Abstract
Repetitive and stereotyped movements (RSMs) in infancy are associated with later diagnoses of autism spectrum disorder (ASD), yet this relationship has not been fully explored in high-risk populations. The current study investigated how RSMs involving object and body use are related to diagnostic outcomes in infant siblings of children with ASD (Sibs-ASD) and typically developing children (Sibs-TD). The rate and number of different types of RSMs were measured at an average of 15 months with follow-up diagnostic evaluations approximately 18 months later. While Sibs-ASD displayed higher rates of RSMs relative to Sibs-TD, rates did not differ according to diagnostic outcome in Sibs-ASD. However preliminary evidence suggests that qualitative differences in RSM type warrant further investigation as early diagnostic markers.
Keywords: Autism spectrum disorders (ASD), Repetitive and stereotyped movements, Infant siblings of children with ASD, Risk marker, Diagnostic outcome
Introduction
Along with impairments in social and communicative functioning, restricted interests and repetitive behaviors are defining features of autism spectrum disorders (ASD) (APA 2000). Yet there is a striking lack of research focused on this symptom domain when compared to the social and communication impairments associated with ASD. Although previous research suggests that repetitive and stereotyped movements (RSMs) are relatively common in young children with ASD (Richler et al. 2007) and can be observed over the course of a relatively brief behavioral sample (Ozonoff et al. 2008), the relation between different types of RSMs and later ASD outcomes in high-risk infant sibling samples has received relatively little attention.
Retrospective studies have compared the early motor behaviors of children with and without ASD by analyzing home videotapes (Baranek 1999; Osterling et al. 2002; Watson et al. 2007; Werner and Dawson 2005) and obtaining parental reports of behavior (Cox et al. 1999; Watson et al. 2007; Werner et al. 2005; Werner et al. 2000). While several studies have found more frequent RSMs in infants later diagnosed with ASD relative to those with a developmental delay (DD) or typical development (TD) (Cox et al. 1999; Osterling et al. 2002), others have failed to find differences between the ASD and DD infants (Baranek 1999; Watson et al. 2007; Werner et al. 2005) or even between the ASD and TD infants (Werner et al. 2000).
Prospective studies of infants at elevated risk for ASD, using varying methods to identify their samples, have also obtained mixed results regarding the association between RSMs in infancy and a later ASD diagnosis. Wetherby and colleagues used a population-based parent-report screening tool, the Communication and Symbolic Behavior Scales (CSBS) Infant-Toddler Checklist (Wetherby and Prizant 2002) to identify children under 24 months at risk for communication disorders. Children scoring in the lower 10th percentile received subsequent face-to-face evaluations using the CSBS Behavior Sample (Wetherby and Prizant 2002; Wetherby et al. 2002). RSMs were then coded from the Behavior Sample when children were 18–24 months old using the Repetitive and Stereotyped Movement Scales (RSMS) (Wetherby and Morgan 2007), which provides specific coding definitions for RSMs involving body actions (e.g., flapping, rubbing, posturing) and RSMs involving objects (e.g., spinning, rolling, collecting). Watt et al. (2008) found that the rate and duration of RSMs with body and RSMs with objects differentiated children diagnosed with ASD from those identified as DD or TD at three to four years of age. Using a simplified and revised version of the RSMS to code the rate and inventory (i.e., number of different types) of RSMs, Morgan et al. (2008) found that the inventory (but not the rate) of RSMs with body, and the rate and inventory of RSMs with objects, differentiated those diagnosed with ASD from those identified as DD or TD at three to four years of age. These results together suggest that some early RSMs may be associated with later diagnostic outcomes of ASD.
The association between RSMs and ASD has also been studied prospectively by comparing later-born siblings of children with ASD (Sibs-ASD) to typically developing infants or to later-born infant siblings of typically developing children (Sibs-TD). Compared to Sibs-TD, Sibs-ASD are known to be at increased risk for developing ASD or behaviors associated with ASD (Folstein and Piven 1991; Veenstra-VanderWeele et al. 2004; Zwaigenbaum et al. 2007); in fact, a recent large-scale study suggests that the recurrence risk for an ASD diagnosis in later-born siblings is 18.7 % (Ozonoff et al. 2011). Accordingly, there has been great interest in the longitudinal study of Sibs-ASD because they offer a window into the earliest prodromal behavioral and neurocognitive features associated with a later ASD diagnosis. In addition, even the Sibs-ASD not later diagnosed with ASD may demonstrate subtle impairments related to ASD symptomatology or more general developmental delay (Folstein and Rutter 1988; Piven et al. 1997; Pickles et al. 2000). Longitudinal studies of high-risk siblings thus have the potential to identify early risk markers for ASD as well as developmental patterns characterizing those siblings with non-ASD outcomes.
In contrast to the population screening research described above, findings from studies of Sibs-ASD are less consistent regarding the extent to which RSMs are present in infants with and without later ASD diagnoses. Ozonoff et al. (2008) found significantly higher rates of repetitive and stereotyped object use in 12-month-old infants later diagnosed with ASD (8 out of 9 of whom were Sibs-ASD), relative to infants whose outcomes were other delays or typical development. However, they did not explicitly compare these behaviors between the Sibs-ASD and Sibs-TD groups. A study by Loh et al. (2007) examined the frequency of occurrence for nine RSMs during videotaped administrations of the Autism Observation Scale for Infants (AOSI) (Bryson et al. 2008), comparing (a) Sibs-ASD with later diagnoses of ASD to Sibs-ASD without later diagnoses of ASD, and (b) Sibs-ASD with later diagnoses of ASD to Sibs-TD. Of the nine RSM items, Sibs-ASD who were later diagnosed with ASD showed more frequent arm waving at 12 months relative to Sibs-TD, and more frequent arm waving at 18 months relative to both Sibs-TD and Sibs-ASD not diagnosed with ASD. In addition, both subgroups of Sibs-ASD (with and without ASD outcomes) showed more “hands to ears” posturing at 18 months when compared to Sibs-TD, yet no differences were found between the two Sibs-ASD subgroups. However, interpretation of these findings is complicated by a significantly lower mean mental age in the Sibs-ASD group relative to Sibs-TD. These group differences in mental age may be important to consider, as previous research has suggested a link between mental age and both object and body RSMs (Bartak and Rutter 1976; Bishop et al. 2006; Campbell et al. 1990; Evans et al. 2004).
