Abstract
Breast cancer tends to arise in older women who are also burdened with comorbidities such as cardiovascular disease (CVD). Increasing numbers of breast cancer survivors and an aging population warrant a better understanding of CVD management and adherence to preventive therapies. We estimated adherence to statins and therapeutic goal lipid values during the year prior to breast cancer diagnosis or baseline, treatment period, and in subsequent years of clinical management among breast cancer survivors.
We sampled women from an existing cohort of 4,221 women diagnosed with incident early stage (I,II) invasive breast cancer from 1990–2008 and enrolled in a large integrated group practice health plan. Among prevalent statin users (N = 1,393) medication adherence and persistence were measured using medication possession ratio (MPR), % adherent (MPR < 0.80) and discontinuation rates (DR). Laboratory data on LDL and HDL were obtained for the coinciding periods.
Mean MPR for statin use (0.78 vs. 0.68; P < 0.001) and proportion adherent (67.0% vs. 51.9%; P < 0.001) declined from baseline to the treatment period. Mean LDL (143 mg/dL baseline vs. 150 mg/dL treatment period; P < 0.001) and proportion not at LDL goal (60.1% vs. 70.8%; P < 0.001) coincided with decreases in adherence. During treatment, non-adherent statin users had the highest mean LDL (160.4 mg/dL) and proportion not at goal LDL (91.8%) overall. Adherence did not return to baseline in subsequent years following treatment although LDL levels did. HDL did not differ by periods of interest or adherence levels.
Adherence to statins in this population was poor, particularly in the treatment period, and lagged in returning to baseline. Understanding the influence of life events such as cancer diagnosis and treatment on management of comorbidities and adherence to preventive therapies are important to the growing population of breast cancer survivors.
Keywords: Breast neoplasm, Medication adherence, Comorbidity, HMG-CoA reductase inhibitors, Dyslipidemias
Introduction
In the United States (US), heart disease and cancer are the leading causes of death, respectively. However, improvements in the treatment of both conditions are leading to a larger and larger cadre of elderly individuals with “multi-morbidity.” In fact, between 2000 and 2020, the number of Americans with multi-morbidity is expected to rise from 60 million to 81 million In light of the aging population and improved survivorship.[1] Oncologists can increasingly expect to be treating patients with multiple morbidities and competing risks for death. This is particularly true for early stage cancers, such as early stage breast cancer where 5-year survival is greater than 75%. For these women, death from CVD is also an important source of morbidity and mortality. Therefore managing their chronic and preventive medications during treatment for early stage breast cancer will be increasingly important. Medications such as HMG-CoA reductase inhibitors or statins, which are demonstrated to reduce CVD events and mortality through improvements in lipid profiles, are paradigmatic examples of important primary and secondary preventive agents.[2–5] However, the effectiveness of statins is tied to adherence.[6] Adherence rates for statins in the general population are estimated at 61% to 82% in the first year of treatment and 10% to 22% three years after initiation.[7] Paucity exists in the literature regarding management of comorbidities during and after breast cancer diagnosis and treatment. Reports on medication adherence are not well indexed in the scientific literature because the number of studies is small and scattered across traditional disease boundaries and disciplines.
Increasing numbers of breast cancer survivors and an aging population at risk for CVD warrant a better understanding of CVD management and adherence to preventive therapies in this population. The purpose of this study was to estimate adherence to statins in the year prior to breast cancer diagnosis, during breast cancer treatment, and in subsequent years of clinical management among a cohort of women diagnosed with early stage invasive breast cancer. Further, we evaluated lipid levels during these same time periods.
Materials and methods
Population and setting
We sampled women from an existing cohort of 4,221 women diagnosed with incident early stage (I, II) invasive breast cancer between 1990 and 2008 at Group Health Cooperative (GH). Women without at least one year of enrollment prior and after breast cancer diagnosis (unless they died) and women with bilateral breast cancer were excluded. GH is a large integrated delivery system that provides comprehensive medical care to approximately 650,000 enrollees in Washington State and parts of Idaho. Incident breast cancers and tumor characteristics were identified through linkage to the Surveillance Epidemiology and End Results Seattle – Puget Sound registry. Women with diagnoses through August 2007 were followed to the earliest of recurrence, death, disenrollment, or end of study period (August 2010). Patient characteristics were obtained through GH automated data files[8], which include laboratory results, inpatient and outpatient diagnoses and procedures, enrollment, pharmacy dispensings, and death (internal records and Washington state death tapes).[9] Information on breast cancer treatment and outcomes (e.g., recurrence) were obtained through review of medical records. For the current study, we selected only women with ≥1 dispensings of statin medication in the year prior to breast cancer diagnosis (N=1,393).
Measures of Medication Adherence
Medication adherence and persistence were measured using medication possession ratio (MPR) and discontinuation rate (DR), respectively. Shorter days’ supply associated with repeated statin dispensings prompted calculation of measures to incorporate both information on oversupply and medication gaps, a more recently validated method using automated pharmacy/claims data.[10] Recent reviews in the scientific literature identify the MPR and DR as the most commonly used and reproducible measures of medication adherence.[11] We defined MPR as the proportion of days’ supply of medication dispensed over the number of days for which the patient had been prescribed statins, or the intended period of statin treatment. For example, in a period of 180 days, four dispensings of 30 days’ supply (120 days) of pravastatin would result in an estimated MPR of 0.67 (120/180). An MPR ≥0.8 was considered the threshold for which women were adherent to chronic statin therapy. DR was calculated using the observed number of discontinuation episodes, defined as a gap of ≥90 days between the end of a previous days’ supply and the subsequent dispensing of statin medication. DR is equal to the proportion of users with ≥1 discontinuation episode per year. Thus, for periods of one year, DR is the one-year cumulative incidence of discontinuation.
