and others have found that agonistic anti-GITR mAb-treated mice demonstrated increased tumor antigen-specific T cell responses and protective tumor immunity [10, 17, 25]. These data indicate that systemic treatment with agonistic anti-GITR mAb, alone or as an adjunct to active immunization, increases the ability to overcome immune tolerance.
CTLA-4, like GITR, is constitutively expressed at high levels on Tregs, but is also up-regulated on activated effector T cells. CTLA-4 is a negative regulator of T-cell activation which suppresses T-cell activity by binding the B7 molecules CD80 and CD86. In contrast to GITR, CTLA-4 is a co-inhibitory immune receptor and CTLA-4 blockade results in improved tumor immunity and tumor regression in several mouse models [7, 26–29]. In 1996, Allison and colleagues reported that the administration of an antagonistic CTLA-4-specific mAb resulted in rejection of pre-established murine tumors [27], an effect also observed in ovarian cancer patients [30]. In clinical studies, treatment with antagonistic CTLA-4-specific mAb induced objective cancer regression in some patients vaccinated with a gp100-derived peptide or with irradiated autologous GM-CSF-secreting tumor cells [4, 31, 32]. In a more recent study in patients with metastatic melanoma, regardless of immunization, treatment with anti-CTLA-4 mAb was found to significantly prolong survival [8]. In the absence of exogenous immunization, the induction of anti-tumor responses by systemic anti-CTLA-4 mAb probably relies on the ability of this mAb to antagonize signals inhibiting quiescent antigen-specific T cells or inhibiting immune regulatory mechanisms that normally control the induction of anti-tumor T cells in response to endogenous tumor antigens in subjects with residual disease.
A major concern with systemically administered immunomodulatory molecules is the induction of autoimmunity. Treatment of mice with anti-GITR mAb exacerbated autoimmune disease in susceptible mice [10, 20, 22]. In our previous study of anti-GITR mAb treated mice, we made similar observations, noting a statistically significant increase in autoimmune hypopigmentation [17].
In a recent study of the anti-CTLA-4 mAb Ipilimumab [8], immune related adverse events, including colitis, were associated with a slightly higher rate of treatment related death. Clinically, anti-CTLA-4 mAb treatment has resulted in severe autoimmune reactions in treated patients[6, 31–35]. In a clinical study in which 19 patients with stage III and IV melanoma were immunized with HLA-A2-restricted melanoma TAA peptides emulsified in adjuvant, co-administration of anti-CTLA-4 mAb led to autoimmunity in eight patients, in a dose dependent fashion [36]. Among 198 patients receiving systemic therapy with the anti-CTLA-4 mAb Ipilimumab, the most common serious toxicity was colitis, occurring in 21% of patients, with 4 patients experiencing colonic perforation, two of whom died. Interestingly, objective tumor response rates to anti-CTLA-4 mAb therapy were significantly higher for subjects who did develop colitis, when compared to those of subjects who did not [37].
Importantly, in our current study using mRNA-transfected DCs to locally deliver these immune modulators, anti-tumor immunity was enhanced, but autoimmunity was not observed in mice in vivo (Figure 1). Furthermore, no increase in non-specific background immune responses against control target cells using human cells in vitro was detected (Figures 3 and 4). Based on the data presented in this report, we anticipate that local delivery of anti-CTLA-4 mAb and soluble GITR-L using mRNA-transfected DCs will circumvent the autoimmune complications associated with systemically administered mAbs, while still augmenting vaccine-induced anti-tumor immune responses in human patients.
Another advantage of the local release of the anti-CTLA4 mAbs and GITR-stimulating molecules provided through mRNA transfection of DCs is the relatively short duration of their secretion at the key location where they are needed, at the site of T cell activation. Previous studies have demonstrated that mRNA has a half-life of less than 4 h after transfection into DCs, suggesting that translation of the mRNA into protein is a transient event in the DCs. In our current study, we confirmed that for both soluble GITR-L and anti-CTLA-4 mAb, most of the secreted proteins were released within 6 h of mRNA transfection, with additional release occurring for up to 24–48 h (Figure 2). Since human CD4+CD25+ Tregs, as well as CD4+CD25- T cells, have been shown to upregulate expression of CTLA-4 within 4 h of activation, this time course of DC secretion of anti-CTLA-4 mAb that we observed should be ideal for blocking CTLA-4 expressed by Tregs and CD4+ T cells [38].
An important observation of our study is that the local GITR stimulation and the CTLA4 inhibition combined to enhance the induction of immune responses; however, the mechanism for this additive effect remains to be fully elucidated. Several mechanisms by which anti-GITR mAb administration augments anti-tumor immunity have been proposed. Cohen et al. observed that anti-GITR mAb treatment led to a significant reduction of tumor infiltrating Foxp3+ Tregs [25]. Conversely, these investigators found that anti-GITR mAb administration did not appear to intrinsically alter the capacity of Tregs and effector T cells, harvested from tumor draining nodes, to exert suppressive effects or to be suppressed by Tregs, respectively [25], suggesting that anti-GITR mAb treatment does not function by globally suppressing Treg activity. In those studies, the augmentation of anti-tumor immunity in response to anti-GITR mAb treatment required GITR expression on both Tregs and effector T cells and required that the mAb be administered several days after tumor inoculation, suggesting that tumor antigen-specific T cell activation may be required in order for anti-GITR mAb treatment to enhance anti-tumor immunity [25]. Recent studies indicate that the enhanced immune response is due to ligation of GITR on the effector cells, making them resistant to Treg-mediated suppression, rather than to a direct negative regulatory effect on Treg function [9, 15, 18–20, 39–46].
Anti-GITR mAb treatment may also be augmenting anti-tumor immunity in response to vaccination by directly co-stimulating effector T cells [10, 24, 42]. We have previously demonstrated that murine DCs transfected with mRNA encoding either anti-GITR mAb or soluble GITR-L alone enhance the anti-tumor response to vaccination with murine melanoma TAA TRP-2 mRNA-transfected DCs in the murine B16 melanoma model [17]. The fact that enhanced anti-tumor immunity was only observed when TAA-loaded and anti-GITR mAb (or GITR-L) mRNA-transfected DCs were co-injected at the same site indicated that local immune modulator secretion mediated this effect, but it remains to be elucidated which cell population mediated this immune enhancement. Tuyaerts, et al., transfected human DCs with mRNA encoding GITR-L, generating DCs that expressed high levels of GITR-L on the cell surface. Such GITR-L-expressing DCs did not down-modulate Treg-mediated suppression, but did demonstrate enhanced T-cell co-stimulation and enhanced the induction of melanoma antigen-specific CD8+ T cells [46]. In our in vitro human experiments, DCs transfected with mRNA encoding soluble GITR-L alone did not suppress the induction of Tregs (data not shown). Only when DCs were transfected with mRNAs encoding both GITR-L and anti-CTLA-4 mAb did they appear to suppress the induction of Tregs (Figure 5A), and such DCs also reduced the suppressive effects of Tregs on CD4+ T cell proliferation (Figure 5B).