The relation between mental age and early RSMs was highlighted in a recent study of repetitive play in Sibs-ASD by Christensen et al. (2010), who found that controlling for mental age considerably influenced the main findings of the study. During an unstructured play assessment, differences in non-functional repetitive play were examined in 18-month-old Sibs-ASD and TD children (comprising both first-born TD children and Sibs-TD). Three Sibs-ASD outcome groups were identified following a diagnostic assessment at 36 months: ASD, Other Delays, and No Delays. Results revealed that Sibs-ASD in all three outcome groups demonstrated significantly more non-functional repetitive play than did the TD group. However, after controlling for group differences in verbal mental age at 18 months, the only group differences in repetitive play that remained were between the No Delays Sibs-ASD group and the TD group (i.e., only the subgroup of Sibs-ASD with no delays had more repetitive play than the TD group). These results point not only to the importance of considering developmental level in interpreting group differences in RSMs, but also to the intriguing possibility that increased RSMs with objects in infancy may be associated with better outcomes (i.e., no signs of developmental delay in toddlerhood) for some subgroups of Sibs-ASD. However, the extent to which these findings would generalize to RSMs with body, to nonverbal measures of mental age, or to other samples, remains to be seen. In particular, due to previous evidence for a negative relationship between mental age and RSMs with body (Bartak and Rutter 1976; Bishop et al. 2006; Campbell et al. 1990), additional research examining the relation of RSMs with body and mental age in Sibs-ASD samples appears warranted.
The purpose of the present study was to provide further clarification regarding the presence and potential diagnostic implications of body and object RSMs in infant siblings of children with ASD. Our primary objective was to determine the extent to which RSMs are elevated in Sibs-ASD with and without later diagnoses of ASD, relative to Sibs-TD, after matching on mental age. The answer to this question ultimately has important implications not only for early identification of ASD, but also for understanding the developmental trajectories of high-risk siblings with and without ASD outcomes. We were also interested in shedding some light on the inconsistent results that have been obtained between prospective population-based studies and infant sibling studies. Toward this end, we utilized the same observational coding system employed by previous population-based studies (i.e., the RSMS; Morgan et al. 2008; Wetherby and Morgan 2007) with an infant sibling sample.
Method
Participant Characteristics
This study included 20 Sibs-ASD and 20 Sibs-TD who were drawn from a database of participants who had previously participated in longitudinal research comparing high-risk and low-risk infant siblings. Informed consent was obtained from all parents. Exclusionary criteria for the Sibs-ASD and Sibs-TD groups were severe sensory or motor impairments and identified genetic or metabolic disorders. Proband diagnoses for the Sibs-ASD group were verified using the Autism Diagnostic Observation Schedule (ADOS) (Lord et al. 2000), the Autism Diagnostic Interview-Revised (ADI-R) (Lord et al. 1994), and DSM-informed clinical judgment from a licensed psychologist. An additional exclusionary criterion for Sibs-TD was any history of ASD in first-degree relatives.
A multi-step process was employed to identify participants eligible for the current study. First, to be considered for inclusion, all children were required to have: (a) a videotaped administration of the Screening Tool for Autism in Two-Year-Olds (STAT) (Stone et al. 2000; Stone et al. 2004; Stone et al. 2008) between the ages of 12 and 24 months (Time 1); (b) scores from the Mullen Scales of Early Learning (MSEL) (Mullen 1995) at Time 1; and (c) a follow-up diagnostic assessment approximately one year later (Time 2) from a licensed psychologist that included the ADOS, the ADI-R and the MSEL. These inclusion requirements yielded a possible sample of 48 Sibs-ASD (3 with an outcome diagnosis of Autistic Disorder, 5 with PDD-NOS, and 40 with no ASD diagnosis) and 30 Sibs-TD (none with an outcome diagnosis of ASD). Second, Sibs-ASD without ASD outcomes (Sibs-ASD/−) were matched to the eight Sibs-ASD with ASD outcomes (Sibs-ASD/+) on Time 1 chronological age (CA) and Time 1 mental age (MA) (within 3 months). Twelve Sibs-ASD/− meeting these criteria were included, yielding a Sibs-ASD group of 20. Third, the Sibs-TD sample was matched on Time 1 CA and MA to the Sibs-ASD group. The only information available to research staff during the matching of Sibs-ASD/+ to Sibs-ASD/− was ID number, diagnostic outcome, Time 1 CA, and Time 1 MA. When matching Sibs-ASD to Sibs-TD, the only information available was ID number, risk group (Sibs-ASD vs. Sibs-TD), Time 1 CA, and Time 1 MA.
Participant characteristics are reported in Table 1. The Sibs-ASD probands had diagnoses of Autistic Disorder (n = 15), PDD-NOS (n = 4), and Asperger’s Disorder (n = 1). The Sibs-ASD group was 65 % male and 90 % Caucasian, and the Sibs-TD group was 70 % male and 95 % Caucasian. At Time 1, no group differences were found between the Sibs-ASD and Sibs-TD for CA, MA, gender, or gestational age (ps >.07). However, significant group differences were found for maternal educational level, χ2 = 10.06, p = .02, with higher levels in the Sibs-TD group. In the Sibs-ASD group, 5 % of mothers had high school degrees, 30 % had partial college attendance, 60 % had college degrees, and 5 % had graduate degrees. In the Sibs-TD group, 5 % of mothers had partial college attendance, 55 % had college degrees, and 40 % had graduate degrees.
Table 1.
