Abstract
A growing body of research indicates connections exist between action, perception, and cognition in infants. In the present study, associated changes between sitting ability and upright face processing were tested in 111 infants. Using the visual habituation “switch” task (Cashon & Cohen, 2004; Cohen & Cashon, 2001), holistic processing of faces was assessed in same-aged non- and near-sitters (22–25 weeks) and same-aged new- and expert-sitters (27–32 weeks). U-shaped relation was found between sitting stage and holistic face processing such that only non-sitters and expert-sitters processed faces holistically. It is posited that the results are due to a reorganization of the upright face processing system resulting from infants’ learning to sit independently and trying to incorporate the meaning of upright faces.
Keywords: infant development, face perception, sitting, posture, U-shaped development, overload, reorganization
It has long been theorized that motor experience, perception, and cognition are intertwined during development (Adolph, 1997; Campos et al., 2000; Gibson, 1988; Piaget, 1952; Thelen, 1990). A resurging interest in this topic and a growing body of literature now supports notions of the interconnectedness between action and other areas of early development (e.g., Campos, Bertenthal, & Kermoian, 1992; Cicchino & Rakison, 2008; Higgins, Campos, & Kermoian, 1996; Meltzoff & Brooks, 2008; Needham, Barrett, & Peterman, 2002; Perone, Madole, Ross-Sheehy, Carey, & Oakes, 2008; Sommerville, Hildebrand, & Crane, 2008; Sommerville, Woodward, & Needham, 2005; Soska, Adolph, & Johnson, 2010; Uchiyama, et al., 2008). These findings highlight the importance of studying the roles that action or physical experience play in other areas of development in infancy (see also Rakison & Woodward, 2008).
A considerable number of studies linking action and other areas of development in infants have focused on locomotive skills (e.g., see Campos et al., 2000). Locomotion has been shown to be related to changes in social cognition (Meltzoff & Brooks, 2008), the encoding of self-propelled motion (Cicchino & Rakison, 2008), and even social interactions (Clearfield, 2011). Recent evidence linking sitting with other developmental changes has emerged as well. Soska, Adolph, and Johnson (2010) found that in conjunction with visual-manual exploration, independent sitting experience was related to the perception of 3D objects 4.5- to 7.5-month-old infants. There is also evidence that sitting is related to manual exploration of objects (Rochat & Goubet, 1995; Spencer, Vereijken, Diedrich, & Thelen, 2000; Soska et al., 2010) and better visuomotor coordination (Bertenthal, Rose, and Bai, 1997). These findings suggest that in addition to the development of locomotion, the development of sitting may be related to changes in other areas and warrants further consideration in developmental research.
One area of development that may be related to learning to sit is infants’ upright face processing. Recent research indicates that during the middle of the first year of life the development of holistic processing of upright faces follows a U-shaped trajectory as the face processing system becomes more attuned to upright over inverted faces (Cashon & Cohen, 2004; Cohen & Cashon, 2001). The regression in performance occurs around 6 months of age -- around the same age infants on average develop independent sitting (World Health Organization, 2006). Given this overlap in age and the growing body of research suggesting that action and perception are interconnected, such as sitting and 3D object perception (Soska et al., 2010), we reasoned that sitting and upright face processing may be interrelated. The goal of the present study was to investigate whether there was a relation between changes in infants’ sitting ability and changes in their processing of upright faces.
Holistic face processing (processing a face as a whole) is thought to be a hallmark of “expert-like” face perception. In adults, upright faces, but not inverted faces, are processed holistically (for review, see Maurer, Le Grand, & Mondloch, 2002). In infants, inversion is shown to affect holistic face processing by 7 months of age (Cohen & Cashon, 2001). The development of holistic face processing for upright faces is curvilinear, specifically quadratic (Cashon & Cohen, 2004): Between 3 and 4 months of age, infants develop holistic processing of upright and inverted faces; around 6 months, they fail to process faces in either orientation holistically; around 7–8 months, infants return to processing upright (but not inverted) face holistically showing the expert-like pattern found in adults.
Reasons for this temporary regression around 6 months are unknown. One hypothesis stems from a set of information-processing principles of infant cognitive and perceptual development (e.g., Cohen, Chaput, & Cashon, 2002; Cohen & Cashon, 2006), which includes the notion that development involves processing the relations between units of information (e.g., holistic processing of faces), but that additional information can cause infants to process at a lower level. Based upon this approach, Cashon and Cohen (2003; 2004) hypothesized that the regression found around 6 months may result from a reorganization of the face-processing system as infants struggle to incorporate new information coming from, for example, improved visual acuity or the addition of social significance of upright faces. There is some indirect evidence to support the idea that upright faces hold more significance than inverted faces around 6–7 months. Gliga et al (2009) found that at 6 months, upright faces hold infants’ attention longer than inverted faces even though faces in either orientation attract infants’ attention equally initially. Kestenbaum and Nelson (1990) found that 7-month-olds categorize expressive faces if the face stimuli are in an upright orientation, but not if they are in an inverted orientation. It is plausible that that through independent sitting, infants gain a new understanding of the social significance, or meaning, of upright faces, which then overloads the upright holistic face processing system as it tries to incorporate this new information.