Regarding the effects of anti-CTLA-4, using a murine B16 melanoma model, Sutmullter, et al., found that the immune response to vaccination as well as survival was further improved when anti-CTLA-4 mAb was administered along with depletion of Tregs, suggesting that anti-CTLA-4 mAb does not primarily function by modulating CTLA-4 on Tregs [47]. Ribas and colleagues evaluated melanoma biopsies from patients treated systemically with anti-CTLA-4 mAb, and noted large numbers of CD8+ CTL in regressing lesions, but did not observe a significant effect on infiltration by Tregs [48]. Similarly, in patients treated at the NCI with Ipilimumab, no effect on peripheral blood Treg numbers or activity either in vitro or in vivo was detectable [31], suggesting that the anti-tumor immune effects of anti-CTLA-4 mAb are not mediated directly by inhibition or depletion of Tregs. In contrast, in those patients treated with the anti-CTLA-4 mAb Ticilimumab who had objective responses, reduced levels of Tregs were noted [49].
In our murine studies, the improved survival seen in TRP-2 mRNA-transfected DC-vaccinated mice when these mice were co-vaccinated with either anti-GITR mAb or anti-CTLA-4 mAb mRNA-transfected DC was superior to that seen with Treg depletion and was not further enhanced by Treg depletion (Supplemental Figure 1). This suggests that local delivery of these immune modulators not only overcomes Treg-mediated negative immune regulatory effects but also further stimulates anti-tumor immunity by other mechanisms.
Additional murine studies have demonstrated that co-administration of anti-GITR and anti-CTLA-4 mAbs have a synergistic effect, leading to eradication of tumors in a murine tumor model [11, 13, 50]. Mitsui et al. attribute the synergistic effect to [a] anti-CTLA-4 mediated increased CD8+ T cell infiltration into tumors and [b] anti-GITR mediated increase in cytokine secretion and Treg resistance, indicating the potential of combining immune modulatory signals that have distinct mechanisms. We observed additive effects in both the B16 mouse melanoma model in vivo (Figure 1) and in human cells in vitro (Figures 3 and 4) using DCs transfected with mRNA encoding immune modulators targeting GITR and CTLA-4.
Based on the results presented in this report, we have initiated a Phase I clinical trial in subjects with metastatic melanoma (ClinicalTrials.gov ID# NCT01216436). In this study, subjects are vaccinated with an intranodal injection of DCs transfected with mRNA encoding melanoma TAAs MART, MAGE-3, gp100 and tyrosinase, in combination with DCs transfected with mRNA encoding soluble human GITR-L and/or anti-CTLA-4 mAb. Since human studies have shown that only a small percentage (1–2%) of DCs migrate to the draining lymph nodes after intradermal injection, and that this migration occurs over a period of several days, our DCs transfected with mRNA encoding soluble GITR-L and/or anti-CTLA-4 mAb would be unlikely to secrete these proteins by the time they migrated to the draining lymph nodes (see Figure 2). To overcome this limitation, in our Phase I clinical trial we are injecting the DCs directly into inguinal and axillary nodes under ultrasound guidance. Of note, Nestle, et al., utilized the same intranodal route for injection of peptide-loaded DCs and noted clinical anti-melanoma responses in a few vaccinated patients, without severe toxicity [51]. We are also planning to evaluate this approach in a similar trial in breast cancer using DCs transfected with the breast cancer TAAs HER2/neu, Muc-1, MAGE-3 and CEA. We anticipate that this novel clinical approach will be safe and that the co-administration of DCs transfected with mRNA encoding soluble immune modulators targeting GITR and CTLA-4 will enhance anti-tumor immunity in response to vaccination, while avoiding the induction of autoimmunity seen with systemic administration of such immune modulators.
Materials and Methods
CLONING AND VALIDATION OF mRNA
Cloning of mRNA
RNA encoding Tumor Associated Antigen (TAA)
The genes encoding the full-length murine melanoma antigen TRP-2, as well as enhanced GFP and murine actin (as controls) have been inserted into the pSP73-Sph/A64 plasmid using PCR and standard molecular biological techniques [17, 52, 53]. The genes encoding human melanoma TAAs MART, tyrosinase, MAGE-3 and gp100 were amplified using polymerase chain reaction and template cDNA generated from RNA isolated from the melanoma cell line DM6, known to express these TAAs, and ligated into pcDNA3.1-64A [54]. Human HER2/neu was cloned using PCR and template cDNA synthesized from RNA isolated from the MCF-7 breast cancer cell line and ligated into pcDNA3.1-64A. The gene for MUC1 (kindly provided by Olivera Finn, University of Pittsburgh) was subcloned into pSP73-Sph/A64. CEACAM 6 was cloned using PCR and template cDNA synthesized from SW403 RNA, then inserted into plasmid pSP73-Sph/A64. SW403 is a CEA+ human tumor cell line that was obtained from ATCC (230-CCL).
RNA encoding anti-mouse GITR mAb, anti-mouse CTLA-4 mAb, soluble mouse GITR-L and rat IgG
Cloning of the H and L chains of control rat IgG and the anti-GITR mAb DTA-1, as well as a soluble mouse GITR-L fusion protein have been previously described [17]. The genes encoding the H and L chains of anti-CTLA-4 mAb 9H10 were cloned by RT-PCR. Total RNA was isolated from the 9H10 anti-mouse CTLA-4 secreting hybridoma cells with the RNeasy mini kit, (Qiagen, Valencia, CA). Five µg of RNA were used in a reverse transcription reaction with the RT primer 5’- ATTCTAGAGGCCGAGGCGGCCGACATG (T-30) VN-3’ and PowerScript RT (Clontech, Mountain View, CA) and the primer 5’- AAGCAGTGGTATCAACGCAGAGTGGCCATATTGGCCrGrGrG. The H chain was amplified from 10% of the RT reaction using Advantage 2 HF PCR mix (Clontech, Mountain View, CA) and the primers 5’- GAAAAGCTTGGCCATTGGGGCCGGTATCAACGCAGAGTGGCCATATTG-3’ and 5’- AAAGAATTCGGCCTTGTTGGCCTCAATTACCCAGAGACCGGGAG-3’. The L chain was amplified with the primers 5’- GAAAAGCTTGGCCATTGGGGCCGGTATCAACGCAGAGTGGCCATATTG-3’ and 5’- AAAGAATTCGGCCTTGTTGGCCCTAACACTCATTCCTGTTCAG-3’. The resulting PCR fragments were digested with SfiI and cloned into the SfiI site of the plasmid pSP73-Sph/PC61-L/A64, which has a T7 promoter and a polyT sequence that allow for the production of in vitro transcribed mRNA with a polyA tail of 64 residues.
