To the Editor
Shwachman-Diamond syndrome (SDS, On-line Mendelian Inheritance in Man (OMIM) #260400) is an autosomal recessive condition, characterized by pancreatic exocrine insufficiency, skeletal abnormalities, bone marrow failure, and an increased risk of myelodysplastic syndrome (MDS) and acute myeloid leukaemia (AML), the latter occurring in 19–36% of patients (Shimamura, 2006). Compound heterozygous mutations in SBDS are identified in the majority of SDS patients. Of the two most frequently found mutations in SBDS, 183-184TA>CT and 258+2T>C, at least one is present in approximately 90% of affected individuals. These mutations are located in exon 2, and result from gene conversion with SBDSP1, the SBDS pseudogene (Boocock et al, 2003). Although its exact function remains unclear, the SBDS protein appears to have a role in ribosome maturation, and might have additional extraribosomal functions (Finch et al, 2011; Johnson & Ellis 2011).
Because of the increased risk of AML, but lack of a clear genotype-phenotype relationship in SDS (Kuijpers et al, 2005), we hypothesized that compound heterozygous SBDS mutations might be present in seemingly sporadic paediatric AML. Furthermore, we hypothesized that heterozygous mutations in SBDS might be present at increased frequency in sporadic AML compared to healthy controls, and might thus be a risk factor for AML development. Given the significant toxicity of standard chemotherapy and transplantation conditioning regimens in SDS patients with MDS or AML (Shimamura, 2006), but the reduction in morbidity after reduced-intensity conditioning regimens (Bhatla et al, 2008), the identification of AML patients carrying SBDS mutations seems clinically relevant.
In leukaemic blast cells derived at diagnosis from 160 paediatric AML patients (median age: 9.6 years (range: 0–18.5 years); 90 (56.3%) male, 70 (43.7%) female), who were enrolled in consecutive Berlin-Frankfürt-Münster, Dutch Childhood Oncology Group/UK Medical Research Council, and Leucemie Aique Myeloide Enfant AML treatment protocols between 1987 and 2008 (Hollink et al, 2011), we specifically amplified SBDS and not SBDSP1, as previously described, and sequenced exon 2 of SBDS (Calado et al, 2007). Germline material of the AML patients was not available, and we assume that SBDS gene variants found in leukaemic blast cells were constitutional and not acquired variants.
Two AML patients carried a heterozygous 258+2T>C mutation (carrier frequency 0.013). This mutation disrupts the donor splice site of intron 2 and results in the use of a cryptic donor splice site in exon 2, leading to a frameshift and premature protein truncation at codon 84 (Boocock et al, 2003). Furthermore, 28 of 160 AML patients carried the silent variant 201A>G (carrier frequency 0.175) (Fig 1). No compound heterozygous mutations in exon 2 of SBDS were detected. Of 168 Dutch blood bank donors, 1 carried the heterozygous 258+2T>C (carrier frequency 0.006). Furthermore, 3 of 168 blood bank donors carried a heterozygous 183-184TA>CT (carrier frequency 0.018), introducing a premature stop codon at amino acid 62. The silent variants 141C>T and 201A>G were present in 2 (carrier frequency 0.012) and 32 (carrier frequency 0.190) controls, respectively (Table I). In previously published controls cohorts, 183-184TA>CT was present in 1 of 70 individuals (carrier frequency 0.014) (Nakashima et al, 2004) and 0 of 100 individuals (Boocock et al, 2003), whereas 258+2T>C was absent in three published controls cohorts of 70, 100 and 276 individuals each (Boocock et al, 2003; Calado et al, 2007; Nakashima et al, 2004).
Figure 1.
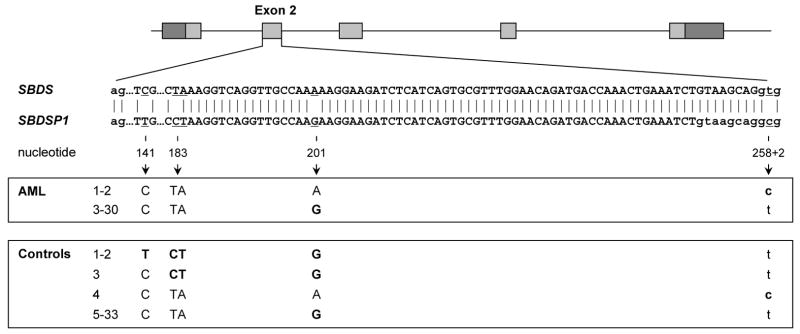
Graphical representation of paediatric AML patients and controls carrying SBDS nucleotide changes, depicted in bold, resulting from gene conversion events with SBDSP1 in and around exon 2. The absence of SBDSP1-like sequences at nucleotide 141, 183-184, and 201 in AML patients, and the absence of SBDSP1-like sequences at nucleotide 141, 183-184, 201, or 258+2 in controls, indicate the specificity of amplicons for SBDS. Figure adapted from Boocock et al ( 2003).
Table I.