In the present study, we tested the hypothesis that sitting ability is related to U-shaped, or quadratic, changes in holistic processing of upright faces. We compared the face processing abilities of infants at four different sitting stages: non-sitters, near-sitters, new-sitters, and expert-sitters. Pilot data indicated that there was substantial overlap in age between non- and near-sitters and between new- and expert-sitters, but not across all four sitting stages: The non- and near-sitters tended to be younger than 6 months, whereas the new- and expert-sitters tended to be older than 6 months. Therefore, we used a partial age-held constant design to control for age by recruiting infants into two age-matched groups: (1) non- and near-sitters (22–25 weeks) and (2) new- and expert-sitters (27–32 weeks) (see Figure 1). Infants’ face processing was tested using the “switch” holistic-face-processing task previously used (Cashon & Cohen, 2004; Cohen & Cashon, 2001). In this paradigm, holistic processing is measured by infants’ ability to notice that the internal features of one habituated face have been combined with the external features of another habituated face, that is, whether infants show a novelty preference for the “switch” face. Based upon our hypothesis that learning to sit leads to an information overload, we predicted that infants who had not begun learning to sit (non-sitters) and those who had mastered sitting (expert-sitters) would process faces holistically, whereas infants who were learning to sit (near- and new-sitters) would struggle to do so.
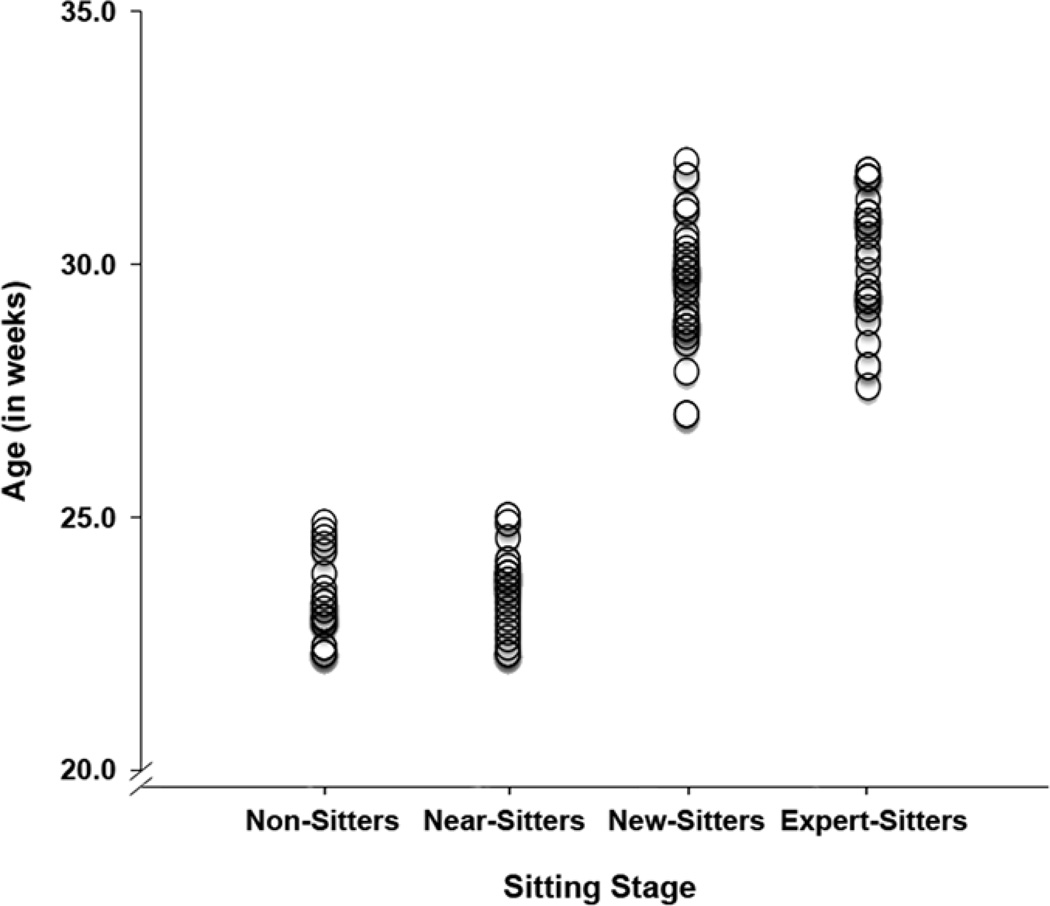
Figure 1.