Sample characteristics
| Sibs-TD (n = 20) | Sibs-ASD (n = 20) | Sibs-ASD/ + (n = 8) | Sibs-ASD/ − (n = 12) | |
|---|---|---|---|---|
| Gestational age (weeks) | ||||
| Mean (SD) | 39.06 (0.97) | 38.21 (1.68) | 38.38 (1.83) | 38.09 (1.64) |
| Range | 37–40 | 35–41 | 35–41 | 36–40 |
| Time 1 CA (month) | ||||
| Mean (SD) | 14.70 (2.41) | 15.4 (3.50) | 15.00 (3.85) | 15.67 (3.39) |
| Range | 12–20 | 12–23 | 12–23 | 12–23 |
| Time 1 MA (month) | ||||
| Mean (SD) | 15.40 (2.16) | 14.76 (3.12) | 14.06 (3.70) | 15.23 (2.73) |
| Range | 11.50–20.25 | 10.50–22.25 | 10.5–22.25 | 11.5–19.75 |
| Time 2 CA (month) | ||||
| Mean (SD) | 32.85 (2.74) | 32.95 (3.38) | 32.25 (2.87) | 33.42 (3.73) |
| Range | 29–38 | 27–42 | 28–37 | 27–42 |
| Time 2 MA (month)* | ||||
| Mean (SD) | 35.18 (5.80) | 29.26 (7.44) | 23.97 (7.59) | 32.79 (4.99) |
| Range | 28.3–44 | 12.5–26.8 | 12.5–35.5 | 29.3–46.8 |
| Months between T1 and T2 | ||||
| Mean (SD) | 18.15 (1.14) | 17.55 (3.50) | 17.25 (3.49) | 17.75 (3.65) |
| Range | 16–21 | 11–25 | 12–22 | 11–25 |
Significant differences were detected in comparisons between the Sibs-ASD and Sibs-TD, between Sibs-ASD/+ and Sibs-ASD/−, and between Sibs-ASD/+ and Sibs-TD
Italic values indicate standard deviations
Of the 12 Sibs-ASD without ASD diagnoses at Time 2, two were identified as having a language impairment, two were identified as having a general developmental delay (see below for definitions), and eight were identified as typically developing. Of the 20 Sibs-TD, two were identified as having a language impairment at Time 2, and 18 were identified as typically developing. Despite the comparability of Time 1 MAs across groups, the mean MA at Time 2 was significantly lower for Sibs-ASD than Sibs-TD, t(38) = −2.81, p = .008, suggesting a slower rate of cognitive development between the ages of approximately 15 and 33 months in the Sibs-ASD group compared to the Sibs-TD group. Follow-up analyses revealed significant differences in Time 2 MA between the Sibs-ASD/+ and Sibs-ASD/− groups, t(18) = −3.15, p = .006, and between the Sibs-ASD/+ and Sibs-TD groups, t(26) = 4.23, p <.001, but not between the Sibs-ASD/− and Sibs-TD groups, t(30) = −1.19, p = .24. This pattern of results suggests that the differences in Time 2 MA between the Sibs-TD and Sibs-ASD groups were driven by the lower Time 2 MAs in the subgroup that received a later diagnosis of ASD (i.e., the Sibs-ASD/+ group).
Measures
Screening Tool for Autism in Two-Year-Olds (STAT) (Stone et al. 2000; 2004)
Repetitive behaviors were coded from videotapes of children participating in Time 1 STAT assessments. The STAT is an interactive, play-based measure that is designed to elicit a range of social-communication behaviors. It takes about 20 min to administer and consists of 12 activities in four domains: play, requesting, directing attention, and motor imitation. Strong screening properties have been reported for children from 24 to 36 months of age (Stone et al. 2004), and its utility has also been demonstrated for children as young as 14 months (Stone et al. 2008). Although originally designed as a screening measure, the STAT has also been used successfully as a structured context within which children’s social-communicative behaviors can be coded (McDuffie et al. 2005; Yoder et al. 2009).
Repetitive and Stereotyped Movement Scales (RSMS) (Morgan et al. 2008; Watt et al. 2008; Wetherby and Morgan 2007)
The RSMS was used to code RSMs from previously recorded videotapes of the Time 1 STAT assessment, using media files that employed ProcoderDV software (Tapp 2003). The RSMS manual provides precise definitions for coding four categories of RSMs with body (i.e., flapping, stiffening, rubbing, patting) and nine categories of RSMs with objects (i.e., spinning, rocking, rolling, collecting, swiping, rubbing, moving, lining, and clutching). Because the RSMS was originally developed for use with the CSBS (Wetherby et al. 2002), slight modifications were made to the original coding definitions to reflect presses and materials that are present in the STAT but not the CSBS.
These coding definitions were then used to derive four separate scores measuring rate and inventory of RSMs with objects and with body. The Object Rate and the Body Rate scores were each calculated by dividing the number of behaviors observed by the duration of the assessment. The Object Inventory and Body Inventory scores represented the number of different categories of RSMs demonstrated during the assessment. Because the number of possible RSM categories differed for the Object Inventory and Body Inventory domains, their scores had different ranges (0–9 for Object and 0–4 for Body). Therefore, the score for each domain was divided by the total possible number of categories for that domain, which resulted in a proportion score that could be compared across domains.
RSM coding was conducted independently by three trained members of the research staff who had no involvement with the previous longitudinal studies and were blind to the risk status and diagnostic outcomes of the sample. Staff were trained using STAT videos of 12–23 month old children who were not participants in the present study. For the initial training period, two members of the research staff independently coded the practice videos and discussed discrepancies, revising the coding manual as needed for clarification in the context of the STAT. Coding of the study videos began after coders demonstrated agreement on scoring, as defined by the average intraclass correlation coefficients (ICCs) above .80 for RSMs with body and object across five consecutive videos. A third coder was then trained to this same level of agreement with the other two coders, using the same standards and the same practice videos.
Mullen Scales of Early Learning (MSEL) (Mullen 1995)
Time 1 and Time 2 MSEL data were obtained from the research database described above. The MSEL is a cognitive assessment comprising four cognitive subscales: Visual Reception, Fine Motor, Receptive Language, and Expressive Language. Each subscale yields a t-score (M = 50, SD = 10) that is used to calculate an Early Learning Composite (ELC; M = 100, SD = 15). The MSEL has strong test–retest reliability, interrater reliability, and concurrent validity with other cognitive and language measures. The Time 1 age equivalents for the four subscales were averaged to derive a MA score for the purpose of matching the groups. In addition, MSEL performance at Time 2 was used to classify outcomes of language impairment (i.e., Receptive or Expressive Language subscale scores more than 1.5 standard deviations below the mean) and developmental delay (ELC more than 1.5 standard deviations below the mean with at least one nonverbal subscale more than 1.5 standard deviations below the mean).