RNA encoding humanized anti-human CTLA-4 mAb
The anti-CTLA-4 hybridoma, clone A3.6B10 was obtained from ATCC (Manassas, VA). Total RNA was isolated with the RNeasy mini kit, (Qiagen, Valencia, CA). Five µg of RNA was used in a reverse transcription reaction with the RT primer 5’- ATTCTAGAGGCCGAGGCGGCCGACATG (T-30) VN-3’ and PowerScript RT (Clontech, Mountain View, CA) and the primer 5’- AAGCAGTGGTATCAACGCAGAGTGGCCATATTGGCCrGrGrG. The H chain was amplified from 10% of the RT reaction using Advantage 2 HF PCR mix (Clontech, Mountain View, CA) and the primers 5’- AAAGAATTCGGCCTTGTTGGCCTCATTTACCCAGAGACCGGGAGATG -3’ and 5’-GAAAAGCTTGGCCATTGGGGCGGTATCAACGCAGAGTGGCCATATTG-3’. The L chain was amplified with the primers 5’- AAGAATTCGGCCTTGTTGGCCTAACACTCATTCCTGTTGAAGCTCTTG -3’ and 5’-GAAAAGCTTGGCCATTGGGGCGGTATCAACGCAGAGTGGCCATATTG-3’. The resulting PCR fragments were digested with HindIII and EcoRI and cloned into the HindIII and EcoRI sites of the plasmid pSP73-Sph/A64.
The V region of the H chain from clone A3.6B10 was then amplified from the plasmid containing the murine H chain cDNA using PCR and appropriate primers. The C region of human IgG2 was amplified by RT-PCR from total RNA isolated from human PBMCs. These two PCR products were then fused using overlapping PCR to produce a gene comprised of the cDNA encoding the VH of the A3.6B10 antibody linked in-frame to the CH of human IgG2. This fragment was digested with appropriate restriction enzymes and then cloned into pSP73-Sph/A64.
The V region of the L chain from clone A3.6B10 was amplified from the plasmid containing the cloned murine L chain gene using PCR and appropriate primers. The C region of human Igκ was amplified by RT-PCR from total RNA isolated from human PBMCs. These two PCR products were then fused using overlapping PCR to produce a gene comprised of the cDNA encoding the VL of the A3.6B10 antibody linked in-frame to the CL of human Igκ. This fragment was digested with appropriate restriction enzymes and then cloned into pSP73-Sph/A64.
RNA encoding soluble human GITR-L human Fc fusion protein
The signal sequence of human gp96 was amplified from a plasmid using PCR primers containing a HindIII site at the 5’ end and a PinAI site at the 3’ end. The hIgG CH2 and CH3 coding region was amplified from the plasmid pFUSE-hIgG1-Fc1 (Invivogen, San Diego CA) with primers: 5’-ATATATACCGGTGACAAAACTCACACATGCCCAC-3’ and 5’-ATATATGGCCGCTAGGGCCAGTTTACCCGGAGACAGGGAGAG-3’ resulting in a fragment with a PinAI site on the 5’ end and an SfiI site at the 3’ end. The hGITR-L extracellular domain was amplified from a plasmid containing the entire hGITR-L coding sequence with primers: 5’-ATATATGGCCCTAGCGGCCGAGACTGCTAAGGAGCCCTG-3’ and 5’-ATATATGTCGACGGCCTAACAGGCCCTAGGAGATGAATTGGGGATTTG-3’encoding an SfiI site at the 5’ end and a SalI site at the 3’ end. The three fragments were ligated into the HindIII and SalI sites of pSP73-Sph/A64//Not and the sequence was confirmed by automated sequencing reactions. The resulting plasmid, pSP73-Sph/hIg-hGITR-L/A64 is digested with SpeI for use as a template for the production of in vitro transcribed mRNA. The accuracy of the fully assembled soluble GITR-L fusion protein-encoding sequence was confirmed by DNA sequencing.
RNA encoding human CTLA-4/CD28 for generating CTLA-4-expressing CHO cells
Human CD4+ cells were isolated from PBMC by negative selection with the EasySep kit (StemCell Technologies, Vancouver, BC, Canada). CD25+ cells were isolated from the CD4+ cells by positive selection with EasySep. 720 ng RNA was used in a reverse transcription reaction with the RT primer 5’-ATTCTAGAGGCCGAGGCGGCCGACATG (T-30) VN-3’ and SMART MMLV RT (Clontech, Mountain View, CA). The extracellular and transmembrane portion of CTLA-4 was amplified with the primers 5’-TATATAAGCTTGCCACCATGGCTTGCCTTGGATTTCAG-3’ and 5’-CAGGAGCCTGCTCCTCTTACTTAGCATTTTGCTCAAAGAAAC-3’ and the intracellular portion of CD28 was amplified with the primers 5’-GTTTCTTTGAGCAAAATGCTAAGTAAGAGGAGCAGGCTCCTG-3’ and 5’-TATATAGAATTCAGGAGCGATAGGCTGCGAAG-3’. The resulting fragments were combined in a PCR with the primers: 5’-TATATAAGCTTGCCACCATGGCTTGCCTTGGATTTCAG-3’ and 5’-TATATAGAATTCAGGAGCGATAGGCTGCGAAG-3’. The product was inserted into the HindIII and EcoRI sites of pSP73-Sph/A64.
RNA encoding human GITR for generating GITR-expressing CHO cells
Human T cells were treated with anti-CD3 and anti-CD28 for 24h before total RNA was isolated using the RNeasy mini kit. Reverse transcription was performed as described for the cloning of CTLA-4/CD28, except 5 µg RNA was used in the reaction. Human GITR was amplified using the Advantage 2 GC PCR kit (Clontech, Mountain View, CA) and the primers: 5’-TATATAAGCTTGCCACCATGGCACAGCACGGGGCGATG-3’ and 5’-TATATAGGATCCTAAGACCCCACCCCATCAG-3’. The resulting fragment was cloned into the HindIII and BamHI sites of pSP73-Sph/A64.