Observation Periods
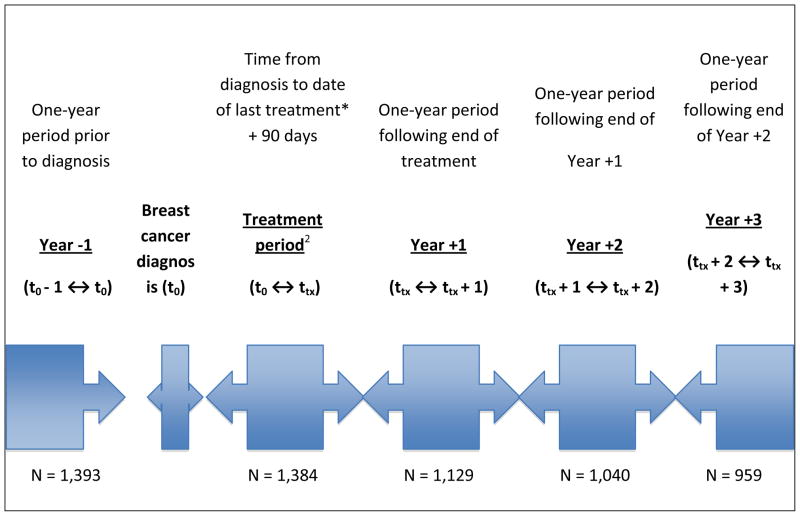
Using dispensing data from the GH automated pharmacy database, MPR and DR were calculated for the one-year period prior to breast cancer diagnosis (Year −1, t0 − 1 year ↔ t0), treatment period (t0 ↔ ttx = trx + 90 d), one-year period following end of treatment (Year +1, tx ↔ tx + 1 year), and two subsequent one-year periods following end of treatment (Figure 1). The treatment period was defined as time from diagnosis to last of surgery, radiation, or chemotherapy plus 90 days. Sensitivity analyses were conducted on the definition of the treatment period. Specifically, we examined no distinction of individual “treatment periods”, meaning observation periods were the same for all women: diagnosis date − 1 year, diagnosis + 1 year, 1–2 years post diagnosis, etc. Results from these sensitivity analyses were similar and therefore only the main results are reported. Women contributed to the four post-diagnosis observation periods only if they were using statins (i.e., no complete discontinuation) in the prior observation period.
Figure 1.
Timeline of observation periods for adherence and persistence of statin use relative to breast cancer diagnosis date and treatment.
1. ≥1 Dispensing of statin medication in the year prior to breast cancer diagnosis
2. Treatment period: SEER diagnosis date to the last incident breast cancer treatment noted in the medical chart (surgery, radiation, or chemotherapy) plus 90 days
Lipid profiles
We obtained laboratory data for statin users on low-density lipoprotein (LDL) and high-density lipoprotein (HDL) within corresponding time periods in which statin adherence was calculated. The proportion of statin users that received ≥ 1 laboratory measurement of LDL and HDL was 67.3%. During the treatment period only 50.7% of statin users had laboratory data on the lipid profile. In subsequent periods, Years +1, +2, and +3, statin users that received ≥ 1 laboratory measurement of LDL and HDL increased and returned to a proportion similar to baseline, 78.3%, 64.4%, and 65.2% respectively. The highest of LDL measurements and lowest of HDL measurements in a given period of interest were used to determine clinical control of dyslipidemia with goals assumed to be: LDL < 130 mg/dL and HDL ≥ 50 mg/dL.[12] We performed sensitivity analyses examining lowest of LDL and highest of HDL measurements when more than one laboratory value was available in a given observation period, as well as mean value of multiple measures. Results from these analyses were not appreciably different from our first approach. On average, less than 10% of those tested had more than one laboratory measure in each period. Thus, we report on only our main analyses.
Results
Demographic and clinical characteristics
Of the 1,393 women on statins at any point during the year prior to breast cancer diagnosis, mean length of time to end of primary treatment (last of surgery, radiation, or chemotherapy) in statin users was 135.1 days (SD, 97.7) (Table 1). Among these women, median age at diagnosis was 65 years, the majority presented with AJCC Stage I tumors (67.4%) and scored zero on the Charlson Comorbidity Index[13] (CCI) (57.2%). Also, 150 (10.8%) statin users had a history of ischemic heart disease in the year prior to diagnosis. Between the year prior to diagnosis and Year +3, 434 women were censored from analysis due to discontinuation of therapy or at first of death, disenrollment, or breast cancer recurrence (Table 2). Most women removed from the analysis in subsequent years were due to discontinuation of therapy or disenrollment (n=300, 69.1%) versus recurrence (n=74, 17.1%) or death (n=60, 13.8%). Of those deaths, less than 10% were cardiovascular-related according to state death records on cause of death.
Table 1.