Scatterplot of age by sitting stage in the younger (22–25 weeks) and older age groups (32–37 weeks).
Method
Participants
One-hundred eleven healthy, full-term infants participated in this study. Participants included 23 “non-sitters” (11 females, M age = 5.37 months, SD = .18), 25 “near-sitters” (13 females, M age = 5.41 months, SD = .19), 35 “new-sitters” (18 females, M age = 6.81 months, SD = .27), and 28 “expert-sitters” (13 females, M age = 6.92 months, SD = .29). Data from an additional 41 infants were excluded for reasons shown in Table 1. This seemingly high number of infants’ data excluded is not uncommon and is similar to or less than those reported by Cashon and Cohen (2004). Participants were recruited from a list of infants born in the local metropolitan area provided by the state birth records department. Parents were sent a letter inviting them to participate followed by a phone call. Participants received either a t-shirt or a bib for their voluntary participation. Participants in the final sample consisted of 89% Caucasian American infants and 9% African American infants.
Table 1.
Reasons for Exclusion by Sitting Stage
| Sitting Stage | ||||
|---|---|---|---|---|
| Reason for exclusion | Non-Sitters | Near-Sitters | New-Sitters | Expert-Sitters |
| Fussiness | 1 | 1 | 2 | 1 |
| Family interaction during testing | 0 | 0 | 0 | 1 |
| Did not meet habituation criteriona | 8 | 2 | 6 | 10 |
| Did not fully habituateb | 1 | 1 | 1 | 4 |
| Fatiguec | 0 | 0 | 1 | 1 |
Looking time during habituation phase did not decrease sufficiently to meet the habituation criterion before the maximum number of habituation trials.
Looking time during the familiar test trial was two standard deviations or greater above the mean.
Looking time during the novel test was less than 2 seconds.
Sitting Classification
Prior to the face-processing task, an experimenter assessed the infants’ sitting abilities. Parents placed their infants in an upright sitting position with their legs out in a C or V on a thin blanket on the floor facing the experimenter. Once the infant appeared to be in a stable position, parents were instructed to remove their support to allow the infant the opportunity to sit unassisted. The length of time infants could sit without support was timed in multiple trials. On at least two trials the experimenter engaged the infants by talking to them; on at least two additional trials, the experimenter showed a toy to them or allowed them to hold a toy. Sitting classification was based on each infant’s best attempt. Infants were classified as follows: (1) “non-sitters” were infants who were unable to sit upright even if in a tripod position (with at least one hand supporting some of their weight) for more than 2 seconds, (2) “near-sitters” were those who could sit upright unassisted or in a tripod position, 2–10 seconds, (3) “new-sitters” were those who could sit upright, without hand support, for more than 10 seconds and whose parents reported they had been doing so for less than 4 weeks, and (4) “expert-sitters” were those who could sit for more than 10 seconds and were reported as being able to do so for more than 4 weeks. If infants clearly met the sitting classification for a new- or expert-sitter, the assessment ended. In the rare case that it was ambiguous as to whether the infant was self-supported, the experimenter provided an additional trial. Each sitting session was videotaped. A second experimenter provided an independent assessment of each infant’s sitting classification for reliability purposes. Disagreements were reviewed by multiple experimenters. If experimenters could not agree unanimously on the classification, that infant’s data were not used (n = 3).
Holistic Face-Processing Task
Stimuli and Apparatus
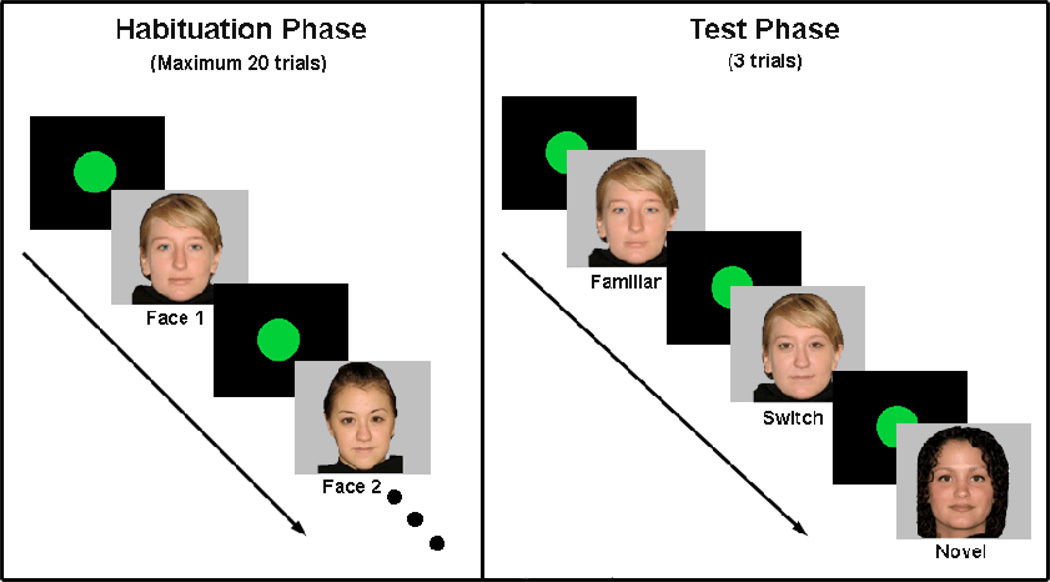
The stimulus set consisted of eight 22 cm × 23 cm color photographs of Caucasian American female faces. Because previous research has shown that infants develop differential processing of own- vs. other-race faces between 3–4 months and 8–9 months of age (Ferguson, Kulfofsky, Cashon, & Casasola, 2009; Kelly et al, 2007), a set of 8 African American stimuli were used for African American infants. All eight faces were used as habituation and test stimuli across participants, although each infant saw only 4 faces (see Figure 2). During the habituation phase, each infant saw two faces presented sequentially in a quasi-random order such that no face would be presented more than 3 times in a row. During the test phase, each infant saw a familiar test face (one of the habituation faces), a switch face, and a novel face. The “switch” face was created using Adobe® Photoshop® by copying the internal features of one habituation face and pasting them onto the other habituation face (see Cohen & Cashon, 2001 for details). Test order was counterbalanced across infants. The attention-getter, a digital movie of an expanding and contracting green ball accompanied by a “dinging” sound, was displayed to orient infants’ attention toward the center of the monitor before each face stimulus presentation.