In vitro transcription of mRNA
All plasmids were digested with SpeI for use as a template for in vitro transcription reactions using the mMESSAGE mMACHINE T7 kit (Ambion, Austin, TX) according to the manufacturer’s protocol. mRNA was purified with the RNeasy mini kit.
Validation of mRNA
RNA transfection
106 CHO cells or mature DCs were electroporated with 10 µg of anti-CTLA-4-encoding H chain mRNA and 5 µg of L chain mRNA or 10 µg of GITR-L/Fc fusion protein mRNA. The supernatant was harvested at various time-points and concentrated by centrifugation through a 50 kD cut-off filter (65–70-fold concentrated). A supernatant sample was collected at 6 h, then at each subsequent time-point, all of the supernatant was harvested and fresh medium containing GM-CSF and IL-4 was added to the mRNA-transfected DCs. The supernatants were analyzed as described below.
Generation of CHO cells expressing CTLA-4 and GITR
106 CHO cells were electroporated with 4 µg of mRNA encoding either human chimeric CTLA-4/CD28 or human GITR. Cells were incubated at 37°C for 24 h before being harvested with Cellstripper, an enzyme-free cell dissociation buffer (Mediatech, Inc, Herndon, VA).
Flow cytometry using CHO-CTLA-4 and CHO-GITR cells
For determining expression of cell-surface CTLA-4 or GITR, commercially available APC-labeled anti-CTLA-4 mAb (BD Pharmingen, San Jose, CA) or anti-GITR mAb (R&D, Minneapolis, MN) was used. Alternatively, CHO-CTLA-4 cells or CHO-GITR cells were treated with 20 µl of concentrated supernatant harvested from CHO cells or DCs transfected with anti-CTLA-4-encoding mRNA or GITR-L fusion protein-encoding mRNA. After washing, the cells were incubated with biotinylated anti-mouse Ig or anti-human Ig, as required (eBioscience, San Diego, CA). The cells were washed and incubated with streptavidin-APC (eBioscience,) before analysis on a FACScalibur flow cytometer (BD biosciences, San Jose, CA).
MURINE IN VIVO EXPERIMENTS
Mice
4–6 weeks old C57BL/6 mice (H-2b) were obtained from the Jackson Laboratory, Bar Harbor, ME. In conducting the research described in this manuscript, the investigators adhered to the "Guide for the Care and Use of Laboratory Animals" as proposed by the committee on care of Laboratory Animal Resources Commission on Life Sciences, National Research Council. The facilities at the Duke Vivarium are fully accredited by the American Association for Accreditation of Laboratory Animal Care, and all studies were approved by the Duke University Institutional Animal Care and Use Committee.
Cell lines and reagents
The F10.9 clone of B16 melanoma (B16/F10.9) of C57BL/6 origin is a highly metastatic, poorly immunogenic and a low class I-expressing cell line. Other cell lines used were DTA-1 cells (anti-murine GITR mAb secreting hybridoma cells, kindly provided by Dr. Shimon Sakaguchi), CHO cells and EL4 thymoma cells. Cells were maintained in DMEM supplemented with 10% FCS, 25 mM HEPES, 2 mM L-glutamine and 1 mM sodium pyruvate.
Generation and electroporation of murine DCs
DCs were generated from bone marrow precursors as described previously [17] in RPMI-5% FCS with GM-CSF (15 ng/ml) and IL-4 (10 ng/ml). GITR expression on bone marrow-derived DCs was low to negative (mature DCs ≤ 15–18% and immature DCs ≤ 10–12%, data not shown). After 7-days in culture, non-adherent cells were harvested as DCs and resuspended in Opti-MEM at 2.5–3×107/ ml. Cells were electroporated in 2-mm cuvettes (200 µl) at 300V for 500µs using an Electro Square Porator (ECM 830, BTX, San Diego, CA). Tumor antigen mRNA was used at 3 µg/106 DCs. For antibody-encoding mRNA transfections, 106 cells were electroporated with 10 µg of H chain antibody mRNA and 5 µg of L chain antibody mRNA or 10 µg of GITR-L/Fc fusion protein mRNA. Transfected DCs were matured for 5 h in GM-CSF+IL4 medium supplemented with LPS (Sigma # L265L, E.coli 026:B6) at 100 ng/ml. Cells were harvested and washed twice prior to use.
B16/F10.9 melanoma tumor immunotherapy model
C57BL/6 (H-2b) mice were implanted with 2.5–3×104 B16/F10.9 tumor cells s.c. in the flank region. Mice were vaccinated once either 2 days or 7 days after tumor implantation. Tumor-bearing mice were immunized s.c. at the base of the ear pinna with 1–1.5×105 mRNA-transfected DCs/ear pinna in 50 µL PBS for a total of 100 µl/mouse. For experiments testing the combination of tumor antigen mRNA-transfected DCs and mAb-encoding mRNA-transfected DCs, mice were immunized with 1–1.5×105 DCs for each group for a combined 2–3×105DCs/ear pinna in 50 µl PBS, for a total of 100 µl/mouse. Tumor growth was evaluated every other day starting on day 10. Mice were sacrificed once the tumor size reached 20-mm. Tumor-free mice were sacrificed after 65–70-days or as indicated in the figure legends.
CTL induction in vivo
C57BL/6 (H-2b) mice were implanted with B16/F10.9 tumor cells s.c. in the flank region and vaccinated once 2 days after tumor implantation, as described above. Cells were harvested from the draining auricular LN 7–10-days after immunization followed by adherence for 1 h. 107 non-adherent lymphocytes were cultured with 2×105 stimulator cells (DCs electroporated with TRP-2-encoding mRNA) in 5 ml of RPMI with 10% FCS, 1 mM sodium pyruvate, 100 IU/ml penicillin, 100 µg/ml streptomycin and 5×10-5 M ß-mercaptoethanol (supplemented RPMI) per well in a 6-well tissue culture plate. Effector T cells were harvested after 5-days followed by a standard europium-release in vitro cytotoxicity assay.