Characteristics of study population at breast cancer diagnosis
| Statin users1 (N = 1,393) | |
|---|---|
| n (%) | |
| Year of breast cancer diagnosis | |
|
| |
| 1990–2000 | 788 (56.6) |
| 2001–2004 | 378 (27.1) |
| 2005–2008 | 271 (16.3) |
|
| |
| Length of cancer treatment (days), time since diagnosis2 | |
|
| |
| Mean (SD) | 135 (98) |
| Interquartile range | 76 to 191 |
| Median | 113 |
|
| |
| Age (years) | |
|
| |
| Mean (SD) | 62.4 (11.0) |
| 18–39 | 12 (0.9) |
| 40–49 | 114 (8.1) |
| 50–59 | 323 (23.2) |
| 60–69 | 451 (32.4) |
| 70–79 | 377 (27.1) |
| 80+ | 116 (8.3) |
|
| |
| Menopausal status | |
|
| |
| Premenopausal | 259 (18.6) |
| Postmenopausal | 1134 (81.4) |
|
| |
| Race3 | |
|
| |
| White | 1,205 (86.8) |
| African American | 52 (3.8) |
| American Indian/Alaska Native | 46 (3.3) |
| Asian/Pacific Islander | 85 (6.1) |
| Unknown | 5 |
|
| |
| Ethnicity3 | |
|
| |
| Not Hispanic | 1313 (94.3) |
| Hispanic | 80 (5.7) |
|
| |
| Education | |
|
| |
| High school or less | 214 (30.9) |
| Some college | 252 (36.4) |
| College or post graduates | 226 (32.7) |
|
| |
| Unknown | 701 |
|
| |
| Body mass index (kg/m2) | |
|
| |
| Mean (SD) | 29.8 (6.7) |
| <18.5 | 14 (1.0) |
| 18.5–24.9 | 330 (23.8) |
| 25.0–29.9 | 447 (32.2) |
| 30.0–34.9 | 317 (22.8) |
| 35.0+ | 281 (20.2) |
| Missing | 4 |
|
| |
| Smoking status | |
|
| |
| Ever | 210 (15.1) |
| Never | 1183 (84.9) |
|
| |
| AJCC stage3 | |
|
| |
| I | 939 (67.4) |
| IIA | 317 (22.8) |
| IIB | 137 (9.9) |
|
| |
| Lymph node status3 | |
|
| |
| Negative | 1004 (72.1) |
| Positive | 252 (18.1) |
| Unknown | 137 (9.8) |
|
| |
| Comorbidities | |
|
| |
| Hypertension | 1213 (87.1) |
| Diabetes mellitus | 504 (36.1) |
| Ischemic heart disease | 150 (10.8) |
|
| |
| Charlson comorbidity index5 | |
|
| |
| 0 | 796 (57.2) |
| 1 | 315 (22.6) |
| 2+ | 169 (12.1) |
| Missing (diagnosis pre-1993) | 113 (8.1) |
|
| |
| Surgical procedure | |
|
| |
| Mastectomy ± radiation | 479 (34.4) |
| Breast conserving, radiation (+) | 777 (55.8) |
| Breast conserving, radiation (−) | 137 (9.8) |
|
| |
| Other treatments | |
|
| |
| Chemotherapy | 399 (28.6) |
| Endocrine therapy4 | 835 (59.9) |
≥ 1 Dispensing of statin medication in the year prior to breast cancer diagnosis
Last date of primary breast cancer treatments (surgery, radiation, or chemotherapy)
From SEER registry or chart when missing from SEER
Aromatase inhibitor or tamoxifen therapy
Deyo RA, Cherkin DC, Ciol MA: Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 45:613–9, 1992
Abbreviations: SD Standard deviation; AJCC American Joint Committee on Cancer
Table 2.
Medication possession ratio and persistence of statin users1 prior to and following breast cancer diagnosis and treatment
| Observation period | N | MPR2 | MPR2 ≥ 0.8 | Discontinuation episodes3 | 1 - DR4 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | IQR | n | (%) | Mean | Median | SD | IQR | n | (%) | |||
| Diagnosis − 1 year | Year −1 | 1,393 | 0.778 | (0.294) | 0.657–0.986 | 934 | 67.0% | 1.003 | 1 | (1.154) | 0–2 | 704 | 50.5% |
| Diagnosis + last treatment | Treatment period5 | 1,384 | 0.678 | (0.336) | 0.500–0.979 | 718 | 51.9% | 0.490 | 0 | (0.604) | 0–1 | 799 | 57.7% |
| Last treatment + 1 year | Year +1 | 1,129 | 0.650 | (0.346) | 0.247–0.986 | 557 | 49.3% | 1.219 | 1 | (1.250) | 0–2 | 469 | 41.5% |
| End of year 1 + 1 year | Year +2 | 1,040 | 0.634 | (0.296) | 0.247–0.986 | 368 | 35.4% | 1.200 | 1 | (1.159) | 0–2 | 414 | 39.8% |
| End of year 2 + 1 year | Year +3 | 959 | 0.686 | (0.318) | 0.329–0.986 | 482 | 50.2% | 1.176 | 1 | (1.192) | 0–2 | 394 | 41.1% |
≥1 Dispensing of statin medication in the year prior to breast cancer diagnosis
Medication possession ratio (MPR): proportion of days’ supply medication dispensed over the observed days of intended use; patients considered adherent at MPR ≥ 0.8, non-adherent at MPR < 0.8
Discontinuation episodes: gaps of ≥90 days between dispensing of subsequent filling
Discontinuation rate (DR): proportion of users with at least one discontinuation episode; 1 - DR = proportion of users with no interruptions in therapy
Treatment period: SEER diagnosis date to the latest of primary cancer treatment (surgery, radiation, or chemotherapy) plus 90 days
Mean MPR from all periods are statistically different (P < 0.05) in comparison to Year-1
Abbreviations: SD Standard deviation; IQR Interquartile range
Medication adherence and persistence
Mean MPR for statin use in the year prior to breast diagnosis (i.e., Year −1) was highest overall, 0.78 (Table 2). In Year −1 there were 934 (63.7%) statin users adherent to medication therapy (i.e., MPR ≥0.8). Mean MPR was lower in the treatment period, 0.68 (P < 0.001) compared to the prior year. Accordingly, statin users considered adherent in the treatment period (50.2%) declined. In the subsequent three years of observation, mean MPR and proportion adherent were similar to the treatment period. The lowest MPR observed was in Year +2, MPR = 0.63 with an observed proportion adherent of 35.9%, although this difference from the treatment period was not statistically significant (P = 0.55). The proportion of users that did not experience a discontinuation episode (DR <1) in Year −1 was 50.5% and greatest in the treatment period (57.7%; P < 0.001). In each of the three years following treatment, the proportion of statin users not experiencing interruptions in therapy significantly declined to lower than that of Year −1 (P < 0.001).