Figure 2.
Examples of stimuli in habituation and test phases. Colored stimuli were used in the actual experiment.
Face stimuli were presented on a 50” Panasonic color plasma monitor. The experimenter observed infants from an adjacent control room via a hidden closed-circuit camera and recorded looking times on each trial. Stimuli were presented and looking times were recorded using Habit X (Cohen, Atkinson, & Chaput, 2004). For reliability purposes, a second experimenter collected data offline while viewing a video of each infant. Inter-observer Pearson correlations were calculated on habituation and test data for each infant. Data were excluded if they were deemed unreliable (i.e., there was at least a one-second difference in looking times recorded by the online and second experimenter on at least one trial). Data from 5 infants were deemed unreliable. The mean Pearson correlation was r = .99 (SD = .01).
Procedure
After sitting ability was assessed, each infant was seated on his or her parent’s lap in a dim room 120 cm away from the monitor. Once the infant’s attention was captured by the attention-getter, the experimenter presented a face stimulus. Each face stimulus stayed on the screen until the infant looked the entire trial length (20 s) or looked away for 1 s after viewing the face for at least 1 s. The attention-getter returned to the screen when either of these criteria was met. This pattern continued until either the infant’s looking time decreased, meeting the habituation criterion (average looking time to 4 consecutive trials is 50% or less than the average of the first 4 trials) or the infant viewed 20 trials (maximum). After the habituation phase, the test phase automatically began and the infant saw the familiar, switch and novel test faces (see Figure 1). The test trial order was counterbalanced and randomly assigned. In this task, significantly longer looking at the switch test face than the familiar test face is taken as evidence of “holistic processing.” The novel test was included to control for infant fatigue. In the event that infants did not look longer at the switch face than the familiar face, it would be important to know if that lack of response were due to overall fatigue or an inability to process holistically.
Results
Preliminary Tests of Age
Mean age in months (with standard deviations) for the four sitting stages are as follows: 5.37 (.18), 5.41 (.19), 6.81 (.27), and 6.92 (.29), respectively. To test whether we were successful in experimentally controlling for age within the younger (22–25 weeks) and older (27–32 weeks) age groups, separate t-tests were conducted. These tests revealed no significant age differences between non-sitters and near-sitters in the younger age group, t (46) = −.76, p = .45, or between new-sitters and expert-sitters in the older age group, t (61) = −1.51, p = .14. These findings provide evidence that we were successful in controlling for age experimentally between sitting groups in each of the two age groups.
To investigate further whether age was controlled for between sitting groups within each age groups, Spearman’s rank order correlations between age in months and sitting ability were computed (see Figure 1). Age and sitting were highly correlated when computed across all four sitting stages (rs = .80, p < .0001). However, when correlations were computed within each of the two age groups, age and sitting ability were not significantly correlated in either age group (rs = .13, p = .39 in the younger age group; rs = .18, p = .16 in the older age group). These preliminary analyses provide clear evidence that age was held constant between sitting groups within the younger age group and within the older age group, thus age of the sitting groups within the older and younger groups was not included in any further analyses.