In vitro cytotoxicity assay
5×106 target cells were labeled with europium for 20 min at 4°C. Ten thousand europium-labeled targets and serial dilutions of effector cells at varying E:T ratios were incubated in 200 µl of CTL stimulation medium with no penicillin-streptomycin in 96-well V-bottom plates. The plates were centrifuged at 500xg for 3 min and incubated at 37°C for 4 h. 50 µl of the supernatant was harvested and added to 150 µl of enhancement solution (Wallac, Perkin-Elmer) in 96-well flat-bottom plates and europium release was measured by time resolved fluorescence using the VICTOR3 Multilabel Counter (Perkin-Elmer). Specific cytotoxic activity was determined using the formula: % specific release = [(experimental release - spontaneous release)/(total release - spontaneous release)]×100. Spontaneous release of the target cells was less than 25% of total release by detergent.
Statistical analysis
For tumor studies, comparison between two groups was performed using the log-rank test (Mantel-Haenszel test). Additional comparisons between groups were done by determining the median survival for each group. Tumor growth curves over time were compared using one-way ANOVA for repeated measures with Bonferroni multiple comparison post-test. Statistical significance in immune assays comparing two groups was done using paired two-tailed Student’s t test. A probability of less than 0.05 (P<0.05) was considered statistically significant.
HUMAN IN VITRO ASSAYS
Human DC generation
Cellular material used in these experiments was obtained from human subjects following informed consent using protocols approved by the Duke University Investigational Review Board. PBMC were isolated from a leukapheresis sample and frozen in a freezing mixture containing 90% autologous plasma and 10% DMSO at 5×107 cells/ml and 2 ml per cryovial. Frozen cells were maintained in a liquid N2 freezer. PBMC were thawed, washed in PBS and resuspended at 2×108 cells in 30 ml AIM-V media (Invitrogen) in T-150 tissue culture flasks. Cells were incubated for 1 h at 37°C, 5% CO2 in a humidified incubator. The non-adherent cells were harvested by rocking the flask from side to side to dislodge them. The adherent cells were replenished with 30 ml AIM-V supplemented with 800 U/ml human GM-CSF and 500 U/ml human IL-4, then incubated at 37°C. DCs were harvested on day 6, by collecting all non-adherent cells, followed by a cold PBS wash. Cells that were still adherent were dissociated with cell dissociation buffer (Invitrogen), 37°C for 20 min. The DCs were washed, counted and maintained on ice until use.
DC maturation
RNA-electroporated DCs (described above) were matured for 6–8 h in AIM-V media containing GM-CSF (800 U/ml), IL-4 (500 U/ml), TNF-α (10 ng/ml), IL-1ß (10 ng/ml), IL-6 (1000 U/ml), and PGE2 (1 µg/ml). All cytokines were obtained from Peprotech, Inc. PGE2 was purchased from Sigma.
In vitro stimulation of T cells with mRNA-transfected DCs
PBMC were thawed and resuspended in PBS and treated with DNase I (Sigma) at 200 U/ml for 20 min at 37°C. DNase I-treated PBMC were incubated for 1 h at 37°C, 5% CO2 in a humidified incubator. Non-adherent cells were harvested and T cells were negatively isolated using the EasySep T cell Enrichment Kit from StemCell Technologies. The isolated T cells were stimulated with mRNA-transfected, matured DCs at a responder T cell to stimulator DC ratio of 10:1 in the presence of 10 ng/ml IL-7. All stimulations were done in RPMI 1640 with 10% FCS, 2 mM L-glutamine, 20 mM HEPES, 1 mM sodium pyruvate, 0.1 mM MEM non-essential amino acids, 100 IU/ml penicillin, 100 µg/ml streptomycin and 5×10-5 M ß-mercaptoethanol (CTL stimulation medium). The responder T-cell concentration was 2×106 cells/ml. IL-2 was added at 100 U/ml on day 3. Cells were incubated at 37°C for 7 days. On day 7, the T cells were restimulated with mRNA-electroporated DCs at a responder to stimulator ratio of 10:1 in complete RPMI-10% FCS in the presence of 50 U/ml IL-2, with the T cells at 1–2×106 cells/ml in CTL stimulation medium. After 6–7 days the T cells were harvested, counted and used as effector T cells in a europium-release CTL assay (described above). Autologous DCs transfected with tumor antigen-encoding mRNA and tumor cells were used as targets.
Assessment of Treg induction
The induction of Tregs by mature DCs was assessed using the methods described by Banerjee, et al [55]. Briefly, monocyte-derived DCs were electroporated with either control mRNA, or with mRNA encoding anti-CTLA-4 mAb and/or mRNA encoding soluble GITR-L, then matured overnight using IL-1β, IL-6, TNF-α and PGE2. Using autologous PBMC, CD4+ cells were isolated by negative magnetic selection (Miltenyi) and were then depleted of CD25+ cells using positive selection (Miltenyi). The mature mRNA transfected DCs and autologous CD4+CD25- T cells were then co-cultured at a 1:10 ratio at 106 T cells/ml in RPMI-1640 supplemented with 10% fetal calf serum for 10 days. Cells were then simultaneously stained with anti-CD25-PE (eBioscience) and anti-CD127-biotin (Miltenyi) followed by staining with streptavidin-FITC (Sigma) to monitor the number of Tregs induced in DC-T cell co-cultures. For FACS analyses of the isolated CD4+CD25- starting population, cells were stained with anti-CD25-PE and anti-CD4-APC.
Assessment of Treg-mediated suppression
Autologous CD4+CD25+ cells induced during autologous DC/CD4+CD25- T cell co-cultures (see above) were isolated using magnetic bead-based negative selection for CD4 and positive selection for CD25 (Miltenyi). To maximize the purity of isolated CD4+CD25+ cells, the amount of anti-CD25 beads used was limited to 2 µL per 107 CD4+ lymphocytes. The purity of isolated CD4+CD25+ T cells as assessed by flow-cytometric analysis was greater than 96%. To determine the inhibitory capacity of these isolated induced CD4+CD25+ cells on the proliferation of CD4+CD25− responder cells, 2.0 × 104 CD4+CD25− T cells were cultured in the presence of anti-CD3 (OKT-3) and anti-CD28 (CD28.2) mAbs (10µg/ml each) with or without indicated numbers of induced CD4+CD25+ T cells at 37°C and 5% CO2 in 96-well U-bottom plates in 200 µL of 1640 RPMI medium supplemented with penicillin/streptomycin, 50µM β-mercaptoethanol and 10% fetal bovine serum. After 4 days, cells were diluted ten-fold in fresh medium and transferred to flat-bottomed 96-well plates, WST-1 (4-[3-(4-iodophenyl)-2-(4-nitrophenyl)-2H-5-tetrazolio]-1,3-benzene disulfonate) (Roche, Indianapolis, IN) was added according to the manufacturer’s recommendations and cells were incubated for 2 h at 37°C and 5% CO2. Absorbance at 450 nm was measured using a Bio-Tek ELISA reader (Winooski, VT).