Lipid profiles
Mean values for LDL and HDL are reported in Table 3. Among statin users with measures of LDL during periods of interest, mean LDL (150.3 mg/dL) and proportion not at goal LDL (70.8%) was highest during the treatment period in comparison to Year −1 (143.3 mg/dL and 60.1%). Among adherent statin users (MPR ≥ 0.8), mean LDL in subsequent years of follow up was significantly lower in comparison to Year−1 except for the treatment period (P = 0.47). In non-adherent statin users (MPR < 0.8), mean LDL was higher in the treatment period (160.4 mg/dL; P < 0.001) and lower in Years +1, +2, and +3 (P < 0.001) compared to Year −1. Mean HDL and proportion not at goal HDL were similar across observation periods and between adherent and non-adherent statin users (Table 3).
Table 3.
Low-density lipoprotein (LDL) and high-density lipoprotein (HDL) measures1 of statin users2 prior to and following breast cancer diagnosis and treatment by adherence status
| All statin users | LDL (mg/dL) | High LDL4 ≥130mg/dL | HDL (mg/dL) | Low HDL4 <50mg/dL | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Observation period | N | Mean | SD | IQR | n | (%) | Mean | SD | IQR | n | (%) | |||||
| Diagnosis − 1 year | Year −1 | 938 | 143.3 | (35.2) | 116– 163 | 564 | 60.1 % | 50.7 | (10.6) | 43–59 | 426 | 45.4 % | ||||
| Diagnosis + last treatment | Treatment period5 | 701 | 150.3 | (35.6) | 122–172 | 496 | 70.8 % | 51.26 | (10.4) | 44–59 | 338 | 48.2 % | ||||
| Last treatment + 1 year | Year +1 | 884 | 143.46 | (36.7) | 114–157 | 519 | 58.7 % | 51.16 | (11.0) | 43–60 | 416 | 47.1 % | ||||
| End of year 1 +1 year | Year +2 | 670 | 139.5 | (35.5) | 114–153 | 356 | 53.1 % | 50.86 | (10.6) | 43–59 | 330 | 49.3 % | ||||
| End of year 2 +1 year | Year +3 | 625 | 141.26 | (37.1) | 110–159 | 321 | 51.4 % | 50.56 | (11.3) | 42–63 | 297 | 47.5 % | ||||
| MPR3 ≥ 0.8 | LDL (mg/dL) | High LDL4 ≥130mg/dL | HDL (mg/dL) | Low HDL4 <50mg/dL | ||||||||||||
| Observation period | N | Mean | SD | IQR | n | (%) | Mean | SD | IQR | n | (%) | |||||
| Diagnosis − 1 year | Year −1 | 326 | 123.1 | (37.6) | 95–144 | 118 | 36.3 % | 53.6 | (9.7) | 47–61 | 111 | 34.0 % | ||||
| Diagnosis + last treatment | Treatment period5 | 238 | 120.76 | (42.4) | 92–135 | 71 | 29.8 % | 53.96 | (9.0) | 47–61 | 74 | 31.1 % | ||||
| Last treatment + 1 year | Year +1 | 278 | 116.3 | (36.1) | 90–130 | 78 | 28.1% | 52.76 | (10.7) | 45–61 | 104 | 37.4 % | ||||
| End of year 1 +1 year | Year +2 | 169 | 112.9 | (37.0) | 86–127 | 42 | 24.9% | 53.76 | (11.1) | 46–64 | 68 | 40.2 % | ||||
| End of year 2 +1 year | Year +3 | 210 | 114.2 | (35.9) | 95–137 | 50 | 23.8 % | 50.6 | (11.6) | 41–62 | 78 | 37.1 % | ||||
| MPR3 < 0.8 | LDL (mg/dL) | High LDL4 ≥130mg/dL | HDL (mg/dL) | Low HDL4 <50mg/dL | ||||||||||||
| Observation period | N | Mean | SD | IQR | n | (%) | Mean | SD | IQR | n | (%) | |||||
| Diagnosis − 1 year | Year −1 | 622 | 143.7 | (40.8) | 112–169 | 446 | 71.7 % | 50.8 | (10.5) | 44–59 | 315 | 50.6 % | ||||
| Diagnosis + last treatment | Treatment period5 | 463 | 160.4 | (40.0) | 131–185 | 425 | 91.8 % | 49.96 | (10.5) | 42–58 | 264 | 57.0 % | ||||
| Last treatment + 1 year | Year +1 | 606 | 137.6 | (39.0) | 119–161 | 441 | 72.7 % | 50.16 | (10.5) | 42–58 | 299 | 49.3 % | ||||
| End of year 1 +1 year | Year +2 | 501 | 134.4 | (39.1) | 105–154 | 314 | 62.7 % | 49.66 | (10.7) | 42–58 | 252 | 50.3 % | ||||
| End of year 2 +1 year | Year +3 | 415 | 133.0 | (39.7) | 117–159 | 247 | 59.5% | 51.86 | (11.8) | 44–60 | 214 | 51.6 % | ||||
Fasting lipid profile measures, highest of LDL and lowest of HDL in the observation period
≥1 Dispensing of statin medication in the year prior to breast cancer diagnosis
Medication possession ratio (MPR): proportion of days’ supply medication dispensed over the observed days of intended use; patients considered adherent at MPR ≥ 0.8, non-adherent at MPR < 0.8
Third report of the National Cholesterol Education Program (NCEP) Expert Panel, Adult Treatment Panel III – Approach to treatment, goals and thresholds: LDL <130 mg/dL and HDL ≥50 mg/dL; Circulation 2002; 106(25): 3143-421.