Tests of Face Processing
To determine whether or not infants in each sitting group processed faces holistically, switch preference scores (equal to looking time during the switch test divided by looking time during the familiar plus switch tests) were calculated and compared to chance (.50). Consistent with our hypothesis, one-sample t-tests revealed that mean switch preference scores were significantly above chance only for the non-sitting and expert-sitting infants (see Table 2). These results are consistent with a quadratic trend and indicate that: (1) in the younger group, non-sitters processed faces holistically, but near-sitters did not, and (2) in the older group, expert-sitters processed face holistically, but new-sitters did not. For comparison purposes and to test for infant fatigue, mean novel preference scores (novel divided by familiar plus novel) were also calculated and analyzed. One-sample t-tests revealed that mean novel preference scores were significantly greater than chance for all four sitting groups (see Table 2), indicating that infants successfully discriminated the novel from the familiar test faces and were not fatigued regardless of sitting stage.
Table 2.
Preference Scores by Sitting Stage Compared to Chance (0.50)
| Sitting Stage | Preference | M | SD | t | Mean Difference |
95% CI |
|---|---|---|---|---|---|---|
| Younger group | ||||||
| Non-sitters | Switch | 0.61** | 0.16 | 3.44 | 0.12 | [0.05, 0.18] |
| (n = 23) | Novel | 0.58* | 0.14 | 2.80 | 0.08 | [0.02, 0.14] |
| New-sitters | Switch | 0.51 | 0.17 | 0.32 | 0.01 | [−0.06, 0.08] |
| (n = 25) | Novel | 0.56* | 0.15 | 2.09 | 0.06 | [0.00, 0.13] |
| Older group | ||||||
| New-sitter | Switch | 0.50 | 0.16 | 0.05 | 0.00 | [−0.05, 0.06] |
| (n = 35) | Novel | 0.62*** | 0.16 | 4.56 | 0.12 | [0.07, 0.17] |
| Expert-sitters | Switch | 0.55* | 0.12 | 2.10 | 0.05 | [0.00, 0.10] |
| (n = 28) | Novel | 0.61** | 0.16 | 3.80 | 0.11 | [0.05, 0.17] |
Note. All tests were two-tailed.
p < .05,
p < .01,
p < .001
To test for a quadratic trend more formally, mean switch preference scores were analyzed in a ANOVA with sitting ability as a between-subjects factor. The ANOVA revealed a significant main effect for sitting stage, F (3, 107) = 2.93, p = .04, partial η2 = .08, but more importantly, polynomial contrasts revealed a significant quadratic trend for sitting stage, t (107) = 2.62, p = .01, d = .51, 95% CI [0.02, 0.13]. This pattern of results was confirmed in a subsequent similar analysis in which age in months was included as a covariate (using the mean age for all four groups). In this analyses, sitting stage was found to be marginally significant, F (3, 106) = 2.38, p = .07, partial η2 = .06. More importantly, a significant quadratic trend for sitting stage remained, t (106) = 2.62, p = .01, d = .51, 95% CI [0.02, 0.14]. Age was not found be a significant covariate (p = .74). A similar set of GLM univariate ANOVAs was also conducted on the mean novel preference scores. Again, age was not found to be significant when included as a covariate. In contrast to the findings with the switch preference scores, these analyses did not reveal a significant main effect for sitting stage or quadratic trend, with or without age as a covariate.
Discussion
The present findings provide evidence for a U-shaped relation between sitting ability and upright face processing in infants in the 5–7 month age range. As predicted, it was found that infants who have not yet begun learning to sit (non-sitters) and those who have already mastered sitting (expert-sitters) process upright faces holistically, whereas those who are in the process of learning to sit (near- and new-sitters) fail to do so. Importantly, evidence of this non-linear relation was found when age was controlled for experimentally with each age group (i.e., by comparing age-matched non- and near-sitters and age-matched new- and expert-sitters) and when age was controlled for statistically across all four sitting stages (i.e., when age was entered as a covariate in the quadratic trend analyses for all sitting groups).
This quadratic pattern found here replicates the age-related U-shaped developmental curve previously found by Cashon and Cohen (2004). Although the present findings do not completely exclude age as a factor (given that we recruited infants into two age ranges), they do suggest that learning to sit is at least one factor involved in the curvilinear trend. Based upon an information-processing approach and the principles put forth by Cohen and colleagues, we believe that learning to sit independently may contribute to their learning about the significance of upright faces, which temporarily causes the face processing system to become overloaded until it reorganizes with the new information about upright faces. Another possibility, however, is that the holistic face processing system is connected to the postural control system. If connected, as sitting develops and the postural control system is temporarily disorganized, the holistic face processing system also would be temporarily disrupted. This explanation is consistent with an embodied cognition or dynamical systems approach (e.g., Anderson, 2003; Barsalou, 1999, 2008; Niedenthal, 2007; Smith & Semin, 2004). At this point, we can only speculate as to which of these factors, or combination of factors, may be involved in the non-linear relation found here between sitting and upright holistic face processing. Future research investigating the role of infants’ understanding of the social meaning of upright faces might help us shed light on which of these explanations is more likely to be correct.