Diagnostic Measures
Diagnostic outcomes at Time 2 were also obtained from the research database. Diagnostic determinations were made by licensed psychologists with autism expertise who were blind with respect to sibling group, based on the ADOS (Lord et al. 2000), ADI-R (Lord et al. 1994), and DSM-IV (APA 2000). The ADOS is a semi-structured observational measure designed to assess communication, social interaction, and play skills. The ADI-R is a structured caregiver interview designed to elicit detailed descriptions of early development and behaviors consistent with ASD.
Analytic Approach
Repeated measures ANOVAs were used to examine the extent to which the four RSM variables at Time 1 differed between the Sibs-TD and all Sibs-ASD (i.e., those with and without later diagnoses of ASD). A follow-up analysis was then conducted to compare Sibs-TD to the subgroup of Sibs-ASD without later ASD diagnoses (Sibs-ASD/−), to determine whether differences were influenced by the subsample of Sibs-ASD with later diagnoses of ASD (i.e., Sibs-ASD/+). Separate ANOVAs were performed for the Rate variables and Inventory variables, with the ANOVA for Inventory variables conducted as an additional exploratory analysis. Exploratory analyses were also conducted to compare the Sibs-ASD/+ to the Sibs-ASD/− on the four RSM variables. In addition, because MA and CA have been previously associated with RSMs in children with and without autism (Bartak and Rutter 1976; Bishop et al. 2006; Campbell et al. 1990; Evans et al. 2004; Thelen 1979), we examined correlations between the four RSM variables and MA and CA at Time 1 and Time 2.
Results
Preliminary Analyses
Interobserver agreement for the coded RSM variables was calculated for 35 % (14/40) of the videos using intraclass correlation coefficients (ICCs). ICCs for Body Rate and Object Rate were .76 and .95, respectively, which reflect ‘excellent’ levels of interobserver agreement (Cicchetti and Sparrow 1981; Fleiss 1986). ICCs for Body Inventory and Object Inventory were .75 and .55, respectively, which are within the range of ‘fair’ to ‘good’ levels of reliability. The relatively low interobserver reliability for Object Inventory suggests that results for this variable should be interpreted with caution.
Because maternal education differed between the Sibs-ASD and Sibs-TD groups, it was considered as a possible covariate for analyses, and correlations between maternal education and the four RSM variables were examined. The only significant correlation was found between maternal education and Object Inventory, for the Sibs-TD group only, r(20) = −.55, p = .01. However, the inclusion of maternal education as a covariate for this analysis did not change the pattern or significance of the results below, thus it was not included in any of the reported analyses.
Correlations between the four RSM variables (which were coded only for Time 1) and Time 1 MA and CA did not attain statistical significance for either the Sibs-ASD or Sibs-TD groups, all ps >.09 (rs ranged from .08 to .39). For the Sibs-TD group, no significant correlations were found between the RSM variables and Time 2 MA and CA, all ps >.06 (rs ranged from −.42 to .24). However, for the Sibs-ASD group, a significant negative correlation was found between Body Inventory and Time 2 MA, r(20) = −.64, p = .002, indicating that a greater variety of Body RSMs at Time 1 was associated with lower MA at Time 2. No other significant correlations were found between the other RSM variables and Time 2 MA or CA for Sibs-ASD, all ps >.63 (rs ranged from −.06 to .11).
RSMs Associated with Sibling Risk Status (Sibs-ASD vs. Sibs-TD)
Rate of RSMs
To examine group differences in the rate of RSMs, a 2 × 2 repeated measures ANOVA with the between-subjects factor of Sibling Group (Sibs-ASD vs. Sibs-TD) and the within-subjects factor of RSM Type (Object Rate vs. Body Rate) was conducted. Significant main effects were obtained for both Sibling Group, F(1,38) = 11.76, p = .001, , and RSM Type, F(1,38) = 36.74, p <.001, . The interaction between Sibling Group and RSM Type was not significant, F(1,38) = 1.61, p = .21, . Sibs-ASD showed significantly higher rates of RSMs than did Sibs-TD, and the overall rate of Object RSMs was higher than the rate of Body RSMs (see Table 2).
Table 2.
Rate per minute of RSMs observed
| Sibs-TD (n = 20) | Sibs-ASD (n = 20) | Sibs-ASD/ + (n = 8) | Sibs-ASD/ − (n = 12) | |
|---|---|---|---|---|
| Body | ||||
| Mean (SD) | 0.02 (.04) | 0.09 (.14) | 0.08 (.17) | 0.09 (.13) |
| Range | 0–0.14 | 0–0.48 | 0–0.48 | 0–0.38 |
| Object | ||||
| Mean (SD) | 0.22 (.16) | 0.40 (.28) | 0.38 (.27) | 0.41 (.29) |
| Range | 0.06–0.65 | 0.05–1.09 | 0.05–0.77 | 0.08–1.09 |
The ANOVA comparing Sibs-TD to Sibs-ASD revealed significant main effects for Sibling Group and RSM Type but no interaction. The ANOVA comparing Sibs-TD to Sibs-ASD/− also revealed significant main effects for Sibling Group and RSM Type but no interaction
Italic values indicate standard deviations
When these analyses were repeated with the siblings later diagnosed with ASD (Sibs-ASD/+) removed from the Sibs-ASD group, the same pattern of results was obtained. Significant main effects were found for Sibling Group, F(1,30) = 9.90, p <.001, , and RSM Type, F(1,30) = 37.17, p <.001, , with Sibs-ASD/− showing higher rates of RSMs than Sibs-TD. These findings suggest that the increased rates of RSMs in Sibs-ASD relative to Sibs-TD were not attributable to the subgroup of Sibs-ASD/+ . Again, the interaction between Sibling Group and RSM Type was not significant, F(1,30) = 1.96, p = .17, .
Inventory of RSMs
To examine group differences in Inventory of RSMs, a 2 × 2 repeated measures ANOVA was conducted with the between-subjects factor of Sibling Group (Sibs-ASD vs. Sibs-TD) and the within-subjects factor of RSM Type (Object Inventory vs. Body Inventory). Significant main effects were found for both Sibling Group, F(1,38) = 5.71, p = .02, , and RSM Type, F(1,30) = 9.90, p = .002, . Sibs-ASD demonstrated a higher inventory of RSMs categories than did Sibs-TD, and the inventory of Object RSMs was higher than the inventory of Body RSMs (see Table 3). The interaction between Sibling Group and RSM Type was not significant, F(1,38) = 0.42, p = .52, .