Supplementary Material
Acknowledgments
We thank David Snyder for his expert technical assistance with the murine immunotherapy studies. This project was partially supported by grant UL1RR024128 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research (SN and DB), and by VA Merit Review grants (SKP, JD, DST).
List of Abbreviations
- (GITR)
glucocorticoid-induced TNFR-related protein
- (TAA)
tumor associated antigen
- (TRP-2)
tyrosinase-related protein-2
Footnotes
Conflict of Interest
The authors have no financial or commercial conflicts of interest.
References
- 1.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, et al. Sipuleucel-T Immunotherapy for Castration-Resistant Prostate Cancer. NEJM. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 2.Kaech SM, Wherry EJ, Ahmed R. Effector and memory T-cell differentiation: implications for vaccine development. Nat Rev Immunol. 2002;2:251–262. doi: 10.1038/nri778. [DOI] [PubMed] [Google Scholar]
- 3.Perez VL, Van Parijs L, Biuckians A, Zheng XX, Strom TB, Abbas AK. Induction of Peripheral T Cell Tolerance In Vivo Requires CTLA-4 Engagement. Immunity. 1997;6:411–417. doi: 10.1016/s1074-7613(00)80284-8. [DOI] [PubMed] [Google Scholar]
- 4.Cranmer LD, Hersh E. The Role of the CTLA4 Blockade in the Treatment of Malignant Melanoma. Cancer Investigation. 2007;25:613–631. doi: 10.1080/07357900701522315. [DOI] [PubMed] [Google Scholar]
- 5.Melero I, Hervas-Stubbs S, Glennie M, Pardoll DM, Chen L. Immunostimulatory monoclonal antibodies for cancer therapy. Nat Rev Cancer. 2007;7:95–106. doi: 10.1038/nrc2051. [DOI] [PubMed] [Google Scholar]
- 6.Hodi FS, Butler M, Oble DA, Seiden MV, Haluska FG, Kruse A, MacRae S, et al. Immunologic and clinical effects of antibody blockade of cytotoxic T lymphocyte-associated antigen 4 in previously vaccinated cancer patients. Proceedings of the National Academy of Sciences. 2008;105:3005–3010. doi: 10.1073/pnas.0712237105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quezada SA, Peggs KS, Curran MA, Allison JP. CTLA4 blockade and GM-CSF combination immunotherapy alters the intratumor balance of effector and regulatory T cells. J. Clin. Invest. 2006;116:935–1945. doi: 10.1172/JCI27745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, et al. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. New England Journal of Medicine. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nocentini G, Riccardi C. GITR: a multifaceted regulator of immunity belonging to the tumor necrosis factor receptor superfamily. European Journal of Immunology. 2005;35:1016–1022. doi: 10.1002/eji.200425818. [DOI] [PubMed] [Google Scholar]
- 10.Cohen AD, Diab A, Perales M-A, Wolchok JD, Rizzuto G, Merghoub T, Huggins D, et al. Agonist Anti-GITR Antibody Enhances Vaccine-Induced CD8+ T-Cell Responses and Tumor Immunity. Cancer Res. 2006;66:4904–4912. doi: 10.1158/0008-5472.CAN-05-2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ko K, Yamazaki S, Nakamura K, Nishioka T, Hirota K, Yamaguchi T, Shimizu J, et al. Treatment of advanced tumors with agonistic anti-GITR mAb and its effects on tumor-infiltrating Foxp3+CD25+CD4+ regulatory T cells. J. Exp. Med. 2005;202:885–891. doi: 10.1084/jem.20050940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Houot R, Levy R. T-cell modulation combined with intratumoral CpG cures lymphoma in a mouse model without the need for chemotherapy. Blood. 2009;113:3546–3552. doi: 10.1182/blood-2008-07-170274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mitsui J, Nishikawa H, Muraoka D, Wang L, Noguchi T, Sato E, Kondo S, et al. Two Distinct Mechanisms of Augmented Antitumor Activity by Modulation of Immunostimulatory/Inhibitory Signals. Clinical Cancer Research. 2010;16:2781–2791. doi: 10.1158/1078-0432.CCR-09-3243. [DOI] [PubMed] [Google Scholar]
- 14.Nomura T, Sakaguchi S. Naturally arising CD25+CD4+ regulatory T cells in tumor immunity. Curr Top Microbiol Immunol. 2005;293:287–302. doi: 10.1007/3-540-27702-1_13. [DOI] [PubMed] [Google Scholar]
- 15.Shevach EM, Stephens GL. The GITR-GITRL interaction: co-stimulation or contrasuppression of regulatory activity? Nat Rev Immunol. 2006;6:613–618. doi: 10.1038/nri1867. [DOI] [PubMed] [Google Scholar]
- 16.Weber J. Review: Anti CTLA-4 Antibody Ipilimumab: Case Studies of Clinical Response and Immune-Related Adverse Events. Oncologist. 2007;12:864–872. doi: 10.1634/theoncologist.12-7-864. [DOI] [PubMed] [Google Scholar]
- 17.Boczkowski D, Lee J, Pruitt S, Nair S. Dendritic cells engineered to secrete anti-GITR antibodies are effective adjuvants to dendritic cell-based immunotherapy. Cancer Gene Ther. 2009;16:900–911. doi: 10.1038/cgt.2009.39. [DOI] [PubMed] [Google Scholar]
- 18.Kanamaru F, Youngnak P, Hashiguchi M, Nishioka T, Takahashi T, Sakaguchi S, Ishikawa I, Azuma M. Costimulation via Glucocorticoid-Induced TNF Receptor in Both Conventional and CD25+ Regulatory CD4+ T Cells. J Immunol. 2004;172:7306–7314. doi: 10.4049/jimmunol.172.12.7306. [DOI] [PubMed] [Google Scholar]
- 19.Nocentini G, Giunchi L, Ronchetti S, Krausz LT, Bartoli A, Moraca R, Migliorati G, Riccardi C. A new member of the tumor necrosis factor/nerve growth factor receptor family inhibits T cell receptor-induced apoptosis. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:6216–6221. doi: 10.1073/pnas.94.12.6216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shimizu J, Yamazaki S, Takahashi T, Ishida Y, Sakaguchi S. Stimulation of CD25+CD4+ regulatory T cells through GITR breaks immunological self-tolerance. Nat Immunol. 2002;3:135–142. doi: 10.1038/ni759. [DOI] [PubMed] [Google Scholar]