Another important issue that should be considered in future research is whether the development of sitting is related to other aspects of face processing or even visual cognition. In the present study, sitting ability was found to be related to infants’ switch preference scores (a measure of face holistic processing) but not their novel preference scores (a measure of infant fatigue, but also a measure of face discrimination). These findings indicate that all aspects of face perception may not be related to sitting, at least in the same way. It is possible, for example, that the differential pattern is due to one face type (i.e., the switch face) being more difficult to process than the other (i.e., the novel face), and therefore more susceptible to information overload brought about by learning to sit. To further explore this issue, future work should include a longitudinal study of the development of different aspects of processing—face, visual, and social—as infants’ sitting abilities develop.
In the larger context, the findings add to the growing list of studies showing connections between action, perception and cognition during development, including sitting (e.g., Soska et al., 2010). However, the present findings are also unique in that they reveal a U-shaped relation. Most developmental changes that have been found to occur with physical changes are positive (but see Berger, 2010; Chen, Metcalfe, Jeka, & Clark, 2007; Corbetta & Bojczyk, 2002; for examples of U-shaped developmental curves within one domain, see Berko, 1958; Brown, 1973; Brown, Cazden, & Bellugi, 1969; Ervin, 1964; Gershkoff-Stowe & Thelen, 2004; Namy, Campbell, & Tomasello, 2004; Thelen & Fisher, 1982; Morton & Johnson, 1991). Corbetta and Bojczyk (2002) and Chen, Metcalfe, Jeka, and Clarke’s (2007) findings are U-shaped and, in this way, more similar to the present findings. Corbetta and Bojczyk found that when infants are learning to walk, they return to reaching with two-hands instead of one. Importantly, their infants were tested while sitting down and not while trying to walk. In the present study, infants’ face processing was associated with sitting ability in general, not their ability to sit independently while viewing faces, as each was supported on a parent’s lap during the face processing task. The authors hypothesized that the temporary return to two-handed reaching may reflect a reorganization of the neuromotor system that occurs while infants are trying to master walking. Similarly, Chen et al. (2007) found that when infants are learning to walk, their sitting ability is briefly (less than 1 month) disrupted displaying greater postural sway when sitting during this time of transition. Chen et al. posited that learning to walk forces the postural control system to recalibrate to include new sensorimotor information from walking. According to Chen et al., changes in postural control will affect other physical skills that involve an internal model of postural control. Regarding the present study, the question remains whether the postural control system may be directly connected to the face processing system in this way as well.
On a final note, we acknowledge that the current results should be interpreted with caution. Because we could not randomly assign infants to groups with different sitting abilities, this was essentially a correlational study. We cannot be certain that learning to sit causes changes in holistic face processing. It is possible that the related changes in sitting and face processing are caused by some other factor, such as maturation. At this point, we believe the best explanation is that learning to sit leads to information overload in the upright face processing system as infants try to incorporate new information about the meaning of upright faces; however, we acknowledge it is possible that an embodied cognition approach may also hold the answer. Either way, we posit that sitting may play a larger role in infants’ cognitive and perceptual development than previously realized and suggest that sitting, as well as other aspects of physical development, should not be overlooked as factors to consider in future developmental research.
Acknowledgments
This research was supported in part by National Institutes of Health Grant P20 RR017702 and University of Louisville Internal Research Incentive Grant 50456. We thank the Kentucky Cabinet for Health and Family Services and the students in the UofL Infant Cognition Laboratory for their help in recruitment and data collection. We thank Guy. O. Dove and Chris A. DeNicola for their comments on previous versions of this manuscript. We are especially grateful to all the parents and infants who participated in this study.
Contributor Information
Cara H. Cashon, Department of Psychological and Brain Sciences, University of Louisville, Louisville, Kentucky, USA
Oh-Ryeong Ha, Department of Psychological and Brain Sciences, Louisville, Kentucky, USA.