Table 3.
Inventory (proportion of behavioral categories) of RSMs observed
| Sibs-TD (n = 20) | Sibs-ASD (n = 20) | Sibs-ASD/ + (n = 8) | Sibs-ASD/ − (n = 12) | |
|---|---|---|---|---|
| Body | ||||
| Mean (SD) | 0.35 (0.59) | 0.75 (0.85) | 1.13 (0.83) | 0.50 (0.80) |
| Range | 0–2 | 0–2 | 0–2 | 0–2 |
| Object | ||||
| Mean (SD) | 1.85 (0.82) | 2.40 (0.75) | 2.13 (0.83) | 2.58 (0.81) |
| Range | 1–3 | 1–4 | 1–3 | 2–4 |
The ANOVA comparing Sibs-TD to Sibs-ASD revealed significant main effects for Sibling Group and RSM Type but no interaction. The ANOVA comparing Sibs-TD to Sibs-ASD/− revealed a significant main effect for RSM Type but no main effect of Sibling Group and no interaction
Italic values indicate standard deviations
When these analyses were repeated with Sibs-ASD/ + subgroup removed, the main effect for Sibling Group was no longer significant, F(1,30) = 2.53, p = .12, . The main effect for RSM Type remained significant, F(1,30) = 20.40, p <.001, , and the interaction between Sibling Group and RSM Type remained non-significant, F(1,30) = 0.50, p = .48, .
Exploratory Analyses: RSMs Associated with a Later ASD Diagnosis (Sibs-ASD/+ vs. Sibs-ASD/−)
Rate of RSMs
To examine group differences in Rate of RSMs, a 2 × 2 repeated measures ANOVA with the between-subjects factor of Outcome Diagnosis (Sibs-ASD/+ vs. Sibs-ASD/−) and the within-subjects factor of RSM Type (Object Rate vs. Body Rate) was used. A significant main effect was obtained for RSM Type, F(1,18) = 13.84, p = .002, , indicating a higher rate of Object RSMs than Body RSMs (see Table 2). However, neither the main effect for Outcome Diagnosis, F(1,18) = 0.14 p = .71, , nor the interaction between Outcome Diagnosis and RSM Type, F(1,18) = 0.40, p = .84, , were significant.
Inventory of RSMs
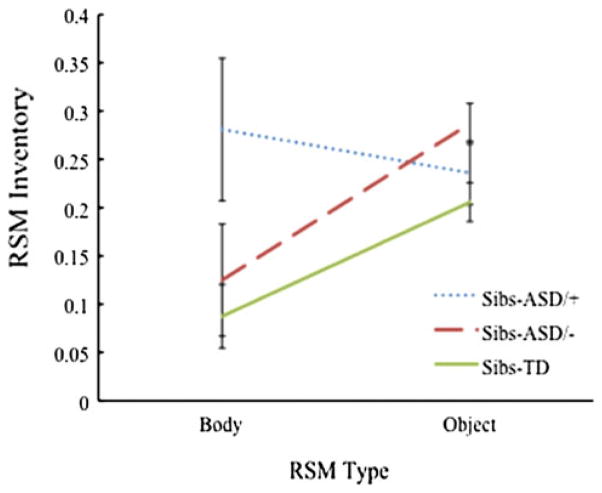
To examine group differences in Inventory of RSMs, a 2 × 2 repeated measures ANOVA with the between-subjects factor of Outcome Diagnosis (Sibs-ASD/+ vs. Sibs-ASD/−) and the within-subjects factor of RSM Type (Object Inventory vs. Body Inventory) was conducted. No main effects were found for Outcome Diagnosis, F(1,18) = 0.96, p = .34, , or RSM Type F(1,18) = 1.62, p = .22, . However, this analysis revealed a significant interaction between Outcome Diagnosis and RSM Type, F(1,18) = 5.07, p = .04, (see Fig. 1). Post hoc analyses examining the simple main effects using Bonferroni’s correction to compare estimated marginal means did not yield statistically significant differences between the Sibs-ASD/+ and Sibs-ASD/− groups for Body Inventory, F(1,18) = 2.84, p = .11, , or Object Inventory, F(1,18) = 1.85, p = .19, . However, dependent t- tests comparing RSM Type within each group revealed significant differences between Body Inventory and Object Inventory for the Sibs-ASD/− group, t(11) = −2.89, p = .02, but not the Sibs-ASD/+ group, t(7) = 0.60, p = .57.
Fig. 1.
Inventory of body and object RSMs for each group, expressed as the proportion of total possible behavior categories observed. Sibs-ASD/+ and Sibs-ASD/− refer to subgroups of Sibs-ASD who either receive a later ASD diagnosis, or do not receive a later ASD diagnosis, respectively. Sibs-TD data included as a reference point only. Error bars represent standard error of the mean
Discussion
The objective of this study was to examine RSMs in infant siblings of children with ASD relative to infant siblings of typically developing children functioning at comparable mental ages. Body and Object RSMs were measured in two ways: (1) as rate of occurrence across the STAT assessment, and (2) as the number of different categories (i.e., inventory) of RSMs observed across the assessment (expressed as the proportion of possible categories). Different patterns of results were found for the rate and inventory measures.
With respect to the rate of Object and Body RSMs, results revealed that: (1) Sibs-ASD demonstrated a significantly higher rate of RSMs than Sibs-TD, a finding that cannot be attributed to mental age differences between the groups; and (2) the group differences remained significant even when the children with later ASD diagnoses (Sibs-ASD/+) were removed from the analysis. These results suggest that elevated rates of RSMs in Sibs-ASD (in this sample, up to four times as high as those seen in Sibs-TD) are not unique to the Sibs-ASD who later develop ASD, but may represent a broader behavioral phenotype that is common in high-risk populations but does not portend a later ASD diagnosis. Exploratory analyses further revealed no significant differences in the rate of RSMs between subgroups of Sibs-ASD with and without later ASD diagnoses. These results are consistent with previous infant sibling studies of RSMs, which have also found elevated levels of RSMs in Sibs-ASD irrespective of outcome diagnoses (Christensen et al. 2010; Loh et al. 2007). Similarly, research involving older, non-affected relatives of individuals with ASD has found evidence of RSMs in the absence of an ASD diagnosis (Bolton et al. 1994; Folstein et al. 1999; Le Couteur et al. 1996; Piven et al. 1997; Spiker et al. 1994).