Treatment period: SEER diagnosis date to the latest of primary cancer treatment (surgery, radiation, or chemotherapy) plus 90 days
Not statistically different (P > 0.05) in comparison to Year-1
Abbreviations: SD Standard deviation; IQR Interquartile range; LDL Low-density lipoprotein; HDL High-density lipoprotein
Discussion
Women with early-stage breast cancer form the majority of contemporary breast cancer survivors in the developed world.[14] Our results suggest that adherence to statin therapies as measured by MPR and DR may be sensitive to timing of breast cancer diagnosis, treatment, and recovery. Medication adherence decreased in the treatment period and lagged in returning to a baseline levels. Furthermore, rates of discontinuation observed in our cohort of women with breast cancer were consistently greater than those reported in more general populations of statin users studied in meta-analyses, rates approximately twice that of the observational studies and four times that of clinical trials.[15] Regardless of statin adherence, LDL levels were lower or returned to pre-diagnosis levels in the years following breast cancer treatment.
Adherence measured in the study period appeared to be correlated with mean LDL; higher adherence (i.e., higher MPR) and persistence (lower DR) were observed in periods where mean LDL was lowest; lower adherence and persistence were observed in periods where mean LDL was greatest. Mean HDL levels remained relatively stable for both those adherent and non-adherent users, although effects of statins to increase catabolism of LDL are more robust versus effects on HDL.[16] Differences in mean LDL were statistically significant for non-adherent statin users between the treatment period and other years of observation, but not among adherent users. This is perhaps suggestive of maintained baseline levels of LDL among adherent statin users and not in non-adherent users.
Relevant literature on medication adherence is limited to statin use in the general population and use of tamoxifen or aromatase inhibitors among breast cancer survivors. To that end, non-adherence to cardioprotective medications among those with coronary heart disease (CHD) is associated with increased risk of cardiovascular hospitalizations and mortality, 12–25% for statin non-adherence.[17,18] In a meta-analysis of CHD primary and secondary prevention studies, medication persistence among initiators and prevalent users was evaluated.[15] In primary prevention studies 21% of statin initiators discontinued treatment in randomized trials versus 41% in observational studies. Differences in our study design and populations from previously published reports and meta-analyses make direct comparison to our population’s statin adherence difficult. Specifically, our study of prevalent users over time is subject to selection biases that would affect medication adherence. We chose statin users from the pre-diagnostic year for the cohort. Thus, our analysis is intended to answer a more specific question about the clinical management experience of breast cancer survivors prior to and following diagnosis and primary treatment. Nonetheless, observed mean MPR values for women in our cohort (MPR 0.63–0.78) were in the range of those studied in the general population of statin users (MPR 0.58–0.79).[15] However, the proportion of our cohort considered non-adherent to therapy was greater than that of the general population after a sharp increase during the treatment period. Although reasons for non-adherence may differ for the general population versus women diagnosed and undergoing treatment for breast cancer, our study lends further evidence to an overall decrease in adherence to statins over time but especially during a stressful life event such as cancer.
Discrepancy in the ranges of discontinuation rates reported in clinical practice settings (31% to 73%) and clinical trials (8% to 28%) compared with that among our population (42% to 60%) may be an indication of the differences in provider support and treatment delivery mechanisms.[19] The Institute of Medicine’s report on cancer survivorship shows that a lack of clear guidelines for caring for patients with a history of cancer creates wide variation in how care is delivered.[20] Cancer survivors may be unclear which provider (oncology or primary care) is primarily responsible for follow-up care. Evidence from the 2009 Behavioral Risk Factor Surveillance System shows that 20% of all cancer survivors continue to use an oncologist or cancer specialist as their primary provider for follow-up care.[21] While discontinuation of statin therapies is clinically warranted in situations that preclude further lipid-lowering treatment, risks that may be mitigated by statin use in breast cancer survivors remain, particularly given the high CVD risk with aging and following cardiotoxic chemotherapies.[22–25] Our observation of decreased LDL among women non-adherent to statin therapies could indicate that other factors beyond the preventive potential of statins on CVD risk are present among breast cancer survivors. These may include healthy behaviors such as diet and exercise following cancer diagnosis and treatment. Additionally, adherence and persistence to statin therapy vary by indication (primary- versus secondary-prevention) and intensity of interaction with a provider for optimization of lipid-lowering treatment. Oncologists, and primary care providers, are well positioned to implement measures to improve adherence to therapies for the management of co-morbid conditions among cancer survivors.[26–29] Recent studies regarding the impact of comorbidity on both treatment decisions and mortality in breast cancer survivors underscore the importance of preventive therapies and care.[1,30] Our research findings should raise further awareness on the need for active involvement of both oncology and primary care providers in the management of comorbidities among breast cancer survivors.