SBDS gene variants resulting from gene conversion in paediatric AML patients and controls. Values represent the number of individuals carrying a variant (carrier frequency).
| Nucleotide change | Amino acid change | AML patients (n=160) | Controls (n=168) |
|---|---|---|---|
| Het. 141C>T | - | - | 2 (0.012) |
| Het. 183-184TA>CT | K62X | - | 3 (0.018) |
| Het. 201A>G | - | 28 (0.175) | 32 (0.190) |
| Het. 258+2T>C | C84fs3 | 2 (0.013) | 1 (0.006) |
AML, acute myeloid leukaemia; Het., heterozygous
We conclude that in a cohort of 160 paediatric AML patients, homozygous or compound heterozygous mutations in SBDS were absent, and heterozygous mutations in SBDS were present at frequencies comparable to healthy controls. Our findings confirm a previous report in which no mutations in exon 2 of SBDS were found in a smaller cohort of 48 children with de novo AML and 48 children with AML in remission (Majeed et al, 2005). Taken together, these results suggest that children with seemingly sporadic AML are unlikely to have underlying SDS.
Acknowledgments
AMA was supported by the KiKa Foundation, Amstelveen, The Netherlands, and the René Vogels Foundation, Oirschot, The Netherlands. This research was supported in part by the NIH (NHLBI) Intramural Research Program.
Footnotes
Authorship contributions
AMA, RTC, SK, NSY, RP, VHJV, MHE conceived and designed the experiments; AMA, SK performed the experiments; AMA, RTC, NSY, CMZ, SK, AB, KG, VH, GJLK, DR, JT, TWK, RP, VHJV, MHE contributed reagents, materials and analysis tools and wrote the paper.
Conflict of interest
The authors have no conflicts of interest to declare.
References
- Bhatla D, Davies SM, Shenoy S, Harris RE, Crockett M, Shoultz L, Smolarek T, Bleesing J, Hansen M, Jodele S, Jordan M, Filipovich AH, Mehta PA. Reduced-intensity conditioning is effective and safe for transplantation of patients with Shwachman-Diamond syndrome. Bone Marrow Transplantation. 2008;42:159–165. doi: 10.1038/bmt.2008.151. [DOI] [PubMed] [Google Scholar]
- Boocock GR, Morrison JA, Popovic M, Richards N, Ellis L, Durie PR, Rommens JM. Mutations in SBDS are associated with Shwachman-Diamond syndrome. Nature Genetics. 2003;33:97–101. doi: 10.1038/ng1062. [DOI] [PubMed] [Google Scholar]
- Calado RT, Graf SA, Wilkerson KL, Kajigaya S, Ancliff PJ, Dror Y, Chanock SJ, Lansdorp PM, Young NS. Mutations in the SBDS gene in acquired aplastic anemia. Blood. 2007;110:1141–1146. doi: 10.1182/blood-2007-03-080044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch AJ, Hilcenko C, Basse N, Drynan LF, Goyenechea B, Menne TF, Gonzalez Fernandez A, Simpson P, D’Santos CS, Arends MJ, Donadieu J, Bellanne-Chantelot C, Costanzo M, Boone C, McKenzie AN, Freund SM, Warren AJ. Uncoupling of GTP hydrolysis from eIF6 release on the ribosome causes Shwachman-Diamond syndrome. Genes & Development. 2011;25:917–929. doi: 10.1101/gad.623011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollink IH, van den Heuvel-Eibrink MM, Arentsen-Peters ST, Pratcorona M, Abbas S, Kuipers JE, van Galen JF, Beverloo HB, Sonneveld E, Kaspers GJ, Trka J, Baruchel A, Zimmermann M, Creutzig U, Reinhardt D, Pieters R, Valk PJ, Zwaan CM. NUP98/NSD1 characterizes a novel poor prognostic group in acute myeloid leukemia with a distinct HOX gene expression pattern. Blood. 2011;118:3645–3656. doi: 10.1182/blood-2011-04-346643. [DOI] [PubMed] [Google Scholar]
- Johnson AW, Ellis SR. Of blood, bones, and ribosomes: is Swachman-Diamond syndrome a ribosomopathy? Genes & Development. 2011;25:898–900. doi: 10.1101/gad.2053011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuijpers TW, Alders M, Tool AT, Mellink C, Roos D, Hennekam RC. Hematologic abnormalities in Shwachman Diamond syndrome: lack of genotype-phenotype relationship. Blood. 2005;106:356–361. doi: 10.1182/blood-2004-11-4371. [DOI] [PubMed] [Google Scholar]
- Majeed F, Jadko S, Freedman MH, Dror Y. Mutation analysis of SBDS in pediatric acute myeloblastic leukemia. Pediatric Blood & Cancer. 2005;45:920–924. doi: 10.1002/pbc.20416. [DOI] [PubMed] [Google Scholar]
- Nakashima E, Mabuchi A, Makita Y, Masuno M, Ohashi H, Nishimura G, Ikegawa S. Novel SBDS mutations caused by gene conversion in Japanese patients with Shwachman-Diamond syndrome. Human Genetics. 2004;114:345–348. doi: 10.1007/s00439-004-1081-2. [DOI] [PubMed] [Google Scholar]
- Shimamura A. Shwachman-Diamond syndrome. Seminars in Hematology. 2006;43:178–188. doi: 10.1053/j.seminhematol.2006.04.006. [DOI] [PubMed] [Google Scholar]