With respect to the inventory of Object and Body RSMs, preliminary results suggest that: (1) Sibs-ASD as a group demonstrated a significantly higher inventory of RSMs (i.e., a higher proportion of different types of RSMs) than Sibs-TD; but (2) these differences were no longer significant when the subgroup with later ASD diagnoses was excluded from the analysis. In addition, exploratory analyses comparing the Sibs-ASD with later ASD diagnoses to those without revealed a significant interaction between outcome diagnosis and RSM Type (Body vs. Object), suggesting different patterns for Object versus Body RSM inventory for the high-risk groups with different diagnostic outcomes. While these analyses were exploratory, the results are consistent with those of Morgan et al. (2008), who found that inventory of Body RSMs, but not rate, was predictive of ASD outcomes in a population-based sample.
Complicating this interpretation is the finding of a significant correlation between inventory of Body RSMs (measured at Time 1) and Time 2 mental age in Sibs-ASD with later ASD diagnoses. Although the groups were matched on mental age at Time 1, the Sibs-ASD/+ had significantly lower mental ages than the Sibs-ASD/− and Sibs-TD at Time 2, suggesting the possibility that a larger inventory of Body RSMs may be associated more generally with atypical developmental trajectories (i.e., slower cognitive growth). The small sample of children with ASD outcomes in the present study precludes definitive conclusions; however, the relation between mental age, Body RSMs, and diagnostic outcome in high risk siblings would seem to be an important area for future investigation.
In an attempt to sort out the discrepant findings between previous population-based screening studies and infant sibling studies regarding RSMs, the same measure used in previous population-based studies (i.e., the RSMS; Morgan et al. 2008; Watt et al. 2008) was employed in the present study. Using the RSMS, previous population-based studies found increased rate and inventory of RSMs with objects and increased inventory of RSMs with body in infants later diagnosed with ASD relative to DD and TD controls (Morgan et al. 2008). In contrast to these prior results, an increased rate and inventory of RSMs in the present study appeared to be more consistent with high-risk status than with diagnostic outcome, suggesting that some behaviors coded with the RSMS may not be as sensitive measures of later ASD diagnosis when used in a high risk sibling sample. That is, the early presence of more frequent RSMs with objects may have different implications for siblings of children with ASD relative to children with no known family history of ASD. For example, it may be the case that infants with a family history of ASD follow a different developmental trajectory than infants without this family history. Indeed, previous research suggests that individuals with ASD from multiplex families (i.e., having more than one child with ASD) can exhibit different behavioral and neurological symptoms compared with those from simplex families (i.e., having only one child with ASD) (Schwichtenberg et al. 2010). Thus the higher rate of RSMs in Sibs-ASD may represent a relatively common, yet transient, phenomenon associated with a familial risk for ASD, whereas a higher rate of RSMs in population-based samples may have longer-term implications for diagnosis.
Alternatively, the different pattern of results between the current study and previous population-based studies may be attributable to the different ages of the samples. The younger ages of the children in the present study (i.e., 12–23 months vs. 18–24 months) may have contributed to the different patterns of results, as previous research has described changes in features of RSMs over time (Bishop et al. 2006; Evans et al. 2004; Thelen 1979). As such, the association of early RSMs with an eventual ASD diagnosis may be stronger when measured at somewhat older ages than those examined in the present study. A final possible explanation for the inconsistent findings across studies may be the nature of the assessment context used (i.e., the STAT vs. the CSBS). For example, the CSBS involves a seated assessment, while the STAT can be conducted on the floor or at a table, allowing more freedom of movement. Although the camera follows the child throughout the assessment, it is possible that there were more instances in the STAT relative to the CSBS in which RSMs were difficult to observe (e.g., when the child turned his/her back to the camera). This factor may also have contributed to the relatively low reliability of the Object Inventory, as it may have been more difficult to reliably identify specific RSMs with objects in such instances. Further research comparing RSMs in Sibs-ASD to high risk samples identified through population screening may serve to clarify the extent to which discrepant findings are attributable to differences between samples, ages, and/or methodologies.
A clear limitation of the current study is the small sample size, particularly for the group of Sibs-ASD infants later diagnosed with ASD. Because the three groups in this study were matched on chronological and mental ages at Time 1 (when the RSM data were collected), the sample sizes of the Sibs-ASD and Sibs-TD groups were reduced, which may have limited the power of the analyses comparing these two groups. However, it was our judgment that the careful matching groups on these variables was necessary in order to draw conclusions about the results, given the potential influence of chronological and mental age on the expression of RSMs in individuals with and without ASD (e.g., Bartak and Rutter 1976; Bishop et al. 2006; Campbell et al. 1990; Evans et al. 2004; Thelen 1979). Although the sample sizes were comparable to (or larger than) previous infant sibling studies of RSMs, replication with larger samples will be critical to substantiate the present findings. In particular, interpretation of the results of comparisons between Sibs-ASD with later ASD diagnoses and Sibs-ASD without later diagnoses should be made cautiously. Another limitation of this study is that RSMs were only measured at Time 1, which prevented us from examining the full relationship between the trajectories of cognitive development (as indexed by mental age) relative to the trajectories of RSMs from Time 1 to Time 2. Finally, the lower reliability of the Object Inventory measure suggests that results of analyses on this measure should be interpreted with caution.
In contrast, strengths of this study include the procedure of matching Sibs-ASD and Sibs-TD on mental age at the time that repetitive behaviors were measured; the use of the same measure of RSMs employed previously to detect group differences in a population-based study; and the examination of both early risk group differences (i.e. sibling diagnostic status) and later diagnostic outcomes in the same sample. There are several important implications of the present results. First, the finding that rate of RSMs may be up to four times higher in Sibs-ASD (including those without a later diagnosis of ASD) relative to Sibs-TD suggests the possibility that the rate of early RSMs may not be associated with later ASD outcomes in the Sibs-ASD population. If supported by future research, this finding may be informative in understanding the early development and outcomes of children at elevated risk for ASD. Second, these results highlight the importance of measuring both rate and inventory of RSMs with body and objects, as these metrics appear to provide different types of information. Third, the results of this study point to the need for continued research on RSMs, using well defined behavioral categories that tap multiple domains, to examine their relation to other symptoms of ASD and the extent to which they are associated with later diagnostic outcomes.