- 21.La SKE, Kwon B. In vivo ligation of glucocorticoid-induced TNF receptor enhances the T-cell immunity to herpes simplex virus type 1. Exp Mol Med. 2005;37:193–198. doi: 10.1038/emm.2005.26. [DOI] [PubMed] [Google Scholar]
- 22.Ramirez-Montagut T, Chow A, Hirschhorn-Cymerman D, Terwey TH, Kochman AA, Lu S, Miles RC, et al. Glucocorticoid-Induced TNF Receptor Family Related Gene Activation Overcomes Tolerance/Ignorance to Melanoma Differentiation Antigens and Enhances Antitumor Immunity. J Immunol. 2006;176:6434–6442. doi: 10.4049/jimmunol.176.11.6434. [DOI] [PubMed] [Google Scholar]
- 23.Tanaka K, Ii K, Ichihara A, Waxman L, Goldberg AL. A high molecular weight protease in the cytosol of rat liver. I. Purification, enzymological properties, and tissue distribution. J. Biol. Chem. 1986;261:15197–15203. [PubMed] [Google Scholar]
- 24.Zhou P, L'Italien L, Hodges D, Schebye XM. Pivotal Roles of CD4+ Effector T cells in Mediating Agonistic Anti-GITR mAb-Induced-Immune Activation and Tumor Immunity in CT26 Tumors. J Immunol. 2007;179:7365–7375. doi: 10.4049/jimmunol.179.11.7365. [DOI] [PubMed] [Google Scholar]
- 25.Cohen AD, Schaer DA, Liu C, Li Y, Hirschhorn-Cymmerman D, Kim SC, Diab A, et al. Agonist Anti-GITR Monoclonal Antibody Induces Melanoma Tumor Immunity in Mice by Altering Regulatory T Cell Stability and Intra-Tumor Accumulation. PLoS ONE. 2010;5:e10436. doi: 10.1371/journal.pone.0010436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abrams SI. Role of anti-CTLA-4 therapies in the treatment of cancer. Current Opinion in Molecular Therapeutics. 2004;6:71–77. [PubMed] [Google Scholar]
- 27.Leach DR, Krummel MF, Allison JP. Enhancement of Antitumor Immunity by CTLA-4 Blockade. Science. 1996;271:1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 28.Santulli-Marotto S, Nair SK, Rusconi C, Sullenger B, Gilboa E. Multivalent RNA Aptamers That Inhibit CTLA-4 and Enhance Tumor Immunity. Cancer Res. 2003;63:7483–7489. [PubMed] [Google Scholar]
- 29.van Elsas A, Hurwitz AA, Allison JP. Combination Immunotherapy of B16 Melanoma Using Anti–Cytotoxic T Lymphocyte–associated Antigen 4 (CTLA-4) and Granulocyte/Macrophage Colony-Stimulating Factor (GM-CSF)-producing Vaccines Induces Rejection of Subcutaneous and Metastatic Tumors Accompanied by Autoimmune Depigmentation. J. Exp. Med. 1999;190:355–366. doi: 10.1084/jem.190.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hodi FS, Mihm MC, Soiffer RJ, Haluska FG, Butler M, Seiden MV, Davis T, et al. Biologic activity of cytotoxic T lymphocyte-associated antigen 4 antibody blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patients. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:4712–4717. doi: 10.1073/pnas.0830997100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maker AV, Yang JC, Sherry RM, Topalian SL, Kammula US, Royal RE, Hughes M, et al. Intrapatient Dose Escalation of Anti-CTLA-4 Antibody in Patients With Metastatic Melanoma. Journal of Immunotherapy. 2006;29:455–463. doi: 10.1097/01.cji.0000208259.73167.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Phan GQ, Yang JC, Sherry RM, Hwu P, Topalian SL, Schwartzentruber DJ, Restifo NP, et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:8372–8377. doi: 10.1073/pnas.1533209100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blansfield JA, Beck KE, Tran K, Yang JC, Hughes MS, Kammula US, Royal RE, et al. Cytotoxic T-Lymphocyte-Associated Antigen-4 Blockage Can Induce Autoimmune Hypophysitis in Patients With Metastatic Melanoma and Renal Cancer. Journal of Immunotherapy. 2005;28:593–598. doi: 10.1097/01.cji.0000178913.41256.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Small EJ, Tchekmedyian NS, Rini BI, Fong L, Lowy I, Allison JP. A Pilot Trial of CTLA-4 Blockade with Human Anti-CTLA-4 in Patients with Hormone-Refractory Prostate Cancer. Clinical Cancer Research. 2007;13:1810–1815. doi: 10.1158/1078-0432.CCR-06-2318. [DOI] [PubMed] [Google Scholar]
- 35.Yang JC, Hughes M, Kammula U, Royal R, Sherry RM, Topalian SL, Suri KB, et al. Ipilimumab (Anti-CTLA4 Antibody) Causes Regression of Metastatic Renal Cell Cancer Associated With Enteritis and Hypophysitis. Journal of Immunotherapy. 2007;30:825–830. doi: 10.1097/CJI.0b013e318156e47e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sanderson K, Scotland R, Lee P, Liu D, Groshen S, Snively J, Sian S, et al. Autoimmunity in a Phase I Trial of a Fully Human Anti-Cytotoxic T-Lymphocyte Antigen-4 Monoclonal Antibody With Multiple Melanoma Peptides and Montanide ISA 51 for Patients With Resected Stages III and IV Melanoma. J Clin Oncol. 2005;23:741–750. doi: 10.1200/JCO.2005.01.128. [DOI] [PubMed] [Google Scholar]
- 37.Beck KE, Blansfield JA, Tran KQ, Feldman AL, Hughes MS, Royal RE, Kammula US, et al. Enterocolitis in Patients With Cancer After Antibody Blockade of Cytotoxic T-Lymphocyte–Associated Antigen 4. Journal of Clinical Oncology. 2006;24:2283–2289. doi: 10.1200/JCO.2005.04.5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jago CB, Yates J, Olsen Saraiva CÂMara N, Lechler RI, Lombardi G. Differential expression of CTLA-4 among T cell subsets. Clinical & Experimental Immunology. 2004;136:463–471. doi: 10.1111/j.1365-2249.2004.02478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim J, Choi WS, Kang H, Kim HJ, Suh J-H, Sakaguchi S, Kwon B. Conversion of Alloantigen-Specific CD8+ T Cell Anergy to CD8+ T Cell Priming through In Vivo Ligation of Glucocorticoid-Induced TNF Receptor. J Immunol. 2006;176:5223–5231. doi: 10.4049/jimmunol.176.9.5223. [DOI] [PubMed] [Google Scholar]