Evidence suggests statins may play a role in decreasing cancer risk and recurrence of aggressive cancers.[31] Statins’ inhibition of HMG-CoA reductase prevents the conversion of HMG-CoA to mevalonate and its downstream products necessary for critical cellular functions including membrane integrity, cell signaling, protein synthesis, and cell cycle progression.[32–34] Disruptions of these processes in neoplastic cells by statins may result in control of tumor initiation, growth, and metastasis.[32–35] Two observational cohort studies have described, though stand to be further replicated and explained, decreased risk of breast cancer recurrence. Among 703 women with stages I-III breast cancer, statin users had a significantly decreased risk of breast cancer recurrence (HR = 0.40, 95% CI 0.24–0.67, P < 0.001).[36] In a Danish prospective cohort of women with stages I–III breast cancer, simvastatin use was associated with 10 fewer breast cancer recurrences per 100 women (10-year risk difference: 0.10, 95% CI 0.08–0.11).[37] As such, adherence to statins may be even more important for women at risk for recurrence and in need of the potential chemopreventive effects of statins.
Strengths and limitations
Several limitations to our study should be noted. Although use of automated pharmacy records provides objective and reproducible adherence measures, this methodology has its drawbacks. First, a dispensed medication does not guarantee patients ingested medication as directed, potentially overestimating adherence. Similarly, patients may receive medications from other sources not captured by health plan data and therefore discontinuation rates may be overestimated. This, however, is unlikely given that approximately 97% of GH enrollees fill their medications at GH-owned or contracted pharmacies.[8,38,39] Issues with regard to external validity make our study’s generalizability circumspect. GH enrollees represent a predominantly White, insured American population in the United States, thereby excluding a proportion of breast cancer survivors. This is noteworthy given that minority and low-income women may be at increased risk of cardiovascular outcomes potentially preventable by statin therapies and have a different likelihood of adherence and discontinuation. Although we can make broad comparisons to statin adherence in the general population from other studies, statin adherence among women in our population without a history of breast cancer would be informative but beyond the scope of this analysis.
Data from the parent study only went back one year prior to breast cancer diagnosis, limiting our ability to evaluate the influence of duration of statin use prior to breast cancer diagnosis on adherence post diagnosis. Analysis of prevalent statin users, women followed from Year −1 versus incident “new users”, may introduce bias. Prevalent users of statins compared with non-users are subject to selection bias because prevalent users have, by definition, survived under treatment. If statin treatment decreased the risk of CVD (or breast cancer) mortality, the group of prevalent users will be progressively enriched with susceptible patients as compared with nonusers or never users. For example, our statin users included 150 (10.7%) women with history of ischemic heart disease in the year prior to diagnosis. It is reasonable to assume that statin use and adherence behavior may be different among these women with a major clinical indication for prevention of CVD mortality, as well as likelihood of being censored from analysis due to CVD death. The mix of incident and prevalent users stands to dilute differences in adherence behavior between those recently starting statin therapy and those on long-term treatment.
However, our study adds to the current literature and has many strengths including a large population-based cohort of women with 1) automated pharmacy records considered to be valid, complete, and used in other epidemiologic studies; 2) long term follow-up; 3) complete capture of cancer and recurrences through the SEER registry and medical charts; 4) cancer and treatment characteristics; and 5) information on diagnoses, laboratory values, and demographics. Also, our approach uses multiple measures of adherence such that comparison to future studies and potential interventions to improve outcomes modifiable by drug therapy are possible.[40] Further studies allowing for comparison of medication adherence to statins in both incident and prevalent users among breast cancer survivors and the general population will be important for understanding any differences in the reasons for non-adherence and the role oncologists may have in managing comorbidities among cancer survivors.
Conclusion
This study provides insight on the role that incident breast cancer diagnosis and its associated characteristics, including treatment and recovery, have on the management of comorbidities. Our study is one of the first of its kind to evaluate adherence to medications used to treat comorbidities among breast cancer survivors immediately antecedent to and following cancer diagnosis and treatment. Given the ability to potentially modify non-adherence, efforts to further establish valid measures of medication adherence, understand multi-morbidity and preventive therapies following cancer diagnosis, and improve self-management are important to the growing population of breast cancer survivors. It will also be important for future studies to understand why LDL levels improve post breast cancer treatment regardless of adherence to statins.
Acknowledgments
SUPPORT: The National Cancer Institute (R01CA120562 to D.M.B) provided funding for this study at the Group Health Research Institute. The National Institutes of Health Cancer Prevention Training Grant in Nutrition, Exercise, and Genetics (R25CA094880) at the University of Washington and Fred Hutchinson Cancer Research Center supported G.S.C.
The National Cancer Institute (R01CA120562) provides funding for this study at the Group Health Research Institute. The National Institutes of Health Cancer Prevention Training Grant in Nutrition, Exercise, and Genetics (R25CA094880) at the University of Washington and Fred Hutchinson Cancer Research Center supported G.S.C.
Footnotes
Authorship contribution: G.S.C. and D.M.B. designed the research plan; G.S.C. assembled and analyzed the data and made tables and figures; all authors contributed substantially to interpretation of the analysis, writing and editing the manuscript, and providing final approval of the manuscript and its content.
The authors have no other potential conflicts of interest, funding, or financial disclosures to report.