Casey L. Allen, Department of Psychological and Brain Sciences, Louisville, Kentucky, USA
A. Cevelle Barna, Department of Psychological and Brain Sciences, Louisville, Kentucky, USA.
References
- Adolph KE. Learning in the development of infant locomotion. Monographs of the Society for Research in Child Development. 1997;62(3) Serial No. 251. [PubMed] [Google Scholar]
- Anderson ML. Embodied cognition: A field guide. Artificial Intelligence. 2003;149:91–130. [Google Scholar]
- Barsalou LW. Perceptual symbol systems. The Behavioral and brain sciences. 1999;22:577–660. doi: 10.1017/s0140525x99002149. [DOI] [PubMed] [Google Scholar]
- Barsalou LW. Grounded cognition. Annual review of psychology. 2008;59:617–645. doi: 10.1146/annurev.psych.59.103006.093639. [DOI] [PubMed] [Google Scholar]
- Berger SE. Locomotor expertise predicts infants’ perseverative errors. Developmental Psychology. 2010;46:326–336. doi: 10.1037/a0018285. [DOI] [PubMed] [Google Scholar]
- Berko J. The child’s learning of English morphology. Word. 1958;14:150–177. [Google Scholar]
- Bertenthal BI, Rose JA, Bai DL. Perception-action coupling in the development of visual control of posture. Journal of Experimental Psychology. 1997;23:1631–1643. doi: 10.1037//0096-1523.23.6.1631. [DOI] [PubMed] [Google Scholar]
- Brown R. A first language. Cambridge, Mass: Harvard University Press; 1973. 1973. [Google Scholar]
- Brown R, Cazden C, Bellugi U. The child’s grammar from I to III. In: Hill JP, editor. Minnesota Symposium on Child Psychology. Minneapolis: University of Minnesota Press; 1969. pp. 28–74. [Google Scholar]
- Campos JJ, Anderson DI, Barbu-Roth MA, Hubbard EM, Hertenstein MJ, Witherington D. Travel broadens the mind. Infancy. 2000;1:149–219. doi: 10.1207/S15327078IN0102_1. [DOI] [PubMed] [Google Scholar]
- Campos JJ, Bertenthal BI, Kermoian R. Early experience and emotional development: The emergence of wariness of heights. Psychological Science. 1992;3:61–64. [Google Scholar]
- Cashon CH, Cohen LB. The construction, deconstruction, and reconstruction of infant face perception. In: Slater A, Pascalis O, editors. The development of face processing in infancy and early childhood. New York: NOVA Science Publishers; 2003. pp. 55–68. [Google Scholar]
- Cashon CH, Cohen LB. Beyond U-shaped development in infants’ processing of faces: An information-processing account. Journal of Cognition and Development. 2004;5:59–80. [Google Scholar]
- Chen C, Metcalfe JS, Jeka JJ, Clarke JE. Two steps forward and one back: Learning to walk affects infants’ sitting posture. Infant Behavior and Development. 2007;30:16–25. doi: 10.1016/j.infbeh.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Cicchino JB, Rakison DH. Producing and processing self-propelled motion in infancy. Developmental Psychology. 2008;44:1232–1241. doi: 10.1037/a0012619. [DOI] [PubMed] [Google Scholar]
- Clearfield MW. Learning to walk changes infants’ social interactions. Infant Behavior and Development. 2011 doi: 10.1016/j.infbeh.2010.04.008. [DOI] [PubMed] [Google Scholar]
- Cohen LB, Atkinson DJ, Chaput HH. Habit X: A new program for obtaining and organizing data in infant perception and cognition studies (version 1.0) Austin, TX: The University of Texas; 2004. [Computer Software] [Google Scholar]
- Cohen LB, Cashon CH. Do 7-month-old infants process independent features or facial configurations? Infant and Child Development. 2001;10:83–92. [Google Scholar]
- Cohen LB, Cashon CH. Infant cognition. In: Damon W, Lerner RM, Kuhn D, Siegler RS, editors. Handbook of child psychology: Vol. 2. Cognition, Perception, and Language. 6th ed. New York: Wiley; 2006. pp. 214–251. (Series Eds.) (Vol. Eds.) [Google Scholar]
- Cohen LB, Chaput HH, Cashon CH. A constructivist model of infant cognition. Cognitive Development. 2002;17:1323–1343. [Google Scholar]
- Corbetta D, Bojczyk KE. Infants return to two-handed reaching when they are learning to walk. Journal of Motor Behavior. 2002;34:83–95. doi: 10.1080/00222890209601933. [DOI] [PubMed] [Google Scholar]
- Ervin SM. Imitation and structural change in children’s language. In: Lenneberg E, editor. New directions in the study of language. Cambridge, MA: MIT Press; 1964. [Google Scholar]
- Ferguson KT, Kulkofsky S, Cashon CH, Casasola M. The development of specialized processing of own-race faces in infancy. Infancy. 2009;14:263–284. doi: 10.1080/15250000902839369. [DOI] [PubMed] [Google Scholar]
- Gershkoff-Stowe L, Thelen E. U-shaped changes in behavior: A dynamic systems perspective. Journal of Cognition and Development. 2004;5:11–36. [Google Scholar]
- Gibson EJ. Exploratory behavior in the development of perceiving, acting, and acquiring of knowledge. Annual Review of Psychology. 1988;39:1–42. [Google Scholar]