- 40.Kohm AP, Carpentier PA, Anger HA, Miller SD. Cutting Edge: CD4+CD25+ Regulatory T Cells Suppress Antigen-Specific Autoreactive Immune Responses and Central Nervous System Inflammation During Active Experimental Autoimmune Encephalomyelitis. J Immunol. 2002;169:4712–4716. doi: 10.4049/jimmunol.169.9.4712. [DOI] [PubMed] [Google Scholar]
- 41.McHugh RS, Whitters MJ, Piccirillo CA, Young DA, Shevach EM, Collins M, Byrne MC. CD4+CD25+ Immunoregulatory T Cells: Gene Expression Analysis Reveals a Functional Role for the Glucocorticoid-Induced TNF Receptor. Immunity. 2002;16:311–323. doi: 10.1016/s1074-7613(02)00280-7. [DOI] [PubMed] [Google Scholar]
- 42.Nishikawa H, Kato T, Hirayama M, Orito Y, Sato E, Harada N, Gnjatic S, et al. Regulatory T Cell-Resistant CD8+ T Cells Induced by Glucocorticoid-Induced Tumor Necrosis Factor Receptor Signaling. Cancer Res. 2008;68:5948–5954. doi: 10.1158/0008-5472.CAN-07-5839. [DOI] [PubMed] [Google Scholar]
- 43.Ronchetti S, Zollo O, Bruscoli S, Agostini M, Bianchini R, Nocentini G, Ayroldi E, Riccardi C. Frontline: GITR, a member of the TNF receptor superfamily, is costimulatory to mouse T lymphocyte subpopulations. European Journal of Immunology. 2004;34:613–622. doi: 10.1002/eji.200324804. [DOI] [PubMed] [Google Scholar]
- 44.Stephens GL, McHugh RS, Whitters MJ, Young DA, Luxenberg D, Carreno BM, Collins M, Shevach EM. Engagement of Glucocorticoid-Induced TNFR Family-Related Receptor on Effector T Cells by its Ligand Mediates Resistance to Suppression by CD4+CD25+ T Cells. J Immunol. 2004;173:5008–5020. doi: 10.4049/jimmunol.173.8.5008. [DOI] [PubMed] [Google Scholar]
- 45.Tone M, Tone Y, Adams E, Yates SF, Frewin MR, Cobbold SP, Waldmann H. Mouse glucocorticoid-induced tumor necrosis factor receptor ligand is costimulatory for T cells. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:15059–15064. doi: 10.1073/pnas.2334901100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tuyaerts S, Van Meirvenne S, Bonehill A, Heirman C, Corthals J, Waldmann H, Breckpot K, et al. Expression of human GITRL on myeloid dendritic cells enhances their immunostimulatory function but does not abrogate the suppressive effect of CD4+CD25+ regulatory T cells. J Leukoc Biol. 2007;82:93–105. doi: 10.1189/jlb.0906568. [DOI] [PubMed] [Google Scholar]
- 47.Sutmuller RPM, van Duivenvoorde LM, van Elsas A, Schumacher TNM, Wildenberg ME, Allison JP, Toes REM, et al. Synergism of Cytotoxic T Lymphocyte-Associated Antigen 4 Blockade and Depletion of CD25+ Regulatory T Cells in Antitumor Therapy Reveals Alternative Pathways for Suppression of Autoreactive Cytotoxic T Lymphocyte Responses. The Journal of Experimental Medicine. 2001;194:823–832. doi: 10.1084/jem.194.6.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ribas A, Comin-Anduix Ba, Economou JS, Donahue TR, de la Rocha P, Morris LF, Jalil J, et al. Intratumoral Immune Cell Infiltrates, FoxP3, and Indoleamine 2,3-Dioxygenase in Patients with Melanoma Undergoing CTLA4 Blockade. Clinical Cancer Research. 2009;15:390–399. doi: 10.1158/1078-0432.CCR-08-0783. [DOI] [PubMed] [Google Scholar]
- 49.Reuben JM, Lee BN, Li C, Gomez-Navarro J, Bozon VA, Parker CA, Hernandez IM, et al. Biologic and immunomodulatory events after CTLA-4 blockade with ticilimumab in patients with advanced malignant melanoma. Cancer. 2006;106:2437–2444. doi: 10.1002/cncr.21854. [DOI] [PubMed] [Google Scholar]
- 50.Houot R, Goldstein MJ, Kohrt HE, Myklebust JH, Alizadeh AA, Lin JT, Irish JM, et al. Therapeutic effect of CD137 immunomodulation in lymphoma and its enhancement by Treg depletion. Blood. 2009;114:3431–3438. doi: 10.1182/blood-2009-05-223958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nestle FO, Alijagic S, Gilliet M, Sun Y, Grabbe S, Dummer R, Burg G, Schadendorf D. Vaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cells [see comments] Nature Medicine. 1998;4:328–332. doi: 10.1038/nm0398-328. [DOI] [PubMed] [Google Scholar]
- 52.Lee J, Dollins CM, Boczkowski D, Sullenger BA, Nair S. Activated B cells modified by electroporation of multiple mRNAs encoding immune stimulatory molecules are comparable to mature dendritic cells in inducing in vitro antigen-specific T-cell responses. Immunology. 2008;125:229–240. doi: 10.1111/j.1365-2567.2008.02833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nair S, Boczkowski D, Fassnacht M, Pisetsky D, Gilboa E. Vaccination against the Forkhead Family Transcription Factor Foxp3 Enhances Tumor Immunity. Cancer Res. 2007;67:371–380. doi: 10.1158/0008-5472.CAN-06-2903. [DOI] [PubMed] [Google Scholar]
- 54.Dannull J, Lesher D-T, Holzknecht R, Qi W, Hanna G, Seigler H, Tyler DS, Pruitt SK. Immunoproteasome Down-Modulation Enhances the Ability of Dendritic Cells to Stimulate Anti-Tumor Immunity. Blood. 2007;110:4341–4350. doi: 10.1182/blood-2007-04-083188. [DOI] [PubMed] [Google Scholar]
- 55.Banerjee DK, Dhodapkar MV, Matayeva E, Steinman RM, Dhodapkar KM. Expansion of FOXP3high regulatory T cells by human dendritic cells (DCs) in vitro and after injection of cytokine-matured DCs in myeloma patients. Blood. 2006;108:2655–2661. doi: 10.1182/blood-2006-03-011353. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.