References
- 1.Ritchie CS, Kvale E, Fisch MJ. Multimorbidity: an issue of growing importance for oncologists. J Oncol Pract. 2011;7(6):371–374. doi: 10.1200/JOP.2011.000460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heart Protection Study Collaborative G. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360(9326):7–22. doi: 10.1016/S0140-6736(02)09327-3. [DOI] [PubMed] [Google Scholar]
- 3.Marchioli R, Marfisi RM, Carinci F, Tognoni G. Meta-analysis, clinical trials, and transferability of research results into practice. The case of cholesterol-lowering interventions in the secondary prevention of coronary heart disease. Arch Intern Med. 1996;156 (11):1158–1172. [PubMed] [Google Scholar]
- 4.Pekkanen J, Linn S, Heiss G, Suchindran CM, Leon A, Rifkind BM, Tyroler HA. Ten-year mortality from cardiovascular disease in relation to cholesterol level among men with and without preexisting cardiovascular disease. N Engl J Med. 1990;322(24):1700–1707. doi: 10.1056/NEJM199006143222403. [DOI] [PubMed] [Google Scholar]
- 5.Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S) Lancet. 1994;344 (8934):1383–1389. [PubMed] [Google Scholar]
- 6.Benner JS, Pollack MF, Smith TW, Bullano MF, Willey VJ, Williams SA. Association between short-term effectiveness of statins and long-term adherence to lipid-lowering therapy. American journal of health-system pharmacy: AJHP: official journal of the American Society of Health-System Pharmacists. 2005;62(14):1468–1475. doi: 10.2146/ajhp040419. [DOI] [PubMed] [Google Scholar]
- 7.Vinker S, Shani M, Baevsky T, Elhayany A. Adherence with statins over 8 years in a usual care setting. The American journal of managed care. 2008;14 (6):388–392. [PubMed] [Google Scholar]
- 8.Saunders KW, Davis RL, Stergachis A. Group Health Cooperative. In: Strom BL, editor. Pharmacoepidemiology. 4. J. Wiley, Chichester; Hoboken, NJ: 2005. pp. 223–239. [Google Scholar]
- 9.Washington State Department of Health, Center for Health Statistics. [Accessed June 15, 2011];Death Data. http://www.doh.wa.gov/ehsphl/CHS/chs-data/death/deatmain.htm.
- 10.Bryson CL, Au DH, Young B, McDonell MB, Fihn SD. A refill adherence algorithm for multiple short intervals to estimate refill compliance (ReComp) Med Care. 2007;45(6):497–504. doi: 10.1097/MLR.0b013e3180329368. [DOI] [PubMed] [Google Scholar]
- 11.Andrade SE, Kahler KH, Frech F, Chan KA. Methods for evaluation of medication adherence and persistence using automated databases. Pharmacoepidemiology and drug safety. 2006;15(8):565–574. doi: 10.1002/pds.1230. discussion 575–567. [DOI] [PubMed] [Google Scholar]
- 12.National Cholesterol Education Program Expert Panel on Detection E, Treatment of High Blood Cholesterol in A. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106 (25):3143–3421. [PubMed] [Google Scholar]
- 13.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45 (6):613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 14.Smigal C, Jemal A, Ward E, Cokkinides V, Smith R, Howe HL, Thun M. Trends in breast cancer by race and ethnicity: update 2006. CA Cancer J Clin. 2006;56 (3):168–183. doi: 10.3322/canjclin.56.3.168. [DOI] [PubMed] [Google Scholar]
- 15.Danaei G, Tavakkoli M, Hernan MA. Bias in observational studies of prevalent users: lessons for comparative effectiveness research from a meta-analysis of statins. Am J Epidemiol. 2012;175(4):250–262. doi: 10.1093/aje/kwr301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature. 1990;343(6257):425–430. doi: 10.1038/343425a0. [DOI] [PubMed] [Google Scholar]
- 17.Ho PM, Magid DJ, Shetterly SM, Olson KL, Maddox TM, Peterson PN, Masoudi FA, Rumsfeld JS. Medication nonadherence is associated with a broad range of adverse outcomes in patients with coronary artery disease. Am Heart J. 2008;155(4):772–779. doi: 10.1016/j.ahj.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 18.Rasmussen JN, Chong A, Alter DA. Relationship between adherence to evidence-based pharmacotherapy and long-term mortality after acute myocardial infarction. JAMA. 2007;297(2):177–186. doi: 10.1001/jama.297.2.177. [DOI] [PubMed] [Google Scholar]
- 19.Murphy CC, Bartholomew LK, Carpentier MY, Bluethmann SM, Vernon SW. Adherence to adjuvant hormonal therapy among breast cancer survivors in clinical practice: a systematic review. Breast cancer research and treatment. 2012;134(2):459–478. doi: 10.1007/s10549-012-2114-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hewitt ME, Ganz P, Institute of Medicine (U.S.), American Society of Clinical Oncology. From cancer patient to cancer survivor: lost in transition. National Academies Press; Washington, D.C: 2006. [Google Scholar]
- 21.Underwood JM, Townsend JS, Stewart SL, Buchannan N, Ekwueme DU, Hawkins NA, Li J, Peaker B, Pollack LA, Richards TB, Rim SH, Rohan EA, Sabatino SA, Smith JL, Tai E, Townsend GA, White A, Fairley TL Division of Cancer P, Control NCfCDP, Health Promotion CfDC, Prevention. Surveillance of demographic characteristics and health behaviors among adult cancer survivors--Behavioral Risk Factor Surveillance System, United States, 2009. Morbidity and mortality weekly report Surveillance summaries. 2012;61 (1):1–23. [PubMed] [Google Scholar]