References
- American Psychiatric Association (APA) Diagnostic and statistical manual of mental disorders: DSM-IV-TR. 4. Washington, DC: American Psychiatric Association; 2000. rev. ed. [Google Scholar]
- Baranek G. Autism during infancy: A retrospective video analysis of sensory-motor and social behaviors at 9–12 months of age. Journal of Autism and Developmental Disorders. 1999;29:213–224. doi: 10.1023/a:1023080005650. [DOI] [PubMed] [Google Scholar]
- Bartak L, Rutter M. Differences between mentally retarded and normally intelligent autistic children. Journal of Autism and Developmental Disorders. 1976;6(2):109–120. doi: 10.1007/BF01538054. [DOI] [PubMed] [Google Scholar]
- Bishop SL, Richler J, Lord C. Association between restricted and repetitive behaviors and nonverbal IQ in children with autism spectrum disorders. Child Neuropsychology. 2006;12(4–5):247–267. doi: 10.1080/09297040600630288. [DOI] [PubMed] [Google Scholar]
- Bolton P, MacDonald H, Pickles A, Rios P, Goode S, Crowson M, et al. A case-control family history study of autism. Journal of Child Psychology and Psychiatry. 1994;35:877–900. doi: 10.1111/j.1469-7610.1994.tb02300.x. [DOI] [PubMed] [Google Scholar]
- Bryson SE, Zwaigenbaum L, Mcdermott C, Rombough V, Brian J. The autism observation scale for infants: Scale development and reliability data. Journal of Autism and Developmental Disorders. 2008;38(4):731–738. doi: 10.1007/s10803-007-0440-y. [DOI] [PubMed] [Google Scholar]
- Campbell M, Locascio JJ, Choroco MC, Spencer EK. Stereotypies and tardive dyskinesia: Abnormal movements in autistic children. Psychopharmacology Bulletin. 1990;26(2):260–266. [PubMed] [Google Scholar]
- Christensen L, Hutman T, Rozga A, Young G, Ozonoff S, Rogers S. Play and developmental outcomes in infant siblings of children with autism. Journal of Autism and Developmental Disorders. 2010;40(8):946–957. doi: 10.1007/s10803-010-0941-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti DV, Sparrow SS. Developing criteria for establishing interrater reliability of specific items: Applications to assessment of adaptive behavior. American Journal of Mental Deficiency. 1981;86:127–137. [PubMed] [Google Scholar]
- Cox A, Klein K, Charman T, Baird G, Baron-Cohen S, Swettenham J, et al. Autism spectrum disorders at 20 and 42 months of age: Stability of clinical and ADI-R diagnosis. Journal of Child Psychology and Psychiatry. 1999;40:719–732. [PubMed] [Google Scholar]
- Evans DW, Lewis MD, Iobst E. The role of the orbitofrontal cortex in normally developing compulsive-like behaviors and obsessive-compulsive disorder. Brain and Cognition. 2004;55(1):220–234. doi: 10.1016/S0278-2626(03)00274-4. [DOI] [PubMed] [Google Scholar]
- Fleiss JL. The design and analysis of clinical experiments. New York: John Wiley; 1986. [Google Scholar]
- Folstein SE, Piven J. Etiology of autism: Genetic influences. Pediatrics. 1991;87:767–773. [PubMed] [Google Scholar]
- Folstein SE, Rutter ML. Autism: Familial aggregation and genetic implications. Journal of Autism and Developmental Disorders. 1988;18(1):3–30. doi: 10.1007/BF02211815. [DOI] [PubMed] [Google Scholar]
- Folstein SE, Santangelo SL, Gilman SE, Piven J, Landa R, Lainhart J, et al. Predictors of cognitive test patterns in autism families. Journal of Child Psychology and Psychiatry. 1999;40:1117–1128. [PubMed] [Google Scholar]
- Le Couteur A, Bailey A, Goode S, Pickles A, Robertson S, Gottesman I, et al. A broader phenotype of autism: The clinical spectrum in twins. Journal of Child Psychology and Psychiatry. 1996;37:785–801. doi: 10.1111/j.1469-7610.1996.tb01475.x. [DOI] [PubMed] [Google Scholar]
- Loh A, Soman T, Brian J, Bryson S, Roberts W, Szatmari P, et al. Stereotyped motor behaviors associated with autism in high-risk infants: A pilot videotape analysis of a sibling sample. Journal of Autism and Developmental Disorders. 2007;37:25–36. doi: 10.1007/s10803-006-0333-5. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Leventhal BL, DiLavore PC, et al. The autism diagnostic observation schedule-generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30(3):205–223. [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism diagnostic interview-revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorder. Journal of Autism and Developmental Disorders. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- McDuffie A, Yoder P, Stone W. Prelinguistic predictors of vocabulary in young children with autism spectrum disorders. Journal of Speech, Language, and Hearing Research. 2005;48(5):1080–1097. doi: 10.1044/1092-4388(2005/075). [DOI] [PubMed] [Google Scholar]
- Morgan L, Wetherby AM, Barber A. Repetitive and stereotyped movements in children with autism spectrum disorders late in the second year of life. Journal of Child Psychology and Psychiatry. 2008;49(8):826–837. doi: 10.1111/j.1469-7610.2008.01904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen E. Mullen scales of early learning. Circle Pines, MN: American Guidance Service, Inc; 1995. [Google Scholar]
- Osterling JA, Dawson G, Munson JA. Early recognition of 1-year-old infants with autism spectrum disorder versus mental retardation. Development and Psychopathology. 2002;14:239–251. doi: 10.1017/s0954579402002031. [DOI] [PubMed] [Google Scholar]