- Gliga T, Elsabbagh M, Andravizou A, Johnson MH. Faces attract infants’ attention in complex displays. Infancy. 2009;14:550–562. doi: 10.1080/15250000903144199. [DOI] [PubMed] [Google Scholar]
- Higgins CI, Campos JJ, Kermoian R. Effect of self-produced locomotion on infant postural compensation to optic flow. Developmental Psychology. 1996;32:836–841. [Google Scholar]
- Kelly DJ, Quinn PC, Slater AM, Lee K, Ge L, Pascalis O. The other-race effect develops during infancy: Evidence of perceptual narrowing. Psychological Science. 2007;18:1084–1089. doi: 10.1111/j.1467-9280.2007.02029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kestenbaum R, Nelson CA. The recognition and categorization of upright and inverted emotional expressions by 7-month-old infants. Infant Behavior and Development. 1990;13:497–511. [Google Scholar]
- Maurer D, Le Grand R, Mondloch CJ. The many faces of face processing. Trends in Cognitive Psychology. 2002;6:255–260. doi: 10.1016/s1364-6613(02)01903-4. [DOI] [PubMed] [Google Scholar]
- Meltzoff AN, Brooks R. Self-experience as a mechanism for learning about others: A training study in social cognition. Developmental Psychology. 2008;44:1257–1265. doi: 10.1037/a0012888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton J, Johnson CONSPEC and CONLERN: A two-process theory of infant face recognition. Psychological Review. 1991;98:164–181. doi: 10.1037/0033-295x.98.2.164. [DOI] [PubMed] [Google Scholar]
- Namy LL, Campbell AL, Tomasello M. The changing role of iconicity in non-verbal symbol learning: A U-shaped trajectory in the acquisition of arbitrary gestures. Journal of Cognition and Development. 2004;5:37–57. [Google Scholar]
- Needham A, Barrett T, Peterman K. A pick-me-up for infants’ exploratory skills: Early simulated experiences reaching for objects using ‘sticky mittens’ enhances young infants’ object exploration skills. Infant Behavior & Development. 2002;25:279–295. [Google Scholar]
- Niedenthal PM. Embodying emotion. Science. 2007;316:1002–1005. doi: 10.1126/science.1136930. [DOI] [PubMed] [Google Scholar]
- Perone S, Madole KL, Ross-Sheehy S, Carey M, Oakes LM. The relation between infants’ activity with objects and attention to object appearance. Developmental Psychology. 2008;44:1242–1248. doi: 10.1037/0012-1649.44.5.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piaget J. The origins of intelligence in children. Oxford, England: International Universities Press; 1952. [Google Scholar]
- Rakison DH, Woodward AL. New perspectives on the effects of action on perceptual and cognitive development. Developmental Psychology. 2008;44:1209–1213. doi: 10.1037/a0012999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochat P, Goubet N. Development of sitting and reaching in 5-to 6-month-old infants. Infant Behavior and Development. 1995;18:53–68. [Google Scholar]
- Smith ER, Semin GR. Socially situated cognition: Cognition in its social context. Advances in Experimental Social Psychology. 2004;36:53–117. [Google Scholar]
- Sommerville JA, Hildebrand E, Crane CC. Experience matters: The impact of doing versus watching on infants' subsequent perception of tool use events. Developmental Psychology. 2008;44:1249–1256. doi: 10.1037/a0012296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommerville JA, Woodward AL, Needham AN. Action experience alters 3-month-old infants’ perception of other’s actions. Cognition. 2005;96:1–11. doi: 10.1016/j.cognition.2004.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soska KC, Adolph KE, Johnson SP. Systems in development: Motor skill acquisition facilitates three-dimensional object completion. Developmental Psychology. 2010;46:129–138. doi: 10.1037/a0014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer JP, Vereijken B, Diedrich FJ, Thelen E. Posture and the emergence of manual skills. Developmental Science. 2000;3:216–233. [Google Scholar]
- Thelen E. Coupling perception and action in the development of skill: a dynamic approach. In: Bloch H, Bertenthal B, editors. Sensory-motor organizations and development in infancy and early childhood. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1990. pp. 39–56. [Google Scholar]
- Thelen E, Fisher DM. Newborn stepping: An explanation for a “disappearing” reflex. Developmental Psychology. 1982;18:760–775. [Google Scholar]
- Uchiyama I, Anderson DI, Campos JJ, Witherington D, Frankel CB, Lejeune L, Barbu-Ruth M. Locomotor experience affects self and emotion. Developmental Psychology. 2008;44:1225–1231. doi: 10.1037/a0013224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization Multicentre Growth Reference Study Group. WHO motor development study: Windows of achievement for six gross motor development milestones. Acta Paediatrica Supplement. 2006;450:86–95. doi: 10.1111/j.1651-2227.2006.tb02379.x. [DOI] [PubMed] [Google Scholar]