- 22.Von Hoff DD, Layard MW, Basa P, Davis HL, Jr, Von Hoff AL, Rozencweig M, Muggia FM. Risk factors for doxorubicin-induced congestive heart failure. Ann Intern Med. 1979;91 (5):710–717. doi: 10.7326/0003-4819-91-5-710. [DOI] [PubMed] [Google Scholar]
- 23.Smith LA, Cornelius VR, Plummer CJ, Levitt G, Verrill M, Canney P, Jones A. Cardiotoxicity of anthracycline agents for the treatment of cancer: systematic review and meta-analysis of randomised controlled trials. BMC Cancer. 2010;10:337. doi: 10.1186/1471-2407-10-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Telli ML, Hunt SA, Carlson RW, Guardino AE. Trastuzumab-related cardiotoxicity: calling into question the concept of reversibility. J Clin Oncol. 2007;25(23):3525–3533. doi: 10.1200/JCO.2007.11.0106. [DOI] [PubMed] [Google Scholar]
- 25.Telli ML, Witteles RM. Trastuzumab-related cardiac dysfunction. Journal of the National Comprehensive Cancer Network: JNCCN. 2011;9 (2):243–249. doi: 10.6004/jnccn.2011.0019. [DOI] [PubMed] [Google Scholar]
- 26.Poluzzi E, Strahinja P, Lanzoni M, Vargiu A, Silvani MC, Motola D, Gaddi A, Vaccheri A, Montanaro N. Adherence to statin therapy and patients’ cardiovascular risk: a pharmacoepidemiological study in Italy. European journal of clinical pharmacology. 2008;64(4):425–432. doi: 10.1007/s00228-007-0428-8. [DOI] [PubMed] [Google Scholar]
- 27.Brookhart MA, Patrick AR, Schneeweiss S, Avorn J, Dormuth C, Shrank W, van Wijk BL, Cadarette SM, Canning CF, Solomon DH. Physician follow-up and provider continuity are associated with long-term medication adherence: a study of the dynamics of statin use. Arch Intern Med. 2007;167(8):847–852. doi: 10.1001/archinte.167.8.847. [DOI] [PubMed] [Google Scholar]
- 28.Ellis JJ, Erickson SR, Stevenson JG, Bernstein SJ, Stiles RA, Fendrick AM. Suboptimal statin adherence and discontinuation in primary and secondary prevention populations. Journal of general internal medicine. 2004;19(6):638–645. doi: 10.1111/j.1525-1497.2004.30516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jackevicius CA, Mamdani M, Tu JV. Adherence with statin therapy in elderly patients with and without acute coronary syndromes. JAMA. 2002;288 (4):462–467. doi: 10.1001/jama.288.4.462. [DOI] [PubMed] [Google Scholar]
- 30.Berglund A, Wigertz A, Adolfsson J, Ahlgren J, Fornander T, Warnberg F, Lambe M. Impact of comorbidity on management and mortality in women diagnosed with breast cancer. Breast cancer research and treatment. 2012;135(1):281–289. doi: 10.1007/s10549-012-2176-4. [DOI] [PubMed] [Google Scholar]
- 31.Demierre MF, Higgins PD, Gruber SB, Hawk E, Lippman SM. Statins and cancer prevention. Nat Rev Cancer. 2005;5(12):930–942. doi: 10.1038/nrc1751. [DOI] [PubMed] [Google Scholar]
- 32.Wong WW, Dimitroulakos J, Minden MD, Penn LZ. HMG-CoA reductase inhibitors and the malignant cell: the statin family of drugs as triggers of tumor-specific apoptosis. Leukemia: official journal of the Leukemia Society of America, Leukemia Research Fund, UK. 2002;16(4):508–519. doi: 10.1038/sj.leu.2402476. [DOI] [PubMed] [Google Scholar]
- 33.Mannello F, Tonti GA. Statins and breast cancer: may matrix metalloproteinase be the missing link. Cancer Invest. 2009;27(4):466–470. doi: 10.1080/07357900802491444. [DOI] [PubMed] [Google Scholar]
- 34.Chan KK, Oza AM, Siu LL. The statins as anticancer agents. Clin Cancer Res. 2003;9 (1):10–19. [PubMed] [Google Scholar]
- 35.Denoyelle C, Vasse M, Korner M, Mishal Z, Ganne F, Vannier JP, Soria J, Soria C. Cerivastatin, an inhibitor of HMG-CoA reductase, inhibits the signaling pathways involved in the invasiveness and metastatic properties of highly invasive breast cancer cell lines: an in vitro study. Carcinogenesis. 2001;22 (8):1139–1148. doi: 10.1093/carcin/22.8.1139. [DOI] [PubMed] [Google Scholar]
- 36.Chae YK, Valsecchi ME, Kim J, Bianchi AL, Khemasuwan D, Desai A, Tester W. Reduced risk of breast cancer recurrence in patients using ACE inhibitors, ARBs, and/or statins. Cancer Invest. 2011;29(9):585–593. doi: 10.3109/07357907.2011.616252. [DOI] [PubMed] [Google Scholar]
- 37.Ahern TP, Pedersen L, Tarp M, Cronin-Fenton DP, Garne JP, Silliman RA, Sorensen HT, Lash TL. Statin prescriptions and breast cancer recurrence risk: a Danish nationwide prospective cohort study. J Natl Cancer Inst. 2011;103(19):1461–1468. doi: 10.1093/jnci/djr291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Buist DS, LaCroix AZ, Brenneman SK, Abbott T., 3rd A population-based osteoporosis screening program: who does not participate, and what are the consequences? J Am Geriatr Soc. 2004;52(7):1130–1137. doi: 10.1111/j.1532-5415.2004.52311.x. [DOI] [PubMed] [Google Scholar]
- 39.Boudreau DM, Doescher MP, Jackson JE, Fishman PA, Saver BG. Impact of healthcare delivery system on where HMO-enrolled seniors purchase medications. Ann Pharmacother. 2004;38(7–8):1317–1318. doi: 10.1345/aph.1D569. [DOI] [PubMed] [Google Scholar]
- 40.Ho PM, Bryson CL, Rumsfeld JS. Medication adherence: its importance in cardiovascular outcomes. Circulation. 2009;119(23):3028–3035. doi: 10.1161/CIRCULATIONAHA.108.768986. [DOI] [PubMed] [Google Scholar]