- Ozonoff S, Macari S, Young G, Goldring S, Thompson M, Rogers S. Atypical object exploration at 12 months of age is associated with autism in a prospective sample. Autism. 2008;12(5):457–472. doi: 10.1177/1362361308096402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Young GS, Carter A, Messinger D, Yirmiya N, Zwaigenbaum Z, et al. Recurrence risk for autism spectrum disorders: A baby siblings research consortium study. Pediatrics. 2011;128(3):e488–e495. doi: 10.1542/peds.2010-2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickles A, Starr E, Kazak S, Bolton P, Papanikolaou K, Bailey A, et al. Variable expression of the autism broader phenotype: Findings from extended pedigrees. Journal of Child Psychology and Psychiatry. 2000;41(4):491–502. [PubMed] [Google Scholar]
- Piven J, Palmer P, Jacobi D, Childress D, Arndt S. Broader autism phenotype: Evidence from a family history study of multiple-incidence autism families. American Journal of Psychiatry. 1997;154:185–190. doi: 10.1176/ajp.154.2.185. [DOI] [PubMed] [Google Scholar]
- Richler J, Bishop SL, Kleinke JR, Lord C. Restricted and repetitive behaviors in young children with autism spectrum disorders. Journal of Autism and Developmental Disorders. 2007;37:73–85. doi: 10.1007/s10803-006-0332-6. [DOI] [PubMed] [Google Scholar]
- Schwichtenberg AJ, Young GS, Sigman M, Hutman T, Ozonoff S. Can family affectedness inform infant sibling outcomes of autism spectrum disorders? Journal of Child Psychology and Psychiatry. 2010;51(9):1021–1030. doi: 10.1111/j.1469-7610.2010.02267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiker DK, Lotspeich L, Kraemer HC, Hallmayer J, McMahon W, Peterson PB, et al. The genetics of autism: Characteristics of affected and unaffected children from 37 multiplex families. American Journal of Medical Genetics. 1994;54:27–35. doi: 10.1002/ajmg.1320540107. [DOI] [PubMed] [Google Scholar]
- Stone WL, Coonrod EE, Ousley OY. Brief report: Screening tool for autism in two-year-olds (STAT): Development and preliminary data. Journal of Autism and Developmental Disorders. 2000;30(6):607–612. doi: 10.1023/a:1005647629002. [DOI] [PubMed] [Google Scholar]
- Stone WL, Coonrod EE, Turner LM, Pozdol SL. Psychometric properties of the STAT for early autism screening. Journal of Autism and Developmental Disorders. 2004;34:691–701. doi: 10.1007/s10803-004-5289-8. [DOI] [PubMed] [Google Scholar]
- Stone WL, McMahon CR, Henderson LM. Use of the screening tool for autism in two-year-olds (STAT) for children under 24 months: An exploratory study. Autism. 2008;12(5):557–573. doi: 10.1177/1362361308096403. [DOI] [PubMed] [Google Scholar]
- Tapp J. ProcoderDV. Nashville, TN: Vanderbilt Kennedy Center; 2003. [Google Scholar]
- Thelen E. Rhythmical stereotypies in normal human infants. Animal Behaviour. 1979;27:699–715. doi: 10.1016/0003-3472(79)90006-x. [DOI] [PubMed] [Google Scholar]
- Veenstra-VanderWeele J, Christian SL, Cook J. Autism as a paradigmatic complex genetic disorder. Annual Review of Genomics and Human Genetics. 2004;5:379–405. doi: 10.1146/annurev.genom.5.061903.180050. [DOI] [PubMed] [Google Scholar]
- Watson LR, Baranek GT, Crais ER, Reznick JS, Dykstra J, Perryman T. The first year inventory: Retrospective parent responses to a questionnaire designed to identify one-year-olds at risk for autism. Journal of Autism and Developmental Disorders. 2007;37:49–61. doi: 10.1007/s10803-006-0334-4. [DOI] [PubMed] [Google Scholar]
- Watt N, Wetherby AM, Barber A, Morgan L. Repetitive and stereotyped behaviors in children with autism spectrum disorders in the second year of life. Journal of Autism and Developmental Disorders. 2008;38(8):1518–1533. doi: 10.1007/s10803-007-0532-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner E, Dawson G. Validation of the phenomenon of autistic regression using home videotapes. Archives of General Psychiatry. 2005;62:889–895. doi: 10.1001/archpsyc.62.8.889. [DOI] [PubMed] [Google Scholar]
- Werner E, Dawson G, Munson J, Osterling J. Variation in early developmental course in autism and its relations with behavioral outcome at 3–4 years of age. Journal of Autism and Developmental Disorders. 2005;35:337–350. doi: 10.1007/s10803-005-3301-6. [DOI] [PubMed] [Google Scholar]
- Werner E, Osterling J, Dawson G, Dino N. Brief report: Recognition of autism spectrum disorders before one year of age—a retrospective study based on home videotapes. Journal of Autism and Developmental Disorders. 2000;30(2):157–162. doi: 10.1023/a:1005463707029. [DOI] [PubMed] [Google Scholar]
- Wetherby AM, Allen L, Cleary J, Kublin K, Goldstein H. Validity and reliability of the communication and symbolic behavior scales developmental profile with very young children. Journal of Speech, Linguae, and Hearing Research. 2002;45:1202–1218. doi: 10.1044/1092-4388(2002/097). [DOI] [PubMed] [Google Scholar]
- Wetherby AM, Morgan L. Repetitive and stereotyped movement scales: Companion to the CSBS. Tallahassee, FL: Florida State University; 2007. [Google Scholar]
- Wetherby A, Prizant B. Communication and symbolic behavior scales developmental profile—first normed edition. Baltimore, MD: Paul H. Brookes; 2002. [Google Scholar]
- Yoder P, Stone W, Walden T, Malesa E. Predicting social impairment and ASD diagnosis in younger siblings of children with autism spectrum disorder. Journal of Autism and Developmental Disorders. 2009;39(10):1381–1391. doi: 10.1007/s10803-009-0753-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwaigenbaum L, Thurm A, Stone W, Baranek G, Bryson S, Iverson J, et al. Studying the emergence of autism spectrum disorders in high-risk infants: Methodological and practical issues. Journal of Autism and Developmental Disorders. 2007;37(3):466–480. doi: 10.1007/s10803-006-0179-x. [DOI] [PubMed] [Google Scholar]